Abstract
Background:
Sand blasted titanium (Ti) is commonly used in designing endosseous dental implants due to its biocompatibility and ability to form bonds with bone tissues. However, titanium implants do not induce strong interactions with teeth bones. To increase strong interactions between Ti disk implants and teeth bones, the l-glutamic acid grafted hydroxyapatite nanorods (nHA) were immobilized on albumin modified Ti disk implants (Ti-Alb).
Methods:
For modification of Ti disk implants by nHA, the l-glutamic acid grafted nHA was synthesized and then immobilized on albumin modified Ti disk implants. Fourier transformed infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscope; energy dispersive spectroscopy and confocal laser scanning microscopy were used to confirm the modification of Ti disk implants. The bioactivity of nHA-modified Ti disk implants was evaluated by seeding MC3T3-E1 cells on Ti-nHA implants.
Results:
Characterization techniques have confirmed the successful modification of Ti disk implants by l-glutamic acid grafted nHA. The nHA-modified Ti disk implants have shown enhanced adhesion, proliferation and cytotoxicity of MC3T3-E1 cells in comparison to pristine Ti implants.
Conclusions:
The modification of Ti implants by l-glutamic acid grafted nHA has produced highly osteogenic Ti disk plants in comparison to pristine Ti disk implants due to the formation of bioactive surfaces by hydroxyapatite nano rods on Ti disk implants. Ti-nHA disk implants showed enhanced adhesion, proliferation, and MC3T3-E1 cells viability in comparison to pristine Ti disk implants. Thus nHA might be to be useful to enhance the osseointegration of Ti implants with teeth bones.
Keywords: Osseointegration, Titanium, Hydroxyapatite, Implants, MC3T3-E1 cells
Introduction
Titanium and its alloys are normally used as endosseous implants due to their excellent mechanical and biocompatibility. Titanium when exposed to air, it immediately within a few seconds forms an oxide layer that provides resistance to chemical attack, and also helps in its integration with bone tissue as well. In physiological conditions, the titanium oxide is hydrated and exhibits anionic properties like hydroxyapatite which help in deposition of polyvalent cations such as, calcium ions as a first step in healing process after implantation. Thus titanium dioxide to a large extent shares many properties with hydroxyapatite and covered with negatively charged oxide groups to perform the ions activity as lattice bound phosphate ions in hydroxyapatite. Despite of sufficient stability and biocompatibility of titanium, the screw-shaped endosseous implants of commercially pure titanium with machined surfaces [1] are found to be disadvantageous due to showing long time for osseointegration in comparison to surface-modified titanium implants, which shown long-term clinical successes.
The surface-modified titanium implants have shown improved biocompatibility and accelerated osseointegration and shorter edentulous period for the patient [2]. The topological surface roughening through sand blasting, acid etching [3], and anodic oxidation processes [4, 5] in implants is found to be effective in getting rapid and stronger osseointegration [1] in comparison to implant modified either with fluoride treatment or by the application of calcium fluoride [6]. The anodic oxidation of titanium not only increased the surface roughness but also produced biologically active thick layer of titanium oxide that found to be responsible for observed enhanced osseointegration by titanium implants [7, 8] comparable to titanium implants coated with hydroxyapatite [9]. In addition to variation in mechanical and chemical structures during anodic oxidation, the topological variation in anodic oxidized titanium surface is found to be more responsive for the observed osteogenic properties in titanium implants than the topological variation caused by sand blasting and/or chemical etching methods [3, 10]. Usually, the surface roughness of about 1.5 μm in implants is found sufficient to induce osseointegration [11] and normally achieved either by sand blasting or chemical etching methods. However, recent studies have suggested that physically roughening together with chemical modification proved to be more promising to make surfaces of implants more biocompatible and osteogenic in comparison to implants, which are able to show biological response either due to physically surface roughening or chemical functionality at their surfaces. The addition of hydrophilicity to physically modified surfaces through coating of peptides [12–14] is proved to be more synergistic in showing higher biocompatibility and osseointegration. These studies have indicated that surface modification of implants might be more interesting and efficient in controlling their biological responses as surface modification includes both topological and chemical aspects to achieve superior and early responses at the interface of implants and teeth bone [15, 16]. Though coating of various forms of calcium phosphate on roughened surface has shown significant improvement in bone osseointegration of the implants [17–19] but such a physical coating layers suffered by the limitations of cohesive delamination as well as due to adhesive failures between coated calcium phosphate and physically roughened surfaces of titanium dental implants [20, 21]. An efficient osseointegration at bone-implant interface is only possible, if implants are able to form a firm and durable connections with teeth bone. To achieve a complete integration between implant and teeth bone, the coating of bone inspired hydroxyapatite on sand blasted titanium disk implant was carried out through chemical bonding to reduce the limitations of delamination of physically coated hydroxyapatite on bone implant surfaces [22].
Our studies have suggested that the chemical modification of sand blasted implant surfaces with hydroxyapatite is likely to enhance calcium deposition more efficiently at the surfaces of titanium implant in comparison to oxidized titanium implants due to a significant difference in number of anionic active sites (–O−) between titanium dioxide and hydroxyapatite. Thus chemical modification of sand blasted titanium disk implants using hydroxyapatite nanorods (nHA) was planned to control the biologically active nanostructured rough surfaces of sand blasted titanium implants to achieve better biological responses for faster osseointegration. The application of nHA in surface modification of sand blasted titanium implants has not only provided nano-structured surface roughness but also created sufficient anionic active sites for enhanced deposition of calcium and better bonding with teeth bones.
Materials and methods
Chemicals
Sand blasted titanium disk implants with 10 mm in diameter and 3.0 mm in thickness were received from Biotem Implant Co. at South Korea. These disk implants were cleaned in ultra-sonic bath using water-alcohol (1:1) mixture for 10 min and then with pure acetone for 10 min before surface modification. 3-Aminopropyltriethoxysilane (APTES) as silane coupling agent (MW, 221.37 g mol−1), l-glutamic acid (GA) (MW, 147.13 g mol−1), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (MW, 155.26 g mol−1), N-hydroxysuccinimide (NHS) (MW, 115.09 g mol−1), fluorescein isothiocyanate (FITC) and bovine serum albumin, phosphate buffered saline (PBS) solution of pH 7.4 (87 × 10−3 mmol Na2HPO4, 14 × 10−3 mmol KH2PO4, 131 × 10−3 mmol NaCl, and 27 × 10−3 mmol KCl) and other chemicals used in experimental work were of high purity reagents and purchased from Sigma-Aldrich Chemical Company, USA. The hydroxyapatite [C10(PO4)6(OH)2] as nanorods having suitable morphology, size and clinical property was prepared in our laboratory using ammonium dihydrogen phosphate (NH4)H2PO4 and calcium nitrate [Ca(NO3)2·4H2O] as reported in our previous studies [23]. The X-ray photoelectron spectra of titanium and hydroxyapatite modified titanium disk implants were recorded using Mg Kα radiation (ESCA, ESCA LAB VIG Microtech, East Grinstead, UK). The scanning electron microscope (SEM) in secondary electron mode under fixed voltage acceleration to produce surface topography has been used to show surface roughness and morphology of adhered cells. Scanning electron microscope (FE-SEM, 400 Hitachi, Tokyo, Japan) in combination with energy dispersive analyzer with a resolution of 133 eV was used to record energy dispersive spectrometer (EDS) to determine weight and atomic percent of elements present at modified surface of titanium disk implants. The specimens were sputter coated by gold–palladium and examined at 85 and 500 times magnification at 10.4 kV. The Fourier transformed infrared spectra (FT-IR) of hydroxyapatite nanorod (nHA)-s and l-glutamic acid-grafted hydroxyapatite nanorods (nHA-GA) were recorded on KBr pallets (FT-IR, Galaxy 7020A; Mattson, Fremont, CA, USA). The mouse pre-osteoblast cells (MC3T3-E1) were purchased from Korea cells bank (Seoul, South Korea) and stored in liquid nitrogen before carrying out cells seeding experiments. The MC3T3-E1 cells were cultured in α-minimum essential medium (α-MEM) (Gibco BRL, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 1.0% penicillin G-streptomycin at 37 °C under 5% CO2 atmosphere. The culture medium was changed every other day. The kinetic microplate reader (EL × 800, Bio-Tek Instruments, Inc., Highland Park, VT, USA) has been used to evaluate the proliferation of cultured cells on the surfaces of sand blasted titanium (Ti) disk implants and Ti disk implants modified with l-glutamic acid-grafted hydroxyapatite nanorods (Ti-nHA). The seeding of MC3T3-E1 cells on Ti and Ti-nHA disks implants were confirmed by recording their FE-SEM micrographs (FE-SEM, 400 Hitachi, Tokyo, Japan). The confocal laser scanning microscopy (CLSM, Zeiss LSM410 Zeiss, Oberkochen, Germany) has been used to measure the fluorescence of calcein-AM and propidium iodide in assaying of MC3T3-E1 cells viability at the surfaces of titanium disk implants.
