Highlights
-
•
ESBL-PE sepsis was predicted by admission at ICU and ESBL-PE colonization.
-
•
Neonates infected with ESBL-PE had significantly high mortality.
-
•
ESBL-producing Klebsiella pneumoniae (ST45) carrying blaCTX-M-15 were predominant.
-
•
Whole genome SNP analysis revealed clonal origin in 50% of ESBL-PE paired cases with similar sequence type.
Keywords: Neonatal sepsis, ESBL-PE, Predictors, Klebsiella pneumoniae, ST45
Abstract
The study was conducted to establish predictors of extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) neonatal sepsis and mortality in a tertiary hospital, Tanzania. Between July and December 2016, blood culture was performed in neonates with clinical features of sepsis and neonates/mothers/guardians were screened for ESBL colonization. Selected isolates underwent whole genome sequencing to investigate relatedness. Logistic regression analysis was performed to determine predictors for ESBL-PE associated neonatal sepsis and mortality. Neonatal ESBL-PE sepsis was detected in 32(10.5%) of the 304 neonates investigated. Neonatal ESBL-PE sepsis was independently predicted by admission at the Intensive care Unit and positive mother and neonate ESBL-PE colonization. Deaths occurred in 55(18.1%) of neonates. Neonates infected with ESBL-PE, admitted at ICU, increased age and those transferred from other centres had significantly high mortality rates. Gram-negative bacteria formed the majority (76%) of the isolates, of which 77% were ESBL-PE. Virulent Klebsiella pneumoniae ST45 carrying blaCTX-M-15 were commonly isolated from neonates. Klebsiella pneumoniae (ST45) were the predominant cause of ESBL-PE neonatal sepsis and mortality. Improved infection control and antibiotic stewardship are crucial in controlling the spread of resistant strains. Rapid diagnostic tests to detect ESBL-PE in low-income countries are needed to guide treatment and reduce ESBL-PE-associated mortality.
1. Introduction
Neonatal sepsis is one of the top three causes of morbidity and mortality during the neonatal period (Bhutta et al., 2010). Diagnosis and management of sepsis pose a great challenge for neonatologists in NICUs especially in low-income countries (Sharma et al., 2016). The increase of multidrug-resistant organisms in neonatal units limits the treatment options and delays effective treatment, resulting in increased morbidity and mortality (Patel and Saiman, 2010). In many low-income countries including Tanzania, extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-PE) are the commonest cause of neonatal sepsis (Christopher et al., 2013; D’Andrea et al., 2013; Ghotaslou et al., 2007; Mshana et al., 2009; Thaver et al., 2009). Infections due to ESBL-PE are associated with increased morbidity and mortality (Blomberg et al., 2005; Kayange et al., 2010; Mhada et al., 2012). The antibiotics of choice for the treatment of neonatal sepsis due to ESBL-PE pathogens are not available in most settings and even when stocked, they are too expensive to be afforded (Vandijck et al., 2008). Varieties of ESBL-PE genotypes have been found in humans, animals and the environment in the city of Mwanza, Tanzania (Mshana et al., 2016). Despite ESBL-PE being prevalent in the city of Mwanza, the transmission pathways and risk factors of these pathogens in relation to neonatal sepsis is not well studied. This study was undertaken to investigate factors associated with ESBL-PE neonatal sepsis and mortality among neonates admitted at the Bugando Medical Centre (BMC), Mwanza, Tanzania. In addition, the study aimed to characterize selected isolates to show their virulence potential and transmission dynamics.
2. Material and methods
2.1. Study duration, area and inclusion criteria
The study was conducted between July and December 2016 at the premature unit and neonatal Intensive care unit (NICU) of BMC. All neonates (0–28 days) with signs and symptoms of sepsis were enrolled. The sample size was estimated using the Kish Leslie formula for the cross sectional studies (Kish, 1965).
2.2. Data collection
Using signs and symptoms published by “The WHO Young infants Study group” (Margolis et al., 1999), a data collection tool was designed and used to obtain socio-demographic and clinical data together with other relevant factors related to risks for neonatal sepsis. At the time of enrollment, clinical data and blood samples were collected. Antenatal history such as use of antibiotics, presence of chronic diseases, febrile illness during pregnancy, labor history, duration of labor, history of premature rupture of membrane, mode of delivery were recorded. Neonates were subjected to a full clinical examination to assess temperature, respiration rate, presence or absence of cyanosis, jaundice, umbilical redness, convulsion, reduced movement and ability to feed.
2.3. Microbiological procedures
Umbilical-rectal swabs were collected using sterile cotton swabs in Amies transport medium (Biolab, HUNGARY) from all neonates to determine colonization status. Analysis of stool samples obtained from mothers/guardians provided data on colonization status. Swab and stool samples were inoculated on MacConkey agar (Oxoid, UK) supplemented with 2 μg/ml cefotaxime (Medochemie Ltd, Cyprus EU) and processed as previously described (Nelson et al., 2014). Using aseptic techniques approximately 1.5–2 ml of blood was obtained and inoculated directly into Brain Heart Infusion broth (BHI) (Oxoid Ltd, UK) in a ratio of blood to BHI of 1:10 and transported to the Catholic University of Health and allied Sciences (CUHAS) Microbiology laboratory for incubation and subsequent processing as previously described (Kayange et al., 2010). All blood culture isolates were tested for their antibiotic susceptibility pattern using disc diffusion methods and interpreted following the Clinical Laboratory Standard Institute guidelines (CLSI, 2015). Confirmation of the ESBL phenotype was performed using the disk approximation method (Mshana et al., 2009). All neonates enrolled in the study were assessed and managed adhering to the BMC neonatal unit protocols. The first line antibiotic treatment option included ampicillin and gentamicin, the second line included cefotaxime followed by the third line that incorporated the use of meropenem. All neonates were evaluated on a daily basis prior to discharge, after which they were monitored for a further 28 days.
2.4. Whole genome sequencing and in silico analyses
Fifty-three (16 from blood and 37 from colonized neonates) ESBL-PE isolates were subjected for whole genome sequencing (WGS). These isolates were serially selected as they were isolated, no other criteria were used, and aim was to sequence the first 50 isolates. DNA was isolated using PureLink® Pro 96 Genomic DNA Purification Kit (Thermo-Fisher Scientific, Dreieich, Germany) according to the manufacturer's instruction. WGS was performed on an Illumina NextSeq 500 instrument (Illumina, San Diego, CA, USA) using an Illumina Nextera XT library with 2 × 150bp paired-end reads. The data was assembled using SPAdes (version 3.6.2) (Bankevich et al., 2012).
Sequences were analyzed for their multi locus sequence types, transferrable resistance genes, plasmid replicon types and pMLST using MLST 1.8, ResFinder, Plasmidfinder and pMLST software of the Center for Genomic Epidemiology (https://cge.cbs.dtu.dk/services/), respectively (Carattoli et al., 2014; Larsen et al., 2012; Zankari et al., 2012). The presence/absence of Klebsiella pneumoniae virulence genes was assessed using blastn following Holt et al (Holt et al., 2015).
The SNP analysis of isolates depicting identical STs was performed using SAMtools (Li, 2011). Regions of high recombination (>50 SNPs per 1 kb) were excluded manually from the analysis.
The raw data of all sequenced ESBL-PE isolates are available at the European Nucleotide Archive (ENA) under the project number PRJEB20875.
2.5. Ethics approval
This study obtained clearance from the Joint CUHAS-BMC Ethics and Scientific Review committee with certificate no: CREC/162/2016. Written informed consent was obtained from parents/guardians of the study participants.
