Abstract
BACKGROUND:
Stem cell therapy requires a serum-free and/or chemically-defined medium for commercialization, but it is difficult to find one that supports long-term expansion of cells without compromising their stemness, particularly for novel stem cells.
METHODS:
In this study, we tested the efficiency of StemPro® MSC SFM Xeno Free (SFM-XF), a serum-free medium, for the long-term expansion of human fetal cartilage-derived progenitor cells (hFCPCs) from three donors in comparison to that of the conventional α-Modified Eagle’s Medium (α-MEM) supplemented with 10% fetal bovine serum (FBS).
RESULTS:
We found that SFM-XF supported the expansion of hFCPCs for up to 28–30 passages without significant changes in the doubling time, while α-MEM with 10% FBS showed a rapid increase in doubling time at 10–18 passages depending on the donor. Senescence of hFCPCs was not observed until passage 10 in both media but was induced in approximately 15 and 25% of cells at passage 20 in SFM-XF and α-MEM with 10% FBS, respectively. The colony forming ability of hFCPCs in SFX-XF was also comparable to that in α-MEM with 10% FBS. hFCPCs expressed pluripotency genes like Oct-4, Sox-2, Nanog, SCF, and SSEA4 at passage 20 and 31 in SFM-XF; however, this was not observed when cells were cultured in α-MEM with 10% FBS. The ability of hFCPCs to differentiate into three mesodermal lineages decreased gradually in both media but it was less significant in SFM-XF. Finally we found no chromosomal abnormality after long-term culture of hFCPCs until passage 17 by karyotype analysis.
CONCLUSION:
These results suggest that SFM-XF supports the long-term expansion of hFCPCs without significant phenotypic and chromosomal changes. This study have also shown that hFCPCs can be mass-produced in vitro, proving their commercial value as a novel source for developing cell therapies.
Keywords: Human fetal cartilage progenitor cells, Serum-free medium, Cell therapy, Pluripotency
Introduction
Cell therapies and regenerative medicine using stem cells are likely to offer viable treatments for many diseases that currently do not have adequate therapies. Cooper and Viswanathan [1] however, to treat patients with stem cells, there needs to be a method for the in vitro expansion of these cells to obtain a large number of cells while maintaining their phenotype. Mesenchymal stem cells (MSCs), one of the most commonly used sources for cell therapies, require 1–4 million cells per treatment and must be expanded for 8–12 weeks to obtain that number [2, 3]. However, MSCs are limited in their ability to proliferate and undergo senescence during long-term culture, resulting in irreversible growth arrest and loss of stem cell phenotypes [4, 5]. Studies have shown that the proliferation of MSCs in vitro is maximal at 3–4 passages, which decreases slightly at 5–6 passages and is rarely maintained at 10–12 passages [6]. Therefore, it is imperative to develop a method for the mass production of clinical grade stem cells with consistent quality [7, 8].
Many studies have attempted to overcome the limitation in the mass production of stem cells, including trying to optimize cell culture conditions, such as culture medium, coating substrate, and seeding density [9]. Among these, the development of a serum-free culture medium and its supplements was one of the most fundamental and imperative issues [10, 11]. The serum-based medium commonly uses fetal bovine serum (FBS), fetal calf serum (FCS) or human serum. These animal derived growth supplements have been used to support consistent stem cell growth and attachment during repeated passages by providing nutrients, attachment factors, and growth factors [12]. However, the use of serum in stem cell therapies has critical regulatory issues due to the inconsistent quality of raw materials and safety concerns due to the presence of animal-derived (xeno) antigens and potential infectious agents that can be delivered to the recipient [9, 13]. Currently, there are many serum-free and/or chemically defined media for the culture of stem cells for both research and clinical use. However, they are not standardized and need further validation and optimization for culturing of each different cell type, particularly cells from novel cell sources.
Previously, we have reported that human fetal cartilage-derived progenitor cells (hFCPCs) are a promising source for stem cells for therapies against cartilage disorders and possibly other diseases. Indeed, hFCPCs performed better than human MSCs and young chondrocytes with respect to the proliferation ability, colony forming ability, and plasticity in forming three mesengenic lineages, chondrocytes, adipocytes, and osteoblasts [14]. We also confirmed that hFCPCs exhibit no tumor forming activity when implanted in the back of nude mice and low toxicity and immune response when injected into the tail vein of rats [15]. In an effort to develop hFCPCs into a commercial product, we wanted to optimize their culture medium to allow for their long-term culture without loss of their phenotypes and consistent outcome for different donors. For this purpose, we have chosen StemPro® MSC SFM Xeno Free (SFM-XF) media that is widely used for MSC culture and compared its performance with that of α-Modified Eagle’s Medium (α-MEM) with 10% FBS that we have been using for hFCPCs culture until now. SFM-XF is a xeno-free medium and incorporates a surface coating substrate (CELLstart). It has been used successfully for large-scale expansion of MSCs from bone marrow or adipose tissue [16, 17].
The aim of the study was to evaluate the performance of SFM-XF in the long-term expansion of hFCPCs from different donors and obtain a general understanding of the characteristics of hFCPCs along with the passages and donor variations. We have cultured hFCPCs from three donors in either α-MEM with 10% FBS or SFM-XF medium until they no longer replicate. The proliferation ability and total yield of hFCPCs were examined by their doubling time and cumulative cell numbers, respectively. We have also examined their self-renewal ability, cellular senescence, gene expression patterns, and multi-lineage differentiation potential intermittently at specific passages to see if these characteristics correlate well with each other along with the number of passages.
