Abstract
Remifentanil is commonly used in operating rooms and intensive care units for the purpose of anesthesia and sedation or analgesia. Although remifentanil may significantly affect the bone regeneration process in patients, there have been few studies to date on the effects of remifentanil on bone physiology. The purpose of this study was to investigate the effects of remifentanil on osteoclast differentiation and bone resorption. Bone marrow-derived macrophages (BMMs) were cultured for 4 days in remifentanil concentrations ranging from 0 to 100 ng/ml, macrophage colony-stimulating factor (M-CSF) alone, or in osteoclastogenic medium to induce the production of mature osteoclasts. To determine the degree of osteoclast maturity, tartrate-resistant acid phosphatase (TRAP) staining was performed. RT-PCR and western blotting analyses were used to determine the effect of remifentanil on the signaling pathways involved in osteoclast differentiation and maturation. Bone resorption and migration of BMMs were analyzed to determine the osteoclastic activity. Remifentanil reduced the number and size of osteoclasts and the formation of TRAP-positive multinuclear osteoclasts in a dose-dependent manner. Expression of c-Fos and NFATC1 was most strongly decreased in the presence of RANKL and remifentanil, and the activity of ERK was also inhibited by remifentanil. In the bone resorption assay, remifentanil reduced bone resorption and did not significantly affect cell migration. This study shows that remifentanil inhibits the differentiation and maturation of osteoclasts and reduces bone resorption.
Keywords: Remifentanil, Osteoclast, Bone resorption, RANKL, ERK
Introduction
Remifentanil is an ultra-short-acting μ-opioid receptor agonist. The characteristics of remifentanil are rather unlike those of other opioids as a result of its very rapid onset and offset of clinical action due to its esterase-based metabolism and minimal accumulation [1, 2]. Although this compound is widely used in a variety of balanced anesthetic techniques, few studies have examined the effects of remifentanil on the bone physiology.
Bone is a dynamic tissue that consists several cell types. Bone remodeling, occurring throughout the entire life in the adult skeleton, is the most important function of bone [3]. Bone homeostasis is solely dependent on the harmonious activity of bone-forming osteoblasts and bone-resorbing osteoclasts [4, 5]. Bone fractures can occur after trauma or as a result of various pathologic conditions, such as bone cancer, metastatic cancer, or osteoporosis [6]. The process of fracture healing is entirely carried out by osteoclasts, osteoblasts, and osteocytes. The goal of bone fracture healing is to reconstruct the structural unity and biomechanical strength of the bone [7]. Bone regeneration consists of a complex process involving inflammation, soft callus, hard callus, and remodeling. Osteoblasts and osteoclasts play an important role during the hard callus phase and the remodeling process [8].
Osteoclasts, derived from monocyte/macrophage lineage precursors, are mainly responsible for bone resorption. Osteoblasts, stromal cells, and lymphocytes provide the two crucial osteoclastogenic factors: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor κB ligand (RANKL) [3, 9]. M-CSF is responsible for the survival of osteoclast precursor cells, and RANKL essentially regulates the osteoclastogenic differentiation of precursor cells [3, 10].
According to recent studies, opioids may have a direct detrimental effect on bone healing. Morphine accelerates sarcoma-induced bone destruction and increases the incidence of spontaneous fractures in mice [11]. However, it is still unknown whether the findings in this model of osteolytic sarcoma can be generalized to other types of fractures or other opioids. Tramadol does not significantly affect human osteoblast activity in vitro [12]. Further, the effects of opioids on osteoclastogenesis are yet to be fully studied.
In this study, we investigated the effect of remifentanil on osteoclastogenesis and bone resorption, as well as signaling pathway of osteoclastogenic gene expression using the RANKL-induced osteoclast differentiation from bone marrow-derived macrophages (BMMs).