2.6. Statistical analysis
Data was entered using Microsoft Excel 2007 and processed using STATA version 13.0 (Stata Corp, College Station, TX, USA). Categorical variables such as history of antibiotic use, history of admission, colonization status etc., were presented proportionally and compared using chi squared test or Fisher’s exact test. Continuous variables such age, gestation age, length of hospital stay, weight etc., were described as medians (inter quartile range/ IQR)). To determine predictors of ESBL-PE neonatal sepsis, ESBL-PE neonatal colonization and the outcome, univariate followed by multivariate logistic regression analysis was performed. All factors with p value of less than 0.2 were further subjected to multivariate logistic analysis and controlled for both age and sex. To estimate survival rates and hazard ratios, a Cox regression model was used. All factors with p value less than 0.05 at 95% confidence interval (CI) were considered statistically significant.
3. Results
3.1. Demographic and clinical characteristics
A total of 304 neonates were enrolled in the study. Male neonates formed the majority 192 (63.2%) of study participants. The median age of the enrolled neonates was 6 days (IQR: 3–9). A total of 94 (30.1%) neonates had birth weights of below 2.5 kg. The median gestation age was 39 weeks (IQR: 37–40) and the median body temperature measured was 37.9 °C (IQR-37-38.2 °C). A total of 57 (14.1%) of all neonates were delivered by cesarean section (C/S) and 16 (5.3%) were delivered at home (Table 1).
Table 1.
Socio-demographic and clinical characteristics of 304 neonates with clinical sepsis.
| Neonate Characteristics | Number | Percent (%)/Median |
|---|---|---|
| Age (days) | 304 | 6 (IQR: 3-9) |
| Body temperature | 304 | 37.85 (IQR:37-38.2) |
| Oxygen Saturation % | 304 | 93 (IQR: 90-96) |
| Birth weight(Kg) | 304 | 2.9 (IQR: 2.2-3.2) |
| Admissions | ||
| BMC | 116 | 38.2 |
| Referral | 188 | 61.8 |
| Sex | ||
| Female | 112 | 36.8 |
| Male | 192 | 63.2 |
| Mode of Delivery | ||
| Cesarean section | 57 | 14.1 |
| Vaginal delivery | 247 | 85.9 |
| Age (days) | ||
| ≤7 | 111 | 36.5 |
| >7 | 193 | 63.5 |
| Birth Weight | ||
| < 2.5 kg | 94 | 30.1 |
| >2.5 kg | 210 | 69.1 |
| Gestation Age | ||
| Full Term | 233 | 76.6 |
| Premature | 71 | 23.4 |
| Body temperature | ||
| < 37.5 °C | 120 | 39.5 |
| ≥37.5 °C | 184 | 60.5 |
| Convulsion | ||
| No | 263 | 86.5 |
| Yes | 41 | 13.5 |
| Sucking | ||
| No | 164 | 53.9 |
| Yes | 140 | 46.1 |
| Skin pustule | ||
| No | 257 | 84.5 |
| Yes | 47 | 15.5 |
| Lethargy | ||
| No | 197 | 64.8 |
| Yes | 107 | 35.2 |
| Cyanosis | ||
| No | 251 | 82.6 |
| Yes | 53 | 17.4 |
| Jaundice | ||
| No | 220 | 72.4 |
| Yes | 84 | 27.6 |
| Umbilical discharge | ||
| No | 243 | 79.9 |
| Yes | 61 | 20.1 |
3.2. ESBL-PE neonatal sepsis, colonization, isolates and genotypes
Of 304 neonates, 32 (10.5%, 95%CI: 7.1–13.9) were infected by ESBL-PE. Neonatal ESBL-PE colonization occurred in 166 cases (54.6%; 95%CI: 49–60.1). In total, 86 (28.3%, 95%CI: 23–33.3) of mothers/guardians were carriers of ESBL-PE. Of the 32 neonates infected by ESBL-PE, Klebsiella pneumoniae was the most common pathogen (65.6%) (Table 2). This was also the case for neonate colonization, where Klebsiella pneumoniae isolates was the most common ESBL-PE (n = 112, 67.5%). On the other hand, of 86 mothers/guardians colonized by ESBL-PE, only 26 (30.2%) were colonized by ESBL-producing Klebsiella pneumoniae. Neonates were significantly more often infected/colonized by Klebsiella pneumoniae than guardians/mothers (P < 0.001). Other bacterial species isolated from the blood of the neonates were Enterococcus spp., Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Acinetobacter baumannii, Salmonella spp., Citrobacter spp., and Enterobacter spp. (Table 2). The proportion of the Klebsiella pneumoniae isolates (n = 26) resistant to ampicillin, amoxicillin/clavulanic, gentamicin, cefotaxime, ciprofloxacin, amikacin and meropenem was 100%, 80%, 84%, 80%, 40%, 60% and 0%, respectively. For the other Gram-negative bacteria (n = 19), the proportion of isolates resistant to ampicillin, gentamicin, cefotaxime, ciprofloxacin, amikacin, amoxicillin/clavulanic and meropenem was 94%, 80%, 79%, 56%, 28%, 70% and 10.5%, respectively. The two isolates that were resistant to meropenem were Acinetobacter baumanii. All of the seven Staphylococcus aureus were methicillin-sensitive, and all three Streptococcus pyogenes isolates were sensitive to penicillin.
Table 2.
Distribution of ESBL-producing isolates from blood, colonizing neonates and those colonizing mothers.
| Isolate | Blood (N = 60) | ESBL from Blood(N = 32) | ESBL Colonizing neonates(N = 178) | ESBL colonizing mothers(N = 86) |
|---|---|---|---|---|
| Klebsiella pneumoniae | 26 (43%) | 21 (65.6%) | 112 (67.5%) | 26 (30.2%) |
| Escherichia coli | 3 (5.0%) | – | 60 (36.1%) | 60 (69.8%) |
| Acinetobacter baumannii | 9 (16%) | 7 (21.9%) | 5 (3.0%) | – |
| Citrobacter spp. | 2 (3.3%) | 2 (6.3%) | – | – |
| Enterobacter spp. | 2 (3.3%) | 2 (6.3%) | 1 (0.6%) | – |
| Pseudomonas aeruginosa | 2 (3.3%) | NA | NA | NA |
| Salmonella spp. | 1 (1.6%) | NA | NA | NA |
| Enterococcus spp. | 5 (8.3%) | NA | NA | NA |
| Staphylococcus aureus | 7 (11.6%) | NA | NA | NA |
| Streptococcus pyogenes | 3 (5%) | NA | NA | NA |
NA = Not applicable.
3.3. Data from whole genome sequencing analysis
Out 296 phenotypically confirmed ESBL isolates, 53(17.9%) were subjected to whole genome sequencing, 38 (68%) were Klebsiella pneumonia of which 15 originated from blood cultures and 23 from neonatal swabs. Klebsiella pneumoniae with the sequence type (ST) ST45 and carrying the blaCTX-M-15 was predominant in blood- (7/15) and from swab- cultures (11/23). Other Klebsiella pneumoniae STs observed were ST348 (3), ST973, ST17, ST20, ST711, ST101, ST14, ST2268 and ST35 (Table 3). All Klebsiella pneumoniae isolates carried the blaCTX-M-15 allele. Quinolone resistance genes was detected in all isolates in various combinations with oqxA and oqxB present. Other quinolone resistance genes detected in Klebsiella pneumoniae isolates were qnrB2, aac(6′)Ib-cr and qnrS1. Apart from Klebsiella pneumoniae ST348, all other Klebsiella pneumoniae isolates harboured aminoglycoside resistance genes. The fosA gene, conferring resistance to fosfomycin, was detected in all Klebsiella pneumoniae isolates.
