Abstract
Background
Recently, several comprehensive genomic analyses demonstrated NOTCH1 and NOTCH3 mutations in head and neck squamous cell carcinoma (HNSCC) in approximately 20% of cases. Similar to other types of cancers, these studies also indicate that the NOTCH pathway is closely related to HNSCC progression. However, the role of NOTCH4 in HNSCC is less well understood.
Methods
We analyzed NOTCH4 pathway and downstream gene expression in the TCGA data set. To explore the functional role of NOTCH4, we performed in vitro proliferation, cisplatin viability, apoptosis, and cell cycle assays. We also compared the relationships among NOTCH4, HEY1 and epithelial mesenchymal transition (EMT) related genes using the TCGA data set and in vitro assays.
Results
HEY1 is specifically up-regulated in HNSCC compared with normal tissues in the TCGA data set. NOTCH4 is more significantly related to HEY1 activation in HNSCC in comparison to other NOTCH receptors. NOTCH4 promotes cell proliferation, cisplatin resistance, inhibition of apoptosis, and cell cycle dysregulation. Furthermore, NOTCH4 and HEY1 up-regulation resulted in decreased E-cadherin expression and increased Vimentin, Fibronectin, TWIST1, and SOX2 expression. NOTCH4 and HEY1 expression were associated with an EMT phenotypes as well as increased invasion and cell migration.
Conclusion
In HNSCC the NOTCH4-HEY1 pathway is specifically up-regulated, induces proliferation and cisplatin resistance, and promotes EMT.
Keywords: Head and neck squamous cell carcinoma, TCGA, NOTCH4, HEY1, EMT
INTRODUCTION
Head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy in the world (1). Despite recent medical progress, HNSCC prognosis has not dramatically improved (2). Thus, defining novel therapeutic target genes and pathways provide an opportunity to elucidate the molecular alterations associated with HNSCC mechanism and improve therapeutic design. Similar to other types of cancers, HNSCC develops through several steps, including the accumulation of genetic and epigenetic alterations, including TP53 (3), CDKN2A (4), EGFR (5), and others.
The Cancer Genome Atlas (TCGA) project aims to examine genetic alterations for a better understanding of cancer pathology and, more importantly, identify signal pathways that can be used as potential targets in cancer treatment (6). Recently, using this data set, several types of cancers, such as lung (7), ovarian and colon cancer (8), were examined by comprehensive pathway analysis. For HNSCC, the comprehensive analysis of somatic genome alterations were also investigated using the TCGA data set (9). In this study, a NOTCH mutation was identified in approximately 20% of patients. In the other two articles, NOTCH1 is the second most frequently mutated gene after TP53 based on whole exome sequencing data (10, 11).
However, the NOTCH pathway changes its functional role depending on specific cancer site or histology. For example, an activated NOTCH pathway in cervical cancer has a poor prognosis (12). In the skin, a tumor suppressor function of NOTCH was reported in mouse keratinocyte tumor development (13). Interestingly, opposing and exclusive roles for the NOTCH pathway are reported in HNSCC (14). NOTCH pathway genes are up-regulated in HNSCC compared with normal or dysplasia tissues (15, 16). Sun et al. showed NOTCH3 was overexpressed in HNSCC tumors compared with normal mucosa and NOTCH1 wild type HNSCC had increased NOTCH downstream genes HES1/HEY1 expression compared with normal mucosa, while NOTCH1 mutated HNSCC do not show up-regulation (17). Inhibition of the NOTCH pathway via a γ-secretase inhibitor decreases cell proliferation and invasion (18). On the other hand, NOTCH mutations in HNSCC are considered as inactivating types, indicating that NOTCH has a tumor suppressor function (17, 19). For example, Grandis et al. showed that more NOTCH1 gene mutations were observed than mutations in the other NOTCH receptor genes and many of NOTCH1 mutations were missense type (17, 19). To further explore the role of specific NOTCH receptors, we examined alterations in NOTCH pathway genes associated with HNSCC compared with normal tissue using TCGA data sets, and found NOTCH4-HEY1 pathway is specifically up-regulated in HNSCC. Furthermore, in this study we explore the functional role of the NOTCH4-HEY1 pathway by using TCGA data set and in vitro experiments.
MATERIALS AND METHODS
TCGA data set
The mRNA expression data of the HNSCC patients were obtained from the TCGA data portal (http://tcga-data.nci.nih.gov/tcga/). We downloaded these data on 05/03/2016. These TCGA data included 520 HNSCC and 46 normal tissues. We used 447 HNSCC cases, excluding 73 tumors with NOTCH mutations (Supplementary Table S1). NOTCH pathway genes included DTX1, JAG1, JAG2, DLL1, DLL3, DLL4, NOTCH1, NOTCH2, NOTCH3, NOTCH4, POU5F1, SOX2, NANOG, CD44 and LMO2. The HES high group was defined as tumors with expression 1 standard deviation greater than the mean of normal tissue for HES1 or HES5. HEY and NOTCH4 high groups were also defined as tumors with expression 1 standard deviation greater than the mean of normal tissue for HEY1 and NOTCH4. Other samples were defined as a low expressing group. mRNA expression was log2-transformed to calculate fold-change. The clinical background and prognosis of these patients was obtained from the firebrowse web site (http://firebrowse.org/).
Cell culture
We used SKN3, Cal27, SCC61 and SCC090 HNSCC cell lines. SKN3 was obtained from the Japanese Collection of Research Bioresource (Ibaraki, Osaka, Japan). SCC61 was obtained from the Weichselbaum Laboratory at the University of Chicago. Cal27 and SCC090 cells were obtained from the Gutkind Laboratory at the University of California San Diego, Moores Cancer Center. SCC090 was established from Human Papilloma Virus (HPV)-positive HNSCC tissues. Other three cells are established from HPV-negative HNSCC tissues. SKN3 was cultured in RPMI-1640 medium (Sigma Aldrich, St. Louis, MO, U.S.A.). Cal27, SCC61 and SCC090 were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma Aldrich). Both mediums were supplemented with 10% fetal bovine serum (FBS) and a penicillin (50 U/ml) and streptomycin (50 μg/ml) cocktail. All cells were cultured under an atmosphere of 5% CO2 at 37°C.
Quantitative real-time PCR
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to validate siRNA-mediated knockdown of NOTCH4 and HEY1 and examine mRNA expression levels in each experiment. Briefly, total RNA was isolated from cells using the RNeasy plus mini kit (Qiagen, Hilden, Germany), and complementary DNA was synthesized using a high-capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Waltham, MA, U.S.A.). We obtained all primers from TaqMan Gene Expression Assays (catalogue number: #4331182. Thermo Fisher Scientific). Each gene ID is described as follows: β-actin (ACTB): Hs01060665_g1; NOTCH4: Hs00965895_g1; HES1: Hs00172878_m1; HEY1: Hs01114113_m1; E-cadherin: Hs01023895_m1; Fibronectin: Hs01549976_m1; Vimentin: Hs00958111_m1; TWIST1: Hs01675818_s1; ALDH1; Hs00946916_m1 and SOX2: Hs01053049_s1. The housekeeping gene ACTB was used as an internal control. qRT-PCR was performed using Quant Studio 6 Flex Real-Time PCR System (Thermo Fisher Scientific).
Western blotting
The following primary antibodies were added to nitrocellulose membrane with 5% non-fat dry milk in Tris-buffered saline and Tween 20: NOTCH4 (#2423, Cell Signaling Technology, Danvers, MA, U.S.A.), HES1 (#sc-25392, Santa Cruz, Dallas, TX, U.S.A.), HEY1 (#ab22614, Abcam, Cambridge, MA, U.S.A.), E-cadherin (#610181, BD Bioscience, San Jose, CA, U.S.A.), Fibronectin (#ab2413, Abcam), Vimentin (#V6630, Sigma Aldrich), TWIST1 (#sc-15393, Santa Cruz) and SOX2 (#2748, Cell Signaling Technology). HRP conjugated goat anti-mouse (#1010-05, 1:20,000 dilution; SouthernBiotech, Birmingham, AL, U.S.A.) or anti-rabbit antibodies (#4010-05, 1:20,000 dilution; SouthernBiotech) were used as secondary antibodies.
Si-RNA and sh-RNA
HNSCC cell lines were transfected with siRNA reagents using Lipofectamine RNAi MAX (Thermo Fisher Scientific) according to the manufacturer’s instructions. All siRNA and si-control reagents used ON-TARGET plus siRNA reagents (GE Dharmacon, Lafayette, CO, U.S.A.). Each catalogue number is described as follows: si-NOTCH4: SMART pool ON-TARGET plus Human NOTCH4 siRNA (L-011883-00-0005); si-HES1: SMART pool ON-TARGET plus Human HES1 siRNA (L-007770-02-0005); si-HEY1: SMART pool ON-TARGET plus Human HEY1 siRNA (L-008709-00-0005); and si-control: ON-TARGET plus Non-targeting Pool (D-001810-10-20). Medium was changed 16 hours after siRNA transfection. mRNA inhibition was observed at a concentration of 30 nM siRNA at 48 hours after transfection. We also used sh-control and sh-NOTCH4 (CSHCTR001-LVRU6MP and HSH011877-LVRU6MP, Genecopoeia, Rockville, MD, U.S.A) to transfect Cal27 cells. The efficiency of down-regulation was validated by calculating mRNA and protein levels (Supplementary Fig. S2A,B, S3B,C,D,E,F). Thus, further analysis was performed under the same conditions.
Viability assay
Cells were seeded in 96-well plates at 1,500 to 9,000 cells/well. For proliferation assays, cell numbers were measured every 24 hours. For cisplatin viability assay, cells were cultured for 24 hours after seeding, and 0.1 to 81 μM cisplatin was added (EMD Millipore, Billerica, MA, U.S.A.). The viability was measured 72 hours after cisplatin exposure. All cell viabilities were measured using Vita Blue Cell Viability Reagent (bimake.com, Houston, TX, U.S.A.). After a 1.5-hour preincubation in the assay solution, the viable cell number in each well was calculated by the fluorescence (Ex = 530–570 nm, Em = 590–620 nm) as measured by a microplate reader (BioTek, Winooski, VT, U.S.A.). The assays were performed three or more times.
