Abstract
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system. Although advances have been made in the treatment of MS, such as the use of IFN-β, glucocorticoids and stem cells, the therapeutic effects of these treatments are not sufficient. In the present study, we evaluated whether the combination of methylprednisolone (MP) and human bone marrow-derived mesenchymal stem cells (BM-MSCs) could enhance the therapeutic effectiveness in experimental autoimmune encephalomyelitis (EAE), a model for MS. EAE was induced by immunizing C57BL/6 mice with myelin oligodendrocyte glycoprotein 35-55 (MOG 35-55). The immunized mice received an intraperitoneal injection of MP (20 mg/kg), an intravenous injection of BM-MSCs (1 × 106 cells) or both on day 14 after immunization. Combination treatment significantly ameliorated the clinical symptoms, along with attenuating inflammatory infiltration and demyelination, compared to either treatment alone. Secretion of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-17) was significantly reduced, and anti-inflammatory cytokines (IL-4, IL-10) was significantly increased by the combination treatment as compared to either treatment alone. Flow cytometry analysis of MOG-reactivated T cells in spleen showed that combination treatment reduced the number of CD4+CD45+ and CD8+ T cells, and increased the number of CD4+CD25+Foxp3+ regulatory T cells. Furthermore, combination treatment enhanced apoptosis in MOG-reactivated CD4+ T cells, a key cellular subset in MS pathogenesis. Combination treatment with MP and BM-MSCs provides a novel treatment protocol for enhancing therapeutic effects in MS.
Electronic supplementary material
The online version of this article (10.1007/s13770-017-0101-y) contains supplementary material, which is available to authorized users.
Keywords: Methylprednisolone, Bone marrow mesenchymal stem cells, Experimental autoimmune encephalomyelitis
Introduction
Multiple sclerosis (MS) is the most common demyelinating disease of the central nervous system (CNS) and is characterized by mononuclear cell infiltration and demyelination [1]. Experimental autoimmune encephalomyelitis (EAE), an animal model of MS, shares many clinical and pathological aspects with human MS [2]. Many mechanisms for the pathogenesis of MS have been reported, with auto-reactive T cells considered as an initiating event [3]. Likewise, CD4+ T helper 1 (Th1) and CD4+ T helper 2 (Th2) cells are recognized as being important in MS immunopathogenesis [4, 5]. The balance between pro-inflammatory and anti-inflammatory cytokines has been reported to be related to the disease process [6]. Furthermore, CD4+CD25+Foxp3+ regulatory T (Treg) cells have been reported to suppress myelin oligodendrocyte glycoprotein (MOG)-specific T cells in EAE [7]. Recently, approved immunomodulatory agents, such as IFN-β, glatiramer acetate, mitoxantrone, natalizumab, and fingolimod, have been used for MS treatment [8–10]. Although many agents have been evaluated as treatments for patients with MS, these therapies remain unsatisfactory [11]. Therefore, developing more effective therapeutic methods for MS is a necessity.
Mesenchymal stem cells (MSCs) are adult multipotent stromal cells that can differentiate into a variety of cell types, including adipocytes, chondrocytes, and osteoblasts [12, 13]. Additionally, MSCs can migrate to the site of lesion [14]. Transplantation of MSCs is a potential therapeutic method that can modulate the immunopathogenic process in autoimmune diseases, such as systemic lupus erythematosus (SLE), graft-versus-host disease (GVHD), and MS [15, 16]. Many studies have shown that human bone marrow-derived mesenchymal stem cells (BM-MSCs) treatment decreased the clinical symptoms of EAE by regulating cytokine expression from Th1 and Th2 cells [17, 18]. Furthermore, BM-MSCs have been shown to regulate activated T lymphocytes in autoimmune disease [19]. These results suggest that BM-MSCs may be an ideal source as a therapeutic for MS treatment.
Methylprednisolone (MP), a synthetic glucocorticoid drug widely used to treat a broad range of autoimmune diseases, has anti-inflammatory effects in MS [2, 20]. MP treatment has been shown to effectively suppress disease progression through the suppression of inflammatory cell infiltration into the CNS in MS [21]. Treatment with MP has been shown to inhibit the production of pro-inflammatory cytokines and increase the expression of anti-inflammatory cytokines in MS [22]. Although diverse therapeutic effects of MP treatment have been identified, high dose or long-term therapy with MP can cause severe side effects, such as telangiectasia, hypertrichosis, osteoporosis, glaucoma, hypertension, and pancreatitis. Consequently, there is an ongoing debate regarding its efficacy [23, 24]. To overcome these problems, combination therapy with MP and other drugs, such as atorvastatin calcium and IFN-β1, has been attempted in MS. These results showed increasing therapeutic effects without side effects of MP [25, 26].
In this study, we first evaluated whether combination treatment with MP and BM-MSCs ameliorates the clinical severity of EAE more than either treatment alone. Our results indicated that combination treatment with MP and BM-MSCs suppresses EAE disease progression compared to either treatment alone. The therapeutic effect of MP and BM-MSC treatment was related to a reduction in demyelination via a decrease in inflammatory cell infiltration in EAE spinal cord. Furthermore, combination treatment modulated cytokine production and the population of MOG-reactivated T cells. Therefore, combination treatment with MP and BM-MSCs may be considered to be a novel treatment method in MS or EAE.
Materials and methods
Cell culture and reagents
Human bone marrow-derived mesenchymal stem cells (BM-MSCs) were purchased from the Catholic Institute of Cell Therapy (CIC, Seoul, Korea). BM-MSCs were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Wisent Bioproducts, St. Bruno, QC, Canada) supplemented with 20% fetal bovine serum (FBS) (Wisent Bioproducts), penicillin, and streptomycin (Gibco, Carlsbad, CA, USA), and maintained at 37 °C with 5% CO2. Methylprednisolone (MP) was purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in PBS, and filter-sterilized.
