Abstract
The purpose of this study was to evaluate the prognostic value of tumour-infiltrating lymphocytes (TILs) in melanoma and to determine whether a simpler numerical scoring system would be more effective. 655 patients presenting to a UK teaching hospital with primary invasive melanoma were analysed. TILs were re-scored using the standard Clark's method and univariable and multivariable analyses of the effect of TILS on overall survival (OS), disease-specific survival (DSS) and metastasis-free survival (MFS) was assessed using Cox regression. 30 (5%) melanomas showed absent, 464 (71%) non-brisk and 161 (24%) brisk TILs. There was a statistically significant relationship between TILs and Breslow thickness, age, melanoma type, mitotic rate and histological regression. TIL grade was a significant predictor of MFS in multivariable analysis (HR 0.44, CI 0.25 to 0.77) but was not significant for OS or DSS. By contrast, when a simple numerical TIL percentage score was employed this was a strong predictor of OS (HR 0.55, CI 0.38 to 0.78), DSS (HR 0.25, CI 0.14 to 0.44) and MFS (HR 0.32, CI 0.21 to 0.51) in multivariable analysis. The percentage TIL score was also significant when adjusted for the prognostic gold standard, AJCC stage: OS (HR0.66, CI 0.46 to 0.95), DSS (HR 0.33, CI 0.19 to 0.60) and MFS (HR 0.41, CI 0.26 to 0.65). The TIL percentage score was subsequently validated in new cases. In summary, this study strongly confirms that higher amounts of TILs are associated with better prognosis and in addition demonstrates the value of a simplified numerical TIL scoring system.
Introduction
Malignant melanoma is a type of skin cancer derived from melanocytes with outcome dependent of clinical stage at diagnosis such that the 93% of patients with early local disease (stage IA) are alive at 10 years, contrasting with only 39% with advanced local disease (stage IIC)1. The American Joint Committee on Cancer (AJCC) version 72 is globally accepted for clinical staging. Nevertheless, stage does not correlate perfectly with outcome so it remains important to identify other factors that might refine its prognostic value.
One histological prognostic feature of potential value but not currently part of staging is the number of tumour infiltrating lymphocytes (TILs). In recent years, the emergence of immune checkpoint inhibitors has fueled interest in TILs because these cells are the biological engine underpinning this therapy. The term, 'tumour-infiltrating lymphocytes', was first coined by Clark and co-workers3, who devised a grading system that has been widely adopted. This recognizes TILs as lymphocytes that directly interact with melanoma cells and is classified as absent, non-brisk or brisk. The latter two can be further regarded as diffuse or peritumoural. Subsequent work has shown that TILs are associated with better prognosis4,5. From a mechanistic perspective, adoptive cell transfer of TILs was associated with tumour regression6. In the context of immune therapies, recent work has sought to gather a more nuanced understanding of TIL sub-populations and their functional state7 and how this relates to the cancer-immunity cycle8.
So far, we know that increased TILs are a good prognostic feature and that the current Clark grading system remains relevant even in the face of analysis of TIL subclasses and gene expression7. However, the standard grading system has potential to be improved. The purpose of the current study was to assess Clark's grading system by completely re-scoring TILs in a cohort of 655 cases with relevant endpoints: overall survival, disease-specific and metastasis-free survival. We then proceded to assess a simple numerical scoring system based on the estimated percentage of tumour cells affected by TILs that was then validated in 101 new cases.
Methods
Study population
Patients were selected from the archives of the University Hospitals of Leicester Cellular Pathology department. Invasive melanoma cases from January 1st 2004 to December 31st 2009 were retrieved and assessed for inclusion and exclusion criteria. For eligibility, patients had to be resident in Leicestershire and have an unambiguous cutaneous invasive primary melanoma. Patients with multiple skin melanoma were excluded. Patients not resident in Leicestershire were excluded because they had thicker melanomas referred for specialist plastic surgery care and were a biased subset. Moreover, these patients were managed at their local hospital so follow up data were patchy and incomplete. Patients were screened for eligibility sequentially creating a retrospective incident cohort. In-situ, non-cutaneous, ambiguous, non-primary and non-Leicestershire cases were filtered out, leaving a total of 683 eligible cases. Of these, 10 were excluded because the patient had multiple primary melanomas, 17 because slides were not filed (which was required for TIL scoring) and 1 because the sections were too faded to score. The remaining 655 cases were designated dataset 1. For validation a new set of cases was selected using the same criteria as dataset 1, except that cases had to be greater than 1mm in Breslow thickness. This extra criterion was added to maximize statistical power from fewer cases, since the power in survival analysis depends on the number of events and the vast majority of these occur in melanomas that are more than 1 mm thick. Selection commenced from 1st January 2010 and continued until 105 cases were found, this number being chosen to ensure we had approximately 100 after any exclusions. From these, 2 cases were excluded because they were small biopsies with no record of full excision in Leicester, 1 case was not in file and 1 turned out to be a local recurrence rather than the primary tumour, leaving a total of 101 cases with 42 deaths, 20 melanoma-specific deaths and 29 developing metastases. This was designated dataset 2.
