Abstract
BACKGROUND. Plasma lipidomic measures may enable improved prediction of cardiovascular outcomes in secondary prevention. The aim of this study is to determine the association of plasma lipidomic measurements with cardiovascular events and assess their potential to predict such events.
METHODS. Plasma lipids (n = 342) were measured in a retrospective subcohort (n = 5,991) of the LIPID study. Proportional hazards regression was used to identify lipids associated with future cardiovascular events (nonfatal myocardial infarction, nonfatal stroke, or cardiovascular death) and cardiovascular death. Multivariable models adding lipid species to traditional risk factors were created using lipid ranking established from the Akaike information criterion within a 5-fold cross-validation framework. The results were tested on a diabetic case cohort from the ADVANCE study (n = 3,779).
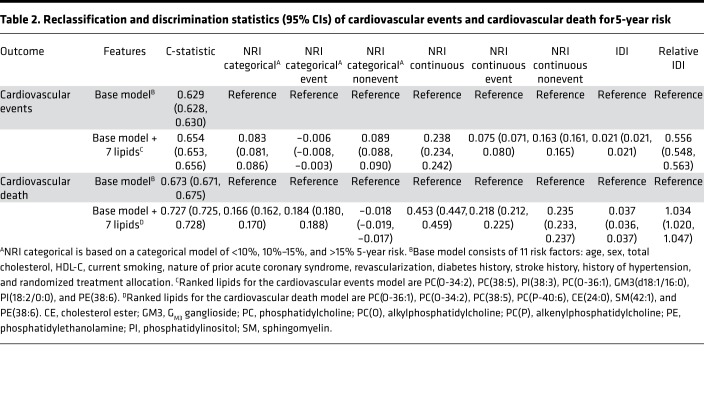
RESULTS. Specific ceramide species, sphingolipids, phospholipids, and neutral lipids containing omega-6 fatty acids or odd-chain fatty acids were associated with future cardiovascular events (106 species) and cardiovascular death (139 species). The addition of 7 lipid species to a base model (11 conventional risk factors) resulted in an increase in the C-statistics from 0.629 (95% CI, 0.628–0.630) to 0.654 (95% CI, 0.653–0.656) for prediction of cardiovascular events and from 0.673 (95% CI, 0.671–0.675) to 0.727 (95% CI, 0.725–0.728) for prediction of cardiovascular death. Categorical net reclassification improvements for cardiovascular events and cardiovascular death were 0.083 (95% CI, 0.081–0.086) and 0.166 (95% CI, 0.162–0.170), respectively. Evaluation on the ADVANCE case cohort demonstrated significant improvement on the base models.
CONCLUSIONS. The improvement in the prediction of cardiovascular outcomes, above conventional risk factors, demonstrates the potential of plasma lipidomic profiles as biomarkers for cardiovascular risk stratification in secondary prevention.
FUNDING. Bristol-Myers Squibb, the National Health and Medical Research Council of Australia (grants 211086, 358395, and 1029754), and the Operational Infrastructure Support Program of the Victorian government of Australia.
Keywords: Cardiology, Metabolism
Keywords: Atherosclerosis, Cardiovascular disease
Plasma lipidomic profiling of almost 10,000 individuals revealed that lipid species improved prediction of cardiovascular outcomes and informed about metabolic pathways involved in disease pathogenesis.
Introduction
Patients with a history of unstable angina and myocardial infarction (MI) are at high risk for recurrent cardiovascular events. The 7-year incidence rates of MI in nondiabetic and diabetic individuals with prior MI at baseline have been reported to be 18.8% and 45.0%, respectively (1). While follow-up and subsequent health care for patients after experiencing a cardiovascular event has improved, identifying stable coronary heart disease patients who are most likely to suffer recurrent fatal and nonfatal cardiovascular events remains a challenge. Such stratification would direct most aggressive management to those at highest risk who are most likely to benefit. In secondary prevention, where the vast majority of individuals have dysfunctional lipid and lipoprotein metabolism, the established risk factors for first cardiovascular events, such as BMI, hypertension, dyslipidemia assessed by traditional clinical lipid profile (total cholesterol, LDL cholesterol [LDL-C], triglycerides, and HDL cholesterol [HDL-C]), smoking, and blood glucose levels may not be sufficiently sensitive to predict future events, perhaps partly because of altered pathophysiology and medication use. In fact, while total cholesterol and LDL-C are strong predictors of both fatal and nonfatal CVD events in primary prevention (2, 3), they are often not significant predictors in individuals with previous history of cardiovascular disease (4).
