Abstract
Background:
Thermogel is an aqueous solution that exhibits a sol-to-gel transition as the temperature increases. Stem cells, growth factors, and differentiating factors can be incorporated in situ in the matrix during the sol-to-gel transition, leading to the formation of a three-dimensional (3D) cell-culture scaffold.
Methods:
The uses of thermogelling polypeptides, such as collagen, Matrigel™, elastin-like polypeptides, and synthetic polypeptides, as 3D scaffolds of cells, are summarized in this paper.
Results:
The timely supply of growth factors to the cells, cell survival, and metabolite removal is to be insured in the cell culture matrix. Various growth factors were incorporated in the matrix during the sol-to-gel transition of the thermogelling polypeptide aqueous solutions, and preferential differentiation of the incorporated stem cells into specific target cells were investigated. In addition, modulus of the matrix was controlled by post-crosslinking reactions of thermogels or employing composite systems. Chemical functional groups as well as biological factors were selected appropriately for targeted differentiation of the incorporated stem cells.
Conclusion:
In addition to all the advantages of thermogels including mild conditions for cell-incorporation and controlled supplies of the growth factors, polypeptide thermogels provide neutral pH environments to the cells during the degradation of the gel. Polypeptide thermogels as an injectable scaffold can be a promising system for their eventual in vivo applications in stem cell therapy.
Keywords: Thermogel, Polypeptide, 3D cell culture, Stem cells, Scaffolds
Introduction
Due to the multipotential nature of adult stem cells, stem cell therapy has been investigated as a novel method for restoring the function of damaged tissues [1]. Scientists have discovered various sources of adult stem cells such as adipose tissue, bone marrow, tonsil tissue, dental pulp, the umbilical cord, synovial fluid, and the placenta [2]. An adequate number of healthy cells and scaffold optimization are crucial factors for successful stem cell therapy. However, there are still problems to be resolved such as dislocation of cells, cell survival, and control of stem cell differentiation into specific target cells [3]. Three-dimensional (3D) cell culture provides fundamental information for understanding cells in a living system. Cell morphology and gene expression are modulated in a different manner under two-dimensional (2D) and 3D environments. 3D environments can affect cell proliferation, differentiation, mechano-responses, and cell survival, those effects are quite different from 2D systems [4–6]. For example, chondrocytes increase expression of type I collagen (COL I) and exhibit a fibroblast morphology in a 2D surface, but they maintain their original spherical morphology with higher type II collagen (COL II) expression in a 3D environment [7, 8]. As another example, breast epithelial cells change to tumor-like cells under 2D conditions, and they reset to their original state in a 3D environment [9].
Hydrogels are considered to be a promising material as an artificial extracellular matrix (ECM) [10]. Both natural and synthetic hydrogels are intended to replicate the natural 3D environment. Cells organize into tissues by maintaining their specialized configurations and morphology [11]. Synthetic hydrogels are often recommended for their reproducibility of production, and compositional control, and low immunogenicity. Synthetic hydrogels have been extensively studied as a 3D culture system for these reasons [12, 13]. A hydrogel that undergoes a sol-to-gel transition with increasing temperature is termed a thermogel. The sol-to-gel transition process is less harmful to cells than chemical or photochemical crosslinking processes used to prepare 3D scaffolds [14]. The simple mixture of cells and growth factors in a solution state is physically crosslinked into a gel by increasing the temperature, and the gel acts as a 3D cell culture matrix incorporating the growth factors. Shear stress during syringe injection of a thick gel is a major concern in terms of cell death, which can be improved by using thermogels [15–18]. Additionally, thermogels can be injected into a target site without complicated surgery and can be formed into any shape [19].
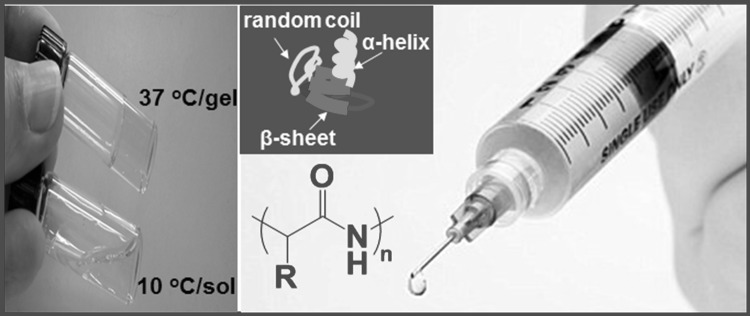
Depending on the amino acid sequence, polypeptides exhibit unique secondary structures such as α-helices, β-sheets, and random coils [20–25]. Polypeptide-based thermogels have great potential in 3D cell culture and tissue engineering applications as in situ gel-forming matrices that incorporate growth factors, cells, and other components (Fig. 1). The major benefits of synthetic polypeptide-based thermogels are controllable degradation and neutral degradation products. Polypeptide hydrogel is degraded by specific enzymes. Ideally, degradation kinetics of scaffold should be matched to the cell proliferation or tissue growth kinetics. The degradation of the hydrogel can be controlled by the composition and molecular weight of the polypeptides. In addition, release kinetics of the incorporated growth factors can be controlled by the degradation of the polypeptides. During degradation, growth factors should be supplied to the cells at an appropriate time in the cell cycle. Therefore, the structure can be designed to meet specific biomedical needs. This review covers the application of polypeptide thermogels in the 3D culture of stem cells and their differentiation to specific cells. We discussed both natural and synthetic polypeptide thermogels as 3D cell culture scaffolds.
Fig. 1.
Thermogelling polypeptide aqueous solution. Polypeptides can form α-helices, β-sheets, or random coils depending on the sequence of the amino acids. The aqueous polypeptide solution undergoes a sol-to-gel transition as the temperature increase from 10 to 37 °C
Collagen and Matrigel™
Collagen is a major protein in the human and is mostly found in fibrous tissues such as tendons, ligaments, bones, blood vessels, and the skin. Collagen has received approval from the Food and Drug Administration (FDA) for clinical applications and has been commercialized by numerous companies including Bioscience, Advanced BioMatrix, Vitrogen and Flexcell [26].
Aqueous collagen solutions (0.2–2 mg/mL) undergo sol-to-gel transition through fibrilogenesis [27, 28]. The properties of gel or fibrilonetworks are sensitive to temperature, pH, and ionic strength because they affect the self-assembly process of collagen. Although collagen is widely used, it remains expensive and has a few limitations including low modulus, and triggering intrinsic immunogenic responses [29]. To improve the mechanical properties of collagen, various methods have been investigated, and are discussed in this section.