Table 3.
Sequence type, beta-lactamases genes, plasmid replicon type and pMLST of 38 ESBL-producing Klebsiella pneumoniae isolates from neonates.
| SNO | SPECIMEN | ST | Beta-lactamases | IncTy | pMLST |
|---|---|---|---|---|---|
| 1 | BLOOD | ST101 | blaCTX-M-15, blaSHV-1, blaTEM-1B | IncFIA, IncFIB, IncR | F-:A16:B- |
| 2 | BLOOD | ST348 | blaCTX-M-15, blaSHV-11, blaTEM-1B | IncFII, IncR | K5:A-:B- |
| 3 | BLOOD | ST348 | blaCTX-M-15, blaSHV-11 | IncFIB, IncR | K5:A-:B- |
| 4 | BLOOD | ST35 | blaCTX-M-15, blaSHV-33, blaTEM-1B, blaSCO-1 | IncFII, IncFIB, IncR | K12:A-:B- |
| 5 | BLOOD | ST35 | blaCTX-M-15, blaSHV-33, blaTEM-1B | IncFII, IncFIB, IncR | K12:A-:B- |
| 6 | BLOOD | ST35 | blaSCO-1, blaSHV-33, blaCTX-M-15, blaTEM-1B | IncFII, IncFIB, IncR | K12:A-:B- |
| 7 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1 | IncHI1B, IncFIB | unknown |
| 8 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1 | IncHI1B, IncFIB | unknown |
| 9 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncHI1B, IncFIB | unknown |
| 10 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1 | IncHI1B, IncFIB | unknown |
| 11 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1, blaOXA-1 | InFIB, IncHI1B | Unknown |
| 12 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1, blaOXA-1 | IncHI1B, IncFIB | unknown |
| 13 | BLOOD | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncHI1B, IncFIB | unknown |
| 14 | BLOOD | ST48 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncFII, IncFIB | K5:A-:B- |
| 15 | BLOOD | ST48 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncFII, IncFIB | K5:A-:B- |
| 16 | SWAB | ST101 | blaCTX-M-15, blaSHV-1, blaTEM-1B | IncFIA, IncFIB, IncR | F-:A16:B- |
| 17 | SWAB | ST14 | blaCTX-M-15, blaSHV-28, blaTEM-1B | InFII, IncR | K1:A-:B- |
| 18 | SWAB | ST17 | blaCTX-M-15, blaSHV-11, blaOXA-1 | IncHI1B, IncFIB | unknown |
| 19 | SWAB | ST20 | blaCTX-M-15, blaSHV-83, blaOXA-1 | IncHI1B, IncFIB | Unknown |
| 20 | SWAB | ST2268 | blaCTX-M-15, blaSHV-1, blaTEM-1B, | IncFIB | Unknown ST |
| 21 | SWAB | ST348 | blaCTX-M-15, blaSHV-11, blaTEM-1B | IncFII, IncR | K5:A-:B- |
| 22 | SWAB | ST348 | blaCTX-M-15, blaSHV-11, blaTEM-1B | IncFII | K5:A-:B- |
| 23 | SWAB | ST35 | blaCTX-M-15, blaSHV-33, blaTEM-1B, blaSCO-1 | IncFII, InFIB, IncR | K12:A-:B- |
| 24 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | InFIB,IncFII, IncFR, IncFIA | K1:A-:B- |
| 25 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | InFIB,IncFII, IncFR, IncFIA | K10:A10:B- |
| 26 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncFII, IncFIB, IncR | K5:A-:B- |
| 27 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncFII, IncFIB, IncR | K5:A-:B- |
| 28 | SWAB | ST45 | blaCTX-M-15, blaSHV-1 | IncHI1B, IncFIB | unknown |
| 29 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaOXA-1 | IncFII,IncHI1B, IncFIB, IncR | K5:A-:B- |
| 30 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncHI1B, IncFIB | Unknown |
| 31 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncHI1B, IncFIB | Unknown |
| 32 | SWAB | ST45 | blaCTX-M-15, blaTEM-1B, blaOXA-1 | IncFII,IncHI1B, IncFIB, IncR | K5:A-:B- |
| 33 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | InFIB,IncFII, IncFR, IncHI1B | K1:A-:B- |
| 34 | SWAB | ST45 | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | IncFII, IncFIB, IncR | K5:A-:B- |
| 35 | SWAB | ST711 | blaCTX-M-15, blaSHV-83, blaTEM-1B, blaOXA-1 | No inc type detected | |
| 36 | SWAB | ST873 | blaCTX-M-15, blaSHV-27, blaTEM-1B | IncFII, IncFIB | K1:A-:B- |
| 37 | SWAB | Unknown | blaCTX-M-15, blaSHV-1, blaTEM-1B, blaOXA-1 | InFIB,IncFII, IncFR, IncHI1A | K5:A-:B- |
| 38 | SWAB | Unknown | blaCTX-M-15, blaTEM-1B, blaOXA-1 | ? |
ST: sequence type, Inc; Incompatibility, pMLST; plasmid multi locus sequence type.
For the 10 Escherichia coli genomes sequenced, ST648 (n = 5) was commonly detected. Other STs detected included ST131 (n = 2), ST 617(n = 1), ST405 (n = 1) and an Escherichia coli isolate with a novel ST designation. All E.coli strains harboured blaCTX-M-15 and aac(6′)Ib-cr genes except for the ST617 isolate which only harboured a blaCTX-M-15 resistance allele.
An Enterobacter cloacae from a swab was typed as ST93, while another isolate obtained from blood was classified as ST116. Both Enterobacter cloacae isolates harboured IncHI2 plasmids were assigned to the plasmid (p) MLST ST1. Resistance genes detected in Enterobacter cloacae included blaACT-7, blaTEM-1B, blaOXA-1 and blaCTX-M-15. The ST93 strain did not harbor a qnrB1 gene but carried sul2, dfrA1 and aadA2 genes that were not present in ST116. The three Acinetobacter baumannii isolates were members of ST1470 and ST405 (n = 2) and carried carbapenemase resistance genes blaNDM-1, blaOXA-58, blaOXA-69, blaCARB-8 and blaOXA-69 (Table 4). IncF plasmids of pMLST K5 and FI were predominant in Klebsiella pneumoniae strains and Escherichia coli respectively, while for Enterobacter spp. the predominant plasmid was IncHI2.
Table 4.
Sequence type, beta-lactamases genes, plasmid replicon type and pMLST of 15 ESBL-producing isolates from neonates.