Materials and methods
Reagents
M-CSF and recombinant RANKL were purchased from PeproTech (Rocky Hill, NJ, USA). Antibodies against phosphorylated extracellular signal-regulated kinase (p-ERK), ERK, phosphorylated Jun N-terminal kinase (p-JNK), JNK, phosphor-p38, and p38 were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against c-Fos and nuclear factor of activated T cell cytoplasmic 1 (NFATc1) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). The Leukocyte Acid Phosphatase (TRAP) kit and anti-β-actin antibody were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cytotoxicity measurement and cell proliferation assay
The stock of remifentanil (Ultiva; Glaxo Smith Kline Pharmaceuticals, UK) was kept frozen at − 20 °C until use and diluted to their concentration with DMEM, when needed. The effects of remifentanil on both cell viability and proliferation were examined by a well-established colorimetric assay using 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT, Sigma-Aldrich). Briefly, BMMs were seeded in 96-well plates with various concentrations of remifentanil for up to 3 days. At the end of culture, cells were further incubated with MTT solution (medium containing 0.5 mg/ml MTT) for more 4 h. Then, the formation of the blue formazan product was measured with a microplate reader at a wavelength of 570 nm.
Isolation of macrophages from mouse bone marrow and in vitro osteoclast differentiation
Mouse whole bone marrow cells were collected from femora and tibiae of 5-week-old ICR mice and cultured overnight on 100-mm dishes in α-modified Eagle Medium (α-MEM; WelGENE Inc., Republic of Korea) supplemented with 10% fetal bovine serum (FBS). After discarding adherent stromal cells, the floating cells were further cultured in the presence of M-CSF (30 ng/ml) on Petri dishes. Cells became adherent after 3–4 days culture and were used as BMMs, the osteoclast precursor cells. Animal experiments were approved by the Committees on the Care and Use of Animals in Research at Pusan National University Dental Hospital (IACUC no. PNUDH-2016-032). To induce osteoclast differentiation, BMMs (4 × 104 cells/48-well plates) were cultured with osteoclastogenic medium (30 ng/ml M-CSF + 100 ng/ml RANKL) for 4 days. After culturing, cells were stained for TRAP activity and the TRAP-positive multinucleated cells with ≥ 3 nuclei were counted as osteoclasts.
Reverse transcriptase polymerase-chain reaction (RT-PCR) analysis
For RT-PCR analysis, total RNAs were isolated using TRIzol reagent (Invitrogen, CA, USA) and 1.5 µg of RNAs were reverse-transcribed with Superscript II (Invitrogen) according to the manufacturer’s protocol. The primer sets used in PCR were as follows: tartrate-resistant acid phosphatase (TRAP), 5′-ACT TCC CCA GCC CTT ACT ACC G-3′ (forward) and 5′-TCA GCA CAT AGC CCA CAC CG-3′ (reverse); NFATc1, 5′-TGC TCC TCCTCC TGC TGC TC-3′ (forward) and 5′-CGT CTT CCA CCT CCA CGT CG-3′ (reverse); c-Fos, 5′-ATG GGC TCT CCT GTC AAC AC-3′ (forward) and 5′-GGC TGC CAA AAT AAA CTC CA-3′ (reverse); calcitonin receptor (CTR), 5′-TGC ATT CCC GGG ATA CAC AG-3′ (forward) and 5′-AGG AAC GCA GAC TTC ACT GG-3′ (reverse); cathepsin K (CTK), 5′-AGG CGG CTA TAT GAC CAC TG-3′ (forward) and 5′-CCG AGC CAA GAG AGC ATA TC-3′ (reverse); Actin, 5′-CGA TGC CCT GAG GCT CTT TT-3′ (forward) and 5′-GGG CCG GAC TCA TCG TAC TC-3′ (reverse).
Western blotting analysis
Western blotting was conducted by following a standard procedure. Briefly, BMMs were lysed in RIPA lysis buffer (50 mM Tris; pH 8.0, 150 mM NaCl, 0.5% sodium deoxycholate, 1.5 mM MgCl2, 1 mM EGTA, 1% Triton X-100, 10 mMNaF and complete protease inhibitor cocktail). The protein concentrations of cell lysates were determined using detergent-compatible protein assay kit (Bio-Rad Laboratories, CA, USA) and 30–45 μg of cell lysates were resolved by 8–10% SDS–polyacrylamide gel electrophoresis. Separated proteins were transferred onto nitrocellulose membranes and membranes were blocked with 5% skim milk for 1 h. After incubation with appropriate primary/secondary antibodies, membrane immunoreactivity was detected with chemiluminescence reagents (Pierce, Rockford, IL, USA).
