Version Changes
Revised. Amendments from Version 1
Our main goal during the writing of the revised version of this article (Version 2) entailed the clarification of concepts that should be considered as ‘hypothetical’, opposite to concepts that should be considered as facts in relation to the possible role that CXCL13 plays in the pathogenesis of MS and the possible therapeutic intervention for this disease by blocking the formation, or the effect, of this chemokine in the CNS. For this purpose, we did intensify the search for evidence of the role CXCL13 could play in the pathogenesis of EAE in the animal model of MS and the possible cellular sources of CXCL13 in the CNS, with special emphasis on the supporting findings involving the meningeal tissue. We included two new sections based on the current knowledge on the role of CXCL13 in other autoimmune diseases and the documented cellular sources of CXCL13 in neuroinflammation caused by diseases other than MS. We also included citations on the adverse effects of chemokine manipulation in the CNS of patients with MS, stressing the fact that the immune response in MS is significantly complex and not completely understood, raising flags of concern when considering extrapolation of therapeutic applications from bench to bedside. Overall, nearly 25% of the citations are new in this updated version of the manuscript.
Abstract
Immunomodulatory therapies available for the treatment of patients with multiple sclerosis (MS) accomplish control and neutralization of peripheral immune cells involved in the activity of the disease cascade but their spectrum of action in the intrathecal space and brain tissue is limited, taking into consideration the persistence of oligoclonal bands and the variation of clones of lymphoid cells throughout the disease span. In animal models of experimental autoimmune encephalomyelitis (EAE), the presence of CXCL13 has been associated with disease activity and the blockade of this chemokine could work as a potential complementary therapeutic strategy in patients with MS in order to postpone disease progression. The development of therapeutic alternatives with ability to modify the intrathecal inflammatory activity of the meningeal tertiary lymphoid organ to ameliorate neurodegeneration is mandatory.
Keywords: multiple sclerosis, chemokines, CXCL13, B cells, tertiary lymphoid organ, meninges
Introduction
Although disease modifying therapy (DMT) agents in multiple sclerosis (MS) have contributed to reduction of neuroinflammation, they have not succeeded in the prevention of progression of disease. Inflammation is the appropriate tissue response to infection, autoimmunity, cancer, injury and allograft transplantation 1. When inflammation does not resolve appropriately, a prolonged immune response persists leading to tissue destruction and loss of function 1. Chronic infiltration by immune cells in the meninges is believed to form transitory lymphoid cell aggregates which simulate secondary lymphoid organs (SLO), and are known as meningeal tertiary lymphoid organs (mTLO) which play an important role in the pathogenesis of autoimmunity 1, 2. The mTLO seem to play a role in the intrathecal activity of immune system cells in MS 3. The SLO, such as lymph nodes, show a cellular organization that includes germinal centers (GC) containing antibody secreting and proliferating B-cells with follicular dendritic cells (FDC), a T-cell zone that incorporates naïve cells from the blood stream, high endothelial venules for extravasation of lymphocytes, and a stromal cell network that provides chemokines and extracellular matrix for cell migration and structural integrity 1. Chemokines are a family of proteins with the specific property of regulating leukocytes in the immune system and they may play a role in neurotransmission and neuromodulation 4. Leukocyte trafficking is mediated by inflammatory chemokines in inflamed tissues and by homeostatic chemokines in lymphoid sites 5 ( Figure 1). In this review, we focus on the role that CXCL13 (also known as B cell attracting chemokine [BCA-1], C-X-C motif ligand 13, or B lymphocyte chemoattractant [BLC]) plays in the organization of the mTLO in MS.
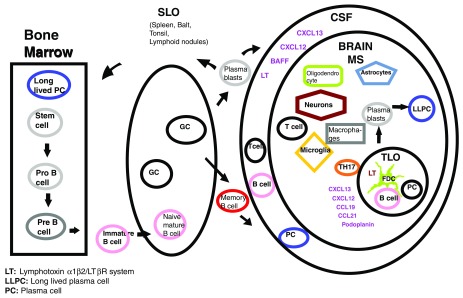
Figure 1. B cells lineage from bone marrow to CNS.
B-cells originating in the bone marrow exit toward the blood stream as immature B-cells; they enter the SLO and specialize in the germinal centers producing memory B cells and plasmablasts, which in pathologic conditions, are able to gain access to the CNS. The TLO is formed in the meninges during chronic inflammation in the deep brain cortical sulci and share organogenesis with the SLO 24. Podoplanin and the Th17 signature cytokine IL-17 have been associated with ectopic lymphoneogenesis in human diseases whereas BAFF is a key factor for mutation and survival of B cells which is produced by astrocytes in the CNS 3, 25. BAFF: B-cell activating factor of the tumor necrosis factor family; Balt: bronchial associated lymphoid tissue; CCL19: chemokine (c-c motif) ligand 19; CSF: cerebrospinal fluid; CXCL13: chemokine (C-X-C motif) ligand 13; FDC: follicular dendritic cells; GC: germinal center; LT: lymphotoxin α1β2/LTβR system; LLPC: long lived plasma cells; MS: multiple sclerosis; PC: plasma cell; SLO: secondary lymphoid organ; TLO: tertiary lymphoid organ.
