Abstract
Platelet-rich fibrin (PRF) has been used in regenerative medicine and dentistry. Recently, its use has been advocated for regenerative periodontics and wound healing. The randomized control trials have assessed the regenerative efficacy of the PRF for restoring intrabony periodontal defects. The objectives are to critically analyze and appraise the currently available literature, focusing on the use of PRF in regenerating periodontal bone defects. An electronic search was conducted (PubMed/MEDLINE, Google Scholar, ISI-WOS). Various combinations of following keywords were used: ‘platelet-rich fibrin’, ‘intrabony’, ‘periodontal’, ‘bone defect’ and ‘guided tissue regeneration’. A secondary search was conducted by analyzing the reference lists of the articles obtained in initial search. The final search resulted in 13 randomized controlled trials being included. In majority of studies, PRF resulted in better clinical/radiographic outcomes than open flap debridement and augmented therapeutic effects of bone grafts. The combination of bovine bone substitutes and PRF resulted in better performance compared to alone. Similarly better outcomes were observed while using PRF in combination with nanohydroxyapatite, metformin and demineralized freeze-dried bone allograft. It can be concluded that PRF produces better outcomes than open flap debridement alone and augments the regenerative effects of bone substitutes.
Keywords: Fibrin, Intrabony defects, Tissue engineering, Regeneration, Periodontal
Introduction
Platelet-rich plasma (PRP) is autologous plasma which has been enriched with platelets and leukocytes in addition to jellifying agents, growth factors, cytokines, bovine thrombin, and anticoagulants [1, 2]. PRP has been employed in regenerative medicine to promote wound healing and tissue regeneration [3, 4]. However, PRP has some reported limitations [5] for example, growth factors are released for only a very short period of time. In addition, there are concerns such as the bovine clotting factors may react with human clotting factors to give rise to bleeding. More recently, a second generation platelet derivative, called platelet-rich fibrin (PRF), has been used in regenerative medicine and dentistry [6–8]. PRF is produced by slow centrifugation of blood and it contains a high number of platelets and leukocytes in addition to the dense fibrin matrix. The fibrin matrix and platelets contribute to wound healing while leukocytes contribute to the anti-bacterial effects. Unlike PRP, PRF contains a fibrin matrix instead of jellifying agents and bovine clotting factors [9]. Hence, the chances of coagulopathies are minimized. Furthermore, PRF exhibits a slow and sustained release of growth factors, such as transforming growth factor-β1, platelet-derived growth factor, and vascular endothelial growth factor which all have been proven to promote the wound healing and tissue regeneration [8, 10].
Guided tissue regeneration (GTR) involves the placement of synthetic and natural barrier membranes and bioactive materials to stimulate the regeneration of periodontal bone and promote healing of periodontal bone defects [11–14]. It has been established in numerous randomized control trials (RCTs) that using biodegradable GTR materials along with open flap debridement (OFD) results in superior outcomes compared to OFD alone [15, 16]. However, these materials have a number of drawbacks including poor biomechanical properties, risk of infection, hypersensitivity reactions, and ethical concerns [17, 18]. Because of its regenerative capabilities, human origin, and absence of animal growth factors, PRF has been used in regenerative dentistry applications [19, 20]. More recently, its use has been advocated for regenerative periodontics and wound healing [21]. Some in vitro and in vivo studies have shown that PRF promotes the proliferation and differentiation of periodontal tissues along with angiogenesis [22, 23]. Furthermore, randomized control trials have assessed the efficacy of PRF for restoring intrabony periodontal defects [24, 25]. The objectives of this review are to critically analyze and appraise the currently available literature, focusing on the use of platelet-rich fibrin and the outcomes in restoring and regeneration of periodontal intrabony defects.
Focus question
In patients with intrabony periodontal defects, what is the effect of using PRF-based grafts on the clinical and radiographic outcomes?
