Abstract
Background:
The tissue engineering and regenerative medicine approach require biomaterials which are biocompatible, easily reproducible in less time, biodegradable and should be able to generate complex three-dimensional (3D) structures to mimic the native tissue structures. Click chemistry offers the much-needed multifunctional hydrogel materials which are interesting biomaterials for the tissue engineering and bioprinting inks applications owing to their excellent ability to form hydrogels with printability instantly and to retain the live cells in their 3D network without losing the mechanical integrity even under swollen state.
Methods:
In this review, we present the recent developments of in situ hydrogel in the field of click chemistry reported for the tissue engineering and 3D bioinks applications, by mainly covering the diverse types of click chemistry methods such as Diels–Alder reaction, strain-promoted azide-alkyne cycloaddition reactions, thiol-ene reactions, oxime reactions and other interrelated reactions, excluding enzyme-based reactions.
Results:
The click chemistry-based hydrogels are formed spontaneously on mixing of reactive compounds and can encapsulate live cells with high viability for a long time. The recent works reported by combining the advantages of click chemistry and 3D bioprinting technology have shown to produce 3D tissue constructs with high resolution using biocompatible hydrogels as bioinks and in situ injectable forms.
Conclusion:
Interestingly, the emergence of click chemistry reactions in bioink synthesis for 3D bioprinting have shown the massive potential of these reaction methods in creating 3D tissue constructs. However, the limitations and challenges involved in the click chemistry reactions should be analyzed and bettered to be applied to tissue engineering and 3D bioinks. The future scope of these materials is promising, including their applications in in situ 3D bioprinting for tissue or organ regeneration.
Keywords: Click chemistry, Hydrogels, 3D bioprinting, Tissue engineering, Regenerative medicine
Introduction
“Click chemistry” is generally defined as the chemical reactions which occurs fast, spontaneous, versatile, extremely selective and which can give high yields of products when two molecular substances or components are mixed or reacted at mild reaction conditions [1, 2]. Among the hydrogels researched, the Cu(I)-catalyzed reactions for hydrogel synthesis were popular and commonly called as Click Chemistry. This specific term was first coined by Sharpless’ group in 2001 [3]. The terms such as bio-orthogonal or bio-click were used to describe the reactions which takes place in presence of macromolecules like proteins or live cells, etc. The main advantage of bio-orthogonal methods is they usually do not affect the other normal biochemical processes [4, 5]. Notable first click chemistry-based hydrogel was reported by Ossipov et al. [6] which was formed using poly(vinyl alcohol) (PVA) polymer. These kinds of hydrogel preparation methods have improved in the recent decade and provide the scientists a valuable tool to modify or synthesize novel polymers with multifunctional properties for numerous applications such as tissue engineering, drug delivery, regenerative medicine and other biomedical applications [7–10]. As explained by Sharpless’ research group, click chemistry reactions needs to be modular, should have wide scope, very high yields, stable at physiological conditions, highly stereo-specific, non-toxic end products, under the simple reaction conditions being started with easily available materials and reagents. Furthermore, the reaction should provide product directly without using chromatographic techniques and toxic solvents [11].
Even though copper-based click chemistry was reported first for hydrogel synthesis using click chemistry, due to its copper ion toxicity and reactive oxygen species generation, this method was recently less preferred in tissue engineering and regenerative medicine [12]. Although scientists use ethylene diamine tetra-acetic acid (EDTA) for removing copper ions from the hydrogels, it may be difficult to use the same method in the cases of injectable hydrogels for regenerative medicine or localized drug delivery [13]. Hence, scientists started preferring copper-free click chemistry for synthesizing hydrogels for tissue engineering and regenerative medicine [14]. The copper-free click chemistry offers various functionalities without using any toxic catalysts or yield any toxic end products after gel formation [1]. The various copper-free click chemistry methods include strain-promoted azide-alkyne cycloaddition (SP-AAC) click hydrogels [14–16], Diels–Alder click chemistry hydrogels [17, 18], thiol-ene [19, 20], oxime [21–23], thiol-yne [24, 25], etc. The other class of click chemistry hydrogel synthesis method includes the pseudo click reactions which also gives high yield of products at moderate and extremely reactive reactions conditions [4]. These click chemistry-based hydrogel materials show immense potentials to be used in the latest technologies like 3D bioprinting for creating tissue and organ structures [26–29]. Even though scaffold-free approaches are investigated for tissue regeneration [30], injectable in situ hydrogels either with or without live cells, growth factors, biomolecules, nanoparticles and microspheres are promising for tissue engineering applications with improved mechanical properties and biocompatibility [31–40].
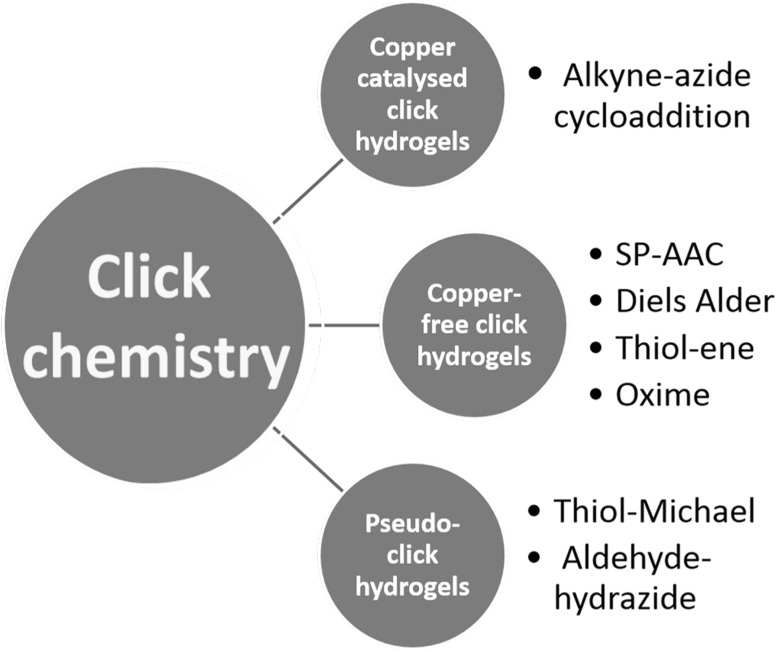
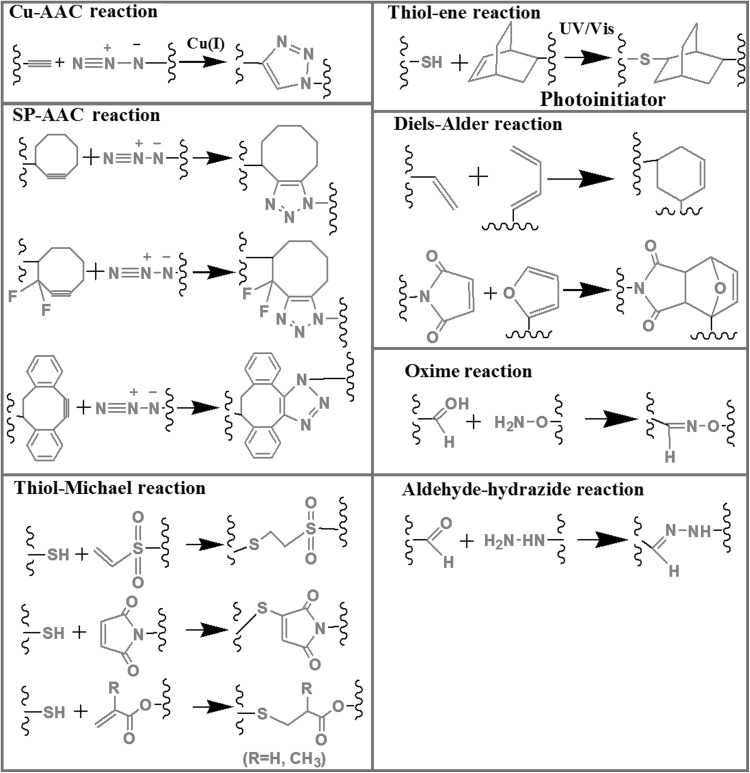
This review article focuses on the tissue engineering and potentially 3D bioprinting applications of click chemistry based on the main topics of copper-catalyzed click hydrogels, copper-free click hydrogels and pseudo-click hydrogels as described in Fig. 1. While copper-catalyzed click chemistry describes on alkyne-azide cycolo-addition reaction, copper-free click chemistry does strain-promoted azide-alkyne cycloaddition (SP-AAC), Diels–Alder, thiol-ene and oxime. Pseudo-click chemistry introduces thiol-Michael and aldehyde-hydrazide reactions. The chemical reaction strategies used in the copper-catalyzed reactions and copper-free click chemistry reactions are summarized in the Fig. 2 and Table 1.
Fig. 1.
Example methods of click chemistry-based hydrogels discussed in this article
Fig. 2.
General click chemistry-related chemical reactions for formation of hydrogels focused in this article.
Adopted and reprinted from [4] Copyright (2018), with permission from Elsevier
Table 1.