Surface modification of Ti disk implants
The surface modification of Ti disk implants has been carried out by following steps.
Amino functionalization of Ti disk implants
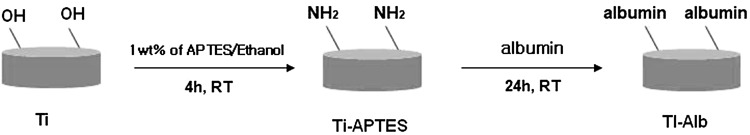
The water-alcohol and acetone-cleaned Ti disk implants were used for amino functionalization using APTES as coupling agent. To carry out amino-functionalization, the Ti disk implants were kept in 1 wt% solution of APTES in 95% ethyl alcohol for 4 h at pH 11.2 at room temperature. Finally, the Ti disk implants were rinsed 3 times with ethanol and deionized water before drying under vacuum. To confirm the anchoring of APTES on the surface of Ti disk implant, the Ti disk implants and APTES coupled Ti implants were treated in dark at 5 °C with freshly prepared FITC solution (1 mg/mL) in anhydrous DMSO. After 8 h, the Ti disk implants were washed with deionized water and dried in vacuum. Subsequently, the FITC-treated Ti disk implant (Ti-FITC), and FITC-treated amine-functionalized Ti disk implant (Ti-APTES-FITC) were used to record their fluorescence images by using confocal laser scanning microscope to confirm the anchoring of APTES on Ti disk implants by comparing the intensity of green florescence of conjugated FITC (λEx = 495 nm, λEm = 519 nm) at a brightness of 100 Cd/mm2. To confirm the anchoring of APTES on Ti disk implants, the EDS of pristine Ti implants and APTES-treated Ti disk implants (Ti-APTES) were recorded using FE-SEM equipped with energy dispersive analyzer. The SEM images of pristine Ti disk implants were also recorded to compare the morphological changes that had taken place after modification of surfaces of Ti disk implants by nHA.
Albumin coating on amino-functionalized Ti disk implants
To prepare albumin-coated amino-functionalized Ti disk implants, 0.5 g of Ti-APTES was added to a 100 mL solution containing albumin (50 mg) and 100 mg each of EDC and NHS as activators. The mixture was allowed to stir at 5 °C. After 6 h, the albumin-anchored Ti disk implants (Ti-Alb) were dialyzed using cellulose acetate membrane (MWCO, 8 kDA) and freeze dried after washing with deionized water. To confirm the anchoring of albumin on Ti disk implants, Ti-Alb were allowed to react with solution of FITC (1 mg/mL) in dark at 5 °C. After 8 h, the FITC-conjugated Ti-Alb (Ti-Alb-FITC) were washed with deionized water and their confocal laser scanning micrographs were recorded to confirm the anchoring of albumin by analyzing the green fluorescence intensity (λEx = 495 nm, λEm = 519 nm) at a brightness of 100 Cd/mm2. Finally, Ti-Alb disk implants were used to couple with nHA-GA.
Synthesis of nHA
The nHA of controlled size and morphology [24] were prepared by using method of chemical precipitation as reported in our previous communication [23]. Briefly, 400 mL solution of (NH4)H2PO3 and 300 mL solution of Ca(NO3)2·4H2O were prepared separately by dissolving 19.75 g of (NH4)H2PO3 and 57.5 g of Ca(NO3)2·4H2O in 400 mL and 300 mL of deionized water, respectively. Before drop-wisely mixing of (NH4)H2PO3 solution with Ca(NO3)2·4H2O, the pH of Ca(NO3)2·4H2O solution was adjusted to 10.4 by adding adequate amount of NH4OH. Finally, after mixing of (NH4)H2PO3, the solution was stirred vigorously at room temperature and resultant white precipitate was aged for about 4 days for the seeding of nHA in solution. The prepared nHA were separated and washed gently with deionized water until pH 7. To avoid the clustering of nHA during drying through vacuum evaporation, the separated nHA were first suspended in 1 butanol. After evaporation vacuum drying of synthesized nHA, the nHA were dried at 80 °C in vacuum oven to remove the remaining traces of solvent. Finally, the dried nHA were annealed at 700 °C for 4 h in hot air oven. To confirm the formation of nHA, the FT-IR spectra (FT-IR-Spectrometer Mattason, Galaxy 7020 A), and X-ray photoelectron spectra (ESCA, ESCA LAB VIG Microtech, Mt 500/1, Etc EAST Grinstead, UK) were recorded.
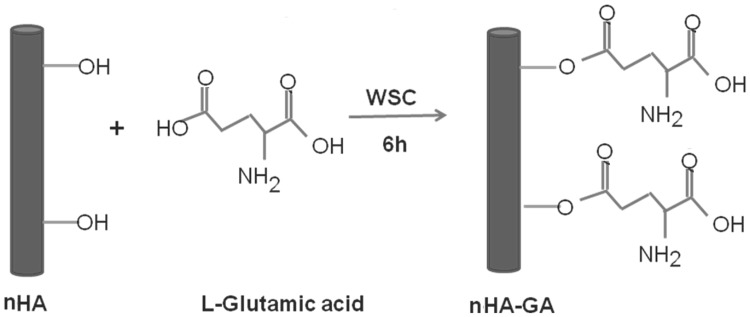
Synthesis of nHA-GA
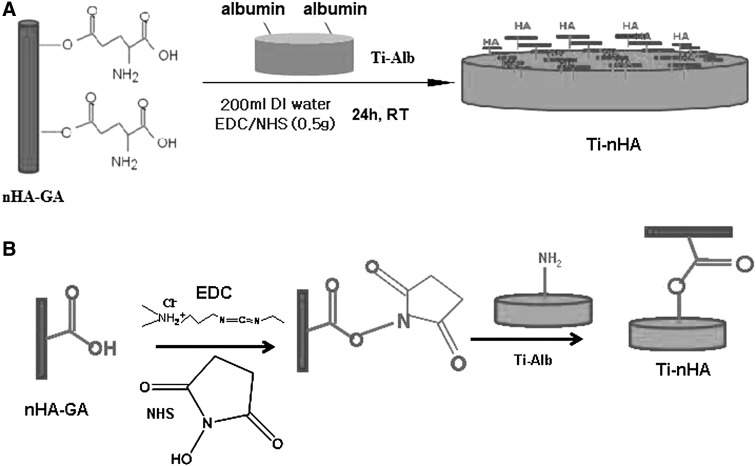
To enhance the chemical interactions of nHA with Ti-APTES disk implants, the nHA were grafted with l-glutamic acid (GA). Briefly, first of all terminal carboxylic acid groups (–COOH) of GA were activated by adding 0.5 g of GA in 200 mL mixture of EDC (0.5 g, 0.25 wt%), and NHS (0.5 g, 0.25 wt%) under constant stirring. After stirring for about 6 h, 0.5 g of nHA was added and solution was further allowed to stir continuously for about 12 h. Finally, the reaction mixture was dialyzed using regenerated cellulose membranes (MWCO, 8 kDA) till pH 7.0. The resultant nHA-GA after washing with PBS was lyophilized, which produced a white powdery solid mass of nHA-GA (Scheme 2). The amount of nHA-GA was determined by titrimetric analysis of remaining l-glutamic acid in solution. To confirm the anchoring of l-glutamic acid on nHA, the FT-IR spectra and X-ray photoelectron spectra were recorded.
