Abstract
To develop decellularized heart valve scaffold from porcine for heart valve regeneration. Porcine heart valves were decellularized with unique optimized approach by using 1% sodium dodecyl sulfate solution and 5% dimethyl sulfoxide for the first time. Effect of decellularization process on scaffold were characterized by hematoxylin–eosin, 4′,6-diamidino-2-phenylindole, Masson’s trichrome, alcian blue staining and scanning electron microscopy for extracellular matrix (ECM) analysis in scaffold. The results showed that developed protocol for decellularization of heart valve scaffold shown complete removal of all cellular components, without changing the properties of ECM. The developed protocol was successfully used for heart valve ECM scaffolds development from porcine. The developed protocol seems to be promising solution for the heart valve tissue engineering application.
Keywords: Decellularization, Heart valve, Tissue engineering, Scaffold, Cardiovascular
Introduction
The loss or failure of cardiac tissue is one of the most devastating and costly problem in human health care system. The regenerative capacity of human heart valves is greatly compromised during pathological condition [1–3]. Heart valve replacement is found the only option for today’s line of treatment [4]. Approximately 290,000 heart valve replacements are performed worldwide per year and the number of patients requiring heart valve replacement is expected to triple by the year 2050 [5]. The shortage of organs for transplantation is an important medical and social problem because transplantation is often the best therapeutic option for end-stage organ failure [6]. The availability of transplantable donor organs is a constant challenge for transplant surgeon to fulfill the overgrowing demand of transplant every year. Importantly transplant related mortality and morbidity is very high observed in patients [7]. A current therapeutic option available are mechanical and bio prosthetic heart valves replacement [8]. Mechanical heart value have disadvantage like life-long anticoagulation therapy is necessary which is associated with a substantial risk of spontaneous bleeding and embolism [9]. Bio prosthetic heart valves suffer from premature degeneration and calcification. This resulted in clinical needs for a substitute in current graft material to replace diseased heart valves.
Tissue engineered heart valves may represent an interesting alternative to current prostheses because of their rapid cellular repopulation, tissue remodeling, and therewith self-repair capacity [3]. Thus, tissue engineering offers the great potential of providing heart valves that can be used to replace diseased and damaged native heart valves [10]. In tissue engineering field there are three important components which are cells, scaffolds and growth factors to generate tissues for functional replacement of damaged or diseased organs upon transplantation [11]. The scaffolds act like natural extracellular matrix (ECM) and supports cell attachment, proliferation, and differentiation [10, 12, 13].
Dimethyl sulfoxide (DMSO) is a small organic molecule containing a hydrophilic sulfoxide group and two hydrophobic methyl groups, which makes it an amphiphilic compound. Several previous studies have reported the ability of DMSO to act as a free radical scavenges which gives it antioxidant properties. In addition, it has been clinically used in the treatment of several pathologies and also proposed for the treatment of Alzheimer’s disease [14]. DMSO prevents intracellular ice formation, recommended for cellular tissues [15], scaffold cryopreservation, which prevents the tissue damage. DMSO also has property of penetration through cell membrane and able to carry other drugs to cross cell membranes barrier [16]. Till date various decellularization techniques are used extensively in an effort to suppress the immunogenic potential and preservation of ECM of biological matrices. Efficiency of cell removal, as well as preservation of the matrix integrity, is highly dependent on the decellularization method used [17]. Among different methods used non-enzymatic detergent-based methods like use of SDS (sodium dodecyl sulphate) had shown effective cell removal and maintaining matrix integrity when compared with decellularization with other more aggressive agents. But at the same time it has a disruptive effect on ECM [18]. Taking this into consideration, the present research proposes the use of unique blend of DMSO as an antioxidant agent along with SDS detergent in custom designed bioreactor for more optimized decellularization without harming the ECM. The bioreactor was made from acrylate material which can be autoclaved which is round in shape in which heart valves fixed into it [19]. The assembly is small, lightweight and can be sterilized by using ethylene oxide. The main benefit of the bioreactor is a uniform fluid flow of detergent which brings effective decellularization process [20, 21].