| SNO | SPECIMEN | Isolate | ST | Beta-lactamases | Inc type | pMLST |
|---|---|---|---|---|---|---|
| 1 | SWAB | A. baumanii | ST405 | blaADC-25, blaOXA-69 | NIL | |
| 2 | SWAB | A. baumanii | ST1470 | blaNDM-1, blaOXA-58 | NIL | |
| 3 | SWAB | A. baumanii | ST405 | blaOXA-69, blaCARB-8,blaADC-25 | NIL | |
| 4 | BLOOD | E. cloacae | ST116 | blaCTX-M-15, blaTEM-1B, blaOXA-1, blaACT | IncHI2A, IncHI2 | ST-1 |
| 5 | SWAB | E. cloacae | ST93 | blaCTX-M-15, blaTEM-1B, blaOXA-1, blaACT-7 | IncHI2A, IncHI2 | ST-1 |
| 6 | SWAB | E. coli | ST131 | blaCTX-M-15 | IncFIA, IncFIB, IncFII | F1:A1:B16 |
| 7 | SWAB | E. coli | ST131 | blaCTX-M-15blaOXA-1 | IncFIA, IncFIB, IncFII | F1:A1:B16 |
| 8 | SWAB | E. coli | ST405 | blaCTX-M-15, blaTEM-1B, blaOXA-1 | IncFII, IncFIA, IncFIB | F1:A1:B16 |
| 9 | SWAB | E. coli | ST617 | blaCTX-M-15 | IncFII, IncFIB, IncFIA | F1:A2:B33 |
| 10 | SWAB | E.coli | ST648 | blaCTX-M-15, blaTEM-1B, blaOXA-1 | IncI2,IncFIA, IncFIB, IncFII | F1:A6:B20 |
| 11 | SWAB | E. coli | ST648 | blaCTX-M-15, blaTEM-1B, blaOXA-1 | IncFII, IncI1, IncFIA | F1:A16:B- |
| 12 | SWAB | E. coli | ST648 | blaCTX-M-15blaTEM-1B, blaOXA-1 | IncI2, Col156, IncFIA, IncFIB, IncFII | F1:A6:B20 |
| 13 | SWAB | E. coli | ST648 | blaCTX-M-15blaTEM-1B, blaOXA-1 | IncQ1, IncFIA, IncY, IncFIB | F-:A1:B32 |
| 14 | SWAB | E. coli | ST648 | blaCTX-M-15blaTEM-1B, blaOXA-1 | IncQ1, IncFIA, IncY, IncFIB | F-:A1:B32 |
| 15 | SWAB | E. coli | Unknown | blaCTX-M-15blaTEM-1B, blaOXA-1 | IncI1, IncQ, IncFIA, IncFIB, Col(BS512) | F1:A1:B49, IncI1=ST 49 |
E. coli; Escherichia coli, E. cloacae; Enterobacter cloacae, A. baumanii; Acinetobacter baumanii, ST: sequence type, Inc; Incompatibility, pMLST; plasmid multi locus sequence type.
3.4. Virulence genes of Klebsiella pneumoniae
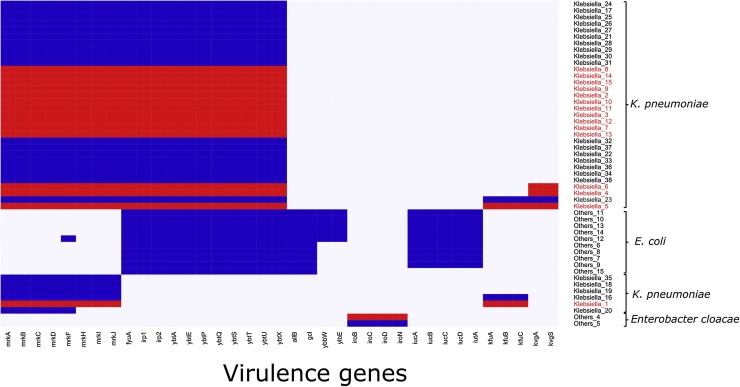
Klebsiella pneumoniae isolates harboured up to four different virulence-associated determinants (Fig. 1). The yersiniabactin operon (ybtAEPQSTUX, irp1, irp2, fyuA) a metallobactin involved in iron acquisition was detected in 32/38 (84.2%) of the examined Klebsiella pneumoniae isolates. Other isolates harbouring yet another operon involved in iron acquisition (kfuABC), which was detected only in 4/38 isolates. Genes associated with pilus formation (mrkABCDFHIJ) were present in all but one of the (42i) Klebsiella pneumoniae examined. Four isolates harboured the kvgAS two-component system involved in sensing iron-limiting conditions and free radical stress.
Fig. 1.
Predicted virulence factors of sequenced ESBL-PE Enterobacteriaceae. Legend: Blue, gene present; grey, gene absent (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
All Klebsiella pneumoniae isolates of sequence types ST14, ST348, ST35, ST45, ST48 and ST873 (n = 30) harboured a combination of yersiniabactin and the pili genes (mrkABCDFHIJ), regardless of whether they had been isolated from blood or umbilical rectal swabs (Supplementary Table S1). The aerobactin genes (iucABCD, iutA), also required for siderophore-based iron uptake, were detected only in Escherichia coli (Fig. 1).
3.5. Transmission of Klebsiella pneumoniae. Isolates to the blood
Twelve pairs of ESBL-PE between neonates blood and neonate swab were detected. In eight neonates, blood culture and neonatal swab isolates depicted identical sequence types. Single nucleotide polymorphisms were identified between these pairs to detect close relationship that indicates a transmission to the blood. Four pairs (50%) depicted very low SNP counts (<20 SNPs) indicating the isolates are of clonal origin. In the other pairs, higher SNP counts were identified, indicating some relationship (Table 5).
Table 5.
SNP analysis of the blood/swab pairs.
| ID NO | BLOOD | ST | Replicon type | ID NO | SWAB | ST | Replicon type | SNP count to blood culture isolate |
|---|---|---|---|---|---|---|---|---|
| 5301 | Kleb.pneu | 45 | IncHI1B, IncFIB | 140/016.ii | E.coli | 131 | IncFIA, IncFIB, IncFII | Other species, not performed |
| 6321 | Kleb.pneu | 48 | IncFII, IncFIB | 6273 | Kleb.pneu | 48 | NA | 13 |
| 6331 | Kleb.pneu | 101 | IncFIA, IncFIB, IncR | 149/016.11 | Kleb.pneu | 101 | IncFIA, IncFIB, IncR | 127 |
| 6353 | Kleb.pneu | 35 | IncFII, IncFIB, IncR | 036/016 | Kleb.pneu | 35 | IncFII, InFIB, IncR | 17 |
| 6854 | Kleb.pneu | 45 | IncHI1B, IncFIB | 153/016 | Kleb.pneu | 101 | InFIB, IncFII, IncFR, IncHI1A | Other sequence type, not performed |
| 6875 | Kleb.pneu | 348 | IncFII, IncR | 47 | Kleb.pneu | 348 | IncFII, IncR | 34 |
| 6881 | Kleb.pneu | 45 | IncHI1B, IncFIB | 154/016 | Kleb.pneu | 348 | IncFII | Other sequence type, not performed |
| 6895 | Kleb.pneu | 45 | InFIB, IncHI1B | 66 | Kleb.pneu | 45 | IncFII,IncHI1B, IncFIB, IncR | 72 |
| 7421 | Kleb.pneu | 45 | IncHI1B, IncFIB | 31 | Kleb.pneu | 45 | IncHI1B, IncFIB | 75 |
| 8824 | Kleb.pneu | 45 | IncHI1B, IncFIB | 58 | Kleb.pneu | 45 | IncHI1B, IncFIB | 6 |
| 8900 | Kleb.pneu | 45 | IncHI1B, IncFIB | 92 | Kleb.pneu | 45 | IncHI1B, IncFIB | 4 |
Kle. pneu: Klebsiella pneumoniae.
3.6. Predictors of ESBL-PE neonatal sepsis
We investigated different factors to establish whether they could predict ESBL-PE neonatal sepsis. The median age of neonates with ESBL-PE neonatal sepsis was 6 days (IQR: 4–8) while that of ESBL-negative neonatal sepsis was 5 days (IQR: 5–10 days), p = 0.139. It was observed that of 39 neonates admitted at NICU, nine (23.1%) had ESBL-PE neonatal sepsis compared to 23 (8.7%) of 269 neonates admitted at premature unit (p = 0.009). Furthermore, it was observed that of 166 neonates colonized by ESBL-PE, 26 (15.7%) were found to be infected by ESBL-PE as compared to only six (4.35%) neonates with no ESBL-PE colonization (p = 0.003). Using multivariable logistic regression analysis, factors that predicted neonatal ESBL-PE sepsis included: admission at NICU (p = 0.021), positive ESBL-PE colonization of the mother/guardian (p = 0.009) and positive neonate ESBL-PE colonization (p = 0.021) (Table 6).
