Abstract
Tumor-associated macrophages are a major constituent of malignant tumors and known to stimulate key steps in tumor progression. In our review in this journal in 2006, we postulated that functionally distinct subsets of these cells exist in different areas within solid tumors. Here, we review the many experimental and clinical studies conducted since then to investigate the function(s), regulation and clinical significance of macrophages in these sites. The latter include three sites of cancer cell invasion, tumor nests, the tumor stroma, and areas close to, or distant from, the tumor vasculature. A more complete understanding of macrophage diversity in tumors could lead to the development of more selective therapies to restore the formidable, anti-cancer functions of these cells.
Keywords: TAMs, Invasion, Perivascular, Stroma, Invasive Edge
Introduction
Tumor-associated macrophages (TAMs) are abundant in most types of malignant tumor and promote tumor angiogenesis, the escape of cancer cells from the tumor into the circulation, and the suppression of anti-tumor immune mechanisms. They also help circulating cancer cells to extravasate at distant sites like the lungs and then promote their survival and persistent growth into metastatic colonies. An increasing number of studies have also shown that TAMs can either antagonize, augment or mediate the antitumor effects of cytotoxic agents, tumor irradiation, anti-angiogenic/vascular damaging agents, and checkpoint inhibitors (1–3).
The origin(s) of these cells is currently a topic for debate. Recent studies have shown that macrophages in many steady-state tissues are not derived from circulating monocytes as originally thought, but rather from embryonic macrophages (particularly from the yolk sac) that are laid down in tissues during development. These progenitors persist into adulthood by local proliferation, and thus maintain themselves independently of the adult hematopoietic system. Alternatively, in some adult tissues like the intestines, the major macrophages populations derive form the bone marrow via monocyte recruitment while others can be chimeric (4). Initially, TAMs in mouse tumors were also thought to be derived largely from blood monocytes (5), however, recent studies have shown that, in some mouse models of brain and pancreatic cancer, they are derived from both blood monocytes and embryonic macrophages. Moreover, the selective depletion of each of these two TAM subtypes showed that only the latter supported the growth of established tumors (6,7). Further studies are required to see if this mixed ontogeny extends to other tumor types.
TAMs often exhibit an array of activation states. In general, they are skewed away from the ‘classically’ activated, tumoricidal phenotype (sometimes referred to as M1) towards an ‘alternatively’ activated tumor-promoting one (M2). However, like macrophages in many other tissues, TAMs show remarkable functional plasticity and often express markers characteristic of both activation states (2,8) making such binary definitions inaccurate. In our review in this journal in 2006 (9), we proposed that TAM functions might, at least in part, be regulated by their location within tumors. We suggested that they exhibited different functions in least three tumor sites; areas of invasion by cancer cells in early tumor development, the stroma, and hypoxic/necrotic areas. Since then, a considerable number of studies have investigated their functions and regulation in these - and other - sites in mouse tumor models, and examined the clinical significance of these spatially distinct TAM subsets (the latter are shown in the Table).
Table. TAMs in different areas of human tumors: correlation with important clinico-pathological features.
CD68 was used to immunolabel TAMs in tumor sections unless otherwise stated. [Abbreviations used: ND, not determined; IF, invasive front of tumor; MVD, microvessel density; PV, perivascular; OS, overall survival; RFS, relapse free survival; PFS, progression free survival; LNM, lymph node metastases; TMEM, tumor microenvironment of metastasis].
| Tumor Type | Tumor Area | Reference | ||||
|---|---|---|---|---|---|---|
| Invasive front | Tumor nests | Stroma | Perivascular | Hypoxic/necrotic area | ||
| Breast | ND | No correlation between TAMs and OS or RFS | High TAMs correlate with reduced OS or RFS | ND | ND | (43) |
| Breast | ND | High TAMs correlate with reduced RFS | High TAMs correlate with reduced RFS (only in ER/PR+ subtype) | ND | ND | (34) |
| Breast | ND | High TAMs correlate with increased tumor MVD, but not mitotic index | High TAMs correlate with increased mitotic index | ND | ND | (104) |
| Breast | ND | ND | ND | ND | High TAMs in poorly vascularised areas correlate with increased tumor MVD and poor RFS & OS | (74) |
| Breast | ND | ND | ND | High ‘TMEMs’ (including PV TAMs) correlate with distant metastases. No correlation with tumor size, grade or LNM | ND | (105) |
| Breast | ND | ND | ND | High HIF-2α+ TAMs correlate with increased tumor MVD, grade, and reduced OS but not RFS | ND | (106) |
| Bladder | ND | ND | High TAMs associate with reduced LNS, and with improved OS in CD163+ tumor | ND | ND | (49) |
| Endometrial | No correlation with RFS | High TAMs correlate with improved RFS | High TAMs correlate with increased LNM but not with RFS | ND | High TAMs correlate with increased myometrial invasion, higher clinical stage and better RFS | (30) |
| Gastric | High S100A8/A9+ TAMs at invasive front and stroma correlate with higher histological grade and LNM but not OS | High S100A8/A9+ TAMs correlate with higher histological grade but not OS | See left | ND | ND | (107) |
| Gastric | High CD163+ TAMs at invasive front and stroma correlate with higher MVD, more significant LNM and tumor invasion, and reduced OS | High CD163+ TAMs correlate with higher histological grade | See left | ND | ND | (44) |
| Gastric | ND | High TAMs correlate with higher frequency of tumor cell apoptosis and improved RFS | ND | ND | ND | (31) |
| Gastric | ND | High TAMs correlate with worse surgical outcome | ND | ND | ND | (108) |
| Gastric | High TAMs correlate with fewer liver metastases | ND | ND | ND | ND | (25) |
| Cervical | High CD163+ TAMs correlate with increased LNM and reduced RFS | High CD163+ TAMs show no correlation with RFS | High CD163+ TAMs show no correlation with RFS | ND | ND | (47) |
| Cervical | ND | ND | ND | ND | High HIF-2α+ TAMs correlate with reduced DFS and higher risk of local recurrence | (75) |
| Cervical | No correlation with lymphatic metastasis | No correlation with lymphatic metastasis | High TAMs correlate with strong lymphatic metastasis | ND | ND | (109) |
| Pancreas | ND | No correlation with OS | High CD163+ (but not CD68+) TAMs correlate with reduced OS | ND | ND | (46) |
| Bile duct | High TAM density correlates with higher recurrence and reduced OS and RFS | ND | ND | ND | ND | (110) |
| Bile duct | ND | ND | High CD163+ TAMs correlate with poorer differentiated histology, nodal metastasis. High CD163+ TAMs in stroma together with low number of CD8+ T cells at cancer nests correlates with reduced OS | ND | ND | (111) |
| Oral | No correlation between the number of CD68+ TAMs and tumor grade | High CD163+ TAMs correlate with higher tumor grade | No correlation between the number of CD68+ TAM and tumor grade | ND | ND | (112) |
| Oral | ND | No correlation | High TAMs correlate with higher tumor grade, increased LNM and reduced OS & DFS | ND | ND | (45) |
| Non-small-cell lung cancer (NSCLC) | ND | High CD163+ TAM density correlates with increased LNM (but not OS) | High CD163+ TAM density correlates with increased LNM (but not OS); | ND | ND | (48) |
| Lung (meta-analysis of 21 studies) | ND | High TAM density correlates with better 3-year OS but not 5-year OS; specifically, high M1(*)-TAMs associate with better 3- and 5-year OS. M2(*)-TAMs was not associated with 3- or 5-year OS | High TAM density correlates with worse 3- and 5-year OS; specifically, high M2-TAMs was associated with reduced 5-year (but not 3-year) OS | ND | ND | (113) |
| Prostatic | ND | High TAMs correlate with poorer tissue differentiation | High TAMs correlate with lower clinical stage | ND | ND | (32) |
| Esophageal | ND | High CD163+ TAMs correlate with higher clinical stage and increased LNM | High CD163+ TAMs correlate with higher clinical stage and increased LNM | ND | ND | (33) |
| Esophageal | ND | High TAMs correlate with higher clinical stage, increased LNM and reduced OS | High TAMs correlate with higher clinical stage, increased LNM and reduced OS | ND | ND | (114) |
| Esophageal | ND | High TAM density correlates with reduced OS | High TAM density correlates with improved OS | ND | ND | (36) |
| Colorectal | High TAMs correlate with fewer liver metastases and improved OS | ND | ND | ND | ND | (27) |
| Colon | High TAMs correlate with improved OS | ND | ND | ND | ND | (21) |
| Liver (hepatocellular carcinoma, HCC) | ND | ND | ND | High CD14+CD16+TIE2+ TAM density correlates with increased MVD | ND | (59) |
| Melanoma | High TAMs correlate with reduced OS | High CD163+ (not CD68+) TAMs correlate with reduced PFS and OS | High CD163+ TAMs correlate with reduced PFS and OS; high CD68+ TAMs with only PFS | ND | ND | (35) |
[‘TAM’, CD68+ cell unless otherwise stated; ‘TMEM’, Tumor microenvironment of metastasis; ‘High TAMs’, high number of TAMs in a given area. *M1-like = CD68+HLA-DR+ cells; M2-like = CD163+ alone, CD204+ alone, CD68+CD163+, CD68+CD206+, or CD68+IL-10+ cells]
In this update we now outline the progress made in understanding TAM behavior in the following tumor sites: three different areas of cancer cell invasion; areas of high cancer cell density (the so-called tumor ‘nests’); the perivascular niche; and poorly vascularized, hypoxic/necrotic tumor areas (Figures 1 & 2). We also discuss the clinical/therapeutic implications of these TAM subsets.