Materials and methods
Isolation and culture of hFCPCs
hFCPCs were isolated from the human fetal cartilage tissue of 12 weeks after gestation, as described previously [14]. The fetal tissues were obtained from three donors following elective abortion with the approval by the institutional review board (IRB) of the Ajou University Medical Center (AJIRB-CRO-16-139). Cartilage tissues were washed with phosphate-buffered saline (PBS; Welgene, Daegu, South Korea), minced into small pieces (< 1 mm3) and treated with 0.1% collagenase type II (Worthington Biochemical Corp, Freehold, NJ, USA) in high-glucose Dulbecco’s modified Eagle medium (DMEM; HyClone, Logan, UT, USA) containing 1% FBS (HyClone) at 37 °C under 5% CO2. After 16 h, isolated cells were cultured in α-MEM (HyClone) supplemented with 10% FBS and 1% penicillin/streptomycin (Gibco, Gaithersburg, MD, USA) at a density of 8 × 103 cells/cm2. Three days after seeding, non-adherent cells were removed and the medium was changed. Cells were passaged at 80% confluence using 0.05% Trypsin–EDTA (Gibco) for 5 min at 37 °C under 5% CO2.
Cell proliferation assay
Two different culture conditions were tested when hFCPCs proliferation was examined from passage 2. The first condition was α-MEM with 10% FBS, the same medium used for cell isolation, and the other condition was SFM-XF, a xeno-free medium from Gibco without serum supplementation. Frozen stocks of hFCPCs at passage one were suspended in each medium and plated in 100 mm dishes at a density of 8 × 103 cells/cm2. Cells were fed with fresh medium every other day and passaged at 80% confluence. The number of viable cells retrieved after each passage was counted on a hemocytometer after trypan blue staining. The doubling time of cells was calculated by using the following formula: DT = {(T − T0) log2}/(logN − logN0) where DT is the doubling time, T − T0 is the culture period, N is the number of cells retrieved after each passage and N0 is the seeding cell number at passage two, which equals 8 × 103 cells/cm2. Subsequently, based on the doubling time obtained, the number of cells that can be obtained was confirmed by calculating the cumulative cell numbers.
Colony forming unit fibroblast (CFU-F) assay
hFCPCs were suspended in α-MEM with 10% FBS and plated in 60 mm dishes at the concentration of 0.45 (10 cells) and 10 (230 cells) cells/cm2. The medium was first changed at 1 week after plating and then replaced every 3 days. At 14 days of culture, the colonies formed were washed twice with PBS and stained with a 5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA) for 20 min at room temperature. The number of colonies was counted and the colony forming efficiency (%) was calculated by {(the number of colonies/the number of seeding cells) × 100}.
RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using the Trizol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions, and the RNA purity and concentration was measured by Nanodrop (JCBIO, Seoul, South Korea). One microgram of total RNA was used for the reverse-transcription into cDNA, and 1 μg of cDNA was used for PCR amplification using a First Strand cDNA Synthesis Kit for RT-PCR (AMV, Roche, Mannheim, Germany).The primer sequences and the reaction conditions are listed in Table 1.
Table 1.
Primers for reverse transcription-polymerase chain reaction (RT-PCR)
| Gene | Primer sequence | Accession number | Size (bp) | Annealing temperature |
|---|---|---|---|---|
| GAPDH | 5′-ACA ACT TTG GTA TCG TGG AA-3′ 5′-AAA TTC GTT GTC ATA CCA GG-3′ |
NM_002046.4 | 458 | 55 |
| Oct-4 | 5′-CGA CCA TCT GCC GCT TTG AG-3′ 5′-CCC CCT GTC CCC CAT TCC TA-3′ |
NM_001173531.1 | 573 | 61 |
| SCF | 5′-CCA TTG ATG CCT TCA AGG AC-3′ 5′-CTT CCA GTA TAA GGC TCC AA-3′ |
NM_000899.4 | 275 | 55 |
| Nanog | 5′-CAGCCCCGATTCTTCCACCAGTCCC-3′ 5′-CGGAAGATTCCCAGTCGGGTTCACC-3′ |
NM_024865.2 | 391 | 58 |
| Sox-2 | 5′-GGCAGCTACAGCATGATGC-3′ 5′-TCGGACTTGACCACCGAAC-3′ |
NM_003106.3 | 237 | 60 |
Flow cytometry
Cells at passage 4 and 20 were analyzed by flow cytometry for the expression of surface markers associated with embryonic stem cells (ESCs) and MSCs. The antibodies used were against CD44, CD73, CD90, CD105, and CD166 for MSC markers, TRA-1-60 and SSEA4 for ESC markers (BD Biosciences, San Jose, CA, USA). Primary antibodies were incubated for 40 min at 4 °C. The dilution factor for each antibody was determined according to the manufacturers’ instructions. Stained cells were analyzed by FACSvantage (Becton–Dickinson, San Jose, CA, USA).
Cellular senescence assay
Senescent cells were determined by senescence-associated-β-galactosidase (SA-β-gal) staining using a Senescence Cell Histochemical Staining kit (Sigma-Aldrich). Cells were seeded in 6-well plates at 1 × 105 cells/well and cultured to 80% confluence. For SA-β-gal staining, cells were first fixed for 10 min at room temperature and then incubated with the staining mixture for 16 h at 37 °C without CO2 supply. Senescent cells were green when observed under a microscope (TE-2000; Nikon, Tokyo, Japan).
Differentiation of hFCPCs
hFCPCs at passage 2, 10, and 20 were subjected to chondrogenic, adipogenic, and osteogenic differentiation in vitro according to the following protocols.
Chondrogenesis
Cells (3 × 105) were centrifuged at 500×g for 5 min in 15 ml tubes, and cell pellets were cultured in a chondrogenic medium consisting of DMEM supplemented with 100 nM dexamethasone (Sigma-Aldrich), 50 μg/ml ascorbate-2 phosphate (Sigma-Aldrich), insulin-transferrin-selenium (ITS) supplement (Sigma-Aldrich), 40 μg/ml proline (Sigma Aldrich), 1.25 mg/ml bovine serum albumin (BSA; Sigma-Aldrich), 100 μg/ml sodium pyruvate (Sigma Aldrich), and 10 ng/ml transforming growth factor-β3 (TGF-β3; R&D Systems). The pellets were cultured at 37 °C with 5% CO2 for 3 weeks, and the culture medium was changed every 3 days. After 3 weeks, the pellets were fixed with 4% formalin. Thin sections of 4 μm were prepared and stained with safranin-O to observe the sulfated glycosaminoglycans (sGAG).
