Abstract
Background:
Polymeric hydrogels are extensively used as promising biomaterials in a broad range of biomedical applications, including tissue engineering, regenerative medicine, and drug delivery. These materials have advantages such as structural similarity to the native extracellular matrix (ECM), multi-tunable physicochemical and biological properties, and biocompatibility.
Methods:
In situ forming hydrogels show a phase transition from a solution to a gel state through various physical and chemical cross-linking reactions. These advanced hydrogel materials have been widely used for tissue regenerative medicine because of the ease of encapsulating therapeutic agents, such as cells, drugs, proteins, and genes.
Results:
With advances in biomaterials engineering, these hydrogel materials have been utilized as either artificial cellular microenvironments to create engineered tissue constructs or as bioactive acellular matrices to stimulate the native ECM for enhanced tissue regeneration and restoration.
Conclusion:
In this review, we discuss the use of in situ cross-linkable hydrogels in tissue engineering and regenerative medicine applications. In particular, we focus on emerging technologies as a powerful therapeutic tool for tissue regenerative medicine applications.
Keywords: Polymeric hydrogels, In situ cross-linkable hydrogels, Tissue engineering, Tissue regenerative medicine
Introduction
Polymeric hydrogels, which are defined as a three-dimensional (3D) hydrophilic network, are promising biomaterials for a broad range of biomedical applications, such as tissue engineering, regenerative medicine, and in drug delivery [1–5]. The 3D hydrophilic networks provide structural frameworks with a large amount of water within the matrices similar to the native extracellular matrix (ECM) [6]. Also, the hydrogel materials can be tailored with various bioactive molecules (e.g., cell-adhesive sites, proteolytic degradable sites, and stimuli-sensitive linkages) to improve their biocompatibility and performance in vitro and in vivo [7–9]. Based upon their unique properties, hydrogel materials have been attractive biomaterials for various biomedical uses. In particular, in situ cross-linkable hydrogels have been widely utilized as therapeutic implants and vehicles for tissue engineering and regenerative medicine [10, 11]. These hydrogels show a phase transition from a solution to a gel state via various physical and chemical stimuli. They can be injected into the target sites using injectable devices and can encapsulate therapeutic agents (e.g., chemical drugs, proteins, cells, and genes) during hydrogel formation. Many types of natural and synthetic biomaterials have been used to create in situ forming hydrogels via numerous cross-linking strategies for tissue engineering and regenerative medicine.
Emerging trends in the design of advanced hydrogel materials include recapitulating the ECM as an artificial ECM or stimulating the native cellular microenvironment as bioactive acellular matrices for enhanced tissue regeneration and repair. The ECM is composed of various proteins and polysaccharides with soluble factors, which provide a structural framework, support cell growth, and regulate cell fate [12]. The natural cellular microenvironment involves various physicochemical cues including nutrients and metabolites, temperature, pH, and oxygen (O2) tension. Growing evidence has demonstrated that these parameters play pivotal roles in cell proliferation, migration, and differentiation in the native cellular microenvironment [13]. Recently, in situ cross-linkable hydrogels have been utilized as artificial cellular microenvironments to create engineered tissue constructs for tissue engineering and regenerative medicine as well as for a better understanding of basic cell biology in healthy and diseased tissues [2, 3, 10, 14]. Also, the advanced hydrogel materials have been used as bioactive acellular matrices to stimulate the native ECM for enhanced tissue regeneration and repair by regulating tissue-material interactions or by delivering bioactive molecules [15–17].
In this review, we introduce the emerging strategies to create in situ cross-linkable hydrogels via various physical and chemical cross-linking reactions. Moreover, we discuss the current uses of in situ cross-linkable hydrogels in tissue engineering and regenerative medicine applications. Specifically, we focus on some of the recently reported techniques for enhanced tissue regeneration.
Strategies to fabricate in situ cross-linkable hydrogels
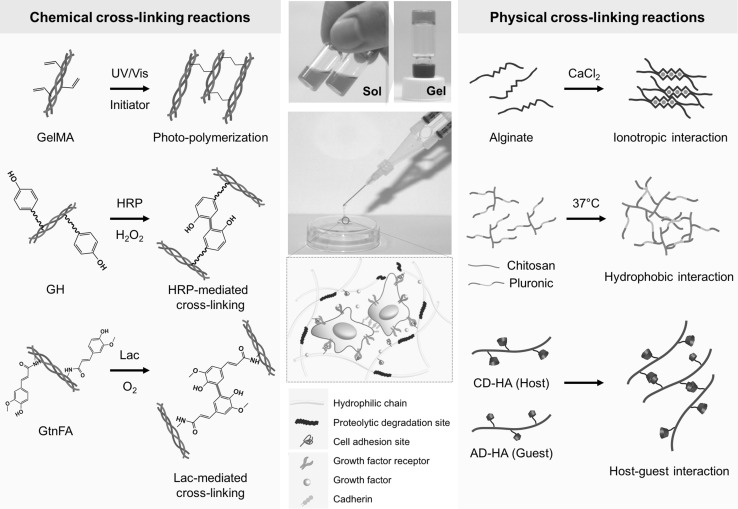
Various cross-linking strategies have been utilized to create in situ forming hydrogels, including physical (e.g., ionotropic interaction, thermo-sensitivity, and host–guest interaction) and chemical cross-linking reactions (e.g., enzyme-mediated cross-linking, photo-cross-linking, and click chemistry) using natural and synthetic polymers [10, 18, 19]. Fig. 1 illustrates the typical cross-linking strategies to fabricate in situ forming hydrogels either as artificial ECM or as acellular matrices. In this section, we discuss the representative cross-linking approaches to fabricate advanced in situ forming hydrogel materials.
Fig. 1.