In normal conditions, the SLO acquire information and prepare for immune defense
The SLO have a genetically determined pattern of development and programming that allows trapping and concentration of foreign antigens to initiate an adaptive immune response 1. Mucosal associated and non-encapsulated lymphoid tissue (including the Peyer’s patches, adenoid tissue of the nasopharynx, tonsils, and the bronchial associated lymphoid tissue), together with lymphoid nodes and spleen, constitute the SLO 6, 7. The lymph node cortex contains clusters or primary follicles that include packaged B cells and FDC, whereas the node para-cortex has a lesser number of dendritic cells (DC) and T cells 6. Generation of B cells with ability to produce auto antibodies usually occur in physiological conditions 8. These auto antibodies are low affinity IgM, which exhibit a wide spectrum of reactivity and strong preference for soluble self-antigens on the cell surface 8. Auto reactive low affinity B cells suffer apoptosis being unlikely they represent danger in normal conditions 8.
Lymphoid cells are able to learn and exchange information at the GC
The GC present remarkable lymphocytic mitosis within SLO follicles 9. Weyand et al. stated the GC are critical in the development of the B-cell normal immune response by driving-in cell division and maturation, B-cell selection with high affinity for immunoglobulin receptors and differentiation of B-cells and plasma cells (PC) 2. Real time imaging technology has allowed visualization of the transit of the B cells from the dark zone to the light zone, and vice versa, during the maturation of the GC 9– 11. The GC light zone displays a predominance of FDC and follicular T-helper (Tfh) cells, whereas the dark zone contains closely packed lymphocytes and stromal cells 9, 12. The chemokine receptor CXCR4 is required for the positioning of the B cells in the dark zone where its ligand, CXCL12, is more abundant and is produced by stromal cells 13. At the light zone, CXCL13 chemokine is concentrated in the FDC processes and, in conjunction with CXCR5, they contribute to the accumulation of B cells in this zone 12, 13. T-cells in the GC are essential to maintain signaling and represent approximately 5–20% of cell population 9. Tfh cells are characterized by the expression of CXCR5 and ICOS, which has an inducible T cell co-stimulatory effect 9, 14. B cell growth and differentiation at the germinal center are supported by IL10 and ICOS 14. Within the light zone, the three possible different outcomes for the centrocytes include death due to apoptosis; differentiation into memory B-cell or long lived plasma cells (LLPC); and re-entrance to the dark zone for a further round of cell mutation and selection 15. The relevant function of the GC is, most likely, the primary production of memory B-cells and LLPC 15, 16 ( Figure 2). Recent studies analyzing IgG heavy chain variable region genes in B cells from MS patients revealed that B cells are able to enter and exit the blood brain barrier (BBB) in order get exposed to somatic hypermutation (SMH) at the GC 17– 21.
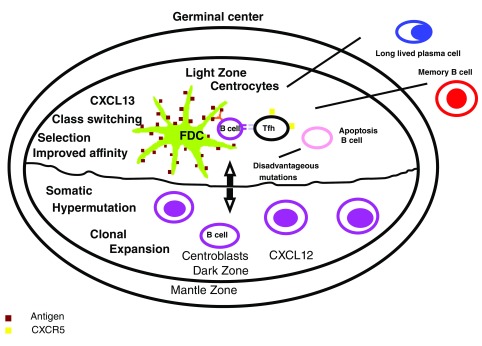
Figure 2. The germinal center.
B-cells enter the dark zone of the germinal center (centroblasts), a step which depends on the expression of CXCR4 in the surface, where the cells go through proliferation and somatic hypermutation (SHM). Subsequently, the cells migrate to the light zone (centrocytes) where they capture antigens through the mutated B cell receptors and are internalized for presentation to the T cells. The centrocytes differentiate from the centroblasts by the level of expression of surface proteins. Centrocytes are CXCR4 low, CD83 high, CD86 high and the centroblasts are CXCR4 high, CD83 low y CD86 low. The fluctuation between centroblasts and centrocytes is part of a synchronized cellular program which permits a temporal separation of the processes of mitosis and SHM from selection. The functional output of the TLO, in comparison to the SLO, could result from the dysregulated nature of their GC response supporting a breakout of autoimmune variants and the development of long lasting humoral autoimmunity characterized by presence of B cells with minimal memory and LLPC 15, 16. FDC: follicular dendritic cells; LLPC: long lasting plasma cells; Tfh: T follicular helper cells.
Chemokines direct traffic of lymphocytes during the cell search for specific information
The induction of lymphoid chemokines, depends on lymphotoxin β (LT-β) and the tumor necrosis factor α (TNF-α) signaling on stromal cells and FDC 22. Lymphotoxin α1β2 (LTα1β2) is expressed in the surface of B and T cells in the adult immune system and ligates to the lymphotoxin β receptor (LTβR) in reticular stromal cells thus inducing expression of lymphoid chemokines, such as CCL19, CCL21 and CXCL13 23. These chemokines regulate the homeostatic traffic of lymphocytes in lymphoid organs and their distribution in the GC 23. Homeostatic chemokines promote secretion of LTα1β2 by T and B cells, establishing a feedback loop that perpetuates the recruitment of lymphocytes and positional organization in the GC 1. The chemokine CXCL13 has the following relevant properties:
-
1.
CXCL13 increases its own production by stimulating the growth of FDC after regulating LTα1β2 on the membrane of B cells 5, 26.
-
2.
CXCL13 is produced in the SLO by FDC and macrophages and is an important chemoattractant to the CNS 27, 28.
-
3.
Follicular stromal cells express CXCL13, which is needed for nesting CXCR5 +B cells and a subset of T cells in the follicular compartment 6.
-
4.
CXCL13 primarily works through CXCR5 expressed in mature B lymphocytes 8, CD4+ Tfh 29, CD4+ Th17 cells 30, minor subset of CD8+ T cells and activated tonsil Treg cells 8, 30.