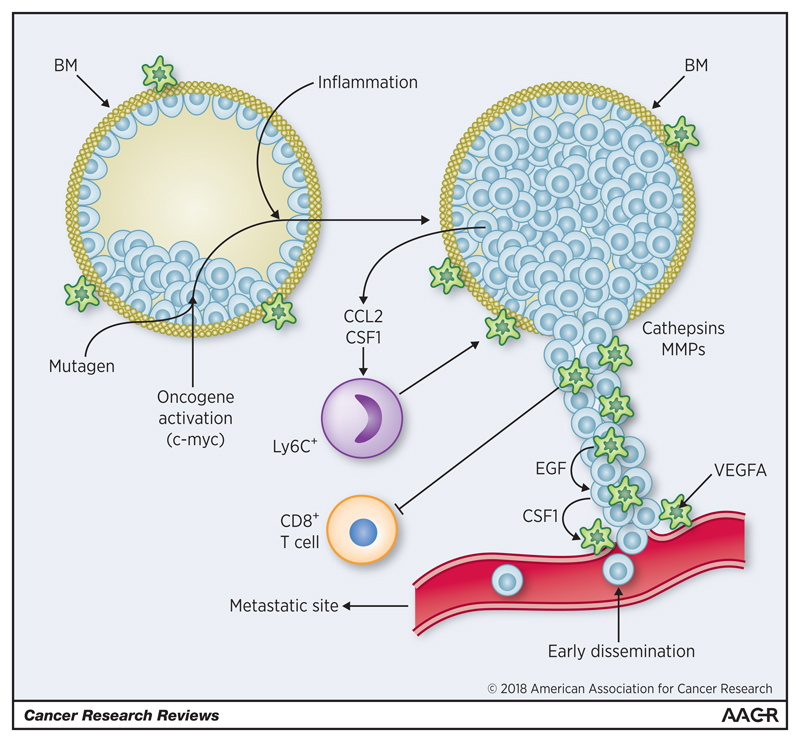
Figure 1. Macrophages in pre-invasive lesions.
Epithelial cells within the basement membrane (BM) undergo transformation in response to a number of stimuli. This causes them to release factors that stimulate both the recruitment of Ly6C+ monocytes and the migration and/or gene expression of surrounding macrophages. These include CSF1, CCL2, EGF, MMPs and cathepsins. Co-operation may takes place between invading cancer cells (expressing CSF1) and macrophages (expressing EGF), to facilitate the movement of cancer cells towards neighboring blood vessels. Perivascular VEGFA+ macrophages then promote the escape of cancer cells into the circulation. Macrophage release of VEGFA also stimulates angiogenesis as pre-invasive lesions progress to invasive ones. Invading cancer cells are protected from anti-tumor immunity by the expression of PD-L1 and IL-23 by macrophages in such sites. (green - macrophages; blue - epithelial/cancer cells; red - blood vessels).
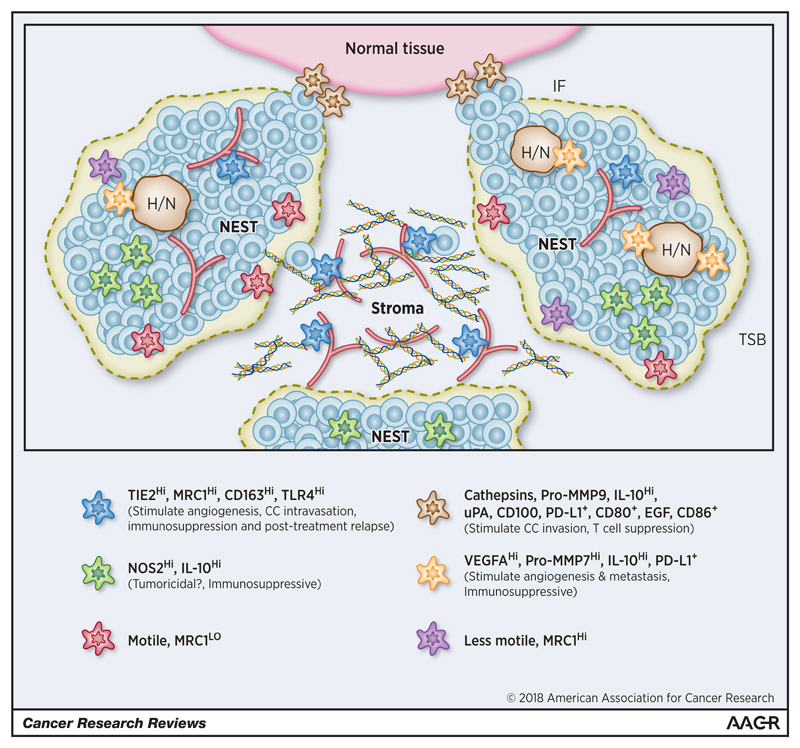
Figure 2. The phenotype of TAMs in different compartments within established primary tumors.
A small sub-compartment within a tumor is shown consisting of 3 tumor ‘nests’ (areas of high cancer cell density) containing hypoxic/necrotic (H/N) areas; the tumor-stroma border (TSB) at the edge of tumor nests (grey dashed line); the stroma (which in most solid tumors is highly vascularized; red); and an invasive front (IF) between this part of the tumor mass and surrounding non-malignant tissue (pink). [Box – cell surface markers, enzymes and cytokines expressed by TAMs in these different regions. The main functions of the various TAM subsets have also been listed].
