Abstract
BACKGROUND:
Mesenchymal stem cells (MSCs) and/or biological scaffolds have been used to regenerate articular cartilage with variable success. In the present study we evaluated cartilage regeneration using a combination of bone marrow (BM)-MSCs, HyalofastTM and/or native cartilage tissue following full thickness surgical cartilage defect in rabbits.
METHODS:
Full-thickness surgical ablation of the medial-tibial cartilage was performed in New Zealand white (NZW) rabbits. Control rabbits (Group-I) received no treatment; Animals in other groups were treated as follows. Group-II: BM-MSCs (1 × 106 cells) + HyalofastTM; Group-III: BMMSCs (1 × 106 cells) + cartilage pellet (CP); and Group-IV: BM-MSCs (1 × 106 cells) + HyalofastTM + CP. Animals were sacrificed at 12 weeks and cartilage regeneration analyzed using histopathology, International Cartilage Repair Society (ICRS-II) score, magnetic resonance observation of cartilage repair tissue (MOCART) score and biomechanical studies.
RESULTS:
Gross images showed good tissue repair (Groups IV > III > Group II) and histology demonstrated intact superficial layer, normal chondrocyte arrangement, tidemark and cartilage matrix staining (Groups III and IV) compared to the untreated control (Group I) respectively. ICRS-II score was 52.5, 65.0, 66 and 75% (Groups I–IV) and the MOCART score was 50.0, 73.75 and 76.25 (Groups II–IV) respectively. Biomechanical properties of the regenerated cartilage tissue in Group IV closed resembled that of a normal cartilage.
CONCLUSION:
HyalofastTM together with BM-MSCs and CP led to efficient cartilage regeneration following full thickness surgical ablation of tibial articular cartilage in vivo in rabbits. Presence of hyaluronic acid in the scaffold and native microenvironment cues probably facilitated differentiation and integration of BM-MSCs.
Keywords: Osteoarthritis, BM-MSCs, Bio-scaffold, Histo-pathology, Biomechanics
Introduction
Osteoarthritis (OA) is a complex disease which is characterized by the loss of structural and biomechanical functions of the extracellular matrix (ECM) [1, 2]. The ensuing changes in the ECM and the cellular components lead to gradual degeneration of the articular cartilage which is marked by cartilage softening, fibrillation and ulceration [1, 2]. OA is a progressive disease, where initial attempt in tissue remodeling and restoration of tissue homeostasis become soon overwhelmed by an imbalance between the anabolic and catabolic events, finally resulting in severe joint dysfunction and disability [3, 4].
The function of the articular cartilage very much depends upon its morphological structure and bio-mechanical properties which in turn relies on the biochemical composition of the cartilage [5]. Articular cartilage is composed of chondrocytes embedded in the ECM which is rich in glycosaminoglycans (GAG), collagen fibrils and water [6]. The microarchitecture of the cartilage with its composition and arrangement of the fibrils, confers the biomechanical properties [6, 7]. These biomechanical effects additionally protect the underlying subchondral bone by facilitating shocks absorption and distribution of loads [8]. Involvement of the subchondral bone and its sclerosis is implicated in alteration of the biomechanical properties, in advanced stages of OA [9, 10].
Injured or damaged articular cartilage has limited ability for self-repair or regeneration. Therefore, with time mild to moderate articular cartilage lesions evolve into full thickness lesions and becomes associated with clinical features of joint pain, swelling and joint dysfunction [11, 12]. Arthroscopic procedures for various conditions have identified multiple degrees of articular cartilage injuries in 63% of patients. Since articular cartilage has limited regeneration capacity, surgical procedures involving marrow stimulation [13] and chondrocyte implantations techniques [14] are being attempted which can help overcome OA by reparative/restorative methods respectively.
Marrow stimulation involved subchondral drilling which recruits stem cells to the site of the lesion and help cartilage repair. However, fibrocartilaginous transformation occurs eventually which fail to function like normal articular cartilage. Autologous chondrocyte implantation (ACI) has progressed through several generations and basically involved initial harvest of chondrocytes; their expansion in culture followed by injection of these cells to the sites of cartilage damage and covering them up either with the periosteal patch (First generation ACI) or with collagen membrane (Second generation ACI). The problems associated with the above two ACI techniques such as periosteal hypertrophy and cell leakage led to the use of matrix associated autologous chondrocyte transplantation (MACT) wherein the harvested chondrocytes were initially seeded onto the matrix, and then the matrix was transplanted to the lesion with fibrin glue [15, 16]. However, it was identified that there was not much difference in the clinical outcomes between ACI and marrow stimulation techniques.
Early diagnosis and treatment is imperative as partial to complete loss of the articular cartilage may lead to long term disability and difficulty in management. Furthermore, unlike other tissues cartilage has inherent poor regeneration capacity and therefore the management of OA is difficult and protracted. Current research therefore focuses more on the use of a matrix together with cells to support faster integration and cartilage regeneration. We in the present study used a combination of bone marrow mesenchymal stem cells (BM-MSCs), native cartilage tissue and a commercial bio-scaffold (Hyalofast™) to understand its efficiency in cartilage regeneration in vivo in New Zealand White rabbits with full thickness cartilage defect.
Materials and methods
The protocol for the use of both BM-MSCs and animals in the present study were approved by the Institutional Review Board (IACUC no. 113-157) and the Research Ethics Committee, King Abdulaziz University (KAU). Informed patient consent was obtained prior to derivation of human BM-MSCs. Animal experiments were performed in strict compliance with the ethical guidelines for humane treatment of animals as defined by KAU.
