Abstract
Stem cell therapy opens a new window in medicine to overcome several diseases that remain incurable. It appears such diseases as cardiovascular disorders, brain injury, multiple sclerosis, urinary system diseases, cartilage lesions and diabetes are curable with stem cell transplantation. However, some questions related to stem cell therapy have remained unanswered. Stem cell imaging allows approval of appropriated strategies such as selection of the type and dose of stem cell, and also mode of cell delivery before being tested in clinical trials. MRI as a non-invasive imaging modality provides proper conditions for this aim. So far, different contrast agents such as superparamagnetic or paramagnetic nanoparticles, ultrasmall superparamagnetic nanoparticles, fluorine, gadolinium and some types of reporter genes have been used for imaging of stem cells. The core subject of these studies is to investigate the survival and differentiation of stem cells, contrast agent’s toxicity and long term following of transplanted cells. The promising results of in vivo and some clinical trial studies may raise hope for clinical stem cells imaging with MRI.
Keywords: Stem cell, MRI, Molecular imaging, Regenerative medicine, Cell therapy
Introduction
The administration of stem cells opens a new window on regenerating many damages in different tissues. The stem cells are capable of self-renew and repairing the damaged tissues with minimal side effects. In spite of promises for regenerative medicine, the stem cell therapy most often are still in the experimental stages, and needs to overcome some challenges before clinical application. However, concerns about some side effects such as failures of cellular therapy remain as important challenges for stem cell therapy. The most important concerns related to therapeutic applications of regenerative medicine include the selection of an appropriated stem cell type, delivery route of stem cells, and dosing regimen. Also, several questions regarding the biology of stem cells in living subjects after transplantation remain to be elucidated. For a successful transplantation, researchers have to overcome these challenges before stem cell therapy [1]. Moreover, it is very critical to understand the biology of transplanted stem cells and their interaction with the host tissue and regeneration of damaged tissue at cellular level.
It seems that providing the new techniques for monitoring the stem cell transplantation fate is an essential requirement for providing new opportunities in regenerative medicine. Recent advances in molecular imaging and production of new probes have provided successful non-invasive tracking of transplanted stem cells in the living subject [2]. Stem cell imaging can be performed through labeling cells with probes that attach to the cells. The signal generated from the probes can be visualized using several imaging systems such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), or magnetic resonance imaging (MRI).
Stem cells in regenerative medicine
New ideas in regenerative medicine must first be tested and the results have to be approved before being tested in clinical trials. Stem cells have a unique potential to develop into different types of cells and tissues, and to maintain cell source during early life and growth. These cells are capable of renewing themselves and also can be induced to tissue specific cells. Due to unique regenerative abilities of stem cells, regenerative medicine has non-negligible potentials for treating diseases such as heart disease, diabetes, kidney failure, stroke, and so on. Different sources of stem cells, such as hematopoietic, mesenchymal, adipose, embryonic, and neural stem cells have been introduced for treatment of various diseases. The bone marrow is the most important source of adult stem cells, containing the hematopoietic and mesenchymal stem cells. The adult tissues, including, adipose, peripheral blood, lung, fetal liver and fallopian tube are another sources for adult stem cells. Moreover, stem cells can be taken from the inner cell mass of blastocysts and umbilical cord blood [2, 3].
Different types of stem cells have been suggested for regenerative medicine. Mesenchymal stem cells (MSCs) are most common candidate for stem cell therapy and clinical application. These cells have good capacity for self-renewal and differentiation while can preserve self-multipotency [4]. A unique feature for MSCs is immunomodulatory properties that reduce probability of transplant rejection [5]. These cells are found in bone marrow and other adult people tissues and are able to differentiate various cell types belonging to skeletal tissues, such as adipocytes, chondrocytes, osteocytes, and cardiomyocytes [6]. Thus, MSCs can be used for different diseases in various tissues such as liver, the urinary, cardiovascular, and gastrointestinal system [7].
The neural stem cells are demonstrated to divide into progenitor cells and to develop into neurons, oligodendrocytes, and astrocytes. These progenitor cells are able to migrate through the brain and spinal cord to maintain neural cells populations. The neural stem cells have been employed for recovery of neurons and oligodendrocytes population within the brain for neurodegenerative diseases, stroke and traumatic brain injury [8]. Clinical and animal studies have been conducted to show the uses of stem cells in cases of spinal cord injury [9, 10].
Other types of stem cells that are able to differentiate to different types of cells are the embryonic stem cells. These cells were used for treatment of different diseases such as autoimmune diabetes mellitus, infertility, retinal blindness, myocardial infarction, etc. [11].
The importance of MRI stem cell imaging
Clinical and animal studies have shown promising results for stem cell regenerative medicine. Nonetheless, the results have been unreliable, resulting in many crucial questions regarding the feasibility of cell-based therapies. Immune responses and cell rejection, carcinogenesis, obtain of tissues other than desired types, and inappropriated functional recovery are important concerns related to stem cell regenerative medicine. Clinical implementation of stem cell therapy will require a better understanding of the fate of stem cell transplantation, differentiate the desired cell type, survive the recipient after transplant, proliferate and generate sufficient quantities of cells for making desired tissue, avoid harming the recipient and function appropriately for the duration of the recipient’s life [1, 12].
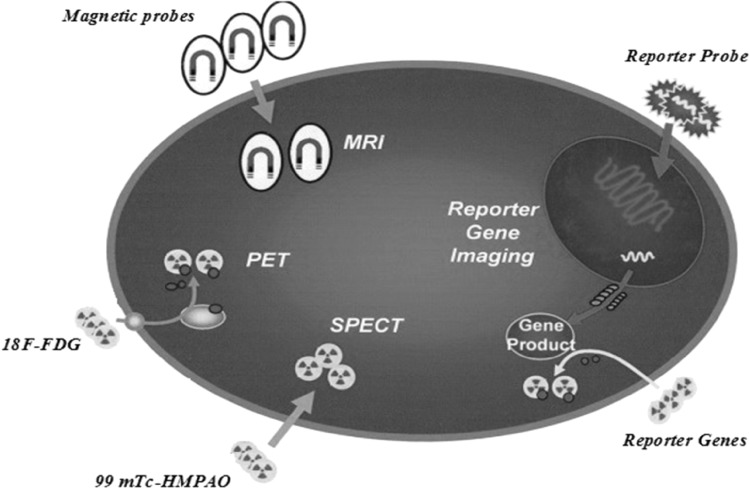
So, along with stem cell research in regenerative medicine, it is necessary to develop an appropriate method to monitor the fate of implanted stem cells after being transplanted to the patients. The best possible way to analyze graft–host interactions and cell survival, proliferation, immune system reactions, and migration is a method for monitoring safety and visualize cells in real-time during hours to days after transplantation. To realize the promise of novel stem cell therapy and overcome pervasive and debilitating diseases, scientists must make different research strategies and possess the mentioned characteristics for an appropriated transplantation method and to quantify their accumulation at the target organ. These can be obtained quantitatively and qualitatively through non-invasive molecular imaging [13, 14]. Moreover, depictions of the efficacy of stem cell regenerative medicine require assessment of in vivo cell tracking and functional recovery. Several molecular imaging techniques are available to follow stem cell fate after transplantation, including PET, SPECT and MRI [15]. Magnetically labeled cells with MRI have several important advantages compared to other imaging technique, including the non-invasive nature of MRI, long-term cell tracking, lack of ionizing radiation and appropriated soft tissue contrast and spatial resolution. Several studies have demonstrated the feasibility and longtime duration of tracking of MRI molecular imaging for stem cell imaging [16]. While, other imaging techniques such as PET and SPECT not allowing a long duration of cell tracking result in short half time of radionuclides. The short half time of probes of these imaging techniques like 6 h for 99 mTc-HMPAO and 1.83 h for 18F-FDG allows tracking of cells for some hours or day after cell injection. Moreover, ionizing radiation of these radionuclides may cause DNA damage and increased risk of cell death or carcinogenesis [17]. The using of MR contrast agents for labeling of stem cells can provide a simple and non-invasive method for tracking of stem cells and monitor accuracy of cell delivery to target tissue for a long time after stem cell transplantation. These characters have made MRI an appropriated choose for stem cell imaging (Fig. 1).
