Abstract
BACKGROUND:
The preservation of stem cell viability and characteristics during cell transport from the bench to patients can significantly affect the success of cell therapy. Factors such as suspending medium, time, temperature, cell density, and container type could be considered for transport conditions.
METHODS:
To establish optimal conditions, human amniotic fluid stem cells’ (AFSCs) viabilities were analyzed under different media {DMEM(H), DMEM/F-12, K-SFM, RPMI 1640, α-MEM, DMEM(L), PBS or saline}, temperature (4, 22 or 37 °C), cell density (1 × 107 cells were suspended in 0.1, 0.5, 1.0 or 2.0 mL of medium) and container type (plastic syringe or glass bottle). After establishing the transport conditions, stem cell characteristics of AFSCs were compared to freshly prepared cells.
RESULTS:
Cells transported in DMEM(H) showed relatively higher viability than other media. The optimized transport temperature was 4 °C, and available transport time was within 12 h. A lower cell density was associated with a better survival rate, and a syringe was selected as a transport container because of its clinical convenience. In compare of stem cell characteristics, the transported cells with established conditions showed similar potency as the freshly prepared cells.
CONCLUSION:
Our findings can provide a foundation to optimization of conditions for stem cell transport.
Keywords: Cell transporting, Medium, Cell density, Amniotic fluid stem cell
Introduction
Cell therapy using stem cells is considered as promising approach to cure degenerative diseases, cancer, damaged tissues, or any disease for which there are very limited therapeutic options. Stem cells can potentially restore the structure and function of injured tissues and organs [1]. In particular, mesenchymal stem cells (MSCs), a population of adult-derived adherent cultured cells, have shown great promise in numerous clinical trials [1–6].
For the clinical application, stem cells should be produced in Good Manufacturing Practice (GMP) according to standard procedures approved by government to ensure the quality and safety of the final cell products [7, 8]. And the produced cells need to be preserved for a period of time until transported to the hospital and then injected into the patient, which may take hours to days [9, 10]. During this transporting period, preservation of cell viability and stem cell characteristics is extremely important because the clinical effect can vary depending on the cell quality. Cells are sensitive to the surrounding environment, and thus require a strictly controlled transport condition to maintain their quality [9].
In Korea, hospitals that capable of carry out cell therapy are usually located in major cities, while most GMP facilities are placed outside of the city. Therefore, cells could be injected into patients approximately 10 h after GMP manufacturing. During this long-term transportation, the cells may lose their viability and stem cell characteristics over time. To overcome suspended cells’ problems, a freezing method could be considered, however, this method has several inconveniences and limitations. During freezing and thawing, cell viability and stem cell characteristics may change [11, 12], and especially cryoprotectant should be removed using reagents and machines. Any remaining cryoprotectant may cause adverse reactions, ranging from mild to severe life-threatening events [13–15]. Thus for clinical application, stem cells are better transported in a suspended state. Therefore, it is necessary to optimize the transport conditions that can maintain cell viability and stem cell characteristics.
The transporting condition may include suspended medium, temperature, time, cell density, container type, and so on [9, 15]. However, little studies have been reported for the combined transporting conditions of stem cells for clinical applications.
In this study, in order to establish optimal transport conditions, we treated amniotic fluid derived stem cell (AFSCs) in various transporting conditions and then compared their viability and stem cell characteristics.
Materials and methods
Human amniotic fluid stem cells (AFSCs) preparation
This study was approved by the Ethics Committee of Kyungpook National University Hospital (IRB No. KNUH 2012-10-018). Amniotic fluids (10 mL) were obtained from 3 women undergoing routine amniocentesis (16–20 weeks gestational age) at Kyungpook National University Hospital. AFSCs were cultured in Chang Medium containing 15% fetal bovine serum (Gibco, Gland Island, NY, USA), 1% l-glutamine, and 1% penicillin/streptomycin with 18% Chang B and 2% Chang C (Irvine Scientific, Irvine, CA, USA) at 37 °C with 5% CO2. Three days later, debris and non-adherent cells were discarded and exchanged with fresh Chang medium. Attached AFSCs were expanded and passaged at 80% confluence. Cells at passage 5 were used for subsequent experiment.
Selection of optimal medium
To select suitable transport medium, Dulbecco’s Modified Eagle Medium high-glucose [DMEM(H)], DMEM low-glucose [DMEM(L)], α-Minimum Essential Medium (MEM), DMEM/F-12, Roswell Park Memorial Institute (RPMI) 1640 and keratinocyte serum-free medium (K-SFM), (Thermo Scientific, Waltham, MA, USA) were tested. Phosphate-buffered saline (PBS) and saline (Thermo Scientific) was used as controls (each group n = 5). Media do not contain fetal bovine serum and antibiotics (penicillin/streptomycin). Other conditions except for the medium were fixed, as 1 × 107 cells/2 mL and stored at 4 °C for 12 h in syringe. Cell viability was measured by the MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay (Promega, Fitchburg, WI, USA) according to the manufacturer’s instructions. Each medium without cells was used as an internal control, and experiments were repeated three times independently.