Tumour infiltrating lymphocyte scoring
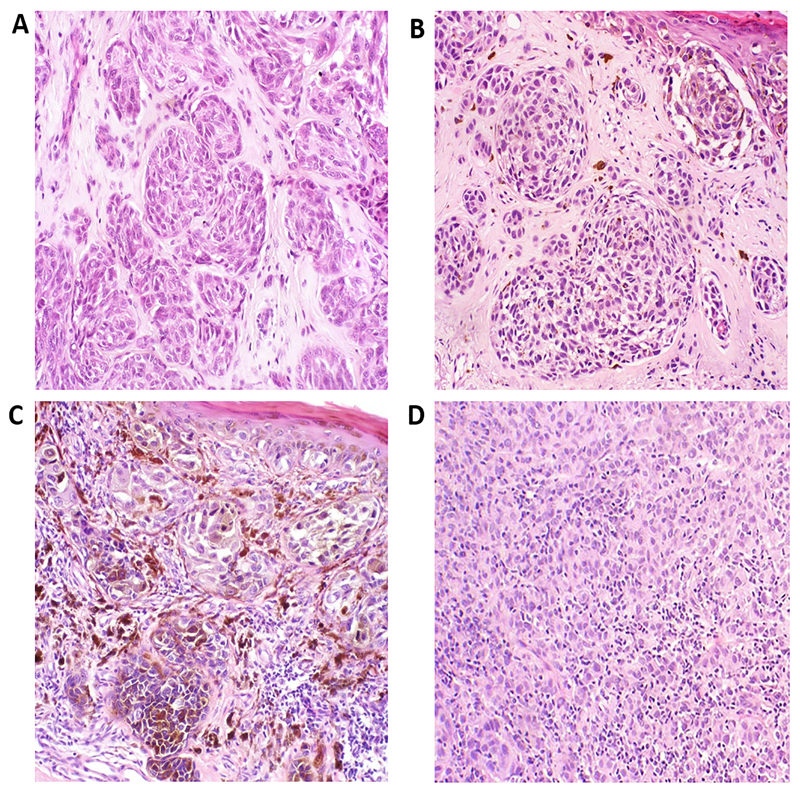
TILs were re-scored. Adherence to the scoring system described by Clark was strictly followed4,5 with guidance also from the Royal College of Pathologists dataset for reporting of melanoma9. The TIL percentage score was measured by estimating the percentage of tumour cells that were disrupted by a lymphocytic infiltrate. As with standard TIL grading, there had to be evidence of disruption of the tumour and/or melanocytic apoptosis. The percentage was estimated from 0-100%, to the nearest 10% (but 5% and 95% were allowed at extremes). This method, by design, at once accounts for TIL intensity and extent such that a light, focal intratumoural or peritumoural scattering of TILs would score only 5%, but the TIL percentage score would necessarily increase as the TIL intensity and extent increased. In cases with purely peritumoural TILs, the score would also depend on tumour nodule size, with the proportion cells disrupted decreasing for larger nodules. The primary rater (KF) was trained to grade TILs and then 20 randomly selected cases were checked for agreement with a dermatopathologist (GS), with a kappa score of 0.92 for Clark scoring and an intraclass correlation coefficient 0.91 for percentage scoring. This indicates excellent agreement. During subsequent scoring, problematic cases were discussed with dermatopathologists (GS/MB). Examples of TIL percentage scores are shown in Figure 1 and a histogram comparing TIL percentage score for non-brisk and brisk categories of Clark scoring are shown in supplementary Figure A1. The TIL percentage scoring for dataset 2 validation cases was done by a different rater (GS). The use of tissue was covered by an NHS research ethics committee favourable opinion.
Figure 1.
Representative photomicrographs of TIL percentage scores from different tertiles (0-5%; 10-20%; >20%). Absent TILS, score 0% (A). TILs 5% (B). TILs 20% (C). TILS 80% (D).
Statistical analyses
Statistical analyses were all performed in R version 3.2.010. For bivariable analysis of TILs and other covariables, Chi-squared and ANOVA tests were used, employing the R Gmisc package11. Survival analyses were performed using the R survival package12 and Kaplan Meier plots generated using the R survminer package13. Time to event analysis was performed with three outcomes, overall survival (OS), disease specific survival (DSS) and metastasis-free survival (MFS). The date of diagnosis was the date of primary sample accession in the pathology database. For OS, failure was death from any cause while for DSS death from melanoma was considered as failure and death from another cause was regarded as censoring. For MFS, the event was the time of first metastasis (either loco-regional or distant). Survival was analysed by the Kaplan Meier method and log rank tests were used to compare survival curves. Univariable and multivariable hazard ratios were determined using Cox proportional hazards method. The proportionality assumption was checked by examining plots of scaled Schoenfeld residuals against transformed time and with a goodness of fit test. Proportionality was not violated. Crude and adjusted hazard ratio tables for Cox regression were generated using the R htmlTable package14 and edited in MS word.