Invasive Areas
There are at least three main sites where the increased invasive behavior of cancer cells has been detected during tumor progression. First, around pre-invasive lesions where the uncontrolled proliferation of newly transformed, neoplastic cells leads to their invasion through the basement membrane into the surrounding normal parenchyma to form a carcinoma. This has been well documented in tissues like the mammary gland where cancer cells invade through the duct or lobule wall to become an invasive carcinoma (Figure 1). Then, in established tumors, at the ‘tumor-stroma border (TSB)’ between cancer cell nests and the stroma within the tumor mass, and at the ‘invasive front (IF)’ where cancer cells invade into surrounding normal tissues (Figure 2).
In 2006 (9), we reviewed the early evidence for macrophages gathering around ducts in adenomas in the mammary glands of MMTV-PyMT mice and promoting their transition to invasive lesions. At the time, this had been demonstrated by crossing MMTV-PyMT mice with a strain carrying a recessive null mutation in the gene encoding colony stimulating factor 1 (CSF-1). The resultant macrophage depletion delayed the progression of pre-invasive lesions into invasive, metastatic carcinomas while early recruitment of macrophages accelerated progression to malignancy characterized by invasion (10). Other studies had suggested that macrophages might promote invasion of newly transformed cancer cells in pre-invasive mammary lesions by releasing the enzymes, cathepsins and matrix metalloproteinases (MMPs), as well as the cytokines, epidermal growth factor (EGF) and tumor necrosis factor alpha (TNFα). These were thought to then remodel the extracellular matrix, promote disruption of the basement membrane, accelerate the motility of cancer cells, and increase the migration of cancer cells. More recently, a number of experimental studies have confirmed the important role of macrophages in the transition of pre-invasive, hyperplastic mammary lesions to early invasive carcinoma. In MMTV-iFGFR1 mice, progression failed to occur when macrophages were depleted in mice bearing hyperplastic lesions (11). Macrophages were also shown to stimulate the progression of pre-invasive lesions in a transplantable, p53-null model of early mammary cancer (12). We also showed that the release of vascular endothelial growth factor A (VEGFA) by macrophages around pre-neoplastic lesions in MMTV-PyMT mice to be essential for the ‘angiogenic switch’ that occurs when these lesions progress to early carcinomas (13,14). Another study showed that macrophages around such preinvasive mammary lesions in mice release CXCR2-binding chemokines, CXCL1 and CXCL5, which promote the migration and invasion of neighboring pre-neoplastic epithelial cells. Here, a subset of macrophages expressing the cell surface proteins, mannose receptor C type 1 (MRC1 or CD206), class A macrophage scavenger receptor (CD204) and major histocompatibility complex II (MHCII), were recruited to ductal hyperplastic lesions. When these cells were depleted using clodronate liposomes, their progression to invasive tumors was markedly delayed (15).
Finally, a recent study in a KRasG12D model of lung cancer has shown that deregulated oncogenes in cancer cells like Myc trigger the transition of indolent lung adenomas to aggressive adenocarcinomas. This is because changes in Myc stimulated an increase in CCL9 and IL-23 expression by lung epithelial cells. CCL9 then stimulated the accumulation of VEGFA+ macrophages (and thus tumor angiogenesis), and their PD-L1-dependent expulsion of T and B cells. Additionally, IL-23 prompted the exclusion of adaptive T and B cells and cytotoxic NK cells (16) (Figure 1).
These findings in mice are supported by clinical studies comparing macrophage levels in low- versus high-grade human ductal invasive in situ carcinomas (DCIS). These lesions are thought to develop into invasive carcinomas of the breast. High-grade DCIS lesions (especially those filled with cancer cells and containing a central area of necrosis, namely ‘comedo DCIS’) are more aggressive and have a greater tendency to become invasive than low-grade DCIS. Higher numbers of CD68+ macrophages have been reported in and around high-grade comedo DCIS than low-grade ones (17). Moreover, analysis of gene expression in 40 cases of DCIS showed that genes upregulated by macrophages following their exposure to a key stimulus upregulated in tumors, CSF-1 were more prevalent in high-grade than low-grade lesions (18).
A number of intravital imaging studies have demonstrated the abundance and characteristics of TAMs in the TSBs of MMTV-PyMT tumors. At least two TAM subsets were present: motile, MRC1- and less motile, MRC1+ (19,20). Interestingly, high numbers of CD68+ TAMs in the TSBs of human colon carcinomas correlate with better overall survival than those with lower numbers (21) (Table). However, their MRC1 status was not investigated in this clinical study – they were labelled with an antibody for the pan macrophage marker, CD68 – so it remains to be seen whether these two subsets were present, and if one or both contributed to the improved prognosis.
It is noteworthy that antibodies against CD68 continue to be used widely to immunolabel TAMs in such human tumors (Table). However, as with many antibodies supposedly labelling individual cell types, those for human CD68 sometimes label cells other than TAMs. For example, a qualitative, immunostaining study reported that some CD68+ cells in human breast tumors fail to express detectable CSF-1 receptor (CSF-1R) or CD45, or markers for epithelial cells, endothelial cells, or mural cells (i.e. vascular smooth muscle cells, pericytes or fibroblasts) (22). The identity of these CD68+ cells, whether they exist in other tumor types, and, indeed, if they label with other CD68 antibodies, is not known.
When it comes to the IF of tumors, TAMs in these regions of mouse RIP1-Tag2 pancreatic tumors have been shown to enhance the invasive potential of cancer cells via their expression of cathepsin B and S, two enzymes regulated by IL-4 released by cancer cells and tumor-infiltrating T cells (23). Further, CD4+ T cells in MMTV-PyMT tumors have been shown to increase the invasiveness of cancer cells via their release of IL-4 which then stimulates TAMs to express EGF release (24).
Together, these experimental data accord well with a previous finding showing that TAMs in the IF of human gastric tumors express the matrix-degrading enzyme, MMP9, and the receptor for the serine protease, urokinase-type plasminogen activator (uPA; which cleaves pro-UPA into its active form) (25). Interestingly, TAMs along the IF of primary human colon carcinomas express CD80 and CD86 (costimulatory signals necessary for T cell activation), suggesting that they may have the potential to help stimulate anti-tumor immunity in this type of cancer (26). This could explain the observation that high CD68+ TAM levels in the IF of human colorectal tumors correlate with a higher relapse-free survival (RFS) (27) (Table). However, various TAM subsets may be present in the IF of tumors with some appearing to be immunosuppressive. For example, TAMs in the IF of human hepatocellular carcinomas (HCCs) express higher levels of the immunosuppressive, negative checkpoint regulator, PD-L1, than those in neighboring cancer nests, and have been linked to poor survival (28). Furthermore, semaphorin 4D (SEMA4D, CD100), a cytokine upregulated in the IF of Colon26 mouse colon tumors, has been shown to stimulate the number of TAMs expressing the immunosuppressive cytokine interleukin 10 (IL-10) in the IF, and thus suppress the number of activated CD8+ T cells in this location. Antibody blockade of SEMA4D suppressed the number of these TAMs at the IF and increased the treatment efficacy of checkpoint inhibitors anti-PD-1 and anti-CTLA4 (29) (Figure 2).
Cancer Nests
The possible function(s) of TAMs in close proximity to cancer cells in tumor ‘nests’ appears to vary with tumor type. For example, TAMs expressing NOS2, an enzyme linked to the cytotoxic potential of TAMs (via its production of nitric oxide), are seen in intimate contact with cancer cells in some human prostate tumors (30), and high numbers of nest TAMs correlate with an improved prognosis in endometrial cancer (31), and a reduced recurrence in gastric cancer (32) (Table). However, high nest TAMs also correlate with reduced overall and RFS in malignant melanomas, as well as breast and esophageal tumors (33–36) (Table). TAMs in the nests of human HCCs preferentially express IL-10 and recruit immunosuppressive FoxP3+ Treg cells (28), although their number has yet to be shown to be associated with outcome in this disease (Figure 2).