Selection of optimal temperature and time
To analyze the effect of transport temperature and time, cells were stored at 4 °C (in a refrigerator), 22 °C (at room temperature), and 37 °C (in an incubator) for 6, 12, 18, 24, 36, and 48 h (each group n = 5). Other conditions except for the temperature and time were fixed, as 1 × 107 cells/2 mL DMEM(H) for 12 h in syringe. For accurate thermography, a digital thermometer (Taylor Precision Products, Oak Brook, IL, USA) was placed beside the plastic syringe containing the cells and the temperature was checked every 6 h during the first day and every 24 h thereafter. At each time point, cell viability was measured by the trypan blue dye exclusion assay and counted with hematocytometer. Each well was tested in duplicate and the experiment was repeated three times independently.
Because cells were formed aggregation depending on the temperature, extracellular matrix (ECM)-related marker expression was analyzed by hematoxylin and eosin (H&E) and immunocytochemistry (ICC). For histological analysis, 4% paraformaldehyde fixed samples were cut into 4 µm thick from paraffin block, attached to a coated slide and treated routine H&E staining. For ICC analysis, samples were processed in 0.2% Triton-X 100 for 10 min and incubated with 200 µL of 5% normal bovine serum in PBS for 2 h. The primary antibody was treated overnight at 4 °C, and the secondary antibody was incubated for 1 h at room temperature. The antibody information was listed in Table 1.
Table 1.
Antibody information in immunocytochemistry
| Primary antibody | Company (Cat. no) | Dilution |
|---|---|---|
| Collagen 1 | Abcam (ab34710) | 1:200 |
| Collagen 4 | Thermo (PA5-37854) | 1:500 |
| Laminin | Novus | 1:500 |
| Fibrinogen | Novus | 1:200 |
| Fibronectin | Abcam (ab2413) | 1:200 |
| Nestin | Abcam (ab6320) | 1:200 |
| a-SMA | Abcam (ab124964) | 1:200 |
| pan Cytokeratin | Abcam (ab7753) | 1:200 |
| Secondary antibody | Company | Dilution |
|---|---|---|
| Alexa Fluor 594 Goat anti-rabbit IgG | Thermo (R37117) | 1:1000 |
| Alexa Fluor 594 Goat anti-mouse IgG | Thermo (A-11032) | 1:1000 |
| FITC Goat anti-mouse IgG | Thermo (A-16079) | 1:1000 |
The H&E and ICC results were confirmed by real-time PCR. Total RNA was isolated from each group using Trizol reagent (Thermo Fisher Scientific), and complementary DNA was synthesized from 1 µg RNA using reverse transcription kits (Applied Biosystems, Foster City, CA, USA). Suitable primers were designed using Primer Express Software (Applied Biosystems). Real-time PCR was performed using the ABI Prism Sequence Detection System 7500 for all genes: 10 min of pre-incubation at 95 °C, followed by 45 cycles for 10 s at 95 °C, 50 s at 58 °C and 20 s at 72 °C. Individual PCRs were carried out in 10 µL volumes in a 96-well reaction plate (Applied Biosystems) containing 2 µL diethylpyrocarbonate-treated water, 1 µL forward and reverse primers (10 pM), and 5 µL SYBR Green Polymerase Chain Reaction Master Mix (all from Applied Biosystems). Beta-actin was used as a control gene and each experiment was repeated in duplicate. To analyze the data, the 2−ΔCt or 2−ΔΔCt method of relative quantification was adapted to estimate copy numbers. Primer information is listed in Table 2.
Table 2.