REMARK criteria
This study adhered to REMARK guidelines15. The features demonstrating REMARK compliance are presented in supplementary Table A1.
Results
After re-scoring TILs in 655 primary melanomas using the standard Clark method, TILs were absent in 30 (5%), non-brisk in 464 (71%) and brisk in 161 (24%). The baseline features for all cases and those within each TIL category are shown in Table 1.
Table 1. Baseline features of melanoma cases.
| Tumour Infiltrating Lymphocytes | |||||
|---|---|---|---|---|---|
| Total | Absent | Brisk | Non brisk | P-value | |
| Age | |||||
| Median (IQR) | 61 (49 - 73) | 61 (47 - 68) | 57 (46 - 67) | 62 (50 - 74) | 0.002 |
| Sex | |||||
| Female | 366 (56%) | 21 (6%) | 88 (24%) | 257 (70%) | 0.29 |
| Male | 289 (44%) | 9 (3%) | 73 (25%) | 207 (72%) | |
| Site | |||||
| Acral | 30 (5%) | 7 (23%) | 2 (7%) | 21 (70%) | < 0.0001 |
| Head & neck | 120 (18%) | 5 (4%) | 27 (22%) | 88 (73%) | |
| Lower limb | 182 (28%) | 9 (5%) | 35 (19%) | 138 (76%) | |
| Trunk | 216 (33%) | 3 (1%) | 63 (29%) | 150 (69%) | |
| Upper limb | 107 (16%) | 6 (6%) | 34 (32%) | 67 (63%) | |
| Breslow Thickness | |||||
| Median (IQR) | 0.9 (0.6 - 2.0) | 0.7 (0.5 - 2.0) | 0.8 (0.5 - 1.3) | 1.1 (0.6 - 2.3) | 0.0006 |
| Ulcer | |||||
| Absent | 553 (84%) | 27 (5%) | 142 (26%) | 384 (69%) | 0.21 |
| Present | 102 (16%) | 3 (3%) | 19 (19%) | 80 (78%) | |
| Mitotic rate | |||||
| Median (IQR) | 1 (0 - 3) | 0 (0 - 2) | 1 (0 - 2) | 1 (0 - 4) | 0.035 |
| Microsatellites | |||||
| Absent | 646 (99%) | 30 (5%) | 160 (25%) | 456 (71%) | 0.65 |
| Present | 9 (1%) | 0 (0%) | 1 (11%) | 8 (89%) | |
| Regression | |||||
| Absent | 519 (79%) | 28 (5%) | 118 (23%) | 373 (72%) | 0.022 |
| Present | 136 (21%) | 2 (1%) | 43 (32%) | 91 (67%) | |
| TILs | |||||
| Absent | 30 (5%) | - | - | - | - |
| Brisk | 161 (25%) | - | - | - | |
| Non brisk | 464 (71%) | - | - | - | |
TILs, Tumour-infiltrating lymphocytes.
The cases in this study were relatively thin melanomas. The median Breslow thickness of 0.9 mm reflects the incident melanomas at the study location, which in turn reflects incident melanomas in the UK in general16. Microscopic satellites were not frequently identified (9% of cases) and regression was present in 21% of cases. TILs had a statistically significant relationship with Breslow thickness, with fewer TILs in thick melanomas. There was an association with age, with brisk TILs being more frequent in younger individuals. Brisk TILs were much less common in acral melanomas. TILs had a significant relationship to mitotic rate, with mitoses being more frequent in the non-brisk and brisk groups compared to absent. TILs were more commonly found when histological regression was also present.