Cross-linking strategies to fabricate in situ forming hydrogels. In situ cross-linkable hydrogels exhibit phase transition from solution to gel state through physical and chemical cross-linking reactions. These advanced hydrogel materials can serve as dynamic cellular or acellular matrices for tissue engineering and regenerative medicine. Abbreviations: GelMA, gelatin-methacryloyl; GH, gelatin-g-hydroxyphenyl propionic acid; HRP, horseradish peroxidase; GtnFA, gelatin-g-ferulic acid; Lac, laccase; CD-HA, b-cyclodextrin-conjugated hyaluronic acid; AD-HA; adamantine-modified hyaluronic acid
Physically cross-linkable hydrogels form hydrogel networks via non-covalent bonds, including inotropic interaction, thermo-sensitive response, and host–guest interactions. Alginate, composed of repeating units of (1-4)-linked β-D-mannuronate (M units) and α-l-guluronate (G units), is the representative natural polymer. The natural polymer can form hydrogel networks in the presence of divalent cations (e.g., calcium, barium, and magnesium) through inotropic interaction between the G units in the polymer backbone [20]. Alginate-based hydrogels have been widely used as injectable matrices in tissue regeneration, wound healing, and drug delivery because of their biocompatibility and easy fabrication [21–23]. Other physical hydrogels include thermo-sensitive hydrogels that can induce 3D hydrogel networks through hydrophobic interactions of amphiphilic polymers (e.g., poloxamers) in aqueous solutions by increasing the temperature. These matrices have been extensively used as injectable carriers for tissue regeneration and drug delivery because of their reversible phase transition and a simple fabrication process [24, 25]. Recently, self-assembled in situ forming hydrogels through host–guest interaction have been developed, which can create hydrogel networks in physiological condition. The physical hydrogels are formed through molecular inclusion between cyclodextrins (CDs) and other guest molecules [e.g., poly(ethylene glycol) (PEG), adamantine, and cholesterols] [26, 27]. The CDs, which are natural cyclic oligosaccharides, have an inner cavity that induces physical cross-linking via molecular inclusion with the guest molecules. These hydrogels are fabricated by simple mixing of two polymer solutions (solution A, CD-modified polymers; solution B, guest molecule-conjugated polymers). Their physicochemical properties (e.g., hydrogel formation time, mechanical properties, and degradation behaviors) can be easily controlled by regulating the pendants and polymer contents [28]. These unique properties allow the hydrogel matrices to be applied to various biomedical applications, including therapeutic delivery carriers, bio-inks, and engineered tissue models for tissue regeneration [26–29]. We introduced some of the representative physical hydrogels. While these hydrogels have some benefits, such as a simple fabrication process and formation in mild conditions, it is still challenging to improve their stability and mechanical strength to extend their potential applications.
Chemically cross-linkable hydrogels form 3D polymeric networks via covalent bonds through various chemical reactions. The chemical cross-linking strategies involve enzyme-mediated cross-linking, click chemistry, and photo-cross-linking reactions [10, 18]. A typical method to create in situ cross-linkable hydrogels is a photo-cross-linking strategy through ultraviolet and visible (UV/vis) light irradiation. For the hydrogel fabrication, methacrylate-conjugated polymer solutions containing photo-initiators (e.g., Irgacure PIs I2959, I184, and I651) were irradiated with UV or visible light to induce a radical cross-linking reaction between the acryl groups in the polymer backbones. These photo-curable hydrogels have been widely used as injectable matrices for tissue regenerative medicine, drug delivery, tissue respiration, and sealant materials [30–33]. Recently, there has been growing interest in creating in situ cross-linkable hydrogels through enzymatic reaction because of the hydrogel formation in physiological conditions and substrate-specific conjugations. Several types of enzyme-mediated cross-linkable hydrogels have been developed using several enzymes [e.g., horseradish peroxidase (HRP), transglutaminase, tyrosinase, lysyl oxidase, and laccase (Lac)] [15–17, 34–39]. Specifically, HRP-mediated cross-linking reactions have attracted attention as a promising chemical cross-linking method because of the biocompatibility and easily controllable physicochemical and biological properties of the hydrogels (e.g., gelation kinetics, mechanical strength, and degradation behaviors) [40, 41]. In the HRP-mediated conjugative reaction, the enzyme catalyzes the di-phenolic formation of phenol-conjugated polymer backbones in the presence of hydrogen peroxide (H2O2) that is converted into water (H2O) and molecular O2 in the process of the hydrogel formation [40]. Growing evidence has demonstrated that the HRP-mediated polymeric hydrogels with excellent bioactivity and tunable physicochemical properties are promising biomaterials in a broad range of biomedical applications, including tissue regenerative medicine, drug delivery, and wound management [42, 43]. Recently, Lac has been used to create in situ cross-linkable hydrogels via O2-consuming cross-linking reaction. For the preparation of O2-controllable hydrogels, ferulic acid (FA)-conjugated polymers were merely mixed with Lac solutions [15, 16, 44]. In the enzymatic cross-linking reaction, Lac catalyzes diferulic acid formation that induces hydrogel networks with O2-consuming reaction. In addition to the enzymatic cross-linking reactions, the click chemistry has been widely utilized as an alternative method to create in situ forming hydrogels as the reaction occurs under physiological conditions in the absence of toxic cross-linking agents [45, 46]. For the hydrogel fabrication, two types of polymers (polymer A, azide-conjugated; polymer B, terminal acetylene-modified polymers) are mixed in the presence of copper (Cu[I]). In this reaction, Cu catalyzes the formation of the 1,2,3-triazole bond that induces 3D hydrogel networks. Because of regioselectivity and rapid hydrogel formation in mild conditions, these hydrogels have been utilized as either engineered matrices or delivery carriers for various biomedical applications as well as tissue regenerative medicine [47–49].
With advances in biomaterials engineering, numerous strategies have been developed to fabricate in situ forming hydrogels through physical and chemical cross-linking reactions. These advanced hydrogel materials hold great potential as either artificial cellular microenvironments to create engineered tissues or bioactive acellular matrices to stimulate the native ECM for improved tissue regeneration and repair.