Fig. 1.
Different imaging modalities for tracing of transplanted stem cells
MR contrast agents for stem cells imaging
Direct labeling using MR contrast agents such as micro-particles or nanoparticles of iron oxide, gadolinium, 19F and reporter genes has the advantages of relatively nontoxic and high spatial resolution compared to labeling of cells by radionuclide agents. Moreover, labeling the cells with MR contrast agents does not affect stem cell differentiation. These properties with MRI labels enable MRI imaging to visualize the data localization and cell fate to detect therapeutic outcome, and help to adjust the dose and deliver route of stem cells to improve the safety and efficacy of stem cell therapy [18].
The stem cell labeling with different MR contrast agents has been used to visualize cellular homing, the efficiency of stem cell transplantation and targeting. Several studies have been conducted for cell labeling with magnetic nanoparticles and have shown that these contrast agents are generally nontoxic and do not affect stem cell division and differentiation capacity [19]. Different factors such as type and particle size are very important for selection of an appropriated contrast agent. In addition to particle size, using an appropriated labeling for contrast agent is very important for stem cell imaging. Saito et al. [20] have suggested that surface coating is more critical than particle size for the optimization of a MR contrast. The most common method for stem cell-labeling before injection is to culture cells with desired contrast agents.
Despite several advantages, most of the contrast agents used in MR stem cell imaging have failed to distinguish individual cells. Thus, for the purposes of cell imaging, such as stem cells used in cell therapy, cells must be labeled with a potent contrast agent to distinguish these cells from the background. Some MR contrast agents have been adopted for verifying the delivery of therapeutic methods after administration of stem cells [21]. The major classes of contrast agents are iron particles, gadolinium and perfluorocarbon (PFC) compounds contain 19F.
Iron particles
Iron particles with some micron in size (4 or 5 micron) have most applications for labeling of the stem cells. These particles produce a potent dark signal void in T2/T2* weighted images. Iron particles in several types and sizes have been used for stem cell tracking. These particles produce a strength signal that facilitates the monitoring of transplanted cells. By contrast to some toxic agents, iron particles have a good biocompatibility due to natural daily requirement of the human body and its biodegradable of iron after entrance to body [22]. Despite their advantages, use of iron particles, in case of overload of iron storage may cause an increase in intracellular unbound iron, resulting in the formation of reactive oxygen species (ROS) through catalyzing the conversion of superoxide and hydrogen peroxide to free-radical ions. The ROS production can lead to oxidative damage and cell death [23, 24]. So, administered iron concentration for MR cell imaging must be in a controlled range. Barrow et al. showed that polymers can reduce toxicity and also protects iron core against degeneration [25]. Paramagnetic nanoparticles, superparamagnetic iron oxide nanoparticles (SPIONs) particles and Ultrasmall superparamagnetic iron oxides (USPIOs) are most important types of iron particles that have been used for this aim.
Recently, non-toxic forms of iron compounds such as ferumoxytol, magneto-endosymbionts, bicycle[6.1.0]nonyne-modified glycol chitosan nanoparticles (BCN-CNPs) have been tested successfully [26–29]. Among them, ferumoxytol has been found as an FDA approved agent for clinical applications [30].
Paramagnetic and superparamagnetic iron oxide nanoparticles (SPIONs)
Superparamagnetic or paramagnetic nanoparticles are iron oxide particles in the range of up to 100 nm coated with biocompatible agents such as proteins, polymers, lipids and polysaccharides, which improve their stability and reduce their aggregation [31]. These particles have magnetization only in an applied magnetic field; also, they are able to form stable colloidal suspensions for biomedical application. The size, shape, and surface nature of SPIONs are controllable by changing the type of the iron salt [32]. In a magnetic field, the SPIONs have a strong magnetic susceptibility and induce fast T2/T2* relaxation.
Images obtained from Iron oxide nanoparticles are highly sensitive for detection of single cells [33]. While low specificity of this technique in regions with low signal leads to reduced ability for in vivo quantification of the signal loss [34]. These properties have made SPIONs as one of the most popular contrast agents used in research and clinical applications for MR imaging of cell therapy. In an applied magnetic field, the individual moments in SPIONs are free to align with the external magnetic field. This feature causes the formation of a single spin, with a net moment at least 4 orders of magnitude more than a comparable ensemble of paramagnetic spins [35]. As regards SPIONs can be delivered to a desired site by a magnetic force, they are good candidates for controlling targeting clinically.
Ultrasmall superparamagnetic iron oxides (USPIOs)
Ultrasmall superparamagnetic nanoparticles are another class of iron MRI contrast agents that were normally used as negative (T2) contrast agents. USPIOs are particles with the size smaller than 50 nm. The size of this particle type will control the T2/T1 relaxivity time and therefore the signal intensity in MRI. So, smaller size of USPIOs results in lower values for the T2 relaxivity time and increases the quality of the diagnosis. Due to small size of USPIOs, these particles are able to avoid fast uptake by the macrophages and the reticulum endothelial system (RES), that sustain prolonged circulation in the bloodstream after intravenous administration [36]. The size of the particles is a substantial factor in clearance by the RES or through renal filtration. Particles with a larger size than 200 nm in diameter are generally cleared via the RES, whereas particles with a smaller diameter than 10 nm are removed through renal clearance system. So, particles with a size between 10 and 100 nm have the greatest circulation time and are appropriated for long time monitoring [37]. Iron particles have shown, insufficient sensitivity for detection of single labeled cells which can be considered as one of the most important disadvantages of this contrast agent. Schellenberger et al. have attempted to detect single transplanted stem cells in vitro and also mice brains. They used very small size of iron oxide nanoparticles and images were obtained using a 7 T MRI instrument. Their results showed that single cell detection can be achieved only after a high number of labeled iron particles to injected cells. However, in an optimized situation the detection rate was lesser than 50% [38].
Fluorine-19 (19F)
19F is another MRI contrast agent that has been used for stem cell tracking in experimental and clinical studies. The 19F is an alternative to iron cell tracking that has been used as an appropriated contrast agent for stem cell imaging. The 19F is able to trace transplanted cells with a higher specificity compared to Iron oxide nanoparticles due to low levels of fluorine in cells [39]. The most important advantages of 19F are the detection of only labeled cells and no background signal from the host’s tissues observed. Perfluorocarbon (PFC), a compound containing 19F, widely has been used in NMR studies. PFC agents are not metabolized by cell and are not degraded by lysosomal enzymes. Also, PFCs do not lead to toxicity even at high doses. A PFC nanoparticle has 200–300 nm diameter and comprises a liquid core encapsulated and a high concentration of 19F atom. PFCs have been used as 19F tracer since the beginning of MRI for various aims such as angiographic and MRS.
For cell imaging, the number of PFC probe is related to the obtained 19F signal intensity and number of cells in regions of interest [40]. Several studies have taken PFC containing 19F as an appropriated MR contrast agent for stem cell tracking. Morawski et al. [39] reported a quantitative assessment of PFC nanoparticles as well as a linear relationship between the measured MR signal and the concentration of targeted PFC nanoparticles. Gaudet et al. have used 19F to detect the feasibility of quantifying human and mice MSCs survival labeled with a 19F in an immune-competent mouse host. Mice were imaged at four time points, at day 0, 3, 9 and 16 after implantation. This study showed the ability of 19F MR contrast agent in measuring the number of transplanted stem cells immediately after transplantation. However, this study has not shown a satisfy signal for later times [41]. A linear relationship between the measured signal intensity and the numbers of targeted nanoparticles that have been investigated within these studies. This is a significant advantage for 19F that can be used for many purposes of stem cell imaging such as selection of an appropriated dose and deliver route of stem cells.
In clinical experience, PFC was tested for acute toxicity. The obtained results didn’t show adverse effects at different using doses. Also, the results have not shown any evidence for active exocytosis of PFC, and the labeled cells preserved from the reticuloendothelial system. These results indicated PFC as an appropriated candidate for long term and non-toxic cell tracking [42]. The use of three dimensional compressed sensing method accelerates 19F MRI data acquisition by at least eightfold for cell tracking without seriously reduction in signal-to-noise ratio (SNR), image degradation and 19F quantification accuracy [43]. A combination of 19F and iron particles has been proposed for detection of viable cells from dead injected cells. Authors stated that imaging of iron-labeled macrophages in proton density images can predict cell rejection [44].