BM-MSC viability and characterization
BM-MSCs and astrocytes were seeded at 5 × 103 cells/well in 96-well plates. The next day, the medium was removed, and cells were treated with various concentrations of MP (0, 1, 5, 10, 20, 40, 80, 160 μM) for 24 h. The viability of cells was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay (Sigma-Aldrich). The optical density (OD) of each well at 570 nm was determined using Spectramax Plus 384 Microplate Reader (Molecular Devices, Sunnyvale, CA, USA). The phenotype of BM-MSCs was analyzed by cell-surface marker staining using flow cytometry (FACS). Antibodies were used to detect mouse anti-human CD34 (8G12, ref. 348057; BD Biosciences, Franklin Lakes, NJ, USA), CD44 (515, ref. 550989; BD Biosciences), CD45 (HI30, ref. 555483; BD Biosciences), CD73 (AD2, ref. 550257; BD Biosciences), CD90 (5E10, ref. 555596; BD Biosciences), and HLA-DR (L243, ref. 347367; BD Biosciences) in BM-MSCs. To examine the potential effect of BM-MSCs on differentiation into adipocytes, osteocytes, and chondrocytes, BM-MSCs were seeded at 1 × 104 cells/well in a 12-well plate. The next day, wells were filled with adipocyte, osteocyte, or chondrocyte differentiation medium (Gibco), respectively. MP (5 μM) was added for 96 h to evaluate the effects. The differentiation medium was carefully changed twice a week. After 19 days, adipocytes, osteocytes, and chondrocytes were detected using 0.3% Oil Red O (Sigma-Aldrich), 2% Alizarin Red S (Sigma-Aldrich), and 1% Alcian Blue (Sigma-Aldrich), respectively. Undifferentiated BM-MSCs were cultured in 10% DMEM medium. The pathotropism of BM-MSCs was identified using an anti-human nuclei antibody in the lumbar spinal cord of EAE mice.
EAE induction and treatment
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), the Catholic University of Korea (Permit Number; 2015-0147-01). Animals were housed under pathogen-free conditions and maintained in compliance with the guidelines of the Department of Laboratory Animals, College of Medicine, Catholic University of Korea. Nine-week-old female C57BL/6 mice were obtained from Orient Bio (Orient Bio Inc., Seongnam, Korea). Hooke kit (EK-2110) was purchased from Hooke Laboratories (Lawrence, MA, USA). Mice were injected subcutaneously at 2 sites using a total of 200 μL of myelin oligodendrocyte glycoprotein 35-55 (MOG 35-55) emulsified in complete Freund’s adjuvant (CFA) containing 6 mg/mL Mycobacterium tuberculosis. Immediately after injection and 24 h later, mice received pertussis toxin (200 ng, intraperitoneally) in 0.1 mL of PBS. To avoid unnecessary suffering, mice were anesthetized by inhalation anesthesia using isoflurane prior to EAE induction. Animals were randomly divided into 4 groups (n = 10 each group): (1) The PBS group received 100 μL PBS by intravenous injection, (2) the MP group received an intraperitoneal injection at a dose of 20 mg/kg, (3) the BM-MSCs group received 1 × 106 cells in 100 μL PBS via intravenous injection and (4) the MP + BM-MSCs group received the same dose of MP and the same number of cells by intraperitoneal and intravenous injections, respectively. All treatments were initiated on day 14 post immunization, a time point at which all mice displayed the clinical signs of EAE. Mice were monitored daily as follows: 0, no clinical signs; 1, limp tail; 2, limp tail and weakness of hind legs; 3, limp tail and complete paralysis of hind legs; 4, limp tail, complete hind leg and partial front leg paralysis; 5, moribund and dead. When animals reach a moribund state, animals were humanely euthanized by overexposure to CO2 gas.
Immunohistopathology
EAE mice from each group were sacrificed on day 30 after immunization. Mice were humanely anesthetized by intraperitoneal injection of zoletil (30 mg/kg) prior to sacrifice. Animals were intracardially perfused with PBS and fixed with 4% paraformaldehyde (PFA) (Millipore, Billerica, MA, USA). The isolated lumbar spinal cords were fixed in 4% PFA and sequentially transferred to 15 and 30% sucrose for 24 h. The fixed spinal cords were embedded in OCT compound (Sakura Finetechnical, Tokyo, Japan). Tissues were cryosectioned in the coronal plane at a thickness of 14 μm onto slides and kept in a deep freezer until staining. Slides were stained with hematoxylin–eosin (H&E) and luxol fast blue (LFB) to evaluate inflammatory infiltration and demyelination, respectively. All images were evaluated using MetaMorph software (Molecular Devices). Inflammatory cell infiltration and remyelination were identified by purified rat anti-mouse CD4/CD8 (BD Biosciences) and polyclonal rabbit anti-mouse myelin basic protein (MBP) monoclonal antibody (Millipore) staining, respectively. CD4 and CD8 antibody staining was visualized with Cy3-conjugated streptavidin antibodies (Jackson ImmunoResearch, West Grove, PA, USA). MBP antibody staining was visualized with goat anti-mouse IgM heavy chain secondary antibody (Invitrogen, Carlsbad, CA, USA). Counterstaining of cell nuclei was carried out by incubating each slide in 4-6-diamidino-2-phenyindole (DAPI) (Roche, Penzberg, Germany) for 15 min. Fluorescence images were obtained using LSM 700 confocal microscope (Carl Zeiss, Oberkochen, Germany) and Zeiss software.
Isolation of splenocytes
Spleens were isolated from each group on day 30 after immunization. Mice were humanely anesthetized by intraperitoneal injection of zoletil (30 mg/kg) prior to sacrifice. A single-cell suspension was achieved by passing the spleen through a 70 μm nylon cell strainer (BD Biosciences). The single cell suspension was washed in PBS and resuspended in red blood cell (RBC) lysis buffer to remove the RBCs. Finally, the suspended cells were filtered through a 40 μm cell strainer (BD Biosciences). Splenocytes were cultured in RPMI 1640 medium (Gibco) supplemented with 10% FBS, penicillin, and streptomycin (Gibco).
Enzyme-linked immunosorbent assay (ELISA)
To identify the pro- and anti-inflammatory cytokine balance, isolated splenocytes (2 × 106 cells) were incubated with MOG (10 μg/mL) (Sigma-Aldrich) in 24-well plates for 7 days. The culture supernatant was used for ELISA analysis. The concentrations of IFN-γ, TNF-α, IL-17, IL-4, and IL-10, were measured using Quantikine immunoassay kit (R&D Systems, Madison, WI, USA), according to the manufacturer’s instructions. The optical density of each well at 450 nm was determined using Spectramax Plus 384 Microplate Reader (Molecular Devices).
FACS analysis
To quantify the percentage of CD4CD45, CD8, CD45R, and Treg positive cells, splenocytes from each group were incubated in MOG (10 μg/mL). After 48 h, splenocytes were washed with PBS and incubated with fluorochrome-labeled relevant antibodies for 20 min at room temperature. After washing with PBS, stained cells were used for FACS analysis and sorting. Splenocytes were first gated on FSC versus SSC to separate debris, and then CD4CD45, CD8, CD45R, and Treg cells were analyzed. Treg cells were analyzed in CD4 gate. The following antibodies were used: CD4 (RM4-5, ref. 06122-60-100; Peprotech), CD45 (30-F11, ref. 07512-50-100; Peprotech), CD8 (2.43, ref. 10112-60-100; Peprotech), CD45R (B220) (RA3-6B2, ref. 07131-50-100; Peprotech), CD25 (PC61.5, ref. 07312-80-100; Peprotech), Foxp3 (3G3, ref. 83412-60-100; Peprotech), and CD4 (RM4-5, ref. 06122-50-100; Peprotech). The stained cells were analyzed by FACS with MoFlo XDP and Summit software (Beckman Coulter, Inc., Fullerton, CA, USA).