Bone resorption experiments
Mature BMMs were cultured on 5-mm-diameter thick dentine prepared using osteocyte dentine discs (ids company, RIA, UK) in the presence or absence of remifentanil and subjected to hematoxylin staining. Histomorphometric analyses for the number of osteoclasts per bone perimeter and eroded surface per bone surface were performed using Image J software.
Boyden chamber migration assay
Transwells with 8 µm porosity polycarbonate membrane inserts were used. BMM cell were suspended in α-MEM media at 1 × 105 cells/100 μL, and were added on the upper chamber remifentanil (10 ng/ml and 50 ng/ml) in α-MEM media was added into the lower chamber. BMMs that migrated through the filter and appeared on the lower side were fixed by carefully immersing the filter into methanol for 1 min, followed by H&E staining and counting three random fields per well. Each experiment was performed in duplicate and three separate experiments were performed in each group.
Statistics
Data were obtained from at least three independent experiments. The Student’s t test was used to determine the significance of differences between two groups. Multiple groups were compared using one-way analysis of variance (ANOVA) followed by a post hoc Tukey test. Differences with p < 0.05 were regarded as significant and denoted with an asterisk (*p < 0.05; **p < 0.01; ***p < 0.001).
Results
Effect of remifentanil treatment on cell viability and proliferation
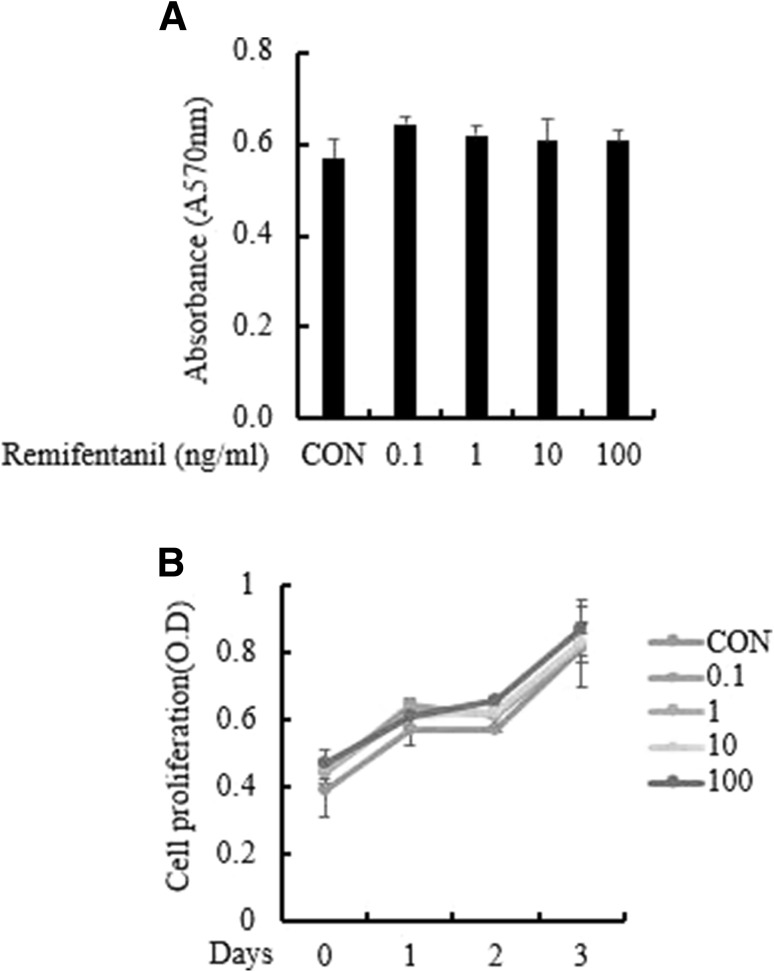
To verify the effect of remifentanil on BMM cell viability and proliferation, we performed an MTT assay. In the MTT assay, the cell viability of BMMs was not affected by treatment with various concentrations of remifentanil (0–100 ng/ml) (Fig. 1A). BMMs were cultured in osteoclastogenic medium with remifentanil for 3 days. There was no significant difference among the different concentrations of remifentanil (Fig. 1B).
Fig. 1.