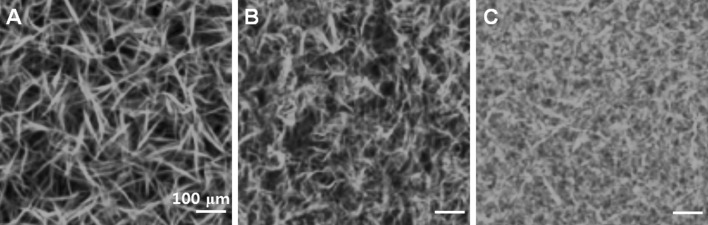
Collagen has been investigated as a 2D as well as 3D cell culture system [30–35]. COL I-coated culture plates promoted cell migration, proliferation, survival, and osteogenic differentiation compared with pristine culture plates [36–40]. In addition, stem cells cultured in 3D collagen hydrogels exhibited higher osteoblasts differentiation compared to stem cells cultured on collagen-coated 2D plates. The rigidity of the collagen hydrogel in a 3D culture affected osteoblast differentiation, and cells grew into mechanically hard, bone-like tissue in a rigid hydrogel [41]. Stem cells have been shown to differentiate into osteoblasts, cardiomyocytes, and neural cells in COL I gels, but differentiate into chondrocytes in COL II gels [42–48]. The chondrogenic gene expression of cells differentiated from adipose-tissue derived mesenchymal stem cells (ADSCs) was higher in COL II gels than in COL I gels, and these cells developed a round-shape morphology [48]. When mesenchymal stem cells (MSCs) were cultured in a COL I gel, they expressed significantly higher amounts of COL II and aggrecan than when in a pellet culture system [49]. Chondrogenic differentiation of ADSCs increased when adding transforming growth factor (TGF) β1 and applying cyclic hydrostatic pressure [50]. COL I gels formed a fibrillary structure with different orientations when varying polymerization temperatures of 4, 21, and 37 °C were used [51] (Fig. 2). Gels consisting of stiffer and shorter fibers suppressed cell spreading, proliferation, and migration and the stem cells exhibited a preference toward adipogenic differentiation. On the other hand, stem cells showed enhanced osteogenic differentiation in the collagen gels made up of long fibers. Moreover, the fact that COL II induces chondrogenic differentiation in a growth medium suggests that chondrocytes bind to COL II through integrin and stimulate differentiation of stem cells into chondrocytes [52]. Composite systems of COL I and COL II supported osteogenic differentiation of bone marrow-derived mesenchymal stem cells (BMSCs) and umbilical cord MSCs [53]. Additionally, chondrocytes preserved their natural spherical morphology and secreted cartilage-specific ECM in the composite systems containing COL I and COL II [54]. Chondrocytes was also shown to maintain their original phenotype and exhibited increased proliferation rate and expression of SOX 9, COL II, and aggrecan over a 3-week period in a composite hydrogel of alginate, hyaluronic acid, and collagen [55]. Hyaline cartilage regeneration has been reported from chondrocytes embedded in a COL II gel [56]. The addition of TGF β1 and hyaluronic acid enhanced cell viability, proliferation, and glycosaminoglycan (GAG) production [57]. Proliferation of chondrocyte is dependent on COL I concentration and exhibited excellent results in 1 mg/ml of COL I [58].
Fig. 2.
Morphologies of COL I gel. A–C The fibers with different length and thickness can be prepared at polymerization temperature of 4, 21, and 37 °C. Reproduced with permission from the American Chemical Society [51]
To improve the mechanical properties, inorganic materials were introduced with hydrogels. Nano hydroxyapatite (nHA) was added to a thermogelling system consisting of poly(ethylene glycol)-polycaprolactone-poly(ethylene glycol) (PEG-PCL-PEG) and collagen, which was used to elicit bone regeneration from stem cells [59]. A COL I/nHA composite scaffold was reported as a biocompatible, mechanically superior, and osteoconductive matrix. The composite scaffold (5.50 kPa) was 18 times stiffer than pure collagen (0.30 kPa) [60]. COL I/nHA was also used as a gene-activated matrix. The scaffold successfully delivered bone morphogenic polypeptide (BMP) 2 in MSCs, which subsequently produced high levels of calcium [61]. The development of bone matrix was confirmed in a nano composite system consisting of MSCs, nHA, and COL I after subcutaneous implantation [62]. The addition of osteogenic growth factor (BMP 2) in a collagen sponge/hydrogel complexes promoted bone generation in a dog with alveolar bone defects [63]. Additionally, rat dermal MSCs turned into osteoblast-like cells and enhanced calcification in a COL I gel. It has been suggested that endogenous TGF β1 secreted from the cells promotes the calcification [64].
Matrigel™ is a natural ECM obtained through decellularization of mouse sarcoma. Matrigel™ is a thermosensitive ECM of which aqueous solutions turn into gels as the temperature increases to 37 °C [65]. The basement membrane components of collagen, laminin, heparan sulfate, and proteoglycan, along with fibroblast growth factor (FGF), epidermal growth factor (EGF), and nerve growth factor (NGF) are the major constituents of Matrigel™ [66]. The aqueous solution of these proteins assembled into a fibrous gel network as the temperature increased [67]. The presence of the above proteins makes Matrigel™ excellent for 3D cell culture; however, lot-to-lot variation and safety concerns prevent it from use in clinical applications [68]. In spite of these limitations, Matrigel™ has been extensively studied for tissue engineering. BMSCs cultured in Matrigel™ survived for a longer time (up to 5 months) compared to those grown in either COL I or control systems injected with phosphate buffered saline (PBS) [69]. Even a growth factor-reduced Matrigel™ stimulated growth of neurite and migration of neuronal progenitor cells [35]. Neural precursor cells combined with Matrigel™ significantly improved sensorimotor and cognitive function in an ischemic stroke model [70]. Embryonic stem cells (ESCs) cultured in Matrigel™ exhibited dopaminergic neuronal differentiation. Neurite outgrowth and branching increased in the presence of retinoic acid [71]. Mouse neuronal stem cell (NSC) encapsulated in Matrigel™ showed oriented neurite growth under the microfluidic device [72]. Subcutaneous injection of tonsil-derived mesenchymal stem cells (TMSCs) incorporated Matrigel™ increased the survival rate of the TMSCs by 40–80%, and they differentiated into parathyroid-like cells and secreted a significant level of parathyroid hormone [73].