Flow cytometry analysis
All flow cytometry analysis such as the NOTCH activity, apoptosis, cell cycle and aldefluor assay were performed using BD FACSCalibur (BD Bioscience). NOTCH activity was examined using a pGreenFire1-Notch plasmid (#TR020PA-1, System Bioscience, Palo Alto, CA, U.S.A.). This lentiviral transfection was performed according to the manufacturer’s protocol. The apoptotic cells were detected using Annexin V-FITC apoptosis detection kit (#C986X37, Sigma Aldrich). Cell cycle phase analysis was carried out using propidium iodide cell staining (#11348639001, Sigma Aldrich) and FlowJo software (ver.10, FLOWJO, Ashland, OR, U.S.A.). To assess ALDH1 activity, we used aldefluor kit (#01700, STEMCELL Technologies, Vancouver, BC, Canada) at a concentration of 50 μlml−1. Diethylaminobenzaldehyde (DEAB) was used to inhibit ALDH1 activity.
Sphere formation assay
Cells were seeded in 6-well ultra-low attachment culture dishes (Corning, Tewksbury, MA, U.S.A.) at 10,000 cells/well. Medium consisted of Repro Stem medium (ReproCELL, Yokohama, Japan) and basic fibroblast growth factor (bFGF: 5 ng/ml) without FBS. After 7 days, photos were obtained (Supplementary Fig. S3A), and sphere cells harvested to extract their mRNA. For qRT-PCR analysis, the adherent and sphere cells mRNA amounts are equalized. NOTCH4 and HEY1 expression were normalized by ACTB expression.
Migration and invasion assay
Migration assays were performed in cell culture insert (24-well 8μm pore size, #353097, Corning). The concentrations of cells were set from 105 to 2×105 cells/ml. Invasion assays were also performed in Corning BioCoat Matrigel invasion chambers (24-well 8μm pore size, #353097, Corning). The concentrations of cells were set from 2×105 to 4×105 cells/ml. Cells were seeded on uncoated or Matrigel-coated inserts in 500 ml of serum-free medium for migration or invasion assays respectively. The lower chambers were filled with 750 μl of 10% FBS-supplemented medium. After 48 h, the cells on the lower surface of the insert were fixed and stained with crystal violet. The number of stained cells was counted at more than three fields under a microscope.
Statistical analysis
All in vitro experiments were performed at least three times. The statistical comparisons of two groups were performed using the Student’s t-test. The TCGA data set analysis of NOTCH pathway genes in the HES and HEY high and low groups was adjusted by false discovery rate using the Benjamini–Hochberg method. Clinical status was compared between two groups using Pearson’s chi-square test. Overall survival was compared using Log-Rank test. Differences were considered significant when P < 0.05. All statistical analyses were performed using JMP 12 software (SAS, Cary, NC, U.S.A.).
RESULTS
NOTCH4-HEY1 is upregulated in HNSCC
First, we examined which NOTCH pathway genes were significantly related NOTCH pathway activation in HNSCC compared with normal tissue using TCGA data sets. We excluded 73 NOTCH mutant samples (Supplementary Table S1). Thus, we examined 447 HNSCC and 46 normal tissues. The NOTCH downstream genes HES1/5 and HEY1 were selected as indicators of downstream NOTCH activity. Two groups were divided according the mRNA expression of these genes compared with normal tissues. No significant difference in NOTCH pathway genes was noted between the HES1/5 high and low groups. On the other hand, HEY1 expression exhibited a significant correlation with several NOTCH pathway genes (DLL4, NOTCH1, NOTCH2, NOTCH3, NOTCH4, and SOX2). All NOTCH receptors were significantly related to HEY1 expression (Supplementary Fig. S1A). Among these receptors, NOTCH4 exhibited the most significant correlation to HEY1 overexpression. NOTCH4 expression in the HEY1 high expressing group was increased approximately 1.58–fold compared with the HEY1 low expressing group (Fig. 1A, Supplementary Fig. S1A). We also compared HES1, HES5 and HEY1 expression between HNSCC and normal tissues using the TCGA data set. HES1 expression in HNSCC was significantly decreased compared with normal tissues (P = 0.0002). HES5 expression was not significantly different between HNSCC and normal tissues (P = 0.1251). On the other hand, HEY1 expression of HNSCC significantly increased compared with normal tissues (P < 0.0001). HEY1 expression in tumor samples was about two times more than that of normal samples (Fig. 1B). In summary, HEY1 was up-regulated compared with normal tissues and was most related to NOTCH4 among the NOTCH receptors in HNSCC. These results suggested that the NOCH4-HEY1 pathway was specifically up-regulated in NOTCH wild type HNSCC compared with normal tissue.
Figure 1. TCGA data set analysis of HES/HEY and NOTCH4 relation.
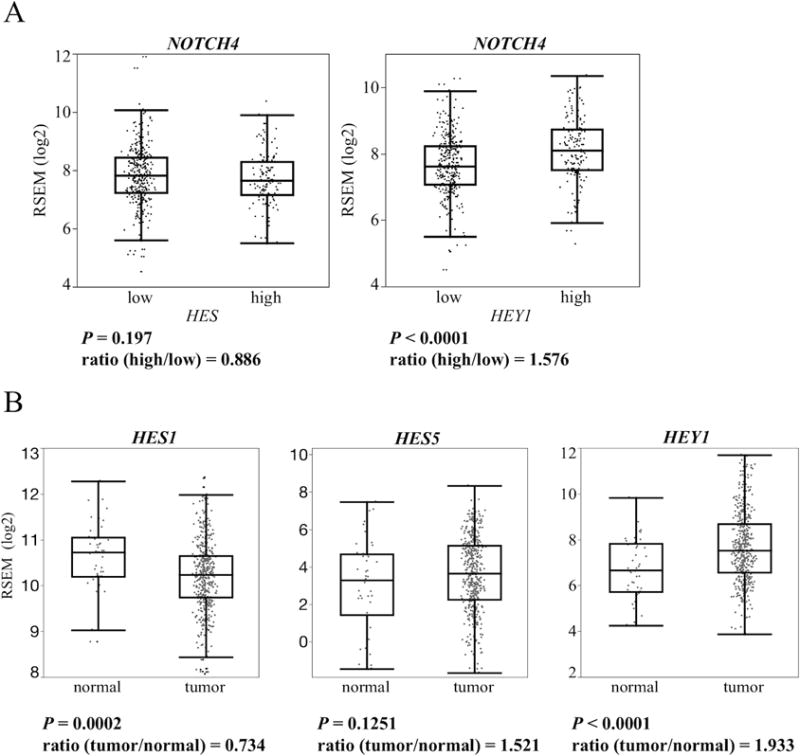
(A) NOTCH4 expression is compared between the HES (HES1+HES5) and HEY1 high/low group using the TCGA data set. Ratio is calculated by dividing the mRNA expression of the HES or HEY1 high group by that of the low group. (B) HES1, HES5 and HEY1 expression are compared between HNSCC and normal tissue using the TCGA data set. Ratio is calculated by dividing the mRNA expression of the tumor samples by that of the normal samples. Whiskers indicate the minimum and maximum values. P value is calculated by using Student’s t-test.
NOTCH4 inhibition inhibits HNSCC and sensitizes HNSCC to cisplatin
To elucidate the properties of the NOTCH4-HEY1 pathway in HNSCC cells, we examined NOTCH4 function in HNSCC cells. First, we examined how NOTCH activity was affected by si-NOTCH4 cells (Supplementary Fig. S2A, S2B) using pGreenFire1-Notch plasmid. This reporter vector shows increased NOTCH activity (GFP+) cells as a result of GFP expression under the binding of a NOTCH specific transcriptional response element (Fig. 2A). Using this vector, we showed si-NOTCH4 cells significantly decreased NOTCH activity in all cell lines (Fig. 2B, Supplementary Fig. S2C). We also compared cell proliferation between si-control and si-NOTCH4 (Fig. 2C). Si-NOTCH4 cells significantly reduced their proliferation compared with si-control cells in all cell lines. In SCC090 cells, si-NOTCH4 cell numbers decreased from day 2 to 3. Compared with si-control, si-NOTCH4 cell numbers were decreased by 20~40% on day 3 (SKN3: 33.3%; Cal27: 24.9%; SCC61: 42.0%; SCC090: 37.5%). Next, we assessed the chemo-resistance properties of si-NOTCH4 cells (Fig. 2D, Supplementary Fig. S2D). We used cisplatin, commonly used for HNSCC chemotherapy. Similar to proliferation assays, si-NOTCH4 cells significantly decreased their cisplatin resistance compared with si-control cells in all cell lines. In comparing IC50, significant differences were noted between the si-control and si-NOTCH4. In particular, Cal27, SCC61 and SCC090 cells decreased IC50 by half to a third (Fig. 2D). These results demonstrated that NOTCH4 inhibition affects NOTCH activity, cell proliferation, and enhances chemo sensitivity in HNSCC cells.
Figure 2. NOTCH activity, proliferation, cisplatin viability, apoptosis assay and cell cycle analysis of si-NOTCH4 cells.
