Abstract
Background:
Several injectable hydrogels have been developed extensively for a broad range of biomedical applications. Injectable hydrogels forming in situ through the change in external stimuli have the distinct properties of easy management and minimal invasiveness, and thus provide the advantage of bypassing surgical procedures for administration resulting in better patient compliance.
Methods:
The injectable in situ-forming hydrogels can be formed irreversibly or reversibly under physiological stimuli. Among several external stimuli that induce formation of hydrogels in situ, in this review, we focused on the electrostatic interactions as the most simple and interesting stimulus.
Results:
Currently, numerous polyelectrolytes have been reported as potential electrostatically interactive in situ-forming hydrogels. In this review, a comprehensive overview of the rapidly developing electrostatically interactive in situ-forming hydrogels, which are produced by various anionic and cationic polyelectrolytes such as chitosan, celluloses, and alginates, has been outlined and summarized. Further, their biomedical applications have also been discussed.
Conclusion:
The review concludes with perspectives on the future of electrostatically interactive in situ-forming hydrogels.
Keywords: Electrostatic interactions, In situ-forming hydrogels, Injectable, Drug delivery, Regenerative medicine
Introduction
Several hydrogels have been developed extensively for a broad range of biomedical applications [1–5]. Most of the hydrogels are composed of hydrophilic materials to form 3-dimensional networks. The 3-dimensional hydrogel networks can be prepared through chemical or physical cross-linking. The hydrogels can generally absorb a large amount of water [6]. Most hydrogels have been prepared by ex vivo prefabrication by pre-designing using synthetic or natural materials.
Recently, biomedical applications of in situ-forming hydrogels have been extensively investigated by hydrogel formation at injected body sites [7–10]. The design of in situ-forming hydrogels is based on the concept that hydrogel materials are prepared as solutions at ambient temperature and then become in situ hydrogels at body temperature.
These hydrogel formulations have an advantage of easy preparation by incorporating several biological factors, such as various cells and growth factors, by simple mixing. Additionally, in situ-forming hydrogel formulations can be easily injected by minimal invasiveness using syringe, and thus, provide the advantage of bypassing surgical procedures for administration resulting in better patient compliance.
The in situ-forming hydrogels can be formed irreversibly or reversibly under physiological stimuli [11–14]. The irreversible in situ-forming hydrogels are prepared by irreversible chemical reactions between functional groups on hydrogels and externally added materials. Meanwhile, reversible hydrogels are prepared by reversible physical stimuli such as temperature, light, electricity, pressure, and sound or reversible interaction between specific functional groups.
In situ-forming hydrogels to respond to temperature among several reversible physical stimuli fall into two main categories: hydrophobic and electrostatic interactions. The formation of reversible in situ-forming hydrogels through hydrophobic interactions between hydrophobic molecules is alternative to irreversible in situ-forming hydrogels. One drawback of in situ-forming hydrogels via hydrophobic interactions is the difficulty in preparing the materials as an aqueous solution.
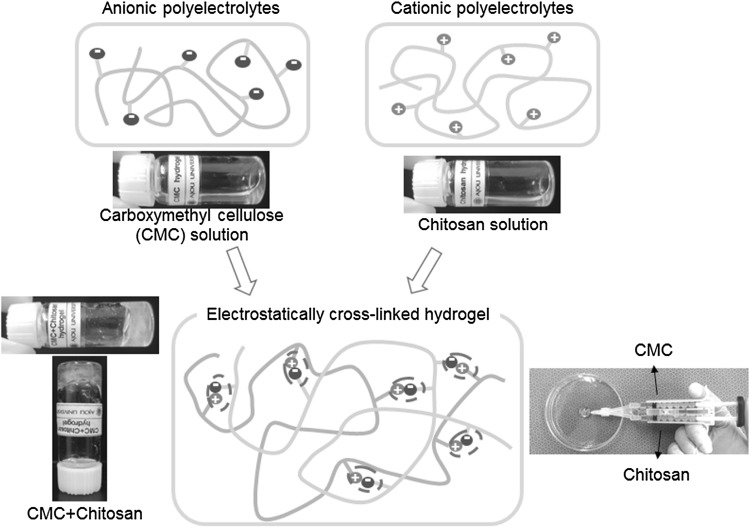
Meanwhile, an electrostatic interaction between charges on certain materials can also create reversible in situ-forming hydrogels [15–18]. The electrostatic materials can be easily prepared as an aqueous solution. The electrostatic material alone without materials of different ionic species allow solution under physiological conditions. The mixing with different ionic materials can easily induce the formation of hydrogel via reversible electrostatic interaction between oppositely charged ionic materials under physiological conditions (Fig. 1).
Fig. 1.
Schematic representative image of electrostatically interactive injectable hydrogels via electrostatic interactions between anionic (e.g. carboxylmethyl cellulose) and cationic (e.g. chitosan) polyelectrolytes. (Image was drawn by Y.B.J. and H.J.J. in the Adobe Photoshop 7.0 software)
This concept is considered in the selection or design of various biomaterials for the formation of electrostatically interactive in situ-forming hydrogels [19–22]. Here, we present a comprehensive and detailed overview of electrostatically interactive in situ-forming hydrogels. We firstly discuss the concept of electrostatically interactive in situ-forming hydrogels, followed by the discussion on potential candidate materials for forming hydrogels and their applications in the biomedical field. Finally, a perspective about future uses of electrostatically interactive in situ-forming hydrogels has been discussed.