Elastin-like polypeptide
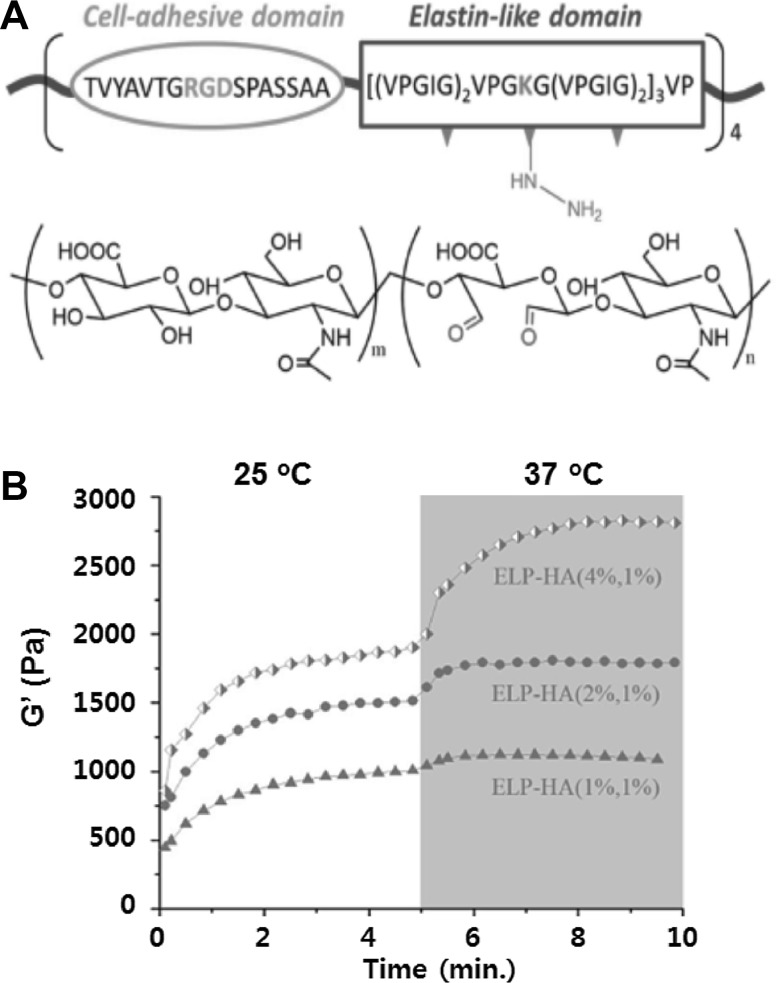
Elastin-like polypeptide (ELP) aqueous solutions also undergoes a sol-to-gel transition as the temperature increases to 34 °C. The most common motif in ELPs is Gly-Xaa-Gly-Val-Pro (GXGVP). Xaa is an arbitrary amino acid. Below the sol-to-gel transition temperature, ELP is a random coil in solution. However, above the transition temperature, the chain hydrophobically folds to β-spiral, involving type II β-turns as the main secondary feature. The hydrophobic association of β-spirals takes on fibrillar form which grow to several hundred nm to form a gel phase [74–76]. ELPs have been broadly investigated for both drug delivery and tissue engineering [77–80]. Human ADSCs incorporated in an ELP upregulated SOX 9 and COL II levels in the growth medium, which was especially pronounced under low-oxygen (5%) compared to high-oxygen (20%) conditions [79]. An RGD-modified ELP hydrogel highly increased cell spreading, attachment, proliferation, and osteogenic differentiation of MSCs [81, 82]. An ELP-poly(ethylene glycol) (ELP-PEG) hybrid hydrogel system was crosslinked with tris(hydroxymethyl) phosphine [83], and human fibroblast cells encapsulated in this hydrogel increased cell viability up to 98% and adopted a more spread-out morphology of cells in the RGD conjugated ELP-PEG thermogel. Tetrakis(hydroxymethyl) phosphonium chloride was added to crosslink an RGD-modified ELP thermogel, and thus the gel modulus increased by up to 2.1 kPa. ELP thermogels enhanced the neurite extension of dorsal root ganglia cells within 7 days [84]. A composite system of collagen and ELP showed an increase in the elastic modulus from 22.0 to 61.8 kPa. At a ratio of 18/6 of ELP/collagen in the composite system, osteogenic activity and deposition of calcium increased [85]. Photo crosslinking reactions increased the ELP modulus to 13 kPa. Cells encapsulated in ELP hydrogels exhibited good cell viability, metabolic activity, and attachment [86]. Photocrosslinked ELP was implanted in the subcutaneous layer of rats, where the gel was stable for more than 56 days [87]. ELP crosslinked with transglutaminase improved cartilage repair. In addition, chondrocytes maintained their phenotype in the ELP hydrogel. The gel increased its modulus from 0.28 to 1.7 kPa due to matrix deposition [88]. A combination of chemical crosslinking and thermogelation stiffened an ELP-hyaluronic acid gel up to 3 kPa by using hydrazine-modified ELP and aldehyde-modified hyaluronic acid gels [89] (Fig. 3). The gel was investigated for chondrogenic, adipogenic and osteogenic differentiation of the incorporated stem cells.
Fig. 3.
ELP-hyaluronic acid conjugate system. A–B Structure and modulus of the gel as a function of time and temperature. Reproduced with permission from Wiley [89]
Synthetic polypeptide thermogels
Synthetic polypeptides can be produced in a large scale or kilogram by ring-opening polymerization of corresponding N-carboxy anhydrides. Degradation kinetics can also be controlled by varying the composition of the polypeptides. In addition, uncertain impurities of biologically-derived materials are concerns due to the fact that they often induce immunogenicity in their eventual in vivo applications [90, 91]. To develop synthetic polypeptide-based thermogels, PEG, poly(N-vinyl pyrrolidone), and Pluronics® (PLX) were used as a hydrophilic block, and polyalanine (PA), poly(alanine-co-phenylalanine) (PAF), and poly(alanine-co-leucine) (PAL) were used as a hydrophobic block [92–94]. Thermogelling polypeptide solutions undergo sol-to-gel transition by dehydration of PEG and changes in secondary structures of polypeptides [92, 94, 95]. As the temperature of polymer aqueous solution increases polypeptide and PEG exhibit following characteristics. α-helix structures of polypeptides are rather stable against temperature as shown in PEG/PAF block copolymers, whereas β-sheet structures of polypeptide are reinforced as shown in PEG/PA block copolymers. In both cases, dehydration of PEG, followed by aggregation of the micelles leads to gel formation, as proved by the collapse of PEG peaks in the 1H-NMR spectra and increase in micellar size in the dynamic light scattering of the aqueous polymer solutions. PEG-PAF thermogel was stable in in vitro but degraded over 2 weeks in the subcutaneous layer of rats [92]. Degradation kinetics of polymers is crucial for their biomedical applications, and it could be controlled based on the composition of the polypeptides. Chondrocytes encapsulated in PA-PLX-PA block copolymer thermogels grew, proliferated, and maintained their spherical phenotype [7, 96]. Chondrogenic gene expression of COL II and GAG was excellent in an initial polymer concentration range of 7–15 wt% [97]. BMSCs, ADSCs and TMSCs expressed chondrogenic genes in both PEG-PA and PEG-PAF thermogels under in vitro and in vivo conditions. These cells maintained their spherical morphology in the PEG-PA or PEG-PAF thermogels, whereas they developed a fibroblast morphology in Matrigel™ [98–100]. TMSCs differentiated into hepatocytes in PEG-PA thermogels with a modulus of approximately 1000 Pa at 37 °C. The cells upregulated expression of the hepatogenic biomarkers of albumin, cytokeratin 18 (CK18), hepatocyte nuclear factor 4 alpha (HNF4α) in the presence of differentiating factors such as hepatocyte growth factor (HGF), basic FGF, and nicotinamide [101]. Moreover, with the incorporation of tauroursodeoxycholic acid (TUDCA) in addition to growth factors (HGF and FGF 4) in PEG-PA thermogels, TMSCs underwent hepatogenic differentiation and significantly elevated expression of the hepatogenic biomarkers of albumin, CK18, HNF4α, glucose 6-phosphatase, cytochrome P450 family 7 subfamily A member 1 (CYP7A1), hepatocyte nuclear factor 3 beta (HNF3β) [102]. The differentiated cells produced approximately 60% more albumin and urea than cells cultured in the HyStem™ gel over the same time period.