Determination of apoptotic cell death
CD4+ T cell subsets were isolated from splenocytes using FACS. In brief, isolated splenocytes from PBS treatment group were incubated with MOG (10 μg/mL) for 48 h. Splenocytes stained with PE-CD4 were sorted by FACS. Isolated CD4 T cells were co-cultured with MP (20 μM) or BM-MSCs (3 × 104 cells) either individually or together for 48 h along with MOG (10 μg/mL). The level of apoptosis of activated CD4 T cells was determined with Caspase-Glo3/7 reagent (Promega, Madison, WI, USA) and FITC Annexin V Apoptosis Dectection Kit (BD Biosciences). The activated splenocytes were incubated with the Caspase-Glo3/7 reagent at 1:1 for 3 h. Caspase3/7 activity in each sample was measured with SpectraMax L luminometer (Molecular Devices). The cells were stained with propiduim (PI) and annexin V, according to the manufacturer’s instructions. The percentage of apoptosis cells was determined by FACS with MoFlo XDP and Summit software (Beckman Coulter).
Statistical analysis
Quantitication was performed by an examiner blinded to the treatment status of each animal. All data are shown as the mean ± SD. The statistical significance of clinical score was analyzed by the Kruskal–Wallis test. The other multiple samples were compared using a one-way ANOVA test with Fisher’s Least Significant Different (LSD) post hoc test. Probability values less than 0.05 were considered statistically significant. All statistic analysis was performed using SPSS 13.0 (SPSS Inc., Chicago, IL).
Results
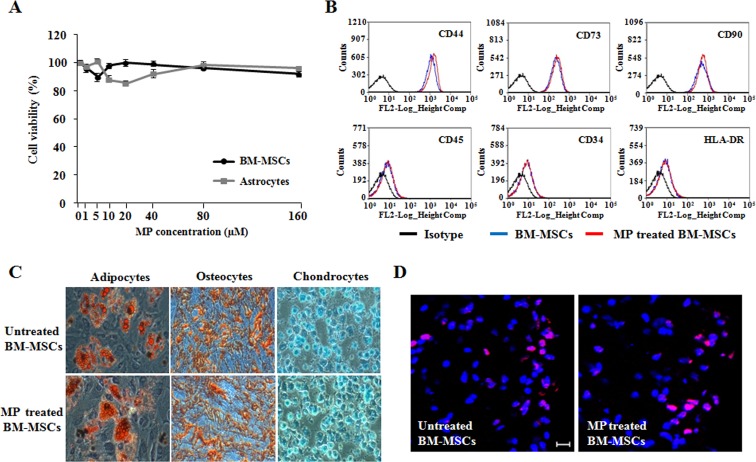
MP does not affect BM-MSC viability, phenotype, differentiation, or pathotropism
We evaluated whether MP affected the characteristics of BM-MSCs, including viability, phenotype, multipotent differentiation, and pathotropism, in vitro. MTT analysis showed that BM-MSCs were not affected by MP (Fig. 1A). FACS analysis of the phenotype demonstrated that BM-MSCs and MP-treated BM-MSCs were negative for CD34, CD45, and HLA-DR, and positive for CD44, CD73, and CD90 (Fig. 1B). Furthermore, the multipotent differentiation potential of BM-MSCs was also not affected by MP (Fig. 1C). The injected BM-MSCs were observed in inflammatory lesion of the lumbar spinal cord (Fig. 1D). These results demonstrated that BM-MSCs are not affected by MP used as a combination treatment for EAE.
Fig. 1.
Effects of MP on BM-MSC stability. A The viabilities of BM-MSCs and astrocytes were analyzed using MTT assay 24 h after MP (0–160 μM) treatment. Point, mean; bars, SD. B FACS analysis of the effects of MP on BM-MSC phenotypes. C The effect of MP (5 μM) on the multi-lineage differentiation potential of BM-MSCs. The differentiation capability of BM-MSCs measured by staining with Oil Red O, Alizarin Red S, and Alcian blue, respectively. Magnification: ×200. D The transplanted BM-MSCs were detected by staining with an anti-human nuclei antibody (red). Counterstaining was performed with DAPI staining (blue). Scale bar, 20 μm. The results are representative of 3 independent experiments
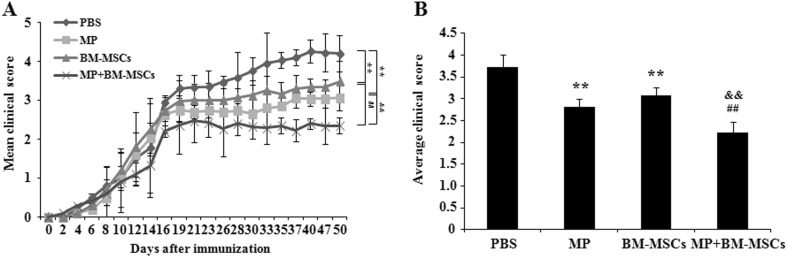
Combination treatment with MP and BM-MSCs attenuates the clinical symptoms in EAE mice
To examine the effect of combination treatment with MP and BM-MSCs on the disease course in EAE mice, we administered PBS, MP (20 mg/kg, i.p.), BM-MSCs (1 × 106 cells, i.v.) or a combination of MP and BM-MSCs to mice (n = 10/group) on day 14 post-immunization. MP or BM-MSCs treatment groups had significantly decreased clinical scores for EAE compared with PBS treatment group (p < 0.01). The combination treatment group also had significantly decreased clinical score compared with MP or BM-MSCs treatment groups (p < 0.01) (Fig. 2A). The average clinical score for MP or BM-MSCs treatment groups were significantly decreased relative to PBS treatment group (p < 0.01). The combination treatment group also had significantly decreased average clinical score compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) (Fig. 2B). These results suggest that combination treatment with MP and BM-MSCs reduced disease progression in EAE mice.
Fig. 2.