Scheme 2.
Synthesis of glutamic acid functionalized of hydroxyapatite nanorods (nHA-GA) by grafting of l-glutamic acid (GA) on hydroxyapatite nanorods (nHA) in presence of water soluble carbodiimide (WSC)
Coupling of nHA-GA on Ti-Alb disk implants
To immobilize nHA-GA on Ti-Alb disk implants, 0.5 g of dried and purified nHA-GA was added to a 200 mL mixture of EDC (0.5 g, 0.25 wt%) and NHS (0.5 g, 0.25 wt%) under constant stirring. After 6 h, the Ti-Alb disk implants were immerged and allowed to react for 24 h. Finally, the nHA immobilized Ti disk implants (Ti-nHA) were separated and rinsed with deionized water to remove the unreacted activating agents and other impurities. To confirm the anchoring of nHA-GA on Ti-Alb disk implants and to determine the weight percent of elements present in Ti-nHA disk implants, the X-ray photoelectron spectra of Ti-nHA disk implants were recorded. To confirm the anchoring of nHA on Ti disk implants, the EDS of Ti-nHA were recorded using FE-SEM equipped with energy dispersive analyzer. To get morphological details of Ti-nHA, the SEM micrographs of Ti-nHA were also recorded to compare their surface morphology with pristine Ti disk implants.
Cellular responses of Ti-nHA disk implants
To determine the effect of nHA on bioactivity of Ti disk implants, the adhesion, proliferation and viability of MC3T3-E1 cells on the surfaces of Ti disk implants were determined by following standard protocols.
Cells adhesion
To assess the biological response of Ti disk implants for the adhesion of MC3T3-E1 cells before and after modification with nHA, the MC3T3-E1 cells (4 × 104 cells/mL) were seeded on the surfaces of Ti and Ti-nHA disk implants in presence of α-MEM medium. To carry out cell seeding, the Ti and Ti-nHA disks implants were kept in 4-wells cell seeding plate and MC3T3-E1 cells at a cell density of 4 × 104 cells/mL were incubated at 37 °C in presence of 5% CO2 in humidified atmospheres in presence of α-MEM medium. After 3 days of incubation, the supernatant was removed and incubated cells were washed with PBS and fixed by using 2.5% glutaraldehyde solution for 10 min. The samples were then dehydrated, dried in a critical point drier before sputter-coating with gold–palladium to record their SEM micrographs.
Cells proliferation
Though colorimetric assaying methods such as MTT, XTTT and MTS are routinely used for the evaluation of cells viability and proliferation by using absorbance corresponding to the number of viable cells but in comparison to these methods, the water soluble tetrazolium salts (WST-1) method is found to be more sensitive in estimating the resultant water soluble formazan corresponding to the number of viable cells than formazan estimated by MTT assay. To determine the effect of surface modification of Ti disk implants by nHA on proliferation of MC3T3-E1, the cells at a density of 2 × 104 cells/mL were seeded on Ti and Ti-nHA disk implants at 37 °C using 100 μL/well α-MEM medium in presence of 5% CO2. For cells seeding experiment, both Ti and Ti-nHA disk implants were fixed in a 4-wells cell seeding plate. The proliferation of MC3T3-E1 cells on disk implants was monitored for incubation time varying from 1 to 3 days. After incubating MC3T3-E1 cells for 1 and 3 days, 10 μL of Premizx WST-1 solution was added to each well to maintain 1:10 dilution ratio of Premizx WST-1 solution in each well, and cells were further allowed to incubate for about 4 h at 37 °C in presence of 5% CO2. After incubation of MC3T3-E1 cells for 4 h in presence of added Premizx WST-1 solution, the absorbance of resultant soluble formazan was recorded at 440 nm using 96 well kinetic microplate reader. The effect of surface modification of Ti disk implant by nHA on MC3T3-E1 cells proliferation was determined by comparing the absorbance of formazan formed in presence of media when cultured on Ti and Ti-nHA disk implants.
Cytotoxicity of Ti and Ti-nHA disk implants
To evaluate the cytotoxicity of Ti and Ti-nHA disk implants, the viability of MC3T3-E1 cells on Ti and Ti-nHA disk implants was evaluated by incubating MC3T3-E1 cells on Ti and Ti-nHA disk implants at 37 °C in α-MEM medium in presence of 5% CO2. After incubation for 2 days, the proliferated cells were stained with calcein-AM and propidium iodide [25]. For staining the proliferated cells, 100 μL PBS solution containing 10 μL calcein-AM (1 mM in dimethyl sulfoxide), and 5μL propidium iodide (1.5 mM in H2O) was used and MC3T3-E1 cells were stained for 15 min at 37 °C. Finally, the cells were examined using CLSM to record green fluorescence at an excitation wavelength of 490 nm to monitor simultaneously the ratio of live and dead cells together.
Statistical analysis
All experimental data were collected in triplicates and presented as means ± standard deviations. The statistical analyses were performed using student’s two tailed test in conjunction with Scheffe’s test for multiple comparison statistics considering p < 0.05, p < 0.01, and p < 0.001 as statistically significant, very significant and extremely significant values, respectively, whereas p > 0.05 is treated as statistically insignificant value.
Results and discussion
Commercially pure titanium and its alloys are important traditional materials for dental implantation due to their useful properties for osseointegration with bones. To enhance its osseointegration properties further, the research and industry has adopted various routes for its surface roughening by using techniques based on mechanical, chemical, electrochemical, and physical methods. The surface roughening provides better opportunities to the implants for interlocking with bone tissues due to enhances surface area and wettability. However, the role of roughening in controlling the osseointegration in dental implantation has been questioned due to the prevalence of peri-implantitis [26]; hence, additive surface modification methods are gaining increasing research interest among scientists to reduce microbial infections of clinically exposed surfaces and to preserve the advantages of surface roughening. The modification of Ti disk implants using nHA has been a step in this direction to obtain a functional roughness by using nHA with high surface area and their chemical properties. The application of l-glutamic acid as spacer in anchoring the nHA on Ti disk implants has contributed significantly in controlling the spatial interactions of immobilized nHA with bone tissues to get better biological response for bone healing. The chemical bonding of nHA with Ti disk implant through APTES has also reduced the limitation of partially or fully delamination of physically coated layer on Ti implant surfaces as usually the case with anodic oxidation or on coating layer physically. The results of surface modification of Ti disk implants by nHA and their biological response for MC3T3-E1 cells are found to be interesting in comparison to pristine Ti disk implants.
Surface modification of Ti disk implants
The Ti disk implants were modified by the reaction of nHA-GA and Ti-Alb disk implants. The schemes of surface modifications of Ti disk implants with nHA are discussed in comparison to pristine Ti disk implants.
Synthesis of Ti-Alb disk implants
To immobilize the nHA on Ti disk implants, the deionized water-alcohol and acetone cleaned Ti disk implants were reacted with APTES in 95% ethyl alcohol for 4 h at pH 11.2 at room temperature as shown in Scheme 1. The anchoring of APTES on Ti disk implant was confirmed by coupling freshly prepared FITC solution (1 mg/mL) in anhydrous DMSO in dark at 5 °C with amino-functionalized Ti disk implants (Ti-APTES) as well as with pristine Ti disk implant. The fluorescence images of FITC-treated Ti-APTES disk implant and pristine Ti disk implants were recorded using confocal laser scanning micrographs (Fig. 1A, B).
Scheme 1.