Interestingly, TAMs have been shown to express the inhibitory receptor signal regulatory protein alpha (SIRPα) at cell surface, which binds to the transmembrane protein, CD47, on cancer cells. When this occurs, it suppresses the ability of TAMs to detect and phagocytose cancer cells. Various studies have shown that blocking CD47 interrupts this ‘don’t-eat-me’ signal and triggers cancer destruction by TAMs in mouse tumors, and high CD47 expression is associated with poor prognosis of bladder cancer, acute myeloid leukemia, non-Hodgkin's lymphoma, and breast cancer (37,38). In this way, cancer cells escape surveillance by TAMs. This would be highly relevant in tumor nests where cancer cells come into close contact with TAMs. It would be interesting to see whether the aforementioned links between high nest TAM numbers and a poor prognosis correlate with the expression of SIRPα and CD47 by TAMs and cancer cells respectively in these sites.
Stroma
In this prominent area of most solid tumors, cancer cells are often sparse or absent. Rather, it consists of a complex network of macromolecules in the extracellular matrix (ECM) including collagen fibrils, laminin, fibronectin, tenascin C and hyaluronic acid (HA). It is often populated by various non-malignant cell populations including fibroblasts, endothelial cells, pericytes, lymphocytes and myeloid cells (39). A number of studies have shown that ECM components (and/or their proteolytic products), such as fibronectin, laminin-10, versican (a chondroitin sulfate proteoglycan), and HA fragments, regulate the phenotype of macrophages (40). Moreover, Pinto and colleagues (41) showed recently that decellularized ECM isolated from human colorectal tumors stimulates macrophages to express a relatively anti-inflammatory, M2-like phenotype with increased expression of IL-10, transforming growth factor β (TGF-β), and decreased C-C chemokine receptor type 7 (CCR7), TNFα and interleukin-6 (IL-6) in vitro. Also, stromal TAMs with higher chemokine (C-C motif) ligand 18 (CCL18) production associates with increased metastasis and reduced survival in breast cancer patients (42). This agrees with a number of studies showing a correlation between high numbers of stromal TAMs in breast, esophageal, gastric, pancreatic, oral and skin tumors and poor overall survival and/or RFS (34,35,43–46) (Table). However, this may depend on tumor type as there is no such correlation in endometrial, cervical, and lung cancer (30,47,48), and in bladder cancer, it even correlates with reduced lymph node metastasis and improved survival (49) (Table).
In addition to the effects of a complex array of components in the ‘matrisome’ of the stroma (i.e. the core ECM proteins including collagens, fibronectins, laminins, proteoglycans, growth factors, chemokines and cytokines, and ECM-remodeling enzymes), the biophysical properties of the stroma also regulate the functions of TAMs. The architecture and stiffness of the ECM have been shown previously to regulate cell behavior (40), and increased substrate stiffness upregulates the expression of various pro-inflammatory genes by macrophages in vitro by activating TLR4 signaling pathways in these cells (50). Possible effects of matrix rigidity on macrophages in the premetastatic niche have also been reported as the cross-linking of collagens and elastins induced by the enzyme, lysyl oxidase (LOX), modifies the recruitment, invasion and retention of myeloid cells (51). In an interesting, recent study, high levels of 22 common matrisome constituents (termed the ‘matrix score’) positively correlated with both tumor stiffness and TAM infiltration in ovarian metastases, although it remains to be seen whether the last two are causally linked (52). To add to this complex picture, it should be noted that different areas of stroma within a given tumor may differ in their chemical and biophysical properties and so regulate TAMs differently (Figure 2).
Interestingly, macrophages in some tissues appear to play an important role on collagen remodeling. Proteolyzed fibrillar collagen recruits macrophages during postpartum mammary involution in rats (53) and macrophages have been shown to facilitate collagen fibrillogenesis in developing mammary glands in mice (54). Given that fibrillar collagen is abundant in stroma of tumors, studies are now warranted to see if this two-way interaction occurs there, and what effects this has, if any, on tumor progression and response to various treatments.
Perivascular Niche
A subset of TAMs lie close to, or on, the abluminal surface of blood vessels in mouse and human tumors (55). These perivascular (PV) cells often express high levels of the M2-associated markers, TIE2 (a major receptor for angiopoietins), MRC1 and CD163, and play a key role in stimulating tumor angiogenesis, metastasis and relapse after frontline treatments for cancer (56). Due to their relatively high expression of TIE2, these cells were initially termed ‘TIE2-expressing monocytes/macrophages (TEMs)’. When co-injected into mice with mouse mammary cancer cells, the resultant tumors were more vascularized than generated with cancer cells alone or cancer cells with TIE2- monocytes (57). Interestingly, the frequency of TEMs has also been shown to positively correlate with MVD in some human tumor types (58,59) (Table).
Genetic deletion of PV TIE2+ TAMs or the pharmacological blockade of the main TIE2 ligand upregulated by the tumor vasculature, angiopoietin 2 (AGPT2), demonstrated the importance of this TAM subset in tumor angiogenesis and growth in various mouse models of cancer (60). The subsequent gene expression profiling of TEMs isolated from mouse tumors revealed their higher expression of a number of tumor-promoting genes including Mmp9, Vegfa, Cxcl12, Tlr4 and Nrp1, than TIE2- TAMs from the same tumors (61).
Intravital imaging studies have shown that some PV TIE2+VEGFA+ TAMs interact closely with both endothelial cells and cancer cells expressing actin binding protein mammalian enabled (MENA). These cell trios have been termed the ‘tumor microenvironment of metastasis’ (TMEM) as they are sites of increased intravasation of cancer cells into the blood. PV TAMs in TMEMs upregulate VEGFA and increase the permeability of neighboring blood vessels (62). Their role in promoting metastasis is supported by the finding that high TMEM frequency correlates with increased risk of distant metastasis in ER+HER2- breast cancer patients (63). Interestingly, a recent study has shown that TMEMs containing TIE2+VEGFA+ PV TAMs are also present in pre-malignant lesions in a mouse model of HER2+ breast cancer and promote the early dissemination of cancer cells (64) (Figure 1).
PV TIE2+ TAMs have also been implicated in the relapse of primary mouse tumors after various forms of treatment. They increase in relapsing glioma after local irradiation, and in lung and mammary tumors after chemotherapy. At such times, they express high levels of CXCR4 and are recruited by upregulated CXCL12 in the perivascular niche (65,66). Our studies showed that this TAM subset then stimulates revascularization and regrowth of tumor via their release of VEGFA (66). A later study confirmed that TIE2 expression at TAMs is required to induce vascularization after chemotherapy in mice (67). Furthermore, a recent paper has also demonstrated that newly recruited monocytes also migrate around untreated tumors in a CXCR4-dependent manner. Flourescently-labelled monocytes were seen to extravasate into untreated PyMT tumor implants through vessels mainly in tumor nests, where they are then exposed to TGF-β released by cancer cells. This stimulates these new recruits to upregulate their expression of CXCR4 and migrate towards CXCL12-expressing fibroblasts around tumor blood vessels in collagen-rich stromal areas. Once they are adjacent to vessels, the monocytes differentiate into the metastasis-assisting, perivascular TAMs reported in TMEMs (62,68).