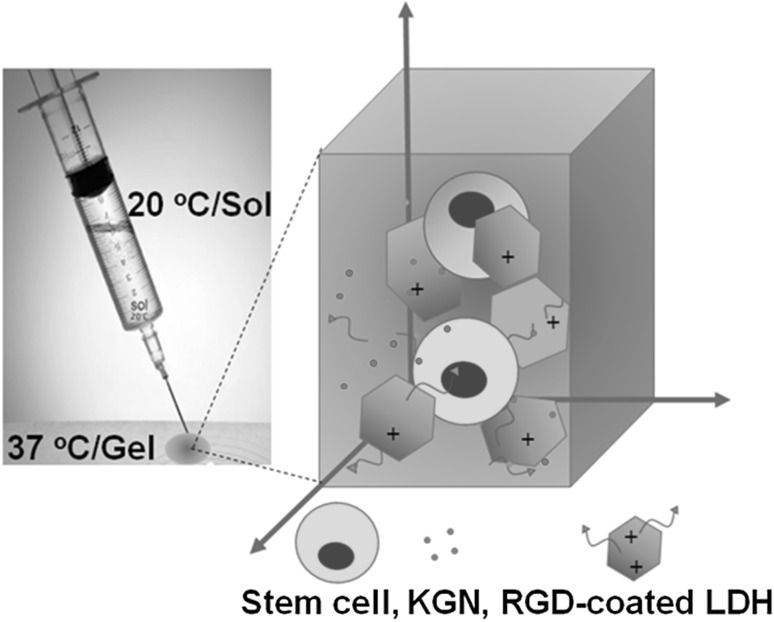
To improve the differentiation of stem cells into target cells in polypeptide thermogels, several composite systems have been investigated as a 3D scaffold of the stem cells. Polystyrene microspheres modified with various functional groups were incorporated in the PEG-PA thermogels. Chondrogenic mRNA expression was significantly higher in thermogels incorporating ammonium or thiol-modified microspheres, but adipogenic and osteogenic mRNA expression was elevated in thermogels incorporating phosphate- and carboxylate-modified microspheres [103]. A composite system of RGD-coated layered double hydroxides (LDHs)/PEG-PA-PD thermogel was prepared as an inorganic/organic hybrid system [104] (Fig. 4). Expression of COL II and SOX 9 at both mRNA and protein levels were significantly increased in the hybrid system compared with a pure PEG-PA thermogel system. The continuous release of kartogenin (KGN) from the hybrid scaffold improved cell aggregation and chondrogenic biomarker expression. Other hybrid systems, including a composite system consisting of graphene oxide (GO) and a PEG-PA thermogel, were also prepared [105]. TMSCs maintained their spherical morphology in the gel hybrid system for up to 21 days. However, the cells became extensively aggregated in the hybrid system, and expression of chondrogenic biomarkers, such as COL II and SOX 9, was significantly increased in the presence of TGF-β3 compared to either pure PEG-PA thermogels or reduced graphene oxide (rGO)/PEG-PA thermogel hybrid systems. In addition, when TMSCs were cultured in an adipogenic induction medium, they more readily differentiated into adipocytes in the GO/PEG-PA hybrid system compared to either pure PEG-PA thermogels or rGO/PEG-PA thermogel hybrid systems [106]. TMSCs differentiated into osteocytes in a composite system consisting of calcium phosphate mesocrystals or nanocrystals and PEG-PAF thermogel [107]. Expression of osteogenic biomarkers, such as BMP 2, osteocalcin, and alkaline phosphatase, was significantly increased in the meso composite system compared to the nano composite system. The hard surface of the mesocrystals provided locations for cell attachments and the thermogel held the cells in place, thus improving osteogenic differentiation of the stem cells. Neuron growth factor-encapsulating alginate microspheres were incorporated in a PEG-PA thermogel for neuronal differentiation of the stem cells [108]. The growth factors were released for up to 4 days and 18 days from the pure thermogel and microsphere-incorporated thermogel, respectively (Fig. 5). Expression of neuronal biomarkers such as nuclear receptor related protein (Nurr-1), neuron specific enolase (NSE), microtubule associated protein (MAP) 2, neurofilament-M (NF-M), and glial fibrillary acidic protein (GFAP) were significantly increased in the composite system. Conjugation of galactose to PEG-PA improved not only hepatogenic differentaion of the incorporated TMSCs but also hepatic biofunctions of the differentiated hepatocytes [109].
Fig. 4.
An inorganic/organic hybrid system consisting of RGD-coated LDH/PEG-PA thermogel incorporating stem cells and kartogenin (KGN). Reproduced with permission from the American Chemical Society [104]
Fig. 5.
A, B Release profiles of the neuronal growth factors from the PEG-PA thermogel (BDNF-GP and NGF-GP) and the microsphere-loaded PEG-PA thermogel (BDNF-MP and NFG-MP). Reproduced with permission from Wiley [108]
Conclusions
Thermogelling polymers aqueous solutions can be sterilizable by microfiltration in a sol state, and form a cell growing matrix by injection into a target site at 37 °C. The characteristics and applications of polypeptide-based thermogels including collagen, Materigel™, ELP, and synthetic polypeptides were summarized in this paper (Table 1). Various growth factors can be incorporated in the matrix to drive preferential differentiation of the incorporated stem cells into specific target cells during the sol-to-gel transition. The timely supply of growth factors to the cells is essential for effective control of the differentiation of the stem cell differentiation, which is provided by composite systems. Cell survival, oxygen and nutrient supply, and metabolite removal should be insured in the matrix. In addition, the modulus of the matrix should be optimized, and chemical functional groups, as well as biological factors, should be selected appropriately for targeted differentiation of the stem cells. Based on information regarding 3D cell culture using thermogels, their eventual application as an injectable tissue engineering scaffold is a promising technology for stem cell therapy.
Table 1.