Gadolinium (Gd)
Gd is a common contrast agent in MRI that has been used in several experimental studies for tracking of transplanted stem cells. While there are some concerns related to potential toxicity of Gd such as nephrogenic systemic fibrosis in some patients, studies have shown satisfactory results for real time imaging of stem cells. Gd is a potent T1-weighted contrast agent with positive signal intensity in MRI images. In contrast to iron particles, positive signal in Gd improves the detection and tracking of cells in low-signal situation. Also, in hemorrhagic situations or necrotic tissues that produce T2-weighted images, use of Gd is preferable to dark signal contrast agents such as iron particles and 19F.
Gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) is the most used type of Gd compound in MRI examinations. However, some various types of Gd compound such as gadolinium rhodamine dextran, gadofluorine M, gadolinium-fullerenol and Gd-DTPA/jetPEI complexes have been examined in several studies. An important disadvantage of Gd-DTPA is its inability to pass through the stem cell membrane. Some studies have made efforts to use other compounds containing Gd for improving cell uptake. Tseng et al. have suggested gadolinium hexanedione nanoparticles (GdH-NPs) to label hMSCs. High hydrophobicity of this compound compared to Gd-DTPA can facilitate its possession through cell membrane and accumulation in hMSCs. As a result, the obtained signal was more powerful [45].
An important advantage of Gd-DTPA compared to some other contrast agents is its fast elimination in dead cells or interstitial spaces. This can allow to separate the viable cells from necrotic or rejected cells [46]. Considering this feature, signal produced by Gd-DTPA is used for detection of dead and live transplanted stem cells. Njen et al. have shown in dead cells labeled with Gd-DTPA and SPIONs, Gd-DTPA against SPIONs releases faster from dead stem cells and away from SPIONs. This results in generation of spots with T1 signal in the vicinity of the dead cells. On the other hand, live cells did not release Gd-DTPA or SPIONs and T1 signal was not observed in the vicinity [47].
In vivo tracking of MSCs labeled with Gd-DTPA/jetPEI have shown real time distribution of MSCs with the hyper-intense signal in injured spinal cord area during 14 days following transplantation. Histological evaluation has confirmed that labeled MSCs by this compound could survive in the injected area. However, there was not any apparent relation between numbers of injected cells and signal intensity [48].
An interesting idea for efficient uptake and high labeling efficiency of contrast particles including Gd-DTPA is using transfection reagent such as viral vectors, liposome, calcium phosphate and others. Using liposomes has shown better concentration and uptake of Gd by the cell membrane of MSCs. Also, liposomes did not have toxic effects on differentiation, viability or proliferation [49]. Shen et al. have shown an effective uptake of Gd-DTPA using a non-liposomal lipid transfection reagent into neural stem cells. This method showed more efficient uptake and less toxicity effect on stem cells compared to other transfection agents such as viral vectors, liposome or calcium phosphate [50]. These studies have shown that Gd-DTPA labeled with different carriers can be good candidate for tracing of injected stem cells. Despite all the advantages of Gd compounds, nephrotoxicity and nephrogenic systemic fibrosis (NSF) are of main side effects for clinical application. However, in recent years, some strategies have been conducted to reduce toxicity of this contrast agent [51, 52]. For example, trimetasphere metallofullerene is a form of Gd compounds with low toxicity which has used for stem cell tracing with success. In this compound Gd core encapsulated in the center of a metallofullerenes [53].
Reporter genes
In molecular biology, reporter genes are used to detect the expression of the gene of interest and changes in transcriptional rate. Also, reporter genes are capable to assess the location of transcriptional activity of a specific protein within living cells. In recent years, different new classes of reporter genes have been encoded for various imaging modalities such as PET, SPECT and optical imaging. MRI reporter genes have unique properties among all reporter genes used with other imaging modalities because these reporter genes are capable to provide information about gene function that can be combined with anatomic and functional information [54]. MRI reporter genes embody serial imaging, which is useful for visualization of dynamic processes. Recent advances in MRI reporter gene techniques have been able to image the cell division, proliferation, migration, and survival. Some recent studies have been conducted to track the survival and proliferation of pluripotent or multipotent cells injected into injured tissue used in cell based regenerative therapies.
Alongside the increased need of molecular imaging for regenerative medicine and stem cell imaging, a sheer number of MRI reporter genes has been developed to visualize the survival and proliferation of stem cells injected into injured tissue. A number of approaches for MRI imaging by reporter genes including, use of constitutive over-expression of iron binding proteins in cells, use of transgenic cells that express the special genes, and use of targeted contrast agents for visualization of engineered cell surface reporter genes has been introduced [55]. Some types of reporter genes include iron homeostasis proteins, reporter enzymes, and chemical exchange saturation transfer (CEST) reporter genes [56].
Overexpression of iron-containing proteins, including transferrin receptor was expected to increase the level of iron in the cells, and thus causes buildup of iron within ferritin. Over-production of the heavy chain of ferritin, which may use in combination with the ferritin or the transferrin receptor, leads to overload of intracellular iron stores. The enzymes such as divalent metal transporter (DMT1) and tyrosinase, β-galactosidase were suggested as reporter gene for MRI. Upregulation of these enzymes results in accumulation of paramagnetic ions and thus generates T2 signal contrast. Reporter genes have been used for imaging of neurogenesis, cardiac, cancer and others [57]. Genetically, labeling of stem cells with one or several reporter genes has unique advantages compared to other labeling methods, because a reporter gene label integrated in the stem cell would be transmitted to its progeny cells, whereas signals resulting from other contrast agents would become weaker with every cell division. This feature allows stem cell tracking regardless of the number of cell divisions. Moreover, the reporter genes are only expressed by viable cells. Thus the reporter gene can be inserted under a specific gene promoter, these contrast agents are only visualized if the stem cell differentiates into desired phenotype [58]. Additionally, the expression of a reporter gene can be made dependent on the differentiation status of a cell. So, the detection of gene reporter correlates with stem cell viability and differentiation ability.
Pereira et al. have investigated the overexpression of ferritin heavy chain-1 (but no transferrin receptor-1) affected the cell’s iron homeostasis. The overexpression of ferritin heavy chain-1 or transferrin receptor-1 didn’t cause remarkable increases in intracellular iron content, but significant increases were seen when these two agents were used in combination. Also, the supplementation with iron sources to obtain contrast is more efficient than the reporter genes [59].
Deans et al. have shown the induction of human transferrin receptor and ferritin in mouse neural stem cell. The transgenic cells have shown a significant increase in T2* at 1.5 and 7 T. The transplantation of these cells into mouse brain showed increase in contrast with surrounding tissue on T2*-weighted images. While the viability of cells was not decreased, increase in ROS was investigated [60]. The adding of iron to the culture medium of transferrin and ferritin reporter has shown that this method is more effective to obtain a more appropriate contrast compared to the use of reporter genes alone [59]. In despite of several advantages of reporter genes, low signal intensity in these contrast agents compared to others is the most important concern (Table 1).
Table 1.
Contrast agents for MR stem cell imaging
| Contrast agents | Advantages | Disadvantages |
|---|---|---|
| Iron particles (include Superparamagnetic iron nanoparticles, Iron oxide nanoparticles and USPIO) | High sensitivity, potent signal | ROS production, decrease in cell proliferation, uptake by the mononuclear phagocyte system, negative signal, partial volume artifact |
| Fluorine-19 | High specificity, linearity relationship to cell numbers | Low sensitivity, low signal to noise ratio per unit scan time |
| Gadolinium | Differentiation between live and dead cells, positive signal | Nephrotoxicity, Nephrogenic systemic fibrosis (NSF) |
| Reporter genes | Long term tracking of cells | Limited Contrast, effect on Iron homeostasis, ROS production |
Because, low contrast is the main disadvantage of reporter genes for tracking of stem cells, scientists have tried to improve MRI signals using novel or dual reporter gene imaging. Development of novel reporter genes such as T cell immunoglobulin and mucin domain containing protein 2, adenoviral vector encoding ferritin heavy chain and magneto-endosymbionts are some example that have been produced in recent years [61–63]. In a study by Guo et al. studied tracing of MSCs in rabbit using two reporter genes including ferritin heavy subunit and transferrin receptor. Their results indicated that dual reporter genes augment the content of produced iron in MSCs without any effect on biological properties of transplanted cells. Also, MRI contrast increased and homing and migration of MSCs detection was improved [64]. In addition, some studies have conducted to provide better understanding of location of transplanted cells using dual modality reporter genes. For example, the tyrosinase reporter gene has been used to trace MSCs to animal infarction cardiac using MRI, ultrasound and PET imaging [65]. Dual-imaging reporter genes have been used for MRI and fluorescence too [66].