Materials and methods
Search methodology
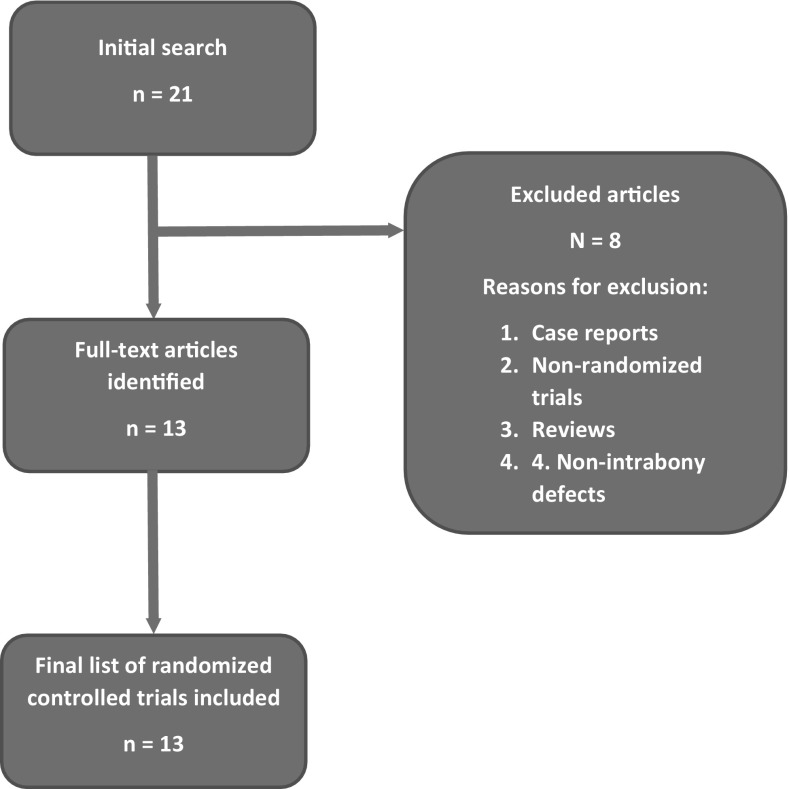
An electronic search was conducted via PubMed/MEDLINE, Google Scholar and ISI Web of Science databases for studies published from 1949 to January 2016. Various combinations of following keywords were used: ‘platelet-rich fibrin’, ‘intrabony’, ‘periodontal’, ‘bone defect’ and ‘guided tissue regeneration’. All the authors conducted the search individually and analyzed the titles and abstracts to select the studies according to the inclusion/exclusion criteria described below. A secondary search was conducted by analyzing the reference lists of the articles obtained in the initial search. Only English language publications were considered. The search methodology is illustrated in Fig. 1.
Fig. 1.
Flow chart of the article selection process for this review
Inclusion and exclusion criteria
The following three inclusion criteria were used: (1) randomized control trials, (2) restoration of bony periodontal defects, and (3) PRF as test intervention. Letters to the editors, commentaries, animal studies, and in vitro studies were excluded.
Quality assessment of randomized control trials
Using the Jadad scale for the quality assessment of control trials [26], the randomized control trials (RCTs) were assigned scores according to blinding, randomization and the description of the patients treated by authors. If the study was double-blinded, a point was given. Randomization of subjects also warranted a point. Additional points were given if methods of blinding and randomization were described. If an account of all patients was provided, a point was given, correspondingly. Hence, a total score out of 5 points was given to each study.
Results
General characteristics and outcomes of clinical studies
The primary search resulted in 21 articles, out of which 13 were randomized control trials that met the inclusion criteria of this review [24, 25, 27–37]. A total of 8 articles were excluded. No additional articles were found after carrying out the secondary search. The number of patients treated were ranged from 10 to 136 and the number of defects ranged from 20 to 120 [24, 25, 27–37]. Only one study included smokers in the treatment groups [30]. Six studies used OFD as the control intervention [24, 31, 33–36], and two studies used PRF as the control [30, 32]. Demineralized freeze-dried bone allograft (DFDBA) was used as the control by two studies [27, 37]. Enamel matrix derivative (EMD) [29], nano-crystalline hydroxyapatite (nHAp) [28], and bovine bone xenograft (BBX) [25] were used by one study each. Eleven studies used PRF at least in one test intervention group [24, 25, 27–29, 31, 33–37], and seven studies used PRF in combination with other GTR materials in the test groups [24, 25, 27, 28, 30, 32, 37]. In addition, the follow-up time ranged from 30 days to 12 months [24, 25, 27–37]. The general characteristics of the studies are detailed in Table 1 and the changes in the clinical and radiographic parameters are summarized in Table 2.
Table 1.