Representative click chemistry reaction methods, reactants, cell culture details, and their advantages and disadvantages. (In table format)
| S.no | Hydrogel preparation methods | Reactants | Cells/in vivo/in vitro | Gelation time and degradation | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|---|
| 1 | Copper-catalyzed click chemistry reactions | HA-hydrazine, collagen, and HA-aldehyde or HA-benzaldehyde, Copper (II) sulfate pentahydrate | hMSCs | Gelation in 5 min | Enhanced focal adhesion, cell spreading, fiber remodeling | Copper ions toxicity, reactive oxygen species generation, regional variation in viscosity due to rapid gelation, non-homogeneous gels | [51] |
| 2 | Diels–Alder click reactions | Furan-linked gelatin against maleimide-linked PEG, chitosan-Pluronic F127) | Cardiomyocyte cells, in vitro and in vivo | Gelation in 2 h, slower degradation rate in vitro | Injectable gels, fully-interpenetrating network, thermosensitive, cell adhesive | Slow gelation, reduced solubility of the functional groups, inability to be injected in vivo in few cases | [59] |
| N-maleoyl alanine terminated F127, furan grafted chondroitin sulfate, oxidized chondroitin sulfate, bone morphogenetic protein-4 | Rat mesenchymal stem cells, bone cells (repair), in vitro and in vivo | Gelation in 3 days, 14 days degradation in vitro | Easily modifiable, improved viscoelastic properties and rheological properties. Swellable, injectable, self-healing ability | [61] | |||
| Sodium alginate, bio glass and modified chondroitin sulphate | Cranial bone defect repair, in vitro and in vivo | Gelation in 3 days, 4 weeks at neutral pH, faster degradation at basic pH in vitro | Triple cross-linked injectable hydrogel, better physio-chemical properties | [60] | |||
| Furfurylamine linked chondroitin sulfate, F127 linked maleimido and PEG-AMI, bone morphogenetic protein-4 | Bone cells (repair) | Gelation in Less than one min | Non-covalent and covalent crosslinking, good biocompatibility | [17] | |||
| Furan linked HA, dexamethasone and maleimide linked HA | Human adipose-derived stem cells, in vitro | Gelation in 60 min, more than 21 days for degradation in vitro | Thermo-responsible hydrogels, dexamethasone-controlled release in local environment, non-cytotoxic, can deliver adipogenic factors | [57] | |||
| HA with furan adipic dihydrazide, HA with furan CHO and followed by addition of dimaleimide PEG | Chondrocyte cells (cartilage), in vitro | Gelation in 5 min | Mechanical properties, tissue adhesive, self-healing, pH responsiveness | [53] | |||
| 3 | Strain-promoted azide-alkyne cycloaddition (SP-AAC) reactions | Azadibenzocyclooctyne-modified dextran and azide-modified dextran | Chondrocyte cells(cartilage), in vitro | Gelation in 1.1 to 10.2 min, slow degradation rate up to 21 days in vitro | Gelation time modifiable using concentration variation and substitution degree of dextran, encapsulation of cells and cell spheroids | Reaction rate is lower than copper catalyzed reactions, alkynes are larger, trigger high perturbation, less ligation rate, high side reactions | [72, 114] |
| Dibenzocyclooctyl (DBCO)-modified HA, 4-arm PEG azide | Chondrocyte cells (cartilage), in vitro and in vivo | Gelation in 10–14 min, slow degradation up to 35 days in vivo | Nontoxic cross linker, good biocompatibility, in situ physical gelation, elastic modulus can be modified by varying concentration | [73] | |||
| Hyperbranched poly(ε-caprolactone) (hyPCL)32-(1R,8S,9 s)-bicyclo[6.1.0]non-4-yn-9-ylmethanol (hyPCL32-BCN) and hyPCL32-azide (hyPCL32-N3) | Bone (MC3T3 preosteoblast) cells | Gelation in 30 min | Injectable hyperbranched PCL, biocompatible, excellent support for cell adhesion and proliferation | [71] | |||
| 4-dibenzocyclooctynol functionalized PEG, 4 arm PEG tetraazide, protein igands (laminin), neurogenic differentiation factor (interferon-γ) | Neural stem cell, In vitro | Gelation in less than 5 min | Additional differentiation factors not required in medium, high cell viability, no UV light required | [16] | |||
| 4 | Thiol-ene-based click chemistry reactions | PEGDA, dithiothreitol, borox | Endothelial cells and neural stem cells, in vitro | Gelation within few min of stirring | Injectable, 3D printable, sacrificial removal enables tubular structure formation | Polymer substrates lack alkenes with electron deficiency, acid condition required for the reaction | [77] |
| 4-arm PEG norbornene, PEG dithiol, Protein array printing of collagen I, collagen III, collagen IV, fibronectin, laminin, elastin and hyaluronan | Smooth muscle cells, in vitro | Cellular stiffness and increased functional contractility, cell attachment and 3D infiltration | UV source needed in radical mediated reactions which may damage cells, oxygen sensitivity may result in disulfide formation; the presence of cysteine and amine group residues may compete with thiol group while hydrogel formation occurs. | [82] | |||
| Alkene-linked (allyl and norbornene residues), poly(oligoethylene glycol methacrylate)), cell adhesive peptides RGD and REDV | Human umbilical vein endothelial cells (HUVECs), in vitro | Photo-patterned peptides at the surface, development of cell array is achieved | [80] | ||||
| Norbornene-linked pectin macromer, monocysteine RGD peptide, biscysteine peptide with matrix metalloproteinase cleavage site | Human neonatal dermal fibroblasts, in vitro | Degradation9 h in enzymatic treatment in vitro | Cell surface receptors in pectin may increase cell attachment and integration, simple, fast, robust, independent modification of the biochemical or biophysical cues in the system is possible | [79] | |||
| 8-arm-PEG thiol macromer, thioester di(vinyl ether), caged thioester catalyst, norbornene RGD | Primary hMSCs, in vitro | Dynamic, modifiable visco-elastic properties, using pH, stoichiometry and crosslinker structure the hydrogel network can be modified, thiol-ester resembles biological reactions | [78] | ||||
| 5 | Oxime based click chemistry reactions | Aminooxy-terminated PEGs, aldehyde modified HA and collagen I | Schwann cells, HMSCs, in vitro | Less toxic to the cells, tunable properties, peptide, protein bonding is easier | Not bio-orthogonal | [22] |
Copper-catalyzed azide-alkyne cycloaddition (Cu-AAC) click chemistry-based hydrogels for tissue engineering
Cu-AAC is one of the commonly used metal-catalyzed methods for synthesizing cross-linked biohydrogels for live cell encapsulation and tissue engineering. The main advantages of the method are quick gelation and high yields of products [1, 2, 4, 41]. However, the limitations of Cu-AAC reactions are non-homogeneous viscosity of the hydrogels with defective regions, improper diffusion of precursors inside the hydrogel and oxidation of copper catalyst leading to lower efficiency [1, 2, 4, 42, 43]. In situ reduction of copper ions were tried using various methods; however, all methods failed to stop the accumulation of excess copper ions inside the hydrogels. Even photochemical method to reduce the copper ions was tried, but this also became less effective as the light exposure was not uniform throughout the hydrogel with less penetration [44, 45].
One of the main problems is the release of copper ions from the catalyst after reaction, which is cytotoxic and curbs the use of this method directly in tissue engineering or regenerative medicine applications [2, 4, 46]. To overcome this challenge, many researchers started using chelating agents or ligands which are water-soluble in nature. Those chelating agents or ligands include bipyridine [47], bis(l-histidine) [48], bis[(tert-butyl triazoyl) methyl]-[(2-carboxy methyl triazoyl)-methyl]-amine [49], tris-(hydroxyl propyl triazolyl methyl) amine [50], etc. Even though these ligands or agents involve in protecting the encapsulated cells and biomolecules from oxidative stress by acting as sacrificial reducing agent, their research results reported that the reduced water solubility after chelation of copper ions also induced toxicity [47]. Recently, Guo et al. used 4-(2-hydroxy ethyl)-1-piperazine ethane sulfonic acid as a chelating agent for copper ions in Cu-AAC reactions to synthesize mussel-inspired citrate-based bio-adhesive hydrogels. Along with the high bio-adhesive properties (223.11 ± 15.94 kPa), these hydrogels possess excellent antimicrobial activity which can be positively considered for invasive applications. As the concentration of periodate was increased, the gelation time decreased slightly, and this may be attributed to the catechol group crosslinking than the click crosslinking [46]. In another interesting work, Lou et al. reported a hydrogel system consisting of interpenetrating hydrogel network resembling extracellular matrices. The high viscoelastic nature of hydrogels shows the immense potential of these hydrogels in stress relaxation, cell distribution, fiber redesigning and formation of focal adhesion points, which are not usually present in other 3D hydrogel systems. Gelation was faster in HA-aldehyde samples and they reached a stable modulus value within 15 min. Amplified modulus was achieved as the concentration of hyaluronic acid (HA) was increased [51]. Even though Cu-AAC-based click chemistry reactions yields high quantity of products with short gelation time, the toxicity associated with copper catalyst poses a big threat for using this method to prepare hydrogels for tissue engineering and regenerative medicine. However, efficient modification of catalysts, chelating agents, and further development in finding alternates for copper catalyst may yield better Cu-AAC based hydrogels with less toxicity for tissue engineering applications [2, 4, 9].