Combination therapy with MP and BM-MSCs alleviates EAE severity. EAE was induced by MOG 35-55 in female C57BL/6 mice. After 14 days of EAE induction, mice were treated with MP (20 mg/kg, i.p.) and BM-MSCs (1 × 106 cells, i.v.) in combination or individually. A The mean daily clinical score of each group was assessed from day 1 until day 50 post-immunization. B The average clinical score of each group was quantified. Columns, mean; bars, SD. ** p < 0.01 compared to PBS treatment group; ## p < 0.01 compared to MP treatment group; && p < 0.01 compared to BM-MSCs treatment group; Kruskal–Wallis test with Fisher’s Least Significant Different (LSD) post hoc test. The results are representative of 3 independent experiments
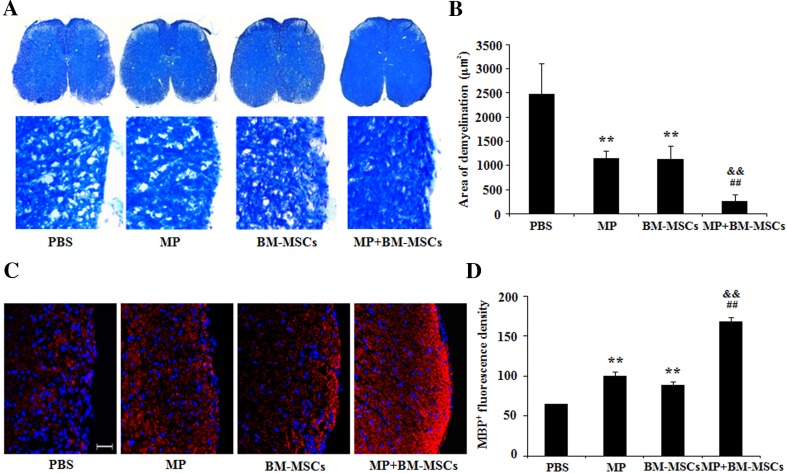
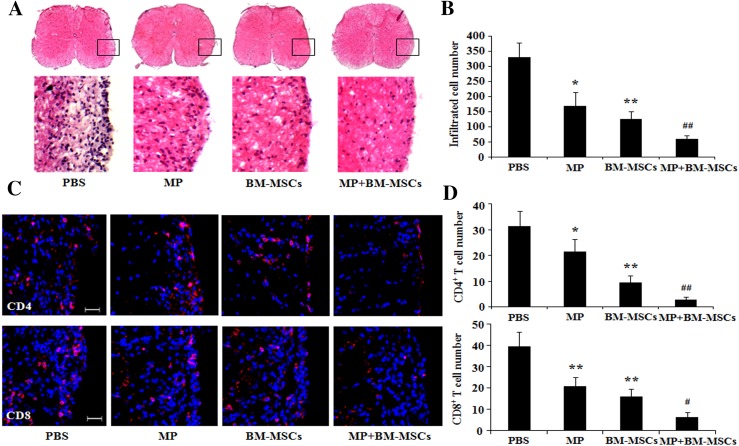
Combination therapy improves histological outcomes in EAE mice
The disease symptoms of EAE are closely related to inflammation in the CNS [27]. To determine whether combination treatment reduced tissue damage in EAE mice, we evaluated demyelination and remyelination in the lumbar spinal cord using LFB and mouse anti-MBP staining, respectively. The results demonstrated that demyelination was significantly decreased in MP and BM-MSCs treatment groups compared with PBS treatment group (p < 0.01). Additionally, the combination treatment group also showed a significant reduction in demyelination compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) (Fig. 3A, B). On the contrary, remyelination was significantly increased in the MP and BM-MCSs treatment group compared with PBS treatment group (p < 0.01). Combination treatment with MP and BM-MSCs also significantly increased the remyelination compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) (Fig. 3C, D). Furthermore, to identify inflammatory cell infiltration, we examined lumbar spinal cord sections from EAE mice. Cell infiltration in the spinal cord was significantly decreased in MP and BM-MSCs treatment groups compared with PBS treatment group (p < 0.05, MP treatment group; p < 0.01, BM-MSCs treatment group). The group receiving the combination of MP and BM-MSCs showed significantly reduced cell infiltration compared with MP and BM-MSCs treated groups, respectively (Fig. 4A, B). The number of infiltrated CD4 and CD8 T cells in the spinal cord was significantly reduced in MP and BM-MSCs treatment groups compared with PBS treatment group (p < 0.05, MP treatment group; p < 0.01, BM-MSCs treatment group). The group receiving a combination of MP and BM-MSCs also had significantly reduced CD4 and CD8 T cells compared with MP treatment group (p < 0.01) (Fig. 4C, D). Taken together, these results suggest that combination treatment decrease demyelination by inhibiting inflammatory cell infiltration in the EAE spinal cord.
Fig. 3.
Combination treatment with MP and BM-MSCs increases remyelination in EAE spinal cords. EAE mice were sacrificed on day 30 after immunization. Staining of the lumbar spinal cords from each group was performed to detect demyelination and remyelination. A Representative image of spinal cord sections stained with LFB. Magnification: ×400. B Demyelinated areas were quantified using MetaMorph image analysis. Columns, mean; bars, SD. C Representative image of sections immunostained with MBP antibody. Scale bar, 25 μm. D The fluorescence density of staining with MBP antibody was quantified using MetaMorph image analysis. Columns, mean; bars, SD. ** p < 0.01 compared to PBS treatment group; ## p < 0.01 compared to MP treatment group; && p < 0.01 compared to BM-MSCs treatment group; One-way ANOVA with LSD post hoc test. The results are representative of 3 independent experiments
Fig. 4.
