Abstract
Stem cell research is one of the most rapidly expanding field of medicine which provides significant opportunities for therapeutic and regenerative applications. Different types of stem cells have been isolated investigating their accessibility, control of the differentiation pathway and additional immunomodulatory properties. Bulk of the literature focus has been on the study and potential applications of adult stem cells (ASC) because of their low immunogenicity and reduced ethical considerations. This review paper summarizes the basic available literature on different types of ASC with special focus on stem cells from dental and orofacial origin. ASC have been isolated from different sources, however, isolation of ASC from orofacial tissues has provided a novel promising alternative. These cells offer a great potential in the future of therapeutic and regenerative medicine because of their remarkable availability at low cost while allowing minimally invasive isolation procedures. Furthermore, their immunomodulatory and anti-inflammatory potential is of particular interest. However, there are conflicting reports in the literature regarding their particular biology and full clinical potentials. Sound knowledge and higher control over proliferation and differentiation mechanisms are prerequisites for clinical applications of these cells. Therefore, further standardized basic and translational studies are required to increase the reproducibility and reduce the controversies of studies, which in turn facilitate comparison of related literature and enhance further development in the field.
Keywords: Orofacial stem cells, Adult stem cell, Regenerative medicine, Stem cell therapy
Introduction to stem cells, types and potential applications
The stem cell engineering is a rapidly growing field in the area of regenerative medicine. Stem cells are being used extensively for understanding development and progression of diseases. Currently, stem cell therapy is one of the bravest and promising moves for successful treatment of various medical conditions. This field is rapidly expanding as different clinical trials reveal their tremendous therapeutic potentials. Stem cells have been investigated as potential therapy for various medical conditions and diseases such as; cerebral ischemia, parkinson’s disease, alzheimer’s disease, retinal disease, diabetes type 1 and 2, myogenic disease [1]. It is also applied for neuronal, cardiovascular and bone regeneration [1–3].
Although various stem cells have been isolated and defined, they share common general features which make them distinctive among other mammalian cells. The main interesting key feature of stem cells is their undifferentiated nature with a potential to either retain their stemness through self-renewal (symmetric division) or give rise to differentiated daughter cells (asymmetric division) [4]. In general, stem cells stay in a quiescent state inside adult tissue, where upon stimulation they enter the cell cycle for division [5, 6]. Two types of cell division mechanism follow the local physiological request, as asymmetric or symmetric cell division [7]. Asymmetric division allows maintenance of a constant stem cell population, while symmetric division is in response to tissue injury or disease conditions [8]. This is controlled by multiple complex biological pathways that maintain the balance; however, the exact mechanism is unknown.
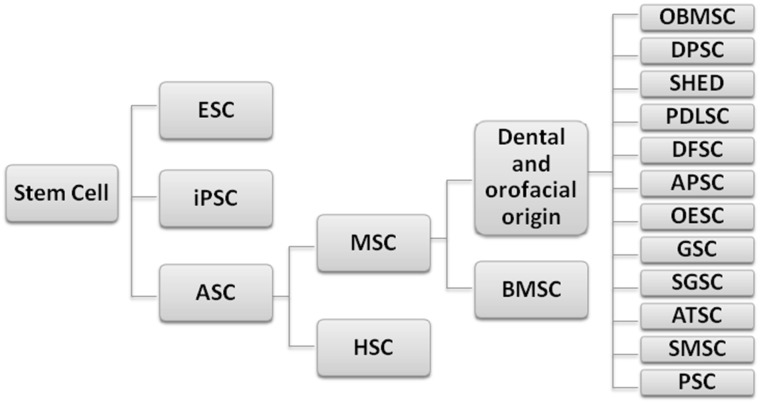
The main three types of stem cells investigated extensively for potential therapeutic and clinical applications in medicine are: embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs), and adult stem cells (ASCs) (Fig. 1). Stem cells are characterized by their ability of self renewal with maintenance of this proliferation potential for a long period, and their unspecialized state with the ability to differentiate (pluripotency) into multiple specialized cell lineages. However, multipotent adult stem cells (ASCs) have lower differentiation potential than pluripotent stem cells (ESCs and iPSCs) [9].
Fig. 1.
Main categories of stem cells. ESC embryonic stem cell, iPSC induced pluripotent stem cell, ASC adult stem cell, MSC mesenchymal stem cell, HSC hematopoietic stem cells, BMSC bone marrow stem cell, OBMSC orofacial bone marrow mesenchymal stem cell, DPSC dental pulp stem cell, SHED exfoliated deciduous teeth stem cell, PDLSC periodontal ligament stem cell, DFSC dental follicle stem cell, APSC adult pulp stem cell, OESC oral epithelium stem cell, GSC gingival stem cell, SGSC salivary gland stem cell, ATSC adipose tissue stem cell, SMSC Schneiderian membrane stem cell, PSC periosteum stem cell
ESCs are originated from the inner cell mass of embryonic blastocyst in the early pre-implantation stage after in vitro fertilization. They can differentiate into most cell types from all three germ layers [9]. A regulatory system of transcription factors maintains ESCs in a pluripotent and unspecialized state as long as they are cultured under appropriate conditions [10]. ESCs offer a great potential for clinical applications but their exact differentiation mechanism is still unclear.
iPSCs are generated through genetic reprogramming of somatic cells by forced expression of genes and transcription factors (i.e. Sox2, c-Myc, and KFL-4) to maintain defined properties of ESCs [11]. However, they differ from ESCs in their cellular epigenetic memory that may divert their differentiation potential toward donor cell lineages [12]. iPSCs are relatively easy to generate and they provide useful tools for drug investigation and in vitro modeling of specific diseases using patient derived cells [13]. However, the viral transfection is used to introduce the reprogramming factors into adult somatic cells which may alter iPSCs in a negative way and limit their applications. This necessitates careful controlling before any clinical applications. Recent studies investigate other non-viral mean of inducing iPSCs using miRNA or small molecules to enhance their stability and transduction efficacy [14, 15].
Stems cells are valuable natural source for therapeutic and regenerative medicine. The main goal is to control the cellular fate by diverting the differentiation pattern to the desired lineage and abolish undifferentiated cells population. However, the ability to control the cellular fate to the lineage of choice is a challenging issue for successful therapeutic applications. The critical drawbacks for clinical use of ESCs and iPSCs are their potential for immune rejection, teratoma formation and critical ethical regulations [11]. Therefore, the extensive body of literature is focused on study of adult stem cells (ASC) and their potential clinical applications. Hereby, we provide a detailed update on different types of adult stem cells, their features and clinical potentials with specific focus on new resources of ASC from dental and orofacial origin.
Adult stem cells
Definition, types, and basic characteristics
It is known that adult stem cells (somatic stem cells or post-natal stem cells) reside in specific location of each tissue in a specialized microenvironment known as the “stem cell niche”. In cell-based regenerative medicine, adult stem cells can be expanded in an undifferentiated state in vivo for a limited number of passages before differentiation into specialized cells of mesodermal origin. These multipotent progenitor cells allow immortalization for desired periods and can express a range of genes after genetic engineering. However, their isolation (from adult tissue and organ of body) and expansion are more difficult than ESCs and they have differentiation potential which is limited to cell range of the original tissue [16].
The two main types of adult stem cells are hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) (Fig. 1). HSCs are blood-derived and they may provide signaling molecules and growth factors that enhance function of other cells through paracrine mechanisms. MSCs were first recognized in bone marrow by Friedenstein, and play a crucial role in tissue regeneration following stress and injury impacts [17]. They are responsible for the maintenance of connective tissues by differentiation into several cell lineages such as; osteoblasts, chondrocytes, adipocytes, and myoblasts [18, 19].
The term MSC may be scientifically inaccurate to be applied to plastic adherent cells isolated from bone marrow or other sources since some of the recognized biological properties of cells may not match the generally accepted criteria for stem cell activity. Therefore, to clarify the terminology and avoid inconsistency between nomenclature and biologic properties, International Society for Cellular Therapy (ISCT) has proposed a new nomenclature “multipotent mesenchymal stromal cells” to be applied to the fibroblast-like plastic-adherent cells, regardless of the tissue of origin. However, the term “Mesenchymal stem cells” should be used only for cells that meet specified stem cell criteria while the commonly used acronym “MSC” can be still applied for both cell populations [20]. Furthermore, for more uniform identification, ISCT has proposed minimal four criteria to characterize human MSC. These criteria are as follow; (1) MSC must adhere to plastic tissue culture plate in standard culture conditions, (2) MSC must express surface definitive markers CD105, CD73 and CD90, (3) MSC should not express CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR, (4) MSC should be able to differentiate in vitro into different cell types of osteoblasts, adipocytes and chondroblasts [21].