Table 6.
Factors associated with ESBL-PE neonatal sepsis among 304 neonates with clinical sepsis at BMC.
| Variable | ESBL Positive | ESBL Negative | OR [95% CI] | p-value | OR [95% CI] | p-value |
|---|---|---|---|---|---|---|
| Median/ n (%) | Median/ n (%) | |||||
| Age in days | 6 IQR (4-8) | 5 IQR (3-10) | 0.94 [0.88 - 1.02] | 0.139 | 0.93 [0.87-1.02] | 0.117 |
| Sex | ||||||
| Female | 17 (8.9) | 175 (91.15) | 1 | |||
| Male | 15 (13.4) | 97 (86.6) | 1.59 [0.76 - 3.33] | 0.217 | 1.5 [0.69-3.4] | 0.286 |
| Gestation age | 38 IQR (36-40) | 39 IQR (37-40) | 1.33 [0.58-3.01] | 0.501 | ||
| Body weight | 2.7 IQR (2.3-3) | 2.9 IQR (2.1-3.3) | 0.79 [0.48-1.29] | 0.348 | ||
| Admission | ||||||
| BMC | 15 (12.9) | 101 (87.1) | 1 | |||
| Referral | 17 (9.04) | 171 (90.9) | 0.7 [0.3-1.4] | 0.286 | ||
| Poor feeding | ||||||
| No | 19 (11.6) | 145 (88.4) | 1 | |||
| Yes | 13 (9.3) | 127 (90.7) | 1.28 [0.61 - 2.69] | 0.516 | ||
| Convulsions | ||||||
| No | 27 (10.3) | 236(89.7) | 1 | |||
| Yes | 5 (12.2) | 36 (87.8) | 1.21 [0.44 - 3.36] | 0.709 | ||
| Skin pustule | ||||||
| No | 27 (10.5) | 230 (89.5) | 1 | |||
| Yes | 5 (10.6) | 42(89.4) | 1.01 [0.36 - 2.78] | 0.978 | ||
| Lethargy | ||||||
| No | 19 (9.6) | 178 (90.3) | 1 | |||
| Yes | 13 (12.2) | 94 (87.9) | 1.3 [0.61 – 2.74] | 0.498 | ||
| Antibiotic use | ||||||
| No | 18 (8.11) | 204 (91.9) | 1 | |||
| Yes | 14 (17.1) | 68 (82.9) | 2.33 [1.1-4.94] | 0.027 | 1.84 [0.82-4.12] | 0.139 |
| Admission | ||||||
| PREM | 23 (8.7) | 242 (91.3) | 1 | |||
| NICU | 9 (23.1) | 30 (79.9 | 3.16 [1.34-7.45] | 0.009 | 3.02 [1.19-7.69] | 0.021 |
| Swab ESBL | ||||||
| No | 6 (4.35) | 132 (95.7) | 1 | |||
| Yes | 26 (15.7) | 140 (84.3) | 4.09 [1.62-10.24] | 0.003 | 3.08 [1.19-7.99] | 0.021 |
| Stool ESBL | ||||||
| No | 15 (6.9) | 203 (93.1) | 1 | |||
| Yes | 17 (19.8) | 69 (80.23) | 3.33 [1.58-7.03] | 0.002 | 2.87 [1.3-6.33] | 0.009 |
| Maternal fever | ||||||
| No | 10 (9.5) | 95 (90.5) | 1 | |||
| Yes | 22 (11.1) | 177 (88.9) | 1.18 [0.53-2.59] | 0.679 | ||
| PROM | ||||||
| No | 22 (10.6) | 186 (89.4) | 1 | |||
| Yes | 10 (10.4) | 86 (89.6) | 0.98 [0.44-2.17] | 0.966 | ||
| Maternal antibiotics | ||||||
| No | 16 (10.1) | 143 (89.9) | 1 | |||
| Yes | 16 (11) | 129 (88.9) | 1.11 [0.53-2.31] | 0.783 |
3.7. Predictors of ESBL-PE neonatal colonization
A total of 166 (54.6%; 95%CI: 49–60.1) neonates were colonized by ESBL-PE. Following univariate analysis, out of the 82 neonates with history of antibiotic use prior being admitted at PREM/NICU, 54(65.9%) were found to be colonized by ESBL-PE as compared to 112 (50.5%) of those without history of antibiotic use (P = 0.017). It was further noted that neonates born from mothers who were colonized by ESBL-PE were more likely to be colonized than those born from mothers with no ESBL-PE colonization (68.6% vs. 49.1%, OR: 2.27, 95%CI 1.34–3.84, P = 0.002). Using multivariable logistic regression analysis, predictors of neonatal ESBL colonization were history of antibiotic use (P = 0.048) and positive ESBL-PE colonization of the mother (P = 0.005) (Table 7).
Table 7.
Factors associated with neonatal ESBL-PE colonization among 304 neonates with clinical sepsis at BMC.
| Variable | ESBL Positive | ESBL Negative | OR [95% CI] | p-value | OR [95% CI] | p-value |
|---|---|---|---|---|---|---|
| Median/ n (%) | Median/ n (%) | |||||
| Age in days | 6 IQR (3-10) | 5 IQR (3-9) | 1.01 [0.98 - 1.05] | 0.529 | ||
| Sex | ||||||
| Female | 60 (53.6) | 52 (46.4) | 1 | |||
| Male | 106 (55.2) | 86 (44.8) | 0.94 [0.59 - 1.49] | 0.782 | ||
| Admission | ||||||
| BMC | 60 (51.7) | 56 (48.3) | 1 | |||
| Referral | 106 (56.4) | 82 (43.6) | 1.2 [0.8-1.9] | 0.428 | ||
| Birth weight (kg) | 2.9 IQR (2.3-3.3) | 2.8 IQR (2.1-3.2) | 1.07 [0.79-1.44] | 0.654 | ||
| Body temperature | 37.85 IQR (37-38) | 37.85 IQR (37-38.3) | 0.96 [0.81-1.15] | 0.662 | ||
| Oxygen Sat (%) | 93 IQR (91-96) | 93 IQR (88-96) | 1.02 [0.99-1.05] | 0.211 | 1.03 [0.99-1.06] | 0.113 |
| Skin pustule | ||||||
| No | 140 (54.5) | 117 (45.5) | 1 | |||
| Yes | 26 (55.3) | 21 (44.7) | 1.03 [0.55 - 1.93] | 0.915 | ||
| Umbilical discharge | ||||||
| No | 132 (54.3) | 111 (45.7) | 1 | |||
| Yes | 34 (55.7) | 27 (44.3) | 1.06 [0.61-1.86] | 0.843 | ||
| History of antibiotic-baby | ||||||
| No | 112 (50.5) | 110 (49.6) | 1 | |||
| Yes | 54 (65.9) | 28 (34.2) | 1.89 [1.12-3.21] | 0.017 | 1.73 [1-2.97] | 0.048 |
| Admission | ||||||
| PREM | 141 (53.2) | 124 (46.8) | 1 | |||
| NICU | 25 (64.1) | 14 (35.9) | 1.57 [0.78-3.15] | 0.205 | 1.89 [0.9-3.95] | 0.092 |
| Maternal fever | ||||||
| No | 56 (53.3) | 49 (46.7) | 1 | |||
| Yes | 110 (55.3) | 89 (44.7) | 1.08 [0.67-1.73] | 0.746 | ||
| PROM | ||||||
| No | 116 (56) | 91 (43.9) | 1 | |||
| Yes | 50 (51.6) | 47 (48.5) | 0.83 [0.51-1.35] | 0.464 | ||
| Maternal antibiotics | ||||||
| No | 82 (51.6) | 77 (48.4) | 1 | |||
| Yes | 84 (57.9) | 61 (42.1) | 1.29 [0.82-2.03] | 0.266 | 1.08 [0.67-1.75] | 0.739 |
| Stool ESBL | ||||||
| No | 107 (49.1) | 111 (50.9) | ||||
| Yes | 59 (68.6) | 27 (31.4) | 2.27 [1.34-3.84] | 0.002 | 2.19 [1.26-3.79] | 0.005 |
3.8. Predictors of the outcome
Out of 304 neonates, 55 (18.1%, 95% CI 13.4–22.4) died. The median oxygen saturation of neonates who died was 92% (IQR 82–95), while that of neonates who were discharged alive was 93%, IQR: 91–96, (P = 0.003). The mortality rate was significantly higher in neonates infected by ESBL-PE than those infected by other bacteria and/or negative cultures (34.4% vs. 16.2%, p = 0.014). On multivariable logistic regression analysis, predictors of death included being referred from other centres (p = 0.004), being admitted at NICU (p = 0.015) and positive ESBL-PE neonatal sepsis (p = 0.011) Table 8.