-
5.
CXCL13 has no relation with CD138+ and CD38+ plasmablasts, and PC 17.
Stromal cells from the T cell zone express the chemokines CCL19 and CCL21, which share the receptor CCR7 that directs naïve, central memory T cells and DC to the T cell compartment 6, 31. CXCR5 is expressed in 20 to 30% of CD4+T cells in blood and CSF, and virtually in all B cells in blood and the majority of B cells in the CSF compartment 32. Mice lacking CXCL13, or its receptor CXCR5, fail to develop peripheral lymph nodes 1. Khademi et al. determined the concentration of CXCL13 in CSF of individuals with MS, other neurological diseases including viral and bacterial infection, and healthy controls finding higher levels of the chemokine in subjects with infections followed to a lesser extent by the patients with MS 33. The levels of CXCL13 correlated negatively with disease span, concluding that early determination of CXCL13 might predict prognosis of disease 33.
Could the TLO become an operation center with ability to magnify an autoimmune response?
By maintaining antibody diversity, B cell differentiation, isotype switching, oligoclonal expansion, and local production of autoreactive PCs, the TLO perpetuate disease in response to environmental inputs 34. Lymphoid organogenesis and formation of mTLO may be facilitated by expression of lymphotoxin α (LT-α) at the external layer of meningeal inflamed vessels leading to the compartmentalization of the immune response in MS 17, 35. It has been postulated that the perpetuation of neuroinflammation and disease progression results from mTLO induced differentiation and maturation of antigen specific effector lymphocytes 28. The identification of independent centroblasts in the CSF of MS patients has suggested that there is an intrathecal B cell differentiation which is not dependent on the immune activity in the blood compartment 17. The TLO, besides SLO, provide a thriving environment where PC differentiate from plasmablasts 6, 28. In the absence of recirculating immune cells from the periphery, the TLO exerts its remarkable ability to remain active for several weeks 36. Therefore, the neutralization of TLO could play a significant role by blocking the re-emergency of auto reactive clones that could be able to drive relapses or resistance to therapy 36. Th17 cells, Tfh and a subtype of activated B cells, which are critical in systemic inflammation related with presence of TLO, are strongly associated with MS progression 37. In an animal model of experimental autoimmune encephalitis (EAE), Peters et al. found the following: 1) Expression of CXCL13 by Th17 cells in the CNS; 2) evidence that IL17 and the surface molecule podoplanin contribute to the development of ectopic lymphoid follicle in target organs; and 3) evidence of GC-like reactions in some of the mTLO as suggested by the presence of CXCL13, PNA- and GL7- positive T and B cells and plasma cells 25. By using next-generation RepSeq analysis, Lehmann-Horn et al. demonstrated the presence of SHM and class switch recombination (CSR) in the mTLO B-cell aggregates which had been initiated by activation of the induced cytidine deaminase enzyme (AICDA) in the Thx2D2 mice animal model of chronic EAE 38. The B-cell repertoires found at the mTLO were different than those found at the SLO suggesting that the mTLO represent autonomous sites of immune activity and that GC activity in the mTLO was antigen driven 38.
In absence of CXCL13, a reduced inflammatory response emerges from studies on animal models and human pathology
Disorganized B cell follicles in SLO have shown reduced capacity to originate natural antibody responses in CXCL13-/- mice 26, 39. Deficiency of CXCL13 results in a moderate course of disease characterized by a better recovery with attenuation of white matter inflammation and gliosis during the acute and chronic stage of EAE 40. Krumbholz et al. showed there was a direct correlation between CXCL13 levels and the number of B cells, T cells and plasmablasts in the CSF of MS patients 5. Clonal expansion and SMH of B cells have been observed in the CSF of patients with MS 41. CXCL13 was upregulated in active MS lesions but not in chronic inactive lesions and, in a similar range, in the serum of patients with relapsing remitting MS (RRMS) and control subjects indicating the intrathecal production of this chemokine 5. CXCL13 was identified by immunohistochemistry in intrameningeal B-cell follicles, but not in the cerebral parenchyma, of chronic active or inactive MS lesions 42. Patients with clinically isolated syndrome, who had shown conversion to clinically definitive MS within 2 years, had high levels of CXCL13 in the CSF 33, 43, 44. Elevated levels of CXCL13 in CSF have also been reported in patients with RRMS compared to controls and the CSF levels have been significantly increased during relapses but declining after initiation of B cell depleting therapy 22, 33, 45.
Facts learned from the role of CXCL13 in other autoimmune diseases
Chronic and active inflammation in target organs such as thymus, thyroid, synovial tissue and salivary gland can be driven by formation of TLO in the corresponding target organs 2. Expression of CXCL13 in pancreatic tissue has been associated with formation of ectopic lymphoid follicles and the induction of cascade events leading to diabetes in a transgenic mice model 46. In patients with Helicobacter pylori the blockade of CXCL13 has prevented the development of mucosa-associated lymphoid tissue (MALT) lymphoma and the propagation of inflammation by CXCL13 have been documented in non-lymphoid tissue 47. Although formation of pulmonary lymphoid follicles has been seen in patients with complicated rheumatoid arthritis, idiopathic pulmonary hypertension and Sjogren’s syndrome, the presence of lymphoid follicles has correlated with positive outcome in patients with lung cancer and infections of the respiratory tract 48. Lung B cells are a major source of CXCL13 and it has a positive role in the lymphoid neogenesis in chronic obstructive pulmonary disease through a LT receptor and toll-like receptor signaling 48.