Copper-free click hydrogels
Diels–Alder (DA) click chemistry hydrogels for tissue engineering
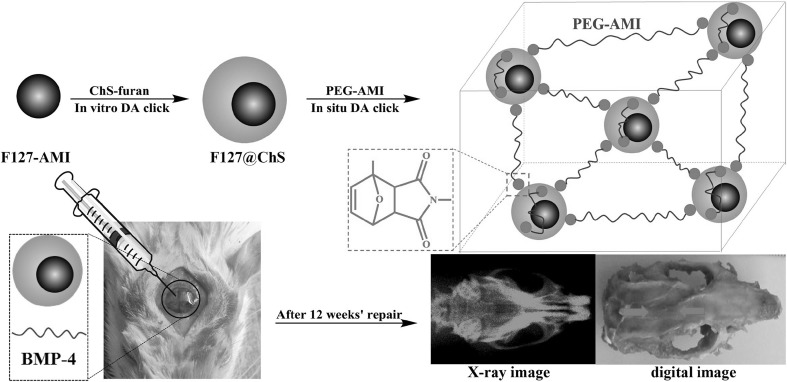
Diels–Alder-based click chemistry reactions occur mainly between the diene and alkene (or dienophile) without the help of a catalyst or coupling agent. These reactions are highly accelerated in presence of water because of the hydrophobic effects [4]. DA reactions occurs in aqueous medium with high reaction rate, versatility, selectivity and efficiency which are characteristics for click chemistry reactions [52, 53]. They also do not yield any toxic end products. Hence, this robust method is highly used for developing cross-linked hydrogels for tissue engineering applications [2, 4]. Koehler et al. synthesized the DA-based hydrogels for drug release and osteogenic differentiation. DA reaction occurred between the maleimide poly(ethylene glycol) (PEG) macromer and furan dexamethasone peptide to form the hydrogels. The dexamethasone release study and osteogenic differentiation of human mesenchymal stem cells (hMSCs) were demonstrated in both 2D and 3D culture environments. The tested hydrogels also showed high alkaline phosphatase activity (6 times more) and increased mineralization [54]. Nimmo et al. reported HA-based furan-modified hydrogels crosslinked using DA reaction via dimaleimide-linked PEG. They suggested that the mechanical and other properties like degradation can be tuned by varying the proportion of furan and maleimide appropriately. Human epithelial cells were used to test the cyto-compatibility and degradation of the hydrogels [55]. Similarly, the Shoichet’s group demonstrated the ability of the furan-modified HA and bis-maleimide PEG hydrogels formed by DA reaction to hold biomolecules (galactose) in spatially defined manner by photo-patterning through a processing of two-photon laser method. They also demonstrated the controlling of porosity and pore size distribution by cryo-gelation and thaw temperature, respectively. The mechanical property of the hydrogel was also controlled by modifying the furan substitution [56]. Yu et al. investigated a tissue-adhesive DA click chemistry-based hydrogels for cartilage tissue engineering. The double-crosslinked network hydrogel was formed between the HA with furan adipic dihydrazide, and HA with furan aldehyde and followed by addition of dimaleimide PEG. The hydrogels showed good mechanical, swelling and self-healing properties as well as cartilage-adhesive properties [53]. Similarly, Fan et al. reported furan-linked HA and maleimide-linked HA as hydrogel components to form biodegradable DA-based click chemistry hydrogels for adipose tissue engineering. The human adipose-derived stem cells were incorporated into the hydrogels to test the cytocompatibility of the hydrogels. The hydrogels promoted stem cell proliferation, however, they did not promote differentiation of the stem cells [57]. Recently Bai et al. reported development of a dual crosslinked injectable self-reinforcing hydrogels for tissue engineering applications. The dual crosslinking was carried out with the help of non-covalent bonding (cyclodextrin, adamantane, poly(N-isopropyl acrylamide) (PNIPAM)) and DA-based click chemistry reaction (furfurylamine-linked chondroitin sulfate and maleimido-linked PEG). This dual crosslinking increased the mechanical strength of the hydrogels significantly and in vivo studies showed promising results for bone repair even without using any cells or growth factors in the hydrogel [58]. They also reported a dual-crosslinked injectable chondroitin sulphate hydrogel loaded with bone morphogenetic protein-4 via DA-based click chemistry for restoration of rat cranial defect. The components of the hydrogels were furfurylamine-linked chondroitin sulfate (ChS-Furan), F127-linked maleimido and PEG-AMI (Fig. 3), which offered both non-covalent and covalent crosslinking. The rat in vivo studies along with BMP-4 revealed the new bone formation in the injected area after 12 weeks of implantation [59].
Fig. 3.
Dual cross-linked injectable chondroitin sulphate-based hydrogel loaded with bone morphogenetic protein-4 via DA based click chemistry for restoration of rat cranial defect.
Reprinted from [17] Copyright (2017), with permission from Elsevier
Bai et al. introduced a triple cross-linked injectable hydrogel for repair of cranial bone defect. The hydrogel components were modified sodium alginate, bioglass and modified chondroitin sulphate. The triple cross-linking was achieved by cross-linking of non-covalent bond, acylhydrazone bond and DA-based click chemistry covalent. The hydrogel combination showed admirable physio-chemical properties and bone regeneration abilities during in vivo studies [60]. Lu et al. reported a similar self-healing and injectable hydrogels for repair of in vivo cranial bone. [61]. Catechol-modified N-(furfural) chitosan (CFC) based dual cross-linked (co-ordination bond and DA click chemistry) hydrogels for tissue engineering application was reported. The co-ordination bonding between iron ions and catechol provide the ability to control the self-healing ability and degree of cross linking. The dual crosslinking also generated very good mechanical properties for the hydrogels [18]. Recently, Smith et al. introduced new click cross-linked hydrogels containing methyl-furan grafted hyaluronan which can form gels at physiological pH unlike hyaluronan-furan which can form gel only at low pH condition. This DA crosslinked hydrogels facilitate the encapsulation of live cells inside the hydrogels for tissue engineering and 3D cell culture applications. Furthermore, they observed hydrogen bonding interactions through computational analysis [62].
Another interesting hydrogel system reported by Abandansari et al. showed interpenetrating hydrogel (furan-linked gelatin against maleimide-linked PEG via DA click chemistry) with in situ gel forming ability and thermo-sensitive (chitosan-Pluronic F127) behavior along with high mechanical and biocompatibility properties for retention of encapsulated cardiac cells. The increased crosslinking degree, reduced gelation time and increased mechanical properties can be achieved by adding equivalent ratios of furan and maleimide. Hence, in this system by varying the PEG and gelatin ratio one can tune the mechanical properties of the hydrogels [59]. As mentioned earlier, the main advantage of DA-based click chemistry is that they do not require any toxic initiators or coupling agents for the reaction. Drugs also can be incorporated for sustained release because of its exceptional site specificity and thermal reversible properties. However, there are some challenges to be addressed for using DA click chemistry hydrogels such as longer gelation time, reduced solubility of the functional groups and in few cases, less effectiveness for usage as injectable gels under physiological environment [2]. Yet, these DA-based click chemistry hydrogels show promising results towards regenerating cartilage, adipose tissue, cranial bone defects and it can be further applied to other tissue engineering applications.
Strain-promoted azide-alkyne cycloaddition (SP-AAC)-based click hydrogels for tissue engineering
To overcome the problems associated with copper-catalyzed click reactions, scientists started developing other methods which does not use copper as catalyst. This lead to the introduction of strain-promoted azide-alkyne cycloaddition click reactions. The method involves reaction of cyclooctyne molecules with azides by means of ring strain and electron withdrawal from fluorine substitutes [63]. “Strain-promoted” concept mainly came from the ring strain which accelerates the reaction between azide and cyclooctyne groups compared to other methods. This SP-AAC can decrease up to 18 kcal/mol of active energy during ring chain reaction because of bond angle distortion [14]. DeForest et al. introduced a robust synthetic hydrogel system consisting of macromolecular precursors (4 arm PEG modified with tetraazide and bis(di-fluorinated cyclooctyne moiety) di-functionalized polypeptide) as reactants for encapsulating cells directly without using copper. They also demonstrated high-resolution patterning of hydrogels with biological functionalities using photo-coupling of orthogonal thiol-ene. The advantages include tailoring the different properties of the hydrogels in situ for creating favorable environment for cells, direct functionalization and photo-patterning in presence of live cells [64]. In their subsequent work, they reported hydrogels containing patterned biomolecules with photo-reversibility. This hydrogel system was developed via SP-AAC reaction, which showed excellent spatiotemporal supremacy over the biomolecules in the 3D hydrogel system with the help of various bio-orthogonal photo-reactions [65]. Kloxin et al. demonstrated similar spatiotemporal control of biological molecules in the hydrogel systems using azide and cyclooctyne groups. This hydrogel with controlled micro-environment may help to understand the tissue morphogenesis and regeneration [66]. Another SP-AAC based hydrogel developed from azide-linked PEG-co-polymer and cyclooctyne-linked PEG was reported by Xu et al. This hydrogel system showed excellent cell viability than the photo-crosslinked PEG when bone marrow stromal cells were encapsulated in the hydrogel [67]. Zheng et al. investigated the ability of the hydrogels formed via SP-AAC reaction to encapsulate human mesenchymal stem cells with high cell viability. The hydrogel was prepared from 3 arm-glycerol exytholate triazide and dibenzocyclooctyne linked PEG. Gelation of the hydrogels with encapsulated cells were achieved by the cumulative effect which occurred because of the molecular interactions between the azide-terminated PEG and strained cyclocotyne units [68].
Takahashi et al. developed an in situ gel forming hyaluronan-based copper-free click chemistry hydrogels for tissue engineering and drug delivery applications. After modifying HA with azide group and cyclooctyne separately, SP-AAC crosslinked gels were obtained by mixing both solutions. In vivo studies revealed the biocompatibility and elimination of hydrogels without affecting the host. The gelation time of the hydrogel formation was less than 5 min. This gelation time was strongly dependent on the polymer concentration used for the preparation. However, when compared with other Schiff’s base reactions, this reaction took more time for gelation. This was attributed to the low degree of HA modification compared to the reported reactions [69]. Jiang et al. reported a fast degrading, injectable hydrogel system made from alkyne-modified PEG and azide-modified PEG cross-linked via SP-AAC reaction. Their results showed excellent properties including in situ gel formation ability under in vivo conditions with mild immunogenic response [70]. Liu et al. revealed poly(ε-caprolactone)-based hyper-branched dendrimers which were cross-linked via SP-AAC reaction exhibiting high mechanical properties and biocompatibility. This hydrophobic dendrimer with multi-functional properties may be used in bone tissue repair and also in biomedical applications [71]. Similarly, Fu et al. described a SP-AAC based hydrogel formed from cyclooctyne-modified HA and azide-modified PEG. This hydrogel also displayed short gelation time, stability, bio-compatibility and slow degradation [15]. Another recent work includes the hydrogel formed from dextran modified with azide and cyclooctyne components using SP-AAC click chemistry. This injectable hydrogel was tested for cartilage tissue engineering by incorporating chondrocytes cells individually and as spheroids. However, spheroids induced higher production of chondrocyte extracellular matrices compared to the individual cells [72].