Recent developments in high-throughput lipidomic technologies may offer new insights into lipid and lipoprotein metabolism. In a cross-sectional study in a high-risk population, several plasma lipid species and classes were found to be associated with and predictive of unstable coronary artery disease (5). In longitudinal studies, plasma lipids species have been shown to improve upon traditional risk factors in predicting cardiovascular events in the general population (6). Few studies have investigated the roles of plasma lipid species in prediction of future cardiovascular events and cardiovascular death in secondary prevention. Here, we report the most detailed lipidomic profiling to date to our knowledge, at baseline in patients from the LIPID cohort, and the analysis of these profiles in relation to future cardiovascular outcomes. Findings were replicated in the ADVANCE (Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation) study.
Results
Baseline characteristics.
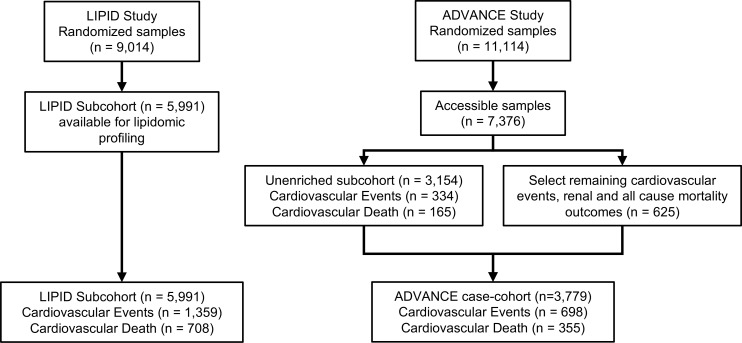
The LIPID subcohort analyzed in this study consisted of 5,991 participants with median age of 64 year (interquartile range [IQR]: 57–69 years). During 8 years of follow-up, 1,359 patients experienced cardiovascular events, of whom 708 died due to cardiovascular disease. Baseline characteristics are shown in Table 1. Individuals who experienced cardiovascular events in the follow-up period were older, had higher systolic blood pressure (SBP), and were more likely to have a history of diabetes and other comorbidities. With the exception of HDL-C, the clinical lipid measures were not significantly different between the two groups. The subcohort closely matched the unanalyzed portion of the LIPID cohort (n = 3,023, Supplemental Table 2; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.121326DS1). The ADVANCE case cohort consisted of 3,779 participants (61.1% male, median age, 67 years [IQR 62–72 years]). There were 698 cardiovascular events in this group, including 355 cardiovascular deaths. Those experiencing a cardiovascular event during the follow-up period were older, had a higher HbA1c and SBP, longer duration of diabetes, and lower HDL-C and estimated glomerular filtration rate (eGFR). As in the LIPID cohort, total cholesterol, LDL-C, and triglycerides were not significantly different between groups (Supplemental Table 3). A consort diagram of the LIPID subcohort and ADVANCE case cohort is shown in Figure 1.
Table 1. Baseline characteristics of the LIPID subcohort.
Figure 1. Consort diagram for lipidomic profiling of the LIPID study and ADVANCE study.
In the LIPID trial, 9,014 participants were randomized to receive pravastatin treatment or placebo. Lipidomic profiling was performed on all participants with baseline samples available (n = 5,991). Of these, 1,359 experienced a cardiovascular event and 708 experienced cardiovascular death. In the ADVANCE study, from 7,376 available baseline samples, a case cohort (n = 3,779) was selected for lipidomic profiling. This consisted of n = 3,154 randomly selected participants and all additional cases of cardiovascular events, renal events, and all-cause mortality (n = 625). Of these, n = 698 experienced a cardiovascular event and 355 experienced cardiovascular death.
Lipidomic analysis.