Isolation and culture
MSCs are commonly isolated from bone marrow aspirate using adherent culture technique. MSCs represent only 1–5 cells in 1 × 104 nucleated cells of bone marrow but they can be expanded in vitro for research in tissue regeneration [22].
Bone marrow aspirate from the iliac crest is the most commonly used and documented procedure for MSC isolation in regenerative medicine. The isolated MSC from the iliac crest or femur proved to have a great potential in bone tissue engineering; however, this procedure is invasive for the patients [23]. Another important factor to consider is increasing donor age that can have a negative impact on biological behaviors of MSC by reducing their proliferation status, viability, multilineage differentiation potentials, expression kinetics, and immunoregulatory features [24, 25]. It has been reported that kinetics of cell growth is slower in MSC derived from old rats (>15 months old) compared with young ones (4 weeks old) [24]. There are many reports about the impact of donor age on clinical efficacy of MSC in bone regeneration where the osteogenic potential of these cells decline with the age increasing [26–28]. Furthermore, in vitro multipotent capability is also reduced by increasing culture period and repeated passaging [23]. In an effort to explain the underlying mechanism of age related impacts, MSC derived from old donors (18 months rat model) were found to have increased susceptibility to reactive oxygen species induced adhesion impairment and apoptosis compared to young donors (8–10 week old rat model). In addition, MSC from old rats donor showed more rapid reduction of survival rate after transplantation into the region of myocardial infarction of rat models [29]. Therefore, the fact that therapeutic capability of MSC is dependent on donor age, remains as a challenge and limitation in therapeutic and regenerative medicine that requires further investigation with special focus on different sources of MSC and their clinical differences. In particular, further studies are required to determine which types of MSC are less dependent on donor age.
Isolation of bone marrow MSC is not limited to iliac crest or femur as the orofacial bone marrow is also a valuable source of MSC (Fig. 1). Orofacial bone marrow stem cells (OBMSC) can be obtained from the bony maxilla or mandible during various intraoral or extraoral surgical procedures such as; dental implant, surgical exodontia of impacted tooth, enucleation of cyst, and orthognathic surgery. OBMSC can be isolated from all ages and it seems that its gene expression pattern is not affected by donor age [30].
It is known that craniofacial bones (membranous bone) provide a better quality bone for autologous grafting compared to other sources of endochondral bones (i.e. iliac crest, rib, femur) [31–33]. This is due to significantly higher reported bone volume, higher bone stability, and lower resorption rate after grafting from membranous bones, which implies that different donor tissues may express different regenerative potentials [34, 35]. The differences in embryonic origin can result in functional variations and behaviors of MSC originated from iliac crest and those of orofacial bones [36]. In fact, the functional difference between MSC of different sources has been well documented in several studies. MSC from orofacial bones demonstrated distinctive differentiation potential and expression pattern and were reported to have higher proliferation rate and osteogenic differentiation potential, are able to produce higher quantity (more volume) and quality (more mineralization) bone with less chondrogenic or adipogenic potential during osteogenesis when compared to those originated from other sources [37–40]. These properties, make OBMSC a better choice than MSC from other sources for craniofacial bone regeneration, however, the main disadvantages is limitation of available and collectable bone marrow volume from orofacial bones (about 0.1–3 ml) that necessitate a reliable in vitro cell expansion technique before their clinical applications [30, 40].
Potential clinical applications
MSCs have been used extensively for transplantation studies in animal model and human therapeutic trials. Their potential advantages include; ability to release bioactive molecules, represent specific receptors on their surface, allow genetic modification, and present low immunogenicity and minimum ethical concerns [41]. However, because MSC are maintained physiologically in a quiescent state within the tissue and organ, their study in the active growing state is more critical compared to pluripotent stem cells. Nevertheless, emerging evidence indicates the presence of both quiescent and active MSCs in several tissues (i.e. hair follicle, gut, bone marrow) in separate locations [6]. Generally, the main disadvantage of MSCs is the absence of definitive in vivo markers thus, they are still not clearly characterized in vitro [22]. Furthermore, when compared to embryonic stem cells, MSCs have limited proliferation and differentiation potential that decrease with passage and age [42].
In tissue engineering and regenerative medicine, MSCs are considered an attractive cell source as they can be rapidly expanded in vitro for several lineages while maintaining their differentiation potential [43]. Furthermore, they can be delivered using different natural or synthetic biomaterials for pre-clinical and clinical studies [44, 45]. It has been reported that MSCs can retain their stemness upon bioencapsulation that is linked to their hypoimmunogenic feature and limited alloantigen expression [46].
MSCs can also play an important role in angiogenesis by promoting the ability of adjacent endothelial cells in migration and tube-like formation [47, 48]. In addition, recent reports have shown their potential for differentiation into tissue-specific cells following systematic infusion [49, 50].
Various bioactive factors are released by MSCs such as cytokines and chemokines that produce paracrine effects. This signaling mechanism may possess an immunomodulatory role by allowing cell homing, migration and attachment of immune cells to injured cells. Currently, the mechanism for immunosuppressive potential of MSCs is not fully understood but it holds a great promise for treatment of auto-immune inflammatory diseases [51].
More recently, direct reprogramming of adult stem cells provided a new horizon as a unique therapeutic strategy in regenerative medicine. The main aim is to instruct adult cells to convert into other required cell types for tissue repair using defined transcription factors in adult organs. This technique allows generating a range of cell types similar to those derived from pluripotent stem cells without reversion [52, 53]. The main advantages of this method are overall simplicity and speed of differentiation process that also allow in situ conversion of cell fate. However, reprogrammed cells have to be characterized in vitro using extracellular matrix (ECM) for survival and growth ability before potential applications [54].
MSC from dental and orofacial origin
MSC from dental tissues
MSC have been isolated and characterized from multiple sources of dental tissues [55] (Fig. 1). MSC from adult dental pulp origin (DPSC) or exfoliated deciduous teeth (SHED) were among the first to be identified [56, 57]. They had similar phenotypic features to those of bone marrow origin (BMSC) such as multipotency and self-renewal capacity [56–58] in addition to their ability to regenerate dentinal pulp complex (Table 1). Furthermore, SHED has shown a distinctive potential in regeneration of bone in critical size defects in vivo by active contribution to osteogenesis and inducing the host cells to differentiate into osteogenic cells [57, 59]. Thus, exfoliated teeth could be a unique resource for stem cell therapy including autologous stem-cell transplantation and tissue engineering.
Table 1.