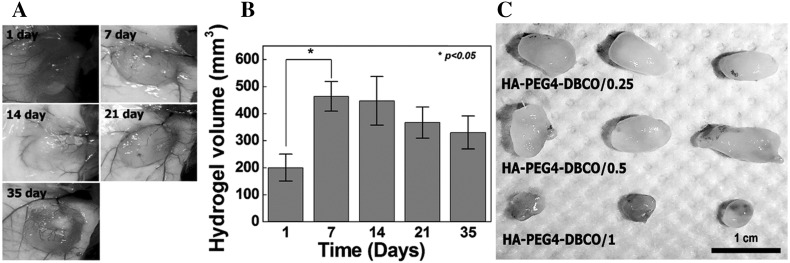
Han et al. reported a biocompatible, in situ cross-linking HA-based injectable hydrogel for cartilage regeneration. The high cross-linking was achieved by SP-AAC mechanism where HA was modified with 4-arm PEG azide and dibenzyl cyclooctyne separately for gel formation. The copper-free click reaction formed gel with the help of reaction between azide and dibenzyl cyclooctyne groups. Different compositions were used to test the various properties required for the injectable hydrogels in vitro. They injected the hydrogels subcutaneously in mice and observed the change in volume of gel at different time periods (Fig. 4A, B). White solid tissue like hydrogels removed from the mice after in vivo experiments for cartilage regeneration were observed (Fig. 4C) [73]. This hydrogel system showed promising results for the regeneration of cartilage in vivo. Recently, a hydrogel system was fabricated using SP-AAC reaction composed of cyclooctyne and azide functionalized PEG-based polymer and protein (laminin) along with a neurogenic differentiation (interferon- γ) factor included in the hydrogel system. In vitro studies with protein-entrapped hydrogels containing neural stem cells showed differentiation without adding any growth factor in the cell culture medium [16]. However, the limitations of SP-AAC based click chemistry are the numerous steps that are involved in creating the cyclooctyne and their very low product yield compared to other methods [4]. Another notable challenge is the production of regio-isomeric combination of triazoles in the SP-AAC reactions. Even though these challenges remained, still this method holds promise for developing alternate hydrogel biomaterials with great degree of spatiotemporal functionalization and cell adhesive properties [14].
Fig. 4.
A Hyaluronic acid-based copper-free click chemistry hydrogels implanted in Balb-c mice for up to 35 days for cartilage regeneration. B Volume of hydrogel in vivo at different time and C White solid tissue like hydrogels with chondrocytes removed from mice.
Reproduced from [73] with permission from the Royal Society of Chemistry
Thiol-ene-based click hydrogels for tissue engineering
Thiol-ene click chemistry reaction takes place between the thiol groups and the alkene groups of the reactants. This method provides many advantages such as high yield, high reaction rates, no initiator for initiation reaction, high selectivity, no sensitivity to oxygen or water and high bio-compatibility, thus forming orthogonal networks [2]. The thiol-ene reaction mechanism comprises of a mixture of step growth reactions and polymer chain growth reactions. The initiator used in the reaction generates radicals first, which then takes up the thiol group protons from the reactants to form the thiyl radicals. Then, carbon-based radicals are formed by thiyl radicals which triggers the carbon–carbon double bond formation. Thus, formed carbon-based radical may either involve in chain transfer or react with new thiol group to form a new thiyl radical or multiply via carbon–carbon double bond [74]. The molecules bearing double bond (carbon–carbon) can be covalently cross-linked with thiol functionalized molecules. Thiol-based reactions are widely considered as safe and non-toxic in organic synthesis and currently, they are researched in the area of click chemistry for synthesizing novel polymeric materials [75].
Among the thiol-based reactions reported, thiol-ene and thiol-yne reactions are vastly used to develop functional hydrogel biomaterials for tissue engineering and regenerative medicine because of its non-toxic nature, where no metal catalysts are used [20]. Initially, Hawker et al. synthesized a PEG-based hydrogel system crosslinked via thiol-ene group with increased mechanical properties compared to that of the normal PEG hydrogel [76]. Poly(ethylene glycol) diacrylate (PEGDA) and dithiothreitol were used to prepare Thiol-ene-based glucose sensitive hydrogel with self-healing ability for neural tissue engineering application. This injectable hydrogel can act as sacrificial material when exposed to glucose to form branched tubular structures with in a 3D construct, where non-sacrificial materials act as the main matrix. Formation of vascularized neural tissue was demonstrated with the help of endothelial cells and neural stem cells after 14 days [77]. Brown et al. reported dynamic, viscoelastic, photo-polymerized hydrogel system from 8 arm PEG thiol macromer, thioester di(vinyl ether) as crosslinker, caged thioester as catalyst and norbornene-RGD for cell adhesion. In vitro experiments with primary hMSCs showed promising results for the hydrogel system. Because of the biological importance of the thiol-esters, it may be highly used in cell scaffold applications. These thiol-ene based photoclick reactions are known to form gels rapidly with homogeneous gelation and highly biocompatible hydrogels at normal physiological conditions [78].
Granja’s group synthesized cellular degradable pectin-based hydrogel system via thiol-ene photo-crosslinking. The cell-instructive hydrogel system consisted of norbornene-functionalized pectin, monocysteine cell-adhesive ligands for integrin attachment and enzymatically cleavable biscysteine peptide crosslinker. Dermal fibroblast cells were encapsulated during the gel formation and in vitro skin tissue formation was evaluated. These thiol-norbornene based hydrogel network formation with high homogeneousness can help formation of tissues by providing enhanced environment. Also, thiol-norbornene based reactions did not involve any strained norbornene group chain growth or homo-polymerization. Further, these reactions yield hydrogels in a shorter time without affecting the cell viability under physiological conditions. Norbornene group modifications are mainly used for their speedy reactivity and high cytocompatibility [79].
Recently, another Thiol-ene-based reaction was used for developing cell arrays with alkene-linked (allyl and norbornene residues), poly(oligoethylene glycol methacrylate) and cell-adhesive peptides RGD and REDV. These systematically patterned biomolecules on the polymer enables us to control the cell adhesion in a controlled way. When human umbilical vein endothelial cells were tested on the patterned structure, RGD-patterned polymers showed higher cell adhesion [80]. Sharma et al. introduced a new microarray system based on thiol-ene photo-clickable peptides that can be used for understanding the cell behavior in 3D micro-environment which combines three technologies, mainly thiol-ene photo click chemistry, microcontact printing and electrospinning. They used thiol-ene method to crosslink the fibrous structures produced via electrospinning and to introduce peptides, a photo-click reaction mediated by norbornene was used in the fibrous matrix. This system with free norbornenes enables any reactant with thiol group to be linked into the 3D construct with high precision. Further, they used a normal contact printer to deposit peptides with cysteine moieties in a microarray model to test the concept with different cell lines [81]. In a similar work, Ding and coworkers demonstrated the ability of such combinatorial, clickable system by enhancing the contractile property of the human smooth muscle cells for vascular tissue engineering. The fibrous hydrogel system showed different biomimetic smooth muscle properties like modifiable structures, composition and mechanical stiffness. With the help of protein array technology and the hydrogels, they demonstrated the in vitro pharmacological responsiveness and contractility of the smooth muscles [82].
Zhou et al. reported a photo-polymerized Thiol-ene-based hydrogel for tissue engineering applications consisting of maleic chitosan, thiol-linked PVA and a biocompatible initiator. This hydrogel showed excellent mechanical properties and fibroblast cell compatibility [83]. Takemoto’s group recently reported the crosslinking of hydrogels with live cells and tissues using azide-alkyne click chemistry reaction. They initially modified the alginate with alkyne group and live cells/tissues were modified synthetically with azide group targeting the sialic acid groups present on the cell surface and followed by incubating both cells with azide group and hydrogels with alkyne group for gel formation. The live cell containing hydrogels showed excellent cell viability [5]. These various works related to Thiol-ene-based click chemistry showed the ability of these reactions to provide hydrogels with optimum properties required for the various tissue engineering applications. However, one should choose the biocompatible reactants and method to develop the successful biomaterial for specific tissue engineering application.
Oxime-based click chemistry hydrogels for tissue engineering
The oxime click chemistry reaction occurs between an aldehyde or ketone group and an amino-oxy group. Reactions are rapid and contain similar orthogonal functionalities that are present in biomolecules and cells. The reaction does not require any toxic catalyst or UV light or no external temperature, and usually the byproduct of the reaction is water [3, 84]. The products obtained from the reactions consists of imine hydrazone and oxime chemical bonds, which are highly stable in physiological conditions [84]. With high stability than thiol groups, this oxime reaction is preferred for modifying different biomacromolecules like peptides, proteins and DNA [85]. Furthermore, they are used in polymer-protein ligation, cell surface modification and, also used to in vivo labeling of tissues [21, 86]. Grover et al. explored the oxime click chemistry-based hydrogels for live encapsulation of MSCs, using 8-arm-amino-oxy-PEG, RGD peptides and glutaraldehyde. They hypothesized that the formation of stable oxime bonds by the reaction of amino-oxy groups in aqueous condition lead to the increased stability of the hydrogels when compared to the normal imine bond formation by amine groups [21].
Amino-oxy modified PEG and aldehyde-modified HA-based oxime-crosslinked biodegradable, biocompatible hydrogels with tunable mechanical properties were evaluated by Hardy et al. for soft nerve tissue engineering. While cytotoxic assay was carried out with Schwann cells, cell adhesion study was performed using human MSCs for the hydrogels. Both showed promising results for the possible application in nerve tissue regeneration when collagen I was incorporated in the hydrogels [22]. DeForest’s research group reported a biorthogonal oxime click chemistry-based hydrogel for 3D cell culture-related applications. In this study, they used mild UV light photo-polymerization for obtaining the cells-encapsulated hydrogel. This photo-mediated oxime reaction enabled them to immobilize proteins with alkoxyamines at specific regions where UV light is exposed. The photo-mediated oxime reactions allow us to polymerize selected areas in the samples based on our interest. The UV irradiated regions in the polymer solution formed gelation except in the photo-masked unexposed regions [23].
Hentzen et al. presented the crosslinking of model collagen peptides through oxime bond formation using 4-oxoacetamido-proline and 4-aminooxy-proline. These peptides were covalently linked, resulting in formation of stable collagen triple helices [87]. Tamura et al. reported a new affinity-guided oxime catalytic system involving pyridinium oxime and N-acyl-N-alkylsulfonamide for protein labelling. This system can be used to selectively label natural proteins present in test tubes and different cell lysates under normal physiological conditions. Cell membrane proteins can be fluorescently tagged and visualized in live cells using this catalytic system. They demonstrated the ability of this system in mouse brain slices [88]. Drawback of using oxime chemistry reaction in bioconjugation is normally oxime reactions require neutral or basic (pH) condition to reduce the possible oxime exchange reaction to occur [1]. Considering the various advantages over the limitations, these oxime-based click chemistry reactions may be used to development novel biomaterials for both in vitro and in vivo applications.