We analyzed 342 individual lipid species in each of the 5,991 participant samples. Batch alignment and assay performance utilized pooled plasma quality control samples (n = 648) spaced evenly throughout the analysis. The median coefficient of variance (CV) of all 342 lipid species was 13.9%, with 90% of lipid species having CV <22.8% (Supplemental Table 4). The same assay performance (median CV of 14%, with 90% of lipid species having CV <23%) was previously reported for the analysis of the ADVANCE case cohort (7).
Association of lipids with future cardiovascular events and cardiovascular death in the LIPID study.
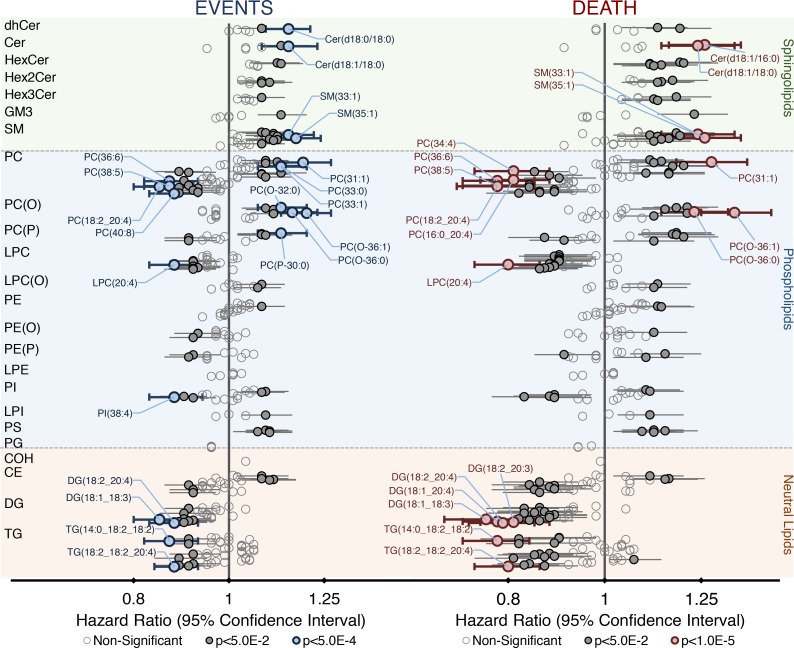
After adjusting for 11 covariates, Cox regression analysis of the total lipid classes and subclasses identified monohexosylceramide, sphingomyelin, and phosphatidylserine as positively associated with future cardiovascular events (P < 0.05, corrected for multiple comparisons). These lipid classes, in addition to trihexosylceramide, were also positively associated with future cardiovascular death, while lysophosphatidylcholine and diacylglycerol were negatively associated with cardiovascular death (Supplemental Figure 1). Importantly, 106 and 139 individual lipid species were significantly associated with future cardiovascular events and cardiovascular death, respectively (Figure 2, Supplemental Table 5, and Supplemental Figure 2), which, in addition to those lipid classes listed above, included species of ceramide, phosphatidylcholine, alkyl- and alkenylphosphatidylcholine, phosphatidylethanolamine, phosphatidylinositol, and triacylglycerol. Lipid species containing saturated and monounsaturated fatty acids were typically positively associated, while species containing polyunsaturated fatty acids were negatively associated. The exceptions to this were the ether and phosphatidylserine species with polyunsaturated fatty acids that were positively associated (Supplemental Table 5). When the 22 covariates were used, 53 and 88 lipid species were associated with cardiovascular events and cardiovascular death, respectively (Supplemental Table 6).
Figure 2. Plasma lipid species associated with future cardiovascular events and cardiovascular death in the LIPID cohort (n = 5,991).