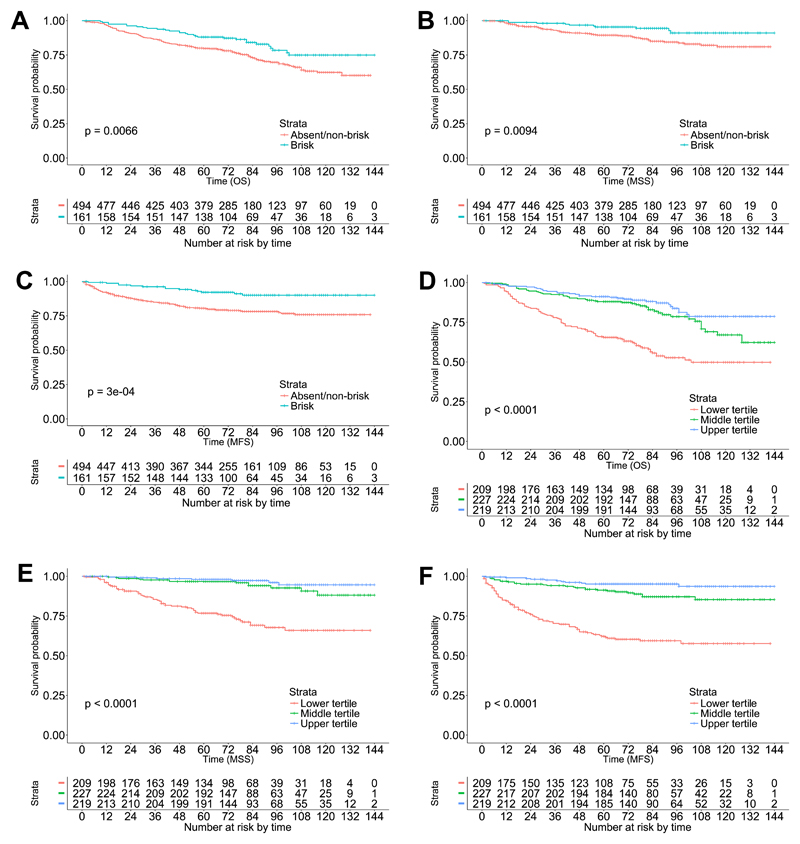
The relationship between TILs and survival was first assessed using Kaplan Meier estimates of the survivor function for 3 outcome events, OS, DSS and MFS. The median follow up was 78 months. With careful re-scoring using strict adherence to standard criteria for TIL grading, there were only 30 cases where TILs were absent. Amongst these, there were only 6 deaths, 3 disease-specific deaths and 5 metastases. In view of the small size of this group and the low number of events, the absent and non-brisk TIL groups were combined. The Kaplan Meier survival plots are shown in Figure 2A-C alongside the number at risk per stratum. The 5 year OS for absent/non-brisk and brisk TILs was 80% and 88% respectively, and corresponding values for DSS were 89% and 95%, and MFS were 81% and 92%. The survival curves for each of these outcomes were significantly different.
Figure 2.
(A-C) Overall, disease-specific and metastasis-free survival respectively for absent/non-brisk and brisk tumour-infiltrating lymphocyte categories, scored according to Clark's method. (D-F) Overall, disease-specific and metastasis-free survival respectively for lower, middle and upper tertiles of tumour-infiltrating lymphocyte percentage score.
A Cox proportional hazards model was created to assess the univariable and multivariable statistical effect of TILs on OS, DSS and MFS. Once again, TILs were analysed as two groups, absent/non-brisk and brisk, with the former as the reference category. The model included standard clinicopathological covariables associated with outcome: Breslow thickness, ulcer, mitotic rate, microscopic satellites, histological regression, age, sex and site. The results are shown in Table 2, where both crude and adjusted hazard ratios are shown. Adjusted hazard ratios for TILs were only significant for the surrogate endpoint of MFS and not for the most objective endpoint, OS, nor for DSS. The only variables that were significant for all outcome events in multivariable analysis were sex, mitotic rate and ulceration. An unabridged version of Table 2 is shown in supplementary Table A2.
Table 2. Cox proportional hazards model for TILs and covariables.
| OS | DSS | MFS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | |||||||
| Variable | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| TILS | ||||||||||||
| Abs/Non brisk | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref |
| Brisk | 0.58** | 0.39 to 0.86 | 0.85 | 0.56 to 1.27 | 0.43* | 0.22 to 0.83 | 0.58 | 0.29 to 1.14 | 0.37*** | 0.21 to 0.65 | 0.44** | 0.25 to 0.77 |
| Regression | ||||||||||||
| Absent | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref |
| Present | 0.55** | 0.35 to 0.87 | 0.65 | 0.41 to 1.03 | 0.21** | 0.07 to 0.56 | 0.31* | 0.11 to 0.86 | 0.25*** | 0.12 to 0.51 | 0.36** | 0.17 to 0.76 |
| Age (years) | 1.06*** | 1.05 to 1.08 | 1.06*** | 1.04 to 1.07 | 1.02** | 1.01 to 1.04 | 1.01 | 0.99 to 1.03 | 1.02*** | 1.01 to 1.03 | 1 | 0.99 to 1.01 |
| Sex | ||||||||||||
| Female | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref |
| Male | 1.79*** | 1.32 to 2.43 | 1.63** | 1.17 to 2.26 | 1.66* | 1.06 to 2.61 | 1.48 | 0.91 to 2.42 | 1.49* | 1.04 to 2.15 | 1.52* | 1.02 to 2.26 |
| Site | ||||||||||||
| Central | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref |
| Peripheral | 0.67* | 0.49 to 0.91 | 0.82 | 0.59 to 1.15 | 0.71 | 0.45 to 1.12 | 0.89 | 0.55 to 1.46 | 0.89 | 0.62 to 1.28 | 0.95 | 0.63 to 1.41 |
| Breslow | 1.14*** | 1.11 to 1.16 | 1.05* | 1.00 to 1.09 | 1.15*** | 1.12 to 1.19 | 1.05 | 0.98 to 1.12 | 1.13*** | 1.11 to 1.16 | 1.07*** | 1.03 to 1.12 |
| Mitotic rate | 1.08*** | 1.06 to 1.10 | 1.03** | 1.01 to 1.05 | 1.10*** | 1.08 to 1.12 | 1.05*** | 1.02 to 1.08 | 1.13*** | 1.11 to 1.15 | 1.08*** | 1.06 to 1.11 |
| Ulcer | ||||||||||||
| Absent | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref |
| Present | 4.83*** | 3.53 to 6.61 | 2.42*** | 1.67 to 3.50 | 6.98*** | 4.43 to 11.00 | 3.25*** | 1.88 to 5.62 | 5.90*** | 4.08 to 8.55 | 2.18** | 1.36 to 3.49 |
| Microsatellites | ||||||||||||
| Absent | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref | 1 | ref |
| Present | 5.28*** | 2.47 to 11.28 | 1.19 | 0.53 to 2.70 | 3.11 | 0.76 to 12.71 | 0.48 | 0.11 to 2.10 | 6.74*** | 2.74 to 16.58 | 0.95 | 0.35 to 2.56 |
OS, overall survival; DSS, disease-specific survival; MFS, metastasis-free survival; TILs, tumour-infiltrating lymphocytes; HR, hazard ratio.