A summary of effects of using platelet-rich plasma on the clinical and radiographic outcomes compared to other interventions
| Authors | Study design | Number (n) | Clinical parameters measured | Control intervention | Test intervention | Follow-up | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| Patients | Smoker | Defects | |||||||
| Thorat et al. [34] | RCT | 40 | 0 | 32 | PI, SBI, PD, CAL, GML | OFD | PRF | 9 months | PD reduction and CAL gain higher in test group |
| Sharma and Pradeep [33] | RCT | 42 | 0 | 56 | PD, CAL, GML, IBD, DF | OFD | PRF | 9 months | Gain in GML and reduction in IBD more in test group |
| Lekovic et al. [30] | RCT* | 17 | 5 | 34 | PD, CAL, DF, HI, REC | PRF | PRF–BBX | 6 months | DF, PD reduction and CAL gain higher in test group |
| Pradeep et al. [31] | RCT* | 54 | 0 | 90 | PD, CAL, IBD, DF | OFD | PRP, PRF | 9 months | All parameters improved more in test groups than control group. Comparable CAL in all groups. Parameters comparable in PRP and PRF |
| Bansal and Bharti [27] | RCT* | 10 | 0 | ∼20 | PD, PI, CAL, GML, SBI, DF, ACR | DFDBA | DFDBA–PRF | 6 months | Only CAL was better in test group. All other parameters comparable in both groups |
| Gupta et al. [29] | RCT | 30 | 0 | 44 | CTBT, PD, CAL, IBD, DR | EMD | PRF | 6 months | Comparable outcomes in both groups. DR better with EMD |
| Pradeep et al. [24] | RCT | 136 | 0 | 120 | PI, SBI, PD, CAL, REC, DF | OFD | PRF, PRF–MF, MF | 9 months | All parameters improved more in test groups than control groups. PRF + MF resulted better outcomes than all other groups |
| Shah et al. [32] | RCT* | 20 | 0 | 40 | PD, RAL, GML | PRF | DFDBA–PRF | 6 months | Results comparable in both groups |
| Elgendy et al. [28] | RCT* | 20 | 0 | 40 | PI, GI, PD, CAL | nHAp | PRF–nHAp | 6 months | Greater PD and CAL observed in test group |
| Ajwani et al. [35] | RCT* | 20 | 0 | 40 | PI, SBI, PD, RAL, GML, IBD | OFD | PRF | 9 months | Greater improvement observed in all parameters except GML in test group |
| Mathur et al. [36] | RCT | 25 | 0 | 38 | PI, GI, PD, REC, CAL, DF, IBD | OFD | PRF, ABG | 6 months | Greater improvement observed in all parameters in test groups than controls. No difference between test groups |
| Gamal et al. [25] | RCT | 30 | 0 | 30 | PI, GI, PD, CAL, IBD, VEGF, PDGF | BBX | PRGF–BBX, PRF–BBX | 30 days | Greater improvement of parameters in test groups. Results comparable in both test groups |
| Agarwal et al. [37] | RCT* | 32 | 0 | 60 | PI, SBI, PD, CAL, REC, RBL, DF | DFDBA | DFDBA–PRF | 12 months | All parameters improved more in test group than control group |
* = split-mouth design
ABG autologous bone graft, ACR alveolar crest resorption, BBX bovine bone xenograft, CAL clinical attachment level, DFDBA demineralized freeze-dried bone allograft, GI gingival index, DF defect fill, DR defect resolution, GML gingival marginal level, IBD intrabony defect depth, MF metformin, nHAp nano-crystalline hydroxyapatite, OFD open-flap debridement, PDGF platelet-derived growth factor, PRF platelet-rich fibrin, PRP platelet rich plasma, RAL relative attachment level, RBL radiographic bone level, SBI sulcular bleeding index, VEGF vascular endothelial growth factor
Table 2.