Artificial extracellular matrices
In situ cross-linkable hydrogels have attracted attention as artificial ECM to create engineered tissues because of their structural similarity to the native ECM and easy encapsulation of target cells within the matrices in the process of the hydrogel formation [1, 7, 8]. The engineered tissue constructs created using the hydrogel materials with the cells have been utilized either as a promising platform for tissue transplantation or as an alternative to animal models for a better understanding of basic cell biology in healthy and diseased tissues. Recently, many researchers have endeavored to create engineered tissue constructs that can precisely recapitulate the native ECM with spatiotemporal complexity. The native microenvironments present various physicochemical and biological properties, including ECM components and remodeling, cell–cell/matrix interactions, soluble factors, pH levels, reactive oxygen/nitrogen species, and O2 tension. In this section, we introduce the most recently reported in situ cross-linkable hydrogels as artificial cellular microenvironments to create engineered tissues for tissue engineering and regeneration.
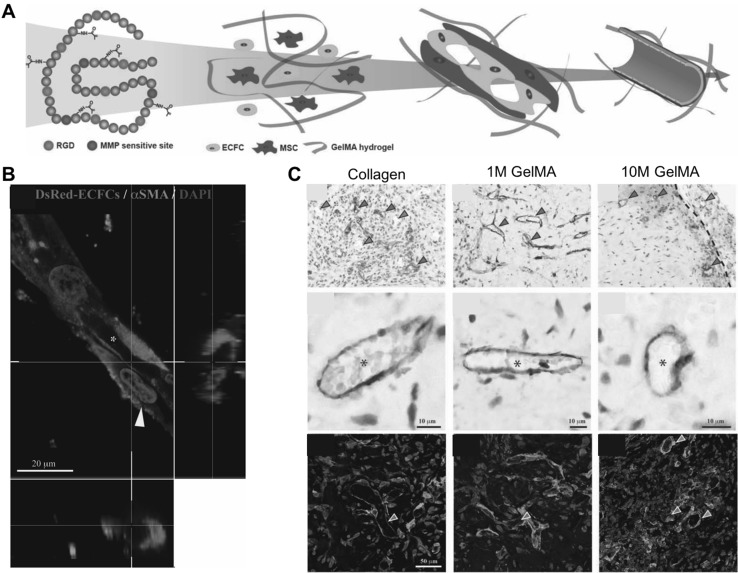
Gelatin-methacryloyl (GelMA) hydrogels, which can induce 3D hydrogel networks through a photo-cross-linking reaction, has been commonly used as a 3D cell culture template [31]. Gelatin is a natural polymer derived from collagen, which is a promising biomaterial as a scaffold for tissue engineering and regeneration. The gelatin possesses bioactive moieties that precisely mimic the native ECM, including cell-adhesive and proteolytic degradable sites [50]. Various bio-fabrication methods have been reported to create cell-laden 3D constructs utilizing the hydrogel materials, including 3D encapsulation, micro-/nano-fabrication, and 3D bio-printing techniques [51]. Daniela et al. developed cell-laden constructs as modular tissue culture platforms [52]. GelMA-based cell-laden hydrogels were fabricated using custom-made and sterilized Teflon molds by UV irradiation with Irgacure 2959 as a photo-initiator [52]. With the advanced hydrogel materials, mature vascular constructs were created using endothelial colony-forming cells (ECFCs) and mesenchymal stem cells (MSCs) (Fig. 2A) [53]. Notably, it was demonstrated that photocurable GelMA hydrogels supported extensive 3D capillary-like vascular networks in vitro (Fig. 2B) [53]. The engineered vasculatures were transplanted into nude mice and, functional anastomoses were observed between the newly formed vascular network and the host vasculature (Fig. 2C) [53]. These results suggested that the GelMA-based engineered vascular constructs hold great potential in the treatment of vascular disorders and tissue regeneration.
Fig. 2.
Engineered vascular constructs using photo-curable GelMA hydrogels. A Schematic representation of the stepwise process of endothelial lumen formation in the engineered microenvironment. B Premature vessel formation of ECFCs surrounded by α-smooth muscle actin (α-SMA)-expressing MSCs (yellow arrow). Scale bar is 20 µm. C Functional vascular formation in vivo. Immunohistochemistry exhibited that the engineered vasculatures were positively stained for human CD31 (red arrow) and murine capillaries (green arrow), carrying murine erythrocytes (asterisks). Fluorescence images show sections stained with rhodamine-conjugated UEA-1 lectin (to mark human ECFC-lined vessels) and fluorescein isothiocyanate-conjugated anti-α-SMA (to mark perivascular cells; red arrowheads). UEA-1 lectin did not bind to the murine vessels (green arrow). Scale bars are 10 µm and 50 µm
(adapted with permission from Ref [53]). (Color figure online)
Recently, there has been growing interest in fabricating in situ forming hydrogels through HRP-mediated cross-linking reaction because of the easy fabrication process with biocompatible and multi-tunable properties. Park and his colleagues developed gelatin-based in situ forming hydrogels through HRP-mediated cross-linking reactions that facilitated angiogenic differentiation and cellular activity of patient-derived human MSCs [54]. For the hydrogel fabrication, hydroxyphenyl propionic acid (GH) polymers were synthesized, which can form hydrogel networks through HRP-mediated conjugative reaction between the phenolic molecules in the polymer backbone. To create engineered vascular tissues, MSCs were encapsulated within the hydrogel matrices during the hydrogel formation. Interestingly, it was demonstrated that the GH hydrogels directed endothelial differentiation of the stem cells through integrin-mediated interaction at the cell–matrix interface, resulting in perusable blood vessel formation in vitro and in vivo [54]. Also, it was found that specific integrin types (α1 and αvβ3) played a critical role in facilitating the angiogenic differentiation of the stem cells [54]. These findings suggested that the purely material-driven effects can regulate endothelial differentiation of MSCs, thereby promoting vascularization of scaffolds for tissue engineering and regenerative medicine.