Possible cellular sources of CXCL13 in the CNS
CXCL13 plays an important role in the formation of the GC in ectopic lymphoid follicles of several organs affected by inflammatory or autoimmune disease, or by infection 1. Important sources of CXCL13 are follicular stromal and FDC 26, 49, 50. In human lung tissue of patients with COPD, Litsious et al. found that stromal cells and DC produce CXCL13 upon stimulation by lymphocytes –mainly B cells- that express LT thus acquiring lymphoid tissue inducer (LTi) cells function in the TLO 48. The LT stimulates the expression of CXCL13 by stromal cells mainly through the LTβ receptor in the SLO 13, 26, 48, 51. Pikor et al. reported the production of CXCL13 by meningeal stromal cells when they are in the presence of LTβR in the relapsing-remmiting model of EAE in SJL/J mice 52. The source of intrathecal production of CXCL13 during neuroinflammation has not been determined with certainty. However, stromal cells within the B cell follicles have been considered to be responsible for the chemokine production and the notion of simple passive transfer from the blood stream to the intrathecal compartment due to dysfunction of the BBB has been detracted 13, 26, 51, 53. According to Essen and collaborators, stromal cells in the meninges could produce CXCL13 in special circumstances and drive the focal accumulation and organization of lymphoid cells in specific sites 51. In their study, they were able to demonstrate that microglia cells, in vivo and in vitro, are the main producers of CXCL13 in acute neuroinflammation induced by the Sindbis virus, which is not associated with demyelination 51. They also found that type-1 interferon could suppress the production of CXCL13 by microglial cells 51. In the rhesus macaque model of neuroborreliosis, Ramesh et al. and Narayan et al. found that infiltrating microglia and macrophage/DC myeloid cells could be one source of CXCL13 in the CNS during inflammation 54, 55. In biopsy specimens from patients with primary CNS lymphoma, Smith and collaborators encountered the following: 1) FDC were not present in the analyzed specimens; 2) there was expression of CXCR5 and CXCL13 in malignant B cells with positive production of BCA-1 (CXCL13) mRNA; and 3) CXCL13 was present in endothelial vascular cells which had a negative production of BCA-1 (CXCL13) mRNA by in situ hybridization, a finding that could be attributed to transcytosis 56. In different types of EAE, CXCL13 and BAFF mRNA transcripts were found to be significantly upregulated in the CNS of mice which developed the relapsing-remitting and the chronic-relapsing courses of disease opposite to those which developed a chronic progressive course. Besides, cells expressing CXCL13 were exclusively found in the brain stem meninges where infiltrating leukocyte proliferation was intense and vascular endothelial cells did not express CXCL13 57. In specimens from patients with giant cell arteritis, arterial TLO with FDC precursors and lymphoid ducts were detected in the medial layer of the temporal arteries expressing CXCL13, BAFF, APRIL, IL 7, IL 17 and LTβ 58.
A forthcoming research task: How early are the mTLO formed in the lifespan of MS?
Pikor et al. conducted studies in the animal model of relapsing-remitting EAE (SJL/J mice) an observed that at the onset of disease the TLO predominantly have T lymphocytes whereas in the acute an relapsing phase, the meningeal aggregates exhibited both T and B cells 52. Meningeal infiltrates can be disperse or well organized encompassing mTLO, whose lifespan is unknown 28, 42. The presence of follicles containing proliferating B cells, T cells, PC and FDC that express CXCL13 in the proximity of inflamed blood vessels in the meninges of patients with secondary progressive MS (SPMS) has been documented 42. The mTLO correlated with neuronal loss, adjacent cortical demyelination and a more rapid progression of disease 22. Patients with SPMS with positive mTLO have shown wide gray matter demyelination associated with loss of neurons, oligodendrocytes, and astrocytes; cortical atrophy, and microglial activation in the outer layer of the cortex 59, 60. It remains to be determined whether the formation of mTLO depends on the subtype of disease or it is the result of inflammation or consequence of chronicity 36.
Could CXCL13 be neutralized by direct action on itself, its receptor (CXCR5) or the lymphotoxin β (LT-β)?