Real-time PCR primer sequences
| Gene | Sequences |
|---|---|
| β-actin | 5′-ATCGTCCACCGCAAATGCT |
| 5′-AAGCCATGCCAATCTCATCT | |
| Collagen 1 | 5′-CTGTTCTGTTCCTTGTGTAACTGTGTT |
| 5′- GCCCGGTGACACATCAA | |
| Collagen 4 | 5′-TGCCACTGCCCTTAACACTG |
| 5′-CGATGTGTCCGTGCCAAAAT | |
| Laminin | 5′-TGGCACTGTGGGATTCTCAT |
| 5′-CAGGGAACACAAGGAGCAAC | |
| Fibrinogen | 5′-AGAAGTGGTGACCTCCGAAG |
| 5′-CCTATGCCAGACAATGTGCC | |
| Fibronectin | 5′-CCAGATGCAAGTGACCGATG |
| 5′-AACTTGAAGGCAGCCACTTG | |
| Oct4 | 5′-GGAGAATTTGTTCCTGCAGT |
| 5′-AGAACCACACTCGGACCACA | |
| SSEA4 | 5′-TCCCAGGTTCAAGCGATTCT |
| 5′-CCAACATGGTGAAACGCAGT | |
| CD44 | 5′-ACACCCCATCCCAGACGAAG |
| 5′-GAGACTTGCTGGCCTCTCCG | |
| Pax7 | 5′-GTTTGCCCCACAGACAAGAT |
| 5′-CTGGTGTGGTCAAGGAAGGT | |
| MAP2 | 5′-GCTCCCGGAGAAGGATTCTG |
| 5′-TCCTCGGTTAGAGACAACAAGCT | |
| UP1a | 5′-GCCGACCAGTACCGTGTATA |
| 5′-AAGACGTCATCCTTGCCTGA |
Selection of optimal cell density
To establish optimal cell density, cells were suspended with 0.1, 0.5, 1 and 2 mL of medium (each group n = 5). Other conditions except for the medium volume were fixed, as 1 × 107 cells/DMEM(H) for 12 h in syringe. Cell viability was determined by the trypan blue dye exclusion assay and counted with hematocytometer. Counting in each well was done in duplicate and the experiment was repeated three times. Percent viability was calculated using the following formula: % viability = (live cell count/total cell count) × 100.
Selection of optimal container type
To select transport container, commonly using plastic syringe and glass bottle were tested. Other conditions except for the container type were fixed, as 1 × 107 cells/2 mL of DMEM(H) for 12 h. Cell viability was determined by the trypan blue dye exclusion assay and counted with hematocytometer. Each well was tested in duplicate and the experiment was repeated three times.
Comparison of stem cell characteristics and multi-differentiation potential between transported cell and freshly prepared cell
Established transport conditions were 1 × 107 cells suspended in 2 mL of DMEM(H) and treated at 4 °C for 12 h. The stem cell characteristics of transported cells were compared with freshly prepared cells (each group n = 5). For real-time PCR, two groups were tested for maintenance of stem cell properties, including inhibition of randomized differentiation. Stem cell markers (Oct4, SSEA4, CD44), and muscle (Pax7), neural (MAP2) and epithelial (UP1a) differentiation markers were analyzed. Primer sequences were listed at Table 2. To assesses whether the transported cells remain multi-potency, cells were treated with each differential medium. For neurogenic differentiation, cells were seeded at a density of 3000 cells/cm2 in DMEM low-glucose medium, antibiotics, supplemented with 2% DMSO, 200 µM butylated hydroxyanisole (BHA, Sigma-Aldrich, Seoul, South Korea), and nerve growth factor (NGF, 25 ng/mL). Two days after, cells were treated with Chang medium with NGF, and NGF was added every 2 days. For myogenic differentiation, cells were seeded at a density of 3000 cells/cm2 with Matrigel (Collaborative Biomedical Products; incubation for 1 h at 37 °C at 1 mg/mL in DMEM) coated dish, and cultured in DMEM low-glucose formulation containing 10% horse serum (Gibco), 0.5% chick embryo extract (Gibco), and antibiotics. One day after, 3 µM 5-aza-2′-deoxycytidine (5-azaC; Sigma-Aldrich) and TGF-β were added, and the cells were cultured up to 2 weeks. For epithelial differentiation, a commercial medium (Cat no. 4101, ScienCell, Carlsbad, CA) was treated for 2 weeks. ICC analysis was carried with nestin, α-smooth actin and pan cytokeratin antibodies (Table 1).
Statistical analysis
The data were expressed as the mean ± standard deviation (SD). Statistically significant differences were determined by t test and one-way analysis of variance with Tukey’s post hoc test. Differences were considered significant when the p value < 0.05.