Table 8.
Factors associated with outcome among 304 neonates admitted with clinical sepsis at BMC.
| Variable | Death | Discharge Alive | OR [95% CI] | p-value | OR [95% CI] | p-value |
|---|---|---|---|---|---|---|
| Median/ n (%) | Median/ n (%) | |||||
| Age in days | 7 IQR (4-10) | 5 IQR (3-9) | 1.03 [0.98 - 1.07] | 0.229 | 1.04[0.99-1.09] | 0.103 |
| Gestation age(weeks) | 38 IQR (35-40) | 39 IQR (37-40) | 0.92[0.85-0.99] | 0.040 | 0.95 [0.87-1.0] | 0.312 |
| Oxygen Sat (%) | 92 IQR (82-95) | 93 IQR (91-96) | 0.95[0.92-0.98] | 0.003 | 1.9 [0.9-3.7] | 0.083 |
| Body weight | 2.8 IQR (2-3.2) | 2.9 IQR (2.3-3.2) | 0.78[0.52-1.14] | 0.188 | ||
| Admission | ||||||
| BMC | 12(10.3) | 104(89.7) | 1 | |||
| Referral | 43(22.9) | 145(77.1) | 2.6[1.3-5.1] | 0.007 | 2.9 [1.4-6.1] | 0.004 |
| Poor feeding | ||||||
| No | 31 (18.9) | 133 (81.1) | 1 | |||
| Yes | 24 (17.1) | 116 (82.9) | 1.13[0.62 - 2.03] | 0.691 | ||
| Convulsions | ||||||
| No | 52 (19.8) | 211(80.2) | 1 | |||
| Yes | 3 (7.3) | 38 (92.7) | 0.32 [0.09 – 1.08] | 0.066 | 0.3[0.1-1.1] | 0.063 |
| Lethargy | ||||||
| No | 33 (16.8) | 164 (83.3) | 1 | |||
| Yes | 22 (20.6) | 85(79.4) | 1.29 [0.71 – 2.34] | 0.411 | ||
| Jaundice | ||||||
| No | 39 (17.7) | 181 (82.3) | 1 | |||
| Yes | 16 (19.1) | 68 (80.9) | 1.09 [0.57 - 2.08] | 0.789 | ||
| Ward | ||||||
| PREM | 40(15.1) | 225(84.9) | 1 | |||
| NICU | 15(38.5) | 24(61.5) | 3.5[1.69-7.28] | 0.001 | 2.7[1.2-6.0] | 0.015 |
| Blood culture | ||||||
| Negative | 34(15.1) | 191(84.9) | 1 | |||
| Positive | 21(26.6) | 58(73.4) | 2.03[1.09-3.77] | 0.024 | ||
| Bacteria/Gram reaction | ||||||
| Negative culture | 37(15.2) | 207(84.8) | 1 | |||
| Gram positive | 5(35.7) | 9(64.3) | 3.12[0.98-9.79] | 0.053 | ||
| Gram negative | 13(28.3) | 33(71.7) | 2.2 [1.06-4.58] | 0.034 | ||
| ESBL-PE Sepsis | ||||||
| No | 44(16.2) | 228(83.8) | 1 | |||
| Yes | 11(34.4) | 21(65.63) | 2.71[1.22-6.03] | 0.014 | 3.2[1.3-7.7] | 0.011 |
| Swab ESBL | ||||||
| No | 21(15.2) | 117(84.8) | 1 | |||
| Yes | 34(20.5) | 132(79.5) | 1.44[0.79-2.61] | 0.237 |
NB: Blood culture and Bacteria/gram reaction were not subjected on multivariable analysis due to collinearity with ESBL-PE sepsis.
On survival analysis, neonates infected with ESBL-PE (HR: 2.2, 95%CI: 1.13–4.26, P = 0.019) and neonates admitted at NICU (HR: 3.07, 95%CI 1.69–5.56, P < 0.001) had significantly higher mortality rates than their counterpart groups. By multivariate cox regression analysis, factors found to predict high rates of mortality were: neonates infected with ESBL-PE (HR:2.4, 95%CI:1.2–4.8, p = 0.014), neonates admitted at NICU (HR:2.2, 95%CI:1.2.2–4.4, P = 0.018), increase in age (HR:1.05, 95%CI:1.01–1.1, P = 0.021) and those referred from other centres (HR:2.4, 95%CI:1.3–4.6, p = 0.008).
4. Discussion
The current study has demonstrated that ESBL-PE contributed to half of the blood culture isolates obtained from neonates in a tertiary hospital in Mwanza, Tanzania. The overall prevalence of the laboratory confirmed sepsis (20%) in the present study is lower than the 50% reported previously from the same centre (Kayange et al., 2010). The difference is probably the result of prophylactic use of broad-spectrum antibiotics in the current cohort. In addition, the proven occurrence of sepsis is also significantly lower than that observed in Mulago National hospital, Kampala Uganda (Mugalu et al., 2006). However, the level of sepsis in the current study is comparable to that observed in Muhimbili national hospital neonatal unit (Mhada et al., 2012).
In low-income countries the major burden of sepsis is borne by children, who are 18 times more likely to die before the age of five than those children in higher-income countries, with infections being a significant source of this disparity (Chan and Lake, 2012). We found that admission to NICU, colonization of neonates, and their mothers/guardians with ESBL-PE were independent predictors of ESBL-PE neonatal sepsis. Neonates infected with ESBL-PE had significantly higher mortality and the majority had severe illness requiring NICU admission. The practice in the neonatal unit/NICU considers carbapenem treatment as the last resort antibiotic. The sad fact of substantial mortality in neonates suffering from blood stream infection due to bacteria with ESBL enzymes that has been demonstrated in the study may very well be a consequence of the unavailability of antimicrobial agents active against those bacteria or due to delay initiation of the appropriate therapy. The slow turnaround times of current blood culture tests lead to a considerable delay in initiating of the appropriate antibiotic treatment and thereby promotes mortality among neonates infected with ESBL-PE. In the current study, the preliminary blood culture results were only obtained after 48 h, with final results for the positive culture given only after 72 h, with incubation for 7 days to confirm negative culture in the majority of cases. The introduction of the semi-automated assays will improve turnaround time of the blood culture and significantly save lives of neonates infected with ESBL-PE. Clinicians should initiate appropriate treatment to high-risk neonates especially those admitted in NICU.