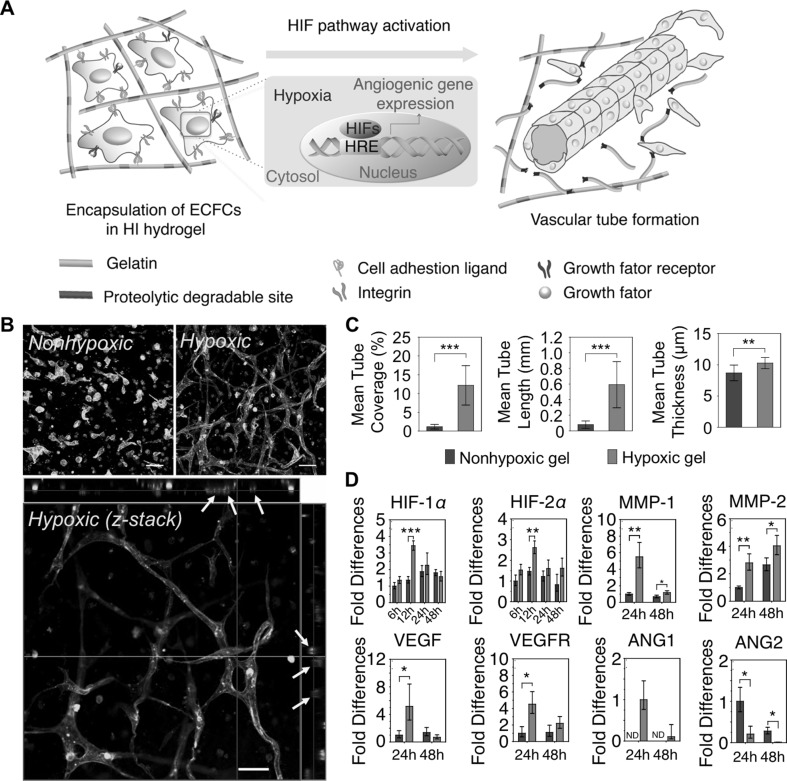
The O2 has been implicated as a pivotal signaling molecule in the regulation of cell growth, migration, and differentiation [55]. Specifically, recent researches have demonstrated that O2 deprivation (defined as hypoxia) plays a critical role in the vascular developmental process (e.g., vasculogenesis and angiogenesis) during tissue development, regeneration, and wound healing [56, 57]. Most recently, Park and Gerecht designed in situ forming hypoxia-inducible (HI) hydrogels as an artificial hypoxic microenvironment to recapitulate the physiological microenvironment [16]. FA-conjugated gelatin (GtnFA) polymers were synthesized, which can form hydrogel networks with O2 consumption in Lac-mediated cross-linking reactions. The physicochemical and biological properties of the HI hydrogels were characterized, including gelation kinetics (2–30 min), tunable mechanical strength (35–370 Pa), controllable oxygen tension within the matrices (0.1–21% pO2), and cytocompatibility (> 90%). It was demonstrated that the optimized hydrogel condition could provide artificial hypoxic microenvironments under 5% pO2 throughout the matrices [16]. To investigate the effect of O2 tension on the vascular differentiation of endothelial progenitor cells, the cells were encapsulated within two types of hydrogels (hypoxic gels, HG vs. non-hypoxic gels, NG) (Fig. 3A). The O2 tension was controlled by varying hydrogel thickness (HG, 2.5 mm in height; NG, 1.25 in mm height in a 96-well plate). Interestingly, the cells encapsulated within HG matrices exhibited more extensive microvasculature through hypoxia-inducible factor (HIF) pathway activation compared to those within the NG matrices (Fig. 3B–D) [16]. The results suggested that the engineered vascular constructs hold great potential as an advanced therapeutic tool for the treatment of vascular disorders and tissue regenerative medicine.
Fig. 3.
Hypoxia-inducible hydrogels to create engineered vasculatures. A Schematic diagram of vascular morphogenesis of ECFCs within artificial hypoxic microenvironment. B Confocal microscopic images of ECFCs cultured within non-hypoxic gels (NG) and hypoxic gels (HG); confocal Z-stacks and orthogonal sections exhibited lumen formation (yellow arrow). Scale bar is 50 µm. C Quantification of vascular tube formation (mean tube coverage, tube length, and tube thickness). D Real-time reverse-transcription polymerase chain reaction for gene expression of ECFCs cultured within two types of hydrogels (NG vs. HG), which is relevant to vascular morphogenesis. Results in C and D are shown as the average value ± s.d. Significance levels were set as *p < 0.05, **p < 0.01, and ***p < 0.001
(adapted with permission from Ref. [16]). (Color figure online)
Bioactive acellular matrices
Recently, in situ forming hydrogels have been designed as bioactive acellular matrices for improved tissue regeneration and repair. These injectable materials stimulate the native ECM by physicochemical changes, such as inducing acute oxidative stress or recruiting endogenous progenitor/stem cells for enhanced tissue regeneration. In this section, we discuss the use of innovative hydrogel materials in tissue regenerative medicine applications.