Materials and methods
Porcine heart valves preparation
20 porcine hearts of 6-month-old pigs collected from slaughterhouse. Heart was dissected and from each heart, aortic valves were dissected and freed from adherent fat and myocardium (Fig. 1A, B). All dissected heart valves rinsed in 70% alcohol followed by normal saline containing antibiotics solution [100 µg/ml streptomycin (Abbott Healthcare), 100 U/ml penicillin (Alembic Pharmaceuticals Ltd), and 2.5 µg/ml Ampholyn (Lyka Labs Ltd)] and stored in freezer at −40 °C until further use.
Fig. 1.
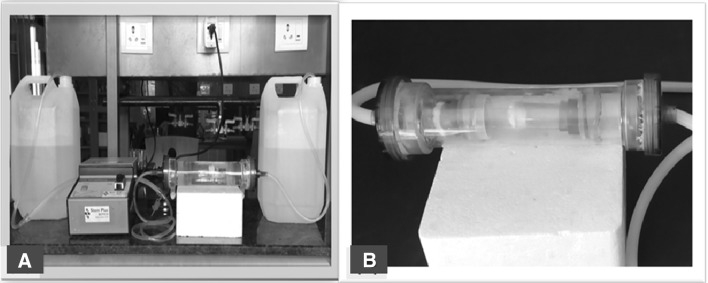
A Bioreactor assembly and B custom made flow bioreactor system designed for decellularized porcine heart valve
Bioreactor assembly
Custom made flow bioreactor system designed for decellularized porcine heart valve (Fig. 2). Flow bioreactor was assembled using transparent acrylic material with translucent and transparent silicon tube of OD-12 mm, ID-10 mm and OD-10 mm, ID-8 mm respectively using peristaltic pump (SAGE Instrument, USA). Decellularization was carried out 1% SDS (Sisco Research Laboratory Pvt Ltd) for 6 h followed by 5% DMSO (Fisher Scientific) solution for 6 h. For each cycle of decellularization done by freshly prepared reagents. Decellularization process was checked by biopsy after completion of each cycle. Samples were fixed in 10% neutral buffered formalin, dehydrated in an ascending alcohol grades and embedded in paraffin. Paraffin blocks were cut into serial sections of 4 µm thickness.
Fig. 2.
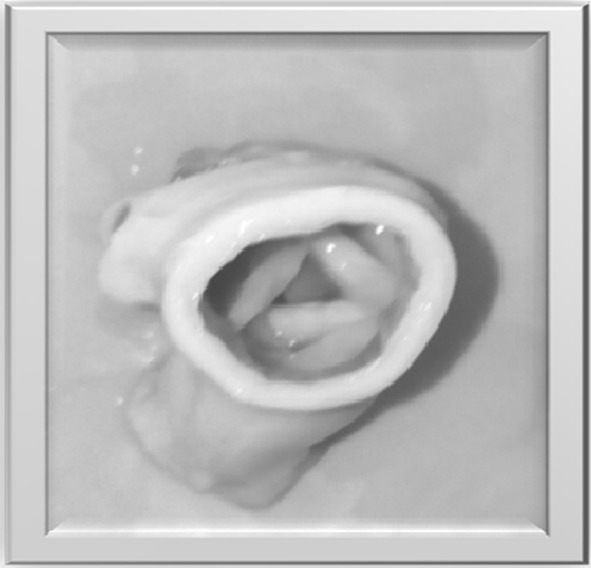
Native porcine heart valve
Decellularization
This method was performed using SDS (Sodium Dodecyl Sulfate) and DMSO with the help of custom made bioreactor. Samples were treated with 1% SDS for 6 h followed by 5% DMSO for 6 h in custom made bioreactor. After 12 h sample were transferred into D/W and stored in deep freezer overnight. It was thawed next morning at room temperature and cycle was repeated again. Many cycles were repeated to achieve complete decellularization of sample.