Derivation of human BM-MSCs
The human BM-MSCs were isolated according to our earlier established protocols [17]. Briefly, bone marrow aspirates collected in heparinized tubes (Becton Dickinson, New Jersey, USA) were directly plated (~ 2 ml) into T175 cm2 flasks (Greiner Bio-one) and cultured using Dulbecco’s modified Eagles medium (Sigma, Missouri, USA) supplemented with 10% gamma irradiated fetal bovine serum (Sigma, Missouri, USA), 2 mM GlutaMax (Invitrogen, Life Technologies, California, USA), and antibiotic solution [penicillin (100 IU/ml; streptomycin (100 µg/ml); Sigma, Missouri, USA]. The monolayer of adherent cells was cultured and propagated under standard culture conditions of 37 °C and 5% carbon dioxide (CO2) in atmospheric air. The derived cells were characterized for MSCs related stemness properties [17].
Preparation of BM-MSCs
Early passages (P2–P4) of BM-MSCs in culture were trypsinized and pelleted. The supernatant was removed, and the cell pellet was resuspended in 10 ml of fresh culture medium and the cells counted. Aliquots of 1 × 106 cells in 1 ml of medium were prepared in 1.5 ml Eppendorf tubes. The cells were washed twice in phosphate buffer saline (PBS) between centrifugation steps and the final cell pellet was resuspended in 50 µl of PBS. The cells were maintained on ice until in vivo animal transplantation into the full thickness cartilage defect area together with cartilage pellet and/or bio-scaffold.
Preparation of cartilage pellet
Cartilage pellet was prepared from the cartilage scrapings obtained from the articular surface of the non-weight bearing area of the same knee joint in the rabbits at the time of surgery. The cartilage scrapings were placed in a petri-dish containing sterile PBS and finely minced with scissors to homogenize the tissue. The tissue homogenate was filtered using a cell strainer and the contents centrifuged at 1000 rpm for 5 min. The cartilage pellet thus obtained was then used for implantation into the defect area together with cells or bio-scaffold (Hyalofast™).
Commercial bioscaffold (Hyalofast™)
Hyalofast™ is a biodegradable hyaluronic acid (HA) based scaffold used as a one-step surgical treatment for chondral and osteochondral defects. It can additionally act as a scaffold for MSCs to adhere, proliferate and promote differentiation into cartilage tissue. This nonwoven scaffold is versatile in nature and can be cut/modified to suit the topography of the joint lesions.
In vivo animal experiment
New Zealand White (NZW) male rabbits Eight mature NZW male rabbits, 9–12 months of age, weighing 3–4 kg were used in this study. They were housed under standard animal housing conditions of 27 °C, with a 12-h dark/ light cycle. Humidity was maintained between 55 and 65%. All rabbits were fed with commercial rabbit feed along with fresh leafy vegetables and water ad libitum. The NZW rabbits were provided 1-week period of adaptation before use in experiments. Each animal was anaesthetized with intramuscular injection of 50 mg/kg ketamine and 50 mg/kg xylazine, followed by 1% lignocaine at the operative site. Prophylactic antibiotic therapy (50 mg/kg cefazolin IM) was given 30 min prior to surgical intervention, and was followed by regular dose for 24 h. All experimental procedures were carried out at the ‘Animal Surgical Research Laboratory’, King Fahd Medical Research Center (KFMRC), King Abdulaziz University (KAU), Jeddah.
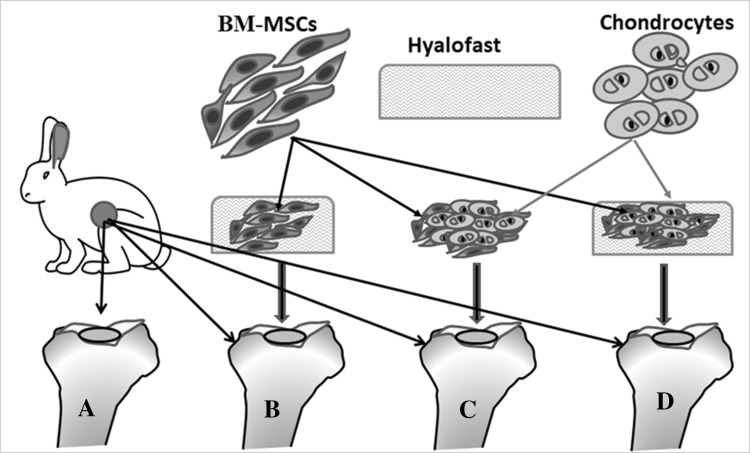
Experimental groups Initially, all the eight NZW rabbits were subjected to full thickness surgical ablation (1 × 1 cm) of the medial tibial cartilage, in both knee joints. The NZW rabbits were then divided into four separate groups based on the treatment regime as follows. Group I—untreated controls; Group II—rabbits treated with BM-MSCs (1 × 106 cells) and Hyalofast™; Group III—rabbits treated with BM-MSCs (1 × 106 cells) and homogenized cartilage pellet; and Group IV—rabbits treated with BM-MSCs (1 × 106 cells), Hyalofast™ and homogenized cartilage pellet (Fig. 1). All animals were then followed up until the end of the study period of 12 weeks and then sacrificed, for evaluation of gross tissue repair, histochemistry, bio-mechanical properties and magnetic resonance imaging (MRI) based scoring for cartilage regeneration.
Fig. 1.