Cox regression models of each lipid species against cardiovascular events (left) and cardiovascular death (right) were created, adjusting for 11 covariates (age, sex, total cholesterol, HDL-C, current smoking, nature of prior acute coronary syndrome, revascularization, diabetes history, stroke history, history of hypertension, and randomized treatment allocation). Hazard ratios per unit standard deviation and 95% CIs are shown. Bolded markers indicate significance (corrected P < 0.05 by Wald test). Colored markers indicate highly significant associations (blue, cardiovascular events, corrected P < 5.0E–4; red, cardiovascular death, corrected P < 1.0E–5 by Wald test). CE, cholesteryl ester; COH, cholesterol; Cer, ceramide; DG, diacylglycerol; dhCer, dihydroceramide; GM3, GM3 ganglioside; HexCer, monohexosylceramide; Hex2Cer, dihexosylceramide; Hex3Cer, trihexosylceramide; LPC, lysophosphatidylcholine; LPC(O), alkylphosphatidylcholine; LPE, lysophosphatidylethanolamine; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PC(O), alkylphosphatidylcholine; PC(P), alkenylphosphatidylcholine; PE, phosphatidylethanolamine; PE(O), alkylphosphatidylethanolamine; PE(P), alkenylphosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin; TG, triacylglycerol.
Multivariable models to predict cardiovascular events and cardiovascular death in the LIPID study.
The ranking of lipid species, using Akaike information criterion–based forward selection of lipids into multivariate models containing 11 covariates, performed by Cox regression (n = 5,991) within a 5-fold cross-validation framework (200 iterations), is shown in Supplemental Table 7. The median and IQR of the selected lipids are also shown in Supplemental Table 7.
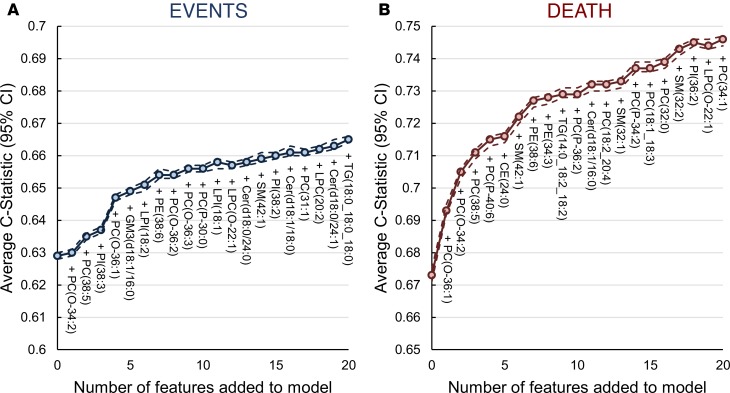
The successive addition of lipid species to the base models resulted in an increase in the C-statistic, which showed an inflection point at 7 lipid species (Figure 3). This was used as the optimal number of features for each outcome to avoid overfitting. The optimal models each contained 11 covariates and 7 lipids species. Detailed results for all the models are shown in Supplemental Tables 8 and 9.
Figure 3. C-statistic for models to predict cardiovascular events and cardiovascular death on the LIPID cohort (n = 5,991).
Cox regression models were used to determine the improvement in C-statistic to predict cardiovascular events (A) and cardiovascular death (B) by the addition of lipids to the base model consisting of 11 covariates (age, sex, total cholesterol, HDL-C, current smoking, nature of prior acute coronary syndrome, revascularization, diabetes history, stroke history, history of hypertension, and randomized treatment allocation). Analysis was performed within a 5-fold cross-validated framework (repeated 200 times). The plots show the average C-statistic (solid line) and 95% CI (dashed lines). Zero features denotes the base model. CE = cholesteryl ester; Cer(d18:0), dihydroceramide; Cer, ceramide; GM3, GM3 ganglioside; LPC, lysophosphatidylcholine; LPC(O), lysoalkylphosphatidylcholine; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PC(O), alkylphosphatidylcholine; PC(P), alkenylphosphatidylcholine; PE, phosphatidylethanolamine; PE(P), alkenylphosphatidylethanolamine; PI, phosphatidylinositol; PS, phosphatidylserine; SM, sphingomyelin; TG, triacylglycerol.