Summaries of polypeptide thermogels for stem cell differentiation
| Thermogel | Characteristics and key properties | References |
|---|---|---|
| Collagen | COL I stimulates osteogenesis of MSCs | [41] |
| Stem cells differentiation into osteoblasts, cardiomyocytes, and neural cells favored in COL I gels, whereas chondrogenic differentiation is preferred in COL II gels | [42–47] | |
| Chondrogenic differentiation of ADSCs was higher in COL II gels than in COL I gels | [48] | |
| Chondrogenic differentiation of ADSCs increased under cyclic hydrostatic pressure | [50] | |
| Gels consisting of stiffer and shorter collagen fibers induced adipogenic differentiation, whereas osteogenic differentiation is preferred in the collagen gels made up of long fibers | [51] | |
| COL I/Nano hydroxyapatite composite system is effective in osteogenic differentiation of MSCs | [60] | |
| Matrigel™ | Matrigel™ stimulated growth of neurite and migration of neuronal progenitor cells | [35] |
| embryonic stem cells cultured in Matrigel™ exhibited dopaminergic neuronal differentiation. | [70] | |
| Neurite outgrowth and branching increased in the presence of retinoic acid | [71] | |
| Subcutaneous injection of tonsil-derived mesenchymal stem cells in Matrigel™ differentiated into parathyroid-like cells | [73] | |
| ELP | ADSCs differentiated into chondrocytes in an ELP gel under low-oxygen (5%) conditions | [79] |
| Mechanical properties of ELP gels increased by chemical crosslinking, photo-crosslinking, or collagen/ELP composite system | [83, 84, 87, 89] | |
| Osteogenic activity and deposition of calcium increased in the ELP/collagen composite system | [85] | |
| Synthetic polypeptide | BMSCs, ADSCs and TMSCs expressed chondrogenic genes in both PEG-PA and PEG-PAF thermogels | [98–100] |
| TMSCs differentiated into hepatocytes in PEG-PA thermogels | [101, 102] | |
| Differentiation of TMSCS into adipocytes, chondrocytes, and osteocytes could be controlled by surface functional groups of the incorporated microspheres in PEG-PA thermogels | [103] | |
| Chondrogenic differentiation of TMSCs improved in RGD-coated layered double hydroxides/PEG-PA-PD thermogels | [104] | |
| Addition of graphene oxide in PEG-PA thermogels enhanced chondrogenic differentiation of TMSCs | [105] | |
| Addition of hydroxyapatite mesocrystals in PEG-PAF thermogels improved osteogenic differentiation of TMSCs | [107] | |
| Sustained release of BDNF and NGF from the incorporated microspheres in PEG-PA thermogels enhanced neuronal differentiation of TMSCs | [108] | |
| Galactose-conjugated PEG-PA thermogels improved hepatogenic differentiation of TMSCs | [109] |
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (2017R1A2B2007356, 2017R1A5A1015365, and 2014M3A9B6034223). MP and HJL equally contributed to this paper.
Conflict of interest
The authors declare no competing financial interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Atala A. Regenerative medicine strategies. J Pediatr Surg. 2012;47:17–28. doi: 10.1016/j.jpedsurg.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Via AG, Frizziero A, Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012;2:154–162. [PMC free article] [PubMed] [Google Scholar]
- 3.Griffith LG, Naughton G. Tissue engineering-current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 4.Baker BM, Chen CS. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125:3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnier F, Keating ME, Wróbel TP, Majzner K, Baranska M, Garcia-Munoz A, et al. Cell viability assessment using the alamar blue assay: a comparison of 2D and 3D cell culture models. Toxicol In Vitro. 2015;29:124–131. doi: 10.1016/j.tiv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Gauvin R, Chen YC, Lee JW, Soman P, Zorlutuna P, Nichol JW, et al. Microfabrication of complex porous tissue engineering scaffolds using 3D projection stereolithography. Biomaterials. 2012;33:3824–3834. doi: 10.1016/j.biomaterials.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi BG, Park MH, Cho SH, Joo MK, Oh HJ, Kim EH, et al. In situ thermal gelling polypeptide for chondrocytes 3D culture. Biomaterials. 2010;31:9266–9272. doi: 10.1016/j.biomaterials.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 8.Jin GZ, Kim HW. Porous microcarrier-enabled three-dimensional culture of chondrocytes for cartilage engineering: a feasibility study. Tissue Eng Regen Med. 2016;13:235–241. doi: 10.1007/s13770-016-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersen OW, Rønnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanza R, Langer R, Vacanti J. Principles of tissue engineering. 4. Atlanta: Elsevier Acad; 2013. [Google Scholar]
- 12.Ko DY, Shinde UP, Yeon B, Jeong B. Recent progress of in situ formed gels for biomedical applications. Prog Polym Sci. 2013;38:672–701. [Google Scholar]
- 13.Loh XJ, Li J. Biodegradable thermosensitive copolymer hydrogels for drug delivery. Expert Opin Ther Pat. 2007;17:965–977. [Google Scholar]
- 14.Moon HJ, Ko du Y, Park MH, Joo MK, Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem Soc Rev. 2012;41:4860–4883. doi: 10.1039/c2cs35078e. [DOI] [PubMed] [Google Scholar]
- 15.Agashi K, Chau DY, Shakesheff KM. The effect of delivery via narrow-bore needles on mesenchymal cells. Regen Med. 2009;4:49–64. doi: 10.2217/17460751.4.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Walker PA, Jimenez F, Gerber MH, Aroom KR, Shah SK, Harting MT, et al. Effect of needle diameter and flow rate on rat and human mesenchymal stromal cell characterization and viability. Tissue Eng Part C Methods. 2010;16:989–997. doi: 10.1089/ten.tec.2009.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai L, Dewi RE, Heilshorn SC. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv Funct Mater. 2015;25:1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chem Soc Rev. 2008;37:1473–1481. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Stewart RJ, Kopecek J. Hybrid hydrogels assembled from synthetic polymers and coiled-coil protein domains. Nature. 1999;397:417–420. doi: 10.1038/17092. [DOI] [PubMed] [Google Scholar]
- 21.Yokoi H, Kinoshita T, Zhang S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc Natl Acad Sci U S A. 2005;102:8414–8419. doi: 10.1073/pnas.0407843102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petka WA, Harden JL, McGrath KP, Wirtz D, Tirrell DA. Reversible hydrogels from self-assembling artificial proteins. Science. 1998;281:389–392. doi: 10.1126/science.281.5375.389. [DOI] [PubMed] [Google Scholar]
- 23.Nowak AP, Breedveld V, Pakstis L, Ozbas B, Pine DJ, Pochan D, et al. Rapidly recovering hydrogel scaffolds from self-assembling diblock copolypeptide amphiphiles. Nature. 2002;417:424–428. doi: 10.1038/417424a. [DOI] [PubMed] [Google Scholar]