Effect of remifentanil treatment on cell viability and proliferation. Remifentanil did not affect cell proliferation and did not have cytotoxic effects on osteoclast precursor cells. A BMMs were cultured in a medium containing the indicated concentrations of remifentanil (0–100 ng/ml) for 24 h. Cell viability was evaluated by an MTT assay. B BMMs were cultured in osteoclastogenic medium for 3 days together with remifentanil (0–100 ng/ml). Cell proliferation was measured by MTT assay. BMMs bone marrow-derived macrophages
Effects of remifentanil treatment on osteoclastogenesis
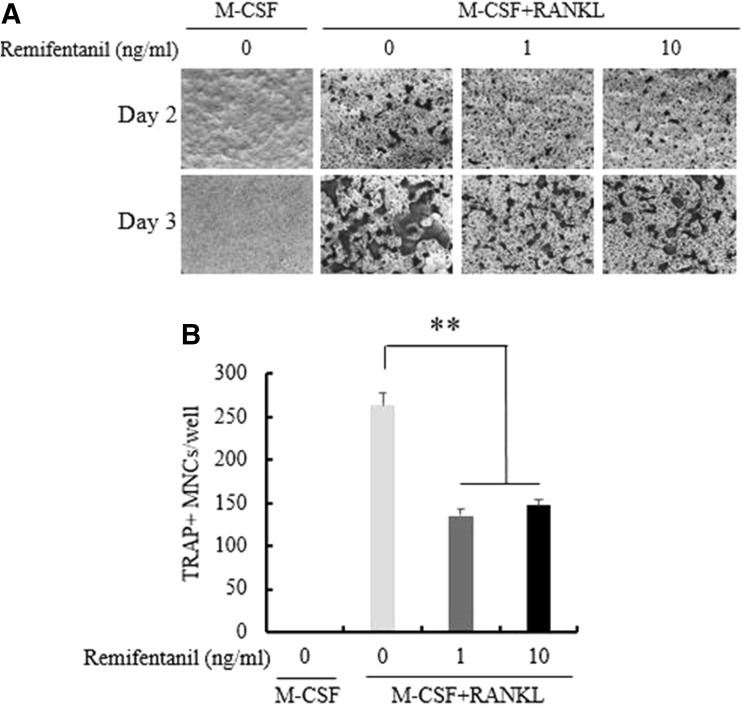
BMMs were cultured with M-CSF alone or osteoclastogenic medium in the presence of remifentanil for 3 days to investigate the formation of mature osteoclasts, which were identified by TRAP staining. Remifentanil decreased the formation of TRAP-positive multinucleated osteoclasts in a dose-dependent manner (Fig. 2A). Adding remifentanil to the BMMs cultured with RANKL decreased the number and size of osteoclasts in a dose-dependent manner (Fig. 2B).
Fig. 2.
Effects of remifentanil treatment on osteoclastogenesis. A BMMs were cultured with M-CSF alone or with M-CSF plus RANKL (osteoclastogenic medium) and remifentanil for 4 days. The number of TRAP-positive multinucleated osteoclasts was determined in each group. B Treating BMMs which were cultured with RANKL with remifentanil decreased the number and size of osteoclasts (*p < 0.05; **p < 0.01; ***p < 0.001). BMMs bone marrow-derived macrophages, M-CSF macrophage-colony stimulating factor, RANKL receptor activator of nuclear factor kappa B ligand, TRAP tartrate-resistant acid phosphatase
Effects of remifentanil treatment on osteoclastogenic transcription factors