Diagrammatic representation showing the in vivo experimental design in knee joints of NZW rabbits of full thickness surgical ablation of the tibial articular surface. A Control rabbit that received no treatment; B BM-MSCs (1 × 106 cells) + Hyalofast™; C cartilage pellet (CP) + Hyalofast™; and D BM-MSCs (1 × 106 cells) + CP + Hyalofast™. A, B showed partial to moderate filling with persistence of the surgical defect. C, D showed good tissue regeneration with near complete filling of the surgical defect. *NZW: New Zealand white, BM-MSCs: bone marrow mesenchymal stem cells
Full thickness surgical ablation of medial tibial cartilage of the knee Briefly, following induction of general anesthesia, both knees were prepared with standard sterilization and draping for aseptic surgery. The rabbits were laid in dorsal recumbent position and each knee joint was opened separately through a medial parapatellar approach. Incision was taken down to the anteromedial joint capsule, with knee flexion to identify the anatomical landmarks. The extensor mechanism was dislocated laterally and the articular surface of the medial tibial cartilage of knee joints were exposed totally. A full thickness surgical ablation of the articular surface (measuring 1 × 1 cm in diameter and 2–3 mm in depth) was then performed using surgical drill and curettage. The surgical defect area was then implanted either with BM-MSCs (1 × 106 cells) and Hyalofast™ (Group II); BM-MSCs (1 × 106 cells) and homogenized cartilage pellet (Group III); and BM-MSCs (1 × 106 cells), Hyalofast™ and homogenized cartilage pellet (Group IV). The controls (Group I) did not receive any implantation. Following implantation of cells, cartilage pellet and scaffold, the extensor mechanism was relocated and the joint capsule was closed medially with 2-0 Vicryl. The skin was then closed by interrupted sutures using 3-0 Prolene and the region cleaned and dressed. Post-operatively, antibiotic therapy (50 mg/kg cefazolin IM) was given thrice daily for 48 h. Animals were allowed unrestricted activity and were closely monitored regularly for signs of wound infection and other complications.
Histological analysis
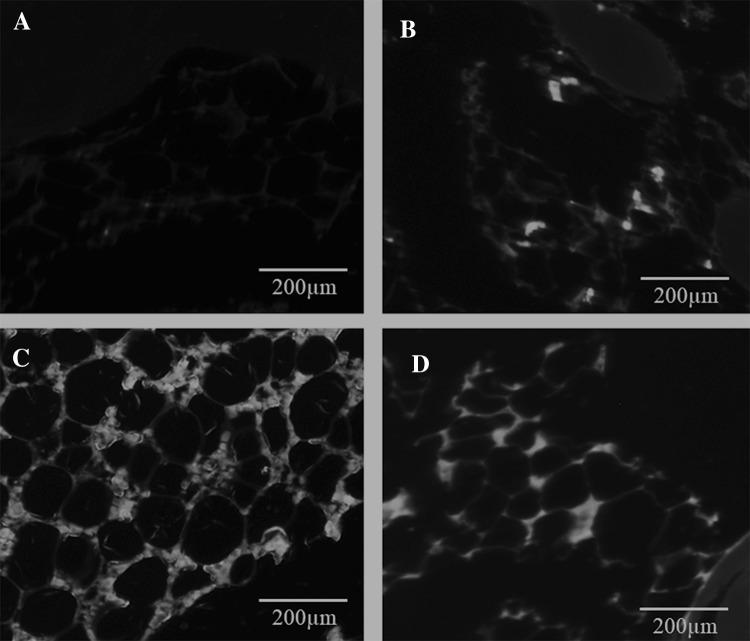
The tibial bone with the articular surface were dissected from all the NZW rabbits in different experimental groups and fixed in 10% buffered neutral formalin. The samples were then decalcified in 4% ethylenediaminetetraacetic acid (EDTA) and subsequently dehydrated using graded series of ethanol, before final embedding in paraffin blocks. Tissue sections were made in coronal plane including normal cartilage and surgical defect area. Separate tissue sections from each experimental group were then stained separately with hematoxylin and eosin (H&E) and toluidine blue using standard histological staining procedures. The histological images obtained using Olympus BX51 light microscope (Olympus Corporation, Tokyo, Japan) were examined by two independent certified pathologists (FA and MS). The International Cartilage Repair Society II (ICRS II) scoring system was used for histological evaluation of cartilage repair [18]. To understand whether the transplanted BM-MSCs survived and contributed to cartilage regeneration, immunohistochemistry for human nuclear antigen was performed. Briefly, the paraffin sections prepared from each group were deparaffinized and antigen retrieval done microwaving the tissue section in Tris/EDTA buffer at 98 °C for 20 min. Following non-specific blocking procedures the sections were incubated with monoclonal anti-human nuclear primary antibody (MAB 1281; Millipore), and stained with secondary antibody (Alexafluor 488) for 30 min and images obtained by using fluorescent microscope (EVOS™ FL Imaging System; ThermoFischer Scientific).
Magnetic resonance imaging
The knee joint of the sacrificed NZW rabbits from the four experimental groups were examined ex-vivo using a 9.4 T high field MRI scanner (BiospecAvance III 9.4 T/30, Bruker Biospin, Ettlingen, Germany). A circular polarized volume coil with receives and transmit configuration, was used. Morphological images of the cartilage were acquired using fat suppressed 3D fast low-angle shot (FLASH) and fat suppressed 2D rapid acquisition with relaxation-enhanced (RARE) sequences.