Comparison of mean changes in radiographic and clinical parameters recorded in the selected studies
| Study | Mean changes | |||
|---|---|---|---|---|
| Defect fill | IBD reduction (mm) | Improvement in PD (mm) | Improvement in CAL (mm) | |
| Thorat et al. [34] | OFD: 28.66% | OFD: 1.24 ± 0.69 | OFD: not stated | OFD: not stated |
| PRF: 46.92% | PRF: 2.12 ± 0.69 | PRF: not stated | PRF: not stated | |
| Sharma and Pradeep [33] | OFD: 1.80 ± 1.56% | OFD: 1.80 ± 1.56% | OFD: 3.21 ± 1.64 | OFD: 3.31 ± 1.76 |
| PRF: 48.26 ± 5.72% | PRF: 48.26 ± 5.72% | PRF: 4.55 ± 1.87 | PRF: 2.77 ± 1.44 | |
| Lekovic et al. [30] | PRF–BBX: not stated | PRF–BBX: Buccal: 4.06 ± 0.87; lingual: 3.94 ± 0.73 | PRF–BBX: Buccal: 4.47 ± 0.78; lingual: 4.29 ± 0.82 | PRF–BBX: Buccal: 3.82 ± 0.78; lingual: 3.71 ± 0.75 |
| PRF: not stated | PRF: Buccal: 2.21 ± 0.68; lingual: 2.06 ± 0.64 | PRF: Buccal: 3.35 ± 0.68; Lingual: 3.24 ± 0.73 | PRF: Buccal: 2.24 ± 0.73; lingual: 2.12 ± 0.68 | |
| Pradeep et al. [31] | OFD: 2.97 ± 0.97 mm, 1.56 ± 15.12% | ODF: 0.13 ± 1.46 | OFD: 2.97 ± 0.93 | OFD: 2.83 ± 0.91 |
| PRP: 3.77 ± 1.77 mm, 56.85 ± 14.01% | PRP: 2.7 ± 0.79 | PRP: 3.77 ± 1.07 | PRP: 2.93 ± 1.08 | |
| PRF: 3.77 ± 1.19 mm, 55.41 ± 11.39% | PRF: 2.8 ± 0.8 | PRF: 3.77 ± 1.19 | PRF: 3.17 ± 1.29 | |
| Bansal and Bharti [27] | DFDBA: 1.93 ± 0.208 | DFDBA: not stated | DFDBA: 3.1 ± 0.738 | DFDBA: 2.3 ± 0.699 |
| DFDBA–PRF: 2.13 ± 1.284 | DFDBA–PRF: not stated | DFDBA–PRF: 4.0 ± 0.816 | DFDBA–PRF: 3.4 ± 0.606 | |
| Gupta et al. [29] | EMD: 2.08 ± 0.78 mm, 43.07 ± 12.21% | EMD: not stated | EMD: 1.80 ± 0.56 | EMD: 2.00 ± 0.54 |
| PRF: 1.6 ± 1.17, 32.41 ± 14.61 | PRF: No stated | PRF: 1.8 ± 0.77 | PRF: 1.87 ± 0.91 | |
| Pradeep et al. [24] | OFD: not stated | OFD: 0.49 ± 0.27 | OFD: 3.00 ± 0.18 | OFD: 2.96 ± 0.18 |
| PRF: not stated | PRF: 2.53 ± 0.30 | PRF: 4.00 ± 0.18 | PRF: 4.03 ± 0.18 | |
| PRF–MF: not stated | PRF–MF: 2.56 ± 0.28 | PRF–MF: 3.93 ± 0.25 | PRF–MF: 3.93 ± 0.25 | |
| OFD–PRF–MF: not stated | OFD–PRF–MF: 2.77 ± 0.30 | OFD–PRF–MF: 4.90 ± 0.30 | OFD–PRF–MF: 4.90 ± 0.30 | |
| Shah et al. [32] | OFD–PRF: not stated | OFD–PRF: not stated | OFD–PRF: 3.67 ± 0.69 | OFD–PRF: 2.97 ± 1.56 |
| DFDBA–PRF: not stated | DFDBA–PRF: not stated | DFDBA–PRF: 3.70 ± 0.68 | DFDBA–PRF 2.97 ± 1.68 | |
| Elgendy et al. [28] | nHAP: not stated | nHAP: not stated | nHAP: not stated | nHAP: not stated |
| PRF–nHAP: not stated | PRF–nHAP: not stated | PRF–nHAP: not stated | PRF–nHAP: not stated | |
| Ajwani et al. [35] | OFD: not stated | OFD: not stated | OFD: not stated | OFD: not stated |
| PRF: not stated | PRF: not stated | PRF: not stated | PRF: not stated | |
| Mathur et al. [36] | OFD + ABG: not stated | OFD + ABG: not stated | OFD + ABG: 2.40 ± 1.06 | OFD + ABG: 2.67 ± 1.63 |
| OFD + PRF: not stated | OFD + PRF: not stated | OFD + PRF: 2.67 ± 1.29 | OFD + PRF: 2.53 ± 1.06 | |
| Gamal et al. [25] | BBX: not stated | BBX: not stated | BBX: not stated | BBX: not stated |
| PRGF–BBX: not stated | PRGF–BBX: not stated | PRGF–BBX: not stated | PRGF–BBX: not stated | |
| PRF–BBX: not stated | PRF–BBX: not stated | PRF–BBX: not stated | PRF–BBX: not stated | |
| Agarwal et al. [37] | DFDBA: not stated | DFDBA: not stated | DFDBA: 3.60 ± 0.51 | DFDBA: 2.61 ± 0.68 |
| DFDBA–PRF: not stated | DFDBA–PRF: not stated | DFDBA–PRF: 4.15 ± 0.84 | DFDBA–PRF: 3.73 ± 0.74 | |
Measurement of clinical and radiographic parameters
In all studies, the clinical and radiographic parameters were recorded at baseline and follow-up along with their mean differences [24, 25, 27–37]. The pocket depth (PD) and clinical/relative attachment levels (CAL/RAL) were measured in all thirteen studies [24, 25, 27–37]. The sulcular bleeding index (SBI) was measured in five studies [25, 27, 34, 35, 37]. The plaque indexed (PI) was measured in eight studies [24, 25, 27, 28, 34–37]. The gingival marginal level and recession (GML/REC) were measured in eight studies [24, 27, 30, 32–35, 37]. The proportion of defect fill (DF) was measured in eight studies [24, 25, 27–37]. The intrabony pocket depth (IBD) was measured in six studies [25, 29, 31, 33, 35, 36]. The radiographic bone levels (RBL), alveolar crest resorption (ACR), and healing index (HI) were measured in one study each [28, 31, 38]. The plaque index was measured in eight studies [24, 25, 27, 28, 34–37]. One study measured the levels of vascular endothelial growth factor (VEGF) and platelet derived growth factor (PGDF) in the gingival crevicular fluid [25].