Different types of adult stem cells from dental and orofacial origin and their main characteristic features compared to bone marrow cells
| Stem cells | Source | Bone regeneration | Periodontium regeneration | Reported phenotypic markers | Advantage | Disadvantage | References |
|---|---|---|---|---|---|---|---|
| BMSC | Bone marrow aspirates, i.e. iliac crest, femur | Yes | None | Oct-4, Nanog, CD73, CD90, CD105, CD106,CD166, SSEA-4, CD9, CD13, CD146, Nestin, Notch-1, STRO-1, CD44,CD24 | Availability | Invasive for patient, Donor age dependant, prolonged culturing reduce in vitro multipotent capability | [23, 26–28] |
| OBMSC | Orofacial bone marrow aspirates, i.e. maxilla, mandible | Yes, produce higher quantity and quality bone than BMSC | None | CD73, CD90, CD105 | Donor age non-dependant, higher proliferation rate, less chondrogenic/adipogenic potential | Limitation of available and collectable bone marrow volume | [30–40] |
| DPSC | Adult dental pulp | Yes | Yes | Oct-4, Nanog, CD73, CD90, CD105, CD166, SSEA-4, CD9, CD13, CD146, Nestin, Notch-1, STRO-1, CD44, CD29 | Similar phenotypic features to BMSC, ability to regenerate dentinal pulp complex | Available after pulp exposure or extraction, requires selective isolation and characterization | [56, 58] |
| SHED | Exfoliated deciduous teeth | Yes | Yes | Oct-4, Nanog, CD73, CD90, CD105, CD166, SSEA-4, CD146, Nestin, STRO-1, CD44 | Similar phenotypic features to BMSC, ability to regenerate dentinal pulp complex | Available after tooth shedding, requires selective isolation and characterization | [57, 59] |
| PDLSC | Periodontal ligament | Yes, potential to repair critical size defects | Yes | Oct-4, CD73, CD90, CD105, CD166, CD9, CD13, CD146, Nestin, STRO-1, CD44, CD29 | Availability, simple procedure | Available after extraction, requires selective isolation and characterization | [59, 61, 62] |
| DFSC | Dental follicle of developing teeth | Yes, higher osteocalcin expression and calcium deposit than BMSC | Yes | CD73, CD90, CD105, CD166, SSEA-4, CD9, CD13, CD146, Nestin, Notch-1, STRO-1, CD44,CD24, CD29 | Availability, simple procedure | Available after extraction, requires selective isolation and characterization | [63, 64, 67] |
| APSC | Apical papilla of developing teeth | Yes | Yes | Oct-4, CD73, CD90, CD105, CD106, CD166, SSEA-4, CD9, CD13, CD146, Nestin,STRO-1, CD44, CD24, CD29, CD80, CD86 | Higher proliferation and regeneration capacity than DPSC | Available in impacted teeth, requires selective isolation and characterization | [65, 66] |
| OESC | Oral epithelium | Yes, stem cells of neural crest origin possess high osteogenic potential in the presence of BMP-2 | Yes | CD73, CD90, CD105, CD44H, | Unlimited availability, oral keratinocyte stem cells regenerate only into oral mucosa ex vivo | Requires selective isolation and characterization | [68–70, 72] |
| GSC | Gingival tissue | Yes | None | Oct-4, Nanog, CD73, CD90, CD105, CD106, SSEA-4, CD9, CD13, CD146, Notch-1, STRO-1, CD44,CD24, CD29 | Unlimited availability, faster proliferation rate than BMSC, immunomodulatory and anti-inflammatory potential | Less effective differentiation potential than PDLSC | [71, 74–77, 106] |
| PSC | Periosteum of maxillofacial bones, i.e. mandible | Yes, show preferential osteogenic differentiation, superior to BMSC in enhancing bone regeneration | None | CD73, CD90, CD105, CD106, CD166, CD9, STRO-1, CD44 | Faster proliferative ability, faster bone remodeling, more sensitive to signaling molecules than BMSC, produce cortical bone compared to BMSC that produce cancellous bone | Available only during surgical exposure | [79, 81–85] |
| ATSC | Adipose tissue, i.e. buccal fat pad | Yes, support accelerated wound healing and bone repair | Yes | CD73, CD90, CD105, CD29, CD34, CD44, CD45, CD14, CD19, CD146, SSEA, CD10, CD13, CD166 | Similar to DPSC but a higher proliferation rate, low donor site morbidity, induce regeneration of dental pulp | Requires selective isolation and characterization | [86–92] |
| SGSC | Salivary glands | Yes | None | CD90, CD117, CD34, CD44, CD29, ALDH, CD166, CD49f | Potential for regeneration of salivary gland is under investigation, isolated progenitor cells from stromal tissue can differentiate into osteoblast, chondrocytes and adipocytes | Requires selective isolation and characterization | [93–97] |
| SMSC | Schneiderian Membrane of maxillary sinus | Yes | None | CD44, VCAM-1, CD146, STRO-1, CD29, CD44, CK19, CD90, CD105, CD73 | Multilineage differentiation capacity into osteoblast, adipocytes and chondrocytes | Available only during surgical exposure, requires selective isolation and characterization | [98–100] |
ALDH aldehyde dehydrogenase, CK cytokeratin, SSEA stage-specific embryonic antigen, STRO-1 stromal precursor antigen-1, VCAM-1 vascular cell adhesion molecule 1
MSC isolated from periodontal ligament (PDLSC) is another type of MSC of dental origin, with the capacity to regenerate periodontium (i.e. cementum, PDL and alveolar bone) in vivo [60]. It has been reported that this potential is also site specific, as PDLSC harvested from alveolar bone surface of PDL when compared to those isolated from root surface, displayed higher proliferation capability, greater osteogenic differentiation potential, higher ALP activity (a marker enzyme of osteoblast differentiation) and mineralization-related markers, and higher bone regeneration ability (Table 1). In addition, they have shown the potential to repair critical size defects of calvaria bone in vivo [61]. Transplantation of these cells, which can also be obtained from extracted teeth, holds a great promise for regeneration of periodontium after periodontal diseases. Furthermore, human PDL can be cryopreserved and recovered subsequently for post-natal stem cells isolation, thus providing a valuable approach in tissue engineering [62].
MSC are also isolated from dental follicle or dental sac (DFSC) [63, 64] and apical papilla (APSC) [65, 66] of developing teeth. DFSC and APSC can be found in impacted teeth, which are commonly discarded as medical waste in dentistry. Interestingly, DFSC showed higher osteocalcin (OC) expression and calcium deposit once compared to bone marrow MSC (4 weeks implantation in mice), that indicates their potency to be as an alternative cell source for bone tissue engineering [67]. Developing dental tissue may provide better source for MSC. It has been reported that compared to DPSC, APSC demonstrate higher proliferation and regeneration capacity upon transplantation in vivo [66]. This could be of interest when a high regeneration capacity is required in critical applications with healing challenges.
MSC from lining mucosa of oral cavity
Another interesting source of adult stem cells is oral mucosa. To date, two types of MSC have been identified and isolated; oral epithelial stem cells (OESC) [68–70] and gingival derived stem cell (GSC) [71]. Oral keratinocyte stem cells could regenerate only into well-organized oral mucosa ex vivo and hold a promise to be used for intraoral grafting procedures [72]. Furthermore, stem cells of neural crest origin may also be located in craniofacial adult tissue as scattered islands of cells in oral tissue. These cells have been isolated in vivo from various oral tissues, i.e. palate, tongue and buccal mucosa. They possess osteogenic potential by differentiation into osteoblast cells, and have expressed high level of ALP enzyme and mineralization profile in the presence of BMP-2 [73], thereby providing a useful source for bone regeneration strategies (Table 1). The gingival overlying alveolar ridge and extracted teeth are frequently discarded but Zhang et al. [74] first characterized GSC which exhibited a stable morphology and characteristics at higher passage and a faster proliferation rate than bone marrow MSC, in addition they are not tumorigenic [75]. The inherent stemness of gingival cells, their multipotency, high reprogramming efficacy into iPSC, ease of isolation, clinical availability, and rapid expansion provide great potential for cell therapy in regenerative medicine and tissue engineering [76]. Interestingly, it has been shown that GSC exhibited fewer inflammatory-related changes during osteogenic differentiation both in vitro and in vivo when compared to PDLSC [77]. Most importantly, GSC were reported to be capable of immunomodulatory functions in experimental colitis animal model by suppression of lymphocyte proliferation and inflammatory cytokines, inducing expression of immunosuppressive anti-inflammatory factors (IL-10 and COX-2) and increasing infiltration of regulatory T cells at the colonic sites [74]. Therefore, GSC may further function as a promising alternative for immunomodulatory, anti-inflammatory and cytotherapeutic applications.
MSC from orofacial bony tissues
Periosteum-derived stem cells (PSC) are other interesting resources of stem cells. This is not only because of their physiological role in fracture repair but is also related to their unique osteogenic potential. The osteogenic capacity of inner layer of periosteum is addressed in multiple other studies after the initial report in 1932 [78]. The isolated heterogeneous cells from the periosteum show preferential osteogenic differentiation, however, they also have adipogenic and chondrogenic potential [79]. Comparative qualitative analysis of tissue-engineered bone comparing bone marrow MSC, alveolar bone cells, and periosteal cells have shown in vivo superiority of periosteal cells in enhancing bone regeneration [80]. Furthermore, histological comparison of newly formed bone after bone marrow and periosteal graft in rat calvarial defects has shown that bone marrow graft induced spongy bone formation, whereas periosteal graft produced cortical bone structure in defect. This finding suggests that quality of bone formation may also be affected by type and source of transplanted cells [81]. It has been found that under normal condition bone marrow MSC are more osteogenic than periosteal cells. However, periosteal cells have faster proliferative ability [82, 83], they are more sensitive to pre-treatment with some signaling molecules (i.e. basic fibroblast growth factor; bFGF and bone morphogenetic protein; BMP-2) before transplant hence, they are more osteogenic [83]. Cultured autogenous periosteal derived stem cells have also been tried clinically for alveolar ridge augmentation or maxillary sinus lift. The results of bone biopsy analysis have indicated prominent recruitment of osteoblasts and osteoclasts along with angiogenesis that suggested faster bone remodeling than conventional autogenous bone grafting. This can reduce postoperative healing phase after bone grafting or dental implant insertion by enhancing osseointegration. Furthermore, expanded periosteum-derived cells can offer a valuable source for cell based bone tissue engineering by reducing the required volume of autogenous bone graft by 40%, allowing less traumatic grafting procedures [84, 85]. Moreover, the concept of bone regeneration can be guided in a desired instructive way based on the use of biomaterials similar to periosteum itself in combination of appropriate construct that mimic ECM of bone.