Synthesis of albumin functionalized Ti disk implants (Ti-Alb) using 3-aminopropyltriethoxysilane (APTES) and albumin
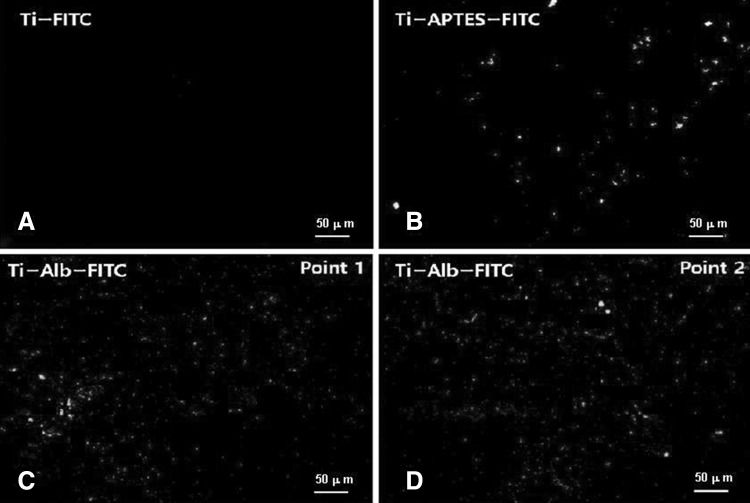
Fig. 1.
A Fluorescence images of FITC conjugated pristine Ti disk implants (Ti-FITC). B FITC conjugated 3-aminopropyltriethoxysilane functionalized APTES modified Ti disk implants (Ti-APTESFITC). C, D Fluorescent images of FITC conjugated albumin modified Ti disk implants (Ti-Alb-FITC) recorded at point 1 and at point 2
The fluorescence image micrograph of FITC-treated Ti-APTES disk implants have shown intense green fluorescence (Fig. 1B) in comparison to micrograph of pristine Ti disk implants (Fig. 1A).
The intense green fluorescence by Ti-APTES disk implants (Fig. 1B) has confirmed the functionalization of Ti disk implants by APTES, which was responsible to couple with FITC, whereas, pristine Ti disk implants could not couple with FITC and did not show green fluorescence (Fig. 1A). The quantitative anchoring of APTES on Ti disk implant was further confirmed by recording EDS of pristine Ti and Ti-APTES disk by using SEM equipped with energy dispersive analyzer (Fig. 2).
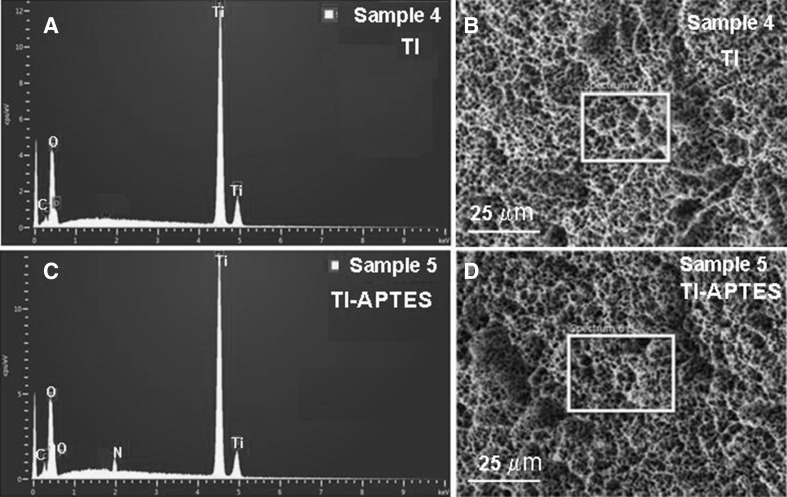
Fig. 2.
A Energy dispersive spectrum (EDS) of pristine Ti disk implants. B FE-SEM images of pristine Ti disk implants (Sample 4). C Energy dispersive spectrum (EDS) of 3-aminopropyltriethoxysilane functionalized Ti disk implants (Ti-APTES). D FE-SEM images of 3-aminopropyltriethoxysilane functionalized Ti disk implants (Sample 5)
The atomic weight percent of elements as determined with EDS analysis of pristine Ti disk implants (Sample 4, Fig. 2A) and Ti-APTES disk implants (Sample 5, Fig. 2C) has confirmed the anchoring of APTES (Table 1, Fig. 2A, C). The Ti-APTES disk implant (Sample 5, Table 1, Fig. 2C) showed a presence of 0.18 wt% nitrogen, which is absent in pristine Ti disk implant (Sample 4, Table 1, Fig. 2A). Thus EDS has confirmed the anchoring of APTES on Ti disk implants.
Table 1.
EDS analysis of surface-modified Ti disk implants
| Element | Ti (Sample 4) | Ti-APTES (Sample 5) | Ti-nHA (Sample 9) | |||
|---|---|---|---|---|---|---|
| wt% | at.% | wt% | at.% | wt% | at.% | |
| C | 0.72 | 2.64 | 1.43 | 4.19 | 1.43 | 4.08 |
| O | 4.40 | 11.68 | 4.48 | 12.16 | 15.66 | 33.6 |
| N | 0.00 | 0.00 | 0.18 | 0.10 | 0.10 | 0.08 |
| P | 0.00 | 0.00 | 0.00 | 0.00 | 4.79 | 5.31 |
| Si | 0.02 | 0.03 | 0.05 | 0.08 | 0.00 | 0.00 |
| Ca | 0.00 | 0.00 | 0.00 | 0.00 | 7.28 | 6.41 |
| Ti | 94.86 | 85.65 | 93.86 | 83.47 | 70.74 | 50.52 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
Pristine Ti disk implants (Sample 4), 3-aminopropyltriethoxysilane modified Ti disk implants (Sample 5), and hydroxyapatite nanorod-modified Ti disk implants (Sample 9)
To enhance the anchoring of nHA on Ti disk implants, the Ti-APTES were treated with albumin (Scheme 1). The immobilization of albumin was confirmed by conjugating FITC and recording florescence images by CLSM (Fig. 1C, D). The fluorescence images of Ti-Alb-FITC disk implants were recorded at different points, which indicated for homogeneous immobilization of albumin on the surfaces of Ti-APTES disk implants (Fig. 1C, D) and for the presence of high density of amino functionality (–NH2) on Ti-Alb disk implants (Fig. 1C, D) in comparison to Ti-APTES disk implants (Fig. 1B). Thus albumin-conjugated Ti disk implants have high density of reactive amine groups, hence likely to provide better opportunity for formation of stable and strong bonding between Ti disk and nHA.
Synthesis of nHA and nHA-GA
To modify the surface of Ti disk implant with nHA, the nHA of controlled size and morphology [24] were prepared using the method as reported in our previous communication [23].
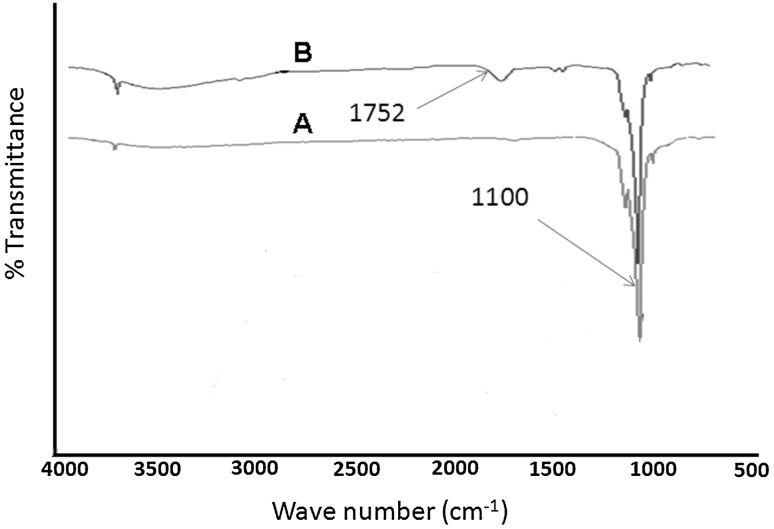
The formation of nHA was confirmed by X-ray analysis and the size measurements were done by TEM analysis as per our previous studies. To enhance the chemical interactions of nHA with Ti-Alb disk implants, the nHA were grafted with l-glutamic acid using 0.5 g nHA in presence of terminal carboxylic acid (–COOH) activated l-glutamic acid (0.5 g) by a mixture of EDC (0.5 g, 0.25 wt%) and NHS (0.5 g, 0.25 wt%) under constant stirring (Scheme 2). The grafting of l-glutamic acid on nHA was confirmed by comparing the FT-IR spectra of nHA (Fig. 3A) with FT-IR spectra of nHA-GA (Fig. 3B). The presence of characteristics absorption bands of l-glutamic acid at 1752 cm−1 and 3500 cm−1 along with characteristic peak of phosphate groups at 1100 cm−1 in FT-IR spectra of nHA-GA has confirmed the formation of l-glutamic acid-grafted nHA (Fig. 3B).