Electrostatically interactive in situ-forming hydrogels: concept and mechanism
In the electrostatically interactive in situ-forming hydrogels, certain materials at room temperature show little or no electrostatic interaction, resulting in the formation of homogeneous solutions. However, with the increase in temperature, electrostatic interaction of certain materials can either form a coacervate or a complex [23]. Hence, the electrostatic interaction should increase to produce viscous and macroscopical hydrogel at body temperature. However, if the electrostatic interaction is too strong, precipitation of certain materials can occur.
Based on this concept, an electrostatic interaction between cationic and anionic electric charges is one of the simple and primary driving forces in the formation of electrostatically interactive in situ-forming hydrogels. Hence, to induce electrostatic interaction, certain materials must be ionized or should bear electric charges, then only electrostatic interactions between opposing charges on certain materials will create reproducible ionic cross-linking.
The formation of electrostatic interactions is influenced by several factors [24]. The properties of electrostatic interactions in the mixture of certain materials can depend primarily on the amount of interactions or ratios between opposite charges.
Firstly, the overall charges in hydrogel network structures can be affected by the molecular weight. Single molecules as electrolytes have one or few electric charges. Meanwhile the ionic polymers as polyelectrolytes are polymerized by monomeric molecules with electric charges. Thus, polyelectrolytes with high molecular weights have large amount of electric charges. The extent of an electrostatic interaction depends significantly on gross densities of electric charges in the polyelectrolytes. The higher the molecular weight of the polyelectrolytes, the higher is the gross charge density, thus the higher the strength of the electrostatic interaction.
Secondly, the electrostatic interaction can be affected by the mobility or flexibility of polyelectrolyte materials. High flexibility can increase the mobility resulting in the increased probability of electrostatic interaction. However, high flexibility can weaken the hydrogel due to the fast mobility of polyelectrolyte backbones. Consequently, the extent of electrostatic interaction depends significantly on the appropriate mobility or flexibility of the polyelectrolytes.
Additionally, since pH changes allow a change in electric charges of polyelectrolytes, the electrostatically interactive in situ-forming hydrogels are affected by pH. The extent of an electrostatic interaction offset reinforces electrostatic interaction in polyelectrolytes according to pH changes under physiological conditions. Thus, there is an optimal pH at which electrostatic interactions allow the formation of the strongest electrostatically interactive in situ-forming hydrogels.
Consequently, the properties of electrostatically interactive in situ-forming hydrogels can offset and reinforce by control of the charge density, mobility or pH changes under physiological conditions.
Materials for electrostatically interactive in situ-forming hydrogels
Recent studies have shown that electrostatically interactive in situ-forming hydrogels are produced by various anionic and cationic polyelectrolytes, such as chitosan, celluloses, and alginates, which are well known to form these hydrogels and are described in the sections below.
Chitosan
Chitin is one of the most popular natural polymers derived from the protective shells of crustaceans. Through alkaline deacetylation of chitin, amine group is added on chitin, forming chitosan; chitosan is available commercially. As deacetylation ratios are above approximately 50%, chitosan becomes soluble by protonation under acidic conditions. Chitosan chains are linear polysaccharides with primary aliphatic amines. Protonated chitosan acts as a pseudonatural cationic polymer in acidic media. The primary ammonium salts on the chitosan chains exhibit properties of typical cationic polyelectrolyte. The ionizable primary ammonium salts on chitosan show the positive zeta potentials [25].
The cationic chitosan polyelectrolyte can interact suitably with anionic materials to produce electrostatically interactive hydrogels. The electrostatically interactive hydrogels can be obtained in various shapes and forms such as gels, powders, beads, films, tablets, capsules, microspheres, microparticles, sponges, nanofibrils, or textile fibers [26–29].
The cationic chitosan polyelectrolyte can interact electrostatically with anionic materials, such as electrolytes including carboxylate salts and phosphate salts with mono or multi anionic groups, and polyelectrolytes including anionic polysaccharides and synthetic anionic polymers with multi anionic groups.
If little or no electrostatic interaction occurs between chitosan and anionic materials at room temperature, their mixture forms a solution. If proper electrostatic interaction occurs at the body temperature, the solution can form electrostatically interactive in situ-forming chitosan hydrogel.
The property of the electrostatic interactions between cationic chitosan polyelectrolyte and anionic materials depends on the density, strength and ratios of ionic charge, and is influenced by solvent, pH, and temperature, as well as the extent of deacetylation and concentration of chitosan [30–33]. The anionic electrolytes freely diffuse throughout the cationic chitosan polyelectrolyte to rapidly form or destroy electrostatic cross-linking. Meanwhile, anionic polyelectrolytes slowly diffuse throughout the cationic chitosan polyelectrolyte to slowly form electrostatically interactive in situ-forming chitosan hydrogel, resulting in relatively strong electrostatic cross-linking.