Scan parameters for FLASH images: repetition time (TR): 40 ms, echo time (TE): 4.6 ms, matrix: 256 × 192 × 128, averages: 1, FOV: 4 × 3 × 2 cm, resolution: 0.0156 cm/pixel in read and 0.117 cm/ pixel in P1 and 0.234 cm/pixel in P2, Flip angle: 40, time of acquisition: 24 min 34 s, slice thickness: 0.16 mm, interspace or gap: 0.16 mm and bandwidth: 50000 Hz. Scan parameters for (RARE) images: TR: 4620 ms; TE: 33 ms; matrix: 256 × 256, averages: 4, FOV: 4 cm, resolution: 0.0156 cm/pixel in read and 0.1117 cm/ pixel in P1, Flip angle: 180 degrees, time of acquisition: 9 min 51 s, slice thickness: 1 mm, gap: 0.5 mm, bandwidth: 89285.7 Hz. Biochemical T2 mapping of the cartilage was acquired using multi-slice multi-echo (MSME) spin echo based protocol.
Regenerated cartilage tissue was evaluated using 2-dimensional magnetic resonance observation of cartilage repair tissue (MOCART) scoring system [19]. Score 0 = no repair and score 100 = excellent repair. The evaluation criteria include nine variables and includes the degree of defect fill; the cartilage interface; cartilage surface; cartilage structure; the signal intensity; the constitution of subchondral lamina; the constitution of subchondral bone; possible adhesions and effusion.
Biomechanical assessment
The load bearing capacity of the regenerated cartilage following full-thickness surgical ablation of the tibial articular and treatment with BM-MSCs, cartilage pellet and Hyalofast™ in NZW rabbits was determined using LRX plus Materials tensile testing machine (AMETEK LLoyd Instruments, Ireland). Briefly, preload stress was calibrated to zero, and then an extension rate of 2 mm/min was applied to all samples and the data was acquired. The compression mode was applied to test the cartilage strength and a maximum load cell of 5 kilo Newton (kN) was applied for each sample. Auto-zero for load and extension was performed after each experiment to ensure accuracy. The results obtained were analyzed using the NEXYGEN™ 4.0 software (AMETEK LLoyd Instruments, Ireland).
Results
Gross appearance
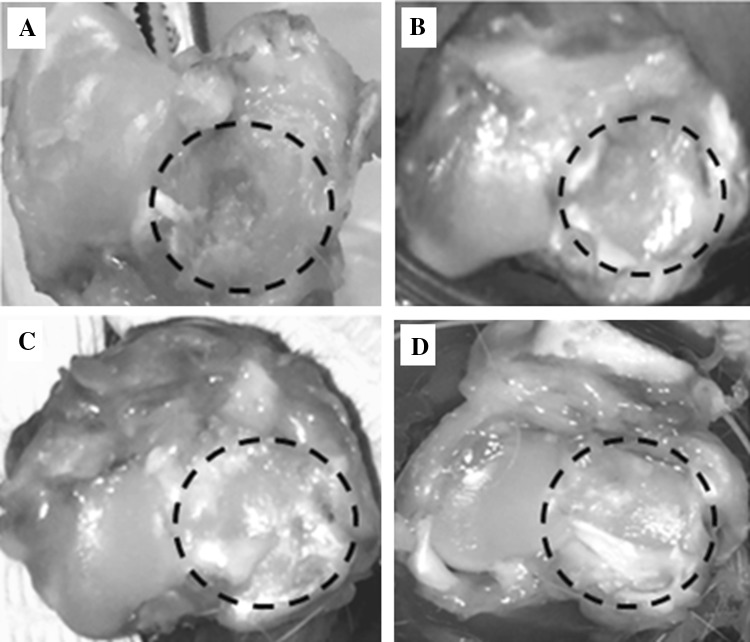
Control rabbits (Group I) showed poor regeneration ability and only contributed to minimal repair of the surgical defect. The experimental group that was transplanted with BM-MSCs and Hyalofast™ (Group II) showed partial tissue regeneration compared to the control. However, treatment with BM-MSCs and homogenized cartilage pellet (Group III); and BM-MSCs, Hyalofast™ and homogenized cartilage pellet (Group IV) demonstrated almost complete filling of the surgical defect area (Fig. 2).
Fig. 2.
Representative gross images of the knee joints of NZW rabbits, showing tissue regeneration and repair at 12 weeks following full thickness surgical ablation of the medial tibial articular surface (within the dotted circle). A Control rabbit that received no treatment; B BM-MSCs (1 × 106 cells) + Hyalofast™; C cartilage pellet (CP) + Hyalofast™; and D BM-MSCs (1 × 106 cells) + CP + Hyalofast™. A, B showed partial to moderate filling with persistence of the surgical defect. C, D showed good tissue regeneration with near complete filling of the surgical defect. *NZW: New Zealand white, BM-MSCs: bone marrow mesenchymal stem cells
Histological analysis
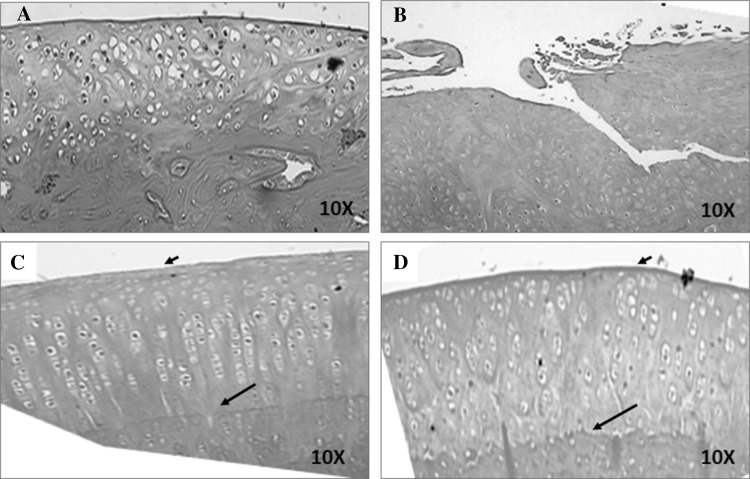
Haematoxylin and eosin staining Cartilage sections at 12 weeks from rabbits in Group I (Control) showed poor repair of the cartilage defect. There was also loss of the superficial and mid-zone layers and the tidemark. In addition, cartilage tissue loss, disorganized chondrocyte pattern and loss of nuclei indicative of cell death was evident. Group II (BM-MSCs and Hyalofast™) also demonstrated marked cartilage loss, with absence of superficial and midzone layers as well as the tidemark. The normal arrangement of chondrocytes was lost, and more fibrous tissue was observed. Group III (BM-MSCs and cartilage pellet) and Group IV (BM-MSCs, cartilage pellet and Hyalofast™) showed marked to complete articular cartilage regeneration with intact superficial layer and orderly arrangement of chondrocytes in the mid and deep zones. Intact tidemark and calcified cartilage zone were evident (Fig. 3).