Decellularized scaffolds characterization
HE staining
Standard histological staining with Hematoxylin and Eosin can serve as a first line of inspection to determine if nuclear structures can be observed.
DAPI
DNA quantification of the scaffolds showed that decellular agent we used caused markedly greater removal of the DNA compared with native DNA in control heart valve. Inspection for the presence of DNA can be performed by staining the specimen with DAPI (Molecular Probes, Oregon, USA) which are fluorescent molecule.
Masson’s trichome
Masson’s trichrome stain used to differentiate collagen and smooth muscle in tumors, and the increase of collagen in disease such as cirrhosis. Routine stain for liver and kidney biopsies. As the name implies, three dyes are employed selectively staining muscle, collagen fibers, fibrin, and erythrocytes. The general rule in tri chrome staining is that the less porous tissue are colored by the smallest dye molecules; Whenever a dye of large molecular size is able to penetrate; it will always do so at the expense of the smaller molecule.
Alcian-blue pH 1.0
Alcian blue staining technique [22] is widely used among developmental biologist to observe the hydrated and viscose matrix of the extracellular glycoproteins usually have bound a large number of glycosaminoglycans (GAGs).
Scanning electron microscopy (SEM)
SEM was performed on cross sections of scaffolds to investigate morphology and porosity. Scanning Electron Microscope (SEM) was done using JEOL JSM. 6360 scanning Electron Microscope model, to image the lumen of the decellularized and rinsed porcine heart valves samples acquired in Department of Physics, Shivaji University, Kolhapur. After dehydration, the porcine heart valve specimen was fixed to the stage using double sided tape. The height of the sample stage 25 need to be adjusted with sample preparation tool before placement of the vessel specimen and stage into the SEM. Proper height adjustment was important to make sure the stage and sample cleared the ceiling of the sample feed and was high enough for the SEM to bring an image of the vessel surface into focus. Several sets of images were taken of each sample at the scales of ×6000.
DNA quantification
At native condition and after decellularization the total amount of DNA was quantified using UV spectrophotometer (UV-1800 UV–Vis Spectrophotometer) at 260 nm. Sample of native and decellularization blood vessels by all four methods were collected at concentration of 1 mg/ml and freeze at −20 °C. After 15 min all samples were crushed with mortar pestle and of D/W was added to it. From above prepared DNA solution 40 µl of solution was taken using Hamilton syringe in 4 ml cuvette to which 3.96 ml of D/W was added. At most care was taken to ensure that solution is air bubble free. Solution was let to stand for 10 min to ensure the complete diffusion of DNA throughout the solution. This represents a 1:100 dilution of the standards and DNA samples. The spectrometer was set at 260 nm and reading was noted down.
Biocompatibility study
Zero h fertilized black leghorn chicken eggs (Gallus gallus) were obtained from local hatchery and cleaned with 70% alcohol. Fertilized chicken eggs were incubated at 37 °C and 80% humidified atmosphere in an incubator. Humidity was monitored regularly by using hygrometer Fertilized eggs were randomly divided into two groups—the control group and experimental groups, and then incubated at 38 °C and 55–65% humidity with automatic rotation in the incubation system. On day 4 of the incubation, a window was opened in the eggs in sterile condition, part of the shell was removed, and to which the tissue engineered scaffold were placed on chorioallantoic membrane (CAM) area. The window was covered by sterile paraffin film and returned to the incubator. On day 8, the eggs were opened the chick embryos were observed. Photographs were taken of CAM of control and experimental. Implanted tissue were removed and collected in 10% neutral buffered formalin. The adjacent CAM is also harvested and kept in 10% neutral buffered formalin for fixation. Both were further processed for hematoxylin eosine staining (HE).