Adipogenesis
Cells (1 × 105) were suspended in adipogenic medium and plated on a 6-well plate. The adipogenic differentiation medium consisted of α-MEM supplemented with 10% FBS, 1 μM dexamethasone, 10 μg/ml insulin (Sigma-Aldrich), 0.5 mM isobutyl-methylxanthine (IBMX; Sigma-Aldrich), and 0.1 mM indomethacin (Sigma-Aldrich). After 3 weeks, cells were stained with oil red O (Sigma-Aldrich) to observe the lipid droplets.
Osteogenesis
Cells (5 × 104) were suspended in osteogenic medium and plated on a 6-well plate. The osteogenic differentiation medium consisted of α-MEM supplemented with 10% FBS, 10 mM β-glycerophosphate (Sigma-Aldrich), 100 nM dexamethasone (Sigma-Aldrich), and 50 μg/ml ascorbate-2 phosphate (Sigma-Aldrich). After 3 weeks, cells were stained with alizarin red (Sigma-Aldrich) to observe the degree of mineralization.
Cytogenic analysis of hFCPCs
Karyotype stability of hFCPCs of passage 5 and 17 was analyzed using GTG banding technique and the karyotype formula by Samkwang Medical Laboratories (Seoul, Korea, https://www.smlab.co.kr).
Results
Long-term proliferation ability of hFCPCs
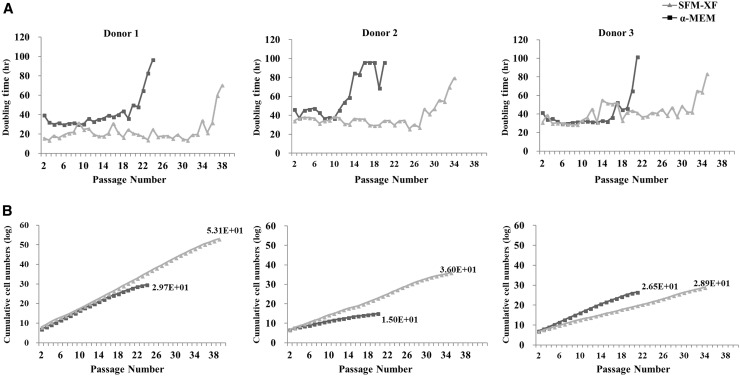
hFCPCs from three different donors (donors 1, 2, and 3) were cultured α-MEM with 10% FBS or Xeno-free medium (SFM-XF). The DT and cumulative cell numbers were assessed from passage 2 until they stopped growing. In the α-MEM with 10% FBS, the three donors showed somewhat different DTs of approximately 20 or 40 h at passage 2, which increased rapidly to more than 18 h after 20, 14, and 17 passages, respectively (Fig. 1A). Cells from the three donors stopped growing at 24, 20, and 21 passages, respectively. In the SFM-XF, DTs at early passages were similar to those in α-MEM with 10% FBS and were maintained at similar levels until 28–30 passages. Cells from the three donors stopped growing at 39, 35, and 34 passages, respectively. Accordingly, the cumulative number of cells obtained from 5 × 105 cells of each donor was 4.58 × 1029 at passage 24, 9.64 × 1014 at passage 20, and 3.10 × 1026 at passage 21 in α-MEM, while it was 1.24 × 1053 at passage 39, 8.97 × 1035 at passage 35, and 7.16 × 1028 at passage 34 in SFM-XF, respectively (Fig. 1B). Therefore, we found that SFM-XF maintained the proliferation ability of hFCPCs for a much longer period and gave a significantly larger cumulative cell yield than α-MEM.
Fig. 1.
The proliferation ability of hFCPCs. Proliferation ability of hFCPCs from three different donors was examined by the doubling time and cumulative cell numbers. hFCPCs were cultured in α-MEM with 10% FBS or in SFM-XF for up to 39 passages. A The doubling time was calculated by the formula: DT = {(T − T0) log2}/(logN − logN0) where DT is the doubling time, T − T0 is the culture period, N is the cell number at the time of assessment, and N0 is an initial cell number which was 5 × 105 cells. B The cumulative cell number was calculated in each donor cells as the number of passages increased
Cellular senescence of hFCPCs
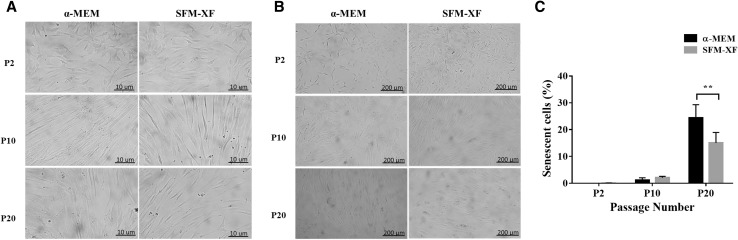
The morphology of hFCPCs in α-MEM with 10% FBS and SFM-XF was similar to each other (Fig. 2A). hFCPCs were polygonal in shape at passage 2 and steadily changed to a fibroblast-like morphology at passage 10, but did not show the flattened morphology of senescent cells even at passage 20. In the SA β-gal staining, the number of senescent cells was not observed at passages 2 and 10 but increased to some extent at passage 20 in both media (Fig. 2B). The number of SA β-gal-positive hFCPCs at passage 20 was significantly lower in SFM-XF than in α-MEM with 10% FBS in the quantitative analysis (Fig. 2C).
Fig. 2.