Results
Selection of transport medium
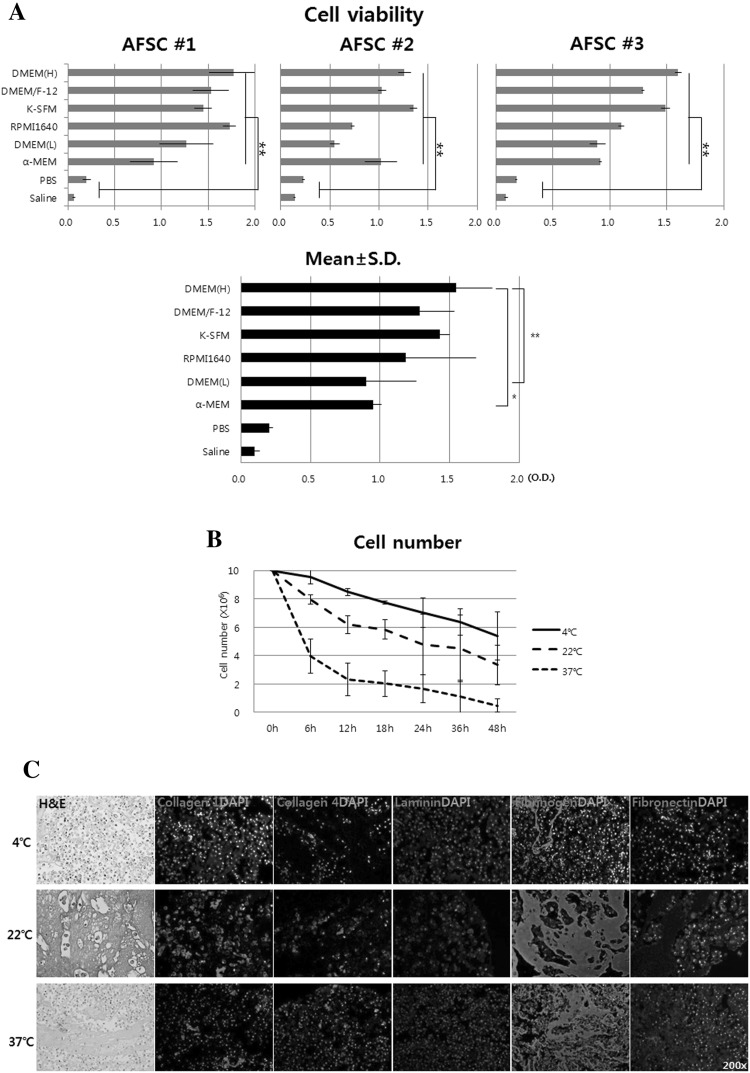
Among the media, cell viability was the highest in DMEM(H), followed by DMEM/F-12, K-SFM, RPMI 1640, DMEM(L), α-MEM, PBS, and saline in sequence. The mean O.D. values obtained for each of the medium were 1.55 ± 0.26, 1.28 ± 0.25, 1.43 ± 0.07, 1.19 ± 0.50, 0.90 ± 0.36, 0.95 ± 0.06, 0.20 ± 0.03 and 0.10 ± 0.04, respectively (Fig. 1A). All of the media showed significant cell viability compared to PBS and saline (p < 0.01). Although the statistical significance among media was seen in DMEM (L) and α-MEM, cell viability was highest in DMEM (L). Based on this result, DMEM(H) was selected as the cell transport medium.
Fig. 1.
Optimization of in vitro cell transport conditions. A Selection of transport medium. Cell viability was the highest in DMEM(H), followed by DMEM/F-12, K-SFM, RPMI 1640, DMEM(L), α-MEM, PBS, and saline in sequence. B Selection of transport temperature and time. Conditions with cell viability more than 80% were temperature of 4 °C and duration of less than 12 h. C Immunocytochemical analysis of the aggregated cell. Cell aggregation was due to the production of various extracellular matrix (ECM), and fibrinogen was dominant. D Real-time PCR analysis of the aggregated cell. The expressions of ECM-related genes were significantly increased at 22 and 37 °C compared to 4 °C. E Cell viability according to medium volume. As medium volume increased, cell viability was proportionally increased. F Living cell number depending on transport container. Even though, glass bottle showed slight high cell viability, there was no statistical difference between the two containers. Ctrl, 1 × 107 cells suspended in 2 mL of DMEM(H) at 4 °C and used immediately, Transported; cell was prepared with same condition and then incubated at 4 °C for 12 h. OD, optical density; *p < 0.05 and **p < 0.01
Selection of transport temperature and time
The viable cell number at 4 °C at each time point (6, 12, 18, 24, 36 and 48 h) was 9.56, 8.48, 7.74, 7.03, 6.35 and 5.39 × 106, respectively. At 22 °C, cell numbers were 7.95, 6.20, 5.84, 4.78, 4.52 and 3.35 × 106, respectively. At 37 °C, cell numbers were 3.96, 2.32, 2.02, 1.66, 1.10 and 0.48 × 106, respectively (Fig. 1B). The temperature and time at which cell viability exceeds 80% were at 4 °C within 12 h. Therefore, the selected temperature and time were 4 °C and within 12 h.