Fig. 3.
A Fourier transformed infrared spectra (FT-IR) showing characteristic phosphate peak (1100 cm−1) of hydroxyapatite nanorods (nHA). B FT-IR spectra of glutamic acid grafted hydroxyapatite nanorods (nHA-GA) showing characteristic peak of glutamic acid peak at 1752 cm−1 in nHA-GA
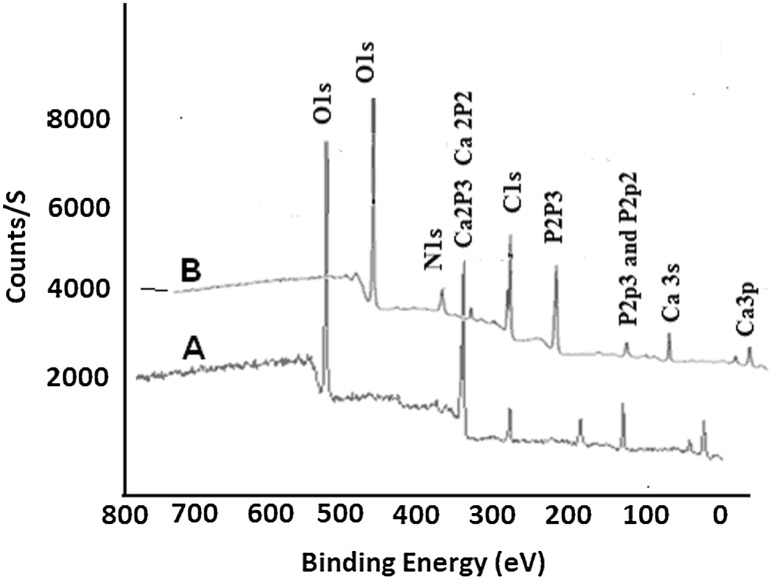
The FT-IR spectrum of nHA has shown a characteristic peak of phosphate groups at 1100 cm−1 and other frequencies of hydroxyapatite (Fig. 3A). The grafting of l-glutamic acid on nHA was also confirmed by comparing the XPS spectra of l-glutamic acid-grafted nHA with pristine nHA (Fig. 4).
Fig. 4.
A X-ray photoelectron spectra showing Ca 2p peaks (346.7 and 350.2 eV) and P 2p peaks (132.5 eV and 133.8 eV) in hydroxyapatite nanorods (nHA). B X-ray photoelectron spectra of glutamic acid grafted hydroxyapatite nanorods (nHA-GA) showing N1s peak of glutamic acid peak at 399.2 eV in nHA-GA
The presence of new peak at 399.2 ± 0.2 eV corresponding to N1s energy level in X-ray photoelectron spectra of has confirmed the grafting of l-glutamic acid on nHA (Fig. 4B). The nHA-GA has shown peaks at 346.7 ± 0.3 eV and 350.2 ± 0.2 eV corresponding to binding energy of Ca 2p3/2 and 2p1/2 energy levels and peaks at 132.5 ± 0.25 eV and 133.8 ± 0.18 eV were corresponding to binding energy of P 2p3/2 and P2p. These peaks are characteristic peaks of calcium and phosphorous of hydroxyapatite and appeared along with nitrogen peaks of l-glutamic acid in XPS spectra of nH-GA (Fig. 4B), which confirmed the formation of nHA-GA. The atomic weight percent of elements from XPS survey spectra has also supported the grafting of l-glutamic acid on nHA (Table 2).
Table 2.
XPS data for the survey of elements (wt%) in nHA and nHA-GA
| Samples | Atomic percent of elements | ||||
|---|---|---|---|---|---|
| C1s | O1s | P2p | Ca 2p | N1s | |
| nHA | 7.50 | 66.52 | 12.76 | 17.87 | – |
| nHA-GA | 8.18 | 67.48 | 11.22 | 12.14 | 2.20 |
The decrease in percentage of calcium from 17.87 to 12.14% and of phosphorous from 12.76 to 11.22% in X-ray photoelectron spectra of nHA-GA also supported the grafting of l-glutamic acid on nHA (Table 2). The X-ray photoelectron survey spectrum of nHA-GA has shown a presence of 2.20% for nitrogen, which confirmed the presence of l-glutamic acid on nHA (Fig. 4B, Table 3). Thus XPS (Fig. 4, Table 2) and FT-IR data (Fig. 3B) both have confirmed the grafting of glutamic acid on nHA.
Table 3.
XPS analysis of pristine Ti and Ti-nHA disk implants
| Samples | Atomic percent of elements | ||||
|---|---|---|---|---|---|
| C1s | O1s | P2p | Ca 2p | Ti 2p | |
| Ti | 17.82 | 59.00 | 0.00 | 0.00 | 22.66 |
| Ti-nHA | 6.45 | 68.98 | 6.10 | 9.94 | 8.53 |
Coupling of nHA-GA on Ti-Alb disk implants
To immobilize the nHA-GA on Ti-Alb disk implants, 0.5 g sample of dried and purified nHA-GA was activated by adding 200 mL mixture of EDC and NHS (0.5 g, 0.25 wt% each). After stirring the mixture for about 6 h, the Ti-Alb disk implants were added and allowed to react for about 24 h at room temperature with EDC and NHS activated nHA-GA (Scheme 3).
Scheme 3.
A Scheme for the coupling of glutamic acid grafted hydroxyapatite nanorods (nHA-GA) with albumin modified Ti disk implants (Ti-Alb) to obtain hydroxyapatite nanorod anchored Ti disk implants (Ti-nHA). B Reaction scheme showing the coupling reaction of glutamic acid grafted hydroxyapatite nanorods (nHA-GA) with albumin modified Ti disk implants (Ti-Alb) in presence of N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) as activators to obtain hydroxyapatite nanorods anchored Ti disk implants (Ti-nHA)
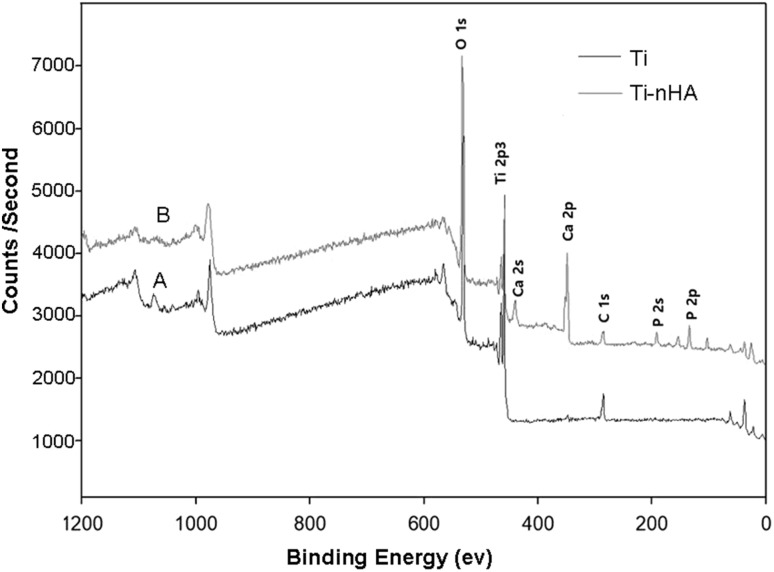
The coupling of nHA-GA with Ti-Alb disk implant was confirmed by comparing the XPS spectra of Ti-nHA (Fig. 5A) with that of pristine Ti disk implants (Fig. 5B). The appearance of Ca 2p (351.0 ± 0.4 eV) and P 2p (133.2 ± 0.4 eV) peaks in XPS spectrum of Ti-nHA along characteristics Ti 2p peak (459.0 ± 0.2 eV) (Fig. 5B) has confirmed the coupling of nHA-GA with Ti-Alb disk implants. The atomic weight percent of elements determined from XPS survey spectra of pristine Ti and Ti-nHA has further confirmed the coupling of nHA-GA with Ti-Alb disk implant (Table 3).