It is well-known that nitric oxide (NO) is involved as a signaling molecule in various biological processes [58, 59]. It plays critical roles in maintaining the natural function of the endothelium and acts as an endogenous vasodilator and natural inhibitor of platelet adhesion and activation [59]. Growing evidence has demonstrated that NO facilitates vascular development process (e.g., vasculogenesis and angiogenesis) and thus, the bioactive molecule has been widely utilized as a therapeutic agent for the treatment of vascular tissue regeneration [60, 61]. Kong and Li developed NO-releasing chitosan hydrogels that promoted endothelial differentiation of mouse embryonic stem (ES) cells [62, 63]. Chitosan-based NO-releasing hydrogels were synthesized by simple mixing of β-galactose-caged NO donor (Gal-NONOate) and chitosan solutions in the presence of CuSO4 and ascorbate [62]. To investigate the effect of NO release on endothelial differentiation, mouse ES cells were cultured on the hydrogel surfaces. Interestingly, the ES cells cultured on the NO-releasing hydrogels showed increased expression of Flk-1 (early endothelial cell marker) and VE-cadherin (mature endothelial marker) under controlled NO-releasing hydrogel systems [63]. The bioactive hydrogels were also applied to diabetic mice with hind-limb ischemia, resulting in enhanced therapeutic angiogenesis in the defect sites [62]. These results demonstrated that the NO-releasing hydrogel matrices hold great promise for the treatment of various vascular disorders, including diabetic wounds and ischemic diseases. Most recently, Thi et al. developed NO-releasing hydrogels with high antibacterial activity through in situ peroxynitrite formation [64]. While NO has been widely used as a therapeutic agent, its short half-life remains a challenge in clinical application in pharmaceutical forms. S-nitrosothiolated gelatin (GelSNO) was incorporated as a NO donor into in situ cross-linkable GH hydrogels to overcome the limitations of NO-controllable hydrogels [64]. NO was released from the matrices through thermal-, visible light-, and oxidizing agent-driven mechanisms and the release behavior was controlled by varying the GelSNO concentration from 0.053 to 2.050 μmol/mL for up to 2 weeks [64]. Notably, the NO-releasing gelatin hydrogels exhibited potent antibacterial effects against both Escherichia coli and Staphylococcus aureus without cytotoxicity on human dermal fibroblasts. The results suggested that HRP-mediated NO-releasing hydrogels may provide an innovative injectable matrix for treating wound infections and tissue regenerative medicine [64].
Reactive oxygen species (ROS) have been implicated as bioactive molecules in regulating cell signaling and maintaining homeostasis [65]. Specifically, it has been demonstrated that H2O2 plays a pivotal role in various therapeutic applications including the treatment of vascular disorders, wound healing and repair, and antibacterial treatment [66, 67]. Park and his colleagues reported an H2O2-releasing hydrogel as a wound dressing material with antibacterial activity against various bacterial strains including clinical isolates of drug-resistant strains [37]. HRP-mediated in situ forming hydrogels were fabricated with excess H2O2 concentrations (1–10 mM). During the hydrogel formation, H2O2 was decomposed into H2O and oxygen, and a certain amount of residual H2O2 was released from the hydrogel matrices in a sustained manner. The amount of H2O2 released was controlled by varying the feed amount of H2O2 (ranging from 2 to 510 μM) [37]. Interestingly, the H2O2-releasing hydrogels exhibited strong killing efficiency toward Gram-positive bacteria including Staphylococcus aureus, Staphylococcus epidermidis, and a clinical isolate of methicillin-resistant Staphylococcus aureus (drug-resistant bacteria) [37]. Also, the hydrogels facilitated skin wound healing and repair in full-thickness defect models. These results suggested that the H2O2-releasing hydrogels have a great potential as antimicrobial dressing materials for wound and infection treatment.
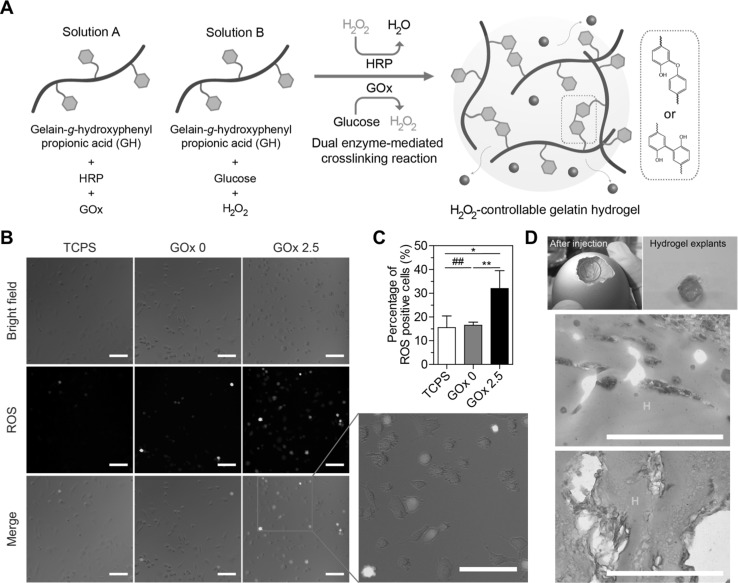
Growing evidence has demonstrated that transient and low levels of H2O2 (ranging from 0.1 to 10 μM) enhanced the angiogenic activities of vascular cells through acute oxidative stress via evaluated intracellular ROS levels in cells or surrounding tissues [68–70]. Lee et al. [17] utilized H2O2-controllable GH hydrogels formed via dual enzyme-mediated cross-linking reaction using HRP and glucose oxidase (GOx) as H2O2-generating enzymes to gradually supply H2O2 that is a substrate in HRP-mediated cross-linking reactions. The H2O2-releasing amount can be accurately controlled by varying the GOx concentrations (ranging from 0 to 10 μM) for up to 48 h [17]. It was demonstrated that the optimal H2O2-releasing condition increased transient intracellular ROS levels in endothelial cells (ECs), enhanced the proliferative activities of vascular cells, and facilitated in ovo neovascularization (Fig. 4) [17]. These results suggested that the H2O2-controllable hydrogels provide injectable and bioactive matrices for vascular tissue regeneration.
Fig. 4.