Results
Flow bioreactor
Flow perfusion of 1% SDS for 6 h followed by 5% DMSO for 6 h subjected over several cycles. Exposing porcine heart valves to SDS and DMSO with shear stresses in flow bioreactor has been shown to enhance uniform decellularization process which was confirmed by HE and DAPI staining after each cycle.
HE staining
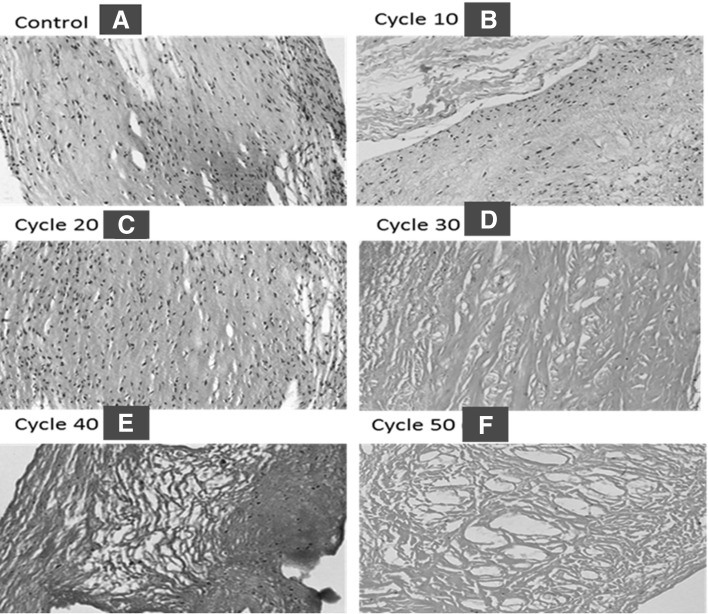
Porcine pulmonary, aortic valves were decellularized by detergent sodium dodecyl sulfate (SDS), dimethyl sulfoxide (DMSO) treatment for 50 cycles. This was monitored by HE staining with hematoxylin and eosin of porcine heart valves which was done after every 10 cycle to keep track of cell removal as shown in Fig. 2. Decellularization efficacy imaging of the H&E stained sections showed a reduction in visible nuclei as decellularization cycle progress. This estimated that complete decellularization was achieved by 50th cycle of decellularization method as shown in Fig. 3.
Fig. 3.
Porcine pulmonary valve A control and B–F decellularized checked by HE staining (20×). H&E stained sections showed a reduction in visible nuclei as decellularization cycle progress
DAPI staining
Scaffolds showing a reduction in DNA and completely remove as decellularization cycle progress. With DAPI the process of decellularization was cross checked after 50th cycle which was a nuclear stain which confirmed the cell removal that finally proved complete decellularization at 50th cycle of method as shown in Fig. 4.
Fig. 4.
Decellularized process confirmed by A control and B–F DAPI staining (20×). Scaffolds showing a reduction in DNA and completely remove as decellularization cycle progress
Masson’s trichrome stain
Masson’s trichrome staining revealed collagen in decellularized heart valve and compared native vessels. Results were promising and appreciable amount of collagen and remain unchanged seen in treated heart valve as shown in Fig. 5.
Fig. 5.

Decellularized porcine heart valve after 50th cycle stained for Masson’s trichrome staining revealed collagen in decellularized heart valve
Alcian-blue pH 1.0
AB staining showed presence of glycosaminoglycans (GAGs) in decellularized heart valve. AB staining demonstrated no obvious disruption to the overall histo-architecture following treatment as shown in Fig. 6.
Fig. 6.