The addition of 7 lipids to the cardiovascular events base model improved the C-statistic from to 0.629 (95% CI, 0.628–0.630) to 0.654 (95% CI, 0.653–0.656), while the addition of 7 lipids to the cardiovascular death base model improved the C-statistic from 0.673 (95% CI, 0.671–0.675) to 0.727 (95% CI, 0.725–0.728). Categorical net reclassification improvement (NRI) improved by 0.083 (95% CI, 0.081–0.086) and 0.166 (95% CI, 0.162–0.170) for cardiovascular events and cardiovascular death, respectively. Continuous NRIs were 0.238 (95% CI, 0.234–0.242) and 0.453 (95% CI, 0.447–0.459) and integrated discrimination indexes (IDIs) were 0.021 (95% CI, 0.021–0.021) and 0.037 (95% CI, 0.036–0.037%) for cardiovascular events and cardiovascular death, respectively (Table 2). Though calibration plots (Supplemental Figure 3) were inconclusive, likelihood ratio tests between the base models without and with the 7 lipid species were significant (Supplemental Table 10). The addition of 7 lipid species to the 22 covariates base model for both cardiovascular events and cardiovascular death models improved the performance of the base models and showed a corresponding lower improvements in C-statistics and categorical NRI upon the addition of the lipid species (Supplemental Tables 11 and 12).
Table 2. Reclassification and discrimination statistics (95% CIs) of cardiovascular events and cardiovascular death for 5-year risk.
The associations of the previously reported ceramide ratios (8) with cardiovascular outcomes were stronger than those of the individual ceramide species (Supplemental Table 13). The Cer(d18:1/18:0)/Cer(d18:1/24:0) ratio gave the strongest hazard ratio for cardiovascular events [1.162 (95% CI, 1.098–1.230)], while Cer(d18:1/16:0)/Cer(d18:1/24:0) gave the strongest hazard ratio for cardiovascular death [1.286 (95% CI, 1.193–1.386)]. The lipid quotient (9) also gave strong hazard ratios [2.578 (95% CI, 1.955–3.399) and 5.345 (95% CI, 3.661–7.805), respectively, per 0.1 unit of the quotient]. However, the addition of the Cer(d18:1/18:0)/Cer(d18:1/24:0) ratio to the base model for cardiovascular events resulted in only marginal improvement in the C-statistic from 0.629 (95% CI, 0.628–0.630) to 0.636 (95% CI, 0.635–0.637), a categorical NRI of 0.027 (95% CI, 0.026–0.029), and a continuous NRI of 0.137 (95% CI, 0.132–0.141). The improvement over the base model for cardiovascular death was similar (Supplemental Table 14). The results for the lipid quotient to predict cardiovascular events were slightly better, with an improvement in the C-statistic from 0.629 (95% CI, 0.628–0.630) to 0.640 (0.639–0.641), a categorical NRI of 0.043 (0.041–0.046) and continuous NRI of 0.189 (0.185–0.194). The improvement over the base model for cardiovascular death was similar (Supplemental Table 14). Further, we included the lipid quotient and ceramide ratios with the individual lipid species and performed feature ranking and model development as described for individual lipid species. Interestingly, the lipid quotient was selected as the top ranked feature, while none of the ceramide ratios were selected in the models (Supplemental Tables 15 and 16). Although the inclusion of the lipid quotient altered the lipid rankings, the performance of the models containing the lipid quotient was almost identical to the original models. The C-statistic for the cardiovascular events model increased from 0.629 (95% CI, 0.628–0.630) to 0.654 (0.652–0.656), with a categorical NRI of 0.084 (0.081–0.088) and continuous NRI of 0.258 (0.252–0.265), when adding the lipid quotient and 6 additional lipid species to the base model.
Evaluation of lipid biomarkers in the ADVANCE study.
Of 106 and 139 lipids significantly associated with cardiovascular events and cardiovascular death in the LIPID study, 97 and 128 were also measured in the ADVANCE study case cohort (7). Weighted Cox regression of lipid species with cardiovascular events and cardiovascular death as outcomes, adjusting for 10 covariates, produced similar hazard ratios for most, but not all, species (Supplemental Table 17 and Supplemental Figure 4), including those selected for the multivariable models (Figure 4).
Figure 4. Association of lipid species that are selected in the predictive models with cardiovascular events and cardiovascular death in the LIPID subcohort (n = 5,991) and ADVANCE case cohort (n = 3,779).