Consequently, the properties of the formed electrostatically interactive in situ-forming chitosan hydrogel depend on the charge density, molecular weight, pH and mixing ratios between the anionic and cationic chitosan polyelectrolyte.
The formulation of cationic chitosan polyelectrolytes with anionic polyelectrolytes can be prepared as solution to permit easy incorporation of several biological factors and cells, and then injected at target tissue sites using syringe, resulting in the formation of electrostatically interactive in situ-forming chitosan hydrogel. The formed electrostatically interactive in situ-forming chitosan hydrogel shows high interconnected network structures to foster cells and tissues at target sites. The degradation of electrostatically interactive in situ-forming chitosan hydrogel occurs mainly through ion exchange by in vivo anionic biological materials or enzyme action [34, 35].
Cellulose derivatives
Celluloses are polysaccharides consisting of a linear chain of several hundred to thousands of glucose units. Celluloses are the most abundant organic biopolymers derived from green plants, many forms of algae, and oomycetes. Thus, they are considered less toxic, biodegradable, environmental-friendly raw biomaterials, and commercially available products.
Carboxymethyl cellulose (CMC) contains carboxylic acid (COOH) groups bound to some of the hydroxyl groups of the cellulose backbone. CMC has been widely used in biomedical and pharmaceutical applications. The carboxylic acid on a conformationally semi-rigid CMC can act as anion group and thus may interact electrostatically with cations [36–39].
Several polyelectrolytes with cationic groups, such as polyethyleneimine, poly(vinyl amine), and chitosan, can interact suitably with CMC resulting in the formation of electrostatically interactive in situ-forming CMC hydrogel [40–42]. In addition, several metal cations such as silver, aluminum, lead, iron, and copper have been used to form electrostatically interactive in situ-forming CMC hydrogel [43, 44].
The electrostatic interactions between anionic CMC and cationic polyelectrolytes are also affected by the ionic strength, molecular weight, chemical compositions and their mixing ratios.
Alginate
Alginates, which are derived from brown algae, are one of the most studied and broadly used materials for many biomedical applications. Additionally, alginates are biocompatible, less toxic, and have relatively low cost as commercially available products. Alginates are linear polysaccharide copolymers with anionic carboxyl groups, which are usually replaced by the monovalent sodium cations.
Anionic alginate polyelectrolyte can interact electrostatically with electrolytes of divalent cations such as calcium, strontium, and barium, or multivalent cations to form an egg-box structure via the anionic stacking on guluronic groups [45–47]. Among divalent cations, magnesium ions do not induce electrostatic interaction.
The formation rate of electrostatically interactive in situ-forming alginate hydrogel depends on alginate concentration and cation type [48]. The gelation by divalent cations is a significant factor in manipulation of the strength of electrostatically interactive in situ-forming alginate hydrogel [49]. Calcium chloride is the most common divalent cation to rapidly form electrostatic cross-linking. However, rapid hydrogelation by calcium chloride causes weak mechanical integrity in irregular structures. Additionally, calcium chloride has high solubility in aqueous solutions and thus is difficult to use during controlled gelation. Calcium sulfate and calcium carbonate have lower water solubilities and thus can slow hydrogelation, resulting in the high mechanical integrity and uniform structures of electrostatically interactive in situ-forming alginate hydrogel [50].
Furthermore, alginate can interact electrostatically with polyelectrolytes such as chitosan, pectin, and ethyl cellulose to form electrostatically interactive in situ-forming alginate hydrogel [51, 52]. The electrostatic hydrogelation is affected by mobility of the polyelectrolytes. The slow electrostatic interaction between alginate and polyelectrolytes forms relative strong electrostatic cross-linking.
Other electrostatically interactive in situ-forming hydrogels
Polyampholytes, with both cationic and anionic repeat groups, which are called zwitterionic groups, can electrostatically interact or repel. There are typical polyampholytes with phosphobetaine, sulfobetaine, and carboxybetaine as zwitterionic groups [53–55]. The polyampholyte solutions can make electrostatically interactive in situ-forming hydrogels. The electrostatic interactions between cationic- and anionic-inside polyampholytes are affected by the native properties and concentration of the zwitterionic groups.
Electrostatically interactive in situ-forming hydrogels: biomedical application
Some electrostatically interactive in situ-forming hydrogels can be utilized in drug delivery systems to sustain therapeutic drug release to treat some diseases.
Cancer is one of the most aggressive diseases leading to death. Most patients undergo surgery initially for removal of cancerous tissues and then are treated with chemotherapeutic agents. The treatment with chemotherapeutic agents has several disadvantages such as poor distribution to the target tumor sites and several toxic side effects, resulting in the nonspecific distribution to healthy normal tissues. Meanwhile, intratumoral injection of chemotherapeutic agents can achieve a high local concentration of the chemotherapeutic agents at the target tumor site with no or minimal distribution to healthy normal tissues [56–58]. The electrostatically interactive in situ-forming hydrogel formulation with chemotherapeutic agents is expected to be easily injected through a syringe needle.