Finally, in metastatic sites like the lungs, a subset of CCR2+Ly6C/Gr1+ macrophages promote the extravasation of cancer cells and their formation of metastases (5). These ‘metastasis-associated macrophages (MAMs)’ have been shown in mouse tumor models to directly tether vascular cell adhesion molecule-1 (VCAM-1) on cancer cells via their α4-integrins, a process that subsequently increases cancer cell survival at such metastatic sites (69). Furthermore, binding of CCL2 to CCR2 on MAMs stimulates their release of CCL3, which binds to CCR1 on cancer cells and facilitates their retention in the lungs (70). These MAMs also promote persistent growth of metastatic lesions through VEGFR1 and CSF-1R signaling (71,72).
Hypoxic/Necrotic Areas
Hypoxia is a hallmark feature in solid tumors and has been linked to increased invasion and metastasis, resistance to therapy, and poor clinical outcome. Hypoxic areas typically have oxygen tensions (pO2 values) below 10 mm Hg and are located more than 150 μm from tumor blood vessels. They form in tumors when the cellular requirement for oxygen outstrips its supply by the poorly organized tumor vasculature. These sites have been identified in tumor sections using hypoxic cell markers, e.g. pimonidazole (PIMO), or immunolabelling for the hypoxia-inducible alpha subunit of the transcription factors, HIFs 1 and 2 (73). High numbers of hypoxia TAMs associate with elevated levels of tumor angiogenesis, metastasis, poor RFS and/or reduced overall survival in breast, endometrial and cervical cancer (30,74,75) (Table & Figure 2).
When TAMs gather in such areas they upregulate HIFs 1 and 2, and various HIF target genes like VEGFA, GLUT1 and MMP7 (76,77). TAMs are recruited into these sites by chemokines upregulated due to hypoxia, including C-X-C motif chemokine 12 (CXCL12), endothelial cell monocyte-activating polypeptide-II (EMAP-II), endothelin 2, VEGFA and SEMA3A (78–80). Hypoxic TAMs become immobilized in hypoxic areas by the direct, inhibitory effect of hypoxia on their mobility (81) and their reduced expression of receptors for tumor-derived chemokines CCR2, CCR5 and NRP1 (79).
Hypoxic TAMs promote tumor angiogenesis, immune evasion and metastasis in various experimental models. For example, they upregulate an array of proangiogenic and immunosuppressive cytokines in hypoxic tumor areas (76,77,82,83), and when their entry into hypoxic tumor areas is impeded by SEMA3A/NRP1 signaling blockade, tumor angiogenesis is markedly reduced, and antitumor immunity restored (80). Hypoxic TAMs are also able to suppress T cell activation in a number of ways including their upregulation of IL-10 and negative checkpoint regulators such as PD-L1 (80). A recent study also showed that macrophages co-cultured with hepatoma cells under hypoxic conditions have increased indoleamine 2, 3-dioxygenase (IDO) expression which suppresses the proliferation of local cytotoxic T cells and expands Treg cells (84).
While exposure to hypoxia per se fails to skew TAMs towards a tumor-promoting, phenotype (85), some studies have shown that a low pH and lactate (which accumulate in poorly vascularized, hypoxic areas due to the poor vascular supply) act in concert to induce a proangiogenic phenotype in TAMs, which, in turn, restores blood perfusion (85–87). Indeed, lactic acid can stimulate expression of VEGFA by macrophages (87). As mentioned previously, this cytokine is not only proangiogenic in tumors but also capable of stimulating the intravasation of cancer cells. It remains to be seen whether VEGFA released by TAMs in poorly vascularized areas (i.e. away from blood vessels) contributes to the latter phenomenon.
Tumor hypoxia can also modulate TAM functions indirectly by stimulating cancer cells to release high-mobility group box 1 protein (HMGB1) that, in turn, stimulates IL-10 production by TAMs. Furthermore, this hypoxia-HMGB1-IL-10 axis has been shown to stimulate metastasis in the murine B16 tumor model (88). Hypoxia also induces metabolic changes in TAMs which then impact directly on the functions of neighboring cells. For example, hypoxia stimulates their expression of REDD1, an mTOR inhibitor and key modulator of metabolism in response to nutrient availability and energy requirement. The resultant inhibition of mTOR in TAMs strongly reduces their glucose uptake and glycolysis, leaving more glucose for neighboring endothelial cells. This results in a more hyperactive and leaky vascular network and the provision of more escape sites for cancer cells into the circulation. So, it is hardly surprising that this mechanism in primary tumors has been shown to drive the formation of distant metastases (89).
Concluding Remarks
A number of experimental studies in mice have now confirmed the ability of different tumor compartments to differentially regulate the phenotype of TAMs. The importance of this is underscored by clinical reports showing that the number and/or phenotype of TAM in specific tumor areas correlate with RFS and/or survival in human tumors (Table).
We are beginning to identify the factors regulating this spatial heterogeneity of TAMs in tumors. As mentioned earlier, genetic changes taking place during early neoplasia can be ‘sensed’ by neighboring macrophages and trigger their tumor-promoting functions. Activation of the oncogene, c-Myc, and mutations in the tumor suppressor gene, p53, in breast epithelial cells are prominent in high-grade DCIS, and regulate the function of macrophages in such in such preinvasive lesions (15,90–92). Later, in established tumors, nest TAMs are exposed to tumor cell-derived factors, hypoxia, low pH and high lactate concentration (due to the tumor vasculature being unable support the metabolic needs of rapidly proliferating tumor cells) (93,94). Alternatively, TAMs in the stroma receive a diverse array of signals, including those released by, or expressed on the surface, of endothelial cells, pericytes, fibroblasts, lymphocytes, other myeloid cells, and ECM constituents. However, it may be over-simplistic to assume that any two similar areas within a tumor (e.g. stromal areas) are identical, and thus regulate TAM behavior in the same way. Furthermore, the phenotype of TAMs in a given area will likely change over time as each site changes within the tumor mass.
It also remains to be seen whether TAMs in different tumor areas are variants of the same monocyte/TAM pool conditioned to perform specific functions in response to local signals, or whether they also have different origins. As described earlier, newly recruited monocytes can migrate from one tumor area to another [e.g. nests to the perivascular niche (68)] and, in doing so, change their phenotype (68). This finding attests to the plasticity - and potential inter-conversion - of TAMs in different sites in tumors. However, this does not exclude the possibility that some TAM subsets may be recruited from distinct subsets of circulating monocytes (95) or from the proliferation of a local TAM progenitor pool (96).
Also mentioned earlier, a recent cell fate-mapping study has shown that TAMs in mouse brain tumors are derived from both resident brain macrophages (microglia) and blood monocytes (97). While they shared a common, tumor-induced gene expression signature, they also exhibited considerable differences in their transcriptional profile, suggesting the retention of certain ontogeny-specific characteristics (6). However, it is not known yet whether this phenomenon is limited to brain tumors, or whether it contributes to the spatial diversity of TAMs in tumors. We also have much to learn about the development of TAM subsets in different areas of metastatic tumors, as virtually every study on this so far has been in the primary setting.
Evidence is also emerging for the role of TAM subsets in certain tumor areas limiting tumor responses to treatment. For example, irradiation, vascular disrupting agents and cytotoxic drugs induce the expansion of the perivascular TAMs, which contributes to tumor angiogenesis and relapse after therapy (69,70,98). Hypoxic TAMs have also been implicated in tumor resistance to several anticancer treatments and to promote relapse (83).