Thiol group-based (Thiol-Michael) pseudo-click hydrogels for tissue engineering
Thiol-Michael pseudo click chemistry involves an addition of thiol group into a double bond of vinyl sulfone, acrylate or maleimide, resulting in thioester bond formation with or without the use of a catalyst [89]. The advantages of this reaction include higher reaction rates, easier access to reagents functionalized with thiol and ene groups and high tolerance towards different functional groups [90–92]. Michael addition reaction-based cross-linked step growth hydrogels were first reported by Hubbell’s group [93]. The reactants used were multi-acrylate linked PEG and dithiol linked PEG or thiol-linked peptides. Different Michael-type addition reaction-based hydrogels were developed in the past decade by various groups around the world. Various biomaterials were used for preparing hydrogels using this method such as chitosan-PEG, fibronectin, HA and gelatin-PEG [19, 94, 95]. Young and Engler reported cross-linked hydrogels, consisting of thiol-HA and PEGDA using Michael addition method for cardiac tissue engineering application [96]. Scientists used highly reactive vinyl sulfone instead of acrylate in the thiol-Michael reactions mainly because of its high electron withdrawing ability and formation of strong bonds [90]. Patterson and Hubbell reported thiol-Michael reaction-based PEG hydrogels for tissue regeneration using 4 arm PEG-linked with vinyl sulfone, cell-adhesive peptides and matrix metalloproteinase degradable peptides [97]. Jin et al. demonstrated the formation of collagen and chondroitin sulphate in the chondrocyte-cultured HA hydrogels prepared from thiol-linked HA and 4-armed PEG with vinyl sulfone, using thiol-Michael addition reaction for cartilage repair [98]. Fibronectin and RGD peptide were incorporated in PEG hydrogels consisting of thiol-maleimide and showed increase in the human fibroblasts cell adhesion and proliferation. The same group developed similar hydrogel with glutathione sensitivity using thiol-Michael reaction. The gel formation slowed down when the pH and temperature was reduced to 6.6 and 4 °C, respectively. This reduction in temperature and pH permitted the proper mixing using vortex machine and for loading samples [99]. Bang et al. reported chondroitin sulphate-based dual cross-linked hydrogels with gelatin grafting for improving the cell-adhesive properties of the chondroitin sulphate. They used the Michael type click chemistry reaction method and N-(3-diethylpropyl)-N-ethylcarbodiimide hydrochloride chemistry for the gel formation and the hydrogels were tested for its application related to tissue engineering and drug delivery [100]. These different works related to thiol-based click chemistry reactions show potentials of this method in developing novel hydrogel biomaterials for tissue engineering applications.
Aldehyde-hydrazide pseudo click hydrogels and amino-yne hydrogels for tissue engineering
Like other click chemistry reactions, aldehyde-hydrazide based pseudo reactions are simple, versatile, and do not generate toxic end products with high reversibility. In general, many polysaccharides were modified with aldehyde and adipic acid dihydrazide (ADH) derivatives to obtain various hydrogels. Bulpitt and Aeschlimann reported the first hydrogel using this method, by using HA-ADH and HA-aldehyde [101]. Tian et al. demonstrated the capability of the aldehyde-hydrazide cross-linked HA-based hydrogels in rat brain repair [102]. This work showed an advanced potential of this method to develop such biomaterials for tissue regeneration. Alginate and HA-based in situ cross-linkable hydrogel was reported by Dahlmann et al. for cardiac tissue engineering. They demonstrated that the hydrogels were able to generate contractile cardiac tissue from rat heart cells [103]. Martinez-Sanz et al. developed an injectable bone morphogenic protein-2 (BMP-2) loaded HA hydrogel for in vivo bone tissue formation. They used synthetic procedure, including amidation and selective oxidation for the formation of stable gels. The gelation occurred within 30 s; however, BMP-incorporated hydrogel samples were placed for curing (3 h) at room temperature. The cured samples were used for in vivo experiments [104]. Similarly, many scientists recently reported different aldehyde-hydrazide hydrogels for tissue engineering applications that include PVA [105, 106], elastin-like protein-HA based hydrogels [107, 108], poly(N-isopropylacrylamide) (PNIPAM), [109, 110], HA-based hydrogels [111], and HA with growth factors for bone tissue engineering [112].
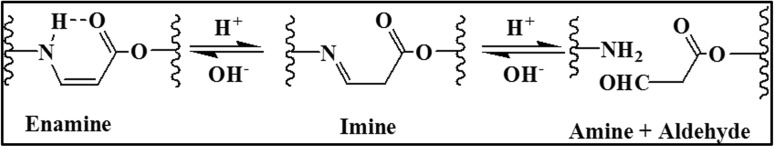
Recently, Huang and Jiang demonstrated the ability of the amino-yne click chemistry to form hydrogels with pH sensitivity for local drug delivery and tissue engineering applications. They reported the carboxymethyl chitosan-PEGDA based pH-responsive, degradable and injectable hydrogels for tissue engineering and biomedical applications without using a catalyst or initiator in normal physiological conditions. The mechanism of amino-yne click chemistry-based hydrogels’ pH sensitivity is depicted in the Fig. 5. In presence of H+ ions, enamine is converted to imine compound. Further, decomposition occurs to form aldehyde and amine group at low pH, ultimately resulting in complete release of drug at pH 2 [113]. Since, this injectable amino-yne based click chemistry hydrogel is simple, less expensive and spontaneously gel-forming, it may provide the much-needed biocompatible hydrogels for tissue engineering applications without using any toxic catalyst or photo initiator.
Fig. 5.
Amino-yne click chemistry-based hydrogels: pH-sensitive mechanism.
Reprinted (redrawn) with permission from [113]. Copyright 2018 American Chemical Society
Application of click chemistry in development of bioinks for 3D bioprinting
Recently numerous novel multifunctional hydrogel biomaterials developed by various reaction methods are reported as bioinks for 3D bioprinting [26]. These hydrogels provide the much-needed microenvironment by biomimicking the extracellular matrices present in the native tissue structures [27]. Different natural and synthetic hydrogels with multifunctional properties like biocompatibility, high mechanical properties, pH sensitivity, temperature sensitivity, etc. and in combination with nanomaterials or composite materials or growth factors, biomolecules, etc. have been applied to create complex 3D structures and functional tissue structures using 3D bioprinting technology for tissue engineering applications [27]. Thus, a multidisciplinary approach involving of stimuli-responsive biomaterials, click chemistry reactions and cutting-edge 3D bioprinting technology may facilitate higher probability to create complex 3D structures which are functional and able to form tissue or organ structures mimicking native tissues [115]. In a review article by Kurzrock and Stewart, they discussed about the combination of click chemistry and 3D bioprinting related to chemical compound development and implications. They also envisioned that the natural proteins can be designed and tested in silico using supercomputers, whereas the click chemistry and 3D bioprinting technology can help us to obtain the desired chemical compound with different structures and high precision [116]. Similar proof-of-concept studies was already reported related to these methods [117, 118].
Likewise, click chemistry reactions have been used to develop hydrogels for 3D bioprinting bioinks [27]. Bertlein et al. reported thiol-ene photo-clickable gelatin-based hydrogels as bioink for fabrication of 3D structures using 3D bioprinting technology [119]. Li et al. demonstrated Cu-AAC based click chemistry reactions for developing peptide functionalized poly(ester urea) scaffolds for bone tissue engineering applications. In this work, they used l-phenylalanine functionalized with propargyl groups-based poly(ester urea) scaffolds with BMP-2 and osteogenic growth peptide. These scaffolds showed increased osteogenic activity and hMSC differentiation than the non-functionalized scaffolds [120]. Stichler et al. reported a similar combination of thiol-ene click chemistry and 3D bioprinting for developing 3D structures recently. They used polyglycidol-based hydrogels which were cross-linked by UV light, and cytotoxicity tests were performed using hMSCs obtained from bone marrow. They added high molecular weight HA to adjust the rheological properties and printed 20 layered structures with high reproducibility [121]. This combinational approach using click chemistry and 3D bioprinting were reported by many scientists recently for various tissue engineering applications [122–126].
Conclusion
In this review, we explained the diverse click chemistry-based hydrogel syntheses, mechanisms, typical hydrogels reported for tissue engineering applications as well as their advantages and disadvantages in details. High reaction rates, spontaneous reactions, selectivity, versatility, high product yields, biocompatibility, in situ gel formation and injectability are some examples of the advantages of click chemistry reactions. Overall, click chemistry-based hydrogels for tissue engineering applications are promising and immensely explored all over the world in the recent years for various biomedical applications like 3D bioprinting, drug delivery, diagnosis, tissue engineering and regenerative medicine, etc. Considering the various advantages and disadvantages of diverse synthesis routes, one should design the experiments for creating novel biomaterials for specific applications. For example, thiol-based hydrogels with residual initiators are reported to induce mild immunogenic response at cellular level. Hence, high care should be taken to remove toxic catalyst or initiators completely during hydrogel synthesis, or cost-effective purification techniques should be employed to obtain better-quality products from the click chemistry reactions. One important criteria for developing such hydrogels with superior properties is the combination of advantages from diverse click chemistry methods or its key biomaterials to obtain multifunctional and biocompatible biomaterials. At the same time, the reactions should not affect or interfere with other processes or it should not have any side effects or cross reactions with unexpected reaction groups. New approaches like the surface modification of live cells with ligands for crosslinking with biocompatible hydrogels are introduced recently, which shows the rapid growth in this area. The use of click chemistry in the synthesis of bioinks for 3D printing are envisioned to provide highly precise 3D tissue structures with live cells embedded in the hydrogels. Future of click chemistry for developing novel multifunctional biomaterials for tissue engineering and regenerative medicine applications are promising and encouraging.
Acknowledgement
This work was supported by the National Research Foundation of Korea (NRF) Grant No. (2015R1A2A1A10054592).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal or human experiments carried out for this article.