H2O2-controllable hydrogels for facilitating neovascularization though transient upregulation of intracellular ROS levels in ECs. A Schematic representation of in situ hydrogel formation via dual enzyme-mediated cross-linking reaction. Newly formed covalent bonds are indicated in red. Transient upregulation of intracellular ROS levels of ECs by sustained release of H2O2 from hydrogels. B Representative optical and fluorescent microscopic images. Scale bar is 100 μm. C Quantitative analysis of fluorescence-positive cells. The results in B are shown as an average ± s.d. Significance levels were set at *p < 0.05, **p < 0.01, and ***p < 0.001. ## indicates not significant. D In ovo angiogenic effect of the hydrogels. Histological sections of hydrogels seven days after injection, stained with α-SMA. Scale bar is 100 μm
(adapted with permission from Ref. [17]). (Color figure online)
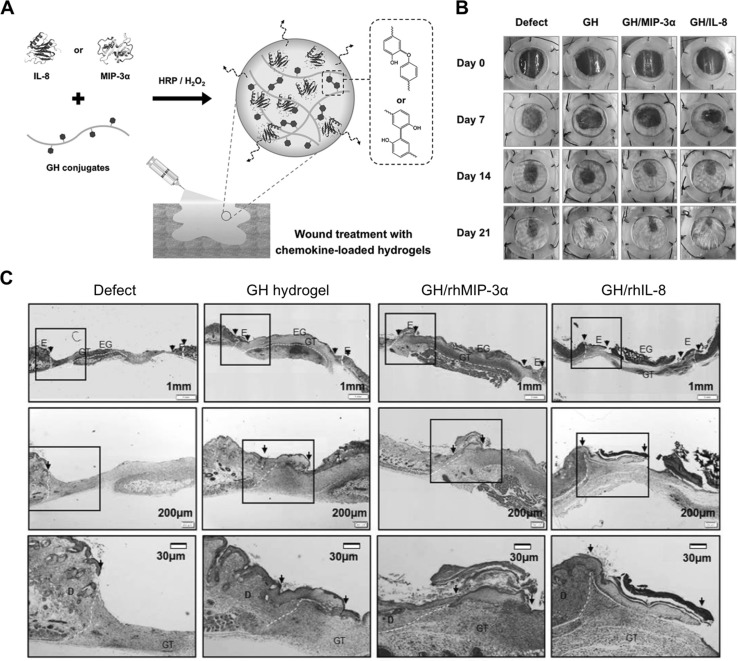
The recruitment of endogenous progenitor or stem cells is essential for tissue regeneration and wound healing [71, 72]. Recently, many researchers have reported hydrogel materials that encapsulate cytokines and chemokines as signaling factors that recruit the endogenous cells from the host tissue to the defect sites. Lee and his colleagues utilized HRP-mediated GH hydrogels that encapsulated cell-recruiting chemokines as injectable and sprayable dressing materials for treatment of a diabetic wound [73]. Cell-recruiting factors (e.g., interleukin (IL)-8, and macrophage inflammatory protein (MIP)-3α) were loaded within GH hydrogels during in situ hydrogel formation. It is well-known that IL-8 and MIP-3 α recruit bone marrow-derived MSCs for tissue regeneration and repair. The wound healing efficacy of the hydrogels was investigated in a streptozotocin-induced diabetic mouse model, showing promoted wound healing and restoration with enhanced re-epithelialization, neovascularization, and thicker granulation [73]. These results suggested that the in situ forming and chemokine-loaded hydrogels can serve as an injectable carrier for skin tissue regeneration (Fig. 5).
Fig. 5.
Chemokine-loaded in situ forming GH hydrogels for enhanced wound healing in diabetic mouse models. A Schematic illustration of in situ GH hydrogel formation through HRP-mediated cross-linking reaction, encapsulating cell-recruiting chemokines (IL-8 or MIP-3α). B Representative digital images depicting wound healing by the chemokine-loaded GH hydrogels in streptozotocin-induced diabetic mice on day 0, 7, 14, and 21. C Re-epithelialization of the regenerative tissues on day 7 after hydrogel treatment. The explants were subjected to Masson’s trichrome staining and showed re-epithelialization of the damaged tissues with the hydrogels. The arrows indicate regenerated edges of the skin wound. D dermis; E epidermis; EG epithelial gap; GT granulation tissue
(adapted with permission from Ref. [73])
Conclusions and future directions
Numerous in situ cross-linkable hydrogels have been explored as artificial cellular microenvironments to create engineered tissues and as bioactive acellular matrices to facilitate tissue regeneration and restoration. Although advanced hydrogel materials are extensively used as engineered matrices, it is still challenging to mimic spatiotemporal complex native tissues more accurately and to improve their biocompatibility, such as reducing inflammatory and immune reactions when transplanted in vivo. In recent years, many researchers have endeavored to create engineered tissues that better mimic the native cellular microenvironments in combination with emerging micro-/nano-fabrication methods, including microfluidic devices and 3D bio-printing techniques. Innovative engineering approaches are developing to reduce inflammation and immune response that may induce functional impairment and tissue or organ failure. These innovative approaches allow the generation of more complex tissue constructs and biocompatible matrices. Thus, the advanced hydrogel materials are a powerful therapeutic tool for successful tissue regeneration and treatment of other diseases.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (NRF-2018R1A2B2004529) and (NRF-2015R1C1A1A01054498).
