Abstract
The Papillomaviridae is a family of small, non-enveloped viruses with double-stranded DNA genomes of 5 748 to 8 607 bp. Their classification is based on pairwise nucleotide sequence identity across the L1 open reading frame. Members of the Papillomaviridae primarily infect mucosal and keratinised epithelia, and have been isolated from fish, reptiles, birds and mammals. Despite a long co-evolutionary history with their hosts, some papillomaviruses are pathogens of their natural host species. This is a summary of the International Committee on Taxonomy of Viruses (ICTV) Report on the taxonomy of the Papillomaviridae, which is available at http://www.ictv.global/report/papillomaviridae.
Keywords: Papillomaviridae, ICTV Report, taxonomy
Virion
The non-enveloped viral capsid is ~600 Å in diameter and consists of 72 pentamers of the major capsid protein, L1, and ~12 molecules of the L2 minor capsid protein (Table 1, Fig. 1) [1].
Table 1. Characteristics of the family Papillomaviridae.
| Typical member: | human papillomavirus 16 (K02718), species Alphapapillomavirus 9, genus Alphapapillomavirus, subfamily Firstpapillomavirinae |
|---|---|
| Virion | Non-enveloped, 55 nm, icosahedral |
| Genome | Circular dsDNA genome of 5 748 to 8 607 bp |
| Replication | Bidirectional (theta) replication |
| Translation | Early and late transcripts, alternative splicing, alternative open reading frames |
| Host Range | Mammals, birds, reptiles and fish |
| Taxonomy | Two subfamilies include >50 genera and >130 species |
Fig. 1.
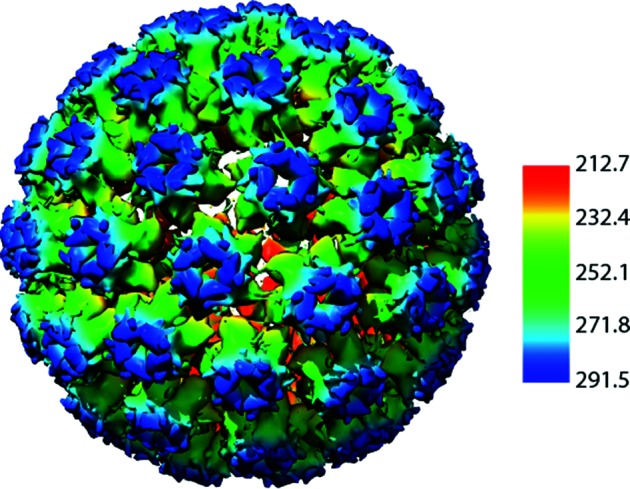
Atomic rendering of a papillomavirus capsid. Derived from an image reconstruction from cryo-electron microscopy of human papillomavirus type 16 at 4.5 Å resolution and colored according to the radial coloring scheme shown (PDB: 5KEP; [7]).
Genome
The viral genome varies from 5 748 to 8 607 bp. The genome comprises three functional regions. The early region encodes proteins involved in transcription, replication, and manipulation of the cellular milieu. The late region encodes the capsid proteins L1 and L2. The upstream regulatory region, located between the L1 and E6 open reading frames, contains the origin of replication as well as binding sites for viral and cellular transcription factors (Fig. 2) [2].
Fig. 2.
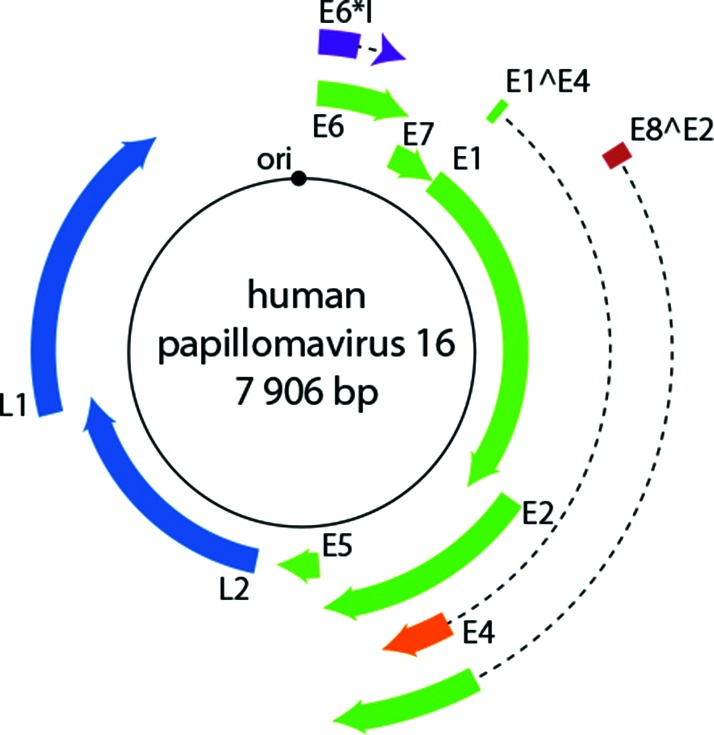
Diagram of the human papillomavirus 16 genome. The viral dsDNA is indicated. The outer boxes indicate the protein-coding open reading frames. Dotted lines represent intron sequences. The black circle represents the viral origin of replication (ori).