MSC from adipose tissues and salivary glands
MSC derived from adipose tissue (ATSC) is another valuable source of progenitor cells in the field of regenerative medicine. ATSC can be easily harvested in large numbers from various sources and related to low donor site morbidity. Although ATSC originate from mesodermal lineages, but their applications can also be extended to ectodermal and endodermal tissues and organs [86]. Although subcutaneous adipose tissue is very abundant, the buccal fat pad could provide an accessible and rich source for stem cells. An animal study by Niada et al. [87] revealed that buccal fat pad contains progenitor cells with the ability to differentiate towards osteogenic lineage with deposition of calcified ECM. Autologous ATSC are applied successfully for orofacial bone reconstruction after jaw bone resections in human. They supported accelerated wound healing and new bone formation following transplantation and further rehabilitation with dental implants [88, 89]. Furthermore, transplanted ATSC induced dental pulp regeneration [90] and regeneration of periodontal tissues including PDL and alveolar bone in extraction sockets of animal models [91]. An animal study comparing ATSC and dental pulp stem cells revealed that although ATSC had a higher proliferation rate and better senescence resistance in culture, but ATSC are very similar and useful as DPSC in regenerative dentistry [92]. Therefore, the use of discarded fat tissue as one of the richest source of adult stem cells in mammals, for isolation and clinical application of stem cells may offer a paradigm shift in providing alternative therapeutic approach in regenerative medicine and dentistry.
Stem cells have also been isolated from salivary glands in human (SGSC) [93]. The primary culture of salivary gland usually contains various cells of different sources including stromal, blood vessel and parenchymal cells. Therefore, selective isolation and characterization is required to obtain the primary cell of interest. Although, the capacity of salivary gland stem cells for regeneration of salivary gland function is under investigation [94–96], but isolated progenitor cells from stromal tissue can be guided to differentiate into osteoblast, chondrocytes and adipocytes [97].
MSC from lining of maxillary sinus
More recently, it was found that the Schneiderian membrane of the maxillary sinus (Schneiderian Membrane stem cell-SMSC) is also a source of MSC. In vitro studies have demonstrated the ability of these cells for high expression of MSC markers (STRO-1, CD29, CD44, CD73, CD90, CD105 and CD146) and multilineage differentiation capacity into osteoblast, adipocytes and chondrocytes [98–100]. Furthermore, SMSC have the capacity to form mineralized bone-like deposits and maintain their MSC features after in vivo transplantation, thus maxillary sinus can also be a candidate of MSC origin for functional bone regeneration [98]. SMSC can be a strong candidate as alternative treatment option to conventional maxillary sinus lifting and bone grafting prior to dental implant insertion. Implementation of these stem cells as native and already present cells for bone regeneration at maxillary sinus floor is a promise for close future. This can significantly reduce the need for bone grafting procedures at maxillary sinus floor and related surgical trauma and cost. Further researches are required to disclose the full characteristics and potentials of SMSC in order to facilitate their application in clinical practice.
Orofacial stem cells; regenerative and immunomodulatoy potentials for clinical applications
The ideal stem cells for regenerative medicine or dentistry should be reliable and safe by allowing complete control of cell fate in the body upon transplantation. Currently, adult MSC are applied clinically for bone or periodontal regeneration. However, the main concerns are the accessibility and feasibility, ease of isolation and characterization, possibility of directing the differentiation pathway into desired cells/tissue of target, and added immunomodulatory properties.
Bone marrow MSC or periosteal derived MSC are suitable sources of progenitor cells for orofacial bony reconstruction because of potential functional match between cell source and target tissue. However, the differentiation capacity of adult MSC is limited to mesenchymal lineages which exclude their application for complex organ regeneration in tissue engineering. Therefore, alternative stem cells such as iPSC may be the choice for complex application; however, the facts of immune rejection, unreliable fate control and ethical issue hinder their clinical applications. The patient-derived autologous iPSC cells may be an alternative approach to overcome these issues. However, in depth knowledge of developmental physiology is required for successful induction of these cells to form desired specific progenitor cells for targeted tissue/organ regeneration.
Introduction of alternative sources of stem cells within the orofacial region holds a significant promise for future clinical applications (Fig. 1). This is because the orofacial sources of stem cells are very rich and accessible and do not require clinician to undergo special training. These cells are not limited to single lineage and are able to regenerate complex organ structures. Among all available reported sources of stem cells in orofacial region, the GSC seems to be the most convenient and accessible source. The gingival tissue can be easily obtained from patient with minimum morbidity and ASCs [75] and iPSCs [76] can be isolated and expanded in vitro to the required cell passage. However, isolation of stem cells from other sources such as bone marrow, periosteum, adipose tissue, salivary glands and dental tissue, are not convenient for clinicians and require more traumatic surgical procedures that cause further donor site morbidity.
In addition to the regenerative potential of stem cells, their immunomodulatory potential has been also an issue of concern. Some other therapeutic effects have been attributed to immunomodulatory properties of stem cells such as angiogenesis, anti-inflammation and anti-apoptosis [101]. The low inherent immunogenicity of MSC, in addition to their immunomodulatory properties is an attractive feature in cell transplant application. A multicentre, phase II experimental study in patients with steroid-resistant, severe, acute graft-versus-host disease (a life-threatening complication after allergenic transplantation with haemopoietic stem cells) confirmed the immunomodulatory capability of MSC. They revealed that infusion of MSC expanded in vitro, irrespective of the donor, might be an effective therapeutic strategy for these patients [102]. Therefore, MSC represent a promising alternative therapy for treatment and prevention of immune-mediated diseases [103].
With this regards, the human orofacial derived MSC have been also reported to possess immunomodulatory properties similar to those of BMSCs [104]. For example, oral mucosal lamina propria progenitor cells are capable of immunomodulation via a dose-independent pathway (unlike other MSC) by release of immunosuppressive molecules that indicates their potential application for wide range of immune-related diseases [105].
In particular, it has been shown that GSCs can function as an immunomodulatory and anti-inflammatory component of the immune system in vivo [74]. Zhang et al. [74] demonstrated that systemic infusion of GSC could home the cutaneous wound site by interaction with host macrophages and inducing their polarization to M2 macrophage (an anti-inflammatory phenotype of macrophages). Furthermore, they reduced the secretion of TNF-α by macrophages, therefore, contributing to a significantly promoted wound repair [106]. Other animal study models revealed that the systemic administration of allogeneic BMSC could prolong the survival of skin and cardiac allograft [107, 108]. This may be related to MSC inhibiting cytokine release and impairing function of natural killer cells and T and B lymphocytes.
Currently, bone marrow is the primary source of MSC, however, orofacial sources of stem cells and in particular, the human GSC have been reported to be superior to BMSC for cell based regenerative therapy [75]. They are reported to lack teratogenic potential and possess several advantages over other sources of MSC, i.e. ease of availability and isolation, homogenous and faster proliferation, stable morphology and characteristic at higher passages, maintenance of normal karyotype and telomerase activity in long-term cultures, high regenerative capacity, and high potential for immune modulation. These properties make them a promising source of stem cells for future clinical cell based therapeutic and regenerative applications [75].
Current challenges and future trend
Different studies investigated the potential clinical applications of stem cells from orofacial origin for regeneration of dental and non-dental tissues as well as cell-based immunotherapy. These include but not limited to- osseous [109–114], neural [115–119], and cardiac muscle regeneration [120], angiogenesis [121, 122], hepatocytes differentiation [123–125], corneal repair [126, 127], treatment of skeletal muscle dystrophy [128, 129] and diabetes mellitus [130, 131]. The interested readers can refer to other reviews on clinical applications of orofacial stem cells [127, 130, 132–134]. The clinical applications of these stem cells will expand in future; however, the current knowledge on their full features and potentials is limited.
Comparing and contrasting of the studies related to stem cells are very challenging because of several conflicting findings on stem cell behavior and phenotypic characteristics in the literature. For example, there are controversial reports with regards to expression of different antigens during stem cell culture. This may be attributed to differences in isolation techniques, culture protocol (culture medium and cell density), passage of stem cells, etc. [135]. It is important to note that younger and older passage of stem cells may also behave differently in their expression of antigen. For example, it is reported that percentages of expression of phenotypic marker are subjected to change in different passages of stem cells, where subsequent passaging may result in increase or decrease of relevant expression markers [136, 137]. With regards to stem cell isolation, the age of donor, body mass index, and exact anatomical location of isolated cell may also influence behavior of stem cells [37, 138, 139]. Furthermore, minor differences in isolation and culture condition may not influences phenotypic expression pattern of primary cells, but it may result in significant impact on differentiation profile of stem cells [140].