Fig. 3.
Representative haematoxylin and eosin histological images of the regenerated cartilage tissue following treatment with Hyalofast™ and/or BM-MSCs/Cartilage pellet in NZW rabbits with full thickness surgical ablation of the medial tibial articular surface. A Control rabbit that received no treatment; B rabbit treated with BM-MSCs (1 × 106 cells) + Hyalofast™; C rabbit treated with cartilage pellet (CP) + Hyalofast™ and D rabbit treated with BM-MSCs (1 × 106 cells) + CP + Hyalofast™. A showed cartilage like tissue with disorganized arrangement of chondrocytes having nuclear debris (blue arrows). B also showed poor cartilaginous tissue with absent chondrocyte pattern and tidemark. Both C and D showed good cartilaginous tissue with intact surface layer (short black arrows), presence of tidemark (long black arrows) and organized chondrocyte pattern. *NZW: New Zealand white, BM-MSCs: bone marrow mesenchymal stem cells
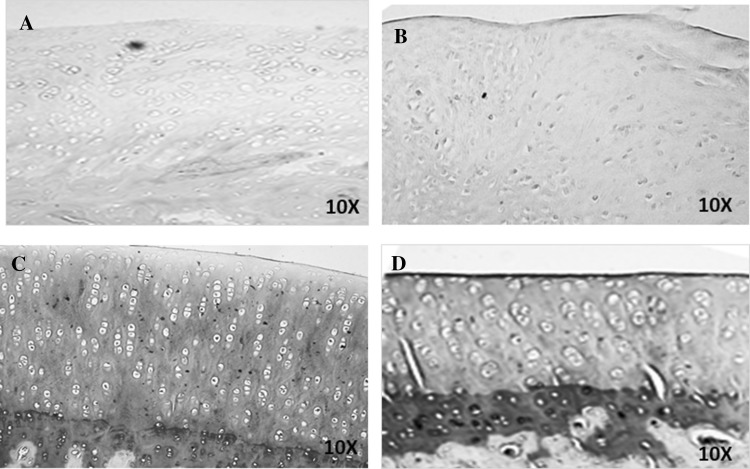
Toluidine blue staining Group I and Group II demonstrated poor cartilage repair with very weak to absent staining of the matrix. Group III and Group IV showed intense hyaline matrix staining up to the superficial layer (Fig. 4).
Fig. 4.
Representative toluidine blue histological images of the regenerated cartilage tissue following treatment with Hyalofast™ and/or BM-MSCs/Cartilage pellet in NZW rabbits with full thickness surgical ablation of the medial tibial articular surface. A Control rabbit that received no treatment; B rabbit treated with BM-MSCs (1 × 106 cells) + Hyalofast™; C rabbit treated with cartilage pellet (CP) + Hyalofast™ and D rabbit treated with BM-MSCs (1 × 106 cells) + CP + Hyalofast™. A, B showed weak to absent cartilage matrix staining. C, D showed intense cartilage matrix staining and presence of superficial, mid and deep zones indicating good cartilage regeneration. *BM-MSCs: bone marrow mesenchymal stem cells, NZW: New Zealand white
ICRS II scoring Evaluation of all parameters by two independent pathologists showed better cartilage regeneration profile in treatment groups compared to the control. The mean percentage value of the overall assessment was 52.5% (Group I), 65.0% (Group II), 66% (Group III) and 75% (Group IV) respectively.
Immunohistochemistry Group I was negative for human anti-nuclear antibody and showed minimal background staining, while Group II, Group III and Group IV were positive (Fig. 5). The BM-MSCs when used together with Hyalofast™ as in Group II (BM-MSCs and Hyalofast™) and Group IV (BM-MSCs, cartilage pellet and Hyalofast™) showed intense staining (Fig. 5B, D) compared to the control.
Fig. 5.