Combination therapy with MP and BM-MSCs decreases infiltration by inflammatory cells in EAE spinal cords. EAE mice were sacrificed on day 30 after immunization. Staining of the lumbar spinal cords from each group was performed to detect inflammatory cell infiltration. A Representative image of spinal cord sections stained with hematoxylin and eosin (H&E). Magnification: ×400. B The number of infiltrated cells was quantified using MetaMorph image analysis. Columns, mean; bars, SD. C Representative image immunostained with anti-CD4 and CD8 antibodies. Scale bar, 25 μm. D The number of CD4 and CD8 T cells was quantified using MetaMorph image analysis. Columns, mean; bars, SD. * p < 0.05, ** p < 0.01 compared to PBS treatment group; # p < 0.05, ## p < 0.01 compared to MP treatment group; One-way ANOVA with LSD post hoc test. The results are representative of 3 independent experiments
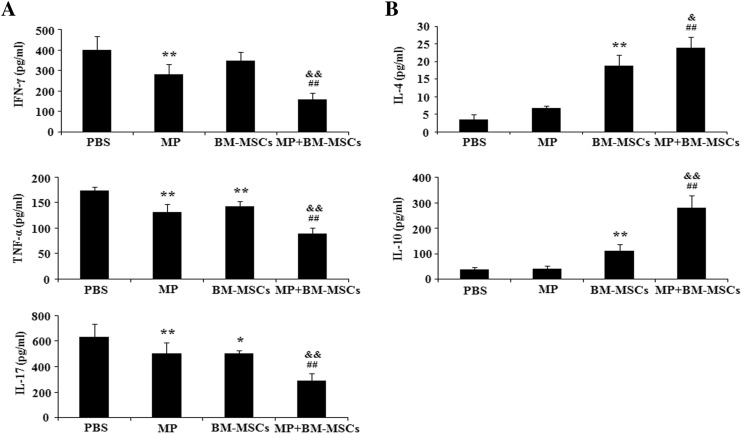
Combination therapy modulates the expression of pro/anti-inflammatory cytokines in EAE mice
The balance of pro/anti-inflammatory cytokines is known to be important in autoimmune disease [28]. To determine whether combination treatment enhanced the immunomodulatory activity, we evaluated the expression of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-17) and anti-inflammatory cytokines (IL-4, IL-10) in supernatants from MOG-reactivated splenocyte cultures. Pro-inflammatory cytokines were significantly decreased by MP treatment compared with PBS treatment (p < 0.01). BM-MSCs treatment also significantly reduced the expression of TNF-α and IL-17 cytokines compared with PBS treatment group (p < 0.05, IL-17; p < 0.01, TNF-α). However, the expression of IFN-γ was not significantly decreased by treatment with BM-MSCs. In the combination treatment group, we observed a significant decrease in the expression of pro-inflammatory cytokines compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) (Fig. 5A). Although the expression of anti-inflammatory cytokines was not significantly altered by MP treatment, treatment with BM-MSCs significantly increased anti-inflammatory cytokine expression compared with PBS treatment (p < 0.01). Combination treatment also significantly increased the expression of anti-inflammatory cytokines compared with the levels in MP treatment group (p < 0.01). Anti-inflammatory cytokines were also more significantly increased by combination treatment than by BM-MSCs treatment (p < 0.05, IL-4; p < 0.01, IL-10) (Fig. 5B). These results suggested that the immunomodulatory effects of combination treatment are mediated by cytokine production from MOG-reactivated lymphocytes.
Fig. 5.
Regulation of cytokine expression by combination treatment. EAE mice were sacrificed on day 30 after immunization. Splenocytes from each treatment group were stimulated with MOG (10 μg/mL) for 7 days. Cell culture supernatants from the splenocytes were examined by ELISA for A Pro-inflammatory (IFN-γ, TNF-α, IL-17) and B Anti-inflammatory (IL-4, IL-10) cytokines. Columns, mean; bars, SD. * p < 0.05, ** p < 0.01 compared to PBS treatment group; ## p < 0.01 compared to MP treatment group; & p < 0.05, && p < 0.01 compared to BM-MSCs treatment group; One-way ANOVA with LSD post hoc test. The results are representative of 3 independent experiments
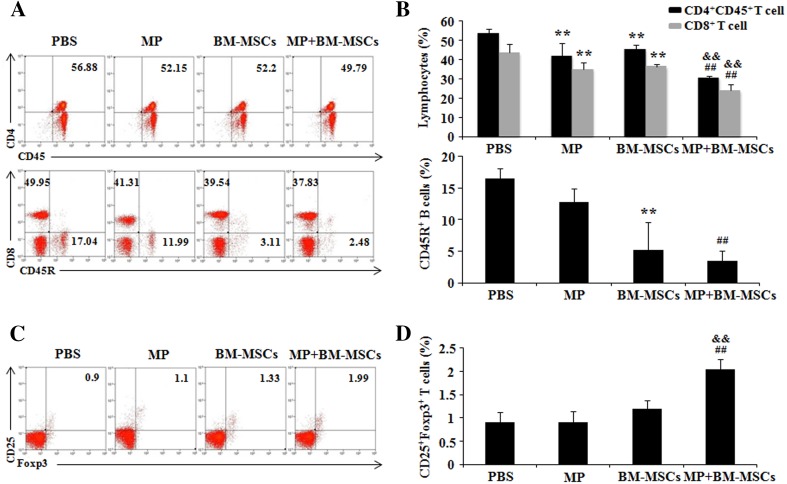
Combination therapy regulates the MOG-reactivated T cells population in EAE mice
Auto-antigen was reported to initiate an encephalitogenic T cell response in EAE mice [29]. We evaluated MOG-reactivated T cells by FACS analysis. Surface staining with relevant markers showed that the populations of MOG-reactivated CD4+CD45+ and CD8+ T cells in MP and BM-MSCs treatment groups were significantly decreased compared with PBS treatment group (p < 0.01). Combination treatment significantly decreased the populations of MOG-reactivated CD4+CD45+ and CD8+ T cells compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) CD45R are surfaces marker of B cells [30]. Although BM-MSCs treatment group had significantly decreased population of MOG-reactivated CD45R+ B cells compared with PBS treatment group (p < 0.01), it was not statistically different compared with combination treatment group (Fig. 6A, B). Recently, it has been reported that Treg cells inhibit the function of multiple immune cell subsets, including CD4 and CD8 T cells [31]. The population of MOG-reactivated CD4+CD25+Foxp3+ Treg cells in MP and BM-MSCs treatment groups was decreased compare to PBS treatment group. The combination treatment group had significantly increased population of MOG-reactivated CD4+CD25+Foxp3+ Treg cells compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) (Fig. 6C, D). These results demonstrated that the combination treatment with MP and BM-MSCs regulates the MOG-reactivated lymphocytes through increasing the MOG-reactivated Treg cells.
Fig. 6.