Next, cell mass, aggregated at 22 and 37 °C, were analyzed with H&E, ICC and real-time PCR to evaluate the type of ECM. Various ECM markers including collagen I (Col1), Col4, laminin, fibrinogen, and fibronectin were used. The ICC results were indicated that cell aggregation was due to the production of various ECM, and fibrinogen was dominant (Fig. 1C). The expressions of ECM-related genes were significantly increased at 22 and 37 °C compared to 4 °C (Fig. 1D). This ECM analysis suggested that cells for vascular injection should be kept at 4 °C to maintain single cell state.
Confirmation of transport cell density
Cells (1 × 107) were treated in various volumes of DMEM(H) from 0.1, 0.5, 1.0 and 2.0 mL at 4 °C for 12 h. Viability of each volume was 57.97 ± 16.79, 75.33 ± 16.59, 95.33 ± 20.87 and 106.8 ± 22.94%, respectively (Fig. 1E). As medium volume increased, cell viability was proportionally increased. Therefore, it is advisable to suspend cells at a maximum medium volume that one time injection capacity, and cell pellet state should be avoid because cells die by 50%.
Selection of transport container
After 1 × 107 cells cultured in 2 mL of DMEM(H) for 12 h, the viable cell number in the glass bottle was 8.70 × 106 and the plastic syringe was 8.48 × 106 (Fig. 1F). Even though, glass bottle showed slight high cell viability, there was no statistical difference between the two containers. Thus the syringe was selected as the cell container for clinical convenience.
Stem cell characteristics and differentiation potential of transported cells
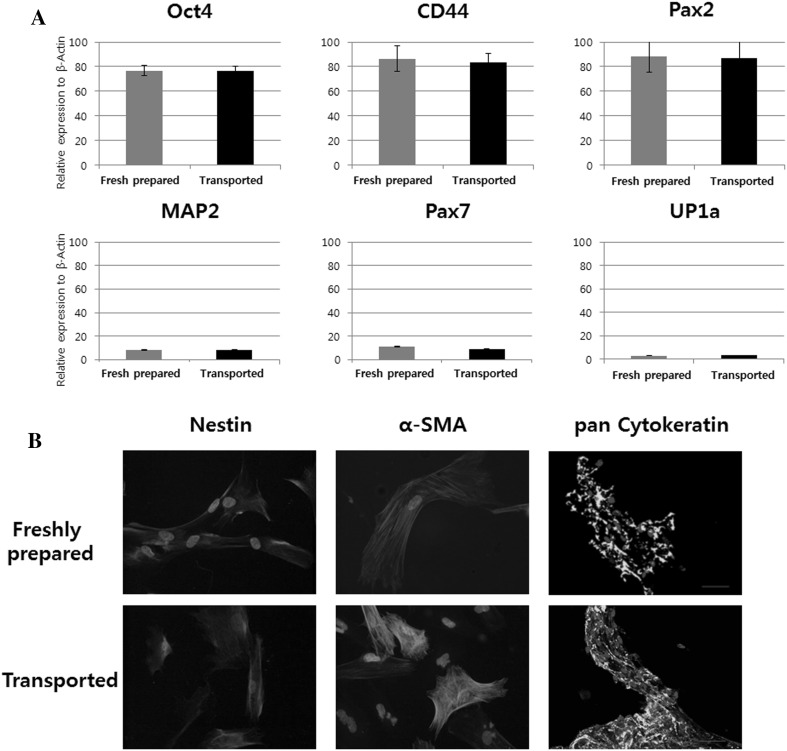
In stem cell and renal progenitor characteristic analysis, Oct4, CD44, and Pax2 expression was well-maintained under the established transport conditions, and spontaneous differentiation into neuron (MAP2), muscle (Pax7), and endothelium (UP1a) cells was effectively inhibited (Fig. 2A) during transport. The transported cells were well differentiated into nerve, muscle and endothelial lineages when treated with each differentiation medium (Fig. 2B).
Fig. 2.
Comparison between transported cells and freshly prepared cells for A maintenance of stem cell and B multi-differentiation potency. Freshly prepared; 1 × 107 cells suspended in 2 mL of DMEM(H) at 4 °C and used immediately, Transported; cell was prepared with same condition and then incubated at 4 °C for 12 h. α-SMA; alpha smooth muscle actin
Discussion
Stem cells show great potential in regenerative medicine and stem cell-based cellular therapeutic strategies have been developed to treat incurable or degenerative diseases [3, 16]. To ensure the safety and potency of cell therapy, cells intended for implantation should be processed under standardized conditions, including for culture, storage, and transportation, because these conditions can significantly affect the clinical results [9]. During these processes, cells need to remain viability and stem cell characteristics [17].