A novel therapeutic monoclonal antibody against CXCL13 (Mab 5261 and Mab 5261-muIg) has been shown to induce functional in vitro inhibition of the chemokine in humans and mice 8. An LTβ receptor blocking immunoglobulin inhibits CXCL13 interactions, suppresses the formation of mTLO in the CNS and ameliorates the symptoms of EAE in rodents 23. In EAE induced by the transfer of myelin-specific Th17 cells (Th17 EAE), Quinn et al. confirmed a role of Tfh cells by blocking Tfh trafficking using antibody against CXCL13 and found that this treatment significantly reduced expression of disease 61. Some DMT available for the treatment for MS ameliorate levels of CXCL13, but the mechanisms by which it occurs are not completely understood. In patients with RRMS treated with natalizumab, a significant reduction in CXCL13 in CSF was observed in comparison to β-interferon 62. In another study, Novakova et al. evaluated the effect of treatment with fingolimod in CSF biomarkers, including CXCL13, of MS patients who had previously been on β-interferon, glatiramer acetate, teriflunomide (and had to switch therapy because of breakthrough disease activity) or natalizumab (who had to switch due to risk of PML) observing significant reduction of CXCL13 in the CSF of patients in both groups 63. Also, Alvarez et al. found that in patients with active RRMS, in spite of treatment with β-interferon or glatiramer acetate, the administration of rituximab led to a normalization of the CSF level of CXCL13 in the majority of patients, thus suggesting that high levels of CXCL13 in CSF at baseline could predict a forthcoming therapeutic response to B cell depletion 64. Piccio et al. found that in patients with RRMS treated with IV rituximab, concomitant with either β-interferon or glatiramer acetate, there was a reduction of CXCL13 and CCL19 in CSF, which correlated with significant reduction of B cells (95%) and T cells (50%) in CSF 32. Perry et al. found intrathecal reduction of CXCL13 (50.4%) and IgG index (13.5%) resulting from inhibition of development of LTi cells in patients with MS treated with daclizumab 65. Braendstrup et al. reported the case of a patient with MS who had undergone allogenic hematopoietic stem cells transplant for treatment of follicular lymphoma and who after two years presented negative determination of oligoclonal bands and detectable CXCL13 in CSF 66. Esen et al. suggested that blockade of CXCL13 could be a possible therapeutic target in EAE with advanced state of inflammation 51. However, past experience manipulating some of the chemokines in the treatment of MS has been unfavorable 67, 68. Atacicept is a recombinant fusion protein that ligands to the cytokines BLys/BAFF and APRIL which are involved in the differentiation, maturation and survival of B cells 67, 68. Although atacicept does not accomplish depletion of progenitor or memory B cells 68, it has the ability to disrupt B cell pathways, thus possibly stimulating a T cell response that leads to the creation of a pro-inflammatory environment 67. In a clinical trial in patients with MS, atacicept was able to reduce the concentration of immunoglobulins and the number of circulating mature B cells correlating with an increment in relapses without changes in the CNS lesion load by MRI 68. Upon discontinuation of atacicept and a 60-week follow-up, laboratory findings and activity of disease normalized 68. In fact, atacicept and infliximab (a TNF blocking drug) have the ability to induce increment of memory B cells in the blood, enhance their ability to enter the CNS, and increase disease relapse rate and lesion load by MRI 69. Also, Badr et al. have reported evidence of synergy between BAFF and CXCL13 which could have important implications for homeostasis of B cells 70. Altogether these findings have led to conclude that the immune response in MS is unpredictable and complex and that additional studies most be conducted with significant focus on patient safety 67, 68.
Would a complementary intrathecal therapy for deactivation of the mTLO be necessary to arrest disease progression?
A self-sustained intrathecal inflammation fostered by CSF chemokines involved in the traffic and survival of inflammatory cells occurs early in disease and is orchestrated by mTLO 3. Studies have shown that lineage of B cells can travel through peripheral blood, cervical lymphoid nodes, and the intrathecal compartment where they can be exposed to SMH in the mTLO and return to peripheral blood 17. As mentioned above, Piccio et al. found that CSF CXCL13 and CCL19 were decreased at week 24 after IV rituximab 32. However, Topping et al. found that therapy with intrathecal rituximab in patients with RRMS and SPMS resulted in no variation of CXCL13 levels in serum and CSF during the period of evaluation 71. Bonnan has hypothesized that, in order to prevent an unwanted generalized immune suppression resulting from systemic targeting of resident TLO, intrathecal immune reset should be attempted with a combination of monoclonal antibodies targeting each cell sub-type and aimed at eliminating simultaneously B cells, T cells, PC and FDC, via the intrathecal route. Excepting rituximab, candidate drugs still require preclinical studies for validation 3. Komori et al. reported that in patients with progressive MS who received therapy with intrathecal rituximab the depletion of B cells in the CSF compartment was transient and incomplete and could be facilitated by complement dependent cytotoxicity and to a lesser degree by antibody dependent celular cytotoxicity 72.
Conclusion
An early neutralization of CXCL13 could interfere with the organization and function of the mTLO thus modifying and reducing inflammation in the CNS of patients with MS. Studies in animal models where CXCL13 has been neutralized, or is not expressed (such as the CXCL13-/- mice), confirm its crucial role maintaining, rather than initiating, inflammation and its manipulation could lead to modification of disease in these models 39. However, any therapeutic strategy unable to neutralize LLPCs or antibody secreting cells will not be successful in an attempt to impede the chronic progression of disease 73. Neutralization of the CXCL13 should be carefully sought as complementary therapy to the DMT in MS.
Data availability
No data is associated with this article.