MOG-reactivated T cell populations in EAE spleens are regulated by combination treatment. EAE mice were sacrificed on day 30 after immunization. Isolated splenocytes from each group were activated with MOG (10 μg/mL) for 48 h before analysis. The percentages of CD4+CD45+, CD8+, CD45R+, and CD4+CD25+Foxp3+ Treg cells were analyzed by FACS. A Representative FACS plots demonstrating the percentages of CD4+CD45+, CD8+, and CD45R+ cells in each group. B CD4+CD45+, CD8+, and CD45R+ cells in each group were quantified. Columns, mean; bars, SD. C Representative FACS plots showing the percentage of CD25+Foxp3+ T cells in each group. Treg cells were analyzed in CD4 gate. D CD25+Foxp3+ T cells were quantified. Columns, mean; bars, SD. ** p < 0.01 compared to PBS treatment group; ## p < 0.01 compared to MP treatment group; && p < 0.01 compared to BM-MSCs treatment group; One-way ANOVA with LSD post hoc test. The results are representative of 3 independent experiments
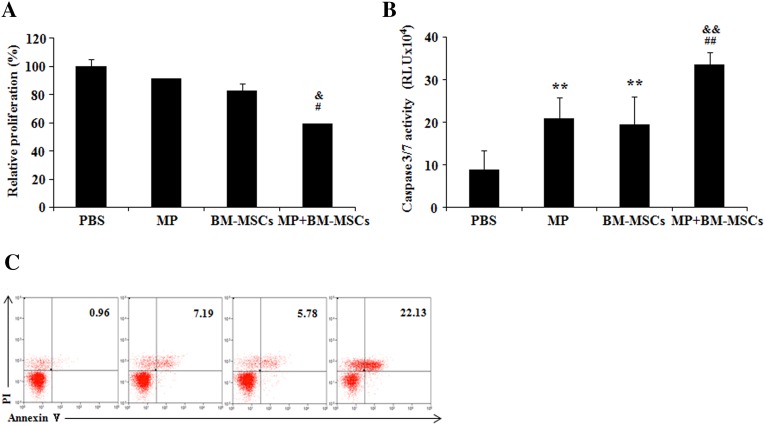
Combination therapy reduces proliferation and promotes apoptosis in MOG-reactivated CD4+ T cells from EAE mice
In EAE, activated T cells are removed by Fas and block Bcl-2 mediated apoptotic pathway [32, 33]. Hence, we examined whether combination treatment modulated the proliferation and apoptosis of MOG-reactivated CD4 T cells. The results showed that the proliferation of MOG-reactivated CD4 T cells in MP and BM-MSCs treatment groups was not significantly affected. However, in the combination treatment group, a significant decrease in proliferation was observed compared with MP and BM-MSCs treatment groups (p < 0.05) (Fig. 7A). Furthermore, significant increase in caspase3/7 activity was seen in MP and BM-MSCs treatment groups compared with PBS treatment group (p < 0.01). In the combination treatment group, significantly increased caspase3/7 activity was observed compared with MP and BM-MSCs treatment groups, respectively (p < 0.01) (Fig. 7B). Annexin V/PI analysis also showed the similar result (Fig. 7C). These data suggest that combination treatment with MP and BM-MSCs reduces the MOG-reactivated T cells by suppressing proliferation and improving the apoptosis pathway.
Fig. 7.
Proliferation and apoptosis of activated CD4 T cells are modulated by combination treatment. EAE mice were sacrificed on day 30 after immunization. MOG-reactivated CD4+ T cells were sorted by FACS. CD4+ T cells were co-cultured with MP (20 μM) or BM-MSCs (3 × 104 cells) in combination or individually for 48 h in the presence of MOG (10 μg/mL). CD4+ T cells were analyzed for A proliferation and B, C apoptosis using EZ-Cytox cell proliferation assay, Caspase-Glo3/7 kit and Annexin V/PI assay in CD4 gate, respectively. Columns, mean; bars, SD; RLU, relative light units. ** p < 0.01 compared to PBS treatment group; # p < 0.05, ## p < 0.01 compared to MP treatment group; & p < 0.05, && p < 0.01 compared to BM-MSCs treatment group; One-way ANOVA with LSD post hoc test. The results are representative of 3 independent experiments
Discussion
Although MS treatment reduces the frequency and length of relapse periods, treatment methods are not effective in all patients with MS [34]. MP treatment has been approved by the Food and Drug Administration (FDA) for the treatment of MS [35]. High dose MP (30–100 mg/kg) therapy is widely used in MS and EAE [36–38]. Despite MP usage in patients with MS, high dose or long-term treatment with MP results in diverse side effects, such as glaucoma, hypertrichosis, and edema [2, 39]. A combination of medications or existing therapies may more greatly affect the disease relative to individual monotherapies and mitigate the adverse events by allowing the use of low-dose drugs [40]. In this regard, we demonstrated that combination treatment with low dose MP (20 mg/kg) and BM-MSCs might improve the therapeutic effects in EAE. Therefore, this method can enhance the safety for treatment of patients with MS.
In general, treatment of patients with MS is initiated after the development of clinical signs. Therefore, we started all treatments on day 14 post-immunization, after the onset of paralysis. Inflammation caused by lymphocyte infiltration is considered to be a cause of tissue damage in MS and EAE. Here, we demonstrated that the combination treatment reduced the clinical score, inflammatory cell infiltration, and demyelination in EAE. A decrease in inflammatory infiltration was associated with a reduction in demyelination and is important in reducing the progression of EAE disease [41]. Moreover, apoptotic cell death in the spinal cord was significantly decreased by combination treatment with MP and BM-MSCs compared with single treatments (Supplementary Fig. S1). Therefore, our results suggest that combination treatment may reduce the symptoms of EAE by decreasing inflammatory cell infiltration and demyelination in the lumbar spinal cord.
The secretion of cytokines from Th1 and Th2 cells is a key determinant of susceptibility or resistance in MS [42]. The susceptibility to EAE is correlated with the expression of the pro-inflammatory cytokines INF-γ, TNF-α, and IL-17 [43]. INF-γ and TNF-α are secreted by Th1 cells [44]. IL-17 is secreted by Th17 cells and plays a crucial role in the pathogenesis of EAE [45]. IL-17 expression was shown to be increased in the chronic lesions of patients with MS and EAE [30]. Combination treatment with MP and BM-MSCs decreased INF-γ, TNF-α, and IL-17 secretion from MOG-reactivated T cells in EAE. Anti-inflammatory cytokines, such as IL-4 and IL-10, are known to regulate the cell-mediated immune response and prevent EAE disease [43, 46]. Anti-inflammatory cytokines increase during remission in patients with MS [47]. Similarly, combination treatment increased IL-4 and IL-10 secretion by MOG-reactivated T cells in EAE. Taken together, these results suggest that the therapeutic effects of combination treatment may alleviate the progression of EAE by modulating Th1, Th17, and Th2 cells.
Auto-antigens recognizing myelin antigens exist in patients with MS and healthy individuals [48]. Activation of auto-antigens induces CNS inflammation and tissue damage [27]. Activated CD4 and CD8 T cells are prominently observed in active acute and chronic MS lesion [49, 50]. In our study, we determined that combination treatment decreases the populations of MOG-reactivated CD4 and CD8 T cells and increased the population of MOG-reactivated CD4+CD25+Foxp3+ Treg cells in EAE.
Treg cells are a special subset of T cells and play a crucial role in immune homeostasis by suppressing deleterious inflammatory responses [51]. Inflammation caused by activated autoreactive T cells in autoimmune disease is regulated by Treg cells [52]. Koutrolos et al. [53] have demonstrated that Treg limit autoimmune inflammation by controlling the effector T cells proliferation and motility within the CNS. Our data showed the increase of Treg cell percentage in spleen of EAE mouse (Fig. 6C, D). In addition, combination treatment significantly increased the apoptosis of CD4+ T cells (Fig. 7B, C). Therefore, the combination treatment may reduce the number of reactivated CD4 and CD8 T cells via increase in the population of reactivated Treg cells.