* p < 0.05; ** p < 0.01; *** p< 0.001
The recognised system for TIL grading attempts to incorporate two features, pattern (peritumoural and diffuse) and intensity (absent, non-brisk and brisk). This could have potential issues, for example brisk peritumoural TILs might only interact with a small percentage of melanoma cells at the edge of a tumour island while a brisk diffuse TIL infiltrate in a tumour island of the same size will interact with a high percentage of melanoma cells. This difference is not recognised within the brisk category. We therefore devised a simple TIL percentage score, where the proportion of melanoma affected by TILs was estimated from H&E stained sections and scored from 0-100%. The mean percentage score was 22%, with a median of 20% and a range of 0% to 90%. In order to perform Kaplan Meier analysis, the TIL percentage score was broken into 3 categories based on tertiles and survival curves were compared. The tertiles corresponded to 0-5%, 10-20% and greater than or equal to 30%. The Kaplan Meier survival plots are shown in Figure 2D-F alongside the number at risk per stratum. The 5 year OS for upper, middle and lower tertiles was 91%, 88% and 66% respectively. Corresponding values for DSS were 98%, 97% and 77% and for MFS were 95%, 91% and 62%. These survival curves differed significantly for all 3 outcomes
The Kaplan Meier estimates of the survivor function showed that the curves for the two higher tertiles substantially overlapped. It therefore seemed sensible to combine these two groups for subsequent analysis using Cox proportional hazards regression, thus creating a group of 0-5% and 10% or more. The proportional hazards regression analysis is shown in Table 3. The crude hazard ratios for all variables in the model except TIL percentage score were shown in Table 2, so are not repeated in Table 3. The TIL percentage crude hazard ratios for OS, DSS and MFS were 0.33 (95% confidence interval 0.24 to 0.44), 0.14 (95% confidence interval 0.08 to 0.23) and 0.17 (95% confidence interval 0.11 to 0.25), and all were statistically significant. An unabridged version of Table 3 is shown in supplementary Table A3. The TIL percentage score tertiles had a statistically significant adjusted hazard ratio for OS, DSS and MFS. In our cohort, the TIL percentage score appears to show evidence of better prognostic value than the standard Clark method of TIL scoring recommended by AJCC. This prompted us to determine whether the TIL percentage score had potential to add value to AJCC staging by creating a Cox proportional hazards regression model containing just TIL percentage score and AJCC stage. The results are shown in Table 4. An unabridged version is shown in supplementary Table A4. A significant reduction in hazard was noted for OS, DSS and MFS when cases were in the middle and upper tertiles for TIL percentage score. This suggests that when as few as 10% of the melanoma cells are involved by TILs the prognosis improves independently of AJCC stage.
Table 3. Cox proportional hazards model for TIL percentage score and covariables.