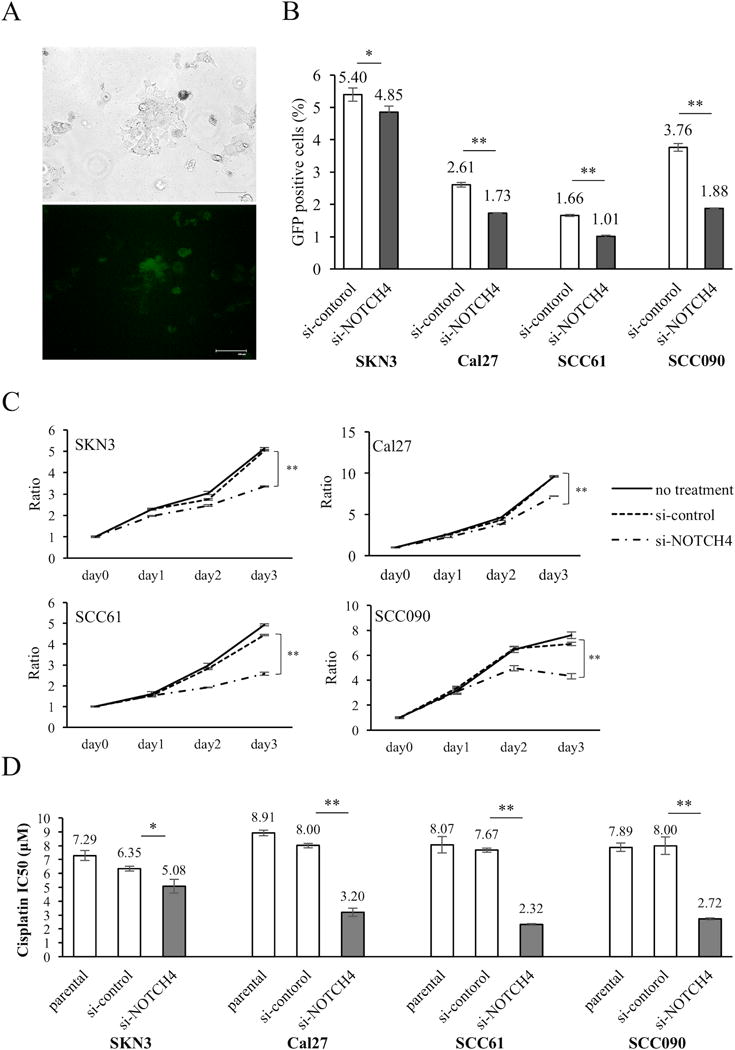
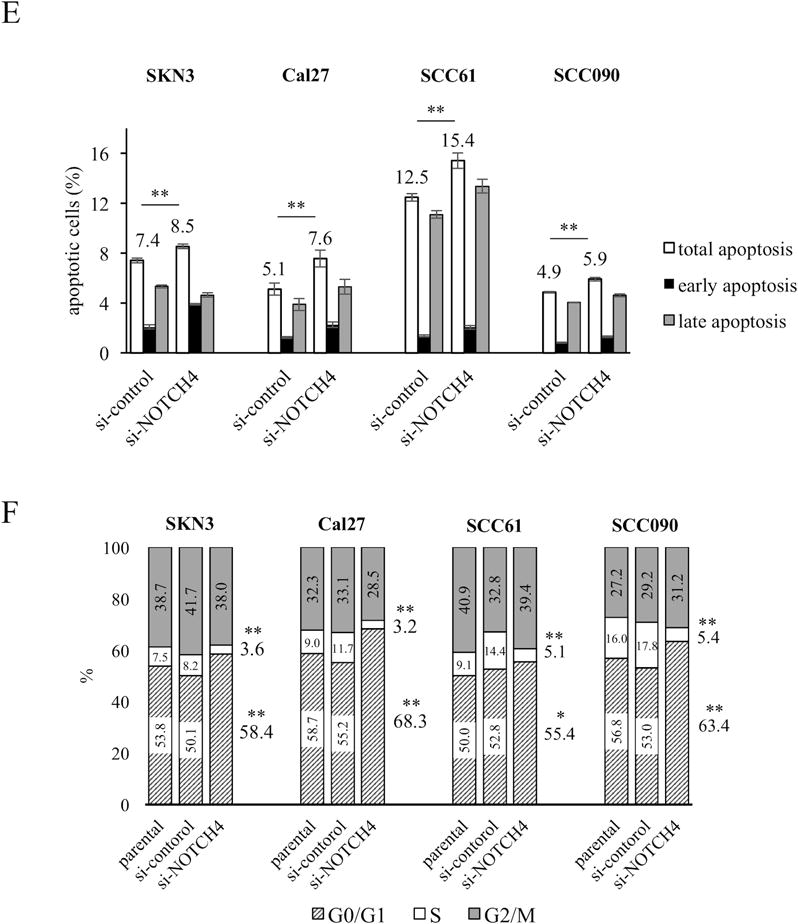
(A) pGreenFire1-Notch plasmid vector was transfected to SKN3 cell. Scale bar indicates 100 μm. (B) NOTCH activity assay of si-control and si-NOTCH4 cells. GFP positive cells have high NOTCH activity. (C) Proliferation assays. si-NOTCH4 cells are compared cell growths to si-control cells on day 3. (D) IC50 of cisplatin in parental, si-control and si-NOTCH4 cells. The IC50 differences between si-control and si-NOTCH4 cells are compared. (E) Apoptosis assays. Total apoptotic fraction is defined as the sum of early and late apoptosis cells. (F) Cell cycle analysis. Each cell cycle phase is compared between si-control and si-NOTCH4 cells. P value is calculated by using Student’s t-test. *: P < 0.05, **: P < 0.01.
NOTCH4 inhibits apoptosis and alters cell cycle
To elucidate what mechanism decreases si-NOTCH4 cell proliferation, we assumed it related to apoptosis and cell cycle alteration. First, we performed apoptosis assays (Supplementary Fig. S2E) and found that all the si-NOTCH4 cells statistically increased the apoptotic cell fraction compared to si-control cells in a modest fashion (Fig. 2E). Furthermore, we performed cell cycle analysis in si-NOTCH4 cells (Supplementary Fig. S2F). The significant increase of G0/G1 phase and decrease of S phase was noted in si-NOTCH4 cells. This result suggested that the si-NOTCH4 cells inhibited cell cycle progression compared to si-control cells (Fig 2F). In these results, we can indicate that NOTCH4 decreases cell proliferation by regulating both apoptosis and cell cycle.
NOTCH4 expression is correlated to EMT related gene expression
We explored other mechanisms related to NOTCH4 and HNSCC properties. NOTCH4 has been noted to induce epithelial mesenchymal transition (EMT) in melanoma (20). EMT promotes cancer proliferation (21) and can be associated with chemo-resistance (22). Thus, we hypothesized that NOTCH4 is also related to EMT in HNSCC and examined the relationship between NOTCH4 and EMT-related genes. Using the TCGA data set, we compared EMT related genes expression between NOTCH4 high and low groups. A significant decrease in the expression of E-cadherin, an epithelial marker, was noted in the NOTCH4 high group compared with the low group (P = 0.001, ratio = 0.760). The expression of mesenchymal markers, such as N-cadherin, Vimentin, and Fibronectin, was significantly increased in the high group compared with the low group (P < 0.0001). The expression of mesenchymal markers of NOTCH4 high group was several times higher than that of low group (N-cadherin: 2.854, Vimentin: 1.937, Fibronectin: 3.266). TWIST1 is known as an EMT-inducing gene, and its expression was significantly increased in the high group (P < 0.0001). The TWIST1 expression of NOTCH4 high group was 1.749 times more than that of NOTCH4 low group (Fig. 3A). SOX2 expression is also related to EMT genes (23). Its expression was also significantly increased in the high group (P < 0.0046). The SOX2 expression of NOTCH4 high group was 1.609 times more than that of NOTCH4 low group by using Student’s t-test (Fig. 3A). These results show an association between NOTCH4 and EMT in HNSCC and raise the possibility that NOTCH4 activation may in part drive EMT in HNSCC.
Figure 3. Comparison of EMT-related genes between the NOTCH4 high and low groups using the TCGA data set. Comparison of NOTCH4 expression between sphere and parental cells.
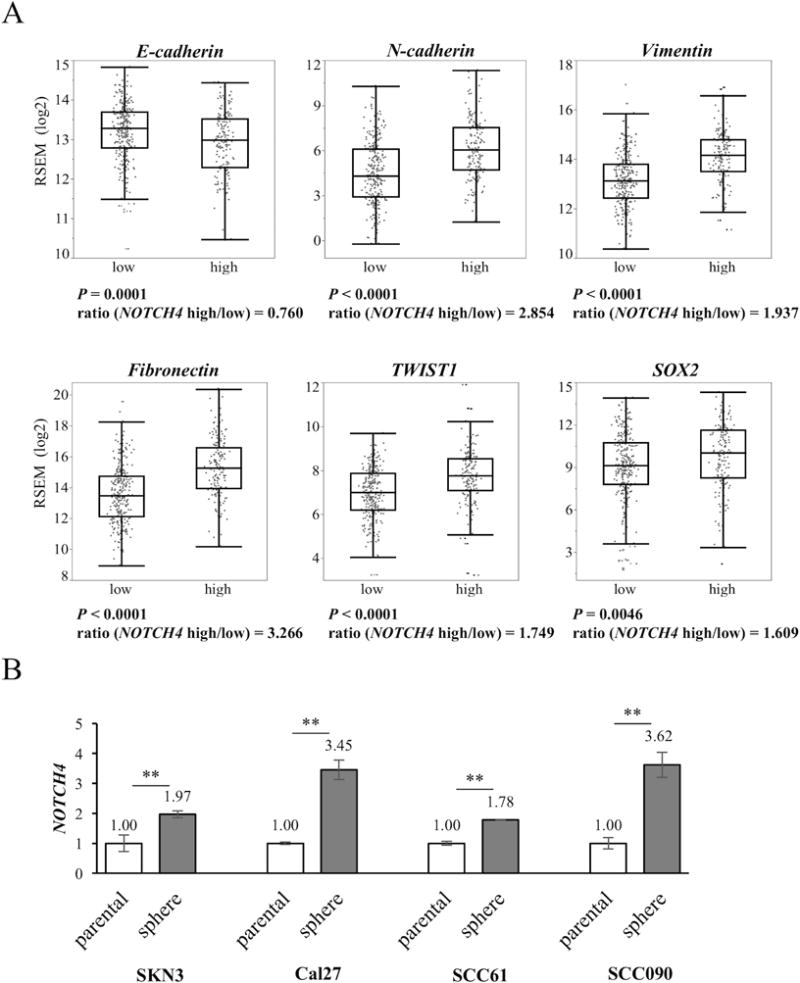
(A) The NOTCH4 high group is defined as tumors with expression 1 standard deviation greater than the mean of normal tissue for NOTCH4. The other tumors are defined as the low group. The boxes represent the interquartile range (25th-75th), and horizontal lines inside the boxes indicate the median. Whiskers indicate the minimum and maximum values. Ratio is calculated by dividing the mRNA expression of the high group by the expression of the low group. (B) NOTCH4 expression is compared between parental and sphere cells using qRT-PCR. P value is calculated by using Student’s t-test. **: P < 0.01.