However, most of studies focused on cell culture and storage process, only few studies have interested for transport conditions. This study was carried out to establish optimal transporting conditions to maintain viability and stem cell characteristics. We optimized the cell transporting medium, temperature, time, density, and container, and then compared maintenance of stem cell characteristics and inhibition of spontaneous differentiation of the transported cells against the freshly prepared cells.
To select the optimal medium, DMEM(H), DMEM/F-12, K-SFM, RPMI 1640, α-MEM, DMEM(L), PBS, and saline were tested. Among them, DMEM(H) showed the highest cell viability. DMEM(H) contains limited nutrients that cells need to survive (not for proliferation), such as inorganic salts, amino acids, and vitamins. Because of these minimum compositions, DMEM(H) is believed to decrease cell metabolism, and this response is believed to be beneficial to survival at low temperatures [18]. Since PBS and saline are composed of only inorganic salts that could not supply the least nutrition, so, most of cells in those media died during the 12 h transport period. The selected DMEM(H) was excluded pH indicators (phenol red or HEPES) and samples of animal origin (xeno). Many of these additives, particularly serum of animal origin, are not acceptable for patients [19] because a lack of tumorigenicity, deformity, and developmental disability effects following long-term exposure in vivo have not been confirmed.
To verify the optimum transport temperature, cell viability was compared at 4 °C, 22 °C, and 37 °C for 48 h. The number of live cells was higher at 4 °C than at 22 °C and 37 °C. For clinical treatment, cell viability is required more than 80% when injected to patients [20]. When the cells were stored at 4 °C within 12 h, cell viability was more than 80%, in contrast, other temperatures such as 22 °C and 37 °C did not satisfy this range. At low temperatures, cells become quiescence which could play a role in increasing cell survival in limited nutrient and oxygen conditions [21], and thus stimulated cells in an inappropriate environment may die. Previous studies demonstrated that cells should be stored under refrigeration [19], and short-term storage of peripheral blood stem cells, peripheral blood mononuclear cells, and bone marrow products should be refrigerated to maintain cell viability [22]. Based on reported studies and the results of this experiment, the transport temperature was selected at 4 °C.
After selecting the temperature, the effect of temperature and medium combination on cell survival was considered. The therapeutic cells are typically transported as a suspension at low temperature for several hours. When cells are stored at low temperature, such as 4 °C, they adapt to the low temperature by decreasing metabolism, similar to animal hibernation This adaption causes reduced membrane fluidity [23], decreased affinity of enzymes for their substrates [24], and increased aqueous viscosity [25]. Additionally, most therapeutic cells show attachment properties, and long-term suspension transport conditions can cause anoikis [26]. In this state, if cells are supplied with a rich nutrient, cell attachment, proliferation, or differentiation can be induced. These cellular responses at low temperatures can cause cell death, and thus minimal nutrient medium is more suitable for maintaining cell viability.
In the transport temperature experiments, cell aggregation was detected at 22 and 37 °C. Cell aggregation carries a clinical risk because vascular injection of stem cells is a commonly executed route in several preclinical settings [27, 28]. When cells are injected intravenously to reach a target site, single cells should be administered. If aggregated cells are injected, cells can attach to vascular endothelial cells and platelets, which may reduce blood flow, interfere with blood circulation, and cause embolism in the micro-capillary [29]. The cell aggregation may cause by ECM components, and those were actively secreted at a room temperature. The ECM components were Col1, Col4, laminin, fibrinogen and fibronectin, and among them, fibrinogen was dominant. The ECM formation during transport can stimulate cells to differentiate, because ECM is a differentiation-stimulating factor [30]. Therefore, during cell transport, low-temperature storage is necessary to prevent cell mass formation.
Cell density is another important factor affecting stem cell viability during transport [22] and should remain low density because of limited nutrient and oxygen availability [31]. In this study, 1 × 107 cells were added to 0.1, 0.5, 1.0 or 2.0 mL of medium for 12 h. The results showed that cell viability was proportional to the amount of culture medium, and 1 × 107 cells should be suspended in more than 1.0 mL of medium to maintain more than 80% cell survival. These results suggest that low cell density is an important factor to maintain cell viability when cell transport.
Transport container types also can affect cell viability and characteristics, and the cell response to a given container may different. Cell responses to container type were examined in plastic syringes and glass bottles. Glass has a naturally polarized surface, providing a suitable surface for cell survival [32]. However, bottles are inconvenient because cells had better be placed in syringes for direct injection into patients. The plastic syringe was composed mainly of polypropylene and has a slight hydrophobic character [33], but its contents can be injected into patients in one step. Our data showed similar cell viability for both containers during storage at 4 °C for 12 h. Thus, the plastic syringe was selected as a suitable transport container because of its convenience in the clinical field.