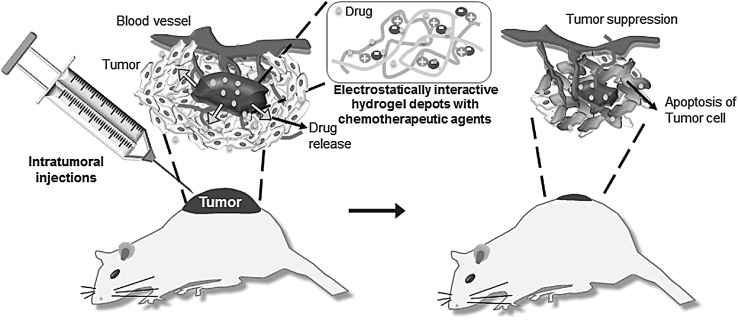
The formulations for intratumoral injection can be prepared easily by mixing of chemotherapeutic agents with electrostatically interactive in situ-forming hydrogel and injected directly into the tumor of xenografted animals (Fig. 2). Intratumoral injection of hydrogel formulation with the chemotherapeutic agents forms a depot at the target tumor site. Thus, the electrostatically formed hydrogel depot with chemotherapeutic agents can achieve a high local concentration inside the target tumor and thus improve efficacy, reduce systemic concentration of the chemotherapeutic agents, and decrease the incidence of side effects compared with traditional administration of the chemotherapeutic agents.
Fig. 2.
Schematic representative image for intratumoral injections of electrostatically interactive injectable hydrogels. (Image was drawn by Y.B.J. and H.J.J. in the Adobe Photoshop 7.0 software)
Rheumatoid arthritis (RA) is a chronic inflammatory disease with a complex multifactorial pathogenesis and a long-lasting, progressive, and irreversible autoimmune disorder in articular joints and bone, resulting in the progressive destruction of articular joints. Several RA drugs are used to slow RA progression and prevent or reduce joint damage. However, the repeat administration of RA drugs has shown adverse effects for gastrointestinal, hematological, pulmonary, and hepatic toxicity. Thus, the intra-articular injection of RA drugs can minimize adverse and side effects by maximizing local anti-inflammatory effects at the injected articular joint site with no or little distribution to healthy normal tissues [59]. The intra-articular injection of the RA drug alone shows comparably rapid clearance of the RA drug at the injected synovial location.
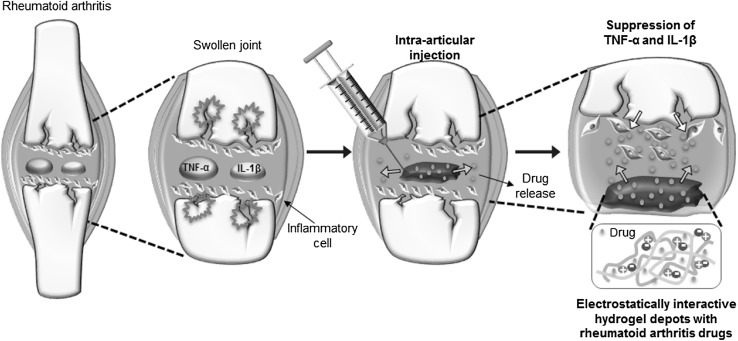
Meanwhile, the electrostatically interactive in situ-forming hydrogel formulation with RA drugs is expected to easily produce depots in the articular joint through a syringe needle (Fig. 3) The intra-articular injection of formulation with RA drugs successfully provides the RA drug depot at the target articular joint. The formed electrostatically interactive in situ-forming hydrogel depots show the long-lasting release of RA drugs in the target articular joint. Thus, RA animals injected intra-articularly with the electrostatically interactive in situ-forming hydrogel formulation and RA drugs suppress TNF-α and IL-1β expression in the articular knee joint [60–62].
Fig. 3.
Schematic representative image for intra-articular injection of electrostatically interactive injectable hydrogels. (Image was drawn by Y.B.J. and H.J.J. in the Adobe Photoshop 7.0 software)
Diabetes mellitus, characterized by hyperglycemia, is a worldwide public health disease. Especially patients with type 1 diabetes have been treated by regular subcutaneous insulin injection. This treatment procedure is quite inconvenient, painful, and with low patient compliance. Additionally, alternative routes such as dermal, rectal, ocular, oral, nasal, and pulmonary methods have been developed, but show low bioavailability of insulin [63]. Meanwhile, electrostatically interactive in situ-forming hydrogel formulation with insulin is expected to easily produce depots after injection through a syringe needle [64–66]. The depots are able to sustain insulin in response to blood glucose levels.
Electrostatically interactive in situ-forming hydrogels can be utilized in tissue engineering. They can easily incorporate various cells and biological factors, such as growth factors, by simple mixing [67]. The electrostatically interactive in situ-forming hydrogel formulation with cells is expected to easily produce hydrogel scaffolds after injection through a syringe needle.
One of the main concerns in the formed hydrogel scaffolds is the long-term survival of the loaded cells in the injected host site. Most of the electrostatically interactive in situ-forming hydrogels show a three-dimensional interconnected structure to support growth of the loaded cells to form tissue, and enable the diffusion of nutrients and growth factors inside hydrogel scaffolds.