Main outcomes of studies
Compared with OFD alone, PRF combined with OFD resulted in significantly improved clinical and radiographic outcomes in six studies [24, 31, 33, 35, 36, 38]. When combined with the bovine bone substitutes, PRF resulted in better performance outcomes as opposed to when used alone [25, 30]. Similar outcomes were observed when PRF was used in combination with DFDBA in one study [37]. However, in another study, no significant differences were observed between PRF and PRF–DFDBA [32]. Better outcomes were observed when PRF-nHAp was used [28]. PRF augmented the effects of ABG in one study [36]. Similar effect was observed when PRF was combined with metformin (MF) [24]. No significant differences were observed between the outcomes of using PRF or PRP [31]. There was no difference between the PD and CAL when PRF and EMD were contrasted and compared but a higher DF percentage was recorded [29]. The mean changes in IBD, PD, DF and CAL are presented in Table 2.
Results of the quality assessment of studies
Only one study scored a perfect 5 points out of 5 [30], 3 points were assigned to one study [34] (35), two studies scored 4 [31, 37], and seven studies scored 2 points [24, 25, 32, 33, 35, 36]. One point was awarded to three studies each [27–29]. Patients were randomized into their respective intervention groups in all studies [24, 25, 27–38] with three studies failing to provide and describe the method of randomization [27–30]. Adequate double-blinding was employed in only three studies [24, 30, 37]. Accounts of all patients treated were provided in only two studies [30, 34]. The results of the quality assessment are shown in Table 3.
Table 3.
Evaluation using the Jadad scores for the included studies
| Study | Randomization | Blinding | An account of all patients | Total score |
|---|---|---|---|---|
| Thorat et al. [38] | 2 | 0 | 1 | 3 |
| Sharma and Pradeep [33] | 2 | 0 | 0 | 2 |
| Lekovic et al. [30] | 2 | 2 | 1 | 5 |
| Pradeep et al. [31] | 2 | 2 | 0 | 4 |
| Bansal and Bharti [27] | 1 | 0 | 0 | 1 |
| Gupta et al. [29] | 1 | 0 | 0 | 1 |
| Pradeep et al. [24] | 2 | 0 | 0 | 2 |
| Shah et al. [32] | 2 | 0 | 0 | 2 |
| Elgendy et al. [28] | 1 | 0 | 0 | 1 |
| Ajwani et al. [35] | 2 | 0 | 0 | 2 |
| Mathur et al. [36] | 2 | 0 | 0 | 2 |
| Gamal et al. [25] | 2 | 0 | 0 | 2 |
| Agarwal et al. [37] | 2 | 2 | 0 | 4 |
Discussion
Autologous plasma derivatives have been used in medicine and dentistry owing to their regenerative abilities [3, 4]. This said, the unique advantage of PRF over conventional plasma derivations such as PRP is that it doesn’t contain any bovine derivatives or jellifying agents [9]. Furthermore, PRF is simpler to prepare and relatively inexpensive. Due to the presence of leukocytes, PRF has also been shown to impose an antibacterial effect [39]. Hence, it is not surprising that significantly better clinical radiographic outcomes were observed when PRF were compared with OFD alone [24, 31, 33–36]. The reduction in PD and IBD upon treatment may be explained by the presence of growth factors present in PRF. However, no difference has been observed between the efficacy and regenerative potential of PRP and PRF [31] even though previous in vitro studies have reported superior results of PRF tested with rat osteoblasts when compared to PRP [40]. From the clinical point of view, the main advantage of PRF over PRP is the superior handling properties than improved efficacy. The PRF can be handled and manipulated similar to conventionally available GTR membranes (Table 3).