AB staining pH 1.0 showed presence of glycosaminoglycan’s (GAGs) after 50th cycle
DNA quantification
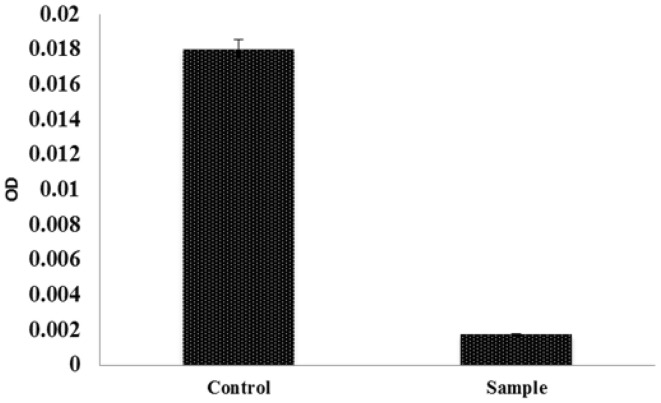
DNA contents in control and decellularized porcine valve compared by DNA quantification. Decellularized valve showed minimal DNA content compared to control. Graphical representation of DNA estimation shown in Fig. 7.
Fig. 7.
Graphical representation of DNA estimation showed minimal DNA content in decellularized heart valve compared to control
SEM analysis
Scanning electron microscopy of scaffold showing the 3-D network of ECM overall morphology (magnification, ×6000) in Fig. 8.
Fig. 8.
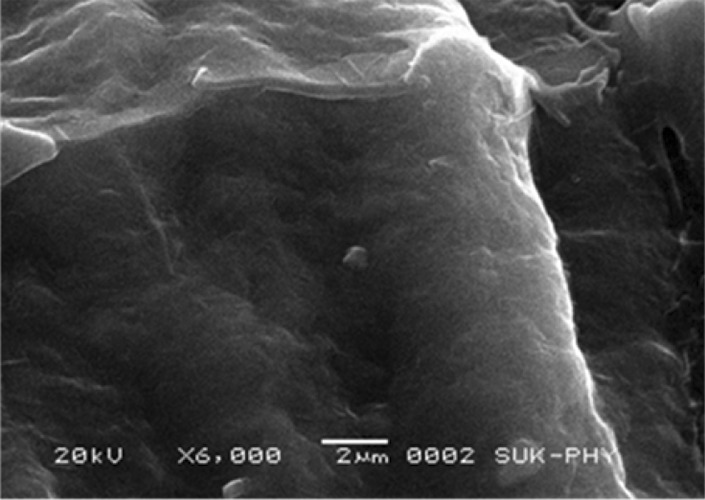
Scanning electron microscopy (magnification, 6000×) of scaffold showing the 3-D network of ECM overall morphology
Biocompability study
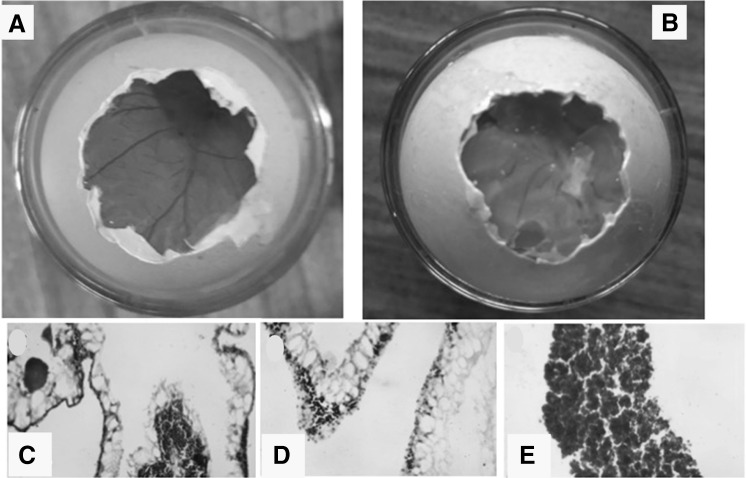
In order to confirm the biocompatibility of the engineered scaffold the chick chorioallantoic membrane (CAM) model was used. Figure 9A represents the CAM area of control and (B) CAM area of decellularized heart valve scaffold grafted at day 4 and visualized at day 8. Figure 9C, D represents the H&E staining of CAM area of control (C), experimental (D) and harvested tissue from the CAM.