| OS | DSS | MFS | ||||
|---|---|---|---|---|---|---|
| Variable | Adjusted HR | 95% CI | Adjusted HR | 95% CI | Adjusted HR | 95% CI |
| TILs % tertile | ||||||
| Lower tertile | 1 | ref | 1 | ref | 1 | ref |
| Mid/upper tertile | 0.55 *** | 0.38 to 0.78 | 0.25 *** | 0.14 to 0.44 | 0.32 *** | 0.21 to 0.51 |
| Regression | ||||||
| Absent | 1 | ref | 1 | ref | 1 | ref |
| Present | 0.73 | 0.46 to 1.16 | 0.44 | 0.16 to 1.26 | 0.47 * | 0.22 to 0.99 |
| Age (years) | 1.05 *** | 1.04 to 1.07 | 1.01 | 1.00 to 1.03 | 1.01 | 0.99 to 1.02 |
| Sex | ||||||
| Female | 1 | ref | 1 | ref | 1 | ref |
| Male | 1.71 ** | 1.23 to 2.39 | 1.65 * | 1.01 to 2.69 | 1.64 * | 1.10 to 2.44 |
| Site | ||||||
| Central | 1 | ref | 1 | ref | 1 | ref |
| Peripheral | 0.8 | 0.57 to 1.11 | 0.82 | 0.50 to 1.35 | 0.9 | 0.60 to 1.34 |
| Breslow | 1.03 | 0.99 to 1.08 | 1.02 | 0.95 to 1.10 | 1.06 * | 1.01 to 1.10 |
| Mitotic rate | 1.02 * | 1.00 to 1.05 | 1.04 ** | 1.01 to 1.07 | 1.07 *** | 1.04 to 1.10 |
| Ulcer | ||||||
| Absent | 1 | ref | 1 | ref | 1 | ref |
| Present | 2.20 *** | 1.51 to 3.20 | 2.62 *** | 1.51 to 4.54 | 1.73 * | 1.08 to 2.77 |
| Microsatellites | ||||||
| Absent | 1 | ref | 1 | ref | 1 | ref |
| Present | 1.05 | 0.46 to 2.40 | 0.41 | 0.09 to 1.82 | 0.99 | 0.36 to 2.72 |
OS, overall survival; DSS, disease-specific survival; MFS, metastasis-free survival; TILs, tumour-infiltrating lymphocytes; HR, hazard ratio.
* p < 0.05; ** p < 0.01; *** p< 0.001
Table 4. Cox proportional hazards model using TIL tertiles and AJCC stage.
| OS | DSS | MFS | ||||
|---|---|---|---|---|---|---|
| Variable | Adjusted HR | 95% CI | Adjusted HR | 95% CI | Adjusted HR | 95% CI |
| TILs % tertlie | ||||||
| Lower tertile | 1 | ref | 1 | ref | 1 | ref |
| Mid/upper tertiles | 0.66 * | 0.46 to 0.95 | 0.33 *** | 0.19 to 0.60 | 0.41 *** | 0.26 to 0.65 |
| AJCC | ||||||
| IA | 1 | ref | 1 | ref | 1 | ref |
| IB | 1.26 | 0.80 to 2.01 | 5.52 ** | 1.63 to 18.68 | 7.88 *** | 2.79 to 22.25 |
| IIA | 1.88 * | 1.05 to 3.39 | 7.66 ** | 2.06 to 28.45 | 14.32 *** | 4.83 to 42.44 |
| IIB | 5.25 *** | 3.12 to 8.85 | 20.15 *** | 5.75 to 70.65 | 25.48 *** | 8.64 to 75.18 |
| IIC | 8.36 *** | 4.97 to 14.05 | 34.14 *** | 9.84 to 118.43 | 43.95 *** | 15.00 to 128.79 |
OS, overall survival; DSS, disease-specific survival; MFS, metastasis-free survival; TILs, tumour-infiltrating lymphocytes; HR, hazard ratio; AJCC, American Joint Committee on Cancer.
* p < 0.05; ** p < 0.01; *** p< 0.001
The 10% cut point for creating the TIL percentage score risk strata emerged from the data as a discovery rather than being specified a priori, therefore we sought to validate the cut-point in dataset 2, a new set of 101 melanomas. These cases were all greater than 1 mm. The results of multivariable analysis are shown in Table 5 alongside the same analysis in a 312 case subset of dataset 1, comprising only cases greater than 1 mm thick. The analysis was limited to this subset to make datasets 1 and 2 comparable. The cut point was significant for OS, DSS and MFS in both the 312 case subset of dataset 1 and in the 101 cases in dataset 2, indicating that the risk strata based on a cut point of 10% can be generalised to new cases.
Table 5. Validation of the TIL percentage score cut point using Cox proportional hazards model with new cases and adjusted for AJCC stage.
| Discovery (n=312) | Validation (n=101) | |||
|---|---|---|---|---|
| Variable | Adjusted HR | 95% CI | Adjusted HR | 95% CI |
| OS | ||||
| Tils % tertile | ||||
| Lower tertile | ref | ref | ||
| Mid/Upper tertiles | 0.54 ** | 0.35 - 0.82 | 0.46 * | 0.24 - 0.88 |
| AJCC | ||||
| IB | ref | ref | ||
| IIA | 1.24 | 0.68 - 2.24 | 0.9 | 0.30 - 2.70 |
| IIB | 3.33 *** | 1.97 - 5.64 | 1.73 | 0.70 - 4.31 |
| IIC | 5.21 *** | 3.08 - 8.81 | 3.46 ** | 1.50 - 7.99 |
| DSS | ||||
| Tils % tertile | ||||
| Lower tertile | ref | ref | ||
| Mid/Upper tertiles | 0.34 *** | 0.18 - 0.64 | 0.30 * | 0.11 - 0.80 |
| AJCC | ||||
| IB | ref | ref | ||
| IIA | 1.15 | 0.50 - 2.63 | 1.02 | 0.17 - 6.14 |
| IIB | 3.00 *** | 1.44 - 6.22 | 1.19 | 0.83 - 12.86 |
| IIC | 5.03 *** | 2.47 - 10.25 | 1.34 | 0.96 - 15.03 |
| MFS | ||||
| Tils % tertile | ||||
| Lower tertile | ref | ref | ||
| Mid/Upper tertiles | 0.48 ** | 0.30 - 0.78 | 0.43 * | 0.19 - 0.96 |
| AJCC | ||||
| IB | ref | ref | ||
| IIA | 1.59 | 0.87 - 2.91 | 4.84 * | 1.25 - 18.76 |
| IIB | 2.82 *** | 1.55 - 5.12 | 3.05 | 0.75 - 12.33 |
| IIC | 4.87 *** | 2.72 - 8.73 | 7.40 ** | 2.01 - 27.27 |
OS, overall survival; DSS, disease-specific survival; MFS, metastasis-free survival; TILs, tumour-infiltrating lymphocytes; HR, hazard ratio; AJCC, American Joint Committee on Cancer.