Acknowledgements
This work was presented, in preliminary version, at the Third annual Americas Committee for Treatment and Research in Multiple Sclerosis ( ACTRIMS Forum 2018) in San Diego, CA, on February 2, 2018. Poster presentation No. P193. Financial support for the on-line publication of this article was provided by The Department of Neurology, MedStar Georgetown University Hospital.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; referees: 2 approved]
References
- 1. Jones GW, Jones SA: Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology. 2016;147(2):141–151. 10.1111/imm.12554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weyand CM, Kurtin PJ, Goronzy JJ: Ectopic lymphoid organogenesis: a fast track for autoimmunity. Am J Pathol. 2001;159(3):787–93. 10.1016/S0002-9440(10)61751-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonnan M: Intrathecal immune reset in multiple sclerosis: exploring a new concept. Med Hypotheses. 2014;82(3):300–309. 10.1016/j.mehy.2013.12.015 [DOI] [PubMed] [Google Scholar]
- 4. Mélik-Parsadaniantz S, Rostène W: Chemokines and neuromodulation. J Neuroimmunol. 2008;198(1–2):62–68. 10.1016/j.jneuroim.2008.04.022 [DOI] [PubMed] [Google Scholar]
- 5. Krumbholz M, Theil D, Cepok S, et al. : Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain. 2006;129(Pt 1):200–211. 10.1093/brain/awh680 [DOI] [PubMed] [Google Scholar]
- 6. Drayton DL, Liao S, Mounzer RH, et al. : Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7(4):344–53. 10.1038/ni1330 [DOI] [PubMed] [Google Scholar]
- 7. Ruddle NH: Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19(2–3):119–125. 10.1007/BF02786481 [DOI] [PubMed] [Google Scholar]
- 8. Klitmatcheva E, Pandina T, Reilly C, et al. : CXCL13 antibody for the treatment of autoimmune disorders. BMC Immunol. 2015;16(1):6. 10.1186/s12865-015-0068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen CD, Okada T, Cyster JG: Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. 10.1016/j.immuni.2007.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hauser AE, Junt T, Mempel TR, et al. : Definition of germinal-center B cell migration in vivo reveals predominant intrazonal circulation patterns. Immunity. 2007;26(5):655–667. 10.1016/j.immuni.2007.04.008 [DOI] [PubMed] [Google Scholar]
- 11. Schwickert TA, Lindquist RL, Shakhar G, et al. : In vivo imaging of germinal centres reveals a dynamic open structure. Nature. 2007;446(7131):83–87. 10.1038/nature05573 [DOI] [PubMed] [Google Scholar]
- 12. Cyster JG, Ansel KM, Reif K, et al. : Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176(1):181–193. 10.1034/j.1600-065x.2000.00618.x [DOI] [PubMed] [Google Scholar]
- 13. Allen CD, Ansel KM, Low C, et al. : Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5(9):943–952. 10.1038/ni1100 [DOI] [PubMed] [Google Scholar]
- 14. Vinuesa CG, Tangye SG, Moser B, et al. : Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5(11):853–865. 10.1038/nri1714 [DOI] [PubMed] [Google Scholar]
- 15. Alsughayyir J, Pettigrew GJ, Motallebzadeh R: Spoiling for a Fight: B Lymphocytes as Initiator and Effector Populations within Tertiary Lymphoid Organs in Autoimmunity and Transplantation. Front Immunol. 2017;8:1639. 10.3389/fimmu.2017.01639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bannard O, Horton RM, Allen CD, et al. : Germinal center centroblasts transition to a centrocyte phenotype according to a timed program and depend on the dark zone for effective selection. Immunity. 2013;39(5):912–924. 10.1016/j.immuni.2013.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blauth K, Owens GP, Bennett JL: The Ins and Outs of B Cells in Multiple Sclerosis. Front Immunol. 2015;6:565. 10.3389/fimmu.2015.00565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bittner S, Ruck T, Wiendl H, et al. : Targeting B cells in relapsing-remitting multiple sclerosis: from pathophysiology to optimal clinical management. Ther Adv Neurol disord. 2017;10(1):51–66. 10.1177/1756285616666741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Von Büdingen HC, Kuo TC, Sirota M, et al. : B cell exchange across the blood-brain-barrier in multiple sclerosis. J Clin Invest. 2012;122(12):4533–43. 10.1172/JCI63842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Palanichamy A, Apeltsin L, Kuo TC, et al. : Immunoglobulin class-switched B cells form an active immune axis between CNS and periphery in multiple sclerosis. Sci Transl Med. 2014;6(248):248ra106. 10.1126/scitranslmed.3008930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stern JN, Yaari G, Vander Heiden JA, et al. : B cells populating the multiple sclerosis brain mature in the draining cervical lymph nodes. Sci Transl Med. 2014;6(248):248ra107. 10.1126/scitranslmed.3008879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Irani DN: Regulated Production of CXCL13 within the Central Nervous System. J Clin Cell Immunol. 2016;7(5): pii: 460. 10.4172/2155-9899.1000460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Columba-Cabezas S, Griguoli M, Rosicarelli B, et al. : Suppression of established experimental autoimmune encephalomyelitis and formation of meningeal lymphoid follicles by lymphotoxin beta receptor-Ig fusion protein. J Neuroimmunol. 2006;179(1–2):76–86. 10.1016/j.jneuroim.2006.06.015 [DOI] [PubMed] [Google Scholar]
- 24. Meinl E, Krumbholz M, Hohlfeld R: B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59(6):880–892. 10.1002/ana.20890 [DOI] [PubMed] [Google Scholar]