Cox regression analysis (LIPID subcohort, circles) or weighted Cox regression analysis (ADVANCE case/cohort, triangles) was performed on those lipid species selected for the optimum models to predict cardiovascular events (A) and cardiovascular death (B) in the LIPID data set. The models were adjusted for 11 covariates (age, sex, total cholesterol, HDL-C, current smoking, nature of prior acute coronary syndrome, revascularization, diabetes history, stroke history, history of hypertension, and randomized treatment allocation) for the analysis of the LIPID subcohort or 10 covariates (age, sex, total cholesterol, HDL-C, current smoking, history of macrovascular disease, coronary artery bypass graft or percutaneous transluminal coronary angioplasty, diabetes duration, current antihypertensive treatment, and statin treatment) for the analysis of the ADVANCE case cohort. Hazard ratios per unit standard deviation and 95% CIs are shown. CE, cholesteryl ester; LPI, lysophosphatidylinositol; PC, phosphatidylcholine; PC(O), alkylphosphatidylcholine; PC(P), alkenylphosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SM, sphingomyelin.
The performance of predictive models in the ADVANCE case cohort is shown in Table 3. The addition of 7 lipids to the ADVANCE base model (10 covariates) to predict cardiovascular events resulted in an increase in the C-statistic to from 0.663 (95% CI, 0.661–0.665) to 0.676 (95% CI, 0.674–0.678) and a categorical NRI of 0.042 (95% CI, 0.038–0.046), while the incorporation of 7 lipids to the base model (10 covariates) to predict cardiovascular death increased the C-statistic from 0.725 (95% CI, 0.722–0.727) to 0.741 (95% CI, 0.738–0.744) and resulted in a categorical NRI of 0.062 (95% CI, 0.057–0.067).
Table 3. Reclassification and discrimination statistics of cardiovascular events and cardiovascular death for 5-year risk in ADVANCE data set.
Interaction of diabetes with plasma lipid species in the LIPID subcohort.
As the ADVANCE case cohort consisted of all diabetes participants, we sought to assess the interaction of diabetes with plasma lipid species in the LIPID subcohort, which contained only approximately 9% of this subcohort had diabetes. Of the 9 lipid species that were utilized in the predictive model of cardiovascular outcomes, only PC(P-40:6) showed a significant interaction with diabetes, with a hazard ratio of 1.420 (95% CI, 1.018–1.982, P = 3.90E–02, Supplemental Table 18).
Discussion
We present data from the lipidomic analysis (342 lipid species) of almost 6,000 individuals with a history of unstable angina or MI enrolled in the LIPID study, making this the single largest lipidomic study thus far reported to our knowledge. Association studies have identified several lipid metabolic pathways involving sphingolipids, ether lipids, and omega-6 fatty acids that appear to be tightly linked with cardiovascular outcomes and, in particular, with cardiovascular death. Our predictive modeling in both the LIPID subcohort and the ADVANCE case cohort further highlights the strength of the associations with cardiovascular death as well as important differences related to diabetes status. Our findings lay the groundwork for the clinical translation of lipidomic biomarkers for cardiovascular risk assessment and have the potential to open new avenues for therapeutic intervention.
Lipid classes associated with cardiovascular outcomes.
At a class level, sphingolipids (monohexosylceramide and sphingomyelin) and phosphatidylserine were positively associated with cardiovascular events. Plasma sphingomyelin levels (measured enzymatically) were previously shown to be positively associated with coronary artery disease (10) and a genetic study of the human lipidome in the population-based San Antonio Family Heart study also reported a genetic (lipid) cluster, dominated by sphingomyelin and ether phospholipid species, as significantly associated with cardiovascular mortality (11). Atherogenic lipoproteins, such as VLDL and LDL, are enriched in sphingolipids, including sphingomyelin, and although LDL-C level was not statistically different between individuals who had cardiovascular events and those who did not, it appears that differences in sphingolipid content of these lipoprotein particles may be related to future risk.
Phosphatidylserine, while a minor plasma phospholipid, is a major component of platelet membranes and is released as microparticles. Circulating levels of phosphatidylserine have been associated with platelet activation which has itself been associated with cardiovascular disease risk (12).
Lipid species associated with cardiovascular outcomes.