All of these variables contribute to controversies in current literature that necessitate development and adherence to standardized protocols for isolation, culture and characterization of stem cells (Table 2). For this purpose, several procedures have been suggested that results in more homogeneity in composition of initial cell culture, such as early washing procedure before cell culture, use of flow cytometric sorting, and use of immunomagnetic separation [141].
Table 2.
The list of important parameters that need to be standardized and fully reported in studies involving stem cells
| Parameter | Detail |
|---|---|
| Donor | |
| Source of tissue | Human, animal, species, genetically modified, purchased, etc. |
| Gender/age | Gender and age of donor at isolation time |
| Anatomical site | Description of exact anatomical location of tissue extracted for stem cell isolation |
| Donor status | General health, nutritional status, BMI, etc. |
| Stem cell | |
| Isolation method | Detailed description of surgical procedure, aspiration strategy, materials, etc. |
| Culture medium | Complete list of ingredients; type/source, batch number, etc. |
| Cell density | Initial cell density, cell density at passage time, etc. |
| Cell passage | Passage number selected for study |
| Characterization method | Detailed description of materials and equipments used to characterize stem cells and expression of their markers |
| Translation | Detailed method of seeding/injection into scaffold before grafting into recipient site |
| Biological signal | List of applied growth factors or proteins, i.e., BMP |
| Cell banking | The impact of banking procedure (i.e., technique and duration) on stem cell behavior |
Although, the main focus of recent studies has been directed toward exploration and characterization of new alternative sources of stem cells, there is a clear need to investigate their particular biology at genetic and cellular levels as well as their potential clinical applications. More specifically, with regards to dental and orofacial stem cells, further studies are necessary to explore the differences in their immunophenotypic characteristics and their relationship with stem cell behaviors. Moreover, identification of a specific phenotypic marker for each type of stem cell is required to help in better isolation and utilization of these cells.
Furthermore, in order to utilize the maximum potential of newly introduced stem cells, it is required to be able to understand and control their differentiation fate. It is specially true for the stem cells of orofacial region because of their potential to regenerate complex tissues. Therefore, a new wheel of research is required to identify the proper method of controlling their fate and guiding them into the desired differentiation track. It has been shown that the orofacial sources of stem cells can differentiate into different cell types and tissues, for example they can differentiate into bone forming cells. However, the characteristic biological features of differentiated cells; osteoblast in this case, should be investigated in more detail compared to the same cells originated from other sources. In addition, the quality and quantity of produced tissues; bone in this case, need to be compared more precisely with the same tissues produced from other cell lines.
Furthermore, the genotype pattern of these new stem cells and the way of their expression in relation to exposure to different external stimuli is not fully investigated in the literature. The impact of extracellular matrix, the properties of cell local microenvironment, topographical features and other external stimuli on the stem cell behavior are other areas to be disclosed in detail. In general, since the appropriate studies at cellular and molecular levels are lacking, it is difficult to conclude about the real impact of these newly identified stem cells in clinical cases. Furthermore, there are other unknown issues such as risk of transmission of infection, rejection rate, quantity of useful stem cells and impact of hereditary disorders and long term storage [142] that need further investigations and comparison to other sources of stem cells [143].
Another interesting area that requires exploration could be the possibility of stem cell banking or tissue banking for future potential applications. In general, all stem cells can undergo standard storage procedure by cryopreservation. However, dental stem cells require special treatment to ensure safety and prevent damage. The usual procedures followed by stem cell banking parties include onsite tooth collection, shipment using tooth transport kit, stem cell processing and cryopreservation.
In summary, clinical applications of stem cells require greater options for regulation of stem cells growth and differentiation profile. For this purpose, several attempts have to be made to understand particular physiology of conventional and newly identified stem cells. The exact self-renewal mechanism of dental and orofacial stem cells needs to be explored in more detail. Furthermore, the mechanism of upregulation and downregulation of different phenotypic markers during proliferation and differentiation of these stem cells require further studies. The interaction between ASC and immune system requires further attention to utilize the benefit of their immunomodulatory potential. Moreover, the interaction between ASC and different microenvironment needs to be understood and further optimized for regeneration of desired tissue. Understanding the particular response of ASC to different signaling molecules and growth factors during deposition of extracellular matrix is another area of further research. Further studies are required to bridge the gap between basic and clinical science related to applications of these stem cells. In this way, the application of stem cells can be optimized based on targeted clinical requirement considering their differentiation potential.
Conclusion
Adult stem cells have interesting capabilities to generate differentiated cells as well as offer immunomodulatory potential. These unique features have motivated extensive studies to define their exact behavior as an initial step toward utilizing their great potentials for clinical applications. Emergence of adult stem cells originated from dental and orofacial tissues provided a novel promising alternative to the traditional procedures and resources of stem cells. These cells could offer full clinical potentials of adult stem cells. Moreover, their ease of access, simplicity, greater availability, and lower cost could provide additional advantages over others sources for cell banking or potential applications. GSC, among the orofacial sources of stem cells, may hold a strong promise because of its remarkable availability and accessibility while simulating other feature of traditional BMSC. However, the full potentials of these new sources and the real clinical differences between them and traditional sources are not yet fully understood. Therefore, further basic and translational studies are required following standardized protocols in isolation, culture and handling of stem cells in particular those of orofacial origin. This helps better understanding of their particular biology and full clinical potentials in therapeutic and regenerative medicine.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Ding DC, Chang YH, Shyu WC, Lin SZ. Human umbilical cord mesenchymal stem cells: a new era for stem cell therapy. Cell Transplant. 2015;24:339–47. [DOI] [PubMed]
- 2.Leite C, Silva NT, Mendes S, Ribeiro A, de Faria JP, Lourenço T, et al. Differentiation of human umbilical cord matrix mesenchymal stem cells into neural-like progenitor cells and maturation into an oligodendroglial-like lineage. PLoS One. 2014;9:e111059. [DOI] [PMC free article] [PubMed]
- 3.Weymann A, Schmack B, Okada T, Soós P, Istók R, Radovits T, et al. Reendothelialization of human heart valve neoscaffolds using umbilical cord-derived endothelial cells. Circ J. 2013;77:207–16. [DOI] [PubMed]
- 4.Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/S0092-8674(00)81692-X. [DOI] [PubMed] [Google Scholar]
- 5.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–194. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez CV, Garcia-Lavandeira M, Garcia-Rendueles ME, Diaz-Rodriguez E, Garcia-Rendueles AR, Perez-Romero S, et al. Defining stem cell types: understanding the therapeutic potential of ESCs, ASCs, and iPS cells. J Mol Endocrinol. 2012;49:R89–111. [DOI] [PubMed]