MR stem cell imaging in different organs
Stem cell therapy has been recommended for treatment of different disorders in various organs such as cardiovascular, nervous, gastrointestinal and urinary systems, and also joints. The most important indications for stem cell therapy and tracking in experimental and clinical studies include stroke, neurodegenerative diseases, trauma, diabetes, multiple sclerosis (MS) and others.
Cardiovascular system
Cardiovascular disorders such as myocardial infarction (MI) are the major cause of morbidity and mortality in the world [67]. Although the most effective treatment is cardiac transplantation, the difference between organ supply and demand restricts its applicability. Another treatment modality is cellular cardiomyoplasty that includes systemic (intravenous) and local (intramyocardial, intracoronary) delivery of skeletal myoblasts, fetal/neonatal cardiomyocytes, embryonic stem cells, hematopoietic stem cells or MSCs. Regenerative medicine is a promising method for functional recovery in MI patients. This modality effort into repopulating the region of infarction enhances cardiac function with viable cardiomyocytes. MRI as a noninvasive imaging technique could use the assessment of migration, survival, and differentiation condition of implanted stem cells in infarcted myocardium [68–70].
The results obtained from preclinical and some clinical studies have indicated that stem cell therapy may improve myocardial function. However, there are questions especially about appropriated method for injection of cells and filtration of stem cells into the infarcted area. Li et al. have evaluated cardiac function after injection of bone marrow stromal cells with a reporter gene in myocardial infarction in rat model. Tracking of delivered cells with MRI has shown that intramyocardial cell implantation compared to intravenous or Intra-aortic implantation results in better localization of cells in the heart. While, signal loss after 48 h was remarkable [71]. Campan and colleagues have reported the use of ferritin heavy chain as a reliable reporter gene to track injected stem cells in a rat model of myocardial infarction. The T2* gradient echo sequence showed iron-accumulating tissue in hearts treated with ferritin reporter gene for 4 weeks after infarction. Prussian blue staining confirmed that the myocardial function and differentiation into cardiac muscle lineage, endothelial, and smooth muscle were not affected by ferritin overexpression [72]. He et al. have detected the noninvasively transplanted MSCs labeled with SPIO for 4 weeks after injection of labeled cells. Histopathological examinations showed that the injected cells were surviving in the MI heart [73] (Fig. 2).
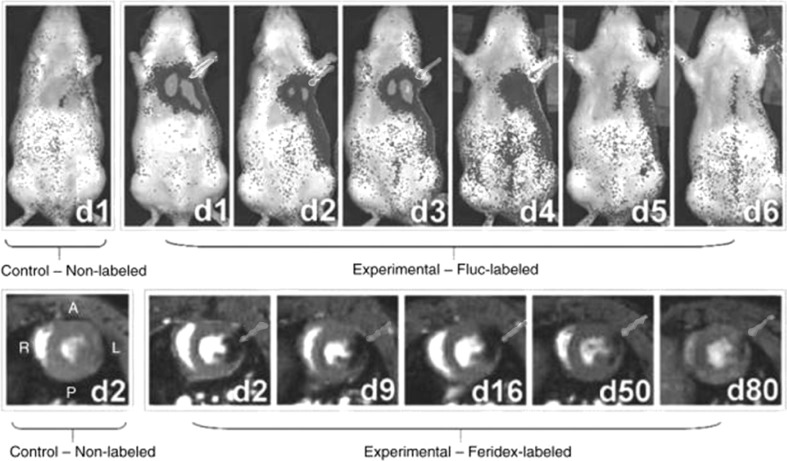
Fig. 2.
Serial bioluminescence and MR imaging of transplanted H9c2 cells. MR imaging indicates a high hypointense signal of transplanted cells in the myocardium. The scope and intensity of signal decreases with time. Transplantation of cells has confirmed by bioluminescence imaging.
Adopted from Cromer Berman et al. [103], with permission
Urinary system
In urology, novel applications of regenerative medicine and tissue engineering were used for many disorders, and the researches in this field have increased dramatically over the past decade. MRI is a complementing and contending modality for studies of stem cell in urology. MRI with a physical labeling method or alone can be applied to assess migration and survival of transplanted stem cells in bladder dysfunction models and prostate cancer. In addition, studies suggest potential efficiency for use on urethral sphincter dysfunction and erectile dysfunction [74–76].
Many studies reported the efficacy of using stem cell in treatment of bladder dysfunction. Yun and Ja reported similar livability of SPION-labeled MSCs compared to unlabeled cells. MSCs labeled with SPIOs underwent normal adipogenic, chondrogenic, and osteogenic differentiation. MRI signal intensity in the regions of SPION-labeled MSCs in rabbit and rat bladders decreased and was limited locally. MRI showed that SPIO-labeled MSCs injected into the bladder could be observed for at least 12 weeks after injection [77]. In another study, Lee et al. demonstrated that MR images were beneficial for monitoring transplanted MSCs in bladder outlet obstruction induced bladder dysfunction. T2-weighted MR images were taken instantly after transplant of MSCs labeled with SPIONs and at 4 weeks after transplantation. T2-weighted MR images demonstrated a clear hypo-intense signal induced by these cells. TGF-β expression and collagen increased after bladder outlet obstruction, and after MSCs transplantation, the expression of both returned to original levels [78]. In another study, Lee et al. [79] used MRI to monitor the migration of genetically modified stem cells after labeling these cells with fluorescent magnetic nanoparticles.
In regarding to urethral sphincter dysfunction, Riviere et al. labeled muscle implants with anionic magnetic nanoparticles. They investigated the biocompatibility of the labeling procedure and its efficiency for MRI follow-up of cell therapy in a model of female pig. These nanoparticles were adsorbed on the implant surface of myogenic precursor cells and were magnetically labeled within the implants. They showed magnetic labeling did not affect cell differentiation or proliferation. In addition, detection of auto graft in vivo by MRI was possible up to 1 month [80].
Song et al. suggested that MRI can be applied to evaluate the long-term therapeutic potential of MSCs for treatment of erectile dysfunction. MSCs labeled with SPIONs injected into the corpus cavernosa of rabbits and rats were evaluated by MRI. MRI signal intensity at the area of these cells in the rabbit and rat corpus cavernosa decreased and was limited locally. MRI showed that the MSCs could be seen for at least 12 weeks’ post- injection into the corpus cavernosum [81].
Nervous system
The experimental studies explored the promising results of stem cell therapy for neurodegenerative diseases and improvement in neural functioning [82]. Stem cell therapy for neural system can alleviate deficits in experimental stroke model in several studies. Molecular imaging of different types of stem cells such as MSCs, bone marrow hematopoietic stem cells and mouse embryonic stem cells (ESCs) through MRI can monitor location, size, tissue repair, stem cell fate, and responses to transplanted stem cell therapy to brain for long time after transplantation [83, 84]. In contrast to other tissues, cellular turnover in the nervous system occurs in a much lower rate and cannot completely restore function. After incidence of stroke, newly generated cells from stem cells migrate to the damaged area of the brain. However, survival of brain stem cells in the damaged site is jeopardized.