In autoimmune disease, the generation of activated T cells is important for the development of disease [54]. Our results showed that the proliferation of MOG-reactivated T cells was inhibited by combination treatment with MP and BM-MSCs. Likewise, other studies have shown that a decrease in activated T cells is associated with an improvement in the symptoms of EAE [30, 55]. Additionally, apoptotic cell death has been primarily observed in EAE [56]. Nguyen et al. [57] demonstrated that dexamethasone (4 mg/mL), a glucocorticoid medication, increased the level of T cell apoptosis in EAE. Similarly, combination treatment with MP and BM-MSCs decreased the MOG activated T cells through a caspase3/7 mediated apoptotic pathway in the spleens of EAE mice. Taken together, combination treatment might reduce disease severity by regulating the proliferation and apoptosis of activated T cells.
In summary, this study demonstrates that combination therapy with low dose MP and BM-MSCs attenuates the clinical score of EAE mice through several mechanisms. Combination treatment has therapeutic effects by suppressing inflammatory cell infiltration and demyelination. These therapeutic effects are associated with the regulation of cytokine production from MOG-reactivated lymphocytes. Furthermore, combination treatment results in a decrease in activated T cells through increasing the population of Treg cells and modulating the proliferation and apoptotic pathways. These findings suggest that combination treatment with low dose MP and BM-MSCs can be used as a promising therapeutic strategy for patients with MS. Additional studies are needed to determine the detailed mechanisms of the therapeutic effects provided by MP and BM-MCSs in EAE.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2054772, 2016R1D1A1B03931146) and by the Ministry of Science, ICT and Future Planning (2014R1A2A2A01004525).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical statement
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC), the Catholic University of Korea (Permit Number; 2015-0147-01).
Contributor Information
Chung Heon Ryu, Phone: 822-2258-7820, Email: ryuch@hit.ac.kr.
Sin-Soo Jeun, Phone: 822-2258-7535, Email: ssjeun@catholic.ac.kr.
References
- 1.El Behi M, Dubucquoi S, Lefranc D, Zéphir H, De Seze J, Vermersch P, et al. New insights into cell responses involved in experimental autoimmune encephalomyelitis and multiple sclerosis. Immunol Lett. 2005;96:11–26. doi: 10.1016/j.imlet.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Reichardt HM, Gold R, Lühder F. Glucocorticoids in multiple sclerosis and experimental autoimmune encephalomyelitis. Expert Rev Neurother. 2006;6:1657–1670. doi: 10.1586/14737175.6.11.1657. [DOI] [PubMed] [Google Scholar]
- 3.Sospedra M, Martin R. Antigen-specific therapies in multiple sclerosis. Int Rev Immunol. 2005;24:393–413. doi: 10.1080/08830180500371256. [DOI] [PubMed] [Google Scholar]
- 4.Hedegaard CJ, Krakauer M, Bendtzen K, Lund H, Sellebjerg F, Nielsen CH. T helper cell type 1 (Th1), Th2 and Th17 responses to myelin basic protein and disease activity in multiple sclerosis. Immunology. 2008;125:161–169. doi: 10.1111/j.1365-2567.2008.02837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeiffer F, Schäfer J, Lyck R, Makrides V, Brunner S, Schaeren-Wiemers N, et al. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011;122:601–614. doi: 10.1007/s00401-011-0883-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Imitola J, Chitnis T, Khoury SJ. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106:163–177. doi: 10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Duffy SS, Lees JG, Moalem-Taylor G. The contribution of immune and glial cell types in experimental autoimmune encephalomyelitis and multiple sclerosis. Mult Scler Int. 2014;2014:285245. doi: 10.1155/2014/285245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartung HP, Gonsette R, König N, Kwiecinski H, Guseo A, Morrissey SP, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- 9.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 10.Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- 11.Goodin DS, Frohman EM, Garmany GP, Jr, Halper J, Likosky WH, Lublin FD, et al. Disease modifying therapies in multiple sclerosis: report of the therapeutics and technology assessment subcommittee of the american academy of neurology and the MS council for clinical practice guidelines. Neurology. 2002;58:169–178. doi: 10.1212/WNL.58.2.169. [DOI] [PubMed] [Google Scholar]
- 12.Gross-Aviv T, Vago R. The role of aragonite matrix surface chemistry on the chondrogenic differentiation of mesenchymal stem cells. Biomaterials. 2009;30:770–779. doi: 10.1016/j.biomaterials.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Lee HK, Lee BH, Park SA, Kim CW. The proteomic analysis of an adipocyte differentiated from human mesenchymal stem cells using two-dimensional gel electrophoresis. Proteomics. 2006;6:1223–1229. doi: 10.1002/pmic.200500385. [DOI] [PubMed] [Google Scholar]
- 14.Aleynik A, Gernavage KM, Mourad YSh, Sherman LS, Liu K, Gubenko YA, et al. Stem cell delivery of therapies for brain disorders. Clin Transl Med. 2014;3:24. doi: 10.1186/2001-1326-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a model of multiple sclerosis (MS) Int J Mol Sci. 2012;13:9298–9331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morando S, Vigo T, Esposito M, Casazza S, Novi G, Principato MC, et al. The therapeutic effect of mesenchymal stem cell transplantation in experimental autoimmune encephalomyelitis is mediated by peripheral and central mechanisms. Stem Cell Res Ther. 2012;3:3. doi: 10.1186/scrt94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Yan Y, Ciric B, Yu S, Guan Y, Xu H, et al. Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity. Am J Pathol. 2010;177:1989–2001. doi: 10.2353/ajpath.2010.091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009;60:1006–1019. doi: 10.1002/art.24405. [DOI] [PubMed] [Google Scholar]
- 20.Brusaferri F, Candelise L. Steroids for multiple sclerosis and optic neuritis: a meta-analysis of randomized controlled clinical trials. J Neurol. 2000;247:435–442. doi: 10.1007/s004150070172. [DOI] [PubMed] [Google Scholar]
- 21.Ratzer R, Romme Christensen J, Romme Nielsen B, Sørensen PS, Börnsen L, Sellebjerg F. Immunological effects of methylprednisolone pulse treatment in progressive multiple sclerosis. J Neuroimmunol. 2014;276:195–201. doi: 10.1016/j.jneuroim.2014.08.623. [DOI] [PubMed] [Google Scholar]