- 24.Jones R. Why nanotechnology needs better polymer chemistry. Nat Nanotechnol. 2008;3:699–700. doi: 10.1038/nnano.2008.349. [DOI] [PubMed] [Google Scholar]
- 25.Deming TJ. Synthetic polypeptides for biomedical applications. Prog Polym Sci. 2007;32:858–875. [Google Scholar]
- 26.Caliari SR, Burdick JA. A practical guide to hydrogels for cell culture. Nat Methods. 2016;13:405–414. doi: 10.1038/nmeth.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higuchi A, Ling QD, Hsu ST, Umezawa A. Biomimetic cell culture proteins as extracellular matrices for stem cell differentiation. Chem Rev. 2012;112:4507–4540. doi: 10.1021/cr3000169. [DOI] [PubMed] [Google Scholar]
- 28.Zhu J, Kaufman LJ. Collagen I self-assembly: revealing the developing structures that generate turbidity. Biophys J. 2014;106:1822–1831. doi: 10.1016/j.bpj.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan A, Khattab A, Islam MA, Hweij KA, Zeitouny J, Waters R, et al. Injectable hydrogels for cardiac tissue repair after myocardial infarction. Adv Sci (Weinh) 2015;2:1500122. doi: 10.1002/advs.201500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lanfer B, Seib FP, Freudenberg U, Stamov D, Bley T, Bornhäuser M, et al. The growth and differentiation of mesenchymal stem and progenitor cells cultured on aligned collagen matrices. Biomaterials. 2009;30:5950–5958. doi: 10.1016/j.biomaterials.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 31.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–1956. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 32.Ward DF, Jr, Salasznyk RM, Klees RF, Backiel J, Agius P, Bennett K, et al. Mechanical strain enhances extracellular matrix-induced gene focusing and promotes osteogenic differentiation of human mesenchymal stem cells through an extracellular-related kinase-dependent pathway. Stem Cells Dev. 2007;16:467–480. doi: 10.1089/scd.2007.0034. [DOI] [PubMed] [Google Scholar]
- 33.Park IS, Han M, Rhie JW, Kim SH, Jung Y, Kim IH, et al. The correlation between human adipose-derived stem cells differentiation and cell adhesion mechanism. Biomaterials. 2009;30:6835–6843. doi: 10.1016/j.biomaterials.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 34.Uemura M, Refaat MM, Shinoyama M, Hayashi H, Hashimoto N, Takahashi JJ. Matrigel supports survival and neuronal differentiation of grafted embryonic stem cell-derived neural precursor cells. J Neurosci Res. 2010;88:542–551. doi: 10.1002/jnr.22223. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki A, Iwama A, Miyashita H, Nakauchi H, Taniguchi H. Role for growth factors and extracellular matrix in controlling differentiation of prospectively isolated hepatic stem cells. Development. 2003;130:2513–2524. doi: 10.1242/dev.00459. [DOI] [PubMed] [Google Scholar]
- 36.Donzelli E, Salvadè A, Mimo P, Viganò M, Morrone M, Papagna R, et al. Mesenchymal stem cells cultured on a collagen scaffold: in vitro osteogenic differentiation. Arch Oral Biol. 2007;52:64–73. doi: 10.1016/j.archoralbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004:24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Yang Q, Mao C, Zhang S. Osteogenic differentiation of bone marrow mesenchymal stem cells on the collagen/silk fibroin bi-template-induced biomimetic bone substitutes. J Biomed Mater Res A. 2012;100:2929–2938. doi: 10.1002/jbm.a.34236. [DOI] [PubMed] [Google Scholar]
- 39.Somaiah C, Kumar A, Mawrie D, Sharma A, Patil SD, Bhattacharyya J, et al. Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS One. 2015;10:e0145068. doi: 10.1371/journal.pone.0145068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naito H, Yoshimura M, Mizuno T, Takasawa S, Tojo T, Taniguchi S, et al. The advantages of three-dimensional culture in a collagen hydrogel for stem cell differentiation. J Biomed Mater Res Part A. 2013;101:2838–2845. doi: 10.1002/jbm.a.34578. [DOI] [PubMed] [Google Scholar]
- 41.Naito H, Dohi Y, Zimmermann WH, Tojo T, Takasawa S, Eschenhagen T, et al. The effect of mesenchymal stem cell osteoblastic differentiation on the mechanical properties of engineered bone-like tissue. Tissue Eng Part A. 2011;17:2321–2329. doi: 10.1089/ten.TEA.2011.0099. [DOI] [PubMed] [Google Scholar]
- 42.George J, Kuboki Y, Miyata T. Differentiation of mesenchymal stem cells into osteoblasts on honeycomb collagen scaffolds. Biotechnol Bioeng. 2006;95:404–411. doi: 10.1002/bit.20939. [DOI] [PubMed] [Google Scholar]
- 43.Di Felice V, Ardizzone NM, De Luca A, Marcianò V, Marino Gammazza A, Macaluso F, et al. OPLA scaffold, collagen I, and horse serum induce a higher degree of myogenic differentiation of adult rat cardiac stem cells. J Cell Physiol. 2009;221:729–739. doi: 10.1002/jcp.21912. [DOI] [PubMed] [Google Scholar]
- 44.Shi C, Li Q, Zhao Y, Chen W, Chen B, Xiao Z, et al. Stem-cell-capturing collagen scaffold promotes cardiac tissue regeneration. Biomaterials. 2011;32:2508–2515. doi: 10.1016/j.biomaterials.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 45.Prabhakaran MP, Venugopal JR, Ramakrishna S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials. 2009;30:4996–5003. doi: 10.1016/j.biomaterials.2009.05.057. [DOI] [PubMed] [Google Scholar]
- 46.Tate CC, Shear DA, Tate MC, Archer DR, Stein DG, LaPlaca MC. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J Tissue Eng Regen Med. 2009;3:208–217. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- 47.Zheng L, Fan HS, Sun J, Chen XN, Wang G, Zhang L, et al. Chondrogenic differentiation of mesenchymal stem cells induced by collagen-based hydrogel: an in vivo study. J Biomed Mater Res A. 2010;93:783–792. doi: 10.1002/jbm.a.32588. [DOI] [PubMed] [Google Scholar]
- 48.Lu Z, Doulabi BZ, Huang C, Bank RA, Helder MN. Collagen type II enhances chondrogenesis in adipose tissue-derived stem cells by affecting cell shape. Tissue Eng Pt A. 2010;16:81–90. doi: 10.1089/ten.TEA.2009.0222. [DOI] [PubMed] [Google Scholar]
- 49.Chang CH, Lin HY, Fang HW, Loo ST, Hung SC, Ho YC, et al. Chondrogenesis from immortalized human mesenchymal stem cells: comparison between collagen gel and pellet culture methods. Artif Organs. 2008;32:561–566. doi: 10.1111/j.1525-1594.2008.00575.x. [DOI] [PubMed] [Google Scholar]
- 50.Ogawa R, Orgill DP, Murphy GF, Mizuno S. Hydrostatic pressure-driven three-dimensional cartilage induction using human adipose-derived stem cells and collagen gels. Tissue Eng Part A. 2014;21:257–266. doi: 10.1089/ten.TEA.2013.0525. [DOI] [PubMed] [Google Scholar]
- 51.Xie J, Bao M, Bruekers SMC, Huck WTS. Collagen gels with different fibrillar microarchitectures elicit different cellular responses. ACS Appl Mater Interfaces. 2017;9:19630–19637. doi: 10.1021/acsami.7b03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosnakovski D, Mizuno M, Kim G, Takagi S, Okumura M, Fujinaga T. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng. 2006;93:1152–1163. doi: 10.1002/bit.20828. [DOI] [PubMed] [Google Scholar]