Neurological disorders such as cerebral ischemia or mentioned neurodegenerative diseases result in a mobilization of progenitor cells and their migration towards the damaged areas. In most cases, the intrinsic response is not sufficient to lead to the functional recovery and to result in a permanent disorder. In the last decade, evidence of neurogenesis probability in the human adult brain has provided the basic scientific hypothesis of (stem) cell transplantation therapy in various neurological disorders including; Parkinson disease, multiple sclerosis (MS), and stroke, to improve neurological defects and relieve disability. It is suggested that regenerative medicine through transplant of stem cells is able to restore the injured area. After transplantation, neural stem cell progeny may survive, proliferate, differentiate a specific lineage and restore the stroke area or die. The fate of transplanted cells to foster long-lasting regeneration is highly dependent on cell delivery, donor cell properties, and graft–host interactions [85, 86].
The MR tracking of injected stem cells in the brain has been conducted by several studies. For the first time, relevant studies were reported in 2007 by Sykova and Jendelova. They followed the embryonic stem cells (ESCs) and MSCs labeled with superparamagnetic contrast agents for imaging the progenitor cells transplanted into rats with a cortical or spinal cord lesion. They considered MR imaging of cell labeled with iron oxide nanoparticles as a useful method for evaluation of migration of transplanted progenitor cells toward a lesion site [87]. Nowadays, imaging of labeled progenitor stem cells with MRI contrast agents such as SPIO or USPIO is already used in experimental models of neurological diseases. In recent years, some studies have been conducted to trace injected stem cells real time and immediately after injection [88–90]. Walczak et al. showed that high-speed MRI can detect the intravascular distribution of SPIO-labeled stem cells. Moreover, they showed that using this instrument can trace homing of injected cells. This property is very important because provide the opportunity for other interventions in the case of unsuccessful homing [91].
Trauma
Zhu et al. have showed cell migration by MRI for neural stem cells injected into patients with brain trauma. Their investigation has shown the presence of neural stem cells (NSCs) for up to 3 weeks after injection [92]. Callera et al. showed that MRI is able to detect administrated labeled-CD34(+) cells with magnetic nanoparticles for 35 days after cell transplantation and also showed the migration of cells toward the damaged site in patients with chronic spinal cord injury [93]. Guzman et al. showed an average of 51.3% of human NSCs at 5 weeks after cell injection. They did not investigate adverse effects of MR contrast agents on survival, migration, and differentiation of NSCs [16].
Stroke
There are two types of stroke, including hemorrhagic and ischemic stroke. Ischemic stroke is the most common type of brain stroke. Stroke is one of the most common causes of human disability caused by irreversible neurological damages. Several studies have reported SPIO-based MRI of grafted cells in the course of migration in stroke experimental models. Stroh et al. have designed an experimental study to track injected mononuclear cells (MNCs) in the ischemic mouse brain using 7 T MRI. Brain ischemia gets filamentous by occlusion of the middle cerebral artery and reperfusion. MNCs were labeled with very small superparamagnetic iron-oxide particles and T2 and T2* sequences were generated and optimized to monitor engraftment and migration of injected cells into the ischemic brain. The region of interest (ROI) data of the experimental animal showed the appearance of the hypointense region in ischemia brain areas [94]. In a clinical study, injection of umbilical cord blood-derived stem cells labeled with SPIO to a patient suffering global cerebral ischemia showed promising results. In this study, transplanted stem cells were traced for 4 months after injection [95].
Pancreas
Diabetes mellitus type 1 or insulin-dependent diabetes is a chronic pancreatic disease that is associated with the autoimmune destruction of the β-cells in the pancreas. The lack of insulin is along with this autoimmune disease that leads to increased blood and urine glucose. Administration of insulin is essential to prevent dangerous effects such as blindness, nephropathy, foot ulcer and amputation. Stem cell therapy is a new strategy for replacement of insulin-producing cells. Adult and embryonic stem cells can be differentiated from β-cells under special conditions. Hematopoietic stem cells, pancreas and liver resident stem cells might give rise to pancreatic endocrine phenotype. The cells derived from adult tissues are differentiated into insulin-secreting cells and alleviate diabetes mellitus in rodents [96, 97]. Dor et al. [98] have investigated that differentiated β-cells in pancreas retain proliferative capacity. Moreover, this cell type can account for turnover and expansion throughout a mouse’s life.
Immune rejection and nonimmunological events such as ischemia, hypoxia and hyperglycemic microenvironment may lead to significant graft loss and fail to make progress in treatment. So, noninvasive monitoring of fate of the transplanted cell and assessing the function of islets graft following transplantation are a crucial issue for diabetes stem cell therapy. Tang et al. have investigated improvement in islet repaired by MSCs differentiation and change in pancreatic microcirculation by in vivo real-time MRI imaging. In this study pig MSCs were cultured and labeled with SPIO. Then, labeled cells were injected into the pancreas of diabetic pigs through targeted intervention. The MR imaging showed the implantation of MSCs can partially repair damaged islet β-cells and restore the function of pancreas in type 1 diabetes [99]. Zhang et al. have revealed that under in vitro situation, β-cells can label with polyvinylpyrrolidone‐coated super paramagnetic iron oxide nanoparticles (PVP‐SPIO) and detected by MRI. In vivo study confirmed cell labeling efficiency after renal subcapsular transplantation [100].
Joints
Cartilage lesions such as osteoarthritis or acute trauma under mechanical or biochemical stress are major clinical problems due to poor intrinsic repair capacity of these tissues. During these situations, the long term upregulation of proinflammatory cytokines and mediators degrades the structure of the articular cartilage. Evidences have proved that stem cell therapy has potential for chondrogenic differentiation and repair of cartilage defect [101]. Jing et al. have shown that MSCs can be efficiently labeled with SPIO to visually track SPIO-labeled MSCs injected into the knee joint of rabbit models for cartilage defects. Then GRE T2*-weighted MR imaging at 1, 4, 8 and 12 weeks after cell injection done. The SPIO contrast agent did not affect cell viability, proliferation and differentiation. Histochemical staining confirmed the data obtained from MRI imaging [102] (Table 2).
Table 2.
Summary of different studies for stem cell tracing using MRI contrast agents
| Route | Transplanted cell type | Contrast agent | Targeted organ | Time of tracing | References |
|---|---|---|---|---|---|
| Chinese mini swine | MSCs | SPIO | Heart | 4 weeks | [73] |
| Rat | Swine cardiac progenitor cells | Ferritin heavy chain | Myocardial | 4 weeks | [72] |
| Rat | MSCs | SPIO | Bladder | 12 weeks | [81] |
| Rat | ESCs and MSCs | Iron oxide nanoparticles | Cortical and spinal cord | More than 1 month | [87] |
| Human | CD34(+) | Magnetic nanoparticles | Spinal cord | 35 days | [93] |
| Mouse | MNCs | SPIO | Brain | 5 weeks | [94] |
| Pig | MSCs | SPIO | Pancreas | 6 weeks | [99] |
| Rabbit | MSCs | SPIO | The knee joint | 12 weeks | [102] |
Conclusion
Stem cell transplantation is currently being evaluated for treatment of many diseases. There is a grave need to determine of cell migration, homing, distribution and differentiation of transplanted stem cells, and finally selection of appropriated methods for delivery of optimal stem cell type. Tracking the injected stem cells with MRI is a non-invasive and relatively safe method and is able to visualize transplanted cells for long term tracing of labeled stem cells. Advances in synthesis of new contrast agent compounds facilitate safe cell tracking for clinical applications. The possible clinical use of stem cell therapy for several diseases can make MR stem cell imaging as a non-negligible modality for evaluation of transplant fate.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal or human experiments carried out for this article.
Contributor Information
Masoud Najafi, Email: masoudnajafi67@yahoo.com.
Elahe Motevaseli, Email: e_motevaseli@tums.ac.ir.