NOTCH4 promotes HNSCC EMT
To confirm this TCGA data set analysis in vitro, we generated sphere colonies (Supplementary Fig. S3A) that were employed to induce enrichment of EMT-related gene expression (24). As noted, the NOTCH4 expression was significantly increased in sphere cells derived from all HNSCC cell lines examined. NOTCH4 expression in sphere cells was increased approximately 1.8- to 3.5–fold compared with parental cells (Fig. 3B). Next, we also compared EMT-related genes between si-NOTCH4 and si-control cells. si-NOTCH4 cells significantly increased E-cadherin expression in SKN3, Cal27 and SCC61 cells. On the contrary, si-NOTCH4 significantly reduced Vimentin (in Cal27, SCC61 and SCC090), Fibronectin (in the all cell lines) and TWIST1 (in Cal27, SCC61 and SCC090) expression. The SOX2 expressions of si-NOTCH4 cells significantly decreased in Cal27, SCC61 and SCC090 cells. However, a portion of si-NOTCH4 cells did not have significant changes in EMT-related genes. For example, E-cadherin and TWIST1 expression did not exhibit significant differences between si-NOTCH4 and si-control cells in SKN3 and SCC090, respectively. Fibronectin expression in si-NOTCH4 cells was significantly increased compared with si-control Cal27 cells. SOX2 expression in si-NOTCH4 cells was also significantly increased compared with si-control SKN3 cells (Fig. 4A). In western blot experiments, elevated E-cadherin expression was also found in Cal27 si-NOTCH4 cells. But, there was no obvious difference of E-cadherin expression between SKN3 si-control and si-NOTCH4 cells. Fibronectin, Vimentin, TWIST1 and SOX2 expression decreased in both SKN3 and Cal27 si-NOTCH4 cells (Fig. 4B). Next, we examined the function of another HNSCC specific NOTCH pathway gene, HEY1.
Figure 4. The expression of EMT-related genes in si-NOTCH4 cells.
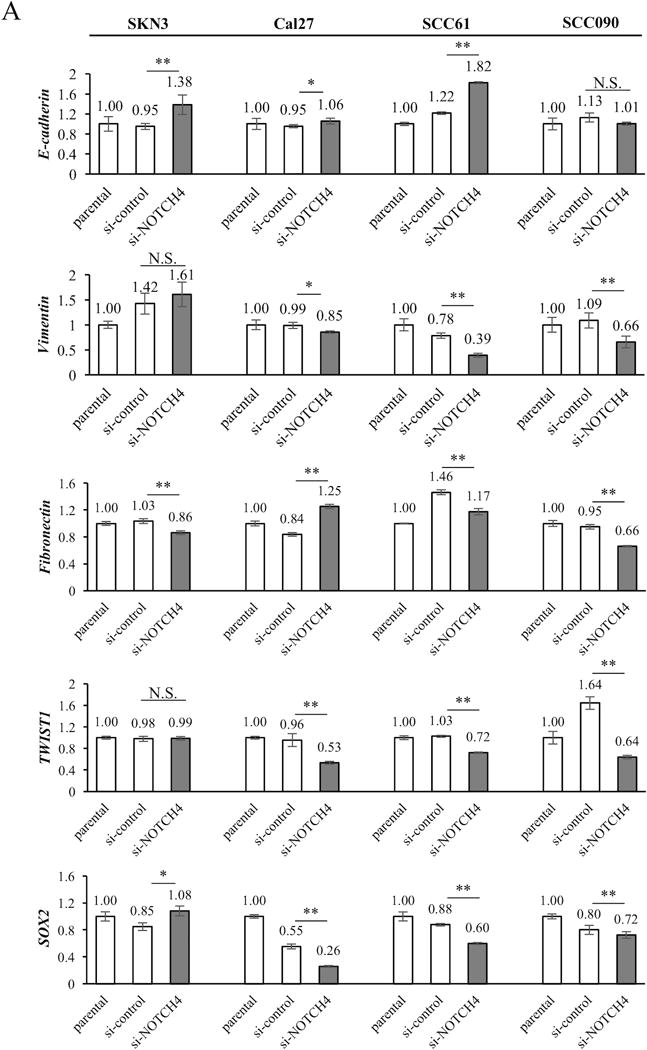
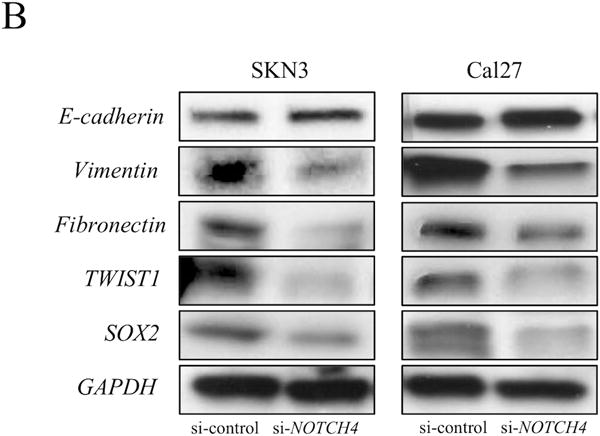
(A) EMT-related gene (E-cadherin, Vimentin, Fibronectin, TWIST1, and SOX2) expression in parental, si-control and si-NOTCH4 cells is measured by qRT-PCR. The expression differences between si-control and si-NOTCH4 cells are compared. P value is calculated by using Student’s t-test. *: P < 0.05, **: P < 0.01, N.S.: not significant. (B) Western blots of EMT-related genes in si-control and si-NOTCH4 of SKN3 and Cal27 cells. GAPDH antibody is used as a control.
NOTCH4 specifically promotes HEY1 expression in HNSCC
Our TCGA data set analysis showed no significant difference in NOTCH pathway genes between the HES high and low expressing tumors. On the other hand, HEY1 expression exhibited a significant correlation with several NOTCH pathway genes (Fig. 1A, and Supplementary Fig. S1A). To confirm these findings in vitro, HES1 and HEY1 expression were compared between si-control and si-NOTCH4 by using qRT-PCR. No significant differences of HES1 expression were noted between si-control and si-NOTCH4 in SKN3, SCC61, and SCC090. In Cal27, HES1 expression was significantly increased in si-NOTCH4 (Fig. 5A). On the other hand, HEY1 expression was significantly decreased in all cell lines with si-NOTCH4 (Fig. 5B). In western blot experiments, we obtained similar results. There were no HES1 expression changes between si-control and si-NOTCH4 cells, but si-NOTCH4 cells had less HEY1 expression than si-control cells (Fig. 5C). Thus, similar to the TCGA data set analysis, our in vitro experiments also showed that NOTCH4 was significantly associated with HEY1 expression.
Figure 5. HES1/HEY1 expression in si-NOTCH4 cells and NOTCH4 expression in si-HEY1 cells.
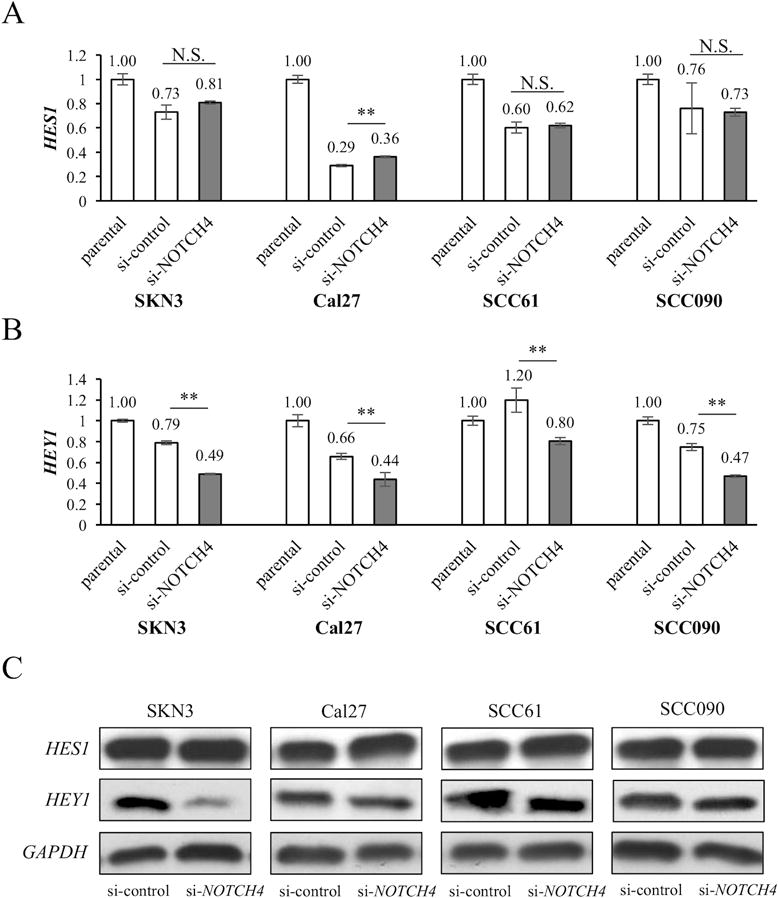
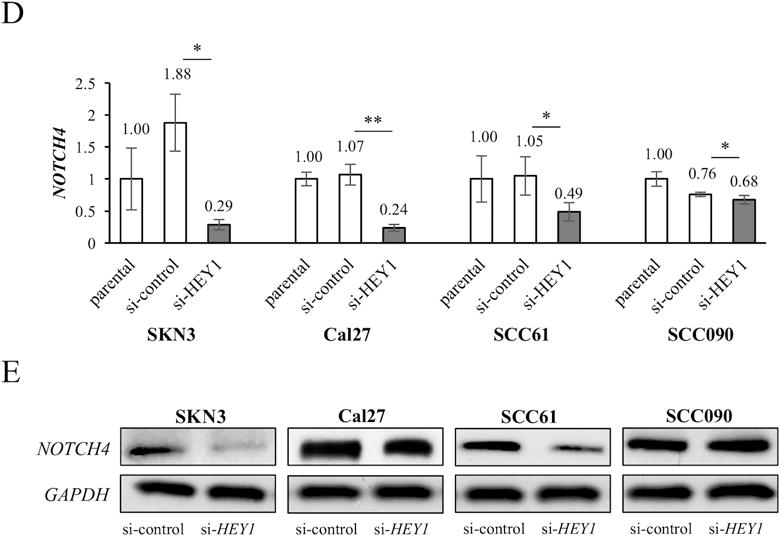
HES1 (A) and HEY1 (B) expression in parental, si-control and si-NOTCH4 cells. mRNA expression is measured by qRT-PCR. The expression differences between si-control and si-NOTCH4 cells are compared. (C) Western blots of HES1 and HEY1 in si-control and si-NOTCH4 cells. (D) NOTCH4 expression of parental, si-control and si-HEY1 cells. mRNA expression is measured by qRT-PCR. The expression differences between si-control and si-HEY1 cells are compared. (E) Western blots of NOTCH4 in si-control and si-HEY1 cells. GAPDH antibody is used as a control. P value is calculated by using Student’s t-test. *: P < 0.05, **: P < 0.01, N.S.: not significant.