- 53.Schneider RK, Puellen A, Kramann R, Raupach K, Bornemann J, Knuechel R, et al. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodeling in three-dimensional collagen scaffolds. Biomaterials. 2010;31:467–480. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 54.Yuan L, Li B, Yang J, Ni Y, Teng Y, Guo L, et al. Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocyte behaviors. Tissue Eng Part A. 2016;22:899–906. doi: 10.1089/ten.TEA.2015.0513. [DOI] [PubMed] [Google Scholar]
- 55.Mahapatra C, Jin GZ, Kim HW. Alginate-hyaluronic acid-collagen composite hydrogel favorable for the culture of chondrocytes and their phenotype maintenance. Tissue Eng Regen Med. 2016;13:538–546. doi: 10.1007/s13770-016-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Funayama A, Niki Y, Matsumoto H, Maeno S, Yatabe T, Morioka H, et al. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci. 2008;13:225–232. doi: 10.1007/s00776-008-1220-z. [DOI] [PubMed] [Google Scholar]
- 57.Kontturi LS, Järvinen E, Muhonen V, Collin EC, Pandit AS, Kiviranta I, et al. An injectable in situ forming type II collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Deliv Transl Res. 2014;4:149–158. doi: 10.1007/s13346-013-0188-1. [DOI] [PubMed] [Google Scholar]
- 58.Jin GZ, Kim HW. Effects of type I collagen concentration in hydrogel on the growth and phenotypic expression of rat chondrocytes. Tissue Eng Regen Med. 2017;14:383–391. doi: 10.1007/s13770-017-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu S, Ni P, Wang B, Chu B, Zheng L, Luo F, et al. Injectable and thermo-sensitive PEG-PCL-PEG copolymer/collagen/n-HA hydrogel composite for guided bone regeneration. Biomaterials. 2012;33:4801–4809. doi: 10.1016/j.biomaterials.2012.03.040. [DOI] [PubMed] [Google Scholar]
- 60.Cunniffe GM, Dickson GR, Partap S, Stanton KT, O’Brien FJ. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med. 2010;21:2293–2298. doi: 10.1007/s10856-009-3964-1. [DOI] [PubMed] [Google Scholar]
- 61.Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, et al. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mater. 2012;24:749–754. doi: 10.1002/adma.201103828. [DOI] [PubMed] [Google Scholar]
- 62.Liao S, Nguyen LT, Ngiam M, Wang C, Cheng Z, Chan CK, et al. Biomimetic nanocomposites to control osteogenic differentiation of human mesenchymal stem cells. Adv Healthc Mater. 2014;3:737–751. doi: 10.1002/adhm.201300207. [DOI] [PubMed] [Google Scholar]
- 63.Kim SK, Cho TH, Han JJ, Kim IS, Park Y, Hwang SJ. Comparative study of BMP-2 alone and combined with VEGF carried by hydrogel for maxillary alveolar bone regeneration. Tissue Eng Regen Med. 2016;13:171–181. doi: 10.1007/s13770-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suyama T, Hatta M, Hata S, Ishikawa H, Yamazaki J. Differentiation of rat dermal mesenchymal cells and calcification in three-dimensional cultures. Tissue Eng Regen Med. 2016;13:527–537. doi: 10.1007/s13770-016-9124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kleinman HK, Martin GR. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 66.Norrby K. In vivo models of angiogenesis. J Cell Mol Med. 2006;10:588–612. doi: 10.1111/j.1582-4934.2006.tb00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Uriel S, Labay E, Francis-Sedlak M, Moya ML, Weichselbaum RR, Ervin N, et al. Extraction and assembly of tissue-derived gels for cell culture and tissue engineering. Tissue Eng Part C Methods. 2009;15:309–321. doi: 10.1089/ten.tec.2008.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koutsopoulos S, Zhang S. Long-term three-dimensional neural tissue cultures in functionalized self-assembling peptide hydrogels, Matrigel and collagen I. Acta Biomater. 2013;9:5162–5169. doi: 10.1016/j.actbio.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Cao F, Sadrzadeh Rafie AH, Abilez OJ, Wang H, Blundo JT, Pruitt B, et al. In vivo imaging and evaluation of different biomatrices for improvement of stem cell survival. J Tissue Eng Regen Med. 2007;1:465–468. doi: 10.1002/term.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin K, Mao X, Xie L, Galvan V, Lai B, Wang Y, et al. Transplantation of human neural precursor cells in Matrigel scaffolding improves outcome from focal cerebral ischemia after delayed postischemic treatment in rats. J Cereb Blood F Met. 2010;30:534–544. doi: 10.1038/jcbfm.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kothapalli CR, Kamm RD. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials. 2013;34:5995–6007. doi: 10.1016/j.biomaterials.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 72.Jang JM, Tran SH, Na SC, Jeon NL. Engineering controllable architecture in matrigel for 3D cell alignment. ACS Appl Mater Interfaces. 2015;7:2183–2188. doi: 10.1021/am508292t. [DOI] [PubMed] [Google Scholar]
- 73.Park YS, Kim HS, Jin YM, Yu Y, Kim HY, Park HS, et al. Differentiated tonsil-derived mesenchymal stem cells embedded in Matrigel restore parathyroid cell functions in rats with parathyroidectomy. Biomaterials. 2015;65:140–152. doi: 10.1016/j.biomaterials.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 74.Kowalczyk T, Hnatuszko-Konka K, Gerszberg A, Kononowicz AK. Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers. World J Microbiol Biotechnol. 2014;30:2141–2152. doi: 10.1007/s11274-014-1649-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rodriguez-Cabello JC, Prieto S, Reguera J, Arias FJ, Ribeiro A. Biofunctional design of elastin-like polymers for advanced applications in nanobiotechnology. J Biomater Sci Polymer Ed. 2007;18:269–286. doi: 10.1163/156856207779996904. [DOI] [PubMed] [Google Scholar]
- 76.Serrano V, Liu W, Franzen S. An infrared spectroscopic study of the conformational transition of elastin-like polypeptides. Biophys J. 2007;93:2429–2435. doi: 10.1529/biophysj.106.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Betre H, Liu W, Zalutsky MR, Chilkoti A, Kraus VB, Setton LA. A thermally responsive biopolymer for intra-articular drug delivery. J Control Release. 2006;115:175–182. doi: 10.1016/j.jconrel.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 78.Xia XX, Wang M, Lin Y, Xu Q, Kaplan DL. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules. 2014;15:908–914. doi: 10.1021/bm4017594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–99. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 80.Nettles DL, Chilkoti A, Setton LA. Applications of elastin-like polypeptides in tissue engineering. Adv Drug Deliv Rev. 2010;62:1479–1485. doi: 10.1016/j.addr.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ravi S, Krishnamurthy VR, Caves JM, Haller CA, Chaikof EL. Maleimide–thiol coupling of a bioactive peptide to an elastin-like protein polymer. Acta Biomater. 2012;8:627–635. doi: 10.1016/j.actbio.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jin E, Lee PT, Jeon WB, Li WJ. Effects of elastin-like peptide on regulation of human mesenchymal stem cell behavior. Regen Eng Transl Med. 2016;2:85–97. [Google Scholar]