Conflict of interest
The authors declare that they have no competing interests.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Zhang Yu Shrike, Khademhosseini Ali. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. doi: 10.1126/science.aaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park KM, Lewis D, Gerecht S. Bioinspired hydrogels to engineer cancer microenvironments. Annu Rev Biomed Eng. 2017;19:109–133. doi: 10.1146/annurev-bioeng-071516-044619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S, Park KM. Engineered polymeric hydrogels for 3D tissue models. Polymers. 2016;8:23. doi: 10.3390/polym8010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2012;64:18–23. doi: 10.1016/j.addr.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13:538–546. doi: 10.1007/s13770-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 7.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 8.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luca A, Butnaru M, Maier SS, Knieling L, Bredetean O, Verestiuc L, et al. Atelocollagen-based hydrogels crosslinked with oxidised polysaccharides as cell encapsulation matrix for engineered bioactive stromal tissue. Tissue Eng Regen Med. 2017;14:539–556. doi: 10.1007/s13770-017-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang JA, Yeom J, Hwang BW, Hoffman AS, Hahn SK. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci. 2014;39:1973–1986. doi: 10.1016/j.progpolymsci.2014.07.006. [DOI] [Google Scholar]
- 11.Bang S, Jung UW, Noh I. Synthesis and biocompatibility characterizations of in situ chondroitin sulfate-gelatin hydrogel for tissue engineering. Tissue Eng Regen Med. 2018;15:25–35. doi: 10.1007/s13770-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hay ED. Cell biology of extracellular matrix. 2. Berlin: Springer; 2013. [Google Scholar]
- 13.Muncie JM, Weaver VM. The physical and biochemical properties of the extracellular matrix regulate cell fate. Curr Top Dev Biol. 2018;130:1–37. doi: 10.1016/bs.ctdb.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosales AM, Anseth KS. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat Rev Mater. 2016;1:15012. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park KM, Blatchley MR, Gerecht S. The design of dextran-based hypoxia-inducible hydrogels via in situ oxygen-consuming reaction. Macromol Rapid Commun. 2014;35:1968–1975. doi: 10.1002/marc.201400369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park KM, Gerecht S. Hypoxia-inducible hydrogels. Nat Commun. 2014;5:4075. doi: 10.1038/ncomms5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee Y, Son JY, Kang JI, Park KM, Park KD. Hydrogen peroxide-releasing hydrogels for enhanced endothelial cell activities and neovascularization. ACS Appl Mater Interfaces. 2018;10:18372–18379. doi: 10.1021/acsami.8b04522. [DOI] [PubMed] [Google Scholar]
- 18.Ko DY, Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci. 2013;38:672–701. doi: 10.1016/j.progpolymsci.2012.08.002. [DOI] [Google Scholar]
- 19.Somekawa S, Mahara A, Masutani K, Kimura Y, Urakawa H, Yamaoka T. Effect of thermoresponsive poly(L-lactic acid)-poly(ethylene glycol) gel injection on left ventricular remodeling in a rat myocardial infarction model. Tissue Eng Regen Med. 2017;14:507–516. doi: 10.1007/s13770-017-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Augst AD, Kong HJ, Mooney DJ. Alginate hydrogels as biomaterials. Macromol Biosci. 2006;6:623–633. doi: 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- 21.García-Astrain C, Avérous L. Synthesis and evaluation of functional alginate hydrogels based on click chemistry for drug delivery applications. Carbohydr Polym. 2018;190:271–280. doi: 10.1016/j.carbpol.2018.02.086. [DOI] [PubMed] [Google Scholar]
- 22.Reakasame S, Boccaccini AR. Oxidized alginate-based hydrogels for tissue engineering applications: a review. Biomacromolecules. 2018;19:3–21. doi: 10.1021/acs.biomac.7b01331. [DOI] [PubMed] [Google Scholar]
- 23.Campbell KT, Hadley DJ, Kukis DL, Silva EA. Alginate hydrogels allow for bioactive and sustained release of VEGF-C and VEGF-D for lymphangiogenic therapeutic applications. PLoS One. 2017;12:e0181484. doi: 10.1371/journal.pone.0181484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/S0169-409X(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 25.Klouda L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur J Pharm Biopharm. 2015;97:338–349. doi: 10.1016/j.ejpb.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 26.Nakahata M, Takashima Y, Yamaguchi H, Harada A. Redox-responsive self-healing materials formed from host-guest polymers. Nat Commun. 2011;2:511. doi: 10.1038/ncomms1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Highley CB, Rodell CB, Burdick JA. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv Mater. 2015;27:5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 28.Rodell CB, Mealy JE, Burdick JA. Supramolecular guest-host interactions for the preparation of biomedical materials. Bioconjug Chem. 2015;26:2279–2289. doi: 10.1021/acs.bioconjchem.5b00483. [DOI] [PubMed] [Google Scholar]
- 29.Rodell CB, Wade RJ, Purcell BP, Dusaj NN, Burdick JA. Selective proteolytic degradation of guest-host assembled, injectable hyaluronic acid hydrogels. ACS Biomater Sci Eng. 2015;1:277–286. doi: 10.1021/ab5001673. [DOI] [PubMed] [Google Scholar]
- 30.Zhao X, Lang Q, Yildirimer L, Lin ZY, Cui W, Annabi N, et al. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv Healthc Mater. 2016;5:108–118. doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assmann A, Vegh A, Ghasemi-Rad M, Bagherifard S, Cheng G, Sani ES, et al. A highly adhesive and naturally derived sealant. Biomaterials. 2017;140:115–127. doi: 10.1016/j.biomaterials.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang YN, Avery RK, Vallmajo-Martin Q, Assmann A, Vegh A, Memic A, et al. A highly elastic and rapidly crosslinkable elastin-like polypeptide-based hydrogel for biomedical applications. Adv Funct Mater. 2015;25:4814–4826. doi: 10.1002/adfm.201501489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Thi P, Lee Y, Nguyen DH, Park KD. In situ forming gelatin hydrogels by dual-enzymatic cross-linking for enhanced tissue adhesiveness. J Mat Chem B. 2017;5:757–764. doi: 10.1039/C6TB02179D. [DOI] [PubMed] [Google Scholar]