- 25. Peters A, Pitcher LA, Sullivan JM, et al. : Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity. 2011;35(6):986–996. 10.1016/j.immuni.2011.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ansel KM, Ngo VN, Hyman PL, et al. : A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406(6793):309–314. 10.1038/35018581 [DOI] [PubMed] [Google Scholar]
- 27. Carlsen HS, Baekkevold ES, Morton HC, et al. : Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104(10):3021–7. 10.1182/blood-2004-02-0701 [DOI] [PubMed] [Google Scholar]
- 28. Mitsdoerffer M, Peters A: Tertiary Lymphoid Organs in Central Nervous System Autoimmunity. Front Immunol. 2016;7:451. 10.3389/fimmu.2016.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fazilleau N, Mark L, McHeyzer-Williams LJ, et al. : Follicular helper T cells: lineage and location. Immunity. 2009;30(3):324–335. 10.1016/j.immuni.2009.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lim HW, Hillsamer P, Kim CH: Regulatory T cells can migrate to follicles upon T cell activation and suppress GC-Th cells and GC-Th cell-driven B cell responses. J Clin Invest. 2004;114(11):1640–9. 10.1172/JCI22325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luther SA, Tang HL, Hyman PL, et al. : Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc Natl Acad Sci U S A. 2000;97(23):12694–12699. 10.1073/pnas.97.23.12694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Piccio L, Naismith RT, Trinkaus K, et al. : Changes in B- and T-lymphocyte and chemokine levels with rituximab treatment in multiple sclerosis. Arch Neurol. 2010;67(6):707–14. 10.1001/archneurol.2010.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khademi M, Kockum I, Andersson ML, et al. : Cerebrospinal fluid CXCL13 in multiple sclerosis: a suggestive prognostic marker for the disease course. Mult Scler. 2011;17(3):335–343. 10.1177/1352458510389102 [DOI] [PubMed] [Google Scholar]
- 34. Corsiero E, Nerviani A, Bombardieri M, et al. : Ectopic Lymphoid Structures: Powerhouse of Autoimmunity. Front Immunol. 2016;7:430. 10.3389/fimmu.2016.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Corcione A, Casazza S, Ferretti E, et al. : Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A. 2004;101(30):11064–9. 10.1073/pnas.0402455101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pitzalis C, Jones GW, Bombardieri M, et al. : Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14(7):447–462. 10.1038/nri3700 [DOI] [PubMed] [Google Scholar]
- 37. Romme Christensen J, Börnsen L, Ratzer R, et al. : Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8(3):e57820. 10.1371/journal.pone.0057820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lehmann-Horn K, Wang SZ, Sagan SA, et al. : B cell repertoire expansion occurs in meningeal ectopic lymphoid tissue. JCI Insight. 2016;1(20):e87234. 10.1172/jci.insight.87234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rainey-Barger EK, Rumble JM, Lalor SJ, et al. : The lymphoid chemokine, CXCL13, is dispensable for the initial recruitment of B cells to the acutely inflamed central nervous system. Brain Behav Immun. 2011;25(5):922–931. 10.1016/j.bbi.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bagaeva LV, Rao P, Powers JM, et al. : CXC chemokine ligand 13 plays a role in experimental autoimmune encephalomyelitis. J Immunol. 2006;176(12):7676–7685. 10.4049/jimmunol.176.12.7676 [DOI] [PubMed] [Google Scholar]
- 41. Colombo M, Dono M, Gazzola P, et al. : Accumulation of clonally related B lymphocytes in the cerebrospinal fluid of multiple sclerosis patients. J Immunol. 2000;164(5):2782–9. 10.4049/jimmunol.164.5.2782 [DOI] [PubMed] [Google Scholar]
- 42. Serafini B, Rosicarelli B, Magliozzi R, et al. : Detection of ectopic B-cell follicles with germinal centers in the meninges of patients with secondary progressive multiple sclerosis. Brain Pathol. 2004;14(2):164–74. 10.1111/j.1750-3639.2004.tb00049.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ferraro D, Galli V, Vitetta F, et al. : Cerebrospinal fluid CXCL13 in clinically isolated syndrome patients: Association with oligoclonal IgM bands and prediction of Multiple Sclerosis diagnosis. J Neuroimmunol. 2015;283:64–69. 10.1016/j.jneuroim.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 44. Brettschneider J, Czerwoniak A, Senel M, et al. : The chemokine CXCL13 is a prognostic marker in clinically isolated syndrome (CIS). PLoS One. 2010;5(8):e11986. 10.1371/journal.pone.0011986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sellebjerg F, Börnsen L, Khademi M, et al. : Increased cerebrospinal fluid concentrations of the chemokine CXCL13 in active MS. Neurology. 2009;73(23):2003–2010. 10.1212/WNL.0b013e3181c5b457 [DOI] [PubMed] [Google Scholar]
- 46. Luther SA, Lopez T, Bai W, et al. : BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12(5):471–481. 10.1016/S1074-7613(00)80199-5 [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto K, Nishiumi S, Yang L, et al. : Anti-CXCL13 antibody can inhibit the formation of gastric lymphoid follicles induced by Helicobacter infection. Mucosal Immunol. 2014;7(5):1244–54. 10.1038/mi.2014.14 [DOI] [PubMed] [Google Scholar]
- 48. Litsious E, Semitekolou M, Galani IE, et al. : CXCL13 production in B cells via Toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(11):1194–1202. 10.1164/rccm.201208-1543OC [DOI] [PubMed] [Google Scholar]
- 49. Sáez de Guinoa J, Barrio L, Mellado M, et al. : CXCL13/CXCR5 signaling enhances BCR-triggered B-cell activation by shaping cell dynamics. Blood. 2011;118(6):1560–9. 10.1182/blood-2011-01-332106 [DOI] [PubMed] [Google Scholar]