The demonstration that TAMs stimulate a number of tumor-promoting mechanisms in mouse tumor models prompted the development of therapeutic approaches to deplete or reprogram them (99). To date, general TAM inhibitors, including those targeting the CSF-1-CSF-1R and the CCL-CCR2 axis, have largely failed to show efficacy in cancer clinical trials as monotherapies (100–102), although they may prove to be effective in combination with other therapeutic agents. Although the CSF1-R inhibitor PLX3397 have shown significant efficacy in tenosynovial giant cell tumors, treatments have also revealed pathologies resulting from the long-term depletion of all macrophages via this inhibitor (103). Targeting specific TAM subsets in tumors may also be a better way forward - in order to deplete or re-educate those that are tumor-promoting, while leaving or increasing those capable of being tumoricidal and/or promoting anti-tumor immunity. Advances in our understanding of how the phenotype of TAM subsets in different tumor areas is influenced by their ontogeny, activation status and complex array of local cues will help to develop this therapeutic approach. Unravelling such a complex array of influences on TAM behavior will likely require a multifaceted approach including cell fate mapping studies, high-dimensional, single-cell analysis techniques, and systems biology/computer modelling. However, this could then lead to personalized approach to the selective targeting of appropriate TAM subsets.
Acknowledgements
The authors acknowledge the support of the following funding bodies for their work on macrophages in cancer: MY & DM (Breast Cancer Now; 2018NovPR102). CEL (Breast Cancer Now; 2016MayPR746 and 2016NovPCC003, Yorkshire Cancer Research; S382, Prostate Cancer-UK (RIA16- ST2-022) and the European Commission – MSCA-ITN-2015-ETN; project acronym, ‘ISPIC’ and H2020- MSCA-RISE-2018; project acronym, ‘Cancer’). JWP (Wellcome Trust (101067/Z/13/Z), MRC Centre grant MR/N022556/1, NIH grant PO1 CA100324).
Footnotes
The authors have not conflicts of interest
References
- 1.Long KB, Collier AI, Beatty GL. Macrophages: Key orchestrators of a tumor microenvironment defined by therapeutic resistance. Mol Immunol. 2017 Dec 19; doi: 10.1016/j.molimm.2017.12.003. pii: S0161-5890(17)30596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14(7):399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guerriero JL. Macrophages: The Road Less Traveled, Changing Anticancer Therapy. Trends Mol Med. 2018;24:472–89. doi: 10.1016/j.molmed.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–61. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Z, Feng X, Herting CJ, Garcia VA, Nie K, Pong WW, et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res. 2017;77:2266–78. doi: 10.1158/0008-5472.CAN-16-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, et al. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity. 2017;47:597. doi: 10.1016/j.immuni.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 10.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwertfeger KL, Xian W, Kaplan AM, Burnett SH, Cohen DA, Rosen JM. A critical role for the inflammatory response in a mouse model of preneoplastic progression. Cancer Res. 2006;66:5676–85. doi: 10.1158/0008-5472.CAN-05-3781. [DOI] [PubMed] [Google Scholar]
- 12.Carron EC, Homra S, Rosenberg J, Coffelt SB, Kittrell F, Zhang Y, et al. Macrophages promote the progression of premalignant mammary lesions to invasive cancer. Oncotarget. 2017;8:50731–46. doi: 10.18632/oncotarget.14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–46. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 14.Lin EY, Li JF, Bricard G, Wang W, Deng Y, Sellers R, et al. Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Mol Oncol. 2007;1:288–302. doi: 10.1016/j.molonc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bohrer LR, Schwertfeger KL. Macrophages promote fibroblast growth factor receptor-driven tumor cell migration and invasion in a CXCR2-dependent manner. Mol Cancer Res. 2012;10:1294–305. doi: 10.1158/1541-7786.MCR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kortlever RM, Sodir NM, Wilson CH, Burkhart DL, Pellegrinet L, Brown Swigart L, et al. Myc Cooperates with Ras by Programming Inflammation and Immune Suppression. Cell. 2017;171:1301–15 e14. doi: 10.1016/j.cell.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esserman LJ, Kumar AS, Herrera AF, Leung J, Au A, Chen YY, et al. Magnetic resonance imaging captures the biology of ductal carcinoma in situ. J Clin Oncol. 2006;24:4603–10. doi: 10.1200/JCO.2005.04.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma M, Beck AH, Webster JA, Espinosa I, Montgomery K, Varma S, et al. Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Res Treat. 2010;123:397–404. doi: 10.1007/s10549-009-0654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyckoff JB, Wang Y, Lin EY, Li JF, Goswami S, Stanley ER, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 2007;67:2649–56. doi: 10.1158/0008-5472.CAN-06-1823. [DOI] [PubMed] [Google Scholar]
- 20.Egeblad M, Ewald AJ, Askautrud HA, Truitt ML, Welm BE, Bainbridge E, et al. Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis Model Mech. 2008;1:155–67. doi: 10.1242/dmm.000596. discussion 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472–9. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 22.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci U S A. 2012;109:2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Migita T, Sato E, Saito K, Mizoi T, Shiiba K, Matsuno S, et al. Differing expression of MMPs-1 and -9 and urokinase receptor between diffuse- and intestinal-type gastric carcinoma. Int J Cancer. 1999;84:74–9. doi: 10.1002/(sici)1097-0215(19990219)84:1<74::aid-ijc14>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 26.Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: a possible antitumor immunity? Lab Invest. 1997;77:231–41. [PubMed] [Google Scholar]
- 27.Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans EE, Jonason AS, Jr, Bussler H, Torno S, Veeraraghavan J, Reilly C, et al. Antibody Blockade of Semaphorin 4D Promotes Immune Infiltration into Tumor and Enhances Response to Other Immunomodulatory Therapies. Cancer Immunol Res. 2015;3:689–701. doi: 10.1158/2326-6066.CIR-14-0171. [DOI] [PubMed] [Google Scholar]
- 30.Shimura S, Yang G, Ebara S, Wheeler TM, Frolov A, Thompson TC. Reduced infiltration of tumor-associated macrophages in human prostate cancer: association with cancer progression. Cancer Res. 2000;60:5857–61. [PubMed] [Google Scholar]
- 31.Ohno S, Ohno Y, Suzuki N, Kamei T, Koike K, Inagawa H, et al. Correlation of histological localization of tumor-associated macrophages with clinicopathological features in endometrial cancer. Anticancer Res. 2004;24:3335–42. [PubMed] [Google Scholar]
- 32.Ohno S, Inagawa H, Dhar DK, Fujii T, Ueda S, Tachibana M, et al. The degree of macrophage infiltration into the cancer cell nest is a significant predictor of survival in gastric cancer patients. Anticancer Res. 2003;23:5015–22. [PubMed] [Google Scholar]