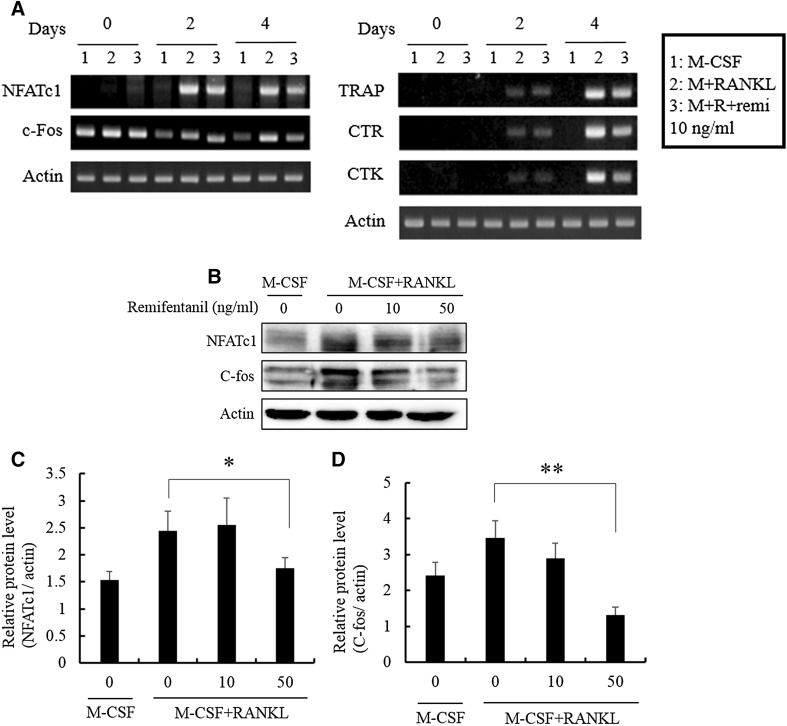
RT-PCR analysis showed that the mRNA expression of c-Fos, NFATc1, TRAP, CTR, and CTK was increased by RANKL (100 ng/ml). Remifentanil reduced the mRNA expression of c-Fos, NFATc1, TRAP, CTR, and CTK at day 2 and 4 (Fig. 3A). The protein expressions of c-Fos and NFATc1, main osteoclastogenic transcription factors, decreased with increasing doses of remifentanil as determined by western blotting (Fig. 3B–D).
Fig. 3.
Effects of remifentanil treatment on osteoclastogenic transcription factors. A As determined by RT-PCR, remifentanil reduced the mRNA expression of NFATc1, c-Fos TRAP, CTR, and CTK at day 2 and 4. B The protein expressions of NFATc1 and c-Fos, main osteoclastogenic transcription factors, decreased with increasing doses of remifentanil as determined by western blotting. C Effect of remifentanil on the expression of NFATc1. D Effect of remifentanil on the expression of c-Fos (*p < 0.05; **p < 0.01; ***p < 0.001). NFATc1 nuclear factor of activated T cell cytoplasmic 1, TRAP tartrate-resistant acid phosphatase, CTR calcitonin receptor, CTK cathepsin K, M-CSF macrophage-colony stimulating factor, RANKL receptor activator of nuclear factor kappa B ligand
Effects of remifentanil treatment on mitogen-activated protein kinases pathways
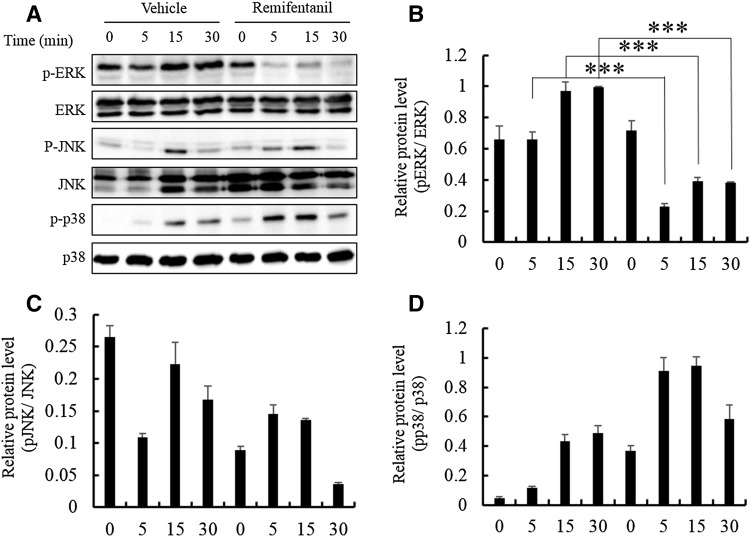
We examined the activation of mitogen-activated protein kinases (MAPKs) which are essential in osteoclastogenesis by western blotting analysis. Remifentanil reduced p-ERK/ERK protein level ratio at 5, 15 and 30 min (Fig. 4A, B). Remifentanil did not have a statistically significant effect on the phosphorylation of JNK and p38 (Fig. 4C, D).