The maintenance of stem cell characteristics including inhibition of unexpected differentiation were compared to the transported cells and freshly prepared cells. The expression of the stem cell and renal progenitor markers (Oct4, CD44, and Pax2) was maintained under the established conditions, and spontaneous differentiation into neuron (MAP2), muscle (Pax7), or endothelium (UP1a) cells was significantly inhibited. In addition, the transported cells remain their multi-potency, which was confirmed to be differentiated when treated with each differentiation medium in vitro. The ICC analysis showed that the cells differentiated for 2 weeks had a significant positive reaction to Nestin, a-SMA, pan Cytokeratin antibody. Thus, the established conditions can maintain stem cell characteristics and multi-differential potency.
In this study, we established the optimal cell transport conditions through analysis of viability and stem cell characteristics. The optimized transport conditions were as follows; the medium was DMEM high-glucose, the temperature was 4 °C, and the limited time was within 12 h. A lower cell density resulted in a better cell survival rate, and a syringe was selected as a suitable container. The transported cells with the established conditions showed similar stem cell characteristics as freshly prepared cells in the in vivo analysis.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and funded by the Korean government, which is funded by the Ministry of Science and ICT (2014M3A9D3034164, 2015R1C1A1A01053509, 2016R1C1B1011180), the Ministry of Education (2015R1D1A3A03020378) and the Ministry of Trade, Industry and Energy (R0005886).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Ethics Committee of Kyungpook National University Hospital (IRB No. KNUH 2012-10-018). This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Contributor Information
Bum Soo Kim, Email: urokbs@knu.ac.kr.
Tae Gyun Kwon, Email: tgkwon@knu.ac.kr.
References
- 1.Joo S, Ko IK, Atala A, Yoo JJ, Lee SJ. Amniotic fluid-derived stem cells in regenerative medicine research. Arch Pharm Res. 2012;35:271–280. doi: 10.1007/s12272-012-0207-7. [DOI] [PubMed] [Google Scholar]
- 2.Rota C, Imberti B, Pozzobon M, Piccoli M, De Coppi P, Atala A, et al. Human amniotic fluid stem cell preconditioning improves their regenerative potential. Stem Cells Dev. 2012;21:1911–1923. doi: 10.1089/scd.2011.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 4.Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Minguell JJ, Erices A. Mesenchymal stem cells and the treatment of cardiac disease. Exp Biol Med (Maywood) 2006;231:39–49. doi: 10.1177/153537020623100105. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus H, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 7.Bosse R, Singhofer-Wowra M, Rosenthal F, Schulz G. Good manufacturing practice production of human stem cells for somatic cell and gene therapy. Stem Cells. 1997;15(Suppl 1):275–280. doi: 10.1002/stem.5530150835. [DOI] [PubMed] [Google Scholar]
- 8.Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–471. doi: 10.1634/stemcells.2004-0331. [DOI] [PubMed] [Google Scholar]
- 9.Sohn HS, Heo JS, Kim H-S, Choi Y, Kim HO. Duration of in vitro storage affects the key stem cell features of human bone marrow-derived mesenchymal stromal cells for clinical transplantation. Cytotherapy. 2013;15:460–466. doi: 10.1016/j.jcyt.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Chen B, Wright B, Sahoo R, Connon CJ. A novel alternative to cryopreservation for the short-term storage of stem cells for use in cell therapy using alginate encapsulation. Tissue Eng Part C Methods. 2013;19:568–576. doi: 10.1089/ten.tec.2012.0489. [DOI] [PubMed] [Google Scholar]
- 11.Pollock K, Sumstad D, Kadidlo D, McKenna DH, Hubel A. Clinical mesenchymal stromal cell products undergo functional changes in response to freezing. Cytotherapy. 2015;17:38–45. doi: 10.1016/j.jcyt.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moll G, Alm JJ, Davies LC, von Bahr L, Heldring N, Stenbeck-Funke L, et al. Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells. 2014;32:2430–2442. doi: 10.1002/stem.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moon JH, Lee JR, Jee BC, Suh CS, Kim SH, Lim HJ, et al. Successful vitrification of human amnion-derived mesenchymal stem cells. Hum Reprod. 2008;23:1760–1770. doi: 10.1093/humrep/den202. [DOI] [PubMed] [Google Scholar]