Fig. 5.
A X-ray photoelectron spectra showing characteristic Ti 2 p peak 459.0 eV in pristine Ti disk implants. B X-ray photoelectron spectra of hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA) showing Ca 2p peaks (351.0 eV), Ti 2p peaks (459.0 eV) in Ti-nHA disk implants
There is a significant increase in phosphate content from zero percent to 6.10% in Ti-nHA disk implants in comparison to pristine Ti disk implant and similar trends were observed for the contents of calcium, which has increased to 9.94 wt%. The decreasing trend in carbon and titanium has further supported the coupling of nHA with Ti disk implants (Table 3).
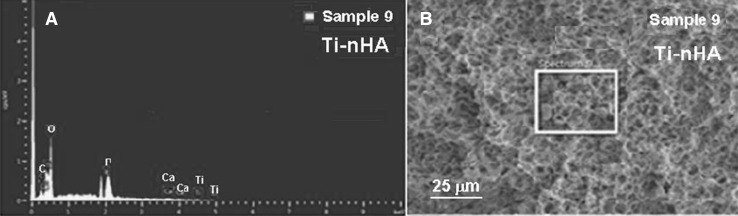
Further quantitative support for the coupling of nHA-GA on Ti-Alb disk implant was obtained from EDS spectra recorded using FE-SEM equipped with energy analyzer (Fig. 6). From the comparison of weight percent amount of elements obtained from the EDS analysis of Ti-Alb disk implant (Sample 4, Table 1, Fig. 2A, B) with the EDS analysis of Ti-nHA disk implants (Sample 9, Table 1, Fig. 9A, B), it is clear that there was a presence of 7.48 wt% of calcium and 4.79 wt% of phosphorous in Ti-nHA disk implants (Sample 9, Fig. 6A), which appeared due to the coupling of nHA-GA and was absent in Ti-Alb disk implants (Sample 4, Table 1, Fig. 2A). Thus EDS data have provided a quantitative support for the coupling of nHA-GA with Ti-Alb disk implants, which was already confirmed from XPS analysis.
Fig. 6.
A Energy dispersive spectrum (EDS) of hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA). B FE-SEM image of hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA)
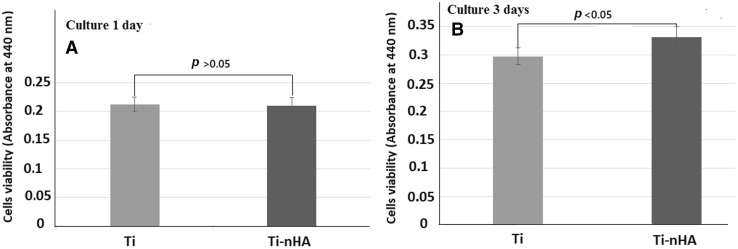
Fig. 9.
A, B Cells viability test by applying WST-1 assay for MC3T3-E1 cells on pristine Ti disk implants and on hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA) after culturing for 1 day and 3 days
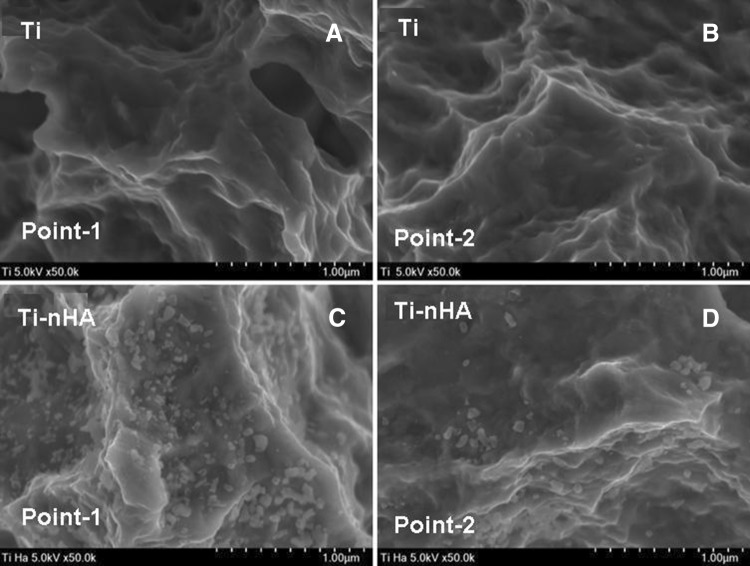
To confirm the presence of nHA on the surface Ti disk implants, the SEM micrographs of Ti-nHA disk implants recorded at different points (Fig. 7C, D) were compared with the SEM micrographs recorded at different points of pristine Ti disk implants (Fig. 7A, B), which provided a clear support for the presence of nHA and formation of nanostructured rough surfaces on Ti-nHA disk implants (Fig. 7C, D) in comparison to volkano microstructured rough surface of pristine Ti disk implants (Fig. 7A, B). The formation of nanostructured surface on Ti disk implants might be considered responsible for the enhanced biological response for the cultured MC3T3E-1 cells in comparison to volcano microstructured rough surface of pristine Ti disk implants.
Fig. 7.
A, B FE-SEM micrographs showing surface morphology of pristine Ti disk implants recorded at point-1 and at point-2. C, D FE-SEM micrographs showing surface morphology of hydroxyapatite modified Ti disk implants (Ti-nHA) recorded at point-1 and at point-2
Cellular responses of Ti-nHA disk implants
To evaluate the biological response of Ti-nHA in comparison to pristine Ti disk implants, the assaying of bioactivity of Ti disk implants was carried for adhesion, proliferation and cytotoxicity of MC3T3-E1 cells by following standard protocols. Since MC3T3-E1 cells are used extensively in cell culture experiments as a model for osteoblasts; hence, for in vitro evaluation of bioactivity of Ti implants, MC3T3-E1 cells were used in these studies [27].
Cell adhesion assay for MC3T3-E1 cells
The biological response of pristine Ti and Ti-nHA disk implants was determined by seeding MC3T3-E1 cells at a fixed density (4 × 104 cells/mL) at 37 °C in presence of 5% CO2 in humidified atmospheres in presence of α-MEM medium. After incubating MC3T3-E1 cells for 1 and 3 days, the seeded MC3T3-E1 cells were fixed using 2.5% glutaraldehyde solution for 10 min after removing α-MEM medium and washing with phosphate-buffered saline (PBS) solution. To record SEM micrographs, the dried and MC3T3-E1 cells fixed titanium disk implants were sputter coated with gold–palladium in vacuum (Fig. 8).