References
- 1.Zou Y, Zhang L, Yang L, Zhu F, Ding M, Lin F, et al. “Click” chemistry in polymeric scaffolds: bioactive materials for tissue engineering. J Control Release. 2018;273:160–179. doi: 10.1016/j.jconrel.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Bratlie KM. Click chemistry and material selection for in situ fabrication of hydrogels in tissue engineering applications. ACS Biomater Sci Eng. 2018;4:2276–2291. doi: 10.1021/acsbiomaterials.8b00230. [DOI] [PubMed] [Google Scholar]
- 3.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed Engl. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35:4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Nagahama K, Kimura Y, Takemoto A. Living functional hydrogels generated by bioorthogonal cross-linking reactions of azide-modified cells with alkyne-modified polymers. Nat Commun. 2018;9:2195. doi: 10.1038/s41467-018-04699-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ossipov DA, Hilborn J. Poly(vinyl alcohol)-based hydrogels formed by “click chemistry”. Macromolecules. 2006;39:1709–1718. [Google Scholar]
- 7.Lee BK, Noh JH, Park JH, Park SH, Kim JH, Oh SH, Kim MS. Thermoresponsive and biodegradable amphiphilic block copolymers with pendant functional groups. Tissue Eng Regen Med. 2018;15:393–402. doi: 10.1007/s13770-018-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee SM, Jang WD. Polyion complex micelle formed from tetraphenylethene containing block copolymer. Biomater Res. 2017;21:17. doi: 10.1186/s40824-017-0103-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi G, Son J, Yoo J, Park C, Koo H. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomater Res. 2018;22:13. doi: 10.1186/s40824-018-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SY, Lee Y, Le Thi P, Oh DH, Park KD. Sulfobetaine methacrylate hydrogel-coated anti-fouling surfaces for implantable biomedical devices. Biomater Res. 2018;22:3. doi: 10.1186/s40824-017-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buwalda SJ, Vermonden T, Hennink WE. Hydrogels for therapeutic delivery: current developments and future directions. Biomacromolecules. 2017;18:316–330. doi: 10.1021/acs.biomac.6b01604. [DOI] [PubMed] [Google Scholar]
- 12.DeForest CA, Anseth KS. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925–931. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Dong M, Zhang Z, Fu G. High elasticity, strength, and biocompatible amphiphilic hydrogel via click chemistry and ferric ion coordination. Polym Adv Technol. 2017;28:1065–1070. [Google Scholar]
- 14.Agard NJ, Prescher JA, Bertozzi CR. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 15.Fu S, Dong H, Deng X, Zhuo R, Zhong Z. Injectable hyaluronic acid/poly(ethylene glycol) hydrogels crosslinked via strain-promoted azide-alkyne cycloaddition click reaction. Carbohydr Polym. 2017;169:332–340. doi: 10.1016/j.carbpol.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zheng J, Wang H, Becker ML, Leipzig ND. Neural stem cell encapsulation and differentiation in strain promoted crosslinked polyethylene glycol-based hydrogels. J Biomater Appl. 2018;32:1222–1230. doi: 10.1177/0885328218755711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai X, Lü S, Cao Z, Ni B, Wang X, Ning P, et al. Dual crosslinked chondroitin sulfate injectable hydrogel formed via continuous Diels–Alder (DA) click chemistry for bone repair. Carbohydr Polym. 2017;166:123–130. doi: 10.1016/j.carbpol.2017.02.062. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Wang L, Yu X, Wang C, Wang Z. Synthesis and characterization of a novel double cross-linked hydrogel based on Diels–Alder click reaction and coordination bonding. Mater Sci Eng C Mater Biol Appl. 2018;82:299–309. doi: 10.1016/j.msec.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Xu K, Cantu DA, Fu Y, Kim J, Zheng X, Hematti P, et al. Thiol-ene Michael-type formation of gelatin/poly(ethylene glycol) biomatrices for three-dimensional mesenchymal stromal/stem cell administration to cutaneous wounds. Acta Biomater. 2013;9:8802–8814. doi: 10.1016/j.actbio.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowe AB. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym Chem. 2010;1:17–36. [Google Scholar]
- 21.Grover GN, Lam J, Nguyen TH, Segura T, Maynard HD. Biocompatible hydrogels by oxime Click chemistry. Biomacromolecules. 2012;13:3013–3017. doi: 10.1021/bm301346e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy JG, Lin P, Schmidt CE. Biodegradable hydrogels composed of oxime crosslinked poly(ethylene glycol), hyaluronic acid and collagen: a tunable platform for soft tissue engineering. J Biomater Sci Polym Ed. 2015;26:143–161. doi: 10.1080/09205063.2014.975393. [DOI] [PubMed] [Google Scholar]
- 23.Farahani PE, Adelmund SM, Shadish JA, DeForest CA. Photomediated oxime ligation as a bioorthogonal tool for spatiotemporally-controlled hydrogel formation and modification. J Mater Chem B. 2017;5:4435–4442. doi: 10.1039/c6tb03400d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macdougall LJ, Truong VX, Dove AP. Efficient in situ nucleophilic thiol-yne click chemistry for the synthesis of strong hydrogel materials with tunable properties. ACS Macro Lett. 2017;6:93–97. doi: 10.1021/acsmacrolett.6b00857. [DOI] [PubMed] [Google Scholar]
- 25.Macdougall LJ, Wiley KL, Kloxin AM, Dove AP. Design of synthetic extracellular matrices for probing breast cancer cell growth using robust cyctocompatible nucleophilic thiol-yne addition chemistry. Biomaterials. 2018;178:435–447. doi: 10.1016/j.biomaterials.2018.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen C, Bang S, Cho Y, Lee S, Lee I, Zhang S, et al. Research trends in biomimetic medical materials for tissue engineering: 3D bioprinting, surface modification, nano/micro-technology and clinical aspects in tissue engineering of cartilage and bone. Biomater Res. 2016;20:10. doi: 10.1186/s40824-016-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:11. doi: 10.1186/s40824-018-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung CS, Kim BK, Lee J, Min BH, Park SH. Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape. Tissue Eng Regen Med. 2018;15:155–162. doi: 10.1007/s13770-017-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aleahmad F, Ebrahimi S, Salmannezhad M, Azarnia M, Jaberipour M, Hoseini M, et al. Heparin/collagen 3D scaffold accelerates hepatocyte differentiation of Wharton’s jelly-derived mesenchymal stem cells. Tissue Eng Regen Med. 2017;14:443–452. doi: 10.1007/s13770-017-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murata D, Akieda S, Misumi K, Nakayama K. Osteochondral regeneration with a scaffold-free three-dimensional construct of adipose tissue-derived mesenchymal stromal cells in pigs. Tissue Eng Regen Med. 2018;15:101–113. doi: 10.1007/s13770-017-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson VJ, Dinnunhan MFK, Turner PR, Faed JM, Cabral JD. A chitosan/dextran-based hydrogel as a delivery vehicle of human bone-marrow derived mesenchymal stem cells. Biomed Mater. 2017;12:035012. doi: 10.1088/1748-605X/aa70f2. [DOI] [PubMed] [Google Scholar]
- 32.Abdi SI, Choi JY, Lee JS, Lim HJ, Lee C, Kim J, et al. In vivo study of a blended hydrogel composed of pluronic F-127-alginate-hyaluronic acid for its cell injection application. Tissue Eng Regen Med. 2012;9:1–9. [Google Scholar]
- 33.Carles-Carner M, Saleh LS, Bryant SJ. The effects of hydroxyapatite nanoparticles embedded in a MMP-sensitive photoclickable PEG hydrogel on encapsulated MC3T3-E1 pre-osteoblasts. Biomed Mater. 2018;13:045009. doi: 10.1088/1748-605X/aabb31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimojo AA, Galdames SE, Perez AG, Ito TH, Luzo ÂC, Santana MH. In vitro performance of injectable chitosan-tripolyphosphate scaffolds combined with platelet-rich plasma. Tissue Eng Regen Med. 2016;13:21–30. doi: 10.1007/s13770-015-9111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barthes J, Mutschler A, Dollinger C, Gaudinat G, Lavalle P, Le Houerou V, et al. Establishing contact between cell-laden hydrogels and metallic implants with a biomimetic adhesive for cell therapy supported implants. Biomed Mater. 2017;13:015015. doi: 10.1088/1748-605X/aa895b. [DOI] [PubMed] [Google Scholar]
- 36.Song WY, Liu GM, Li J, Luo YG. Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. Tissue Eng Regen Med. 2016;13:750–761. doi: 10.1007/s13770-016-9123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JH, Choi YJ, Yi HG, Wang JH, Cho DW, Jeong YH. A cell-laden hybrid fiber/hydrogel composite for ligament regeneration with improved cell delivery and infiltration. Biomed Mater. 2017;12:055010. doi: 10.1088/1748-605X/aa7b51. [DOI] [PubMed] [Google Scholar]
- 38.Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13:538–546. doi: 10.1007/s13770-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin H, Yan Z, Bauer RJ, Peng J, Schieker M, Nerlich M, et al. Functionalized thermosensitive hydrogel combined with tendon stem/progenitor cells as injectable cell delivery carrier for tendon tissue engineering. Biomed Mater. 2018;13:034107. doi: 10.1088/1748-605X/aaadd1. [DOI] [PubMed] [Google Scholar]
- 40.Kashte S, Jaiswal AK, Kadam S. Artificial bone via bone tissue engineering: current scenario and challenges. Tissue Eng Regen Med. 2017;14:1–14. doi: 10.1007/s13770-016-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Braun AC, Gutmann M, Lühmann T, Meinel L. Bioorthogonal strategies for site-directed decoration of biomaterials with therapeutic proteins. J Control Release. 2018;273:68–85. doi: 10.1016/j.jconrel.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Azagarsamy MA, McKinnon DD, Alge DL, Anseth KS. Coumarin-based photodegradable hydrogel: Design, synthesis, gelation, and degradation kinetics. ACS Macro Lett. 2014;3:515–519. doi: 10.1021/mz500230p. [DOI] [PubMed] [Google Scholar]