References
- 1.Ikehara S. Grand challenges in stem cell treatments. Front Cell Dev Biol. 2013;1:2. doi: 10.3389/fcell.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choumerianou DM, Dimitriou H, Kalmanti M. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008;14:53–60. doi: 10.1089/teb.2007.0216. [DOI] [PubMed] [Google Scholar]
- 3.Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9. doi: 10.1186/1756-9966-30-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mahla RS. Stem cells applications in regenerative medicine and disease therapeutics. Int J Cell Biol. 2016;2016:6940283. doi: 10.1155/2016/6940283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells. Diabetes. 2008;57:1759–1767. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- 7.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volarevic V, Erceg S, Bhattacharya SS, Stojkovic P, Horner P, Stojkovic M. Stem cell-based therapy for spinal cord injury. Cell Transplant. 2013;22:1309–1323. doi: 10.3727/096368912X657260. [DOI] [PubMed] [Google Scholar]
- 9.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, et al. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salazar DL, Uchida N, Hamers FP, Cummings BJ, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One. 2010;5:e12272. doi: 10.1371/journal.pone.0012272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wobus AM, Boheler KR. Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 2005;85:635–678. doi: 10.1152/physrev.00054.2003. [DOI] [PubMed] [Google Scholar]
- 12.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17:11–22. doi: 10.1016/j.stem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Spiriev T, Sandu N, Schaller B. Molecular imaging and tracking stem cells in neurosciences. Methods Mol Biol. 2013;1052:195–201. doi: 10.1007/7651_2013_27. [DOI] [PubMed] [Google Scholar]
- 14.Sandu N, Momen-Heravi F, Sadr-Eshkevari P, Schaller B. Molecular imaging for stem cell transplantation in neuroregenerative medicine. Neurodegener Dis. 2012;9:60–67. doi: 10.1159/000330713. [DOI] [PubMed] [Google Scholar]
- 15.McColgan P, Sharma P, Bentley P. Stem cell tracking in human trials: a meta-regression. Stem Cell Rev. 2011;7:1031–1040. doi: 10.1007/s12015-011-9260-8. [DOI] [PubMed] [Google Scholar]
- 16.Guzman R, Uchida N, Bliss TM, He D, Christopherson KK, Stellwagen D, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci U S A. 2007;104:10211–10216. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava AK, Kadayakkara DK, Bar-Shir A, Gilad AA, McMahon MT, Bulte JW. Advances in using MRI probes and sensors for in vivo cell tracking as applied to regenerative medicine. Dis Model Mech. 2015;8:323–336. doi: 10.1242/dmm.018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngen EJ, Artemov D. Advances in monitoring cell-based therapies with magnetic resonance imaging: future perspectives. Int J Mol Sci. 2017;18:E198. doi: 10.3390/ijms18010198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito S, Tsugeno M, Koto D, Mori Y, Yoshioka Y, Nohara S, et al. Impact of surface coating and particle size on the uptake of small and ultrasmall superparamagnetic iron oxide nanoparticles by macrophages. Int J Nanomedicine. 2012;7:5415–5421. doi: 10.2147/IJN.S33709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muja N, Bulte JW. Magnetic resonance imaging of cells in experimental disease models. Prog Nucl Magn Reson Spectrosc. 2009;55:61–77. doi: 10.1016/j.pnmrs.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin R, Lin B, Li D, Ai H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: design considerations and clinical applications. Curr Opin Pharmacol. 2014;18:18–27. doi: 10.1016/j.coph.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Gutteridge JM, Rowley DA, Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of ‘catalytic’ iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982;206:605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emerit J, Beaumont C, Trivin F. Iron metabolism, free radicals, and oxidative injury. Biomed Pharmacother. 2001;55:333–339. doi: 10.1016/s0753-3322(01)00068-3. [DOI] [PubMed] [Google Scholar]
- 25.Barrow M, Taylor A, Murray P, Rosseinsky MJ, Adams DJ. Design considerations for the synthesis of polymer coated iron oxide nanoparticles for stem cell labelling and tracking using MRI. Chem Soc Rev. 2015;44:6733–6748. doi: 10.1039/c5cs00331h. [DOI] [PubMed] [Google Scholar]
- 26.Lee NK, Kim HS, Yoo D, Hwang JW, Choi SJ, Oh W, et al. Magnetic resonance imaging of ferumoxytol-labeled human mesenchymal stem cells in the mouse brain. Stem Cell Rev. 2017;13:127–138. doi: 10.1007/s12015-016-9694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brewer KD, Spitler R, Lee KR, Chan AC, Barrozo JC, Wakeel A, et al. Characterization of magneto-endosymbionts as MRI cell labeling and tracking agents. Mol Imaging Biol. 2017 doi: 10.1007/s11307-017-1093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S, Yoon HI, Na JH, Jeon S, Lim S, Koo H, et al. In vivo stem cell tracking with imageable nanoparticles that bind bioorthogonal chemical receptors on the stem cell surface. Biomaterials. 2017;139:12–29. doi: 10.1016/j.biomaterials.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Ho C. Mesenchymal Stem Cell Preparation and Transfection-free Ferumoxytol Labeling for MRI Cell Tracking. Curr Protoc Stem Cell Biol. 2017;43:2B.7.1–2B.7.14. doi: 10.1002/cpsc.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khurana A, Nejadnik H, Chapelin F, Lenkov O, Gawande R, Lee S, et al. Ferumoxytol: a new, clinically applicable label for stem-cell tracking in arthritic joints with MRI. Nanomedicine (Lond) 2013;8:1969–1983. doi: 10.2217/nnm.12.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthiah M, Park IK, Cho CS. Surface modification of iron oxide nanoparticles by biocompatible polymers for tissue imaging and targeting. Biotechnol Adv. 2013;31:1224–1236. doi: 10.1016/j.biotechadv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Wang YX, Xuan S, Port M, Idee JM. Recent advances in superparamagnetic iron oxide nanoparticles for cellular imaging and targeted therapy research. Curr Pharm Des. 2013;19:6575–6593. doi: 10.2174/1381612811319370003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by MRI. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 34.Heyn C, Bowen CV, Rutt BK, Foster PJ. Detection threshold of single SPIO-labeled cells with FIESTA. Magn Reson Med. 2005;53:312–320. doi: 10.1002/mrm.20356. [DOI] [PubMed] [Google Scholar]
- 35.Bulte JW, Duncan ID, Frank JA. In vivo magnetic resonance tracking of magnetically labeled cells after transplantation. J Cereb Blood Flow Metab. 2002;22:899–907. doi: 10.1097/00004647-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Di Marco M, Sadun C, Port M, Guilbert I, Couvreur P, Dubernet C. Physicochemical characterization of ultrasmall superparamagnetic iron oxide particles (USPIO) for biomedical application as MRI contrast agents. Int J Nanomedicine. 2007;2:609–622. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Zhao H, Chen Z, Lan M. Ultrasmall superparamagnetic iron oxide nanoparticles for magnetic resonance imaging contrast agent. J Nanosci Nanotechnol. 2014;14:210–220. doi: 10.1166/jnn.2014.9192. [DOI] [PubMed] [Google Scholar]
- 38.Ariza de Schellenberger A, Kratz H, Farr TD, Löwa N, Hauptmann R, Wagner S, et al. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int J Nanomedicine. 2016;11:1517–1535. doi: 10.2147/IJN.S101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morawski AM, Winter PM, Yu X, Fuhrhop RW, Scott MJ, Hockett F, et al. Quantitative “magnetic resonance immunohistochemistry” with ligand-targeted 19F nanoparticles. Magn Reson Med. 2004;52:1255–1262. doi: 10.1002/mrm.20287. [DOI] [PubMed] [Google Scholar]