Replication
Papillomaviruses primarily infect epithelial cells. Following a micro-abrasion, the incoming virion complexes with extracellular heparin sulfate proteoglycans on the basement membrane. This interaction results in conformational changes in the L1 and L2 capsid proteins, in turn allowing for transfer of the virion to an unknown entry receptor. Following furin cleavage of L2, the virion becomes internalised using a process that shares similarities with macropinocytosis. The L2-DNA complex traffics to the trans-Golgi network, until mitosis. By metaphase, the viral DNA can be seen to be associated with host chromosomes [3]. The viral replication cycle consists of three distinct phases of replication. Initial limited viral DNA amplification is supported by the viral E1 and E2 replication proteins. This initial burst of replication is followed by maintenance replication, during which the viral genome is maintained at a relatively low, but constant copy number in the proliferating cells of a lesion. Finally, as an infected cell completes cellular differentiation there is a switch towards differentiation-dependent genome amplification, and eventual generation of progeny virions [4]. During maintenance replication, the viral E6 and E7 proteins are able to usurp the cellular environment, allowing for viral replication in differentiated cells [5]. In the top layers of the differentiated epithelia, viral DNA is amplified to a high copy number and the capsid proteins self-assemble into particles encapsidating the viral DNA. As the cells slough off into the environment, infectious virions are released, completing the viral replication cycle.
Taxonomy
Classification of the Papillomaviridae is based on sequence identity across the L1 open reading frame [6]. The family includes two subfamilies, Firstpapillomavirinae, which includes >50 genera and >130 species, and Secondpapillomavirinae, with a single genus and species. Genera are named according to the Greek alphabet (e.g. Alphapapillomavirus), with the prefixes ‘Dyo-’ and ‘Treis-’ indicating additional cycles through the alphabet (i.e. Greek for ‘a second or third time’).
Resources
Full ICTV Online (10th) Report: http://www.ictv.global/report/papillomaviridae.
The Papillomavirus Episteme (PaVE): http://pave.niaid.nih.gov.
The human papillomavirus reference center: http://www.hpvcenter.se.
The animal papillomavirus reference center: http://www.animalpv.org.
Funding information
Production of this summary, the online chapter, and associated resources was funded by a grant from the Wellcome Trust (WT108418AIA). The work in the McBride lab is in part supported by the Intramural Research Program of the NIAID, NIH.
Acknowledgements
Current and previous members of the ICTV papillomavirus study group. Members of the ICTV Report Consortium are Elliot J. Lefkowitz, Andrew J. Davison, Stuart G. Siddell, Peter Simmonds, Sead Sabanadzovic, Donald B. Smith, Richard J. Orton and Balázs Harrach.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Finnen RL, Erickson KD, Chen XS, Garcea RL. Interactions between papillomavirus L1 and L2 capsid proteins. J Virol. 2003;77:4818–4826. doi: 10.1128/JVI.77.8.4818-4826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Doorslaer K. Evolution of the Papillomaviridae. Virology. 2013;445:11–20. doi: 10.1016/j.virol.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Campos SK. Subcellular trafficking of the papillomavirus genome during initial infection: the remarkable abilities of minor capsid protein L2. Viruses. 2017;9:370. doi: 10.3390/v9120370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McBride AA. Replication and partitioning of papillomavirus genomes. Adv Virus Res. 2008;72:155–205. doi: 10.1016/S0065-3527(08)00404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harden ME, Munger K. Human papillomavirus molecular biology. Mutat Res Rev Mutat Res. 2017;772:3–12. doi: 10.1016/j.mrrev.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Villiers EM, Fauquet C, Broker TR, Bernard HU, Zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 7.Guan J, Bywaters SM, Brendle SA, Ashley RE, Makhov AM, et al. Cryoelectron microscopy maps of human papillomavirus 16 reveal L2 densities and heparin binding site. Structure. 2017;25:253–263. doi: 10.1016/j.str.2016.12.001. [DOI] [PubMed] [Google Scholar]