Currently, basic and preclinical researches of electrostatically interactive in situ-forming hydrogels using various cells, like stem cells, have been developed for the treatment of damaged or diseased tissue in animal models for bone and cartilage regeneration and vascular autografts. Although a number of electrostatically interactive in situ-forming hydrogels are evaluated, the clinical research must continue to elucidate the appropriate tissue development inside the hydrogel scaffold.
Despite the potential biomedical applications for these electrostatically interactive in situ-forming hydrogels, quality assurances of various polyelectrolytes are required to fully expand their in vivo biomedical application. Additionally, it is noteworthy to say that the mechanical property and biodegradability of electrostatically interactive in situ-forming hydrogels should reflect features of the extracellular matrices under physiological conditions. The mechanical strength and biodegradability are very important when electrostatically interactive in situ-forming hydrogels are applied as drug depot or scaffold. The degradation rate of drug depot or scaffold needs to be correlated with maintaining of mechanical strength during the appropriate time frame to sustain drug release or guide the neo-tissue formation in damaged or diseased tissue. The in vivo degradation of electrostatically interactive in situ-forming hydrogels depends on the charge density, molecular weight, pH and mixing ratios between the anionic and cationic polyelectrolytes. Thus the electrostatically interactive in situ-forming hydrogels designed to in vivo persist from few days to few months are a better option for biomedical application.
Summary and outlook of electrostatically interactive in situ-forming hydrogels
Research on electrostatically interactive in situ-forming hydrogels is increasingly gaining attention for biomedical applications. Presently, these hydrogels have been successfully applied in animal models. The important research for enabling human applications must focus on easy handing, sterilization, injectability, and in vivo depot formation rate of electrostatically interactive in situ-forming hydrogels. Furthermore, the human applicable goals for the development of these hydrogels minimize immune response. Thus, ideal electrostatically interactive in situ-forming hydrogels must induce transplant tolerance or minimal side effects. Additionally, detailed and accurate investigation of these hydrogels is needed in the clinically applicable issues such as immune response, host tissue integration, and target tissue regeneration. Ultimately, researchers in various fields such as pharmaceutical, material sciences, and biotechnology must put in a united effort to develop and realize the electrostatically interactive in situ-forming hydrogels.
Acknowledgement
This work was supported by the Pukyong National University Research Abroad Fund in 2014 (C-D-2014-0713).
Conflicts of interest
The authors have no financial conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Zhang Z. Injectable biomaterials for stem cell delivery and tissue regeneration. Expert Opin Biol Ther. 2016;17:49–62. doi: 10.1080/14712598.2017.1256389. [DOI] [PubMed] [Google Scholar]
- 2.Kim DY, Kwon DY, Kwon JS, Kim JH, Min BH, Kim MS. Stimuli-responsive injectable in situ-forming hydrogels for regenerative medicines. Polym Rev (Phila Pa) 2015;55:407–452. doi: 10.1080/15583724.2014.983244. [DOI] [Google Scholar]
- 3.Yang J, Zhang YS, Yue K, Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luca A, Butnaru M, Maier SS, Knieling L, Bredetean O, Verestiuc L, et al. Atelocollagen-based hydrogels crosslinked with oxidised polysaccharides as cell encapsulation matrix for engineered bioactive stromal tissue. Tissue Eng Regen Med. 2017;14:539–556. doi: 10.1007/s13770-017-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia T, Liu W, Yang L. A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine. J Biomed Mater Res A. 2017;105:1799–1812. doi: 10.1002/jbm.a.36034. [DOI] [PubMed] [Google Scholar]
- 6.Kim H, Jeong H, Han S, Beack S, Hwang BW, Shin M, et al. Hyaluronate and its derivatives for customized biomedical applications. Biomaterials. 2017;123:155–171. doi: 10.1016/j.biomaterials.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 7.Vedadghavami A, Minooei F, Mohammadi MH, Khetani S, Rezaei Kolahchi A, Mashayekhan S, et al. Manufacturing of hydrogel biomaterials with controlled mechanical properties for tissue engineering applications. Acta Biomater. 2017;62:42–63. doi: 10.1016/j.actbio.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Zhou K, Ghosh D, Jha NN, Singh PK, Jacob RS, et al. Implantable amyloid hydrogels for promoting stem cell differentiation to neurons. NPG Asia Mater. 2016;8:e304. doi: 10.1038/am.2016.116. [DOI] [Google Scholar]