- 33.Hu JM, Liu K, Liu JH, Jiang XL, Wang XL, Yang L, et al. The increased number of tumor-associated macrophage is associated with overexpression of VEGF-C, plays an important role in Kazakh ESCC invasion and metastasis. Exp Mol Pathol. 2017;102:15–21. doi: 10.1016/j.yexmp.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Gwak JM, Jang MH, Kim DI, Seo AN, Park SY. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One. 2015;10:e0125728. doi: 10.1371/journal.pone.0125728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–7. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- 36.Xu B, Chen L, Li J, Zheng X, Shi L, Wu C, et al. Prognostic value of tumor infiltrating NK cells and macrophages in stage II+III esophageal cancer patients. Oncotarget. 2016;7:74904–16. doi: 10.18632/oncotarget.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao MP, Weissman IL, Majeti R. The CD47-SIRPalpha pathway in cancer immune evasion and potential therapeutic implications. Curr Opin Immunol. 2012;24:225–32. doi: 10.1016/j.coi.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willingham SB, Volkmer JP, Gentles AJ, Sahoo D, Dalerba P, Mitra SS, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci U S A. 2012;109:6662–7. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci. 2012;125:5591–6. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 40.Varol C, Sagi I. Phagocyte-extracellular matrix crosstalk empowers tumor development and dissemination. FEBS J. 2018;285:734–51. doi: 10.1111/febs.14317. [DOI] [PubMed] [Google Scholar]
- 41.Pinto ML, Rios E, Silva AC, Neves SC, Caires HR, Pinto AT, et al. Decellularized human colorectal cancer matrices polarize macrophages towards an anti-inflammatory phenotype promoting cancer cell invasion via CCL18. Biomaterials. 2017;124:211–24. doi: 10.1016/j.biomaterials.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55. doi: 10.1016/j.ccr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park JY, Sung JY, Lee J, Park YK, Kim YW, Kim GY, et al. Polarized CD163+ tumor-associated macrophages are associated with increased angiogenesis and CXCL12 expression in gastric cancer. Clin Res Hepatol Gastroenterol. 2016;40:357–65. doi: 10.1016/j.clinre.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Ni YH, Ding L, Huang XF, Dong YC, Hu QG, Hou YY. Microlocalization of CD68+ tumor-associated macrophages in tumor stroma correlated with poor clinical outcomes in oral squamous cell carcinoma patients. Tumour Biol. 2015;36:5291–8. doi: 10.1007/s13277-015-3189-5. [DOI] [PubMed] [Google Scholar]
- 46.Hu H, Hang JJ, Han T, Zhuo M, Jiao F, Wang LW. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol. 2016;37:8657–64. doi: 10.1007/s13277-015-4741-z. [DOI] [PubMed] [Google Scholar]
- 47.Carus A, Ladekarl M, Hager H, Nedergaard BS, Donskov F. Tumour-associated CD66b+ neutrophil count is an independent prognostic factor for recurrence in localised cervical cancer. Br J Cancer. 2013;108:2116–22. doi: 10.1038/bjc.2013.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carus A, Ladekarl M, Hager H, Pilegaard H, Nielsen PS, Donskov F. Tumor-associated neutrophils and macrophages in non-small cell lung cancer: no immediate impact on patient outcome. Lung Cancer. 2013;81:130–7. doi: 10.1016/j.lungcan.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 49.Aljabery F, Olsson H, Gimm O, Jahnson S, Shabo I. M2-macrophage infiltration and macrophage traits of tumor cells in urinary bladder cancer. Urol Oncol. 2018;36:159 e19–e26. doi: 10.1016/j.urolonc.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 50.Previtera ML, Sengupta A. Substrate Stiffness Regulates Proinflammatory Mediator Production through TLR4 Activity in Macrophages. PLoS One. 2015;10:e0145813. doi: 10.1371/journal.pone.0145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce OMT, Delaine-Smith RM, Maniati E, Nichols S, Wang J, Bohm S, et al. Deconstruction of a Metastatic Tumor Microenvironment Reveals a Common Matrix Response in Human Cancers. Cancer Discov. 2018;8:304–19. doi: 10.1158/2159-8290.CD-17-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Brien J, Lyons T, Monks J, Lucia MS, Wilson RS, Hines L, et al. Alternatively activated macrophages and collagen remodeling characterize the postpartum involuting mammary gland across species. Am J Pathol. 2010;176:1241–55. doi: 10.2353/ajpath.2010.090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ingman WV, Wyckoff J, Gouon-Evans V, Condeelis J, Pollard JW. Macrophages promote collagen fibrillogenesis around terminal end buds of the developing mammary gland. Dev Dyn. 2006;235:3222–9. doi: 10.1002/dvdy.20972. [DOI] [PubMed] [Google Scholar]
- 55.Venneri MA, De Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–85. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 56.Lewis CE, Harney AS, Pollard JW. The Multifaceted Role of Perivascular Macrophages in Tumors. Cancer Cell. 2016;30:18–25. doi: 10.1016/j.ccell.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Ji J, Zhang G, Sun B, Yuan H, Huang Y, Zhang J, et al. The frequency of tumor-infiltrating Tie-2-expressing monocytes in renal cell carcinoma: its relationship to angiogenesis and progression. Urology. 2013;82:974 e9–13. doi: 10.1016/j.urology.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 59.Matsubara T, Kanto T, Kuroda S, Yoshio S, Higashitani K, Kakita N, et al. TIE2-expressing monocytes as a diagnostic marker for hepatocellular carcinoma correlates with angiogenesis. Hepatology. 2013;57:1416–25. doi: 10.1002/hep.25965. [DOI] [PubMed] [Google Scholar]
- 60.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–26. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 61.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, et al. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–14. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 62.Harney AS, Arwert EN, Entenberg D, Wang Y, Guo P, Qian BZ, et al. Real-Time Imaging Reveals Local, Transient Vascular Permeability, and Tumor Cell Intravasation Stimulated by TIE2hi Macrophage-Derived VEGFA. Cancer Discov. 2015;5:932–43. doi: 10.1158/2159-8290.CD-15-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rohan TE, Xue X, Lin HM, D'Alfonso TM, Ginter PS, Oktay MH, et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linde N, Casanova-Acebes M, Sosa MS, Mortha A, Rahman A, Farias E, et al. Macrophages orchestrate breast cancer early dissemination and metastasis. Nat Commun. 2018;9:21. doi: 10.1038/s41467-017-02481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes R, Qian BZ, Rowan C, Muthana M, Keklikoglou I, Olson OC, et al. Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 2015;75:3479–91. doi: 10.1158/0008-5472.CAN-14-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L, Li J, Wang F, Dai C, Wu F, Liu X, et al. Tie2 Expression on Macrophages Is Required for Blood Vessel Reconstruction and Tumor Relapse after Chemotherapy. Cancer Res. 2016;76:6828–38. doi: 10.1158/0008-5472.CAN-16-1114. [DOI] [PubMed] [Google Scholar]
- 68.Arwert EN, Harney AS, Entenberg D, Wang Y, Sahai E, Pollard JW, et al. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Reports. 2018;23:1239–48. doi: 10.1016/j.celrep.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell. 2011;20:538–49. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kitamura T, Qian BZ, Soong D, Cassetta L, Noy R, Sugano G, et al. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J Exp Med. 2015;212:1043–59. doi: 10.1084/jem.20141836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Qian BZ, Zhang H, Li J, He T, Yeo EJ, Soong DY, et al. FLT1 signaling in metastasis-associated macrophages activates an inflammatory signature that promotes breast cancer metastasis. J Exp Med. 2015;212:1433–48. doi: 10.1084/jem.20141555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Celus W, Di Conza G, Oliveira AI, Ehling M, Costa BM, Wenes M, et al. Loss of Caveolin-1 in Metastasis-Associated Macrophages Drives Lung Metastatic Growth through Increased Angiogenesis. Cell Rep. 2017;21:2842–54. doi: 10.1016/j.celrep.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hughes VS, Wiggins JM, Siemann DW. Tumor oxygenation and cancer therapy-then and now. Br J Radiol. 2018 doi: 10.1259/bjr.20170955. 20170955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–9. [PubMed] [Google Scholar]