Fig. 4.
Effects of remifentanil treatment on mitogen-activated protein kinases pathways. A Remifentanil reduced p-ERK/ERK protein level ratios at min 5, 15, and 30. B Effect of remifentanil on the phosphorylation of ERK. C Effect of remifentanil on the phosphorylation of JNK. D Effect of remifentanil on the phosphorylation of p38 (*p < 0.05; **p < 0.01; ***p < 0.001). ERK extracellular signal-regulated kinase, JNK Jun N-terminal kinase
Effects of remifentanil treatment on bone resorption and the migration of BMMs
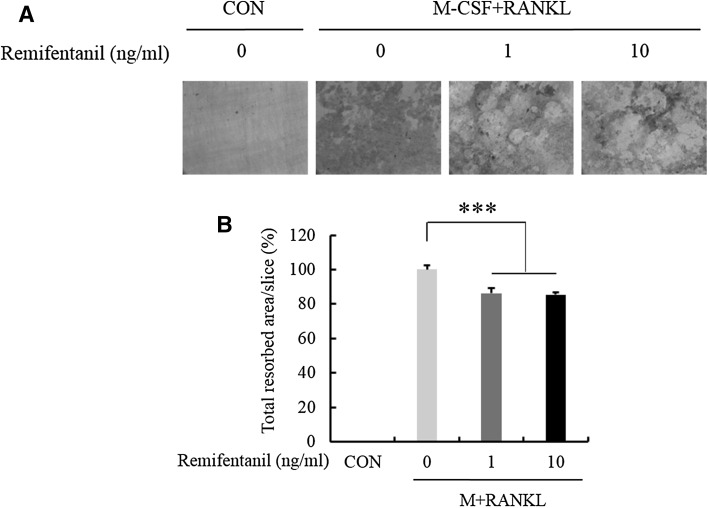
To evaluate the role of remifentanil in the bone resorption activity of osteoclasts, mature osteoclasts were cultured on dentin discs for 5 days in the presence of various concentrations of remifentanil (0, 1, 10 ng/ml). Bone resorption assays showed that remifentanil decreased the number of resorption pits on dentin discs (Fig. 5A, B).
Fig. 5.
Effects of remifentanil treatment on bone resorption. The resorption pits were visualized and quantified by a laser scanning of cell-removed dentin discs. A Bone resorption assay showed that remifentanil decreased resorption pits on dentin discs. B Effect of remifentanil on bone resorbed area (*p < 0.05; **p < 0.01; ***p < 0.001). CON control, M-CSF macrophage-colony stimulating factor, RANKL receptor activator of nuclear factor kappa B ligand
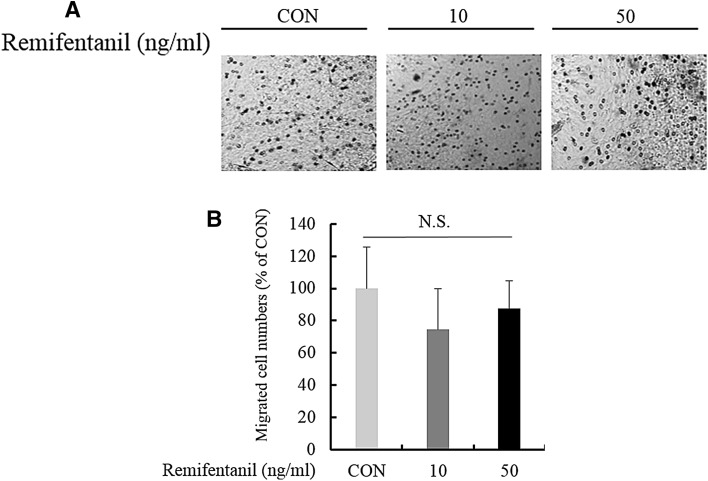
We evaluated the effect of remifentanil on BMM migration. There was no significant difference among the concentrations of remifentanil (0, 10, 50 ng/ml) (Fig. 6A, B). Therefore, remifentanil did not affect BMM migration.
Fig. 6.