Representative immunohistochemical images of the regenerated cartilage tissue following staining with primary monoclonal human anti-nuclear antibody and Alexafluor 488 secondary antibody. The tissue sections were prepared following treatment with Hyalofast™ and/or BM-MSCs/Cartilage pellet in NZW rabbits with full thickness surgical ablation of the medial tibial articular surface. A Control rabbit that received no treatment; B rabbit treated with BM-MSCs (1 × 106 cells) + Hyalofast™; C rabbit treated with cartilage pellet (CP) + Hyalofast™ and D rabbit treated with BM-MSCs (1 × 106 cells) + CP + Hyalofast™. A showed no staining indicting absence of human cells (Negative). B–D showed intense positive staining indicating the presence of transplanted human BM-MSCs. *BM-MSCs: bone marrow mesenchymal stem cells, NZW: New Zealand white
MRI analysis
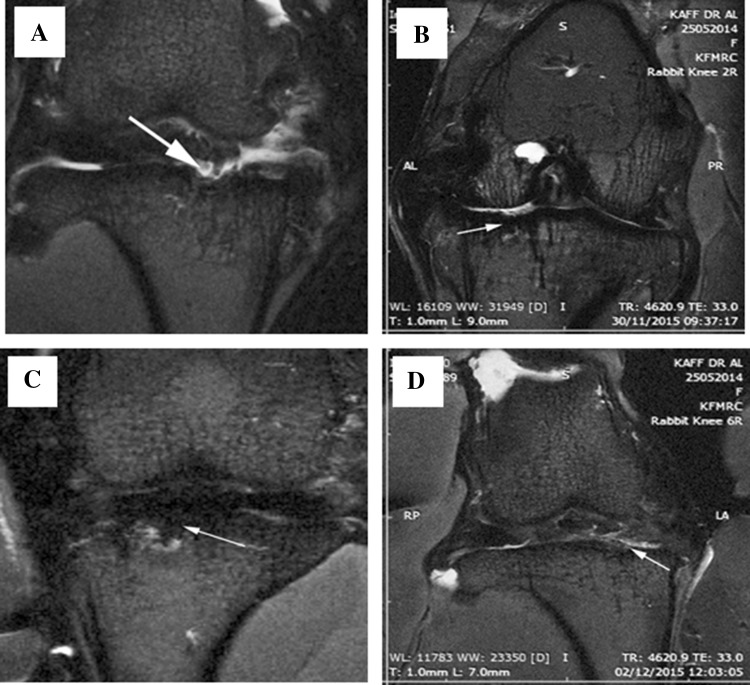
MRI of the knee joints of the treated rabbits showed varying grades of repair and filling of the defect. There was irregular destruction of the articular surfaces which correlated well with that of the histological findings. The MOCART score was 50 ± 5.66; 73.75 ± 3.75 and 76.25 ± 4.27 in Group II, Group III and Group IV respectively compared to the untreated control (Fig. 6).
Fig. 6.
Representative coronal T2 images showing medial tibial plateau osteochondral defect (white arrows) following treatment with Hyalofast™ and/or BM-MSCs/Cartilage pellet in NZW rabbits with full thickness surgical ablation of the medial tibial articular surface. A Control; B BM-MSCs (1 × 106 cells) + Hyalofast™; C cartilage pellet (CP) + Hyalofast™ and D BM-MSCs (1 × 106 cells) + CP + Hyalofast™. MOCART score was 50±5.66; 73.75±3.75 and 76.25±4.27 in B–D respectively compared to A. *BM-MSCs: bone marrow mesenchymal stem cells, NZW: New Zealand white
Biomechanical properties
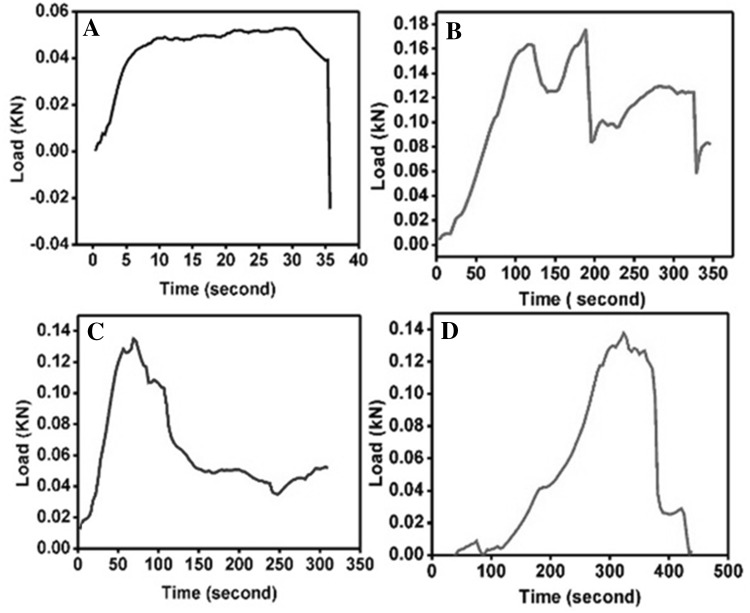
The load bearing capacity of the regenerated cartilage showed mild improvement in the treated groups, compared to the untreated control. Samples in Group I showed maximal load bearing up to 520 N and had sustained resistance to maximal load thrice before finally dropping down. Samples in Group II developed fractures around 160 N but showed resistance up to maximum of 170 N. Group III demonstrated load bearing capacity up to a maximum of 135 N and development of fine fissures. Group IV demonstrated the least resistance against the external load with maximal load bearing up to 34 N. Unlike other samples there was a complete drop in the resistance that was almost linear after reaching the breaking point (Fig. 7).
Fig. 7.
Representative load bearing capacity of the regenerated cartilage tissue determined using LRX plus Materials tensile testing machine following treatment with Hyalofast™ and/or BM-MSCs/cartilage pellet in NZW rabbits with full thickness surgical ablation of the medial tibial articular surface. A Control rabbit that received no treatment and B rabbit treated with BM-MSCs (1 × 106 cells) + Hyalofast™ showed sustained load bearing with plateau. C rabbit treated with cartilage pellet (CP) + Hyalofast™ and D rabbit treated with BM-MSCs (1 × 106 cells) + CP + Hyalofast™ showed gradual increase of load bearing pattern that returned to baseline indicative of a normal cartilage-like response. *BM-MSCs: bone marrow mesenchymal stem cells, NZW: New Zealand white
Discussion
The present study highlights the effectiveness of a commercially available biological scaffold, when used with or without cells in cartilage regeneration. In the settings of a full thickness complete tibial articular cartilage ablation in rabbits, a combination of MSCs, cartilage pellet and Hyalofast™ demonstrated better cartilage regeneration compared to the use of Hyalofast™ alone or its combinations with MSCs or cartilage pellet.