- 10.Higuchi A, Ling QD, Hsu ST, Umezawa A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem Rev. 2012;112:4507–40. [DOI] [PubMed]
- 11.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 12.Kim K, Zhao R, Doi A, Ng K, Unternaehrer J, Cahan P, et al. Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:1117–9. [DOI] [PMC free article] [PubMed]
- 13.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1:39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Anokye-Danso F, Trivedi CM, Juhr D, Gupta M, Cui Z, Tian Y, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell. 2011;8:376–88. [DOI] [PMC free article] [PubMed]
- 15.Valamehr B, Abujarour R, Robinson M, Le T, Robbins D, Shoemaker D, et al. A novel platform to enable the high-throughput derivation and characterization of feeder-free human iPSCs. Sci Rep. 2012;2:213. [DOI] [PMC free article] [PubMed]
- 16.Van Damme A, Thorrez L, Ma L, Vandenburgh H, Eyckmans J, Dell’Accio F, et al. Efficient lentiviral transduction and improved engraftment of human bone marrow mesenchymal cells. Stem Cells. 2006;24:896–907. [DOI] [PubMed]
- 17.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–247. doi: 10.1097/00007890-196803000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. [DOI] [PubMed]
- 19.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. [DOI] [PubMed]
- 20.Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–5. [DOI] [PubMed]
- 21.Fernández Vallone VB, Romaniuk MA, Choi H, Labovsky V, Otaegui J, Chasseing NA. Mesenchymal stem cells and their use in therapy: what has been achieved? Differentiation. 2013;85:1–10. [DOI] [PubMed]
- 22.Väänänen HK. Mesenchymal stem cells. Ann Med. 2005;37:469–479. doi: 10.1080/07853890500371957. [DOI] [PubMed] [Google Scholar]
- 23.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–165. doi: 10.1023/B:ABME.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 24.Wu LW, Wang YL, Christensen JM, Khalifian S, Schneeberger S, Raimondi G, et al. Donor age negatively affects the immunoregulatory properties of both adipose and bone marrow derived mesenchymal stem cells. Transpl Immunol. 2014;30:122–7. [DOI] [PubMed]
- 25.Marędziak M, Marycz K, Tomaszewski KA, Kornicka K, Henry BM. The influence of aging on the regenerative potential of human adipose derived mesenchymal stem cells. Stem Cells Int. 2016;2016:2152435. [DOI] [PMC free article] [PubMed]
- 26.Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, et al. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–20. [DOI] [PubMed]
- 27.Nishida S, Endo N, Yamagiwa H, Tanizawa T, Takahashi HE. Number of osteoprogenitor cells in human bone marrow markedly decreases after skeletal maturation. J Bone Miner Metab. 1999;17:171–177. doi: 10.1007/s007740050081. [DOI] [PubMed] [Google Scholar]
- 28.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82:583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 29.Li L, Guo Y, Zhai H, Yin Y, Zhang J, Chen H, et al. Aging increases the susceptivity of MSCs to reactive oxygen species and impairs their therapeutic potency for myocardial infarction. PLoS One. 2014;9:e111850. [DOI] [PMC free article] [PubMed]
- 30.Han J, Okada H, Takai H, Nakayama Y, Maeda T, Ogata Y. Collection and culture of alveolar bone marrow multipotent mesenchymal stromal cells from older individuals. J Cell Biochem. 2009;107:1198–1204. doi: 10.1002/jcb.22224. [DOI] [PubMed] [Google Scholar]
- 31.Crespi R, Vinci R, Capparè P, Gherlone E, Romanos GE. Calvarial versus iliac crest for autologous bone graft material for a sinus lift procedure: a histomorphometric study. Int J Oral Maxillofac Implants. 2007;22:527–532. [PubMed] [Google Scholar]
- 32.Borstlap WA, Heidbuchel KL, Freihofer HP, Kuijpers-Jagtman AM. Early secondary bone grafting of alveolar cleft defects. A comparison between chin and rib grafts. J Craniomaxillofac Surg. 1990;18:201–205. doi: 10.1016/S1010-5182(05)80411-1. [DOI] [PubMed] [Google Scholar]
- 33.Koole R, Bosker H, van der Dussen FN. Late secondary autogenous bone grafting in cleft patients comparing mandibular (ectomesenchymal) and iliac crest (mesenchymal) grafts. J Craniomaxillofac Surg. 1989;17:28–30. [DOI] [PubMed]
- 34.Carinci F, Farina A, Zanetti U, Vinci R, Negrini S, Calura G, et al. Alveolar ridge augmentation: a comparative longitudinal study between calvaria and iliac crest bone grafrs. J Oral Implantol. 2005;31:39–45. [DOI] [PubMed]
- 35.Mertens C, Decker C, Seeberger R, Hoffmann J, Sander A, Freier K. Early bone resorption after vertical bone augmentation—a comparison of calvarial and iliac grafts. Clin Oral Implants Res. 2013;24:820–5. [DOI] [PubMed]
- 36.Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–9. [DOI] [PubMed]
- 37.Igarashi A, Segoshi K, Sakai Y, Pan H, Kanawa M, Higashi Y, et al. Selection of common markers for bone marrow stromal cells from various bones using real-time RT-PCR: effects of passage number and donor age. Tissue Eng. 2007;13:2405–17. [DOI] [PubMed]
- 38.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–68. [DOI] [PubMed]
- 39.Aghaloo TL, Chaichanasakul T, Bezouglaia O, Kang B, Franco R, Dry SM, et al. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 2010;89:1293–8. [DOI] [PMC free article] [PubMed]
- 40.Matsubara T, Suardita K, Ishii M, Sugiyama M, Igarashi A, Oda R, et al. Alveolar bone marrow as a cell source for regenerative medicine: differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399–409. [DOI] [PubMed]
- 41.Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;174:249–282. doi: 10.1007/3-540-31265-X_11. [DOI] [PubMed] [Google Scholar]
- 42.Fu RH, Wang YC, Liu SP, Huang CM, Kang YH, Tsai CH, et al. Differentiation of stem cells: strategies for modifying surface biomaterials. Cell Transplant. 2011;20:37–47. [DOI] [PubMed]
- 43.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Si YL, Zhao YL, Hao HJ, Fu XB, Han WD. MSCs: biological characteristics, clinical applications and their outstanding concerns. Ageing Res Rev. 2011;10:93–103. [DOI] [PubMed]
- 45.Ren G, Chen X, Dong F, Li W, Ren X, Zhang Y, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1:51–8. [DOI] [PMC free article] [PubMed]
- 46.Barry FP, Murphy JM, English K, Mahon BP. Immunogenicity of adult mesenchymal stem cells: lessons from the fetal allograft. Stem Cells Dev. 2005;14:252–265. doi: 10.1089/scd.2005.14.252. [DOI] [PubMed] [Google Scholar]
- 47.Bidarra SJ, Barrias CC, Barbosa MA, Soares R, Granja PL. Immobilization of human mesenchymal stem cells within RGD-grafted alginate microspheres and assessment of their angiogenic potential. Biomacromolecules. 2010;11:1956–1964. doi: 10.1021/bm100264a. [DOI] [PubMed] [Google Scholar]
- 48.Moon JJ, West JL. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr Top Med Chem. 2008;8:300–310. doi: 10.2174/156802608783790983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med. 2012;1:44–50. doi: 10.5966/sctm.2011-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hao L, Sun H, Wang J, Wang T, Wang M, Zou Z. Mesenchymal stromal cells for cell therapy: besides supporting hematopoiesis. Int J Hematol. 2012;95:34–46. doi: 10.1007/s12185-011-0991-8. [DOI] [PubMed] [Google Scholar]
- 51.Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128–134. doi: 10.1016/j.molmed.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Chambers SM, Studer L. Cell fate plug and play: direct reprogramming and induced pluripotency. Cell. 2011;145:827–830. doi: 10.1016/j.cell.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A, et al. Direct conversion of human fibroblasts to multilineage blood progenitors. Nature. 2010;468:521–6. [DOI] [PubMed]
- 55.Akiyama K, Chen C, Gronthos S, Shi S. Lineage differentiation of mesenchymal stem cells from dental pulp, apical papilla, and periodontal ligament. Methods Mol Biol. 2012;887:111–121. doi: 10.1007/978-1-61779-860-3_11. [DOI] [PubMed] [Google Scholar]
- 56.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100:5807–12. [DOI] [PMC free article] [PubMed]
- 58.Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29:532–539. doi: 10.1016/S8756-3282(01)00612-3. [DOI] [PubMed] [Google Scholar]
- 59.Seo B, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, et al. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14:428–34. [DOI] [PMC free article] [PubMed]
- 60.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55. [DOI] [PubMed]
- 61.Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, et al. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng A. 2011;17:1015–26. [DOI] [PubMed]
- 62.Seo BM, Miura M, Sonoyama W, Coppe C, Stanyon R, Shi S. Recovery of stem cells from cryopreserved periodontal ligament. J Dent Res. 2005;84:907–12. [DOI] [PubMed]