- 40.Ahrens ET, Zhong J. In vivo MRI cell tracking using perfluorocarbon probes and fluorine-19 detection. NMR Biomed. 2013;26:860–871. doi: 10.1002/nbm.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaudet JM, Ribot EJ, Chen Y, Gilbert KM, Foster PJ. Tracking the fate of stem cell implants with fluorine-19 MRI. PLoS One. 2015;10:e0118544. doi: 10.1371/journal.pone.0118544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahrens ET, Helfer BM, O’Hanlon CF, Schirda C. Clinical cell therapy imaging using a perfluorocarbon tracer and fluorine-19 MRI. Magn Reson Med. 2014;72:1696–1701. doi: 10.1002/mrm.25454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong J, Mills PH, Hitchens TK, Ahrens ET. Accelerated fluorine-19 MRI cell tracking using compressed sensing. Magn Reson Med. 2013;69:1683–1690. doi: 10.1002/mrm.24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaudet JM, Hamilton AM, Chen Y, Fox MS, Foster PJ. Application of dual 19F and iron cellular MRI agents to track the infiltration of immune cells to the site of a rejected stem cell transplant. Magn Reson Med. 2017;78:713–720. doi: 10.1002/mrm.26400. [DOI] [PubMed] [Google Scholar]
- 45.Tseng CL, Shih IL, Stobinski L, Lin FH. Gadolinium hexanedione nanoparticles for stem cell labeling and tracking via magnetic resonance imaging. Biomaterials. 2010;31:5427–5435. doi: 10.1016/j.biomaterials.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Ludemann L, Wurm R, Zimmer C. Pharmacokinetic modeling of Gd-DTPA extravasation in brain tumors. Investig Radiol. 2002;37:562–570. doi: 10.1097/00004424-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Ngen EJ, Wang L, Kato Y, Krishnamachary B, Zhu W, Gandhi N, et al. Imaging transplanted stem cells in real time using an MRI dual-contrast method. Sci Rep. 2015;5:13628. doi: 10.1038/srep13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, He ZJ, Xu B, Wu QZ, Liu G, Zhu H, et al. Evaluation of cell tracking effects for transplanted mesenchymal stem cells with jetPEI/Gd-DTPA complexes in animal models of hemorrhagic spinal cord injury. Brain Res. 2011;1391:24–35. doi: 10.1016/j.brainres.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 49.Guenoun J, Koning GA, Doeswijk G, Bosman L, Wielopolski PA, Krestin GP, et al. Cationic Gd-DTPA liposomes for highly efficient labeling of mesenchymal stem cells and cell tracking with MRI. Cell Transplant. 2012;21:191–205. doi: 10.3727/096368911X593118. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Cheng LN, Zhong XM, Duan XH, Guo RM, Hong GB. Efficient in vitro labeling rabbit neural stem cell with paramagnetic Gd-DTPA and fluorescent substance. Eur J Radiol. 2010;75:397–405. doi: 10.1016/j.ejrad.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 51.Xiao Y, Liu Y, Yang S, Zhang B, Wang T, Jiang D, et al. Sorafenib and gadolinium co-loaded liposomes for drug delivery and MRI-guided HCC treatment. Colloids Surf B Biointerfaces. 2016;141:83–92. doi: 10.1016/j.colsurfb.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 52.Guo C, Sun L, She W, Li N, Jiang L, Luo K, et al. A dendronized heparin–gadolinium polymer self-assembled into a nanoscale system as a potential magnetic resonance imaging contrast agent. Polym Chem. 2016;7:2531–2541. [Google Scholar]
- 53.Murphy SV, Hale A, Reid T, Olson J, Kidiyoor A, Tan J, et al. Use of trimetasphere metallofullerene MRI contrast agent for the non-invasive longitudinal tracking of stem cells in the lung. Methods. 2016;99:99–111. doi: 10.1016/j.ymeth.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gilad AA, Jr Winnard PT, van Zijl PC, Bulte JW. Developing MR reporter genes: promises and pitfalls. NMR Biomed. 2007;20:275–290. doi: 10.1002/nbm.1134. [DOI] [PubMed] [Google Scholar]
- 55.Vandsburger MH, Radoul M, Cohen B, Neeman M. MRI reporter genes: applications for imaging of cell survival, proliferation, migration and differentiation. NMR Biomed. 2013;26:872–884. doi: 10.1002/nbm.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vande Velde G, Himmelreich U, Neeman M. Reporter gene approaches for mapping cell fate decisions by MRI: promises and pitfalls. Contrast Media Mol Imaging. 2013;8:424–431. doi: 10.1002/cmmi.1590. [DOI] [PubMed] [Google Scholar]
- 58.Kraitchman DL, Bulte JW. Imaging of stem cells using MRI. Basic Res Cardiol. 2008;103:105–113. doi: 10.1007/s00395-008-0704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pereira SM, Moss D, Williams SR, Murray P, Taylor A. Overexpression of the MRI reporter genes ferritin and transferrin receptor affect iron homeostasis and produce limited contrast in mesenchymal stem cells. Int J Mol Sci. 2015;16:15481–15496. doi: 10.3390/ijms160715481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deans AE, Wadghiri YZ, Bernas LM, Yu X, Rutt BK, Turnbull DH. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn Reson Med. 2006;56:51–59. doi: 10.1002/mrm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patrick PS, Rodrigues TB, Kettunen MI, Lyons SK, Neves AA, Brindle KM. Development of Timd2 as a reporter gene for MRI. Patrick PS, Rodrigues TB, Kettunen. 2016;75:1697–1707. doi: 10.1002/mrm.25750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dai HY, He R, Zhang Y, Wu RH, Xiao YY. Adenoviral vector mediated ferritin over-expression in mesenchymal stem cells detected by 7T MRI in vitro. PLoS One. 2017;12:e0185260. doi: 10.1371/journal.pone.0185260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brewer KD, Spitler R, Lee KR, Chan AC, Barrozo JC, Wakeel A, et al. Characterization of magneto-endosymbionts as MRI cell labeling and tracking agents. Mol Imaging Biol. 2017 doi: 10.1007/s11307-017-1093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo R, Li Q, Yang F, Hu X, Jiao J, Guo Y, et al. In vivo MR imaging of dual MRI reporter genes and Deltex-1 gene-modified human mesenchymal stem cells in the treatment of closed penile fracture. Mol Imaging Biol. 2017 doi: 10.1007/s11307-017-1128-0. [DOI] [PubMed] [Google Scholar]
- 65.Liu M, Wang Y, Li M, Zhang Y, Lan X. Using the tyrosinase gene as a tri-modality reporter gene for monitoring transplanted stem cells in acute myocardial infarction. J Nucl Med. 2017;58:167. [Google Scholar]
- 66.Wu MR, Liu HM, Lu CW, Shen WH, Lin IJ, Liao LW, et al. Organic anion-transporting polypeptide 1B3 as a dual reporter gene for fluorescence and magnetic resonance imaging. FASEB J. 2017 doi: 10.1096/fj.201700767R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reddy KS, Yusuf S. Emerging epidemic of cardiovascular disease in developing countries. Circulation. 1998;97:596–601. doi: 10.1161/01.cir.97.6.596. [DOI] [PubMed] [Google Scholar]
- 68.Kraitchman DL, Heldman AW, Atalar E, Amado LC, Martin BJ, Pittenger MF, et al. In vivo magnetic resonance imaging of mesenchymal stem cells in myocardial infarction. Circulation. 2003;107:2290–2293. doi: 10.1161/01.CIR.0000070931.62772.4E. [DOI] [PubMed] [Google Scholar]
- 69.Hua P, Wang YY, Liu LB, Liu JL, Liu JY, Yang YQ, et al. In vivo magnetic resonance imaging tracking of transplanted superparamagnetic iron oxide-labeled bone marrow mesenchymal stem cells in rats with myocardial infarction. Mol Med Rep. 2015;11:113–120. doi: 10.3892/mmr.2014.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim YJ, Huh YM, Choe KO, Choi BW, Choi EJ, Jang Y, et al. In vivo magnetic resonance imaging of injected mesenchymal stem cells in rat myocardial infarction; simultaneous cell tracking and left ventricular function measurement. Int J Cardiovasc Imaging. 2009;25:99–109. doi: 10.1007/s10554-008-9407-0. [DOI] [PubMed] [Google Scholar]