Morphology change and cellular senescence of hFCPCs as the number of passages increased. hFCPCs from donor 2 were cultured in α-MEM with 10% FBS or in SFM-XF. A The morphology of hFCPCs at passages 2, 10, and 20 are presented. Scale bars = 10 μm. B Cells possessing a senescent phenotype were examined by SA-β-gal staining shown in green. Scale bars = 200 μm. C The number of senescent cells was quantified using the ImageJ program. Data are presented as mean with standard deviation (SD) from three independent experiments (n = 3). **p < 0.01
Colony forming ability of hFCPCs
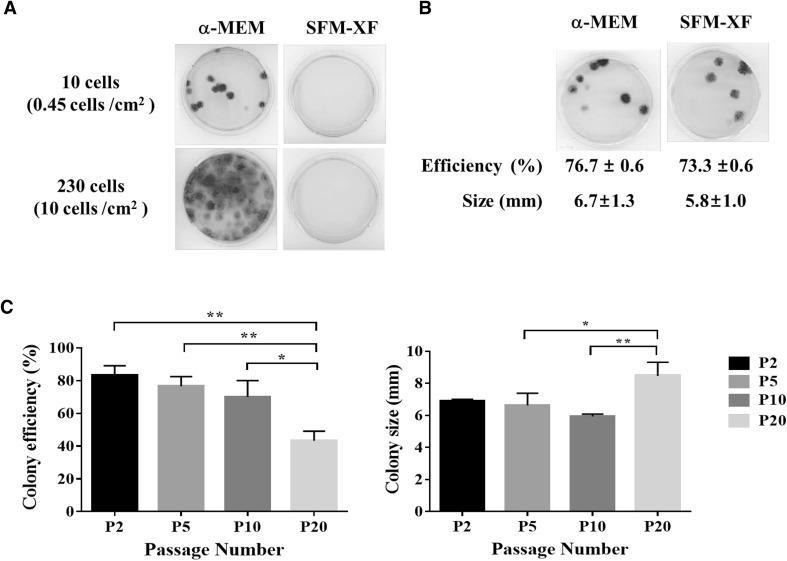
The ability of hFCPCs at passage 2 to self-renew under the two culture conditions (α-MEM with 10% FBS or SFM-XF) was compared by the CFU-F assay. When hFCPCs were first plated at 10 and 230 cells in a 35-mm dish, such that the cell density was 0.45 and 10 cells/cm2, respectively, they showed a colony forming efficiency of > 70% at 10 cells and too many colonies that were difficult to count were observed at 230 cells in α-MEM with 10% FBS (Fig. 3A). Unexpectedly, SFM-XF did not support colony formation, so we tried switching the media back to α-MEM with 10% FBS at the fifth passage just for the CFU-F assay (Fig. 3B). This resulted in a colony forming efficiency of 76.7 ± 0.6% for the α-MEM group and 73.3 ± 0.6% in the SFM-XF group. This shows that there was no statistically significant difference between the two experimental groups. The two groups also showed no significant difference in the mean diameter of colonies (6.7 ± 1.3 mm in the α-MEM group vs. 5.8 ± 1.0 mm in SFM-XF group). Subsequently, we observed changes in CFU-F for hFCPCs in α-MEM with 10% FBS at passage 2, 5, 10, and 20 to determine if it decreases with the decrease in proliferation ability over the passages. As shown in Fig. 3C, the colony forming efficiency was 83.3 ± 15.3% at the second passage and decreased gradually at passage 5 (76.7 ± 5.8%), and passage 10 (70.0 ± 10.0%). At passage 20, it dropped significantly to 43.3 ± 5.8% efficiency when the DT increased dramatically. The size of the colonies was measured as 6.9 ± 0.1 mm at passage 2, 6.6 ± 0.8 mm at passage 5, 5.9 ± 0.2 mm at passage 10, and 8.5 ± 0.9 mm at passage 20, which showed similar values until passage 10 but increased at passage 20.
Fig. 3.
Colony formation ability of hFCPCs. Colony formation ability of hFCPCs from donor 2 was examined by the CFU-F assay. A hFCPCs cultured in α-MEM with 10% FBS or SFM-XF were plated at 10 or 230 cells per 35 mm dish and subjected to the CFU-F assay at passage 2 for 14 days. Unexpectedly, there was no colony obtained in SFM-XF medium. B hFCPCs cultured in α-MEM with 10% FBS or SFM-XF were both suspended in α-MEM with 10% FBS and plated at 10 cells per 35 mm dish at passage 5 for the CFU-F assay. The colony forming efficiency and the diameter of the colonies were represented as the mean value with S.D. from three independent experiments (n = 3). C The CFU-F assay was performed with hFCPCs cultured in α-MEM with 10% FBS at passages 2, 5, 10, and 20. The colony forming efficiency and diameter of colonies were measured as above and presented in the graphs (n = 3). *p < 0.05; **p < 0.01
Expression of stem cell-related genes in hFCPCs
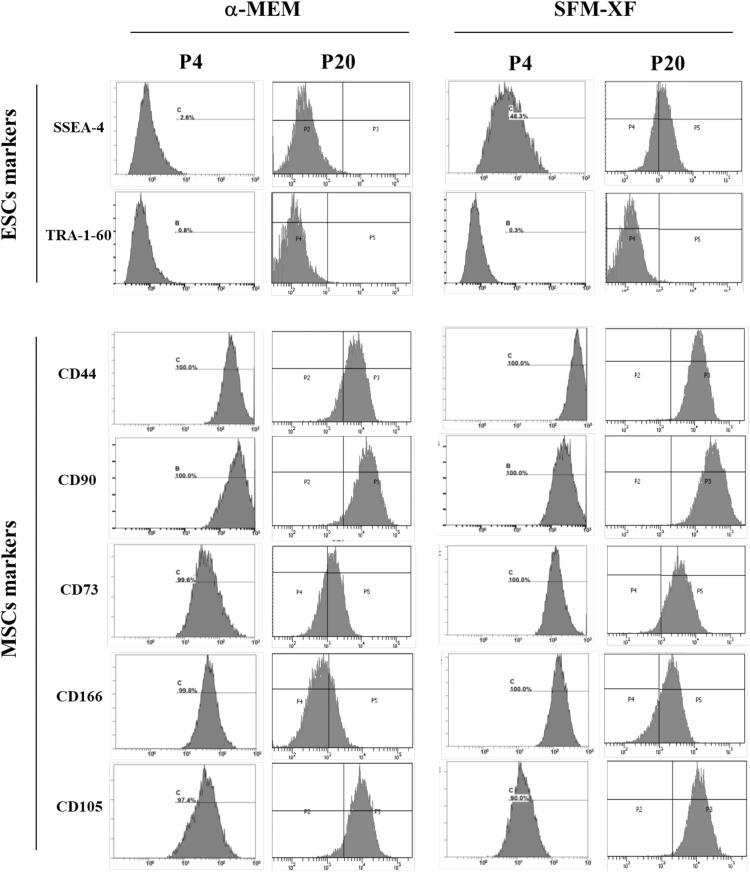
We have previously shown that hFCPCs express MSC markers and some ESC-related genes (21). In this study, we first compared the expression of selected surface markers for MSCs (CD44, CD73, CD90, CD105, and CD166) and ESCs (SSEA4 and TRA-1-60) in hFCPCs cultured in α-MEM with 10% FBS or SFM-XF at passages 4 and 20 by flow cytometry. At passage 4, both groups showed more than 90% expression of all MSC markers. At passage 20, some MSC markers such as CD44, CD73, and CD166 showed reduced expression particularly in the α-MEM group, while CD90 and CD105 maintained a high level of expression in both groups. This result showed that hFCPCs expressed MSC markers at high levels both in α-MEM and SFM-XF groups at passage 2 but was better able to maintain this expression in the SFM-XF group (Fig. 4).