- 63.Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C. Dental follicle stem cells and tissue engineering. J Oral Sci. 2010;52:541–552. doi: 10.2334/josnusd.52.541. [DOI] [PubMed] [Google Scholar]
- 64.Morsczeck C, Götz W, Schierholz J, Zeilhofer F, Kühn U, Möhl C, et al. Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol. 2005;24:155–65. [DOI] [PubMed]
- 65.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, C, Zhang et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006;1:e79. [DOI] [PMC free article] [PubMed]
- 66.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. [DOI] [PMC free article] [PubMed]
- 67.Park BW, Kang EJ, Byun JH, Son MG, Kim HJ, Hah YS, et al. In vitro and in vivo osteogenesis of human mesenchymal stem cells derived from skin, bone marrow and dental follicle tissues. Differentiation. 2012;83:249–59. [DOI] [PubMed]
- 68.Izumi K, Tobita T, Feinberg SE. Isolation of human oral keratinocyte progenitor/stem cells. J Dent Res. 2007;86:341–346. doi: 10.1177/154405910708600408. [DOI] [PubMed] [Google Scholar]
- 69.Nakamura T, Endo K, Kinoshita S. Identification of human oral keratinocyte stem/progenitor cells by neurotrophin receptor p75 and the role of neurotrophin/p75 signaling. Stem Cells. 2007;25:628–638. doi: 10.1634/stemcells.2006-0494. [DOI] [PubMed] [Google Scholar]
- 70.Jones KB, Klein OD. Oral epithelial stem cells in tissue maintenance and disease: the first steps in a long journey. Int J Oral Sci. 2013;5:121–129. doi: 10.1038/ijos.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du L, Yang P, Ge S. Culturing and characterization of human gingival mesenchymal stem cells and their chemotactic responses to stromal cell-derived factor-1. Hua Xi Kou Qiang Yi Xue Za Zhi. 2015;33:238–243. doi: 10.7518/hxkq.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003;32:188–197. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 73.Ono M, Suzawa T, Takami M, Yamamoto G, Hosono T, Yamada A, et al. Localization and osteoblastic differentiation potential of neural crest-derived cells in oral tissues of adult mice. Biochem Biophys Res Commun. 2015;464:1209–14. [DOI] [PubMed]
- 74.Zhang Q, Shi SS, Liu Y, Uyanne J, Shi Y, Shi SS, et al. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol. 2009;183:7787–98. [DOI] [PMC free article] [PubMed]
- 75.Tomar GB, Srivastava RK, Gupta N, Barhanpurkar AP, Pote ST, Jhaveri HM, et al. Human gingiva-derived mesenchymal stem cells are superior to bone marrow-derived mesenchymal stem cells for cell therapy in regenerative medicine. Biochem Biophys Res Commun. 2010;393:377–83. [DOI] [PubMed]
- 76.Egusa H, Okita K, Kayashima H, Yu G, Fukuyasu S, Saeki M, et al. Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One. 2010;5:e12743. [DOI] [PMC free article] [PubMed]
- 77.Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, et al. Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials. 2013;34:7033–47. [DOI] [PubMed]
- 78.Fell HB. The osteogenic capacity in vitro of periosteum and endosteum isolated from the limb skeleton of fowl embryos and young chicks. J Anat. 1932;66:157–80. [PMC free article] [PubMed]
- 79.Wang Q, Huang C, Zeng F, Xue M, Zhang X. Activation of the Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo. Am J Pathol. 2010;177:3100–11. [DOI] [PMC free article] [PubMed]
- 80.Zhu SJ, Choi BH, Huh JY, Jung JH, Kim BY, Lee SH. A comparative qualitative histological analysis of tissue-engineered bone using bone marrow mesenchymal stem cells, alveolar bone cells, and periosteal cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:164–9. [DOI] [PubMed]
- 81.Ueno T, Honda K, Hirata A, Kagawa T, Kanou M, Shirasu N, et al. Histological comparison of bone induced from autogenously grafted periosteum with bone induced from autogenously grafted bone marrow in the rat calvarial defect model. Acta Histochem. 2008;110:217–23. [DOI] [PubMed]
- 82.Rosales-Rocabado JM, Kaku M, Kitami M, Akiba Y, Uoshima K. Osteoblastic differentiation and mineralization ability of periosteum-derived cells compared with bone marrow and calvaria-derived cells. J Oral Maxillofac Surg. 2014;72:694.e1–694.e9. doi: 10.1016/j.joms.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 83.Agata H, Asahina I, Yamazaki Y, Uchida M, Shinohara Y, Honda MJ, et al. Effective bone engineering with periosteum-derived cells. J Dent Res. 2007;86:79–83. [DOI] [PubMed]
- 84.Nagata M, Hoshina H, Li M, Arasawa M, Uematsu K, Ogawa S, et al. A clinical study of alveolar bone tissue engineering with cultured autogenous periosteal cells: coordinated activation of bone formation and resorption. Bone. 2012;50:1123–9. [DOI] [PubMed]
- 85.Schmelzeisen R, Schimming R, Sittinger M. Making bone: implant insertion into tissue-engineered bone for maxillary sinus floor augmentation-a preliminary report. J Craniomaxillofac Surg. 2003;31:34–39. doi: 10.1016/S1010-5182(02)00163-4. [DOI] [PubMed] [Google Scholar]
- 86.Mizuno H, Tobita M, Uysal AC. Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804–810. doi: 10.1002/stem.1076. [DOI] [PubMed] [Google Scholar]
- 87.Niada S, Ferreira LM, Arrigoni E, Addis A, Campagnol M, Broccaioli E, et al. Porcine adipose-derived stem cells from buccal fat pad and subcutaneous adipose tissue for future preclinical studies in oral surgery. Stem Cell Res Ther. 2013;4:148. [DOI] [PMC free article] [PubMed]
- 88.Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, et al. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201–9. [DOI] [PubMed]
- 89.Kulakov AA, Goldshtein DV, Grigoryan AS, Rzhaninova AA, Alekseeva IS, Arutyunyan IV, et al. Clinical study of the efficiency of combined cell transplant on the basis of multipotent mesenchymal stromal adipose tissue cells in patients with pronounced deficit of the maxillary and mandibulary bone tissue. Bull Exp Biol Med. 2008;146:522–5. [DOI] [PubMed]
- 90.Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M. Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials. 2012;33:2109–2118. doi: 10.1016/j.biomaterials.2011.11.056. [DOI] [PubMed] [Google Scholar]
- 91.Tobita M, Mizuno H. Adipose-derived stem cells for periodontal tissue regeneration. Methods Mol Biol. 2011;702:461–470. doi: 10.1007/978-1-61737-960-4_34. [DOI] [PubMed] [Google Scholar]
- 92.Hung CN, Mar K, Chang HC, Chiang YL, Hu HY, Lai CC, et al. A comparison between adipose tissue and dental pulp as sources of MSCs for tooth regeneration. Biomaterials. 2011;32:6995–7005. [DOI] [PubMed]
- 93.Sato A, Okumura K, Matsumoto S, Hattori K, Hattori S, Shinohara M, Endo F. Isolation, tissue localization, and cellular characterization of progenitors derived from adult human salivary glands. Cloning Stem Cells. 2007;9:191–205. doi: 10.1089/clo.2006.0054. [DOI] [PubMed] [Google Scholar]
- 94.Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3:e2063. [DOI] [PMC free article] [PubMed]
- 95.Rotter N, Schwarz S, Jakob M, Brandau S, Wollenberg B, Lang S. Salivary gland stem cells: can they restore radiation-induced salivary gland dysfunction? HNO. 2010;58:556–563. doi: 10.1007/s00106-010-2111-0. [DOI] [PubMed] [Google Scholar]
- 96.Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis. 2011;17:143–153. doi: 10.1111/j.1601-0825.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 97.Gorjup E, Danner S, Rotter N, Habermann J, Brassat U, Brummendorf TH, et al. Glandular tissue from human pancreas and salivary gland yields similar stem cell populations. Eur J Cell Biol. 2009;88:409–21. [DOI] [PubMed]
- 98.Guo J, Weng J, Rong Q, Zhang X, Zhu S, Huang D, et al. Investigation of multipotent postnatal stem cells from human maxillary sinus membrane. Sci Rep. 2015;5:11660. [DOI] [PMC free article] [PubMed]
- 99.Berbéri A, Al-Nemer F, Hamade E, Noujeim Z, Badran B, Zibara K. Mesenchymal stem cells with osteogenic potential in human maxillary sinus membrane: an in vitro study. Clin Oral Investig. 2017;21:1599–609. [DOI] [PubMed]
- 100.Kim SW, Lee IK, Yun KI, Kim CH, Park JU. Adult stem cells derived from human maxillary sinus membrane and their osteogenic differentiation. Int J Oral Maxillofac Implants. 2009;24:991–8. [PubMed]
- 101.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 102.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86. [DOI] [PubMed]
- 103.Zhao S, Wehner R, Bornhäuser M, Wassmuth R, Bachmann M, Schmitz M. Immunomodulatory properties of mesenchymal stromal cells and their therapeutic consequences for immune-mediated disorders. Stem Cells Dev. 2010;19:607–614. doi: 10.1089/scd.2009.0345. [DOI] [PubMed] [Google Scholar]