Although PRF can mimic and be handled like a GTR membrane, its main disadvantage is that it resorbs in approximately 7 days [30] which is substantially less than the 4–6 weeks required for most periodontal regeneration applications [41]. Additionally, due to its fast resorption rate, its ability of space maintenance is compromised. Studies that have investigated the combined use of PRF and bone substitutes have observed better clinical outcomes than PRF alone [27, 28, 30, 36]. This may be also attributed to osteoconductive effect of the hydroxyapatite present in such bone substitutes [42]. However, the study by Gamal et al. [25] failed to observe any significant difference between the amount of vascular endothelial growth factor (VEGF) as well as plasma derived growth factor (PDGF) released in the defects restored with a combination of PRF and bovine bone. This observation suggests that combined usage of bovine bone and PRF does not have a significant advantage over using bovine bone alone. Nevertheless, the study by Gamal et al. was carried out for only 30 days and on a relatively small sample size. This is why more long-term and large-scale studies are required to further investigate and explain these findings.
An addition of 1% MF to PRF has shown substantial advantage in improving clinical and radiographic outcomes after 9 months [24]. Previous studies have also shown that a topical application of 1% MF along with scaling and root planning (SRP) has shown to be more effective in treating periodontitis in smokers than SRP alone [43]. It is noteworthy, however that in the study by Pradeep et al. [24] no statistical difference was observed between the clinical parameters following either MF application or restoring the defect with PRF. That said, the improved efficacy of MF + PRF may be attributed to the superior bone-fill compared to MF or PRF alone. In the study by Gupta et al. [29] CBCT imaging suggests that EMD is superior compared to PRF in terms of defect resolution. This could be because of the propylene-glycol alginate (PGA) carrier which contains EMD [44]. PGA may provide greater space maintenance than PRF due to its synthetic polymeric structure and, hence, a higher defect resolution. However, more long-term studies are required to investigate this hypothesis. Conversely, a comparison between autologous bone graft (ABG) and PRF has yielded no significant difference between their efficacies albeit more crestal bone loss was observed with ABG [36]. Nevertheless, the short follow-up period (6 months) warrants long-term studies to compare and contrast them.
A major shortcoming among the studies included in this review might be the lack of adequate of follow-up. No study followed-up the patients for more than 12 months [24, 25, 27–37]. Moreover, in none of the studies histological or microbial investigations were conducted. Hence, relying solely on the results of the studies reviewed, the long-term efficacy of PRF and PRF-based combinations cannot be concluded. Additionally, only one of the studies included smokers in the treatment groups which might have led to favorable outcomes in those studies which did not include. Due to short term follow up period of included studies, no significant quantitative data regarding the improvement of intrabony defect can be reported. The quality assessment of the studies revealed a lack of adequate blinding which can be a source of bias in RCTs [26]. Hence, RCTs with longer follow-up periods and better blinding protocols are definitely required to ascertain the long-term efficacy of PRF.
Conclusion
The platelet-rich fibrin when combined with open-flap debridement, produces better outcomes compared to the open flap debridement alone. The regenerative potential of platelet-rich fibrin results in better augmentation and regeneration of periodontal bone defects. In addition, PRF may augment the regenerative potential of bone grafts. However, more long-term and well-designed clinical trials are needed to ascertain the clinical efficacy of platelet-rich fibrin and platelet-rich fibrin containing bone grafts.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. 2013;41:356–364. doi: 10.1177/0363546512471299. [DOI] [PubMed] [Google Scholar]