- 50. Bajénoff M: Stromal cells control soluble material and cellular transport in lymph nodes. Front Immunol. 2012;3:304. 10.3389/fimmu.2012.00304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Esen N, Rainey-Barger EK, Huber AK, et al. : Type-I interferons suppress microglial production of the lymphoid chemokine, CXCL13. Glia. 2014;62(9):1452–1462. 10.1002/glia.22692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pikor NB, Astarita JL, Summer-Deluca L, et al. : Integration of Th17- and Lymphotoxin-Derived Signals Initiates Meningeal-Resident Stromal Cell Remodeling to Propagate Neuroinflammation. Immunity. 2015;43(6):1160–1173. 10.1016/j.immuni.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 53. Lalor SJ, Segal BM: Lymphoid chemokines in the CNS. J Neuroimmunol. 2010;224(1–2):56–61. 10.1016/j.jneuroim.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ramesh G, Borda JT, Gill A, et al. : Possible role of glial cells in the onset and progression of Lyme neuroborreliosis. J Neuroinflammation. 2009;6:23. 10.1186/1742-2094-6-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Narayan K, Dail D, Li L, et al. : The nervous system as ectopic germinal center: CXCL13 and IgG in lyme neuroborreliosis. Ann Neurol. 2005;57(6):813–823. 10.1002/ana.20486 [DOI] [PubMed] [Google Scholar]
- 56. Smith JR, Braziel RM, Paoletti S, et al. : Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101(3):815–821. 10.1182/blood-2002-05-1576 [DOI] [PubMed] [Google Scholar]
- 57. Magliozzi R, Columba-Cabezas S, Serafini B, et al. : Intracerebral expression of CXCL13 and BAFF is accompanied by formation of lymphoid follicle-like structures in the meninges of mice with relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148(1–2):11–23. 10.1016/j.jneuroim.2003.10.056 [DOI] [PubMed] [Google Scholar]
- 58. Ciccia F, Rizzo A, Maugeri R, et al. : Ectopic expression of CXCL13, BAFF, APRIL and LT-β is associated with artery tertiary lymphoid organs in giant cell arteritis. Ann Rheum Dis. 2017;76(1):235–243. 10.1136/annrheumdis-2016-209217 [DOI] [PubMed] [Google Scholar]
- 59. Magliozzi R, Howell OW, Reeves C, et al. : A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477–493. 10.1002/ana.22230 [DOI] [PubMed] [Google Scholar]
- 60. Howell OW, Reeves CA, Nicholas R, et al. : Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain. 2011;134(Pt 9):2755–2771. 10.1093/brain/awr182 [DOI] [PubMed] [Google Scholar]
- 61. Quinn JL, Kumar G, Agasing A, et al. : Role of TFH cells in Promoting T Helper 17-Induced Neuroinflammation. Front Immunol. 2018;9:382. 10.3389/fimmu.2018.00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Novakova L, Axelsson M, Khademi M, et al. : Cerebrospinal fluid biomarkers as a measure of disease activity and treatment efficacy in relapsing-remitting multiple sclerosis. J Neurochem. 2017;141(2):296–304. 10.1111/jnc.13881 [DOI] [PubMed] [Google Scholar]
- 63. Novakova L, Axelsson M, Khademi M, et al. : Cerebrospinal fluid biomarkers of inflammation and degeneration as measures of fingolimod efficacy in multiple sclerosis. Mult Scler. 2017;23(1):62–71. 10.1177/1352458516639384 [DOI] [PubMed] [Google Scholar]
- 64. Alvarez E, Piccio L, Mikesell RJ, et al. : Predicting optimal response to B-cell depletion with rituximab in multiple sclerosis using CXCL13 index, magnetic resonance imaging and clinical measures. Mult Scler J Exp Transl clin. 2015;1:2055217315623800. 10.1177/2055217315623800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Perry JS, Han S, Xu Q, et al. : Inhibition of LTi cell development by CD25 blockade is associated with decreased intrathecal inflammation in multiple sclerosis. Sci Transl Med. 2012;4(145):145ra106. 10.1126/scitranslmed.3004140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Braendstrup P, Langkilde AR, Schreiber K, et al. : Progression and CSF Inflammation after Eradication of Oligoclonal Bands in an MS Patient Treated with Allogeneic Hematopoietic Cell Transplantation for Follicular Lymphoma. Case Rep Neurol. 2012;4(2):101–106. 10.1159/000339738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bible E: Multiple sclerosis: Atacicept increases relapse rates in multiple sclerosis. Nat Rev Neurol. 2014;10(4):182. 10.1038/nrneurol.2014.48 [DOI] [PubMed] [Google Scholar]
- 68. Kappos L, Hartung HP, Freedman MS, et al. : Atacicept in multiple sclerosis (ATAMS): a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Neurol. 2014;13(4):353–63. 10.1016/S1474-4422(14)70028-6 [DOI] [PubMed] [Google Scholar]
- 69. Baker D, Marta M, Pryce G, et al. : Memory B Cells are Major Targets for Effective Immunotherapy in Relapsing Multiple Sclerosis. EBioMedicine. 2017;16:41–50. 10.1016/j.ebiom.2017.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Badr G, Borhis G, Lefevre EA, et al. : BAFF enhances chemotaxis of primary human B cells: a particular synergy between BAFF and CXCL13 on memory B cells. Blood. 2008;111(5):2744–54. 10.1182/blood-2007-03-081232 [DOI] [PubMed] [Google Scholar]
- 71. Topping J, Dobson R, Lapin S, et al. : The effects of intrathecal rituximab on biomarkers in multiple sclerosis. Mult Scler Relat Disord. 2016;6:49–53. 10.1016/j.msard.2016.01.001 [DOI] [PubMed] [Google Scholar]
- 72. Komori M, Lin YC, Cortese I, et al. : Insufficient disease inhibition by intrathecal rituximab in progressive multiple sclerosis. Ann Clin Transl Neurol. 2016;3(3):166–179. 10.1002/acn3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Eggers EL, Michel BA, Wu H, et al. : Clonal relationships of CSF B cells in treatment-naive multiple sclerosis patients. JCI Insight. 2017;2(22): pii: 92724. 10.1172/jci.insight.92724 [DOI] [PMC free article] [PubMed] [Google Scholar]