Fig. 4.
Expression of surface markers on hFCPCs by flow cytometry. hFCPCs from donor 2 were cultured in α-MEM with 10% FBS and in SFM-XF and subjected to flow cytometric analysis for surface markers of ESCs (SSEA-4 and TRA-1-60), and MSCs (CD44, CD73, CD90, CD105, and CD166) at passages 4 and 20
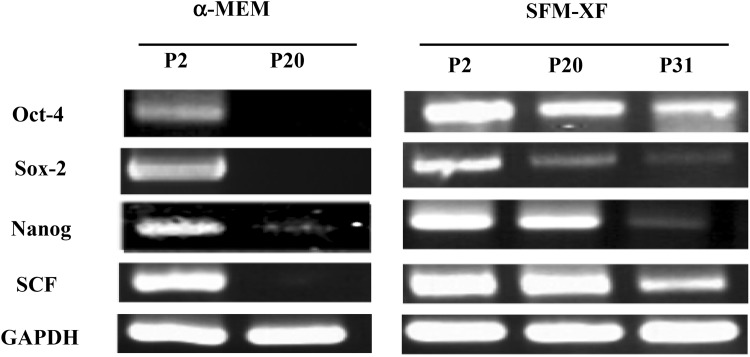
In the case of the ESC markers, SSEA4 was somewhat similarly expressed at passage 4 (48.0%) and 20 (68%) in the SFM-XF group but not in the α-MEM group. TRA-1-60 was not expressed at any passage in both groups. We then performed RT-PCR analysis to examine the expression of pluripotency-related genes (Oct-4, Sox-2, Nanog, and SCF). In the α-MEM group, expression of Oct-4 was clear but relatively weak, however the other three genes were expressed quite strongly at passage 2. None of the genes were expressed at passage 20 (Fig. 5). In SFM-XF group, all four genes, including Oct-4, showed a significant level of expression at passage 2 and some of them maintained their expression even at passage 20 and 31. Oct-4 and SCF showed strong expression at passage 2, which decreased gradually over the subsequent passages but was clearly observed even at passage 31. Sox-2 was clearly, yet weakly, expressed at passage 2, which decreased significantly at passage 20 and was not detected at passage 31. Nanog showed a high level expression at passage 2, which decreased over the passages but was still strong at passage 20, and had almost disappeared at passage 31. This showed that the expression of some ESCs and pluripotency markers in hFCPCs was more significant and better maintained over the passages in the SFM-XF group than in the α-MEM group.
Fig. 5.