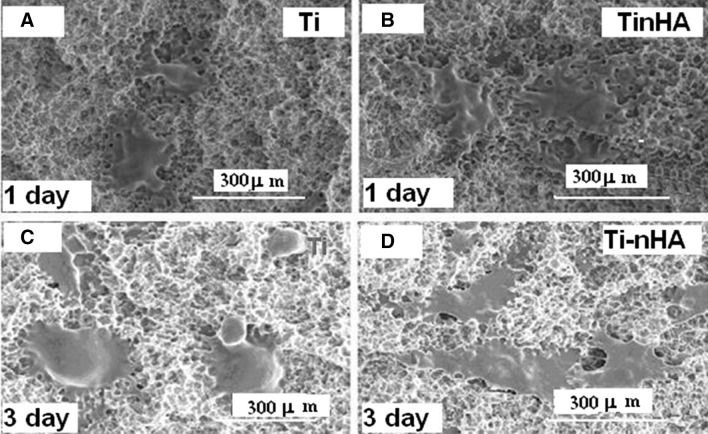
Fig. 8.
A, B FE-SEM micrographs of MC3T3-E1 cells cultured for 1 day on Ti disk implants and on hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA). C, D FE-SEM images of MC3T3-E1 cells cultured for 3 days on Ti disk implant and on hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA)
On comparing the SEM micrographs recorded to evaluate the adhesion of MC3T3-E1 cells on pristine Ti (Fig. 8C) and Ti-nHA disk implants (Fig. 8D), it has been found that MC3T3-E1 cells have shown better spreading on Ti-nHA disk implants (Fig. 8D) in comparison to spreading of MC3T3-E1 cells on pristine Ti disk implants (Fig. 8C). This has clearly indicated that modification of titanium surface with nHA has helped in spreading of MC3T3-E1 cells than the surface of pristine Ti disk implants. This might be due to the presence of bioactive and hydrophilic hydroxyapatite nanorods at the surface of titanium implants, which provided significantly a larger surface area [23] to MC3T3-E1 cells to adhere to the surfaces modified Ti disks implants. The pristine Ti disk implants were less hydrophilic and were having microstructural morphology that did not allow sufficient adhesion to MC3T3-E1 cells. Thus Ti-nHA disk implants would provide faster osseointegration of titanium implant and will reduce the edentulous period for the fixation of implants with bone tissues in dental patients. The adhesion of MC3T3-E1 cells on Ti-nHA implants was also evaluated for a seeding period of 1 day on Ti disk implant (Fig. 8A) and on Ti-nHA disk implant (Fig. 8B) but MC3T3-E1 cells seeded for 3 days have shown better adhesion on surfaces of both pristine Ti and Ti-nHA disk implants as indicated by their SEM micrographs (Fig. 8C, D).
Proliferation assay for MC3T3-E1 cells
The strategy to improve the bioactivity of titanium dental implants by surface modification with nHA was further evaluated by assaying the proliferation activity of MC3T3-E1cells on pristine Ti and Ti-nHA disk implants. To evaluate the proliferation of MC3T3E-1 cells more accurately, the water soluble tetrazolium salts (WST-1) method was applied as it is more sensitive in estimating the number of viable cells than conventionally used MTT assaying method. On applying the water soluble tetrazolium salts (WST-1) method to determine the proliferation of MC3T3-E1 cells at fixed cells density (2 × 104 cells/mL) on pristine Ti and Ti-nHA disk implants, the absorbance of resultant soluble formazan was recorded at 440 nm using 96 wells kinetic microplate reader for cell seeding period of 1 day and 3 days. Thus on monitoring the absorbance of resultant formazan on seeding MC3T3-E1 cells on pristine Ti and Ti-nHA disk implants (Fig. 9) it was apparent that MC3T3-E1 cells proliferated efficiently on the surfaces of Ti-nHA disk implants in comparison to the surfaces of pristine Ti disk implants.
These results have clearly suggested that nanostructured nHA together with albumin at the surface of Ti-nHA disk implants have played a significant role in providing a better biological environment to MC3T3-E1 cells in comparison to surfaces of pristine Ti disk implants. The nanosized rods of hydroxyapatite on Ti disk implants were able to form interconnected channels for the proliferation and vascularization to MC3T3-E1 cells, which was missing on the surface of pristine Ti disk implants. The extent of MC3T3-E1 cells proliferation has shown an increasing trend on increasing the seeding time from 1 to 3 days (Fig. 9A, B).
The statistical analysis of proliferation data at p < 0.05 for the proliferation of MC3T3-E1 cells for 3 days have shown a significant difference in proliferation activity of MC3T3-E1 cells on Ti-nHA disk implants in comparison to proliferation activity of MC3T3-E1 cells on pristine Ti disk implants (Fig. 9). The difference in number of MC3T3-E1 cells proliferated after 1 day on pristine Ti and Ti-nHA disk implants was found to be insignificant at a confidence level of p > 0.05. Thus it is clear that MC3T3-E1 cells were able to proliferate efficiently after incubation period of 3 days on Ti-nHA disk implants but pristine Ti disk implants were poor in showing their bioactivity to MC3T3-E1 cells even after seeding these cells for 3 days (Fig. 9).
Cytotoxicity of pristine Ti and Ti-nHA disk implants
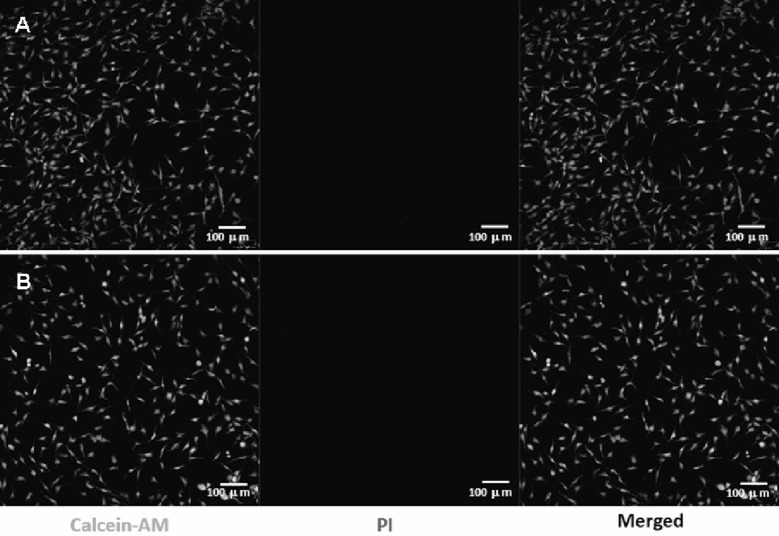
The assaying of dental material for cytotoxicity is an essential need to screen the harmful effect of material to oral tissues. The assaying of cytotoxicity provides a measure of cell death caused by the cytotoxic effect of materials or their extracts. Although, different types of cytotoxicity assaying tests are available but evaluation of in vitro toxicity of the material is a first step to investigate the biocompatibility of materials for dental applications. In this study the confocal laser scanning microscopy (CLSM) has been used to acquire the image of cells, which were stained using calcein-AM and propidium iodide on selected area at the implant surfaces. The calcein-AM green (λEx 620 nm—λEm 650 nm) and propidium iodide red (λEx 535 nm, λEm 617 nm) fluorescence emission intensity [25] were used to integrate the ratio of live and dead cells in recorded images of cells. The evaluation of cytotoxicity by CLSM has provided an accurate index of the cells viability and biocompatibility with dental implant materials. The cytotoxicity of pristine Ti and Ti-nHA disk implants was determined by evaluating the viability of MC3T3-E1 cells from the intensity of green/red fluorescence ratio in CLSM images of MC3T3-E1 cells seeded on pristine Ti and Ti-nHA disk implants (Fig. 10).
Fig. 10.