- 9.Jin GZ, Kim HW. Effects of type I collagen concentration in hydrogel on the growth and phenotypic expression of rat chondrocytes. Tissue Eng Regen Med. 2017;14:383–391. doi: 10.1007/s13770-017-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae JW, Choi JH, Lee Y, Park KD. Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J Tissue Eng Regen Med. 2015;9:1225–1232. doi: 10.1002/term.1917. [DOI] [PubMed] [Google Scholar]
- 11.Van Nieuwenhove I, Tytgat L, Ryx M, Blondeel P, Stillaert F, Thienpont H, et al. Soft tissue fillers for adipose tissue regeneration: From hydrogel development toward clinical applications. Acta Biomater. 2017;63:37–49. doi: 10.1016/j.actbio.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Saludas L, Pascual-Gil S, Prósper F, Garbayo E, Blanco-Prieto M. Hydrogel based approaches for cardiac tissue engineering. Int J Pharm. 2017;523:454–475. doi: 10.1016/j.ijpharm.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 13.Song WY, Liu GM, Li J, Luo YG. Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. Tissue Eng Regen Med. 2016;13:750–761. doi: 10.1007/s13770-016-9123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Z, Gao X, Ullah MW, Li S, Wang Q, Yang G. Electroconductive natural polymer-based hydrogels. Biomaterials. 2016;111:40–54. doi: 10.1016/j.biomaterials.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhao LZ, Zhou CH, Wang J, Tong DS, Yu WH, Wang H. Recent advances in clay mineral-containing nanocomposite hydrogels. Soft Matter. 2015;11:9229–9246. doi: 10.1039/C5SM01277E. [DOI] [PubMed] [Google Scholar]
- 16.Park SH, Kim DY, Panta P, Heo JY, Lee HY, Kim JH, et al. An intratumoral injectable, electrostatic, cross-linkable curcumin depot and synergistic enhancement of anticancer activity. NPG Asia Mater. 2017;9:e397. doi: 10.1038/am.2017.102. [DOI] [Google Scholar]
- 17.Lee JY, Kang YM, Kim ES, Kang ML, Lee B, Kim JH, et al. In vitro and in vivo release of albumin from an electrostatically crosslinked in situ-forming gel. J Mater Chem. 2010;20:3265–3271. doi: 10.1039/b922614a. [DOI] [Google Scholar]
- 18.Shinya S, Fukamizo T. Interaction between chitosan and its related enzymes: a review. Int J Biol Macromol. 2017;104:1422–1435. doi: 10.1016/j.ijbiomac.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira NM, Reis RL, Mano JF. The potential of liquid marbles for biomedical applications: a critical review. Adv Healthc Mater. 2017;6:1700192. doi: 10.1002/adhm.201700192. [DOI] [PubMed] [Google Scholar]
- 20.Cho KH, Singh B, Maharjan S, Jang Y, Choi YJ, Cho CS. Local delivery of CTGF siRNA with poly(sorbitol-co-PEI) reduces scar contraction in cutaneous wound healing. Tissue Eng Regen Med. 2017;14:211–220. doi: 10.1007/s13770-017-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borges J, Mano JF. Molecular interactions driving the layer-by-layer assembly of multilayers. Chem Rev. 2014;114:8883–8942. doi: 10.1021/cr400531v. [DOI] [PubMed] [Google Scholar]
- 22.Raftery R, O’Brien FJ, Cryan SA. Chitosan for gene delivery and orthopedic tissue engineering applications. Molecules. 2013;18:5611–5647. doi: 10.3390/molecules18055611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jho Y, Yoo HY, Lin Y, Han S, Hwang DS. Molecular and structural basis of low interfacial energy of complex coacervates in water. Adv Colloid Interface Sci. 2017;239:61–73. doi: 10.1016/j.cis.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Elsaid N, Jackson TL, Elsaid Z, Alqathama A, Somavarapu S. PLGA microparticles entrapping chitosan-based nanoparticles for the ocular delivery of ranibizumab. Mol Pharm. 2016;13:2923–2940. doi: 10.1021/acs.molpharmaceut.6b00335. [DOI] [PubMed] [Google Scholar]
- 25.Duque Sánchez L, Brack N, Postma A, Pigram PJ, Meagher L. Surface modification of electrospun fibres for biomedical applications: a focus on radical polymerization methods. Biomaterials. 2016;106:24–45. doi: 10.1016/j.biomaterials.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Frost SJ, Mawad D, Higgins MJ, Ruprai H, Kuchel R, Tilley RD, et al. Gecko-inspired chitosan adhesive for tissue repair. NPG Asia Mater. 2016;8:e280. doi: 10.1038/am.2016.73. [DOI] [Google Scholar]
- 27.Sobhani A, Rafienia M, Ahmadian M, Naimi-Jamal MR. Fabrication and characterization of polyphosphazene/calcium phosphate scaffolds containing chitosan microspheres for sustained release of bone morphogenetic protein 2 in bone tissue engineering. Tissue Eng Regen Med. 2017;14:525–538. doi: 10.1007/s13770-017-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou HY, Jiang LJ, Cao PP, Li JB, Chen XG. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr Polym. 2015;117:524–536. doi: 10.1016/j.carbpol.2014.09.094. [DOI] [PubMed] [Google Scholar]
- 29.Tahrir FG, Ganji F, Ahooyi TM. Injectable thermosensitive chitosan/glycerophosphate-based hydrogels for tissue engineering and drug delivery applications: a review. Recent Pat Drug Deliv Formul. 2015;9:107–120. doi: 10.2174/1872211308666141028145651. [DOI] [PubMed] [Google Scholar]