Fig. 9.
Biocompatibility study of heart valve scaffold in chick chorioallantoic membrane (CAM). A The CAM area of control and B CAM area of decellularized heart valve scaffold grafted at day 4 and visualized at day 8. B Clearly suggests the grafted scaffold had no toxic effect and normal angiogenesis pattern is seen with compared to control. C, D The H& E staining of CAM area of control C and experimental D. The H& E staining reveals the uniform nuclear and cytoplasm structure with no observed necrosis. E The H& E staining of transplanted tissue harvested from CAM area shows normal recruitment of cells. This clearly reveals the biocompability of the scaffold
Discussion
This study was designed to generate decellularized porcine heart valves using flow bioreactor assembly. The extracellular matrix in scaffold represents a good substrate in terms of biochemical properties able to drive cell adhesion, proliferation and differentiation; moreover, decellularized scaffolds usually shows good biomechanical properties [23]. The rationale for use of the detergents in the present decellularization protocols was based on the decellularization agent’s effectiveness and destabilization of ECM. Detergents that are strongly ionic or hydrophobic or zwitterionic are commonly used and may be effective against one or two of the protein–protein interactions or/protein–lipid interactions but not all. Therefore, a mixture of detergents will be required for efficient removal of cellular material from the grafts. The use of SDS in our initial experiments did not give satisfactory results; we therefore decided to try other chemicals and choose DMSO, which is a very common organic solvent used for dissolving lipophilic substances. At the same time, DMSO is known to be cytotoxic at high concentration. We therefore combined the actions of DMSO together with SDS to achieve a gentle and efficient decellularization process. As per our best of knowledge we report for the first time the use of DMSO for decellularization of heart wall. We found that treatment with DMSO + SDS successfully preserved the three-dimensional architecture and native matrix composition of the tissue. Cell removal and ECM properties of decellularized porcine heart valves were investigated and compared to native tissue. The process showed good results; no residual cells were observed in HE staining and this condition was confirmed by DAPI analysis where no lasting nuclei were marked. Furthermore, the HE staining revealed a well maintained structure of the valve with preserved and organized laminae. The residual DNA content after decellularization was far low from the quantity found in native tissue; by now it is unclear whether this few residual DNA could elicit or not an adverse response from the host and indeed many commercial products with positive clinical outcomes contain remnant DNA and it seem unlikely that residual fragments could play a role in any host response [14]. It is very interesting to note that the low levels residual DNA confirms the removal of antigenicity which is very essential criteria for successful decellularization process. SEM analysis reported a preserved ECM of valve leaflet without evidence of any damaged area. This reserved basal layer is very important for tissue remodeling in the valve field since this avoids the promotion of early thrombi and the molecular signaling of the ECM can promote the re-endothelialization [24, 25]. The present methods enable to preserve basal layer and may facilitate the process of recellularization. In addition to this the last aspect takes even more importance when considering the possibility of naked implants (decellularized scaffold implanted without cell seeding) as suggested by Dahl et al. [26]; investigating whether a prior to grafting endothelial seeding is necessary or not becomes intriguing in the presence of a single rich surface like the decellularized one. A proper characterization of the decellularized leaflet, considering the however, the loss of the cellular component could introduce some alteration in the ECM and its interactions, which could have undesirable impact on cell adhesion, cell proliferation and there differentiation. The biocompability study of prepared scaffold is revealed in Fig. 9A, B clearly suggests the grafted scaffold had no toxic effect and normal angiogenesis pattern is seen with compared to control. The H&E staining reveals (Fig. 9C, D) the uniform nuclear and cytoplasm structure with no observed necrosis. Figure 9E. The H&E staining of transplanted tissue harvested from CAM area shows normal recruitment of cells. This clearly reveals the biocompability of the scaffold. Figure 10 represent the final decellularized valve scaffold by using combination of antioxidant and detergent agents. The complete removal of cellular and nuclear matter provides a promising substrate for recellularization process. This method reaches a key importance in the heart valves replacement field where few clinical substitutes are available. The aim of this work was to assess the outcome on porcine heart valves decellularization protocol by using SDS and DMSO combination for the first time and proving efficacy. The combination facilitates complete removal of cells uniformly within all layers of the tissue. Results of Masson’s trichrome staining, GAGs staining, and SEM analysis showed optimum native ECM retention. Thus proposed method successfully demonstrates the use of SDS in combination of DMSO for complete cell removal, and preserved ECM structure and holds a huge potential in heart valve tissue engineering application and transplantation.