- 83.Wang H, Cai L, Paul A, Enejder A, Heilshorn SC. Hybrid elastin-like polypeptide-polyethylene glycol (ELP-PEG) hydrogels with improved transparency and independent control of matrix mechanics and cell ligand density. Biomacromolecules. 2014;15:3421–3428. doi: 10.1021/bm500969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9:5590–5599. doi: 10.1016/j.actbio.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gurumurthy B, Bierdeman PC, Janorkar AV. Composition of elastin like polypeptide–collagen composite scaffold influences in vitro osteogenic activity of human adipose derived stem cells. Dent Mater. 2016;32:1270–1280. doi: 10.1016/j.dental.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang YN, Avery RK, Vallmajo-Martin Q, Assmann A, Vegh A, Memic A, et al. A highly elastic and rapidly crosslinkable elastin-like polypeptide-based hydrogel for biomedical applications. Adv Funct Mater. 2015;25:4814–4826. doi: 10.1002/adfm.201501489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Madl CM, Katz LM, Heilshorn SC. Bio-orthogonally cross-linked, engineered protein hydrogels with tunable mechanics and biochemistry for cell encapsulation. Adv Funct Mater. 2016;26:3612–3620. doi: 10.1002/adfm.201505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–1779. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 89.Wang H, Zhu D, Paul A, Cai L, Enejder A, Yang F, et al. Covalently adaptable elastin-like protein–hyaluronic acid (ELP-HA) hybrid hydrogels with secondary thermoresponsive crosslinking for injectable stem cell delivery. Adv Funct Mater. 2017;27:1605609. doi: 10.1002/adfm.201605609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Mater Today (Kidlington) 2010;13:14–22. [Google Scholar]
- 91.Jonker AM, Löwik DWPM, van Hest JCM. Peptide- and protein-based hydrogels. Chem Mater. 2012;24:759–773. [Google Scholar]
- 92.Jeong Y, Joo MK, Bahk KH, Choi YY, Kim HT, Kim WK, et al. Enzymatically degradable temperature-sensitive polypeptide as a new in situ gelling biomaterial. J Control Release. 2009;137:25–30. doi: 10.1016/j.jconrel.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 93.Choi YY, Joo MK, Sohn YS, Jeong B. Significance of secondary structure in nanostructure formation and thermosensitivity of polypeptide block copolymer. Soft Matter. 2008;4:2383–2387. [Google Scholar]
- 94.Moon HJ, Choi BG, Park MH, Joo MK, Jeong B. Enzymatically degradable thermogelling poly(alanine-co-leucine)-poloxamer-poly(alanine-co-leucine) Biomacromol. 2011;12:1234–1242. doi: 10.1021/bm101518c. [DOI] [PubMed] [Google Scholar]
- 95.Kim JY, Park MH, Joo MK, Lee SY, Jeong B. End groups adjust molecular nano-assembly pattern and thermal gelation of polypeptide block copolymer aqueous solution. Macromolecules. 2009;42:3147–3151. [Google Scholar]
- 96.Lee H, Choi BG, Moon HJ, Choi J, Park K, Jeong B, et al. Chondrocyte 3D-culture in RGD-modified crosslinked hydrogel with temperature-controllable modulus. Macromol Res. 2012;20:106–111. [Google Scholar]
- 97.Choi BG, Park MH, Cho SH, Joo MK, Oh HJ, Kim EH, et al. Thermal gelling polyalanine-poloxamine-polyalanine aqueous solution for chondrocytes 3D culture: initial concentration effect. Soft Matter. 2011;7:456–462. [Google Scholar]
- 98.Park MH, Moon HJ, Park JH, Shinde UP, Ko du Y, Jeong B. PEG-Poly(l-alanine) thermogel as a 3D scaffold of bone-marrow-derived mesenchymal stem cells. Macromol Biosci. 2015;15:464–472. doi: 10.1002/mabi.201400426. [DOI] [PubMed] [Google Scholar]
- 99.Park MH, Yu Y, Moon HJ, Ko du Y, Kim HS, Lee H, et al. 3D culture of tonsil- derived mesenchymal stem cells in poly (ethylene glycol)-poly(l-alanine-co-l-phenyl alanine) thermogel. Adv Healthc Mater. 2014;3:1782–1791. doi: 10.1002/adhm.201400140. [DOI] [PubMed] [Google Scholar]
- 100.Yeon B, Park MH, Moon HJ, Kim SJ, Cheon YW, Jeong B. 3D culture of adipose-tissue-derived stem cells mainly leads to chondrogenesis in poly (ethylene glycol)-poly (l-alanine) diblock copolymer thermogel. Biomacromol. 2013;14:3256–3266. doi: 10.1021/bm400868j. [DOI] [PubMed] [Google Scholar]
- 101.Kim SJ, Park MH, Moon HJ, Park JH, Ko du Y, Jeong B. Polypeptide thermogels as a three dimensional culture scaffold for hepatogenic differentiation of human tonsil-derived mesenchymal stem cells. ACS Appl Mater Inter. 2014;6:17034–17043. doi: 10.1021/am504652y. [DOI] [PubMed] [Google Scholar]
- 102.Hong JH, Lee HJ, Jeong B. Injectable polypeptide thermogel as a tissue engineering system for hepatogenic differentiation of tonsil-derived mesenchymal stem cells. ACS Appl Mater Interfaces. 2017;9:11568–11576. doi: 10.1021/acsami.7b02488. [DOI] [PubMed] [Google Scholar]
- 103.Kye EJ, Kim SJ, Park MH, Moon HJ, Ryu KH, Jeong B. Differentiation of tonsil-tissue-derived mesenchymal stem cells controlled by surface-functionalized microspheres in PEG polypeptide thermogels. Biomacromolecules. 2014;15:2180–2187. doi: 10.1021/bm500342r. [DOI] [PubMed] [Google Scholar]
- 104.Lee SS, Choi GE, Lee HJ, Kim Y, Choy JH, Jeong B. Layered double hydroxide and polypeptide thermogel nanocomposite system for chondrogenic differentiation of stem cells. ACS Appl Mater Interfaces. 2017;9:42668–42675. doi: 10.1021/acsami.7b17173. [DOI] [PubMed] [Google Scholar]
- 105.Park J, Kim IY, Patel M, Moon HJ, Hwang SJ, Jeong B. 2D and 3D hybrid systems for enhancement of chondrogenic differentiation of tonsil-derived mesenchymal stem cells. Adv Funct Mater. 2015;25:2573–2582. [Google Scholar]
- 106.Patel M, Moon HJ, Ko du Y, Jeong B. Composite system of graphene oxide and polypeptide thermogel as an injectable 3D scaffold for adipogenic differentiation of tonsil- derived mesenchymal stem cells. ACS Appl Mater Interfaces. 2016;8:5160–5169. doi: 10.1021/acsami.5b12324. [DOI] [PubMed] [Google Scholar]
- 107.Moon HJ, Patel M, Chung H, Jeong B. Nanocomposite versus mesocomposite for osteogenic differentiation of tonsil-derived mesenchymal stem cells. Adv Healthc Mater. 2016;5:353–363. doi: 10.1002/adhm.201500558. [DOI] [PubMed] [Google Scholar]
- 108.Patel M, Moon HJ, Jung BK, Jeong B. Microsphere-incorporated hybrid thermogel for neuronal differentiation of tonsil derived mesenchymal stem cells. Adv Healthc Mater. 2015;4:1565–1574. doi: 10.1002/adhm.201500224. [DOI] [PubMed] [Google Scholar]
- 109.Moon HJ, Lee HJ, Patel M, Park S, Chang SH, Jeong B. Hepatogenic supported differentiation of mesenchymal stem cells in a lactobionic acid-conjugated thermogel. ACS Macro Lett. 2017;6:1305–1309. doi: 10.1021/acsmacrolett.7b00802. [DOI] [PubMed] [Google Scholar]