- 75.Kawanaka T, Kubo A, Ikushima H, Sano T, Takegawa Y, Nishitani H. Prognostic significance of HIF-2alpha expression on tumor infiltrating macrophages in patients with uterine cervical cancer undergoing radiotherapy. J Med Invest. 2008;55:78–86. doi: 10.2152/jmi.55.78. [DOI] [PubMed] [Google Scholar]
- 76.Burke B, Giannoudis A, Corke KP, Gill D, Wells M, Ziegler-Heitbrock L, et al. Hypoxia-induced gene expression in human macrophages: implications for ischemic tissues and hypoxia-regulated gene therapy. Am J Pathol. 2003;163:1233–43. doi: 10.1016/S0002-9440(10)63483-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Murdoch C, Lewis CE. Macrophage migration and gene expression in response to tumor hypoxia. Int J Cancer. 2005;117:701–8. doi: 10.1002/ijc.21422. [DOI] [PubMed] [Google Scholar]
- 79.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–35. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, et al. Impeding macrophage entry into hypoxic tumor areas by Sema3A/Nrp1 signaling blockade inhibits angiogenesis and restores antitumor immunity. Cancer Cell. 2013;24:695–709. doi: 10.1016/j.ccr.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Turner L, Scotton C, Negus R, Balkwill F. Hypoxia inhibits macrophage migration. Eur J Immunol. 1999;29:2280–7. doi: 10.1002/(SICI)1521-4141(199907)29:07<2280::AID-IMMU2280>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 82.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192:150–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 83.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126:3672–9. doi: 10.1172/JCI84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia XF, et al. Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis. Cancer Res. 2016;76:818–30. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 85.Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Res. 2014;74:24–30. doi: 10.1158/0008-5472.CAN-13-1196. [DOI] [PubMed] [Google Scholar]
- 86.Carmona-Fontaine C, Deforet M, Akkari L, Thompson CB, Joyce JA, Xavier JB. Metabolic origins of spatial organization in the tumor microenvironment. Proc Natl Acad Sci U S A. 2017;114:2934–9. doi: 10.1073/pnas.1700600114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Huber R, Meier B, Otsuka A, Fenini G, Satoh T, Gehrke S, et al. Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages. Sci Rep. 2016;6 doi: 10.1038/srep29914. 29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wenes M, Shang M, Di Matteo M, Goveia J, Martin-Perez R, Serneels J, et al. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016;24:701–15. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 90.Done SJ, Eskandarian S, Bull S, Redston M, Andrulis IL. p53 missense mutations in microdissected high-grade ductal carcinoma in situ of the breast. J Natl Cancer Inst. 2001;93:700–4. doi: 10.1093/jnci/93.9.700. [DOI] [PubMed] [Google Scholar]
- 91.Cooks T, Pateras IS, Jenkins LM, Patel KM, Robles AI, Morris J, et al. Mutant p53 cancers reprogram macrophages to tumor supporting macrophages via exosomal miR-1246. Nat Commun. 2018;9:771. doi: 10.1038/s41467-018-03224-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aulmann S, Bentz M, Sinn HP. C-myc oncogene amplification in ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2002;74:25–31. doi: 10.1023/a:1016061327812. [DOI] [PubMed] [Google Scholar]
- 93.Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462–72. doi: 10.1016/j.ccell.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kobayashi H. Cancer Chemotherapy Specific to Acidic Nests. Cancers (Basel) 2017;9 doi: 10.3390/cancers9040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kiss M, Van Gassen S, Movahedi K, Saeys Y, Laoui D. Myeloid cell heterogeneity in cancer: not a single cell alike. Cell Immunol. 2018 doi: 10.1016/j.cellimm.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5. doi: 10.1126/science.1252510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bowman RL, Klemm F, Akkari L, Pyonteck SM, Sevenich L, Quail DF, et al. Macrophage Ontogeny Underlies Differences in Tumor-Specific Education in Brain Malignancies. Cell Rep. 2016;17:2445–59. doi: 10.1016/j.celrep.2016.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Invest. 2011;121:1969–73. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoves S, Ooi CH, Wolter C, Sade H, Bissinger S, Schmittnaegel M, et al. Rapid activation of tumor-associated macrophages boosts preexisting tumor immunity. J Exp Med. 2018;215:859–76. doi: 10.1084/jem.20171440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–8. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 101.von Tresckow B, Morschhauser F, Ribrag V, Topp MS, Chien C, Seetharam S, et al. An Open-Label, Multicenter, Phase I/II Study of JNJ-40346527, a CSF-1R Inhibitor, in Patients with Relapsed or Refractory Hodgkin Lymphoma. Clin Cancer Res. 2015;21:1843–50. doi: 10.1158/1078-0432.CCR-14-1845. [DOI] [PubMed] [Google Scholar]
- 102.Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy Foundation Early Phase Clinical Trials Consortium phase II study. Neuro Oncol. 2016;18:557–64. doi: 10.1093/neuonc/nov245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med. 2015;373:428–37. doi: 10.1056/NEJMoa1411366. [DOI] [PubMed] [Google Scholar]
- 104.Ch'ng ES, Tuan Sharif SE, Jaafar H. In human invasive breast ductal carcinoma, tumor stromal macrophages and tumor nest macrophages have distinct relationships with clinicopathological parameters and tumor angiogenesis. Virchows Arch. 2013;462:257–67. doi: 10.1007/s00428-012-1362-4. [DOI] [PubMed] [Google Scholar]
- 105.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res. 2009;15:2433–41. doi: 10.1158/1078-0432.CCR-08-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, et al. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002;62:1326–9. [PubMed] [Google Scholar]
- 107.Liu JY, Peng CW, Yang GF, Hu WQ, Yang XJ, Huang CQ, et al. Distribution pattern of tumor associated macrophages predicts the prognosis of gastric cancer. Oncotarget. 2017;8:92757–69. doi: 10.18632/oncotarget.21575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Okumura H, Matsumoto M, et al. Tumor-associated macrophage (TAM) infiltration in gastric cancer. Anticancer Res. 2003;23:4079–83. [PubMed] [Google Scholar]
- 109.Ding H, Cai J, Mao M, Fang Y, Huang Z, Jia J, et al. Tumor-associated macrophages induce lymphangiogenesis in cervical cancer via interaction with tumor cells. APMIS. 2014;122:1059–69. doi: 10.1111/apm.12257. [DOI] [PubMed] [Google Scholar]
- 110.Atanasov G, Hau HM, Dietel C, Benzing C, Krenzien F, Brandl A, et al. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. 2015;15:790. doi: 10.1186/s12885-015-1795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Miura T, Yoshizawa T, Hirai H, Seino H, Morohashi S, Wu Y, et al. Prognostic Impact of CD163+ Macrophages in Tumor Stroma and CD8+ T-Cells in Cancer Cell Nests in Invasive Extrahepatic Bile Duct Cancer. Anticancer Res. 2017;37:183–90. doi: 10.21873/anticanres.11304. [DOI] [PubMed] [Google Scholar]
- 112.Mori K, Hiroi M, Shimada J, Ohmori Y. Infiltration of m2 tumor-associated macrophages in oral squamous cell carcinoma correlates with tumor malignancy. Cancers (Basel) 2011;3:3726–39. doi: 10.3390/cancers3043726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu P, Wu D, Zhao L, Huang L, Chen G, Shen G, et al. Inverse role of distinct subsets and distribution of macrophage in lung cancer prognosis: a meta-analysis. Oncotarget. 2016;7:40451–60. doi: 10.18632/oncotarget.9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang XL, Liu K, Liu JH, Jiang XL, Qi LW, Xie YF, et al. High infiltration of CD68-tumor associated macrophages, predict poor prognosis in Kazakh esophageal cancer patients. Int J Clin Exp Pathol. 2017;10:10282–92. [PMC free article] [PubMed] [Google Scholar]