- 43.Pérez JM, Cano R, Ramón DJ. Multicomponent azide–alkyne cycloaddition catalyzed by impregnated bimetallic nickel and copper on magnetite. RSC Adv. 2014;4:23943–23951. [Google Scholar]
- 44.Tasdelen MA, Yagci Y. Light-induced click reactions. Angew Chem Int Ed Engl. 2013;52:5930–5938. doi: 10.1002/anie.201208741. [DOI] [PubMed] [Google Scholar]
- 45.Li KW, Cen L, Zhou C, Zhang AK, Yao F, Tan LH, et al. Well-defined poly(ethylene glycol) hydrogels with enhanced mechanical performance prepared by thermally induced copper-catalyzed azide–alkyne cycloaddition. Macromol Mater Eng. 2016;301:1374–1382. [Google Scholar]
- 46.Guo J, Kim GB, Shan D, Kim JP, Hu J, Wang W, et al. Click chemistry improved wet adhesion strength of mussel-inspired citrate-based antimicrobial bioadhesives. Biomaterials. 2017;112:275–286. doi: 10.1016/j.biomaterials.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo J, Meng F, Jing X, Huang Y. Combination of anti-biofouling and ion-interaction by click chemistry for endotoxin selective removal from protein solution. Adv Healthc Mater. 2013;2:784–789. doi: 10.1002/adhm.201200157. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy DC, McKay CS, Legault MC, Danielson DC, Blake JA, Pegoraro AF, et al. Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J Am Chem Soc. 2011;133:17993–18001. doi: 10.1021/ja2083027. [DOI] [PubMed] [Google Scholar]
- 49.Besanceney-Webler C, Jiang H, Zheng T, Feng L, Soriano del Amo D, Wang W, et al. Increasing the efficacy of bioorthogonal click reactions for bioconjugation: a comparative study. Angew Chem Int Ed Engl. 2011;50:8051–8056. doi: 10.1002/anie.201101817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hong V, Presolski SI, Ma C, Finn MG. Analysis and Optimization of Copper-Catalyzed Azide-Alkyne Cycloaddition for Bioconjugation. Angew Chem Int Ed Engl. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lou J, Stowers R, Nam S, Xia Y, Chaudhuri O. Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials. 2018;154:213–222. doi: 10.1016/j.biomaterials.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Yigit S, Sanyal R, Sanyal A. Fabrication and functionalization of hydrogels through “click” chemistry. Chem Asian J. 2011;6:2648–2659. doi: 10.1002/asia.201100440. [DOI] [PubMed] [Google Scholar]
- 53.Yu F, Cao X, Du J, Wang G, Chen X. Multifunctional hydrogel with good structure integrity, self-healing, and tissue-adhesive property formed by combining Diels–Alder click reaction and acylhydrazone bond. ACS Appl Mater Interfaces. 2015;7:24023–24031. doi: 10.1021/acsami.5b06896. [DOI] [PubMed] [Google Scholar]
- 54.Koehler KC, Alge DL, Anseth KS, Bowman CN. A Diels–Alder modulated approach to control and sustain the release of dexamethasone and induce osteogenic differentiation of human mesenchymal stem cells. Biomaterials. 2013;34:4150–4158. doi: 10.1016/j.biomaterials.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nimmo CM, Owen SC, Shoichet MS. Diels-Alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromolecules. 2011;12:824–830. doi: 10.1021/bm101446k. [DOI] [PubMed] [Google Scholar]
- 56.Owen SC, Fisher SA, Tam RY, Nimmo CM, Shoichet MS. Hyaluronic acid click hydrogels emulate the extracellular matrix. Langmuir. 2013;29:7393–7400. doi: 10.1021/la305000w. [DOI] [PubMed] [Google Scholar]
- 57.Fan M, Ma Y, Zhang Z, Mao J, Tan H, Hu X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering. Mater Sci Eng C Mater Biol Appl. 2015;56:311–317. doi: 10.1016/j.msec.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Bai X, Lü S, Cao Z, Gao C, Duan H, Xu X, et al. Self-reinforcing injectable hydrogel with both high water content and mechanical strength for bone repair. Chem Eng J. 2016;288:546–556. [Google Scholar]
- 59.Abandansari HS, Ghanian MH, Varzideh F, Mahmoudi E, Rajabi S, Taheri P, et al. In situ formation of interpenetrating polymer network using sequential thermal and click crosslinking for enhanced retention of transplanted cells. Biomaterials. 2018;170:12–25. doi: 10.1016/j.biomaterials.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 60.Bai X, Lü S, Liu H, Cao Z, Ning P, Wang Z, et al. Polysaccharides based injectable hydrogel compositing bio-glass for cranial bone repair. Carbohydr Polym. 2017;175:557–564. doi: 10.1016/j.carbpol.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 61.Lü S, Bai X, Liu H, Ning P, Wang Z, Gao C, et al. An injectable and self-healing hydrogel with covalent cross-linking in vivo for cranial bone repair. J Mater Chem B. 2017;5:3739–3748. doi: 10.1039/c7tb00776k. [DOI] [PubMed] [Google Scholar]
- 62.Smith LJ, Taimoory SM, Tam RY, Baker AE, Binth Mohammad N, Trant JF, et al. Diels–Alder click-cross-linked hydrogels with increased reactivity enable 3D cell encapsulation. Biomacromolecules. 2018;19:926–935. doi: 10.1021/acs.biomac.7b01715. [DOI] [PubMed] [Google Scholar]
- 63.Prescher JA, Dube DH, Bertozzi CR. Chemical remodelling of cell surfaces in living animals. Nature. 2004;430:873–877. doi: 10.1038/nature02791. [DOI] [PubMed] [Google Scholar]
- 64.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.DeForest CA, Anseth KS. Photoreversible patterning of biomolecules within click-based hydrogels. Angew Chem Int Ed Engl. 2012;51:1816–1819. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kloxin AM, Lewis KJR, DeForest CA, Seedorf G, Tibbitt MW, Balasubramaniam V, et al. Responsive culture platform to examine the influence of microenvironmental geometry on cell function in 3D. Integr Biol (Camb). 2012;4:1540–1549. doi: 10.1039/c2ib20212c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J, Filion TM, Prifti F, Song J. Cytocompatible poly(ethylene glycol)-co-polycarbonate hydrogels cross-linked by copper-free, strain-promoted click chemistry. Chem Asian J. 2011;6:2730–2737. doi: 10.1002/asia.201100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng J, Smith Callahan LA, Hao J, Guo K, Wesdemiotis C, Weiss RA, et al. Strain-promoted cross-linking of PEG-based hydrogels via copper-free cycloaddition. ACS Macro Lett. 2012;1:1071–1073. doi: 10.1021/mz3003775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi A, Suzuki Y, Suhara T, Omichi K, Shimizu A, Hasegawa K, et al. In situ cross-linkable hydrogel of hyaluronan produced via copper-free click chemistry. Biomacromolecules. 2013;14:3581–3588. doi: 10.1021/bm4009606. [DOI] [PubMed] [Google Scholar]
- 70.Jiang H, Qin S, Dong H, Lei Q, Su X, Zhuo R, et al. An injectable and fast-degradable poly (ethylene glycol) hydrogel fabricated via bioorthogonal strain-promoted azide–alkyne cycloaddition click chemistry. Soft Matter. 2015;11:6029–6036. doi: 10.1039/c5sm00508f. [DOI] [PubMed] [Google Scholar]
- 71.Liu X, Miller AL, Fundora KA, Yaszemski MJ, Lu L. Poly(ε-caprolactone) dendrimer cross-linked via metal-free click chemistry: injectable jydrophobic platform for tissue engineering. ACS Macro Lett. 2016;5:1261–1265. doi: 10.1021/acsmacrolett.6b00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang X, Li Z, Shi T, Zhao P, An K, Lin C, et al. Injectable dextran hydrogels fabricated by metal-free click chemistry for cartilage tissue engineering. Mater Sci Eng C Mater Biol Appl. 2017;73:21–30. doi: 10.1016/j.msec.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 73.Han SS, Yoon HY, Yhee JY, Cho MO, Shim HE, Jeong JE, et al. In situ cross-linkable hyaluronic acid hydrogels using copper free click chemistry for cartilage tissue engineering. Polym Chem. 2018;9:20–27. [Google Scholar]
- 74.Rydholm AE, Bowman CN, Anseth KS. Degradable thiol-acrylate photopolymers: polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials. 2005;26:4495–4506. doi: 10.1016/j.biomaterials.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 75.Xi W, Scott TF, Kloxin CJ, Bowman CN. Click chemistry in materials science. Adv Funct Mater. 2014;24:2572–2590. [Google Scholar]
- 76.Lin CC, Raza A, Shih H. PEG hydrogels formed by thiol-ene photo-click chemistry and their effect on the formation and recovery of insulin-secreting cell spheroids. Biomaterials. 2011;32:9685–9695. doi: 10.1016/j.biomaterials.2011.08.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tseng TC, Hsieh FY, Theato P, Wei Y, Hsu SH. Glucose-sensitive self-healing hydrogel as sacrificial materials to fabricate vascularized constructs. Biomaterials. 2017;133:20–28. doi: 10.1016/j.biomaterials.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 78.Brown TE, Carberry BJ, Worrell BT, Dudaryeva OY, McBride MK, Bowman CN, et al. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials. 2018;178:496–503. doi: 10.1016/j.biomaterials.2018.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pereira RF, Barrias CC, Bártolo PJ, Granja PL. Cell-instructive pectin hydrogels crosslinked via thiol-norbornene photo-click chemistry for skin tissue engineering. Acta Biomater. 2018;66:282–293. doi: 10.1016/j.actbio.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 80.Colak B, Di Cio S, Gautrot JE. Biofunctionalized patterned polymer brushes via thiol–ene coupling for the control of cell adhesion and the formation of cell arrays. Biomacromolecules. 2018;19:1445–1455. doi: 10.1021/acs.biomac.7b01436. [DOI] [PubMed] [Google Scholar]