- 30.Shimojo AAM, Galdames SEM, Perez AGM, Ito TH, Luzo ACM, Santana MHA. In vitro performance of injectable chitosan-tripolyphosphate scaffolds combined with platelet-rich plasma. Tissue Eng Regen Med. 2016;13:21–30. doi: 10.1007/s13770-015-9111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KS, Lee JH, Ahn HH, Lee JY, Lee B, Lee HB, et al. The osteogenic differentiation of rat muscle-derived stem cells in vivo within in situ-forming chitosan scaffolds. Biomaterials. 2008;29:4420–4428. doi: 10.1016/j.biomaterials.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Cho MH, Kim KS, Ahn HH, Kim MS, Kim SH, Khang G, et al. Chitosan gel as an in situ-forming scaffold for rat bone marrow mesenchymal stem cells in vivo. Tissue Eng Part A. 2008;14:1099–1108. doi: 10.1089/ten.tea.2007.0305. [DOI] [PubMed] [Google Scholar]
- 33.Junter GA, Thébault P, Lebrun L. Polysaccharide-based antibiofilm surfaces. Acta Biomater. 2016;30:13–25. doi: 10.1016/j.actbio.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Song L, Li L, He T, Wang N, Yang S, Yang X, et al. Peritoneal adhesion prevention with a biodegradable and injectable N,O-carboxymethyl chitosan-aldehyde hyaluronic acid hydrogel in a rat repeated-injury model. Sci Rep. 2016;6:37600. doi: 10.1038/srep37600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez-Lorenzo C, Blanco-Fernandez B, Puga AM, Concheiro A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv Drug Deliv Rev. 2013;65:1148–1171. doi: 10.1016/j.addr.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 36.Abeer MM, Mohd Amin MC, Martin C. A review of bacterial cellulose-based drug delivery systems: their biochemistry, current approaches and future prospects. J Pharm Pharmacol. 2014;66:1047–1061. doi: 10.1111/jphp.12234. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Liu X, Li Y, Wang Y, Bao C, Chen Y, et al. A postoperative anti-adhesion barrier based on photoinduced imine-crosslinking hydrogel with tissue-adhesive ability. Acta Biomater. 2017;62:199–209. doi: 10.1016/j.actbio.2017.08.047. [DOI] [PubMed] [Google Scholar]
- 38.Kim MS, Kim JH, Min BH, Chun HJ, Han DK, Lee HB. Polymeric scaffolds for regenerative medicine. Polym Rev (Phila Pa). 2011;51:23–52. doi: 10.1080/15583724.2010.537800. [DOI] [Google Scholar]
- 39.Udoetok IA, Wilson LD, Headley JV. Quaternized cellulose hydrogels as sorbent materials and pickering emulsion stabilizing agents. Materials (Basel) 2016;9:E645. doi: 10.3390/ma9080645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y, Wang L, Qing Y, Yan N, Tian C, Huang Y. A green route to prepare fluorescent and absorbent nano-hybrid hydrogel for water detection. Sci Rep. 2017;7:4380. doi: 10.1038/s41598-017-04542-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Zhang X, Teng A, Liu A. Mechanical reinforcement of gelatin hydrogel with nanofiber cellulose as a function of percolation concentration. Int J Biol Macromol. 2017;103:226–233. doi: 10.1016/j.ijbiomac.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 42.Dolan GK, Yakubov GE, Bonilla MR, Lopez-Sanchez P, Stokes JR. Friction, lubrication, and in situ mechanics of poroelastic cellulose hydrogels. Soft Matter. 2017;13:3592–3601. doi: 10.1039/C6SM02709A. [DOI] [PubMed] [Google Scholar]
- 43.Kim KS, Kang YM, Lee JY, Kim ES, Kim CH, Min BH, et al. Injectable CMC/PEI gel as an in vivo scaffold for demineralized bone matrix. Biomed Mater Eng. 2009;19:381–390. doi: 10.3233/BME-2009-0603. [DOI] [PubMed] [Google Scholar]
- 44.Nguyen MK, Alsberg E. Bioactive factor delivery strategies from engineered polymer hydrogels for therapeutic medicine. Prog Polym Sci. 2014;39:1235–1265. doi: 10.1016/j.progpolymsci.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giri TK, Thakur D, Alexander A, Ajazuddin, Badwaik H, Tripathi DK. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: present status and applications. Curr Drug Deliv. 2012;9:539–555. doi: 10.2174/156720112803529800. [DOI] [PubMed] [Google Scholar]
- 46.Mun CH, Hwang JY, Lee SH. Microfluidic spinning of the fibrous alginate scaffolds for modulation of the degradation profile. Tissue Eng Regen Med. 2016;13:140–148. doi: 10.1007/s13770-016-9048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams PA, Campbell KT, Silva EA. Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis. J Biomed Mater Res A. 2018;106:138–146. doi: 10.1002/jbm.a.36217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bauer A, Gu L, Kwee B, Li WA, Dellacherie M, Celiz AD, et al. Hydrogel substrate stress-relaxation regulates the spreading and proliferation of mouse myoblasts. Acta Biomater. 2017;62:82–90. doi: 10.1016/j.actbio.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regan Med. 2016;13:538–546. doi: 10.1007/s13770-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Yan X, Zhao J, Feng H, Li P, Tong Z, et al. Preparation of the chitosan/poly(glutamic acid)/alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohydr Polym. 2018;191:8–16. doi: 10.1016/j.carbpol.2018.02.065. [DOI] [PubMed] [Google Scholar]