HEY1 inhibition decreases NOTCH4 expression in HNSCC
HEY1 is generally activated by NOTCH receptors. However, si-HEY1 cells significantly decreased NOTCH4 mRNA expression in all HNSCC cell lines (Fig. 5D and Supplementary Fig. S3B, S3C). We also found less NOTCH4 expression in si-HEY1 cells of SKN3, Cal27 and SCC61 by western blot (Fig. 5E). These results may indicate that HEY1 also reciprocally regulates NOTCH4 expression in HNSCC.
HEY1 is expression is associated with EMT genes in HNSCC
Next, we hypothesized that HEY1 was also related to EMT in HNSCC similar to NOTCH4. Thus, using a TCGA data set similar to that used for NOTCH4 analysis, EMT-related genes were compared between HEY1 high and low groups (Fig. 6A). In contrast to NOTCH4, E-cadherin expression was not significantly decreased in the HEY1 high group (P = 0.10, ratio = 0.825). However, the expressions of N-cadherin (P = 0.0038), Vimentin (P = 0.0068), Fibronectin (P = 0.0002), TWIST1 (P = 0.0025), and SOX2 (P < 0.0001) were significantly increased in the HEY1 high group (Fig. 6A). The expression of other EMT related genes of HEY1 high group were several times more than that of low group (N-cadherin: 2.332, Vimentin: 1.249, Fibronectin: 1.720, TWIST1: 1.228, SOX2: 3.724).
Figure 6. Comparison of HEY1 and EMT-related genes using TCGA data set. Comparison of HEY1 expression between sphere and parental cells.
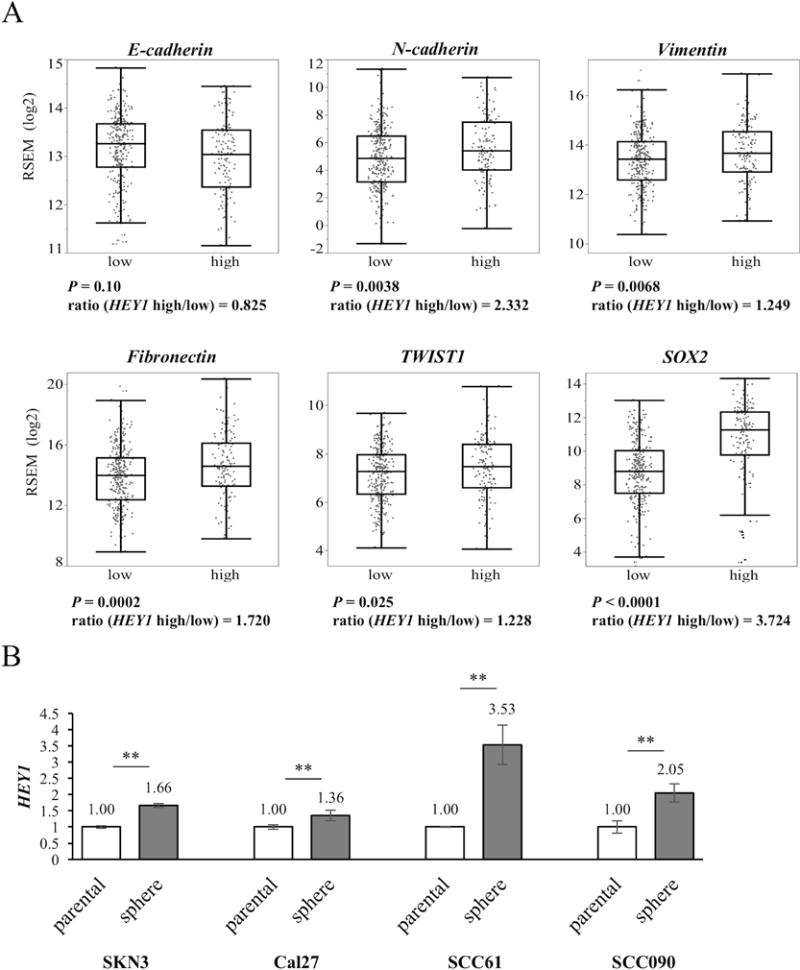
(A) Comparison of EMT-related genes between HEY1 high and low groups using the TCGA data set. The boxes represent the interquartile range (25th-75th), and horizontal lines inside the boxes indicate median. Whiskers indicate the minimum and maximum values. Ratio is calculated by dividing the mRNA expression of the high group by the expression of the low group. (B) HEY1 expression is compared between parental and sphere cells using qRT-PCR. P value is calculated by using Student’s t-test. **: P < 0.01.
Similar to the previous NOTCH4 experiment, we compared HEY1 expression between parental and sphere cells. HEY1 expression significantly increased in sphere cells of all HNSCC cell lines. HEY1 expression in sphere cells was increased approximately 1.4- to 3.5–fold compared with parental cells (Fig. 6B). To summarize our sphere cells experiments as shown Fig. 3B and 6B, sphere cells were enriched in both NOTCH4 and HEY1 expression.
We also ascertained the relation of HEY1 and EMT genes in vitro. As shown in Fig. 7A, si-HEY1 cells significantly increased E-cadherin expression in the all cell lines. On the contrary, si-NOTCH4 significantly reduced Vimentin, Fibronectin and TWIST1 expression in the all cell lines. The SOX2 expressions of si-HEY1 cells significantly decreased in SKN3, SCC61 and SCC090 cells. However, only si-HEY1 Cal27 cells did not have significant changes of SOX2 expression (Fig. 7A). In western blot experiments, we also noted that si-HEY1 cells had higher E-cadherin expression and less mesenchymal marker gene (Vimentin, Fibronectin, TWIST1 and SOX2) expression than si-control cells. Only Cal27 cells lacked an obvious difference of E-cadherin expression (Fig. 7B). Furthermore, to assess whether these expression changes affect the cell phenotype, we performed migration and invasion assays. We found significant decrease of migrated and invaded cells in si-NOTCH4 and si-HEY1 cells compared to si-control cells (Fig. 7C, 7D and 7E). In these results, we concluded that the NOTCH4-HEY1 pathway induces EMT in HNSCC.
Figure 7. HEY1 relates to EMT gene expression and EMT functions.