- 104.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry—part I: stem cell sources. J Prosthodont Res. 2012;56:151–165. doi: 10.1016/j.jpor.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 105.Davies LC, Lönnies H, Locke M, Sundberg B, Rosendahl K, Götherström C, et al. Oral mucosal progenitor cells are potently immunosuppressive in a dose-independent manner. Stem Cells Dev. 2012;21:1478–87. [DOI] [PMC free article] [PubMed]
- 106.Zhang QZ, Su WR, Shi SH, Wilder-Smith P, Xiang AP, Wong A, et al. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells. 2010;28:1856–68. [DOI] [PMC free article] [PubMed]
- 107.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–8. [DOI] [PubMed]
- 108.Zhou HP, Yi DH, Yu SQ, Sun GC, Cui Q, Zhu HL, et al. Administration of donor-derived mesenchymal stem cells can prolong the survival of rat cardiac allograft. Transpl Proc. 2006;38:3046–51. [DOI] [PubMed]
- 109.Yu BH, Zhou Q, Wang ZL. Periodontal ligament versus bone marrow mesenchymal stem cells in combination with Bio-Oss scaffolds for ectopic and in situ bone formation: a comparative study in the rat. J Biomater Appl. 2014;29:243–53. [DOI] [PubMed]
- 110.Park SY, Kim KH, Gwak EH, Rhee SH, Lee JC, Shin SY, et al. Ex vivo bone morphogenetic protein 2 gene delivery using periodontal ligament stem cells for enhanced re-osseointegration in the regenerative treatment of peri-implantitis. J Biomed Mater Res A. 2015;103:38–47. [DOI] [PubMed]
- 111.Liu HC, E LL, Wang DS, Su F, Wu X, Shi ZP, Lv Y, Wang JZ. Reconstruction of alveolar bone defects using bone morphogenetic protein 2 mediated rabbit dental pulp stem cells seeded on nano-hydroxyapatite/collagen/poly(L-lactide) Tissue Eng A. 2011;17:2417–2433. doi: 10.1089/ten.tea.2010.0620. [DOI] [PubMed] [Google Scholar]
- 112.Maraldi T, Riccio M, Pisciotta A, Zavatti M, Carnevale G, Beretti F, et al. Human amniotic fluid-derived and dental pulp-derived stem cells seeded into collagen scaffold repair critical-size bone defects promoting vascularization. Stem Cell Res Ther. 2013;4:53. [DOI] [PMC free article] [PubMed]
- 113.Annibali S, Bellavia D, Ottolenghi L, Cicconetti A, Cristalli MP, Quaranta R, et al. Micro-CT and PET analysis of bone regeneration induced by biodegradable scaffolds as carriers for dental pulp stem cells in a rat model of calvarial “critical size” defect: Preliminary data. J Biomed Mater Res B Appl Biomater. 2014;102:815–25. [DOI] [PubMed]
- 114.d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. [DOI] [PubMed]
- 115.Nicola FC, Rodrigues LP, Crestani T, Quintiliano K, Sanches EF, Willborn S, et al. Human dental pulp stem cells transplantation combined with treadmill training in rats after traumatic spinal cord injury. Braz J Med Biol Res. 2016;49:e5319. [DOI] [PMC free article] [PubMed]
- 116.Nicola FD, Marques MR, Odorcyk F, Arcego DM, Petenuzzo L, Aristimunha D, et al. Neuroprotector effect of stem cells from human exfoliated deciduous teeth transplanted after traumatic spinal cord injury involves inhibition of early neuronal apoptosis. Brain Res. 2017;1663:95–105. [DOI] [PubMed]
- 117.Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012;122:80–90. [DOI] [PMC free article] [PubMed]
- 118.Zhang J, Lu X, Feng G, Gu Z, Sun Y, Bao G, et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell Tissue Res. 2016;366:129–42. [DOI] [PubMed]
- 119.Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. [DOI] [PubMed]
- 120.Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2008;26:638–45. [DOI] [PubMed]
- 121.Yeasmin S, Ceccarelli J, Vigen M, Carrion B, Putnam AJ, Tarle SA, et al. Stem cells derived from tooth periodontal ligament enhance functional angiogenesis by endothelial cells. Tissue Eng A. 2014;20:1188–96. [DOI] [PMC free article] [PubMed]
- 122.Yoo CH, Na HJ, Lee DS, Heo SC, An Y, Cha J, et al. Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials. 2013;34:8149–60. [DOI] [PubMed]
- 123.Hirata M, Ishigami M, Matsushita Y, Ito T, Hattori H, Hibi H, et al. Multifaceted therapeutic benefits of factors derived from dental pulp stem cells for mouse liver fibrosis. Stem Cells Transl Med. 2016;5:1416–24. [DOI] [PMC free article] [PubMed]
- 124.Yamaza T, Alatas FS, Yuniartha R, Yamaza H, Fujiyoshi JK, Yanagi Y, et al. In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res Ther. 2015;6:171. [DOI] [PMC free article] [PubMed]
- 125.Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Fushimi N, Mitev V, et al. Novel management of acute or secondary biliary liver conditions using hepatically differentiated human dental pulp cells. Tissue Eng A. 2015;21:586–93. [DOI] [PMC free article] [PubMed]
- 126.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Intravitreally transplanted dental pulp stem cells promote neuroprotection and axon regeneration of retinal ganglion cells after optic nerve injury. Investig Ophthalmol Vis Sci. 2013;54:7544–7556. doi: 10.1167/iovs.13-13045. [DOI] [PubMed] [Google Scholar]
- 127.Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. Concise review: dental pulp stem cells: a novel cell therapy for retinal and central nervous system repair. Stem Cells. 2017;35:61–67. doi: 10.1002/stem.2398. [DOI] [PubMed] [Google Scholar]
- 128.Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Zucconi E, Fonseca SA, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: local or systemic? J Transl Med. 2008;6:35. [DOI] [PMC free article] [PubMed]
- 129.Yang R, Chen M, Lee CH, Yoon R, Lal S, Mao JJ. Clones of ectopic stem cells in the regeneration of muscle defects in vivo. PLoS One. 2010;5:e13547. [DOI] [PMC free article] [PubMed]
- 130.Guimarães ET, Cruz Gda S, Almeida TF, Souza BS, Kaneto CM, Vasconcelos JF, et al. Transplantation of stem cells obtained from murine dental pulp improves pancreatic damage, renal function, and painful diabetic neuropathy in diabetic type 1 mouse model. Cell Transplant. 2013;22:2345–54. [DOI] [PubMed]
- 131.Kanafi MM, Rajeshwari YB, Gupta S, Dadheech N, Nair PD, Gupta PK, et al. Transplantation of islet-like cell clusters derived from human dental pulp stem cells restores normoglycemia in diabetic mice. Cytotherapy. 2013;15:1228–36. [DOI] [PubMed]
- 132.Park YJ, Cha S, Park YS. Regenerative applications using tooth derived stem cells in other than tooth regeneration: a literature review. Stem Cells Int. 2016;2016:9305986. [DOI] [PMC free article] [PubMed]
- 133.Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015;9:1205–1216. doi: 10.1002/term.1899. [DOI] [PubMed] [Google Scholar]
- 134.Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K. Stem cells in dentistry—part II: Clinical applications. J Prosthodont Res. 2012;56:229–248. doi: 10.1016/j.jpor.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 135.Suga H, Matsumoto D, Eto H, Inoue K, Aoi N, Kato H, et al. Functional implications of CD34 expression in human adipose-derived stem/progenitor cells. Stem Cells Dev. 2009;18:1201–10. [DOI] [PubMed]
- 136.Mitchell JB, McIntosh K, Zvonic S, Garrett S, Floyd ZE, Kloster A, et al. Immunophenotype of human adipose-derived cells: temporal changes in stromal-associated and stem cell-associated markers. Stem Cells. 2006;24:376–85. [DOI] [PubMed]
- 137.Farré-Guasch E, Martí-Pagès C, Hernández-Alfaro F, Klein-Nulend J, Casals N. Buccal fat pad, an oral access source of human adipose stem cells with potential for osteochondral tissue engineering: an in vitro study. Tissue Eng C Methods. 2010;16:1083–1094. doi: 10.1089/ten.tec.2009.0487. [DOI] [PubMed] [Google Scholar]
- 138.van Harmelen V, Skurk T, Röhrig K, Lee YM, Halbleib M, Aprath-Husmann I, et al. Effect of BMI and age on adipose tissue cellularity and differentiation capacity in women. Int J Obes Relat Metab Disord. 2003;27:889–95. [DOI] [PubMed]
- 139.Jurgens WJ, Oedayrajsingh-Varma MJ, Helder MN, Zandiehdoulabi B, Schouten TE, Kuik DJ, et al. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: implications for cell-based therapies. Cell Tissue Res. 2008;332:415–26. [DOI] [PMC free article] [PubMed]
- 140.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10:320–330. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 141.Griesche N, Luttmann W, Luttmann A, Stammermann T, Geiger H, Baer PC. A simple modification of the separation method reduces heterogeneity of adipose-derived stem cells. Cells Tissues Organs. 2010;192:106–115. doi: 10.1159/000289586. [DOI] [PubMed] [Google Scholar]
- 142.Ballen K, Broxmeyer HE, McCullough J, Piaciabello W, Rebulla P, Verfaillie CM, et al. Current status of cord blood banking and transplantation in the United States and Europe. Biol Blood Marrow Transplant. 2001;7:635–45. [DOI] [PubMed]
- 143.Sullivan MJ. Banking on cord blood stem cells. Nat Rev Cancer. 2008;8:823. [DOI] [PubMed]