Despite being a cross-sectional study, the study has highlighted important factors that might predict ESBL-PE neonatal sepsis. Previous studies (Kritsotakis et al., 2011; Le et al., 2008; Mshana et al., 2016; Sandiumenge et al., 2006) have demonstrated that exposure to antibiotics can lead to infections/colonization with ESBL-PE, this was also the case in the present study.
As observed in previous studies (Chacha et al., 2014; Kayange et al., 2010), Klebsiella pneumoniae was the predominant species isolated. However, unlike a previous study where ST14 was predominant (Mshana et al., 2013), in the current study ST45 has been found to be the most common clone. We conclude that the majority of ESBL-PE sepsis are endogenous since isolates of Klebsiella pneumoniae ST45 was both a predominant colonizer of the neonate microflora as well as causing infections at the same time. These data show that most ESBL-PE neonatal sepsis within a given time period might be caused by clonally related isolates as observed in the current study as evidenced by the fact that 50% of paired isolates (blood: neonatal swab) with similar sequence types were clonal. It should be noted that, these isolates could also originate from guardians/mothers, environment or contaminated hands of healthcare workers, underscoring a follow-up exploration study for other sources of neonates colonization. It should be emphasized that hand hygiene can reduce healthcare-associated infections, including those caused by ESBL-PE (Allegranzi and Pittet, 2009). Interestingly, ST45 isolates either colonizing the neonates or associated with neonate infections, harboured different plasmid replicon types and indicate that these strains might have either evolved separately or promiscuously acquired plasmids from different sources. Thus, blood isolates harboured IncFIB and IncHI1 plasmids of hitherto unclassified pMLST types, while ST45 strains colonizing the neonates had IncFII, IncFIB and IncR plasmids.
The majority of Klebsiella pneumoniae isolates harboured genes for siderophores involved in sequestering iron from the environment. The presence of siderophores have been linked to community infections (Holt et al., 2015) emphasizing the broad occurrence of ESBL-PE in our setting. Surprisingly only two Escherichia coli strains, both of which were not ESBL, were isolated from the blood despite the fact that about 70% and 36% of guardians/mothers and neonates were colonized by ESBL-producing Escherichia coli. The intrinsic ability of Klebsiella pneumoniae to survive in the environment and its ability to survive in the serum, through siderophores-dependent iron acquisition, could partially account for its transmission to neonates and development of blood stream infections (Podschun and Ullmann, 1998). Indeed in the majority of the isolates sequenced, virulence factors predisposing to infections were detected.
As in previous studies (Bhutta et al., 2010; Mshana et al., 2011, 2016; Seni et al., 2016) in the hospital and community among humans and animals, 90% of isolates were found to carry blaCTX-M-15. Acinetobacter baumannii carrying a blaNDM-1 gene was detected as a colonizing isolate. This is worrisome because the inappropriate use of meropenem could promote selective growth and hence increase challenges in the management of the neonatal sepsis in low-income countries. Furthermore the blaNDM-1 might translocate to conjugative plasmids and be transmitted to other Enterobacteriaceae through horizontal genetic transfer (Struelens et al., 2010).
The limitations of this study include the following: Because of the low number of culture-positive blood samples we decided to compare those with ESBL-PE neonatal sepsis and other neonates assuming that all neonates were, from the onset, equally at risk of getting ESBL-PE neonatal sepsis. Being a single center, the results may not be generalizable. A prospective multicenter cohort study would have been more suitable. Another limitation was the lack of colonization and mortality data from neonates without signs and symptoms of neonatal sepsis for comparative analysis. Finally, not all isolates underwent molecular characterization including those colonizing the mother/guardians. Future studies should consider whole genome sequencing of larger numbers of isolates from neonates, guardian/mothers and health care workers to reveal dynamics of transmission and other source of ESBL-PE.
5. Conclusion and recommendation
The majority of septic neonates has been shown to be colonized by ESBL producers and among those colonized with strains secreting ESBL enzymes; the majority was shown to be colonized by ESBL-producing strains of K. pneumoniae. ESBL-producing Klebsiella pneumoniae was the most frequent isolated from blood of neonates at BMC neonatal unit. The virulent isolates of the Klebsiella pneumoniae ST45 carrying blaCTX-M-15 were predominantly infecting and colonizing neonates. Maternal and neonatal ESBL-PE colonization are important factors predicting ESBL neonatal sepsis. Our study demonstrates that neonatal mortality is significantly high in neonates infected with ESBL-PE. There is a need to improve and emphasize on infection control interventions and antimicrobial stewardship because these are cheap inexpensive strategies to control the increase of multi-resistant gram-negative bacteria. Furthermore, rapid diagnostic tests to detect ESBL-PE from neonates’ blood are highly needed in the low-income countries in order to reduce the mortality with ESBL-PE neonatal sepsis. In addition, there is a need to improve turnaround time of blood culture assays for the neonatal units in order to guide antibiotic treatment.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors acknowledge the support provided by Department of Pediatric and Child Health-BMC, Technical support from Mr. Vitus Silago and we appreciate the technical assistance by Christina Gerstmann and Natalia Lest at the Institute of Medical Microbiology in Giessen.
This study was supported by a grant from the Wellcome Trust (WT087546MA) to SACIDS and also by grants from the Bundesministerium fuer Bildung und Forschung (BMBF, Germany) within the German Center for Infection research (DZIF/grant number 8000 701–3 [HZI] to TC and CI and TI06.001/8032808811 to TC).