Expression of stem cell-related genes in hFCPCs. Expression of selected ESCs markers (Oct-4, Sox-2, and Nanog) and SCF in hFCPCs from donor 2 cultured in both α-MEM with 10% FBS and SFM-XF at passages 2, 20, and 31 was examined by RT-PCR. Gapdh expression was used as an internal control
Multi-linage differentiation ability of hFCPCs
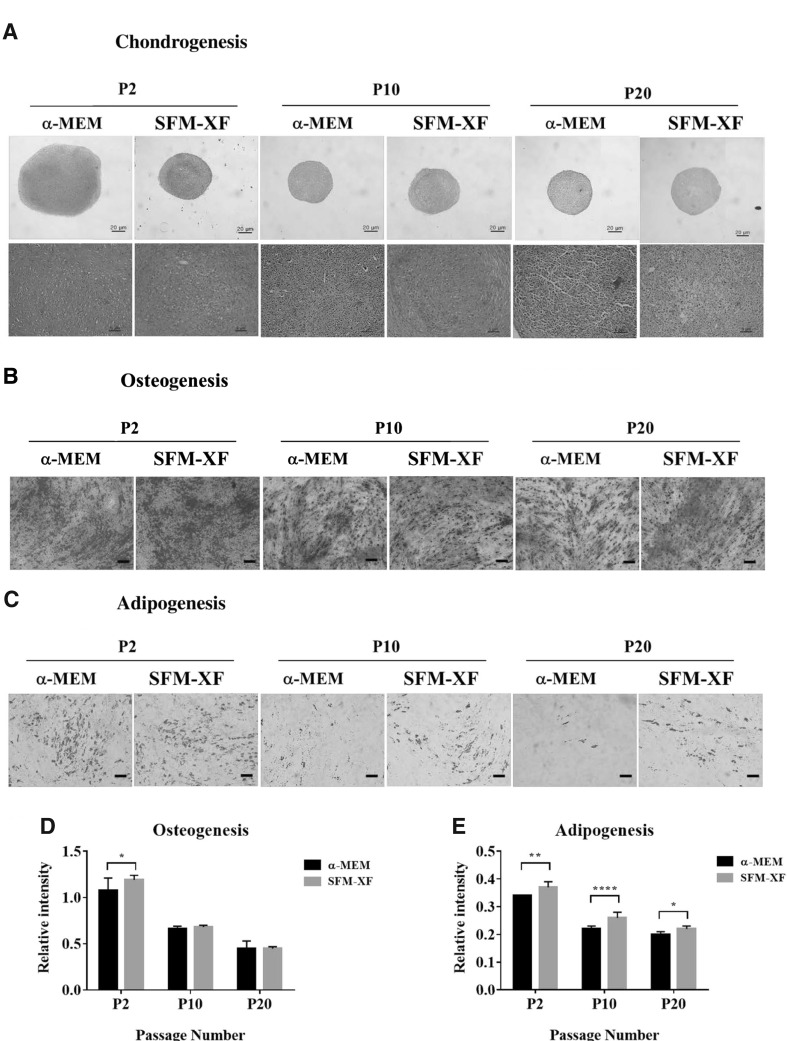
We have previously shown that hFCPCs have better chondrogenic, adipogenic, and osteogenic abilities than human MSCs from the bone marrow and human adult chondrocytes (21). In this study, their multi-lineage differentiation ability was compared in the α-MEM and SFM-XF groups at passages 2, 10, and 20. In chondrogenic differentiation, both α-MEM and SFM-XF groups showed strong positive signals during safranin-O staining but the size of the pellet was much larger in the α-MEM group at passage 2 (Fig. 6A). In contrast, the α-MEM group almost completely lost its chondrogenic ability at passages 10 and 20, while the SFM-XF group maintained it to some extent at passage 10. In oil-red O staining, adipogenic differentiation of hFCPCs was similar in both groups at passage 2, and it decreased with increasing number of passages. This decrease was more dramatic in the α-MEM group than in the SFM-XF group (Fig. 6B). The osteogenic differentiation of hFCPCs, shown by alizarin staining, was also strongest at passage 2 and decreased with increasing passages, but osteogenesis was maintained to some extent even at passage 20 in both groups (Fig. 6C). There was no significant difference in osteogenic ability between the two groups.
Fig. 6.
Differentiation ability of hFCPCs into three mesengenic cell lineages. hFCPCs from donor 2 were cultured in α-MEM with 10% FBS and in SFM-XF and subjected to A chondrogenic, B osteogenic, and C adipogenic differentiation in vitro at passages 2, 10, and 20. After 3 weeks, samples were stained with safranin-O for chondrogenesis, alizarin S for osteogenesis, and oil-red O for adipogenesis. Scale bars = 20 μm (upper panels) and 5 μm (lower panels) for A, and = 100 μm for B, C. The signal intensities of D osteogenic and E adipogenic differentiation were presented quantitatively from three independent experiments. *p < 0.05; **p < 0.01; ****p < 0.0001
Cytogenic analysis of hFCPCs
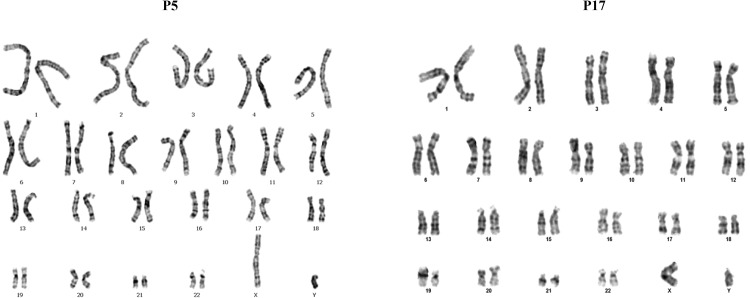
Cytogenetic analysis was performed at passage 5 and 17, to provide information on the genetic stability of the long term culture. The hFCPCs were stable at two the passages tested. The hFCPCs showed normal diploid karyotypes, 46 XY, without aneuploidy or polyploidy, chromosome structural abnormalities were not detected (Fig. 7).
Fig. 7.
Cytogenic analysis of hFCPCs. Cytogenic analysis of hFCPCs from donors 2 was examined by GTG banding technique. The hFCPCs were cultured at passages 5 and 17 in α-MEM with 10% FBS
Discussion
In this study, we found that hFCPCs were successfully expanded in vitro for more passages in SFM-XF (StemPro® MSC SFM Xeno Free from Fisher Scientific) than in α-MEM with 10% FBS. The DT of hFCPCs was similar in the two culture media in the beginning but showed significantly different patterns as the number of passages increased. Indeed, the DT was stable without an irreversible drift in SFM-XF for up to 28–30 passages for all three donors tested, while culture in α-MEM with 10% FBS produced an increase in DT after 10–18 passages depending on the donor. Accordingly, the yield of cells was significantly different between the SFM-XF and the α-MEM groups at a given passage and in terms of the total number of cells obtained at the final passage. The characteristics of hFCPCs were also maintained well in SFM-XF at levels equivalent to or better than in α-MEM with 10% FBS, such as the colony forming ability, expression of stem cell-related genes, resistance to senescence, and the multi-lineage differentiation ability. Considering the high performance and donor stability, we think that SFM-XF can be used to culture and expand hFCPCs for cell therapy. SFM-XF is current good manufacturing practice (cGMP) compliant and could be used for commercial development in the future.