The underlying pathology in OA is mainly articular cartilage damage and destruction, which by itself has inherent poor regeneration capacity. Current pharmacological therapies provide only symptomatic relief and advanced stages may require surgical intervention to help functional restoration. Recently stem cells and/or their derivatives are identified to help regenerate/restore damaged cartilage. Both autologous and allogeneic MSCs are used in various surgical procedures such as ACI and MACT to bring about cartilage regeneration [15, 16]. Direct intraarticular injections of MSCs derived from bone marrow, fat or other sources have demonstrated less synovial thickening, reduced osteophyte formation and delayed OA progression [20, 21]. However, in the absence of complete cure for OA, there is a continuous search for novel therapeutics.
The use of Hyalofast™ and BM-MSCs and/or cartilage pellet enhanced cartilage regeneration. The main composition of Hyalofast™ is hyaluronan (HA) which is one of the principal component of the articular cartilage matrix. Umbilical cord Wharton’s jelly derived MSCs when cultured with two commercially available HA acid based hydrogels namely HyStem and HyStem-C resulted in increased production of collagen type II and differentiation into chondrocytes [22]. Pro-inflammatory cytokine interleukin-1 beta (IL-1β) is associated with cartilage degradation via activation of the matrix metalloproteinases (MMPs), and HA demonstrated suppression of the MMPs that were stimulated with IL-1β and mechanical load, in an osteochondral explant tissue model derived from OA patients [23]. A multicenter randomized controlled clinical trial study has reported that intra-articular administration of HA together with culture expanded autologous BM-MSCs led to functional improvement of knee OA and also the quality of life [24]. Above studies indicate that HA has both chondro-inductive and chondro-protective effects, and Hyalofast™, being mainly composed of HA probably facilitated BM-MSCs attachment and helped chondrocyte differentiation in the presence of native cartilage tissue.
Gross and microscopic analysis revealed better cartilage repair with Hyalofast™ and BM-MSCs and/or cartilage pellet as observed in the present study. Presence of intact superficial layer, organized arrangement of the chondrocytes, the tidemark and production of cartilage matrix indicated successful cartilage regeneration. Singh et al. reported that direct intra-articular injection of BM-MSCs alone into the OA knee joint following anterior cruciate ligament transection in NZW rabbits helped cartilage regeneration [25]. Transplantation of human BM-MSCs that were earlier differentiated into chondrocytes in vitro together with a biphasic composite (platelet rich fibrin glue-hydroxyapatite) demonstrated superior hyaline-like cartilage formation at 8 weeks than with undifferentiated BM-MSCs [26]. Similarly, intra-articular injections of equine umbilical cord derived MSCs into NZW rabbits with medial meniscal release model of OA reported lesser cartilage fibrillation, reduction in MMPs, anti-catabolic and anti-inflammatory activities by acting upon the synovium [27]. Above studies indicate that xenogeneic MSCs can integrate with host tissue and contribute to cartilage tissue repair, which were similar to the results of our study. However, the same studies did not highlight the type and nature of the regenerated cartilage nor the biomechanical properties which are crucial for functional restoration of OA joint. In the present study too, we had used human BM-MSCs for transplantation into NZW rabbits, and the immunohistochemistry for human anti-nuclear antigen demonstrated the presence of transplanted BM-MSCs indicating that these cells survived and integrated in the host tissue and probably contributed cartilage regeneration in tandem with the available microenvironmental cues.
In the present study MRI imaging and the MOCART score showed improvement in the experimental groups than the control. MOCART scoring system was specifically designed to evaluate the knee repair and serves as an index of tissue regeneration [19]. MRI imaging and its ability to understand the soft-tissue contrast enables direct visualization of the cartilage defect and/or their regeneration. Cartilage matrix that embeds the chondrocytes, especially comprising of collagen II and proteoglycans provide the tensile strength and contribute to the biomechanical properties. Importantly, HA forms a coat around the chondrocytes, and being negatively charged absorbs water molecules which in turn contributes to the resilience of the cartilage [28]. Biomechanical properties of cartilage are more reliably obtained using indentation testing and subsequent analysis of stress-relaxation curve as used in the present study. It has the unique advantage whereby load is tested without causing displacement of tissue; however, the orientation of the specimen is important to overcome the inherent anisotropic properties [29]. A recent prospective study where autologous bone marrow aspirate concentrate and Hyalofast™ were utilized to treat full thickness chondral defects in patients more than 45 years old reported effective functional restoration and decrease in pain. Therefore, it is evident that Hyalofast™ composed mainly of HA probably contributed as a right support for the stem cells to adhere and undergo cartilage differentiation efficiently.
Increase in the numbers of animals, immunostaining for collagen type and gene expression studies for cartilage specific genes would have added additional strength and more precise information to our study. Similarly, an interval and follow-up MRI after cells transplantation would have provided insights into direct in vivo cartilage regeneration status and functional outcome. The above limitations will be carefully addressed in our subsequent studies.
Acknowledgements
We acknowledge the financial support provided by the “Sheik Salem Bin Mahfouz Scientific Chair for Treatment of Osteoarthritis by Stem Cells”; the stem cell laboratory facility at CEGMR and the animal facility at King Fahd Medical Research Centre, King Abdulaziz University.
Conflict of interest
All the authors declare that they have no conflict of interest.