* p < 0.05; ** p < 0.01; *** p< 0.001
Discussion
This is a large single centre retrospective cohort study assessing the prognostic significance of TILs in melanoma prognosis. The strong aspects of this study are the large numbers of patients (655), the fact that these represent a true unbiased incident cohort, that the TIL grade was re-scored for all tissue samples in order to avoid inter-observer bias, that we assessed three important end points (OS, DSS and MFS), that we included important covariables in regression analysis and that we validated the cut point for stratifying TIL percentage score with new cases. Most crucially we show that the prognostic value of TILs assessment can be enhanced by simply estimating the percentage of tumour cells involved by TILs and that this remains significant in multivariable analysis even when standard TIL grading using Clark's system does not. These findings are very timely, given the known role of the immune system in determining cancer behaviour and the emerging role of immune checkpoint inhibition in melanoma care.
While this study has several strong points, there were some issues. It should be recognised that this is a single centre study so there is a need to demonstrate that the findings can be generalised. On the other hand, the general demographic features of the patients were consistent with those seen in the UK and western countries. There is also the concern that the TILs in dataset 1 were scored by a single rater, so there is a risk that the rater rather than the grading system is being tested. However, the rater was intensively trained in TIL scoring, discussed difficult cases with dermatopathologists (GS and MB) and had a low threshold for deciding if discussion was required. In addition the agreement score for the rater and a dermatopathologist (GS) for TIL grade was very high and a different scorer was used for the validation case scoring. A final issue was that sentinel node biopsy was not a part of standard care during the recruitment period in the study centre, so there was no possibility to use sentinel node status as an outcome measure. This was mitigated by the fact that we were able to assess three other outcome events, OS, DSS and MFS.
It has been nearly 40 years since TILs were first recognised as a prognostic factor for melanoma17 and since then evidence has accumulated steadily. The present study strongly supports the idea that increasing amounts of TILs are associated with better outcome. Internationally, TILs scoring has been standardized using Clark's system of absent, non-brisk and brisk, but there have been attempts to improve this. The most notable example, because of the large sample size and analysis of the association between TIL score and sentinel node status, was the 4 tier system used by Azimi18. This system attempted to amalgamate the pattern of infiltration (focal, multifocal, diffuse) with the intensity (absent, mild, moderate, marked) which could be cross-tabulated into a grade (0 to 4). Using this system, TIL grade was an independent prognostic factor for disease specific survival, recurrence-free survival and for predicting sentinel node status. A big advantage of this novel grading system was that it addressed a problem with Clark grading, in particular involvement of the entire base of tumour by peritumoural TILs is labelled brisk, yet only a small proportions of tumour cells might be interacting with TILs because in cases with large tumour nodules most melanoma cells reside in the central part of a tumour mass. Evidence suggests that this is not merely a theoretical problem, because in the grading system described by Azimi and co-workers brisk peritumoural TILs ranged from a low grade of 1 to high grade of 3 (see Table A2 from that article18). A potential problem with Azimi's system is that there is a subjective element in deciding whether the TIL intensity is mild, moderate or marked. The system we employed also has a subjective element, being semi-quantitative, but is very simple and requires no cross tabulation of pattern and intensity. One merely estimates the proportion of dermal melanoma disrupted by TILs and assigns a percentage to the nearest 10%. This system had very strong inter-observer agreement. Importantly, this percentage TIL score was strongly significant in multivariable analysis for OS, DSS and MFS with eight other covariables, which was not the case for TIL grading by the Clark system. Furthermore the novel scoring system showed independent prognostic value in a prognostic model that included the gold standard for prognosis, AJCC stage.