- 22.Correale J, Arias M, Gilmore W. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- 23.Pozzilli C, Marinelli F, Romano S, Bagnato F. Corticosteroids treatment. J Neurol Sci. 2004;223:47–51. doi: 10.1016/j.jns.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 24.Tischner D, Reichardt HM. Glucocorticoids in the control of neuroinflammation. Mol Cell Endocrinol. 2007;275:62–70. doi: 10.1016/j.mce.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Li XL, Zhang ZC, Zhang B, Jiang H, Yu CM, Zhang WJ, et al. Atorvastatin calcium in combination with methylprednisolone for the treatment of multiple sclerosis relapse. Int Immunopharmacol. 2014;23:546–549. doi: 10.1016/j.intimp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Ravnborg M, Sørensen PS, Andersson M, Celius EG, Jongen PJ, Elovaara I, et al. Methylprednisolone in combination with interferon beta-1a for relapsing-remitting multiple sclerosis (MECOMBIN study): a multicentre, double-blind, randomised, placebo-controlled, parallel-group trial. Lancet Neurol. 2010;9:672–680. doi: 10.1016/S1474-4422(10)70132-0. [DOI] [PubMed] [Google Scholar]
- 27.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 29.Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- 30.Lv J, Du C, Wei W, Wu Z, Zhao G, Li Z, et al. The antiepileptic drug valproic acid restores T cell homeostasis and ameliorates pathogenesis of experimental autoimmune encephalomyelitis. J Biol Chem. 2012;287:28656–28665. doi: 10.1074/jbc.M112.356584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowther DE, Hafler DA. Regulatory T cells in the central nervous system. Immunol Rev. 2012;248:156–169. doi: 10.1111/j.1600-065X.2012.01130.x. [DOI] [PubMed] [Google Scholar]
- 32.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 33.Ivanov VN, Nikolić-Zugić J. Biochemical and kinetic characterization of the glucocorticoid-induced apoptosis of immature CD4+ CD8+ thymocytes. Int Immunol. 1998;10:1807–1817. doi: 10.1093/intimm/10.12.1807. [DOI] [PubMed] [Google Scholar]
- 34.Goldenberg MM. Multiple sclerosis review. P T. 2012;37:175–184. [PMC free article] [PubMed] [Google Scholar]
- 35.Berkovich R. Treatment of acute relapses in multiple sclerosis. Neurotherapeutics. 2013;10:97–105. doi: 10.1007/s13311-012-0160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milligan NM, Newcombe R, Compston DA. A double-blind controlled trial of high dose methylprednisolone in patients with multiple sclerosis: 1 clinical effects. J Neurol Neurosurg Psychiatry. 1987;50:511–516. doi: 10.1136/jnnp.50.5.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu YQ, Yang Y, Wang YG, Dai YQ, Xiao L, Qiu W, et al. Combined therapy with methylprednisolone and ulinastatin in experimental autoimmune encephalomyelitis. Chin Med J (Engl) 2013;126:3439–3445. [PubMed] [Google Scholar]
- 38.Wei ZS, Hong MF, Su QX, Wang XH, Yu QY, Peng ZX, et al. Super-high-dose methylprednisolone does not improve efficacy or induce glucocorticoid resistance in experimental allergic encephalomyelitis. Neuroimmunomodulation. 2011;18:28–36. doi: 10.1159/000314736. [DOI] [PubMed] [Google Scholar]
- 39.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 40.Paintlia AS, Paintlia MK, Singh I, Skoff RB, Singh AK. Combination therapy of lovastatin and rolipram provides neuroprotection and promotes neurorepair in inflammatory demyelination model of multiple sclerosis. Glia. 2009;57:182–193. doi: 10.1002/glia.20745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, et al. Mesenchymal stem cells generate a CD4+ CD25+ Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodríguez-Sáinz Mdel C, Sánchez-Ramón S, de Andrés C, Rodríguez-Mahou M, Muñoz-Fernández MA. Th1/Th2 cytokine balance and nitric oxide in cerebrospinal fluid and serum from patients with multiple sclerosis. Eur Cytokine Netw. 2002;13:110–114. [PubMed] [Google Scholar]
- 43.Nagelkerken L. Role of Th1 and Th2 cells in autoimmune demyelinating disease. Braz J Med Biol Res. 1998;31:55–60. doi: 10.1590/S0100-879X1998000100007. [DOI] [PubMed] [Google Scholar]
- 44.Steinman L. Mixed results with modulation of TH-17 cells in human autoimmune diseases. Nat Immunol. 2010;11:41–44. doi: 10.1038/ni.1803. [DOI] [PubMed] [Google Scholar]
- 45.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt-Weber CB, Alexander SI, Henault LE, James L, Lichtman AH. IL-4 enhances IL-10 gene expression in murine Th2 cells in the absence of TCR engagement. J Immunol. 1999;162:238–244. [PubMed] [Google Scholar]
- 47.Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK, et al. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol. 2007;190:139–145. doi: 10.1016/j.jneuroim.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–187. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 49.Abdul-Majid KB, Wefer J, Stadelmann C, Stefferl A, Lassmann H, Olsson T, et al. Comparing the pathogenesis of experimental autoimmune encephalomyelitis in CD4-/- and CD8-/- DBA/1 mice defines qualitative roles of different T cell subsets. J Neuroimmunol. 2003;141:10–19. doi: 10.1016/S0165-5728(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 50.Friese MA, Fugger L. Pathogenic CD8(+) T cells in multiple sclerosis. Ann Neurol. 2009;66:132–141. doi: 10.1002/ana.21744. [DOI] [PubMed] [Google Scholar]
- 51.Valencia X, Lipsky PE. CD4+ CD25+ FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Podojil JR, Luo X, Miller SD. Intrinsic and induced regulation of the age-associated onset of spontaneous experimental autoimmune encephalomyelitis. J Immunol. 2008;181:4638–4647. doi: 10.4049/jimmunol.181.7.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koutrolos M, Berer K, Kawakami N, Wekerle H, Krishnamoorthy G. Treg cells mediate recovery from EAE by controlling effector T cell proliferation and motility in the CNS. Acta Neuropathol Commun. 2014;2:163. doi: 10.1186/s40478-014-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galligan CL, Pennell LM, Murooka TT, Baig E, Majchrzak-Kita B, Rahbar R, et al. Interferon-beta is a key regulator of proinflammatory events in experimental autoimmune encephalomyelitis. Mult Scler. 2010;16:1458–1473. doi: 10.1177/1352458510381259. [DOI] [PubMed] [Google Scholar]
- 55.Makar TK, Trisler D, Bever CT, Goolsby JE, Sura KT, Balasubramanian S, et al. Stem cell based delivery of IFN-beta reduces relapses in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;196:67–81. doi: 10.1016/j.jneuroim.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Tabi Z, McCombe PA, Pender MP. Antigen-specific down-regulation of myelin basic protein-reactive T cells during spontaneous recovery from experimental autoimmune encephalomyelitis: further evidence of apoptotic deletion of autoreactive T cells in the central nervous system. Int Immunol. 1995;7:967–973. doi: 10.1093/intimm/7.6.967. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen KB, McCombe PA, Pender MP. Increased apoptosis of T lymphocytes and macrophages in the central and peripheral nervous systems of Lewis rats with experimental autoimmune encephalomyelitis treated with dexamethasone. J Neuropathol Exp Neurol. 1997;56:58–69. doi: 10.1097/00005072-199701000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.