Confocal laser scanning micrographs (CLSM) of A MC3T3-E1 cells culture on Ti disk implants and on B hydroxyapatite nanorod-modified Ti disk implants (Ti-nHA) for 2 days after staining with calcein-AM and propidium iodide for live and dead assay
The calcein-AM stained fluorescence images of Ti-nHA disk implants seeded with MC3T3-E1 cells (Fig. 10B) were found to be almost same to pristine Ti disk implant (Fig. 10A) without any spot of red fluorescence. However, Ti-nHA disk implants have shown high biocompatibility and maintained more than 96% cells viability as compared to pristine Ti disk implants after a contact time of 2 days. This has suggested that modification of Ti disk implants by nHA did not add any cytotoxicity to pristine Ti disk surfaces; hence Ti-nHA disk implants could be suitable for dental applications without causing any harm to dental tissue.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2016R1C1B1016513). The work reported in this manuscript was carried out by students of Master of Engineering Degree and being submitted for publication after evaluating and discussing the contents. One of the authors, Prof. K.C. Gupta, would like to thank Prof. Inn-Kyu Kang for sponsoring his visit to Department of Polymer Science and Engineering at Kyungpook National University as visiting Professor for collaborative research.
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
There are no animal experiments carried out for this article.
Contributor Information
So Jung Park, Phone: +82 53 950 5630.
Bo Su Kim, Phone: +82 53 950 5630.
Kailash Chandra Gupta, Phone: +91 132 285325, Email: kcgptfcy@iitr.ac.in.
Inn-Kyu Kang, Phone: + 82 53 950 5629, Email: ikkang@knu.ac.kr.
References
- 1.Yeo IS, Han JS, Yang JH. Biomechanical and histomorphometric study of dental implants with different surface characteristics. J Biomed Mater Res B Appl Biomater. 2008;87:303–311. doi: 10.1002/jbm.b.31104. [DOI] [PubMed] [Google Scholar]
- 2.Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010;25:63–74. [PubMed] [Google Scholar]
- 3.Al-Nawas B, Groetz KA, Goetz H, Duschner H, Wagner W. Comparative histomorphometry and resonance frequency analysis of implants with moderately rough surfaces in a loaded animal model. Clin Oral Implants Res. 2008;19:1–8. doi: 10.1111/j.1600-0501.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 4.Nicu EA, Van Assche N, Coucke W, Teughels W, Quirynen M. RCT comparing implants with turned and anodically oxidized surfaces: a pilot study, a 3-year follow-up. J Clin Periodontol. 2012;39:1183–1190. doi: 10.1111/jcpe.12022. [DOI] [PubMed] [Google Scholar]
- 5.Lee HJ, Yang IH, Kim SK, Yeo IS, Kwon TK. In vivo comparison between the effects of chemically modified hydrophilic and anodically oxidized titanium surfaces on initial bone healing. J Periodontal Implant Sci. 2015;45:94–100. doi: 10.5051/jpis.2015.45.3.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong YS, Kim MJ, Han JS, Yeo IS. Effect of hydrophilicity and fluoride surface modifications to titanium dental implants on early osseointegration: an in vivo study. Implant Dent. 2014;23:529–533. doi: 10.1097/ID.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 7.Glauser R, Zembic A, Ruhstaller P, Windisch S. Five-year results of implants with an oxidized surface placed predominantly in soft quality bone and subjected to immediate occlusal loading. J Prosthet Dent. 2007;97:S59–S68. doi: 10.1016/S0022-3913(07)60009-2. [DOI] [PubMed] [Google Scholar]
- 8.Larsson C, Thomsen P, Aronsson BO, Rodahl M, Lausmaa J, Kasemo B, et al. Bone response to surface-modified titanium implants: studies on the early tissue response to machined and electropolished implants with different oxide thicknesses. Biomaterials. 1996;17:605–616. doi: 10.1016/0142-9612(96)88711-4. [DOI] [PubMed] [Google Scholar]
- 9.Zechner W, Tangl S, Fürst G, Tepper G, Thams U, Mailath G, et al. Osseous healing characteristics of three different implant types. Clin Oral Implants Res. 2003;14:150–157. doi: 10.1034/j.1600-0501.2003.140203.x. [DOI] [PubMed] [Google Scholar]
- 10.Koh JW, Yang JH, Han JS, Lee JB, Kim SH. Biomechanical evaluation of dental implants with different surfaces: removal torque and resonance frequency analysis in rabbits. J Adv Prosthodont. 2009;1:107–112. doi: 10.4047/jap.2009.1.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wennerberg A, Hallgren C, Johansson C, Danelli S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin Oral Implants Res. 1998;9:11–19. doi: 10.1034/j.1600-0501.1998.090102.x. [DOI] [PubMed] [Google Scholar]
- 12.Shin H, Jo S, Mikos AG. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353–4364. doi: 10.1016/S0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhao H, Huang Y, Zhang W, Guo Q, Cui W, Sun Z, et al. Mussel-inspired peptide coatings on titanium implant to improve osseointegration in osteoporotic condition. ACS Biomater Sci Eng. 2018;4:2505–2515. doi: 10.1021/acsbiomaterials.8b00261. [DOI] [PubMed] [Google Scholar]
- 14.Werner S, Huck O, Frisch B, Vautier D, Elkaim R, Voegel JC, et al. The effect of microstructured surfaces and laminin-derived peptide coatings on soft tissue interactions with titanium dental implants. Biomaterials. 2009;30:2291–2301. doi: 10.1016/j.biomaterials.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Ellingsen JE. Surface configurations of dental implants. Periodontol 2000. 2000;17:36–46. doi: 10.1111/j.1600-0757.1998.tb00121.x. [DOI] [PubMed] [Google Scholar]
- 16.Alghamdi HS, Cuijpers VM, Wolke JG, van den Beucken JJ, Jansen JA. Calcium-phosphate-coated oral implants promote osseointegration in osteoporosis. J Dent Res. 2013;92:982–988. doi: 10.1177/0022034513505769. [DOI] [PubMed] [Google Scholar]
- 17.Koh JW, Kim YS, Yang JH, Yeo IS. Effects of a calcium phosphate-coated and anodized titanium surface on early bone response. Int J Oral Maxillofac Implants. 2013;28:790–797. doi: 10.11607/jomi.2783. [DOI] [PubMed] [Google Scholar]
- 18.Saber-Samandari S, Alamara K, Saber-Samandari S, Gross KA. Micro-Raman spectroscopy shows how the coating process affects the characteristics of hydroxylapatite. Acta Biomater. 2013;9:9538–9546. doi: 10.1016/j.actbio.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 19.You C, Yeo IS, Kim MD, Eom TK, Lee JY, Kim S. Characterization and in vivo evaluation of calcium phosphate coated cp-titanium by dip-spin method. Curr Appl Phys. 2005;5:501–506. doi: 10.1016/j.cap.2005.01.020. [DOI] [Google Scholar]
- 20.Surmenev RA, Surmeneva MA, Ivanova AA. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—a review. Acta Biomater. 2014;10:557–579. doi: 10.1016/j.actbio.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Otsuka Y, Kojima D, Mutoh Y. Prediction of cyclic delamination lives of plasma-sprayed hydroxyapatite coating on Ti–6Al–4V substrates with considering wear and dissolutions. J Mech Behav Biomed Mater. 2016;64:113–124. doi: 10.1016/j.jmbbm.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res. 2001;58:570–592. doi: 10.1002/jbm.1056. [DOI] [PubMed] [Google Scholar]
- 23.Haider A, Gupta KC, Kang IK. PLGA/HA hybrid nanofiber scaffold as a nanocargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res Lett. 2014;9:314. doi: 10.1186/1556-276X-9-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan ZY, Liu JQ, Peng LM. Morphosynthesis of vesicular mesostructured calcium phosphate under electron irradiation. Langmuir. 2002;18:2450–2452. doi: 10.1021/la011518z. [DOI] [Google Scholar]
- 25.Koo TH, Borah JS, Xing ZC, Moon SM, Jeong Y, Kang IK. Immobilization of pamidronic acids on the nanotube surface of titanium discs and their interaction with bone cells. Nanoscale Res Lett. 2013;8:124. doi: 10.1186/1556-276X-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Atieh MA, Alsabeeha NH, Faggion CM, Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84:1586–1598. doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 27.Sudo H, Kodama HA, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96:191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]