- 2.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 3.Albanese A, Licata ME, Polizzi B, Campisi G. Platelet-rich plasma (PRP) in dental and oral surgery: from the wound healing to bone regeneration. Immun Ageing. 2013;10:23. doi: 10.1186/1742-4933-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sommeling CE, Heyneman A, Hoeksema H, Verbelen J, Stillaert FB, Monstrey S. The use of platelet-rich plasma in plastic surgery: a systematic review. J Plast Reconstr Aesthet Surg. 2013;66:301–311. doi: 10.1016/j.bjps.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–e44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e45–e50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Dohan Ehrenfest DM, Bielecki T, Mishra A, Borzini P, Inchingolo F, Sammartino G, et al. In search of a consensus terminology in the field of platelet concentrates for surgical use: platelet-rich plasma (PRP), platelet-rich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr Pharm Biotechnol. 2012;13:1131–1137. doi: 10.2174/138920112800624328. [DOI] [PubMed] [Google Scholar]
- 9.Zumstein MA, Berger S, Schober M, Boileau P, Nyffeler RW, Horn M, et al. Leukocyte-and platelet-rich fibrin (L-PRF) for long-term delivery of growth factor in rotator cuff repair: review, preliminary results and future directions. Curr Pharm Biotechnol. 2012;13:1196–1206. doi: 10.2174/138920112800624337. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak HF, Harvey VS, Estrella P, Brown LF, McDonagh J, Dvorak AM. Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest. 1987;57:673–686. [PubMed] [Google Scholar]
- 11.Najeeb S, Zafar MS, Khurshid Z, Siddiqui F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J Prosthodont Res. 2016;60:12–19. doi: 10.1016/j.jpor.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Najeeb S, Khurshid Z, Matinlinna JP, Siddiqui F, Nassani MZ, Baroudi K. Nanomodified peek dental implants: bioactive composites and surface modification—a review. Int J Dent. 2015; 2015:381759. [DOI] [PMC free article] [PubMed]
- 13.Sheikh Z, Najeeb S, Khurshid Z, Verma V, Rashid H, Glogauer M. Biodegradable materials for bone repair and tissue engineering applications. Materials (Basel) 2015;8:5744–5794. doi: 10.3390/ma8095273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zafar M, Khurshid Z, Almas K. Oral tissue engineering progress and challenges. Tissue Eng Regen Med. 2015;12:387–397. doi: 10.1007/s13770-015-0030-6. [DOI] [Google Scholar]
- 15.Jepsen S, Eberhard J, Herrera D, Needleman I. A systematic review of guided tissue regeneration for periodontal furcation defects. What is the effect of guided tissue regeneration compared with surgical debridement in the treatment of furcation defects? J Clin Periodontol. 2002;29:103–116. doi: 10.1034/j.1600-051X.29.s3.6.x. [DOI] [PubMed] [Google Scholar]
- 16.Murphy KG, Gunsolley JC. Guided tissue regeneration for the treatment of periodontal intrabony and furcation defects. A systematic review. Ann Periodontol. 2003;8:266–302. doi: 10.1902/annals.2003.8.1.266. [DOI] [PubMed] [Google Scholar]
- 17.Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TG, Kowolik MJ, et al. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—a materials perspective. Dent Mater. 2012;28:703–721. doi: 10.1016/j.dental.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 18.Milella E, Ramires P, Brescia E, La Sala G, Di Paola L, Bruno V. Physicochemical, mechanical, and biological properties of commercial membranes for GTR. J Biomed Mater Res. 2001;58:427–435. doi: 10.1002/jbm.1038. [DOI] [PubMed] [Google Scholar]
- 19.Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–234. doi: 10.1016/j.tibtech.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Carlson NE, Roach RB Jr. Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc. 2002;133:1383–6. [DOI] [PubMed]
- 21.Femminella B, Iaconi MC, Di Tullio M, Romano L, Sinjari B, D’Arcangelo C, et al. Clinical comparison of platelet-rich fibrin and a gelatin sponge in the management of palatal wounds after epithelialized free gingival graft harvest: a randomized clinical trial. J Periodontol. 2016;87:103–113. doi: 10.1902/jop.2015.150198. [DOI] [PubMed] [Google Scholar]
- 22.Kawase T, Okuda K, Wolff LF, Yoshie H. Platelet-rich plasma-derived fibrin clot formation stimulates collagen synthesis in periodontal ligament and osteoblastic cells in vitro. J Periodontol. 2003;74:858–864. doi: 10.1902/jop.2003.74.6.858. [DOI] [PubMed] [Google Scholar]
- 23.Hatakeyama I, Marukawa E, Takahashi Y, Omura K. Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng Part A. 2014;20:874–882. doi: 10.1089/ten.tea.2013.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN. Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2015;86:729–37. [DOI] [PubMed]
- 25.Gamal AY, Abdel Ghaffar KA, Alghezwy OA. Crevicular fluid growth factors release profile following the use of platelet-rich fibrin and plasma rich growth factors in treating periodontal intrabony defects: a randomized clinical trial. J Periodontol. 2016;87:654–662. doi: 10.1902/jop.2016.150314. [DOI] [PubMed] [Google Scholar]