- 35.Tan H, Guo S, Dinh ND, Luo R, Jin L, Chen CH. Heterogeneous multi-compartmental hydrogel particles as synthetic cells for incompatible tandem reactions. Nat Commun. 2017;8:663. doi: 10.1038/s41467-017-00757-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoang Thi TT, Lee Y, Ryu SB, Nguyen DH, Park KD. Enhanced tissue adhesiveness of injectable gelatin hydrogels through dual catalytic activity of horseradish peroxidase. Biopolymers. 2018;109:e23077. doi: 10.1002/bip.23077. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y, Choi KH, Park KM, Lee JM, Park BJ, Park KD. In situ forming and H2O2-releasing hydrogels for treatment of drug-resistant bacterial infections. ACS Appl Mater Interfaces. 2017;9:16890–16899. doi: 10.1021/acsami.7b03870. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Lee SH, Lee JE, Park SJ, Kim K, Kim IS, et al. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials. 2018;178:401–412. doi: 10.1016/j.biomaterials.2018.04.057. [DOI] [PubMed] [Google Scholar]
- 39.Kim SH, An YH, Kim HD, Kim K, Lee SH, Yim HG, et al. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int J Biol Macromol. 2018;110:479–487. doi: 10.1016/j.ijbiomac.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 40.Lee F, Bae KH, Kurisawa M. Injectable hydrogel systems crosslinked by horseradish peroxidase. Biomed Mater. 2015;11:014101. doi: 10.1088/1748-6041/11/1/014101. [DOI] [PubMed] [Google Scholar]
- 41.Bae JW, Choi JH, Lee Y, Park KD. Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J Tissue Eng Regen Med. 2015;9:1225–1232. doi: 10.1002/term.1917. [DOI] [PubMed] [Google Scholar]
- 42.Choi MY, Kim JT, Lee WJ, Lee Y, Park KM, Yang YI, et al. Engineered extracellular microenvironment with a tunable mechanical property for controlling cell behavior and cardiomyogenic fate of cardiac stem cells. Acta Biomater. 2017;50:234–248. doi: 10.1016/j.actbio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 43.Park KM, Son JY, Choi JH, Kim IG, Lee Y, Lee JY, et al. Macro/Nano-gel composite as an injectable and bioactive bulking material for the treatment of urinary incontinence. Biomacromolecules. 2014;15:1979–1984. doi: 10.1021/bm401787u. [DOI] [PubMed] [Google Scholar]
- 44.Lewis DM, Park KM, Tang V, Xu Y, Pak K, Eisinger-Mathason TS, et al. Intratumoral oxygen gradients mediate sarcoma cell invasion. Proc Natl Acad Sci U S A. 2016;113:9292–9297. doi: 10.1073/pnas.1605317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Desai RM, Koshy ST, Hilderbrand SA, Mooney DJ, Joshi NS. Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials. 2015;50:30–37. doi: 10.1016/j.biomaterials.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 47.Pereira RF, Barrias CC, Bártolo PJ, Granja PL. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018;66:282–293. doi: 10.1016/j.actbio.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 48.Grim JC, Marozas IA, Anseth KS. Thiol-ene and photo-cleavage chemistry for controlled presentation of biomolecules in hydrogels. J Control Release. 2015;219:95–106. doi: 10.1016/j.jconrel.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anseth KS, Klok HA. Click chemistry in biomaterials, nanomedicine, and drug delivery. Biomacromolecules. 2016;17:1–3. doi: 10.1021/acs.biomac.5b01660. [DOI] [PubMed] [Google Scholar]
- 50.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48:222–272. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 51.Klotz BJ, Gawlitta D, Rosenberg AJWP, Malda J, Melchels FPW. Gelatin-methacryloyl hydrogels: towards biofabrication-based tissue repair. Trends Biotechnol. 2016;34:394–407. doi: 10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loessner D, Meinert C, Kaemmerer E, Martine LC, Yue K, Levett PA, et al. Functionalization, preparation and use of cell-laden gelatin methacryloyl-based hydrogels as modular tissue culture platforms. Nat Protoc. 2016;11:727–746. doi: 10.1038/nprot.2016.037. [DOI] [PubMed] [Google Scholar]
- 53.Chen YC, Lin RZ, Qi H, Yang Y, Bae H, Melero-Martin JM, et al. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv Funct Mater. 2012;22:2027–2039. doi: 10.1002/adfm.201101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee Y, Balikov DA, Lee JB, Lee SH, Lee SH, Lee JH, et al. In situ forming gelatin hydrogels-directed angiogenic differentiation and activity of patient-derived human mesenchymal stem cells. Int J Mol Sci. 2017;18:E1705. doi: 10.3390/ijms18081705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 56.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nat Rev Mol Cell Biol. 2008;9:285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang Y, Qi PK, Yang ZL, Huang N. Nitric oxide based strategies for applications of biomedical devices. Biosurf Biotribol. 2015;1:177–201. doi: 10.1016/j.bsbt.2015.08.003. [DOI] [Google Scholar]
- 59.Kumar S, Singh RK, Bhardwaj TR. Therapeutic role of nitric oxide as emerging molecule. Biomed Pharmacother. 2017;85:182–201. doi: 10.1016/j.biopha.2016.11.125. [DOI] [PubMed] [Google Scholar]
- 60.Zhao Y, Vanhoutte PM, Leung SW. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Papapetropoulos A, García-Cardeña G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Q, Zhang J, Song L, Ji Q, Yao Y, Cui Y, et al. Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis. Biomaterials. 2013;34:8450–8458. doi: 10.1016/j.biomaterials.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 63.Nie Y, Zhang K, Zhang S, Wang D, Han Z, Che Y, et al. Nitric oxide releasing hydrogel promotes endothelial differentiation of mouse embryonic stem cells. Acta Biomater. 2017;63:190–199. doi: 10.1016/j.actbio.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 64.Hoang Thi TT, Lee Y, Le Thi P, Park KD. Nitric oxide-releasing injectable hydrogels with high antibacterial activity through in situ formation of peroxynitrite. Acta Biomater. 2018;67:66–78. doi: 10.1016/j.actbio.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 65.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bretón-Romero R, Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Byon CH, Heath JM, Chen Y. Redox signaling in cardiovascular pathophysiology: a focus on hydrogen peroxide and vascular smooth muscle cells. Redox Biol. 2016;9:244–253. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Marinho HS, Real C, Cyrne L, Soares H, Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2:535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 70.Yasuda M, Ohzeki Y, Shimizu S, Naito S, Ohtsuru A, Yamamoto T, et al. Stimulation of in vitro angiogenesis by hydrogen peroxide and the relation with ETS-1 in endothelial cells. Life Sci. 1999;64:249–258. doi: 10.1016/S0024-3205(98)00560-8. [DOI] [PubMed] [Google Scholar]
- 71.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 73.Yoon DS, Lee Y, Ryu HA, Jang Y, Lee KM, Choi Y, et al. Cell recruiting chemokine-loaded sprayable gelatin hydrogel dressings for diabetic wound healing. Acta Biomater. 2016;38:59–68. doi: 10.1016/j.actbio.2016.04.030. [DOI] [PubMed] [Google Scholar]