Fig. 10.
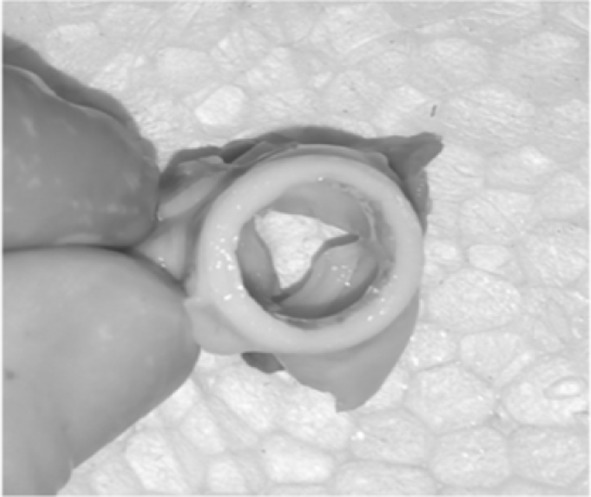
Decellularized porcine heart valve designed by novel approach by using combination of antioxidant and detergent agents
Acknowledgements
The authors thank D. Y. Patil University for financial support. Authors are also thankful for department of physics Shivaji University Kolhapur for extending SEM facility. Dr. Meghnad G Joshi acknowledge Department of Science and Technology (DST), Govt. of India (SB/SO/HS/0198/2013).
Conflicts of interest
The authors have no financial conflicts of interest related to this study.
Ethical Statement
All authors declare that they have no ethical issues for human and animal right in the ethical statement section. The present study we do not have any ethical issues for human and animal right. The present study does not involve use of human and animal subjects. The heart valves were collected from slaughter house. The study protocol was approved by the Institutional Research committee. The approval number is DMCK 93/2017.
References
- 1.Coffey S, Cairns BJ, Iung B. The modern epidemiology of heart valve disease. Heart. 2016;102:75–85. doi: 10.1136/heartjnl-2014-307020. [DOI] [PubMed] [Google Scholar]
- 2.Syedain Z, Reimer J, Lahti M, Berry J, Johnson S, Tranquillo RT. Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun. 2016;7:12951. doi: 10.1038/ncomms12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Driessen-Mol A, Emmert MY, Dijkman PE, Frese L, Sanders B, Weber B, et al. Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep. J Am Coll Cardiol. 2014;63:1320–9. [DOI] [PubMed]
- 4.Torella M, Torella D, Nappi G, Chiodini P, Torella M, De Santo LS. Oral anticoagulation after mechanical heart valve replacement: low intensity regimen can make the difference. J Clin Exp Cardiol. 2014;5:319. [Google Scholar]
- 5.Yacoub MH, Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med. 2005;2:60–61. doi: 10.1038/ncpcardio0112. [DOI] [PubMed] [Google Scholar]
- 6.Citerio G, Cypel M, Dobb GJ, Dominguez-Gil B, Frontera JA, Greer DM, et al. Organ donation in adults: a critical care perspective. Intensive Care Med. 2016;42:305–315. doi: 10.1007/s00134-015-4191-5. [DOI] [PubMed] [Google Scholar]
- 7.Rosen A, Ison MG. Screening of living organ donors for endemic infections: understanding the challenges and benefits of enhanced screening. Transpl Infect Dis. 2017;19:e12633. doi: 10.1111/tid.12633. [DOI] [PubMed] [Google Scholar]
- 8.Tillquist MN, Maddox TM. Cardiac crossroads: deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer Adherence. 2011;5:91–99. doi: 10.2147/PPA.S16420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guglielmetti LC, Sorabella R, Chiuzan C, Najjar M, Castillero E, Lambert D, et al. Bridging anticoagulation after mechanical aortic heart valve replacement: a questionable routine. Ann Thorac Surg. 2016;102:48–54. doi: 10.1016/j.athoracsur.