In AJCC staging, the Breslow depth, ulceration and microscopic satellites are all recognised histological features1. More recently, evidence about mitotic rate19–21 has led to its inclusion in AJCC staging too. The evidence from the present study and previous data strongly support the inclusion of TILs grade in pathology reports. Routine inclusion is important so that further studies of TILs in melanoma prognosis can be enabled. Indeed, inclusion of TIL grading is required in pathology reports in the UK by the Royal College of Pathologists9 and inclusion is recommended in UK guidelines for melanoma management22. TIL grade is also supported as a data item by the American Academy of Dermatology23 and TILs are an essential feature of the Armed Forces Institute of Pathology eight-year survival prognostic model24. More recently, TILs have become important because they may have a role in determining the response to immune therapy. In this regard, TIL response in metastases is likely to be more important than primary melanoma but nevertheless a clearer understanding of TILs in primary melanoma is likely to add to our overall understanding of this phenomenon. The present study has focused on the simple histological scoring of TILs, but dissecting the fine detail of TIL subpopulations and their complex interactions with melanoma cells will become increasingly important as a predictive biomarker for immune therapy and is an area of intense research.
In summary, this study strongly supports earlier data showing that increased amounts of TILs are associated with better prognosis. The study evaluated important outcome measures and demonstrates the prognostic value of a novel and simple numerical TIL scoring system.
Supplementary Material
Conflicts of interest and sources of funding
KF was supported by the Association of Clinical Pathologists ACP Student Research Fund. For remaining authors none declared.
References
- 1.Balch CM, Gershenwald JE, Soong S-j, et al. Final version of 2009 AJCC melanoma staging and classification. Journal of Clinical Oncology. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of Surgical Oncology. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 3.Clark WH, Jr, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29:705–27. [PubMed] [Google Scholar]
- 4.Clark WH, Jr, Elder DE, Guerry D, 4th, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 5.Clemente CG, Mihm MC, Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–10. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 7.Lee N, Zakka LR, Mihm MC, Jr, et al. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology. 2016;48:177–87. doi: 10.1016/j.pathol.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Slater D, Walsh M. Standards and datasets for reporting cancers: Dataset for the histological reporting of primary cutaneous malignant melanomas and regional lymph nodes. royal college of pathologists; 2014. [Google Scholar]
- 10.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Available at: https://www.R-project.org/ [Google Scholar]
- 11.Gordon M. Gmisc: Descriptive statistics, transition plots, and more. 2016 Available at: https://CRAN.R-project.org/package=Gmisc.
- 12.Therneau T. A package for survival analysis in s, version 2.38. 2015 Available at: http://CRAN.R-project.org/package=survival.
- 13.Kassambara A, Kosinski M. Survminer: Drawing survival curves using ’ggplot2’. 2016 Available at: https://CRAN.R-project.org/package=survminer.
- 14.Gordon M. HtmlTable: Advanced tables for markdown/HTML. 2016 Available at: https://CRAN.R-project.org/package=htmlTable.
- 15.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumour mARKer prognostic studies (rEMARK) Br J Cancer. 2005;93:387–91. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CRUK. Skin cancer incidence statistics. Cancer Research UK; 2015. [Google Scholar]
- 17.Larsen TE, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 3. The relation between the tumour-associated lymphocyte infiltration and age and sex, tumour cell type, pigmentation, cellular atypia, mitotic count, depth of invasion, ulceration, tumour type and prognosis. Acta Pathologica Et Microbiologica Scandinavica. Section A, Pathology. 1978;86A:523–530. [PubMed] [Google Scholar]
- 18.Azimi F, Scolyer RA, Rumcheva P, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 19.Azzola MF, Shaw HM, Thompson JF, et al. Tumor mitotic rate is a more powerful prognostic indicator than ulceration in patients with primary cutaneous melanoma: An analysis of 3661 patients from a single center. Cancer. 2003;97:1488–1498. doi: 10.1002/cncr.11196. [DOI] [PubMed] [Google Scholar]
- 20.Francken AB, Shaw HM, Thompson JF, et al. The prognostic importance of tumor mitotic rate confirmed in 1317 patients with primary cutaneous melanoma and long follow-up. Annals of Surgical Oncology. 2004;11:426–433. doi: 10.1245/ASO.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JF, Soong S-J, Balch CM, et al. Prognostic significance of mitotic rate in localized primary cutaneous melanoma: An analysis of patients in the multi-institutional American Joint Committee on Cancer melanoma staging database. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology. 2011;29:2199–2205. doi: 10.1200/JCO.2010.31.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsden J, Newton-Bishop J, Burrows L, et al. Revised U.K. guidelines for the management of cutaneous melanoma 2010. British Journal of Dermatology. 2010;163:238–256. doi: 10.1111/j.1365-2133.2010.09883.x. [DOI] [PubMed] [Google Scholar]
- 23.Bichakjian CK, Halpern AC, Johnson TM, et al. Guidelines of care for the management of primary cutaneous melanoma. Journal of the American Academy of Dermatology. 2011;65:1032–1047. doi: 10.1016/j.jaad.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Elder DE. Melanocytic Tumors of the Skin. 12th ed. edition. Washington, D.C.: American Registry of Pathology; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.