- 81.Sharma S, Floren M, Ding Y, Stenmark KR, Tan W, Bryant SJ. A photoclickable peptide microarray platform for facile and rapid screening of 3-D tissue microenvironments. Biomaterials. 2017;143:17–28. doi: 10.1016/j.biomaterials.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 82.Ding Y, Xu X, Sharma S, Floren M, Stenmark K, Bryant SJ, et al. Biomimetic soft fibrous hydrogels for contractile and pharmacologically responsive smooth muscle. Acta Biomater. 2018;74:121–130. doi: 10.1016/j.actbio.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Zhao S, Zhang C, Liang K, Li J, Yang H, et al. Photopolymerized maleilated chitosan/thiol-terminated poly(vinyl alcohol) hydrogels as potential tissue engineering scaffolds. Carbohydr Polym. 2018;184:383–389. doi: 10.1016/j.carbpol.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Kalia J, Raines RT. Hydrolytic stability of hydrazones and oximes. Angew Chem Int Ed Engl. 2008;47:7523–7636. doi: 10.1002/anie.200802651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Christman KL, Broyer RM, Schopf E, Kolodziej CM, Chen Y, Maynard HD. Protein nanopatterns by oxime bond formation. Langmuir. 2011;27:1415–1418. doi: 10.1021/la103978x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. Visualizing enveloping layer glycans during zebrafish early embryogenesis. Proc Natl Acad Sci U S A. 2010;107:10360–10365. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hentzen NB, Smeenk LEJ, Witek J, Riniker S, Wennemers H. Cross-linked collagen triple helices by oxime ligation. J Am Chem Soc. 2017;139:12815–12820. doi: 10.1021/jacs.7b07498. [DOI] [PubMed] [Google Scholar]
- 88.Tamura T, Song Z, Amaike K, Lee S, Yin S, Kiyonaka S, et al. Affinity-guided oxime chemistry for selective protein acylation in live tissue systems. J Am Chem Soc. 2017;139:14181–14191. doi: 10.1021/jacs.7b07339. [DOI] [PubMed] [Google Scholar]
- 89.Mather BD, Viswanatan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31:487–531. [Google Scholar]
- 90.Chatani S, Nair DP, Bowman CN. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol-Michael addition reaction and polymerization. Polym Chem. 2013;4:1048–1055. [Google Scholar]
- 91.Hoyle CE, Lowe AB, Bowman CN. Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chem Soc Rev. 2010;39:1355–1387. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- 92.Li GZ, Randev RK, Soeriyadi AH, Rees G, Boyer C, Tong Z, et al. Investigation into thiol-(meth)acrylate michael addition reactions using amine and phosphine catalysts. Polym Chem. 2010;1:1196–1204. [Google Scholar]
- 93.Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- 94.Kim MS, Choi YJ, Noh I, Tae G. Synthesis and characterization of in situ chitosan-based hydrogel via grafting of carboxyethyl acrylate. J Biomed Mater Res A. 2007;83:674–682. doi: 10.1002/jbm.a.31278. [DOI] [PubMed] [Google Scholar]
- 95.Yu Y, Deng C, Meng F, Shi Q, Feijen J, Zhong Z. Novel injectable biodegradable glycol chitosan-based hydrogels crosslinked by Michael-type addition reaction with oligo(acryloyl carbonate)-b-poly(ethylene glycol)-b-oligo(acryloyl carbonate) copolymers. J Biomed Mater Res A. 2011;99:316–326. doi: 10.1002/jbm.a.33199. [DOI] [PubMed] [Google Scholar]
- 96.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–7845. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 98.Jin R, Moreira Teixeira LS, Krouwels A, Dijkstra PJ, van Blitterswijk CA, Karperien M, et al. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6:1968–1977. doi: 10.1016/j.actbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 99.Baldwin AD, Kiick KL. Reversible maleimide-thiol adducts yield glutathione-sensitive poly(ethylene glycol)-heparin hydrogels. Polym Chem. 2013;4:133–143. doi: 10.1039/C2PY20576A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bang S, Jung UW, Noh I. Synthesis and biocompatibility characterizations of in situ chondroitin sulfate–gelatin hydrogel for tissue engineering. Tissue Eng Regen Med. 2018;15:25–35. doi: 10.1007/s13770-017-0089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 102.Tian WM, Zhang CL, Hou SP, Yu X, Cui FZ, Xu QY, et al. Hyaluronic acid hydrogel as Nogo-66 receptor antibody delivery system for the repairing of injured rat brain: in vitro. J Control Release. 2005;102:13–22. doi: 10.1016/j.jconrel.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 103.Dahlmann J, Krause A, Möller L, Kensah G, Möwes M, Diekmann A, et al. Fully defined in situ cross-linkable alginate and hyaluronic acid hydrogels for myocardial tissue engineering. Biomaterials. 2013;34:940–951. doi: 10.1016/j.biomaterials.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 104.Martínez-Sanz E, Ossipov DA, Hilborn J, Larsson S, Jonsson KB, Varghese OP. Bone reservoir: injectable hyaluronic acid hydrogel for minimal invasive bone augmentation. J Control Release. 2011;152:232–240. doi: 10.1016/j.jconrel.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 105.Karvinen J, Joki T, Ylä-Outinen L, Koivisto JT, Narkilahti S, Kellomäki M. Soft hydrazone crosslinked hyaluronan- and alginate-based hydrogels as 3D supportive matrices for human pluripotent stem cell-derived neuronal cells. React Funct Polym. 2018;124:29–39. [Google Scholar]
- 106.Alves MH, Young CJ, Bozzetto K, Poole-Warren LA, Martens PJ. Degradable, click poly(vinyl alcohol) hydrogels: characterization of degradation and cellular compatibility. Biomed Mater. 2012;7:024106. doi: 10.1088/1748-6041/7/2/024106. [DOI] [PubMed] [Google Scholar]
- 107.Zhu D, Wang H, Trinh P, Heilshorn SC, Yang F. Elastin-like protein-hyaluronic acid (ELP-HA) hydrogels with decoupled mechanical and biochemical cues for cartilage regeneration. Biomaterials. 2017;127:132–140. doi: 10.1016/j.biomaterials.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krishna UM, Martinez AW, Caves JM, Chaikof EL. Hydrazone self-crosslinking of multiphase elastin-like block copolymer networks. Acta Biomater. 2012;8:988–997. doi: 10.1016/j.actbio.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li S, Xia Y, Qiu Y, Chen X, Shi S. Preparation and property of starch nanoparticles reinforced aldehyde–hydrazide covalently crosslinked PNIPAM hydrogels. J Appl Polym Sci. 2018;135:45761. [Google Scholar]
- 110.Patenaude M, Hoare T. Injectable, mixed natural-synthetic polymer hydrogels with modular properties. Biomacromolecules. 2012;13:369–378. doi: 10.1021/bm2013982. [DOI] [PubMed] [Google Scholar]
- 111.Wang LL, Highley CB, Yeh YC, Galarraga JH, Uman S, Burdick JA. 3D extrusion bioprinting of single-and double-network hydrogels containing dynamic covalent crosslinks. J Biomed Mater Res A. 2018;106:865–875. doi: 10.1002/jbm.a.36323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yan HJ, Casalini T, Hulsart-Billström G, Wang S, Oommen OP, Salvalaglio M, et al. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials. 2018;161:190–202. doi: 10.1016/j.biomaterials.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 113.Huang J, Jiang X. Injectable and degradable pH-responsive hydrogel via spontaneous amino-yne click reaction. ACS Appl Mater Interfaces. 2018;10:361–370. doi: 10.1021/acsami.7b18141. [DOI] [PubMed] [Google Scholar]
- 114.Aioub AG, Dahora L, Gamble K, Finn MG. Selection of natural peptide ligands for copper-catalyzed azide–alkyne cycloaddition catalysis. Bioconjug Chem. 2017;28:1693–1701. doi: 10.1021/acs.bioconjchem.7b00161. [DOI] [PubMed] [Google Scholar]
- 115.Jang J, Park JY, Gao G, Cho DW. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials. 2018;156:88–106. doi: 10.1016/j.biomaterials.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 116.Kurzrock R, Stewart DJ. Click chemistry, 3D-printing, and omics: the future of drug development. Oncotarget. 2016;7:2155–2158. doi: 10.18632/oncotarget.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Symes MD, Kitson PJ, Yan J, Richmond CJ, Cooper GJ, Bowman RW, et al. Integrated 3D-printed reactionware for chemical synthesis and analysis. Nat Chem. 2012;4:349–354. doi: 10.1038/nchem.1313. [DOI] [PubMed] [Google Scholar]
- 118.Içten E, Giridhar A, Taylor LS, Nagy ZK, Reklaitis GV. Dropwise additive manufacturing of pharmaceutical products for melt-based dosage forms. J Pharm Sci Exp Pharmacol. 2015;104:1641–1649. doi: 10.1002/jps.24367. [DOI] [PubMed] [Google Scholar]
- 119.Bertlein S, Brown G, Lim KS, Jungst T, Boeck T, Blunk T, et al. Thiol–ene clickable gelatin: a platform bioink for multiple 3D biofabrication technologies. Adv Mater. 2017;29:1703404. doi: 10.1002/adma.201703404. [DOI] [PubMed] [Google Scholar]
- 120.Li S, Xu Y, Yu J, Becker ML. Enhanced osteogenic activity of poly(ester urea) scaffolds using facile post-3D printing peptide functionalization strategies. Biomaterials. 2017;141:176–187. doi: 10.1016/j.biomaterials.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 121.Stichler S, Jungst T, Schamel M, Zilkowski I, Kuhlmann M, Böck T, et al. Thiol-ene clickable polyglycidol hydrogels for biofabrication. Ann Biomed Eng. 2017;45:273–285. doi: 10.1007/s10439-016-1633-3. [DOI] [PubMed] [Google Scholar]
- 122.Yin R, Zhang N, Wang K, Long H, Xing T, Nie J, et al. Material design and photo-regulated hydrolytic degradation behavior of tissue engineering scaffolds fabricated via 3D fiber deposition. J Mater Chem B. 2017;5:329–340. doi: 10.1039/c6tb02884e. [DOI] [PubMed] [Google Scholar]
- 123.Leijten J, Seo J, Yue K, Santiago GT, Tamayol A, Ruiz-Esparza GU, et al. Spatially and temporally controlled hydrogels for tissue engineering. Mater Sci Eng R Rep. 2017;119:1–35. doi: 10.1016/j.mser.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yeh YC, Ouyang L, Highley CB, Burdick JA. Norbornene-modified poly (glycerol sebacate) as a photocurable and biodegradable elastomer. Polym Chem. 2017;8:5091–5099. [Google Scholar]
- 125.Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.You S, Li J, Zhu W, Yu C, Mei D, Chen S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. J Mater Chem B. 2018;6:2187–2197. doi: 10.1039/C8TB00301G. [DOI] [PMC free article] [PubMed] [Google Scholar]