Despite relatively few lipid classes showing association with cardiovascular events, there was a plethora of individual lipid species associated. Thus, within some classes, including phosphatidylcholine, alkenylphosphatidylcholine, phosphatidylinositol, diacylglycerol, and triacylglycerol, we observed some species positively associated with a given outcome, while others were negatively associated, and as a consequence, class association was not significant. While in other classes (dihydroceramide, ceramide, and mono-, di-, and trihexosylceramide) only some species show association, and, as a consequence, at a class level these were often not significant. Several previous studies have also identified some of these lipid species associated with secondary prevention outcomes (8, 9, 13), although these studies typically measured fewer lipids, adjusted for fewer covariates, and, in some cases, did not correct for multiple comparisons. The concordance of these previous studies with the current analysis is shown in Table 4.
Table 4. Summary of lipid species identified in previous studies.
Sphingolipids indicate ceramide synthase associations with cardiovascular risk.
While multiple sphingolipid species were associated with future cardiovascular death and cardiovascular events, including dihydroceramide; ceramide; mono-, di-, and trihexosylceramide; and GM3 ganglioside, those containing either 16:0 or 18:0 as the acyl chain showed the strongest associations. Although the association of saturated fatty acids with cardiovascular outcomes has been recognized for many years, these results suggest regulation of sphingolipid biosynthesis at the level of ceramide synthase (CerS), which has multiple forms that show specificity for different acyl chains. CerS2 is the predominant form in liver and shows specificity for the longer chain acyl species C20:0, C22:0, C24:0, and C24:1, none of which were associated with future events in this study. CerS1 is specific for the synthesis of Cer(d18:0/18:0)/Cer(d18:1/18:0), and its expression in muscle is inversely associated with alterations in glucose tolerance in a mouse model of insulin resistance (14). We have also reported on the association of dihydroceramide and ceramide species containing 18:0 (but not 16:0) with prediabetes and diabetes (15). CerS5 and CerS6 have specificity for the C16:0 acyl chain and are expressed primarily in lung and intestine/kidney, respectively (16). Thus, our observations may reflect differential regulation of these pathways in liver and/or other tissues.
Several other studies have also reported associations of ceramide and other sphingolipid species with cardiovascular outcomes in secondary prevention (Table 4). We also observed positive associations for many ceramide species [Cer(d18:1/16:0), Cer(d18:1/18:0), and LacCer(d18:1/18:0)], while some species, such as Cer(d18:1/24:0), were not significant in our analyses after correction for multiple comparisons. The differences in these results likely relate to the size of the cohorts, the specific populations, and the 11 covariates used in the LIPID trial analysis. Corresponding to our findings, inhibition of the glycosphingolipid pathway decreased atherosclerosis in mice and rabbits by reducing plasma cholesterol (17), highlighting the therapeutic potential of these observations.
Dysregulation of desaturases leads to an imbalance in fatty acid composition in multiple lipid classes.
Many phospholipid species containing omega-6 fatty acids (in particular 20:4) were negatively associated with cardiovascular outcomes. This finding was in agreement with a previous analysis of the LURIC study (9) (Table 4). Further, a negative association (adjusted for traditional risk factors) of the omega-6–containing lysophosphatidylcholine species LPC(20:4) (also reported here), as well as LPC(16:0), was observed in the population-based Malmo Diet and Cancer study (18). Since phospholipids are the major source of arachidonic acid for the biosynthesis of the clinically important and inflammatory prostaglandins and other eicosanoids, it is tempting to speculate that the negative association is relate to increased production of these proinflammatory phospholipid species, which have been associated with incident cardiovascular disease (19). However, we also observed negative associations with cholesteryl ester species containing the omega-6 fatty acids 18:3 and 20:4 but not their biosynthetic precursors 18:2 and 20:3 (Supplemental Table 5). These associations then appear to relate more to metabolism of the omega-6 fatty acids via the desaturases FADS2 and FADS1, respectively, as has been reported previously (20).