- 51.Wei Z, Zhao J, Chen YM, Zhang P, Zhang Q. Self-healing polysaccharide-based hydrogels as injectable carriers for neural stem cells. Sci Rep. 2016;6:37841. doi: 10.1038/srep37841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lym JS, Nguyen QV, da Ahn W, Huynh CT, Jae HJ, Kim YI, et al. Sulfamethazine-based pH-sensitive hydrogels with potential application for transcatheter arterial chemoembolization therapy. Acta Biomater. 2016;41:253–263. doi: 10.1016/j.actbio.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Liu L, Wang X, Ok YS, Elliott JAW, Chang SX, et al. Flexible and self-healing aqueous supercapacitors for low temperature applications: polyampholyte gel electrolytes with biochar electrodes. Sci Rep. 2017;7:1685. doi: 10.1038/s41598-017-01873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shim SW, Kwon DY, Park JH, Kim JH, Chun HJ, Koh YJ, et al. Preparation of zwitterionic sulfobetaine end-functionalized poly(ethylene glycol)-b-poly(caprolactone) diblock copolymers and examination of their thermogelling properties. J Polym Sci A Ploym Chem. 2014;52:2185–2191. doi: 10.1002/pola.27230. [DOI] [Google Scholar]
- 55.Jung BK, Oh E, Hong J, Lee Y, Park KD, Yun CO. A hydrogel matrix prolongs persistence and promotes specific localization of an oncolytic adenovirus in a tumor by restricting nonspecific shedding and an antiviral immune response. Biomaterials. 2017;147:26–38. doi: 10.1016/j.biomaterials.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Kim DY, Kwon DY, Kwon JS, Park JH, Park SH, Oh HJ, et al. Synergistic anti-tumor activity through combinational intratumoral injection of an in situ injectable drug depot. Biomaterials. 2016;85:232–245. doi: 10.1016/j.biomaterials.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Wang X, Dong K, Luo J, Zhang Q, Cheng Y. Injectable and responsively degradable hydrogel for personalized photothermal therapy. Biomaterials. 2016;104:129–137. doi: 10.1016/j.biomaterials.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Kanazawa T, Tamano K, Sogabe K, Endo T, Ibaraki H, Takashima Y, et al. Intra-articular retention and anti-arthritic effects in collagen-induced arthritis model mice by injectable small interfering RNA containing hydrogel. Biol Pharm Bull. 2017;40:1929–1933. doi: 10.1248/bpb.b17-00481. [DOI] [PubMed] [Google Scholar]
- 59.Cheng OT, Souzdalnitski D, Vrooman B, Cheng J. Evidence-based knee injections for the management of arthritis. Pain Med. 2012;13:740–753. doi: 10.1111/j.1526-4637.2012.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park JH, Park SH, Lee HY, Lee JW, Lee BK, Lee BY, et al. An injectable, electrostatically interacting drug depot for the treatment of rheumatoid arthritis. Biomaterials. 2018;154:86–98. doi: 10.1016/j.biomaterials.2017.10.055. [DOI] [PubMed] [Google Scholar]
- 61.Kim K, Park JH, Park SH, Lee HY, Kim JH, Kim MS. An injectable, click-cross-linked small intestinal submucosa drug depot for the treatment of rheumatoid arthritis. Adv Healthc Mater. 2016;5:3105–3117. doi: 10.1002/adhm.201601040. [DOI] [PubMed] [Google Scholar]
- 62.Wang P, Zhuo X, Chu W, Tang X. Exenatide-loaded microsphere/thermosensitive hydrogel long-acting delivery system with high drug bioactivity. Int J Pharm. 2017;528:62–75. doi: 10.1016/j.ijpharm.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 63.Zhao F, Wu D, Yao D, Guo R, Wang W, Dong A, et al. An injectable particle-hydrogel hybrid system for glucose-regulatory insulin delivery. Acta Biomater. 2017;64:334–345. doi: 10.1016/j.actbio.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 64.Shen YI, Cho H, Papa AE, Burke JA, Chan XY, Duh EJ, et al. Engineered human vascularized constructs accelerate diabetic wound healing. Biomaterials. 2016;102:107–119. doi: 10.1016/j.biomaterials.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Tendulkar S, Mirmalek-Sani SH, Childers C, Saul J, Opara EC, Ramasubramanian MK. A three-dimensional microfluidic approach to scaling up microencapsulation of cells. Biomed Microdevices. 2012;14:461–469. doi: 10.1007/s10544-011-9623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong X, Yang F. Recent progress in developing injectable matrices for enhancing cell delivery and tissue regeneration. Adv Healthc Mater. 2018;7:e1701065. doi: 10.1002/adhm.201701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee BH, Shirahama H, Kim MH, Lee JH, Cho NJ, Tan LP. Colloidal templating of highly ordered gelatin methacryloyl-based hydrogel platforms for three-dimensional tissue analogues. NPG Asia Mater. 2017;9:e412. doi: 10.1038/am.2017.126. [DOI] [Google Scholar]