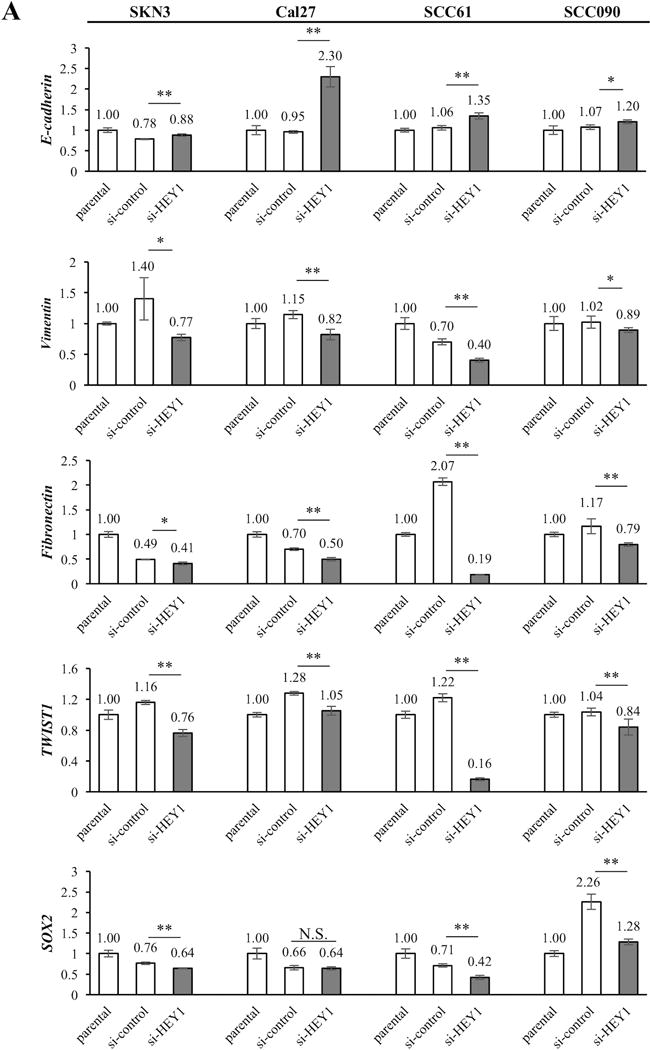
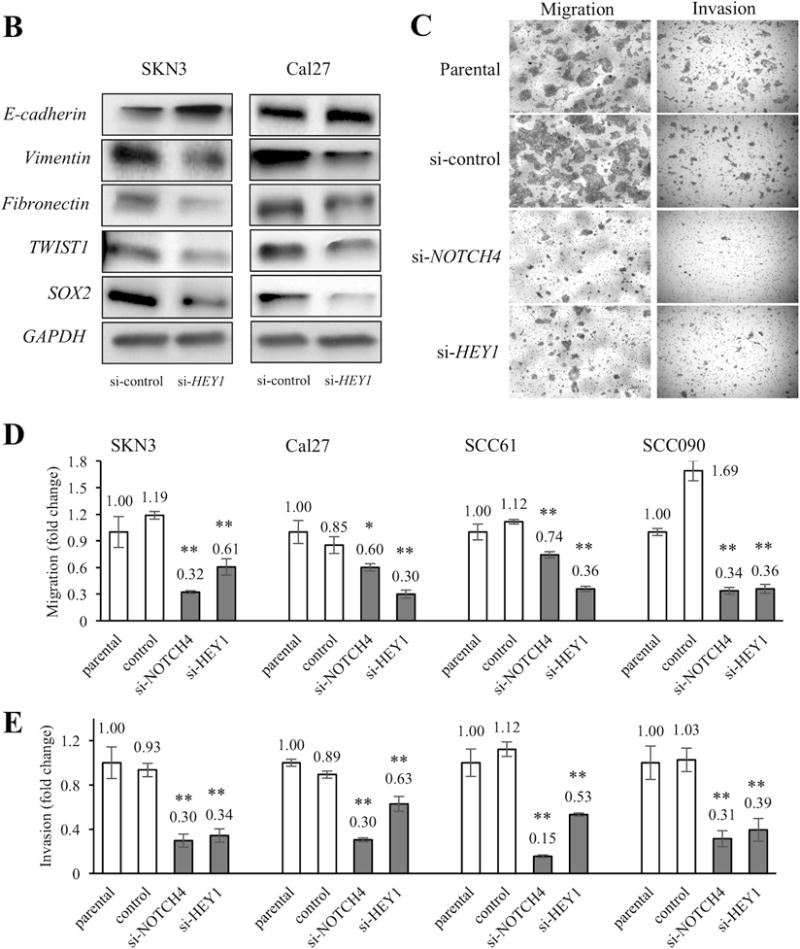
(A) The comparisons of EMT-related gene (E-cadherin, Vimentin, Fibronectin, TWIST1 and SOX2) expressions among parental, si-control and si-HEY1 cells. The expression differences between si-control and si-HEY1 cells are compared. (B) Western blots of EMT-related genes in si-control and si-HEY1 cells. (C) Representative images of migration and invasion assays. The cell line is SCC090. (D, E) Migration and invasion assays in parental, si-control, si-NOTCH4 and si-HEY1 cells. The migration and invasion indexes were calculated by deviding the number of parental cells thorough the chamber. The differences between si-control and si-NOTCH4/si-HEY1 cells are compared. P value is calculated by using Student’s t-test. *: P < 0.05, **: P < 0.01, N.S.: not significant.
Stable transfectants using sh-NOTCH4 were created in Cal27 cells, however, despite RNA knockdown, protein levels of NOTCH4 were unchanged, indicating that NOTCH4 expression is obligate for survival in cell line systems (Supplementary Fig. S3D, S3E, S3F).
DISCUSSION
The NOTCH pathway is highly conserved through evolution and plays important roles during embryonic development (25). The NOTCH pathway also affects normal tissue cell proliferation and inhibits apoptosis (26). In mammals, the NOTCH pathway has four receptors (NOTCH1, 2, 3 and 4) and five ligands (JAG1 and 2, DLL1, 3 and 4), all of which are type 2 transmembrane proteins (27). This pathway is activated by a ligand binding to a NOTCH receptor. Subsequently, the γ-secretase complex releases the intracellular domain of the NOTCH receptor, which moves to the nucleus, resulting in the transcriptional activation of NOTCH target genes, such as the HES/HEY family (27, 28).
The NOTCH pathway is also an attractive cancer therapeutic target. For instance, inhibition of the NOTCH pathway by the γ-secretase inhibitor (GSI) decreases cell proliferation and invasion (18). Thus, several clinical trials use GSI for cancer treatment (29–32). However, GSI exhibits toxicity in normal stem cells and clinically results in gastrointestinal toxicity, diarrhea, hepatotoxicity and nephrotoxicity (33–37). Wu et al. considered that these adverse events resulted from GSI nonspecific effect for NOTCH pathway. Thus, they showed that inhibition of NOTCH1 or NOTCH2 alone mildly affected intestinal morphology and some goblet cell metaplasia, but that inhibition of both NOTCH1 and 2 caused severe intestinal toxicity in their mouse model (36). Furthermore, many studies assumed that GSIs used for clinical trails have biological equivalent effect for each NOTCH receptors. But, Ran et al. examined the NOTCH inhibition potential of several GSIs and showed these GSIs had different effect for each NOTCH receptors. For instance, not all GSIs had sufficient pharmacological effect for NOTCH4 (38). Harrison et al. also showed that two GSIs (DAPT and Dibenzazepine) had no effect on NOTCH4 in breast cancer cells (39).
Our TCGA data set analysis showed that that only HEY1 is up-regulated among the NOTCH downstream genes compared with normal tissues, in addition NOTCH4 is the most significantly associated with HEY1 activation in HNSCC. Next, we used pGreenFire1-Notch plasmid in order to examine to what extent NOTCH4 related to NOTCH activity. Wicha et al. used this vector to assess the NOTCH activity and show that NOTCH activity is related to tumorigenicity, cancer stem cells (CSCs), and poor prognosis in lung adenocarcinoma and breast cancer (40, 41). NOTCH4 receptor was expressed at 3-5 fold higher levels in the NOTCH high activity cells compared to NOTCH low activity breast cancer cell lines. (41). We also show that si-NOTCH4 cells significantly decreased NOTCH activity in all cell lines. NOTCH4 is known to promote mouse mammary epithelial transformation and tumorigenesis (42). This report is the first study linking NOTCH4 and a cancer phenotype. Soriano et al. also demonstrated that normal mammary cells exhibited altered shape and promoted an invasive and tumorigenic phenotype by NOTCH4 (43). Thus, we next examined NOTCH4 function in HNSCC and showed NOTCH4 affected HNSCC cell proliferation and cisplatin resistance in vitro. There are several papers indicates that the NOTCH pathway affects cell cycle and apoptosis. For instance, Demarest et al. indicates that the NOTCH pathway promotes cell cycle progression and inhibits apoptosis by using T-ALL cells (44). As a result of our apoptosis and cell cycle analysis, we can suggest that NOTCH4 decreases cell growth by regulating both apoptosis and the cell cycle.
Lombardo et al. showed that the EMT phenotype was induced by NOTCH4 in breast cancer cells (45). NOTCH4 is also known as an EMT trigger and promotes the metastasis of melanoma cells (20). EMT promotes cancer migration, invasion, metastasis (46) and is also related to poor prognosis. Thus, EMT represents one of the most important phenotypes in cancer therapy. In the melanoma study, NOTCH4 was also related to SOX2 expression and cell invasion. Clinically, approximately 60% of melanoma tissues express high levels of NOTCH4 protein. High NOTCH4 expression is related to metastasis and poor prognosis (20). We also demonstrated that NOTCH4 was related to EMT gene expression in HNSCC using the TCGA data set. By qRT-PCR and western blot analysis, not all cell lines exhibited a significant change in EMT-related genes upon NOTCH4 knockdown.
Using the TCGA data set, we demonstrated that HEY1 was significantly up-regulated in tumors compared with normal tissue among the NOTCH downstream genes. qRT-PCR and western blot analysis revealed the same result. In general, HEY1 is related to the development of the heart, neurogenesis and osteogenesis (47–51). In cardiovascular studies, HEY1 regulates endocardia EMT in septum and valve development (52). In heart development, HEY1 cooperates with TWIST1 to promote EMT (53). In cancer studies, HEY1 is an indicator of poor clinical prognosis in several cancer types, such as pancreas (54), colon (55), esophagus (56), and thyroid (57). In a thyroid cancer study, HEY1 expression was also related to recurrence and metastasis (57). Lung metastasis of osteosarcoma cells was also promoted by HEY1 in a nude mouse model (58). However, few studies about EMT and HEY1 in cancers have been performed. Our current study is the first HNSCC study that assesses the relationship between HEY1 and EMT. In the TCGA data set analysis, TWIST1 and mesenchymal genes such as N-cadherin, Vimentin and Fibronectin were significantly increased in the HEY1 high group. But, E-cadherin expression was not significantly decreased in the HEY1 high group (Fig. 6A). However, this finding may result from the method used to divide the groups. For example, if the group was divided by the average of HNSCC HEY1 expression, E-cadherin had significant differences similar to other EMT-related genes (Supplementary Fig. S4A). Furthermore, our in vitro experiments showed that E-cadherin, Vimentin, Fibronectin and TWIST1 expressions exhibit significant differences between si-HEY1 and si-control cells in all cell lines. We also revealed that the NOTCH4-HEY1 pathway was significantly correlated with SOX2 in the TCGA data set and in vitro. SOX2 is a marker gene of tissue stem cells (59), CSCs (60) as well as EMT (23) in the head and neck region. Furthermore, SOX2 is reported to co-expressed with HEY1/HEY2 in the inner ear (61) and regulated by the NOTCH pathway in the developing inner ear. During inner ear development, HEY1 expression is significantly increased in the regulation of SOX2 (62). The NOTCH pathway is also necessary to maintain SOX2-positive stem cells in the pituitary gland (63). In our TCGA results, the HEY1 high group exhibited 3.72–fold increased SOX2 expression (Fig. 6A). In in vitro experiments, inhibition of HEY1 resulted in significantly decreased SOX2 expression. In these contexts, we suggest that HEY1 may regulate SOX2 as well as other EMT related genes in HNSCC (Fig. 7A, 7B). EMT is known to enhance cell migration and invasion (64). We also showed that both NOTCH4 and HEY1 promoted migration and invasion properties (Fig. 7C, 7D and 7E). Thus, we concluded that the NOTCH4–HEY1 pathway induces EMT in HNSCC.