This study showed that SFM-XF, developed for the expansion of MSCs, could support the expansion of hFCPCs that have strong chondrogenic phenotypes. Therefore, we might expect that SFM-XF can be used for other types of adult and fetal stem/progenitor cells. The successful use of SFM-XF in hFCPC expansion for a significant number of passages was probably because hFCPCs and MSCs share fibroblastic phenotypes and their expansion depends on the regulation of the universal cell cycle machinery. When compared with MSCs, hFCPCs possess better proliferation and colony forming abilities, and SFM-XF allowed these abilities to be maintained for a greater number of passages. Many studies on MSCs have shown that the average DT for human MSCs is approximately 45 h up to passage 5 and then the cells lose their proliferation ability rapidly after passage 6. The colony forming ability of human MSCs is also much more limited compared to that of hFCPCs. For example, a previous study showed that only 50 colonies were formed from 5 × 105 MSCs [18].
In the surface marker analysis, hFCPCs were positive for most of the MSC markers tested (CD44, CD73, CD90, CD105, and CD166) and it did not changed significantly between passages 4 and 20 in both SFM-XF and α-MEM with 10% FBS. However, these markers are also expressed in most fibroblastic and plastic-adhesive cells of other types [19, 20] and might not be the best indicator of stemness of hFCPCs. Among the pluripotency genes, hFCPCs expressed SSEA-4, Oct-4, Sox-2, Nanog, and SCF but not Tra-1-60, and their expression was more prominent in SFM-XF. Flow cytometry analysis revealed that the expression of SSEA-4 was observed only in the SFM-XF group. The percent positive for SSEA-4 was less than 50% at passage 4 and it was maintained at passage 20. Among the four other genes examined via RT-PCR analysis, the expression of Oct-4 and SCF decreased as the number of passages increased but remained at significant levels up to passage 31. Conversely, the expression level of Sox-2 and Nanog were very low at passage 20 and 31, respectively. It is not clear why hFCPCs show this expression pattern for the pluripotency genes, but the expression of some of the essential pluripotency genes in hFCPCs demonstrates their primitive and stem cell-like characteristics. Nanog, Sox-2, and Oct-4 are essential transcription factors that maintain the pluripotency of ESCs. Sox-2 and Oct-4 are known to cooperate to induce multiple subsets of pluripotency genes, including Nanog and themselves, which constitutes complex transcriptional networks [21–23]. Nanog also regulates the pluripotency and differentiation of ESCs via its interaction with other transcription factors, such as Oct-4, Sox-2, Klf4, and Slug3/4 [24]. SCF is a ligand of the c-kit tyrosine kinase receptor and plays a crucial role in the proliferation, differentiation, and survival of pluripotent progenitor cells [25]. SCF is known to upregulate the expression of some stem cell markers, including Oct-4 and Nanog, in these progenitor cells. Studies have shown that MSCs express SSEA-4, Oct-4, and SCF but the expression of Sox-2 and Nanog in MSCs has been rarely reported. Typically, SCF is expressed by bone marrow stromal cells and supports hematopoietic cell survival and proliferation [25].
In this study, there was no strict correlation between the proliferation ability, cellular senescence, self-renewal, and differentiation plasticity of hFCPCs in SFM-XF and α-MEM with 10% FBS. Our results using hFCPCs from donor 2 showed that the proliferation ability of hFCPCs did not decrease even at passage 20 in SFM-XF, when the percentage of senescent cells was approximately 15% and their differentiation ability was significantly impaired. The proliferation of cells depends on cell cycle progression and cell division whose inhibition for long period is involved in cellular senescence. It is likely that 15% of senescent cells did not have a significant effect on the expansion of the whole population of cells. We also speculate that the differentiation ability of stem/progenitor cells might not strictly correlate with their proliferation ability as the number of passages increases. Loss of their ability to differentiate appeared to correlate well with a decrease in self-renewal ability in α-MEM as the passages increased. Self-renewal of cells generally entails unlimited and asymmetric cell division of stem cells. However, the CFU-F assay used in this study measures the ability of a single cell to survive and grow into a colony [26], which has different implications than cell proliferation ability.
This study has some technical issues to consider. First, we could not obtain any colony in the CFU-F assay using SFM-XF and had to change the medium back to α-MEM with 10% FBS at the time of performing the CFU-F assay. We were concerned that this might have hampered cell viability and eventually the colony forming ability of hFCPCs, but the CFU-F assay shows that this was not the case. It is not clear why no colony formed in SFM-XF. SFM-XF uses a coating substrate on the culture dish called CELLstart. We tested CFU-F assays using combinations of SFM-XF and α-MEM with 10% FBS with or without CELLstart and found that the combination of α-MEM with 10% FBS and CELLstart still forms colonies though at a slightly lower efficiency than α-MEM with 10% FBS alone (data not shown). Therefore, the main cause for the inability of hFCPCs to form colonies in SFM-XF is likely SFM-XF itself and not the coating material.
Acknowledgement
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI17C2191).
Conflict of interest
The authors have no conflict of interest to declare regarding this study.
Ethical statement
This study was approved by the institutional review board (IRB) of the Ajou University Medical Center (IRB No.: AJIRB-CRO-16-139).
Contributor Information
Byoung-Hyun Min, Phone: 82-31-219-4444, Email: dr.bhmin@gmail.com.
Byung Hyune Choi, Phone: 82-32-890-0939, Email: bryan@inha.ac.kr.