- 71.Li SH, Lai TY, Sun Z, Han M, Moriyama E, Wilson B, et al. Tracking cardiac engraftment and distribution of implanted bone marrow cells: comparing intra-aortic, intravenous, and intramyocardial delivery. J Thorac Cardiovasc Surg. 2009;137:1225–1233. doi: 10.1016/j.jtcvs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Campan M, Lionetti V, Aquaro GD, Forini F, Matteucci M, Vannucci L, et al. Ferritin as a reporter gene for in vivo tracking of stem cells by 1.5-T cardiac MRI in a rat model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;300:H2238–H2250. doi: 10.1152/ajpheart.00935.2010. [DOI] [PubMed] [Google Scholar]
- 73.He G, Zhang H, Wei H, Wang Y, Zhang X, Tang Y, et al. In vivo imaging of bone marrow mesenchymal stem cells transplanted into myocardium using magnetic resonance imaging: a novel method to trace the transplanted cells. Int J Cardiol. 2007;114:4–10. doi: 10.1016/j.ijcard.2005.11.112. [DOI] [PubMed] [Google Scholar]
- 74.Sumino Y, Mimata H. Regenerative medicine as a new therapeutic strategy for lower urinary tract dysfunction. Int J Urol. 2013;20:670–675. doi: 10.1111/iju.12137. [DOI] [PubMed] [Google Scholar]
- 75.Lin CS. Advances in stem cell therapy for the lower urinary tract. World J Stem Cells. 2010;2:1–4. doi: 10.4252/wjsc.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Adamowicz J, Kloskowski T, Tworkiewicz J, Pokrywczyńska M, Drewa T. Urine is a highly cytotoxic agent: does it influence stem cell therapies in urology? Transplant Proc. 2012;44:1439–1441. doi: 10.1016/j.transproceed.2012.01.128. [DOI] [PubMed] [Google Scholar]
- 77.Song YS, Ku JH. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol Urodyn. 2007;26:584–593. doi: 10.1002/nau.20351. [DOI] [PubMed] [Google Scholar]
- 78.Lee HJ, Won JH, Doo SH, Kim JH, Song KY, Lee SJ, et al. Inhibition of collagen deposit in obstructed rat bladder outlet by transplantation of superparamagnetic iron oxide-labeled human mesenchymal stem cells as monitored by molecular magnetic resonance imaging (MRI) Cell Transplant. 2012;21:959–970. doi: 10.3727/096368911X627516. [DOI] [PubMed] [Google Scholar]
- 79.Lee HJ, Doo SW, Kim DH, Cha YJ, Kim JH, Song YS, et al. Cytosine deaminase-expressing human neural stem cells inhibit tumor growth in prostate cancer-bearing mice. Cancer Lett. 2013;335:58–65. doi: 10.1016/j.canlet.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 80.Rivière C, Lecoeur C, Wilhelm C, Péchoux C, Combrisson H, Yiou R, et al. The MRI assessment of intraurethrally—delivered muscle precursor cells using anionic magnetic nanoparticles. Biomaterials. 2009;30:6920–6928. doi: 10.1016/j.biomaterials.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 81.Song YS, Ku JH, Song ES, Kim JH, Jeon JS, Lee KH, et al. Magnetic resonance evaluation of human mesenchymal stem cells in corpus cavernosa of rats and rabbits. Asian J Androl. 2007;9:361–367. doi: 10.1111/j.1745-7262.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 82.Aghayan HR, Soleimani M, Goodarzi P, Norouzi-Javidan A, Emami-Razavi SH, Larijani B, et al. Magnetic resonance imaging of transplanted stem cell fate in stroke. J Res Med Sci. 2014;19:465–471. [PMC free article] [PubMed] [Google Scholar]
- 83.Jendelová P, Herynek V, Urdzikova L, Glogarová K, Kroupová J, Andersson B, et al. Magnetic resonance tracking of transplanted bone marrow and embryonic stem cells labeled by iron oxide nanoparticles in rat brain and spinal cord. J Neurosci Res. 2004;76:232–243. doi: 10.1002/jnr.20041. [DOI] [PubMed] [Google Scholar]
- 84.Syková E, Jendelová P. Magnetic resonance tracking of transplanted stem cells in rat brain and spinal cord. Neurodegener Dis. 2006;3:62–67. doi: 10.1159/000092095. [DOI] [PubMed] [Google Scholar]
- 85.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders—how to make it work. Nat Med. 2004;10:S42–S50. doi: 10.1038/nm1064. [DOI] [PubMed] [Google Scholar]
- 86.Sugaya K. Potential use of stem cells in neuroreplacement therapies for neurodegenerative diseases. Int Rev Cytol. 2003;228:1–30. doi: 10.1016/s0074-7696(03)28001-3. [DOI] [PubMed] [Google Scholar]
- 87.Sykova E, Jendelova P. In vivo tracking of stem cells in brain and spinal cord injury. Prog Brain Res. 2007;161:367–383. doi: 10.1016/S0079-6123(06)61026-1. [DOI] [PubMed] [Google Scholar]
- 88.Zhao JY, Chen G, Gu YP, Cui R, Zhang ZL, Yu ZL, et al. Ultrasmall magnetically engineered Ag2Se quantum dots for instant efficient labeling and whole-body high-resolution multimodal real-time tracking of cell-derived microvesicles. J Am Chem Soc. 2016;138:1893–1903. doi: 10.1021/jacs.5b10340. [DOI] [PubMed] [Google Scholar]
- 89.Kubo T, Baba T, Ikezaki K, Sekiguchi H, Nishino Y, Miyazawa A, et al. Realtime single molecular motion analysis of nicotinic acetylcholine receptor Alpha 7 by diffracted X-Ray tracking method. Biophys J. 2016;110:222a. [Google Scholar]
- 90.Odeleye AOO, Castillo-Avila S, Boon M, Martin H, Coopman K. Development of an optical system for the non-invasive tracking of stem cell growth on microcarriers. Biotechnol Bioeng. 2017;114:2032–2042. doi: 10.1002/bit.26328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Walczak P, Wojtkiewicz J, Nowakowski A, Habich A, Holak P, Xu J, et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J Cereb Blood Flow Metab. 2017;37:2346–2358. doi: 10.1177/0271678X16665853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhu J, Zhou L, XingWu F. Tracking neural stem cells in patients with brain trauma. N Engl J Med. 2006;355:2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]
- 93.Callera F, de Melo CM. Magnetic resonance tracking of magnetically labeled autologous bone marrow CD34+ cells transplanted into the spinal cord via lumbar puncture technique in patients with chronic spinal cord injury: CD34+ cells’ migration into the injured site. Stem Cells Dev. 2007;16:461–466. doi: 10.1089/scd.2007.0083. [DOI] [PubMed] [Google Scholar]
- 94.Stroh A, Zimmer C, Werner N, Gertz K, Weir K, Kronenberg G, et al. Tracking of systemically administered mononuclear cells in the ischemic brain by high-field magnetic resonance imaging. Neuroimage. 2006;33:886–897. doi: 10.1016/j.neuroimage.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 95.Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, et al. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS One. 2014;9:e97631. doi: 10.1371/journal.pone.0097631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hussain MA, Theise ND. Stem-cell therapy for diabetes mellitus. Lancet. 2004;364:203–205. doi: 10.1016/S0140-6736(04)16635-X. [DOI] [PubMed] [Google Scholar]
- 97.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 98.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 99.Tang K, Xiao X, Liu D, Shen Y, Chen Y, Wang Y, et al. Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin-induced diabetes in miniature pigs: Real-time tracing with MRI in vivo. Int J Mol Med. 2014;33:1469–1476. doi: 10.3892/ijmm.2014.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang B, Jiang B, Chen Y, Huang H, Xie Q, Kang M, et al. Detection of viability of transplanted beta cells labeled with a novel contrast agent–polyvinylpyrrolidone-coated superparamagnetic iron oxide nanoparticles by magnetic resonance imaging. Contrast Media Mol Imaging. 2012;7:35–44. doi: 10.1002/cmmi.461. [DOI] [PubMed] [Google Scholar]
- 101.Orth P, Rey-Rico A, Venkatesan JK, Madry H, Cucchiarini M. Current perspectives in stem cell research for knee cartilage repair. Stem Cells Cloning. 2014;7:1–17. doi: 10.2147/SCCAA.S42880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jing XH, Yang L, Duan XJ, Xie B, Chen W, Li Z, et al. In vivo MR imaging tracking of magnetic iron oxide nanoparticle labeled, engineered, autologous bone marrow mesenchymal stem cells following intra-articular injection. Joint Bone Spine. 2008;75:432–438. doi: 10.1016/j.jbspin.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 103.Cromer Berman SM, Walczak P, Bulte JW. Tracking stem cells using magnetic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3:343–355. doi: 10.1002/wnan.140. [DOI] [PMC free article] [PubMed] [Google Scholar]