NOTCH4 expression is also reported to increase in breast CSCs (65). EMT is closely correlated with CSCSs (66). In HNSCC, CD10 (67), CD44 (68) and ALDH1 (69) are defined as CSCs markers. Thus, the expressions of these markers were compared between NOTCH4 and HEY1 high/low groups using the TCGA data set. Significant differences in ALDH1 and CD10 but not CD44 expression were noted between NOTCH4 high and low groups (Supplementary Fig. S5A). The ALDH1 expression also significantly increased in the HEY1 high group compared with the HEY1 low group. CD10 and CD44 expression did not exhibit a significant difference between HEY1 high and low groups (Supplementary Fig. S5B). Regarding ALDH1 expression, the NOTCH4 high group exhibited 1.63-fold increased expression and the HEY1 high group exhibited 5.87-fold increased expression compared with each low group. To elucidate the relationships between NOTCH4-HEY1 and ALDH1 in vitro, ALDH1 expression was compared by using qRT-PCR, and significantly increased in both si-NOTCH4 and si-HEY1 of all HNSCC cell lines except Cal27 si-NOTCH4 cells (Supplementary Fig. S5C). We also performed aldefluor assays and found similar results, with both si-NOTCH4 and si-HEY1 cells showing and increased ALDH1+ fraction (Supplementary Fig. S5D, S5E). Young et al. show ALDH1 regulates NOTCH1 expression in ovarian cancer cells (70). In our results, we also suggest that ALDH1 can regulate NOTCH4-HEY1 pathway.
Wicha et al. showed high NOTCH4 and HEY1 expression in primary breast cancer patient samples correlated with poor overall survival using a TCGA data set (41). Simões et al. also showed NOTCH4 high breast cancer had high HEY1 expression and worse clinical prognosis, such as overall survival and metastasis free survival (65). Thus, we also compared the clinical background and prognosis between the NOTCH4/HEY1 high and low group using a TCGA data set (Supplementary Fig. S6A, S6B and Supplementary Table S2). We noted is no significant difference between the NOTCH4/HEY1 high and low group except the age of the HEY1 high/low group (Supplementary Table S2), as well as no difference in overall survival between the NOTCH4/HEY1 high and low group. (Supplementary Fig. S6A, S6B).
HEY1 is generally activated by NOTCH receptors. However, we demonstrate that si-HEY1 cells significantly decreased NOTCH4 expression in all HNSCC cell lines (Fig. 5D, 5E). Whether this effect is direct or indirect is not known; however, this result may indicate that HEY1 also regulates NOTCH4 expression. In other words, the NOTCH4-HEY1 pathway may create a positive feedback loop in HNSCC.
In this study, we used one HPV-positive HNSCC cell line, SCC090. The gene expression and pathways of HPV-positive HNSCC differs from HPV-negative HNSCC (71, 72). Regarding cancer therapy, HPV-positive HNSCC is more sensitive to radiation and chemotherapy than HPV-negative cancer (73). However, in our experiments, there were no differences in the results between SCC090 and the other HPV-negative HNSCC cell lines. This finding indicates that the function of the NOTCH4-HEY1 pathway does not change regardless of HPV status.
In conclusion, we demonstrate that the NOTCH4-HEY1 pathway of HNSCC is specifically up-regulated and promotes EMT. NOTCH4 is also related to proliferation, chemoresistance, apoptosis inhibition, and cell cycle alteration in HNSCC. Thus, this pathway may represent a novel target for HNSCC therapy or may serve as a target to improve chemotherapeutic sensitivity.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
The identification of HNSCC specific genes and pathways may be essential for targeted cancer therapy. Recently, several comprehensive genomic analyses reveal that the NOTCH pathway is closely related to HNSCC progression. However, defining which NOTCH pathway predominantly affects HNSCC development is not well examined and understood. In this study, we examined the role of NOTCH4 using the TCGA data set and in vitro experiments. Consequently, we demonstrate that the NOTCH4-HEY1 pathway is specifically up-regulated in HNSCC compared with normal tissue. We also demonstrate that NOTCH4 promotes HNSCC proliferation, cisplatin resistance, a reduction in apoptosis and cell cycle alterations. Finally, we indicate that the NOTCH4-HEY1 pathway promotes EMT by examining EMT-related genes such as E-cadherin, Vimentin, Fibronectin, TWIST1 and SOX2. This finding has great potential for expanding our knowledge regarding the NOTCH pathway in cancer biology and may provide guidance in the development of novel specific HNSCC therapies.
Acknowledgments
Financial Support
This study was supported by National Institute of Dental and Craniofacial Research (NIDCR, number: R01DE023347). J.A.Califano received this grant.
Footnotes
Disclosure of Potential Conflict of Interest
The authors declare no potential conflicts of interest.
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. The Lancet. 2008;371:1695–709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo WL, Kao SY, Chi LY, Wong YK, Chang RC. Outcomes of oral squamous cell carcinoma in Taiwan after surgical therapy: factors affecting survival. J Oral Maxillofac Surg. 2003;61:751–8. doi: 10.1016/s0278-2391(03)00149-6. [DOI] [PubMed] [Google Scholar]
- 3.Poeta ML, Manola J, Goldenberg D, Forastiere A, Califano JA, Ridge JA, et al. The Ligamp TP53 Assay for Detection of Minimal Residual Disease in Head and Neck Squamous Cell Carcinoma Surgical Margins. Clin Cancer Res. 2009;15:7658–65. doi: 10.1158/1078-0432.CCR-09-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demokan S, Chuang A, Suoglu Y, Ulusan M, Yalniz Z, Califano JA, et al. Promoter methylation and loss of p16(INK4a) gene expression in head and neck cancer. Head Neck. 2012;34:1470–5. doi: 10.1002/hed.21949. [DOI] [PubMed] [Google Scholar]
- 5.Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 6.de Castro G, Jr, Negrao MV. The Cancer Genome Atlas findings in head and neck cancer: a renewed hope. Curr Opin Oncol. 2014;26:245–6. doi: 10.1097/CCO.0000000000000075. [DOI] [PubMed] [Google Scholar]
- 7.Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–25. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dellinger AE, Nixon AB, Pang H. Integrative Pathway Analysis Using Graph-Based Learning with Applications to TCGA Colon and Ovarian Data. Cancer Inform. 2014;13:1–9. doi: 10.4137/CIN.S13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cancer Genome Atlas N. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517:576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agrawal N, Frederick MJ, Pickering CR, Bettegowda C, Chang K, Li RJ, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–7. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stransky N, Egloff AM, Tward AD, Kostic AD, Cibulskis K, Sivachenko A, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–60. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yousif NG, Sadiq AM, Yousif MG, Al-Mudhafar RH, Al-Baghdadi JJ, Hadi N. Notch1 ligand signaling pathway activated in cervical cancer: poor prognosis with high-level JAG1/Notch1. Arch Gynecol Obstet. 2015;292:899–904. doi: 10.1007/s00404-015-3694-1. [DOI] [PubMed] [Google Scholar]
- 13.Dotto GP. Notch tumor suppressor function. Oncogene. 2008;27:5115–23. doi: 10.1038/onc.2008.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yap LF, Lee D, Khairuddin A, Pairan MF, Puspita B, Siar CH, et al. The opposing roles of NOTCH signalling in head and neck cancer: a mini review. Oral Dis. 2015;21:850–7. doi: 10.1111/odi.12309. [DOI] [PubMed] [Google Scholar]
- 15.Hijioka H, Setoguchi T, Miyawaki A, Gao H, Ishida T, Komiya S, et al. Upregulation of Notch pathway molecules in oral squamous cell carcinoma. Int J Oncol. 2010;36:817–22. doi: 10.3892/ijo_00000558. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Hong HS, Liu ZX, Kim RH, Kang MK, Park NH, et al. TNFalpha enhances cancer stem cell-like phenotype via Notch-Hes1 activation in oral squamous cell carcinoma cells. Biochem Biophys Res Commun. 2012;424:58–64. doi: 10.1016/j.bbrc.2012.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun W, Gaykalova DA, Ochs MF, Mambo E, Arnaoutakis D, Liu Y, et al. Activation of the NOTCH pathway in head and neck cancer. Cancer Res. 2014;74:1091–104. doi: 10.1158/0008-5472.CAN-13-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao J, Duan L, Fan M, Wu X. Gamma-secretase inhibitors exerts antitumor activity via down-regulation of Notch and Nuclear factor kappa B in human tongue carcinoma cells. Oral Dis. 2007;13:555–63. doi: 10.1111/j.1601-0825.2006.01334.x. [DOI] [PubMed] [Google Scholar]
- 19.Egloff AM, Grandis JR. Molecular pathways: context-dependent approaches to Notch targeting as cancer therapy. Clin Cancer Res. 2012;18:5188–95. doi: 10.1158/1078-0432.CCR-11-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin X, Sun B, Zhu D, Zhao X, Sun R, Zhang Y, et al. Notch4+ cancer stem-like cells promote the metastatic and invasive ability of melanoma. Cancer Sci. 2016;107:1079–91. doi: 10.1111/cas.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 22.Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang N, Hui L, Wang Y, Yang H, Jiang X. Overexpression of SOX2 promotes migration, invasion, and epithelial-mesenchymal transition through the Wnt/beta-catenin pathway in laryngeal cancer Hep-2 cells. Tumour Biol. 2014;35:7965–73. doi: 10.1007/s13277-014-2045-3. [DOI] [PubMed] [Google Scholar]