Reference
- 1.Cooper K, Viswanathan C. Establishment of a mesenchymal stem cell bank. Stem Cells Int. 2011;2011:905621. doi: 10.4061/2011/905621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao K, Liu Q. The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. J Hematol Oncol. 2016;9:46. doi: 10.1186/s13045-016-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Estrada JC, Torres Y, Benguria A, Dopazo A, Roche E, Carrera-Quintanar L, et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death Dis. 2013;4:e691. doi: 10.1038/cddis.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turinetto V, Vitale E, Giachino C, Janmaleki M, Teymoori M. Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 2016 doi: 10.3390/ijms17071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbani M, Tafazzoli-Shadpour M, Shokrgozar MA, Janmaleki M, Teymoori M. Cyclic stretch effects on adipose-derived stem cell stiffness, morphology and smooth muscle cell gene expression. Tissue Eng Regen Med. 2017;14:279–286. doi: 10.1007/s13770-017-0033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osipova EY, Shamanskaya TV, Kurakina OA, Nikitina VA, Purbueva BB, Ustugov AY, et al. Biological characteristics of mesenchymal stem cells during ex vivo expansion. Br J Med Med Res. 2011;1:85–95. doi: 10.9734/BJMMR/2011/282. [DOI] [Google Scholar]
- 7.Chun SY, Park GB, Kwon TG, Choi SH. Analysis of stability of human urine derived stem cells during serial subcultures. Tissue Eng Regen Med. 2015;12 Suppl 2:122–131. doi: 10.1007/s13770-015-0438-z. [DOI] [Google Scholar]
- 8.Son Y. Recent advances in stem cell researches and their future perspectives in regenerative medicine. Tissue Eng Regen Med. 2017;14:641–642. doi: 10.1007/s13770-017-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomopoulos A, van Deen WK, Manansala AR, Lacey PN, Tomakili TA, Ziman A, et al. Optimization of human mesenchymal stem cell manufacturing: the effects of animal/xeno-free media. Sci Rep. 2015;13:16570. doi: 10.1038/srep16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agata H, Watanabe N, Ishii Y, Kubo N, Ohshima S, Yamazaki M, et al. Feasibility and efficacy of bone tissue engineering using human bone marrow stromal cells cultivated in serum-free conditions. Biochem Biophys Res Commun. 2009;382:353–358. doi: 10.1016/j.bbrc.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Patrikoski M, Juntunen M, Boucher S, Campbell A, Vemuri MC, Mannerström B, Miettinen S. Development of fully defined xeno-free culture system for the preparation and propagation of cell therapy-compliant human adipose stem cells. Stem Cell Res Ther. 2013;4:27. doi: 10.1186/scrt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usta SN, Scharer CD, Xu J, Frey TK, Nash RJ. Chemically defined serum-free and xeno-free media for multiple cell lineages. Ann Transl Med. 2014;2:97. doi: 10.3978/j.issn.2305-5839.2014.09.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiskanen A, Satomaa T, Tiitinen S, Laitinen A, Mannelin S, Impola U, et al. N-glycolylneuraminic acid xenoantigen contamination of human embryonic and mesenchymal stem cells is substantially reversible. Stem Cells. 2007;25:197–202. doi: 10.1634/stemcells.2006-0444. [DOI] [PubMed] [Google Scholar]
- 14.Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant. 2016;25:449–461. doi: 10.3727/096368915X688641. [DOI] [PubMed] [Google Scholar]
- 15.Lee SJ, Oh HJ, Truong MD, Lee KB, Kim J, Kim YJ, et al. Therapeutic possibility of human fetal cartilage-derived progenitor cells in rat arthritis model. Tissue Eng Regen Med. 2015;12 Suppl 2:147–154. doi: 10.1007/s13770-015-0441-4. [DOI] [Google Scholar]
- 16.Santos Fd, Andrade PZ, Abecasis MM, Gimble JM, Chase LG, Campbell AM, et al. Toward a clinical-grade expansion of mesenchymal stem cells from human sources: a microcarrier-based culture system under xeno-free conditions. Tissue Eng Part C Methods. 2011;17:1201–1210. doi: 10.1089/ten.tec.2011.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chase LG, Yang S, Zachar V, Yang Z, Lakshmipathy U, Bradford J, et al. Development and characterization of a clinically compliant xeno-free culture medium in good manufacturing practice for human multipotent mesenchymal stem cells. Stem Cells Transl Med. 2012;1:750–758. doi: 10.5966/sctm.2012-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samsonraj RM, Rai B, Sathiyanathan P, Puan KJ, Rötzschke O, Hui JH, et al. Establishing criteria for human mesenchymal stem cell potency. Stem Cells. 2015;33:1878–1891. doi: 10.1002/stem.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mafi P, Hindocha S, Mafi R, Griffin M, Khan WS. Adult mesenchymal stem cells and cell surface characterization—a systematic review of the literature. Open Orthop J. 2011;5 Suppl 2:253–260. doi: 10.2174/1874325001105010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrobback K, Wrobel J, Hutmacher DW, Woodfield TB, Klein TJ. Stage-specific embryonic antigen-4 is not a marker for chondrogenic and osteogenic potential in cultured chondrocytes and mesenchymal progenitor cells. Tissue Eng Part A. 2013;19:1316–1326. doi: 10.1089/ten.tea.2012.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amini S, Fathi F, Mobalegi J, Sofimajidpour H, Ghadimi T. The expressions of stem cell markers: Oct4, Nanog, Sox2, nucleostemin, Bmi, Zfx, Tcl1, Tbx3, Dppa4, and Esrrb in bladder, colon, and prostate cancer, and certain cancer cell lines. Anat Cell Biol. 2014;47:1–11. doi: 10.5115/acb.2014.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, et al. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- 23.Miyazawa K, Tanaka T, Nakai D, Morita N, Suzuki K. Immunohistochemical expression of four different stem cell markers in prostate cancer: high expression of NANOG in conjunction with hypoxia-inducible factor-1alpha expression is involved in prostate epithelial malignancy. Oncol Lett. 2014;8:985–992. doi: 10.3892/ol.2014.2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang W, Sui Y, Ni J, Yang T. Insights into the Nanog gene: a propeller for stemness in primitive stem cells. Int J Biol Sci. 2016;12:1372–1381. doi: 10.7150/ijbs.16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma Y, Liang D, Liu J, Axcrona K, Kvalheim G, Giercksky KE, et al. Synergistic effect of SCF and G-CSF on stem-like properties in prostate cancer cell lines. Tumour Biol. 2012;33:967–978. doi: 10.1007/s13277-012-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]