Ethical statement
This manuscript has not been published previously in any other journal or language. The study protocol was approved by the Institutional Review Board (IACUC no. 113-157).
References
- 1.Keuttner K, Goldberg VM. Osteoarthritic disorders. Rosemont: American Academy of Orthopedic Surgeons; 1995. pp. 21–23. [Google Scholar]
- 2.Creamer P, Hochberg MC. Osteoarthritis. Lancet. 1997;16:503–508. doi: 10.1016/S0140-6736(97)07226-7. [DOI] [PubMed] [Google Scholar]
- 3.Arden NK, Leyland KM. Osteoarthritis year 2013 in review: clinical. Osteoarthritis Cartilage. 2013;21:1409–1413. doi: 10.1016/j.joca.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 4.Permuy M, Guede D, López-Peña M, Muñoz F, Caeiro JR, González-Cantalapiedra A. Effects of diacerein on cartilage and subchondral bone in early stages of osteoarthritis in a rabbit model. BMC Vet Res. 2015;11:143. doi: 10.1186/s12917-015-0458-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed] [Google Scholar]
- 6.Venn M, Maroudas A. Chemical composition and swelling of normal and osteoarthritic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977;36:121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klisch SM, Asanbaeva A, Oungoulian SR, Masuda K, Thonar EJ, Davol A, et al. A cartilage growth mixture model with collagen remodeling: validation protocols. J Biomech Eng. 2008;130:031006. doi: 10.1115/1.2907754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Haaften EE, Ito K, van Donkelaar CC. The initial repair response of articular cartilage after mechanically induced damage. J Orthop Res. 2017;35:1265–1273. doi: 10.1002/jor.23382. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Q, Lu S, Du Z, Friis T, Yao J, Crawford R, et al. Characterization of nano-structural and nano-mechanical properties of osteoarthritic subchondral bone. BMC Musculoskelet Disord. 2016;17:367. doi: 10.1186/s12891-016-1226-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ficklin T, Thomas G, Barthel JC, Asanbaeva A, Thonar EJ, Masuda K, et al. Articular cartilage mechanical and biochemical property relations before and after in vitro growth. J Biomech. 2007;40:3607–3614. doi: 10.1016/j.jbiomech.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002;402:21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Chubinskaya S, Haudenschild D, Gasser S, Stannard J, Krettek C, Borrelli J., Jr Articular cartilage injury and potential remedies. J Orthop Trauma. 2015;29(Suppl 12):S47–S52. doi: 10.1097/BOT.0000000000000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 14.Gillogly SD. Treatment of large full-thickness chondral defects of the knee with autologous chondrocyte implantation. Arthroscopy. 2003;19(Suppl 1):147–153. doi: 10.1016/j.arthro.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 15.Steinwachs M. New technique for cell-seeded collagen matrix-supported autologous chondrocyte transplantation. Arthroscopy. 2009;25:208–211. doi: 10.1016/j.arthro.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid-to long-term clinical outcomes. Orthop J Sports Med. 2017;5:2325967117709250. doi: 10.1177/2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalamegam G, Abbas M, Gari M, Alsehli H, Kadam R, Alkaff M, et al. Pelleted bone marrow derived mesenchymal stem cells are better protected from the deleterious effects of arthroscopic heat shock. Front Physiol. 2016;7:180–192. doi: 10.3389/fphys.2016.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mainil-Varlet P, Van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38:880–890. doi: 10.1177/0363546509359068. [DOI] [PubMed] [Google Scholar]
- 19.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. doi: 10.1186/ar3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, et al. Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in Rabbit. Knee. 2011;18:71–75. doi: 10.1016/j.knee.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Aleksander-Konert E, Paduszyński P, Zajdel A, Dzierżewicz Z, Wilczok A. In vitro chondrogenesis of Wharton’s jelly mesenchymal stem cells in hyaluronic acid-based hydrogels. Cell Mol Biol Lett. 2016;21:11. doi: 10.1186/s11658-016-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pohlig F, Guell F, Lenze U, Lenze FW, Mühlhofer HM, Schauwecker J, et al. Hyaluronic acid suppresses the expression of metalloproteinases in osteoarthritic cartilage stimulated simultaneously by interleukin 1β and mechanical load. PLoS One. 2016;11:e0150020. doi: 10.1371/journal.pone.0150020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II) J Transl Med. 2016;14:246. doi: 10.1186/s12967-016-0998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A, Goel SC, Gupta KK, Kumar M, Arun GR, Patil H, et al. The role of stem cells in osteoarthritis. An experimental study in rabbits. Bone Joint Res. 2014;3:32–37. doi: 10.1302/2046-3758.32.2000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang KM, Lee JH, Park CM, Song HR, Wang JH. Xenotransplantation of human mesenchymal stem cells for repair of osteochondral defects in rabbits using osteochondral biphasic composite constructs. Knee Surg Sports Traumatol Arthrosc. 2014;22:1434–1444. doi: 10.1007/s00167-013-2426-y. [DOI] [PubMed] [Google Scholar]
- 27.Saulnier N, Viguier E, Perrier-Groult E, Chenu C, Pillet E, Roger T, et al. Intra-articular administration of xenogeneic neonatal Mesenchymal Stromal Cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 2015;23:122–133. doi: 10.1016/j.joca.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 28.Griffin M, Premakumar Y, Seifalian A, Butler PE, Szarko M. Biomechanical characterization of human soft tissues using indentation and tensile testing. J Vis Exp. 2016 doi: 10.3791/54872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y, Zheng S, Szarko M, Lee J. Anisotropic properties of bovine nasal cartilage. Microsc Res Tech. 2012;75:300–306. doi: 10.1002/jemt.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]