- 24.Han XY, Wei B, Fang JF, Zhang S, Zhang FC, Zhang HB, et al. Epithelial-mesenchymal transition associates with maintenance of stemness in spheroid-derived stem-like colon cancer cells. PLoS One. 2013;8:e73341. doi: 10.1371/journal.pone.0073341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7:678–89. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 26.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 27.Gordon WR, Arnett KL, Blacklow SC. The molecular logic of Notch signaling–a structural and biochemical perspective. J Cell Sci. 2008;121:3109–19. doi: 10.1242/jcs.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. doi: 10.1146/annurev.pathmechdis.3.121806.154300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strosberg JR, Yeatman T, Weber J, Coppola D, Schell MJ, Han G, et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur J Cancer. 2012;48:997–1003. doi: 10.1016/j.ejca.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek MA, Dasari A, et al. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs. 2014;32:739–45. doi: 10.1007/s10637-014-0083-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee SM, Moon J, Redman BG, Chidiac T, Flaherty LE, Zha Y, et al. Phase 2 study of RO4929097, a gamma-secretase inhibitor, in metastatic melanoma: SWOG 0933. Cancer. 2015;121:432–40. doi: 10.1002/cncr.29055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Gong L, Ou R, Zheng Z, Chen J, Xie F, et al. Sequential combination therapy of ovarian cancer with cisplatin and gamma-secretase inhibitor MK-0752. Gynecol Oncol. 2016;140:537–44. doi: 10.1016/j.ygyno.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, et al. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–16. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 34.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 35.Garber K. Notch emerges as new cancer drug target. J Natl Cancer Inst. 2007;99:1284–5. doi: 10.1093/jnci/djm148. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–7. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 37.Purow B. Notch inhibition as a promising new approach to cancer therapy. Adv Exp Med Biol. 2012;727:305–19. doi: 10.1007/978-1-4614-0899-4_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ran Y, Hossain F, Pannuti A, Lessard CB, Ladd GZ, Jung JI, et al. gamma-Secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct. EMBO Mol Med. 2017;9:950–66. doi: 10.15252/emmm.201607265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison H, Farnie G, Howell SJ, Rock RE, Stylianou S, Brennan KR, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–18. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hassan KA, Wang L, Korkaya H, Chen G, Maillard I, Beer DG, et al. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin Cancer Res. 2013;19:1972–80. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D’Angelo RC, Ouzounova M, Davis A, Choi D, Tchuenkam SM, Kim G, et al. Notch reporter activity in breast cancer cell lines identifies a subset of cells with stem cell activity. Mol Cancer Ther. 2015;14:779–87. doi: 10.1158/1535-7163.MCT-14-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallahan D, Callahan R. The mouse mammary tumor associated gene INT3 is a unique member of the NOTCH gene family (NOTCH4) Oncogene. 1997;14:1883–90. doi: 10.1038/sj.onc.1201035. [DOI] [PubMed] [Google Scholar]
- 43.Soriano JV, Uyttendaele H, Kitajewski J, Montesano R. Expression of an activated Notch4(int-3) oncoprotein disrupts morphogenesis and induces an invasive phenotype in mammary epithelial cells in vitro. Int J Cancer. 2000;86:652–9. doi: 10.1002/(sici)1097-0215(20000601)86:5<652::aid-ijc8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Demarest RM, Ratti F, Capobianco AJ. It’s T-ALL about Notch. Oncogene. 2008;27:5082–91. doi: 10.1038/onc.2008.222. [DOI] [PubMed] [Google Scholar]
- 45.Lombardo Y, Faronato M, Filipovic A, Vircillo V, Magnani L, Coombes RC. Nicastrin and Notch4 drive endocrine therapy resistance and epithelial to mesenchymal transition in MCF7 breast cancer cells. Breast Cancer Res. 2014;16:R62. doi: 10.1186/bcr3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 47.Sakamoto M, Hirata H, Ohtsuka T, Bessho Y, Kageyama R. The basic helix-loop-helix genes Hesr1/Hey1 and Hesr2/Hey2 regulate maintenance of neural precursor cells in the brain. J Biol Chem. 2003;278:44808–15. doi: 10.1074/jbc.M300448200. [DOI] [PubMed] [Google Scholar]
- 48.Kokubo H, Miyagawa-Tomita S, Nakazawa M, Saga Y, Johnson RL. Mouse hesr1 and hesr2 genes are redundantly required to mediate Notch signaling in the developing cardiovascular system. Dev Biol. 2005;278:301–9. doi: 10.1016/j.ydbio.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 49.Kokubo H, Tomita-Miyagawa S, Hamada Y, Saga Y. Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development. 2007;134:747–55. doi: 10.1242/dev.02777. [DOI] [PubMed] [Google Scholar]
- 50.Sharff KA, Song WX, Luo X, Tang N, Luo J, Chen J, et al. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J Biol Chem. 2009;284:649–59. doi: 10.1074/jbc.M806389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salie R, Kneissel M, Vukevic M, Zamurovic N, Kramer I, Evans G, et al. Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone. 2010;46:680–94. doi: 10.1016/j.bone.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 52.Fischer A, Steidl C, Wagner TU, Lang E, Jakob PM, Friedl P, et al. Combined loss of Hey1 and HeyL causes congenital heart defects because of impaired epithelial to mesenchymal transition. Circ Res. 2007;100:856–63. doi: 10.1161/01.RES.0000260913.95642.3b. [DOI] [PubMed] [Google Scholar]
- 53.Luna-Zurita L, Prados B, Grego-Bessa J, Luxan G, del Monte G, Benguria A, et al. Integration of a Notch-dependent mesenchymal gene program and Bmp2-driven cell invasiveness regulates murine cardiac valve formation. J Clin Invest. 2010;120:3493–507. doi: 10.1172/JCI42666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mann CD, Bastianpillai C, Neal CP, Masood MM, Jones DJ, Teichert F, et al. Notch3 and HEY-1 as prognostic biomarkers in pancreatic adenocarcinoma. PLoS One. 2012;7:e51119. doi: 10.1371/journal.pone.0051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Candy PA, Phillips MR, Redfern AD, Colley SM, Davidson JA, Stuart LM, et al. Notch-induced transcription factors are predictive of survival and 5-fluorouracil response in colorectal cancer patients. Br J Cancer. 2013;109:1023–30. doi: 10.1038/bjc.2013.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forghanifard MM, Taleb S, Abbaszadegan MR. Notch Signaling Target Genes are Directly Correlated to Esophageal Squamous Cell Carcinoma Tumorigenesis. Pathol Oncol Res. 2015;21:463–7. doi: 10.1007/s12253-014-9849-8. [DOI] [PubMed] [Google Scholar]
- 57.Jung CW, Kong JS, Seol H, Park S, Koh JS, Lee SS, et al. Expression of activated Notch1 and HEY1 in papillary thyroid carcinoma. Histopathology. 2016 doi: 10.1111/his.13065. [DOI] [PubMed] [Google Scholar]
- 58.Tsuru A, Setoguchi T, Matsunoshita Y, Nagao-Kitamoto H, Nagano S, Yokouchi M, et al. Hairy/enhancer-of-split related with YRPW motif protein 1 promotes osteosarcoma metastasis via matrix metallopeptidase 9 expression. Br J Cancer. 2015;112:1232–40. doi: 10.1038/bjc.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hume CR, Bratt DL, Oesterle EC. Expression of LHX3 and SOX2 during mouse inner ear development. Gene Expr Patterns. 2007;7:798–807. doi: 10.1016/j.modgep.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim YC, Oh SY, Cha YY, Kim SH, Jin X, Kim H. Cancer stem cell traits in squamospheres derived from primary head and neck squamous cell carcinomas. Oral Oncol. 2011;47:83–91. doi: 10.1016/j.oraloncology.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 61.Benito-Gonzalez A, Doetzlhofer A. Hey1 and Hey2 control the spatial and temporal pattern of mammalian auditory hair cell differentiation downstream of Hedgehog signaling. J Neurosci. 2014;34:12865–76. doi: 10.1523/JNEUROSCI.1494-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Neves J, Parada C, Chamizo M, Giraldez F. Jagged 1 regulates the restriction of Sox2 expression in the developing chicken inner ear: a mechanism for sensory organ specification. Development. 2011;138:735–44. doi: 10.1242/dev.060657. [DOI] [PubMed] [Google Scholar]
- 63.Zhu X, Tollkuhn J, Taylor H, Rosenfeld MG. Notch-Dependent Pituitary SOX2(+) Stem Cells Exhibit a Timed Functional Extinction in Regulation of the Postnatal Gland. Stem Cell Reports. 2015;5:1196–209. doi: 10.1016/j.stemcr.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–92. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simões Bruno M, O’Brien Ciara S, Eyre R, Silva A, Yu L, Sarmiento-Castro A, et al. Anti-estrogen Resistance in Human Breast Tumors Is Driven by JAG1-NOTCH4-Dependent Cancer Stem Cell Activity. Cell Reports. 2015;12:1968–77. doi: 10.1016/j.celrep.2015.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–51. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukusumi T, Ishii H, Konno M, Yasui T, Nakahara S, Takenaka Y, et al. CD10 as a novel marker of therapeutic resistance and cancer stem cells in head and neck squamous cell carcinoma. Br J Cancer. 2014;111:506–14. doi: 10.1038/bjc.2014.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385:307–13. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 70.Young MJ, Wu YH, Chiu WT, Weng TY, Huang YF, Chou CY. All-trans retinoic acid downregulates ALDH1-mediated stemness and inhibits tumour formation in ovarian cancer cells. Carcinogenesis. 2015;36:498–507. doi: 10.1093/carcin/bgv018. [DOI] [PubMed] [Google Scholar]
- 71.Mirghani H, Ugolin N, Ory C, Goislard M, Lefevre M, Baulande S, et al. Comparative analysis of micro-RNAs in human papillomavirus-positive versus -negative oropharyngeal cancers. Head Neck. 2016;38:1634–42. doi: 10.1002/hed.24487. [DOI] [PubMed] [Google Scholar]
- 72.Suarez E, Gonzalez L, Perez-Mitchell C, Ortiz AP, Ramirez-Sola M, Acosta J, et al. Pathway Analysis using Gene-expression Profiles of HPV-positive and HPV-negative Oropharyngeal Cancer Patients in a Hispanic Population: Methodological Procedures. P R Health Sci J. 2016;35:3–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