Effects of remifentanil treatment on the migration of BMMs. A Boyden chamber migration assay results showed that remifentanil treatment did not affect the migration of BMMs. B Effect of remifentanil on migrated cell nembers. BMMs bone marrow-derived macrophages
Discussion
The object of the current study was to determine the effect of remifentanil on RANKL-induced osteoclast differentiation, bone resorption, and BMM migration. This study provides three principal findings. First, we showed that remifentanil treatment did not affect cell viability or the proliferation of BMMs.
Second, remifentanil treatment decreased osteoclastogenesis in this study. We showed that remifentanil reduced the formation of mature osteoclasts during in vitro osteoclast differentiation with RANKL. Using RT-PCR and western blotting analysis, we also demonstrated that remifentanil decreased the expression of NFATc1, c-Fos, TRAP, CTR, CTK, and p-ERK, which are associated with osteoclastogenesis.
RANKL plays important roles in osteoclastogenesis. RANKL binds to the RANK receptor which is expressed on osteoclast precursors and mediates osteoclast activation and also guarantees survival in mature osteoclasts [13]. NFATc1 is potently induced by RANKL and this process is essential for successful osteoclast differentiation. It is known that the induction of NFATc1 by RANKL is regulated by intracellular calcium oscillation and c-Fos, another transcription factor [14, 15]. As a main transcription factor of osteoclastogenesis, NFATc1 regulates various osteoclastogenesis-related genes such as TRAP, CTR, and CTK [9, 16, 17]. MAPK (ERK, JNK, and p38) pathways also are activated by RANKL in osteoclasts and osteoclast precursors [18]. These MAPK signaling pathways have critical functions in osteoclastogenesis because they induce c-Fos and NFATc1 which are key transcription factors for osteoclast differentiation [19, 20].
Third, remifentanil treatment reduced bone resorption activity of osteoclasts but did not affect the migration of BMMs. The size of osteoclasts is increased by cell–cell fusion-mediated multi-nucleation during osteoclastogenesis, and the fusion of pre-osteoclasts allows the formation of mature osteoclasts. Mature osteoclasts perform the resorption of bone tissue [21]. Therefore, osteoclast maturation is indispensable in bone resorption. Our third finding implies that remifentanil decreases bone resorption as well as osteoclast maturation.
Several studies in animal and human cells show that opioids can reduce bone mineral density by directly interfering with osteoblast activity [22–24]. Opioid receptors in osteoblasts and proenkephalin-derived peptides may inhibit the activity of alkaline phosphatase in rat osteosarcoma-derived cell lines [23]. Similarly, in humans, it is thought that opioids reduce the growth of osteoblasts since they decrease the levels of serum osteocalcin, which is a main marker of mature osteoblasts [24]. Unlike previous studies, our recent study revealed that remifentanil pretreatment increases the proliferation and differentiation of osteoblasts in hypoxia-reoxygenation injury [25] and the current study showed the negative effects of remifentanil treatment on osteoclastogenesis. Therefore, we can suggest that remifentanil has positive effects on bone-forming osteoblasts and negative effects on bone-resorbing osteoclasts during the bone healing process.
In this study, we documented that remifentanil treatment negatively regulates RANKL-induced osteoclast differentiation and bone resorption by inhibiting c-Fos/NFATc1 expression. No functional studies were performed to investigate the effects of remifentanil on osteoclastogenesis. Therefore, although the findings of this study are limited to an in vitro interpretation, we suggest that remifentanil may have a beneficial effect in bone healing.
Conflict of interest
The authors have no financial conflicts of interest.
Ethical standard
Animal experiments were approved by the Committees on the Care and Use of Animals in Research at Pusan National University Dental Hospital (IACUC No. PNUDH-2016-032).