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Cheung DY, Duan B, Butcher JT. Current progress in tissue engineering of heart valves: multiscale problems, multiscale solutions. Expert Opin Biol Ther. 2015;8:1155–1172. doi: 10.1517/14712598.2015.1051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey BM, Zeisberger M, Hoerstrup SP. Tissue engineering and regenerative medicine - new initiatives for individual treatment offers. Transfus Med Hemother. 2016;43:318–319. doi: 10.1159/000450716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S, Liu X, Yeung KWK, Liu C, Yang X. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng R Rep. 2014;80(1):1. doi: 10.1016/j.mser.2014.04.001. [DOI] [Google Scholar]
- 13.Mendelson K, Schoen FJ. Heart valve tissue engineering: concepts, approaches, progress, and challenges. Ann Biomed Eng. 2006;34:1799–1819. doi: 10.1007/s10439-006-9163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du T, Chao L, Zhao S, Chi L, Li D, Shen Y, et al. Successful cryopreservation of whole sheep ovary by using DMSO-free cryoprotectant. J Assist Reprod Genet. 2015;32:1267–1275. doi: 10.1007/s10815-015-0513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu ZW, Quinn PJ. Dimethyl sulphoxide: a review of its applications in cell biology. Biosci Rep. 1994;14:259–281. doi: 10.1007/BF01199051. [DOI] [PubMed] [Google Scholar]
- 16.Roosens A, Somers P, De Somer F, Carriel V, Van Nooten G, Cornelissen R. Impact of detergent-based decellularization methods on porcine tissues for heart valve engineering. Ann Biomed Eng. 2016;44:2827–2839. doi: 10.1007/s10439-016-1555-0. [DOI] [PubMed] [Google Scholar]
- 17.Liao J, Joyce EM, Sacks MS. Effects of decellularization on the mechanical and structural properties of the porcine aortic valve leaflet. Biomaterials. 2008;29:1065–1074. doi: 10.1016/j.biomaterials.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.König F, Hollweck T, Pfeifer S, Reichart B, Wintermantel E, Hagl C. A pulsatile bioreactor for conditioning of tissue-engineered cardiovascular constructs under endoscopic visualization. J Funct Biomater. 2012;3:480–496. doi: 10.3390/jfb3030480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naruse K, Yamada T, Sokabe M. Involvement of SA channels of cultured endothelial cells. Am J Physiol. 1998;274:H1532–1538. doi: 10.1152/ajpheart.1998.274.5.H1532. [DOI] [PubMed] [Google Scholar]
- 21.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Correia CR, Moreira-Teixeira LS, Moroni L, Reis RL, van Blitterswijk CA, Karperien M, et al. Chitosan scaffolds containing hyaluronic acid for cartilage tissue engineering. Tissue Eng Part C Methods. 2011;17:717–730. doi: 10.1089/ten.tec.2010.0467. [DOI] [PubMed] [Google Scholar]
- 23.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2011;13:27–53. [DOI] [PMC free article] [PubMed]
- 24.Pellegata AF, Asnaghi MA, Stefani I, Maestroni A, Maestroni S, Dominioni T, et al. Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed Res Int. 2013;2013:918753. [DOI] [PMC free article] [PubMed]
- 25.Peterse TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:222–231. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dahl SL, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant. 2003;12:659–666. doi: 10.3727/000000003108747136. [DOI] [PubMed] [Google Scholar]