- 26.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 27.Bansal C, Bharti V. Evaluation of efficacy of autologous platelet-rich fibrin with demineralized-freeze dried bone allograft in the treatment of periodontal intrabony defects. J Indian Soc Periodontol. 2013;17:361–366. doi: 10.4103/0972-124X.115663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elgendy EA, Abo Shady TE. Clinical and radiographic evaluation of nanocrystalline hydroxyapatite with or without platelet-rich fibrin membrane in the treatment of periodontal intrabony defects. J Indian Soc Periodontol. 2015;19:61–65. doi: 10.4103/0972-124X.148639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta SJ, Jhingran R, Gupta V, Bains VK, Madan R, Rizvi I. Efficacy of platelet-rich fibrin vs. enamel matrix derivative in the treatment of periodontal intrabony defects: a clinical and cone beam computed tomography study. J Int Acad Periodontol. 2014;16:86–96. [PubMed] [Google Scholar]
- 30.Lekovic V, Milinkovic I, Aleksic Z, Jankovic S, Stankovic P, Kenney E, et al. Platelet-rich fibrin and bovine porous bone mineral vs. platelet-rich fibrin in the treatment of intrabony periodontal defects. J Periodontal Res. 2012;47:409–417. doi: 10.1111/j.1600-0765.2011.01446.x. [DOI] [PubMed] [Google Scholar]
- 31.Pradeep A, Rao NS, Agarwal E, Bajaj P, Kumari M, Naik SB. Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of 3-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2012;83:1499–1507. doi: 10.1902/jop.2012.110705. [DOI] [PubMed] [Google Scholar]
- 32.Shah M, Patel J, Dave D, Shah S. Comparative evaluation of platelet-rich fibrin with demineralized freeze-dried bone allograft in periodontal infrabony defects: a randomized controlled clinical study. J Indian Soc Periodontol. 2015;19:56–60. doi: 10.4103/0972-124X.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A, Pradeep AR. Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial. J Periodontol. 2011;82:1705–1712. doi: 10.1902/jop.2011.110075. [DOI] [PubMed] [Google Scholar]
- 34.Thorat M, Pradeep A, Pallavi B. Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol. 2011;38:925–932. doi: 10.1111/j.1600-051X.2011.01760.x. [DOI] [PubMed] [Google Scholar]
- 35.Ajwani H, Shetty S, Gopalakrishnan D, Kathariya R, Kulloli A, Dolas RS, et al. Comparative evaluation of platelet-rich fibrin biomaterial and open flap debridement in the treatment of two and three wall intrabony defects. J Int Oral Health. 2015;7:32–37. [PMC free article] [PubMed] [Google Scholar]
- 36.Mathur A, Bains VK, Gupta V, Jhingran R, Singh GP. Evaluation of intrabony defects treated with platelet-rich fibrin or autogenous bone graft: a comparative analysis. Eur J Dent. 2015;9:100–108. doi: 10.4103/1305-7456.149653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal A, Gupta ND, Jain A. Platelet rich fibrin combined with decalcified freeze-dried bone allograft for the treatment of human intrabony periodontal defects: a randomized split mouth clinical trail. Acta Odontol Scand. 2016;74:36–43. doi: 10.3109/00016357.2015.1035672. [DOI] [PubMed] [Google Scholar]
- 38.Thorat SB, Diaspro A, Salerno M. In vitro investigation of coupling-agent-free dental restorative composite based on nano-porous alumina fillers. J Dent. 2014;42:279–286. doi: 10.1016/j.jdent.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Bielecki TM, Gazdzik TS, Arendt J, Szczepanski T, Król W, Wielkoszynski T. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br. 2007;89:417–420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 40.He L, Lin Y, Hu X, Zhang Y, Wu H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:707–713. doi: 10.1016/j.tripleo.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 41.Lindhe J, Karring T, Lang NP. Clinical periodontology and implant dentistry. Copenhagen: Blackwell Munksgaard; 2003. pp. 650–703. [Google Scholar]
- 42.Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials. 2001;22:87–96. doi: 10.1016/S0142-9612(00)00174-5. [DOI] [PubMed] [Google Scholar]
- 43.Rao NS, Pradeep A, Kumari M, Naik SB. Locally delivered 1% metformin gel in the treatment of smokers with chronic periodontitis: a randomized controlled clinical trial. J Periodontol. 2013;84:1165–1171. doi: 10.1902/jop.2012.120298. [DOI] [PubMed] [Google Scholar]
- 44.Sculean A, Schwarz F, Becker J, Brecx M. The application of an enamel matrix protein derivative (Emdogain) in regenerative periodontal therapy: a review. Med Princ Pract. 2007;16:167–180. doi: 10.1159/000100386. [DOI] [PubMed] [Google Scholar]