- 14.Özkavukcu S, Erdemli E. Cryopreservation: basic knowledge and biophysical effects. J Ankara Med School. 2002;24:187–196. [Google Scholar]
- 15.Shu Z, Heimfeld S, Gao D. Hematopoietic SCT with cryopreserved grafts: adverse reactions after transplantation and cryoprotectant removal before infusion. Bone Marrow Transplant. 2014;49:469–476. doi: 10.1038/bmt.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 17.Hagell P, Brundin P. Cell survival and clinical outcome following intrastriatal transplantation in Parkinson disease. J Neuropathol Exp Neurol. 2001;60:741–752. doi: 10.1093/jnen/60.8.741. [DOI] [PubMed] [Google Scholar]
- 18.Berggren M, Laudon H, Jonsson A, Jansson M. Nutrient constraints on metabolism affect the temperature regulation of aquatic bacterial growth efficiency. Microb Ecol. 2010;60:894–902. doi: 10.1007/s00248-010-9751-1. [DOI] [PubMed] [Google Scholar]
- 19.Burger SR, Hubel A, McCullough J. Development of an infusible-grade solution for non-cryopreserved hematopoietic cell storage. Cytotherapy. 1999;1:123–133. doi: 10.1080/0032472031000141250. [DOI] [PubMed] [Google Scholar]
- 20.Gálvez-Martín P, Hmadcha A, Soria B, Calpena-Campmany AC, Clares-Naveros B. Study of the stability of packaging and storage conditions of human mesenchymal stem cell for intra-arterial clinical application in patient with critical limb ischemia. Eur J Pharm Biopharm. 2014;86:459–468. doi: 10.1016/j.ejpb.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Olson WC, Smolkin ME, Farris EM, Fink RJ, Czarkowski AR, Fink JH, et al. Shipping blood to a central laboratory in multicenter clinical trials: effect of ambient temperature on specimen temperature, and effects of temperature on mononuclear cell yield, viability and immunologic function. J Transl Med. 2011;9:26. doi: 10.1186/1479-5876-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kao GS, Kim HT, Daley H, Ritz J, Burger SR, Kelley L, et al. Validation of short-term handling and storage conditions for marrow and peripheral blood stem cell products. Transfusion. 2011;51:137–147. doi: 10.1111/j.1537-2995.2010.02758.x. [DOI] [PubMed] [Google Scholar]
- 23.Graumann P, Marahiel MA. Some like it cold: response of microorganisms to cold shock. Arch Microbiol. 1996;166:293–300. doi: 10.1007/s002030050386. [DOI] [PubMed] [Google Scholar]
- 24.Gerday C, Aittaleb M, Arpigny JL, Baise E, Chessa JP, Garsoux G, et al. Psychrophilic enzymes: a thermodynamic challenge. Biochim Biophys Acta. 1997;1342:119–131. doi: 10.1016/S0167-4838(97)00093-9. [DOI] [PubMed] [Google Scholar]
- 25.D'amico S, Collins T, Marx JC, Feller G, Gerday C. Psychrophilic microorganisms: challenges for life. EMBO Rep. 2006;7:385–389. doi: 10.1038/sj.embor.7400662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gilmore AP. Anoikis. Cell Death Differ. 2005;12(Suppl 2):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 27.Cui LL, Kerkelä E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015 doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui LL, Kinnunen T, Boltze J, Nystedt J, Jolkkonen J. Clumping and viability of bone marrow derived mesenchymal stromal cells under different preparation procedures. Stem Cells Int. 2016;2016:1764938. [DOI] [PMC free article] [PubMed]
- 29.Furlani D, Ugurlucan M, Ong L, Bieback K, Pittermann E, Westien I, et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc Res. 2009;77:370–376. doi: 10.1016/j.mvr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Linsley C, Wu B, Tawil B. The effect of fibrinogen, collagen type I, and fibronectin on mesenchymal stem cell growth and differentiation into osteoblasts. Tissue Eng Part A. 2013;19:1416–1423. doi: 10.1089/ten.tea.2012.0523. [DOI] [PubMed] [Google Scholar]
- 31.Radisic M, Euloth M, Yang L, Langer R, Freed LE, Vunjak-Novakovic G. High-density seeding of myocyte cells for cardiac tissue engineering. Biotechnol Bioeng. 2003;82:403–414. doi: 10.1002/bit.10594. [DOI] [PubMed] [Google Scholar]
- 32.Guseva NV, Dessus-Babus S, Moore CG, Whittimore JD, Wyrick PB. Differences in Chlamydia trachomatis serovar E growth rate in polarized endometrial and endocervical epithelial cells grown in three-dimensional culture. Infect Immun. 2007;75:553–564. doi: 10.1128/IAI.01517-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith ER, Howlin BJ, Hamerton I. Using POSS reagents to reduce hydrophobic character in polypropylene nanocomposites. J Mater Chem A. 2013;1:12971–12980. doi: 10.1039/c3ta80001f. [DOI] [Google Scholar]