References
- Allegranzi B., Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect. 2009;73:305–315. doi: 10.1016/j.jhin.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta Z.A., Chopra M., Axelson H., Berman P., Boerma T., Bryce J., Bustreo F., Cavagnero E., Cometto G., Daelmans B. Countdown to 2015 decade report (2000–10): taking stock of maternal, newborn, and child survival. The Lancet. 2010;375:2032–2044. doi: 10.1016/S0140-6736(10)60678-2. [DOI] [PubMed] [Google Scholar]
- Blomberg B., Jureen R., Manji K.P., Tamim B.S., Mwakagile D.S., Urassa W.K., Fataki M., Msangi V., Tellevik M.G., Maselle S.Y. High rate of fatal cases of pediatric septicemia caused by gram-negative bacteria with extended-spectrum beta-lactamases in Dar es Salaam, Tanzania. J. Clin. Microbiol. 2005;43:745–749. doi: 10.1128/JCM.43.2.745-749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carattoli A., Zankari E., García-Fernández A., Larsen M.V., Lund O., Villa L., Aarestrup F.M., Hasman H. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob. Agents Chemother. 2014;58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacha F., Mirambo M.M., Mushi M.F., Kayange N., Zuechner A., Kidenya B.R., Mshana S.E. Utility of qualitative C-reactive protein assay and white blood cells counts in the diagnosis of neonatal septicaemia at Bugando Medical Centre, Tanzania. BMC Pediatr. 2014;14:248. doi: 10.1186/1471-2431-14-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M., Lake A. Towards ending preventable child deaths. The Lancet. 2012;379:2119. doi: 10.1016/S0140-6736(12)60908-8. [DOI] [PubMed] [Google Scholar]
- Christopher A., Mshana S.E., Kidenya B.R., Hokororo A., Morona D. Bacteremia and resistant gram-negative pathogens among under-fives in Tanzania. Ital. J. Pediatr. 2013;39:27. doi: 10.1186/1824-7288-39-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI . Clinical and Laboratory Standards Institute; Wayne, PA: 2015. Performance Standards for Antimicrobial Susceptibility Testing; Twentfith Information Supplement CLSI Document M100-S25. [Google Scholar]
- D’Andrea M.M., Arena F., Pallecchi L., Rossolini G.M. CTX-M-type β-lactamases: a successful story of antibiotic resistance. Int. J. Med. Microbiol. 2013;303:305–317. doi: 10.1016/j.ijmm.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Ghotaslou R., Ghorashi Z., Nahaei M. Klebsiella pneumoniae in neonatal sepsis: a 3-year-study in the pediatric Hospital of Tabriz Iran. Japan. J. Infect. Dis. 2007;60:126. [PubMed] [Google Scholar]
- Holt K.E., Wertheim H., Zadoks R.N., Baker S., Whitehouse C.A., Dance D., Jenney A., Connor T.R., Hsu L.Y., Severin J. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc. Natl. Acad. Sci. 2015;112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayange N., Kamugisha E., Mwizamholya D.L., Jeremiah S., Mshana S.E. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatr. 2010;10:39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kish L. Survey sampling New York. J. Wiley Sons. 1965;643:16. [Google Scholar]
- Kritsotakis E.I., Tsioutis C., Roumbelaki M., Christidou A., Gikas A. Antibiotic use and the risk of carbapenem-resistant extended-spectrum-β-lactamase-producing Klebsiella pneumoniae infection in hospitalized patients: results of a double case–control study. J. Antimicrob. Chemother. 2011;66:1383–1391. doi: 10.1093/jac/dkr116. [DOI] [PubMed] [Google Scholar]
- Larsen M.V., Cosentino S., Rasmussen S., Friis C., Hasman H., Marvig R.L., Jelsbak L., Pontén T.S., Ussery D.W., Aarestrup F.M. Multilocus sequence typing of total genome sequenced bacteria. J. Clin. Microbial. 2012;06094-06011 doi: 10.1128/JCM.06094-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Nguyen T., Okamoto M., McKamy S., Lieberman J.M. Impact of empiric antibiotic use on development of infections caused by extended-spectrum β-lactamase bacteria in a neonatal intensive care unit. Pediatr. Infect. Dis. J. 2008;27:314–318. doi: 10.1097/INF.0b013e3181606850. [DOI] [PubMed] [Google Scholar]
- Li H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics. 2011;27:2987–2993. doi: 10.1093/bioinformatics/btr509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis P., Mulholland E., Harallel F., Gove S. Clinical prediction of serious bacterial infections in young infants in developing countries. Pediatr. Infect. Dis. J. 1999;18:S23–S31. doi: 10.1097/00006454-199910001-00005. [DOI] [PubMed] [Google Scholar]
- Mhada T.V., Fredrick F., Matee M.I., Massawe A. Neonatal sepsis at Muhimbili National Hospital, Dar es Salaam, Tanzania; aetiology, antimicrobial sensitivity pattern and clinical outcome. BMC Public Health. 2012;12:904. doi: 10.1186/1471-2458-12-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshana S.E., Kamugisha E., Mirambo M., Chakraborty T., Lyamuya E.F. Prevalence of multiresistant gram-negative organisms in a tertiary hospital in Mwanza, Tanzania. BMC Res. Notes. 2009;2:49. doi: 10.1186/1756-0500-2-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshana S., Imirzalioglu C., Hain T., Domann E., Lyamuya E., Chakraborty T. Multiple ST clonal complexes, with a predominance of ST131, of Escherichia coli harbouring blaCTX‐M-15 in a tertiary hospital in Tanzania. Clin. Microbiol. Infect. 2011;17:1279–1282. doi: 10.1111/j.1469-0691.2011.03518.x. [DOI] [PubMed] [Google Scholar]
- Mshana S.E., Hain T., Domann E., Lyamuya E.F., Chakraborty T., Imirzalioglu C. Predominance of Klebsiella pneumoniae ST14 carrying CTX-M-15 causing neonatal sepsis in Tanzania. BMC Infect. Dis. 2013;13:466. doi: 10.1186/1471-2334-13-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshana S.E., Falgenhauer L., Mirambo M.M., Mushi M.F., Moremi N., Julius R., Seni J., Imirzalioglu C., Mecky M., Chakraborty T. Predictors of blaCTX-M-15 in varieties of Escherichia coli genotypes from humans in community settings in Mwanza, Tanzania. BMC Infect. Dis. 2016 doi: 10.1186/s12879-016-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugalu J., Nakakeeto M., Kiguli S., Kaddu–Mulindwa D.H. Aetiology, risk factors and immediate outcome of bacteriologically confirmed neonatal septicaemia in Mulago hospital, Uganda. Afr. Health Sci. 2006;6:120–126. doi: 10.5555/afhs.2006.6.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E., Kayega J., Seni J., Mushi M.F., Kidenya B.R., Hokororo A., Zuechner A., Kihunrwa A., Mshana S.E. Evaluation of existence and transmission of extended spectrum beta lactamase producing bacteria from post-delivery women to neonates at Bugando Medical Center, Mwanza-Tanzania. BMC Res. Notes. 2014;7:279. doi: 10.1186/1756-0500-7-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.J., Saiman L. Antibiotic resistance in neonatal intensive care unit pathogens: mechanisms, clinical impact, and prevention including antibiotic stewardship. Clin. Perinatol. 2010;37:547–563. doi: 10.1016/j.clp.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podschun R., Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandiumenge A., Diaz E., Rodriguez A., Vidaur L., Canadell L., Olona M., Rue M., Rello J. Impact of diversity of antibiotic use on the development of antimicrobial resistance. J. Antimicrob. Chemother. 2006;57:1197–1204. doi: 10.1093/jac/dkl097. [DOI] [PubMed] [Google Scholar]
- Seni J., Falgenhauer L., Simeo N., Mirambo M.M., Imirzalioglu C., Matee M., Rweyemamu M., Chakraborty T., Mshana S.E. Multiple ESBL-producing Escherichia coli sequence types carrying quinolone and aminoglycoside resistance genes circulating in companion and domestic farm animals in Mwanza, Tanzania, harbor commonly occurring plasmids. Front. Microbial. 2016;7 doi: 10.3389/fmicb.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Kumar C., Pandita A., Pratap O.T., Dasi T., Murki S. Bacteriological profile and clinical predictors of ESBL neonatal sepsis. J. Matern.-Fetal Neonatal Med. 2016;29:567–570. doi: 10.3109/14767058.2015.1011118. [DOI] [PubMed] [Google Scholar]
- Struelens M., Monnet D., Magiorakos A., O’Connor F.S., Giesecke J. New Delhi metallo-beta-lactamase 1-producing Enterobacteriaceae: emergence and response in Europe. Eurosurveillance. 2010 doi: 10.2807/ese.15.46.19716-en. [DOI] [PubMed] [Google Scholar]
- Thaver D., Ali S.A., Zaidi A.K. Antimicrobial resistance among neonatal pathogens in developing countries. Pediatr. Infect. Dis. J. 2009;28:S19–S21. doi: 10.1097/INF.0b013e3181958780. [DOI] [PubMed] [Google Scholar]
- Vandijck D.M., Depaemelaere M., Labeau S.O., Depuydt P.O., Annemans L., Buyle F.M., Oeyen S., Colpaert K.E., Peleman R.P., Blot S.I. Daily cost of antimicrobial therapy in patients with intensive care unit-acquired, laboratory-confirmed bloodstream infection. Int. J. Antimicrob. Agents. 2008;31:161–165. doi: 10.1016/j.ijantimicag.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Zankari E., Hasman H., Cosentino S., Vestergaard M., Rasmussen S., Lund O., Aarestrup F.M., Larsen M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012;67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]