References
- 1.Elliott P, O’Hare R, Bill KM, Phillips AS, Gibson FM, Mirakhur RK. Severe cardiovascular depression with remifentanil. Anesth Analg. 2000;91:58–61. doi: 10.1213/00000539-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Ouattara A, Boccara G, Kockler U, Lecomte P, Leprince P, Leger P, et al. Remifentanil induces systemic arterial vasodilation in humans with a total artificial heart. Anesthesiology. 2004;100:602–607. doi: 10.1097/00000542-200403000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 4.Nakashima T, Takayanagi H. Osteoimmunology: crosstalk between the immune and bone systems. J Clin Immunol. 2009;29:555–567. doi: 10.1007/s10875-009-9316-6. [DOI] [PubMed] [Google Scholar]
- 5.Kular J, Tickner J, Chim SM, Xu J. An overview of the regulation of bone remodelling at the cellular level. Clin Biochem. 2012;45:863–873. doi: 10.1016/j.clinbiochem.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Freeman KT, Koewler NJ, Jimenez-Andrade JM, Buus RJ, Herrera MB, Martin CD, et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108:473–483. doi: 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 7.Aro HT, Wippermann BW, Hodgson SF, Chao EY. Internal remodeling of periosteal new bone during fracture healing. J Orthop Res. 1990;8:238–246. doi: 10.1002/jor.1100080213. [DOI] [PubMed] [Google Scholar]
- 8.Cruess RL, Dumont J. Fracture healing. Can J Surg. 1975;18:403–413. [PubMed] [Google Scholar]
- 9.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 10.Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.King T, Vardanyan A, Majuta L, Melemedjian O, Nagle R, Cress AE, et al. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain. 2007;132:154–168. doi: 10.1016/j.pain.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matziolis G, Rau HM, Klever P, Erli HJ, Paar O. Modification of human osteoblasts by various analgesics. Unfallchirurg. 2002;105:527–531. doi: 10.1007/s00113-001-0382-3. [DOI] [PubMed] [Google Scholar]
- 13.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 14.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 15.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Q, Wang X, Liu Y, He A, Jia R. NFATc1: functions in osteoclasts. Int J Biochem Cell Biol. 2010;42:576–579. doi: 10.1016/j.biocel.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Choi HJ, Park YR, Nepal M, Choi BY, Cho NP, Choi SH, et al. Inhibition of osteoclastogenic differentiation by Ikarisoside A in RAW 264.7 cells via JNK and NF-kappaB signaling pathways. Eur J Pharmacol. 2010;636:28–35. doi: 10.1016/j.ejphar.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Lee ZH, Kim HH. Signal transduction by receptor activator of nuclear factor kappa B in osteoclasts. Biochem Biophys Res Commun. 2003;305:211–214. doi: 10.1016/S0006-291X(03)00695-8. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Chang EJ, Ryu J, Lee ZH, Lee Y, Kim HH. Induction of c-Fos and NFATc1 during RANKL-stimulated osteoclast differentiation is mediated by the p38 signaling pathway. Biochem Biophys Res Commun. 2006;351:99–105. doi: 10.1016/j.bbrc.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Huang H, Ryu J, Ha J, Chang EJ, Kim HJ, Kim HM, et al. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-kappaB transactivation by RANKL. Cell Death Differ. 2006;13:1879–1891. doi: 10.1038/sj.cdd.4401882. [DOI] [PubMed] [Google Scholar]
- 21.Ishii M, Saeki Y. Osteoclast cell fusion: mechanisms and molecules. Mod Rheumatol. 2008;18:220–227. doi: 10.3109/s10165-008-0051-2. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Castrillón JL, Olmos JM, Gómez JJ, Barrallo A, Riancho JA, Perera L, et al. Expression of opioid receptors in osteoblast-like MG-63 cells, and effects of different opioid agonists on alkaline phosphatase and osteocalcin secretion by these cells. Neuroendocrinology. 2000;72:187–194. doi: 10.1159/000054586. [DOI] [PubMed] [Google Scholar]
- 23.Rosen H, Bar-Shavit Z. Dual role of osteoblasticproenkephalin derived peptides in skeletal tissues. J Cell Biochem. 1994;55:334–339. doi: 10.1002/jcb.240550310. [DOI] [PubMed] [Google Scholar]
- 24.Rico H, Costales C, Cabranes JA, Escudero M. Lower serum osteocalcin levels in pregnant drug users and their newborns at the time of delivery. Obstet Gynecol. 1990;75:998–1000. [PubMed] [Google Scholar]
- 25.Baik SW, Park BS, Kim YH, Kim YD, Kim CH, Yoon JY, et al. Effects of remifentanil preconditioning on osteoblasts under hypoxia-reoxygenation condition. Int J Med Sci. 2015;12:583–589. doi: 10.7150/ijms.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]