Interestingly, we also saw significant associations of omega-6 fatty acid–containing species of diacyl- and triacylglycerols. While all diacylglycerol species showed a nonsignificant or negative association with cardiovascular outcomes, the strength of the association varied depending on the fatty acid composition. Thus, while DG(18:1_18:2), DG(18:1_18:3), DG(18:1_20:3), and DG(18:1_20:4) were all negatively associated with cardiovascular death, DG(18:1_18:3) and DG(18:1_20:4) showed stronger hazard ratios and lower P values than DG(18:1_18:2) and DG(18:1_20:3) (Supplemental Table 5). Similarly, most species of triacylglycerol containing an 18:2 fatty acid were negatively associated with cardiovascular death, but those also containing a 20:4 fatty acid typically showed a stronger association (Supplemental Table 5).
In contrast diacyl- and triacylglycerols containing primarily saturated or monounsaturated fatty acids typically showed no association or a positive trend, with TG(18:0_18:0_18:0) showing a significant positive association with cardiovascular death (Supplemental Table 5). In addition to this, we observed negative associations of DG(16:1_16:1) and TG(16:1_16:1_16:1) with cardiovascular death; since palmitoleate (16:1) is primarily the product of stearoyl-CoA desaturase (SCD1), which acts on saturated fatty acids (16:0 and 18:0), this suggests a downregulation of this desaturase in addition to FADS1 and FADS2 described above. The net result of this is a proportional increase in saturated fats, leading to an imbalance in the saturated/polyunsaturated ratio. This imbalance is also evident in the phospholipids (saturated species showing positive associations and polyunsaturated species show negative associations with cardiovascular outcomes) and so has the potential to effect membrane properties and, potentially, cellular function.
Odd-chain fatty acid metabolism is altered in those at increased risk of cardiovascular events.
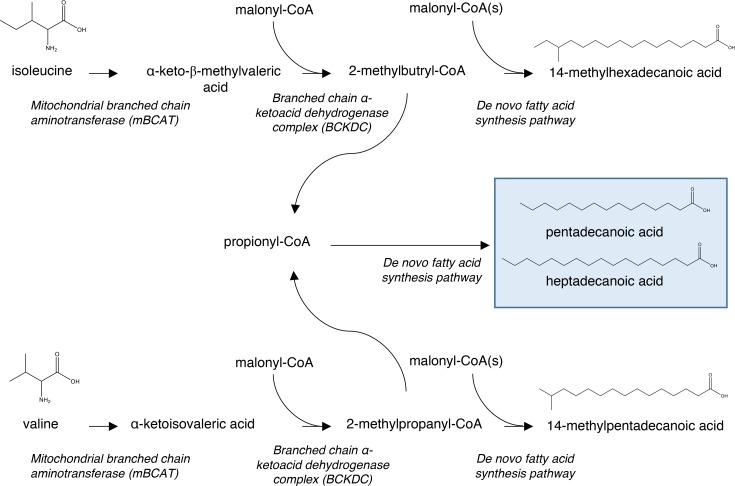
Of relevance are the positive associations between species of sphingomyelin-, phosphatidylcholine-, phosphatidylethanolamine-, and cholesteryl ester–containing odd-chain fatty acids and cardiovascular outcomes (Figure 2 and Supplemental Table 5). These represent some of the strongest associations and indicate a further alteration in fatty acid metabolism. Odd-chain fatty acids (which include branched-chain fatty acids), long thought to be derived solely from diet, are now also recognized as a product of branched-chain amino acid catabolism within mitochondria in multiple tissues, including adipose and muscle (Figure 5) (21, 22). In humans, branched-chain amino acid catabolism is thought to play a role in adipocyte metabolism and differentiation (23). Branched-chain amino acids are positively associated with insulin resistance and type 2 diabetes (T2D) (24, 25), while odd-chain fatty acids have been shown to be negatively associated with T2D (15). Mendelian randomization studies have identified PPM1K, an activator of branched-chain α-ketoacid dehydrogenase (BCKD), a key enzyme in branched-chain amino acid catabolism, as associated with both branched-chain amino acid levels and incident T2D (26). In primary prevention, branched-chain amino acids have also been positively associated with cardiovascular risk (27). Thus, the positive associations of odd-chain fatty acids with cardiovascular outcomes described here is at odds with an insulin resistance/diabetes explanation and represents an as-yet uncharacterized aspect of branched-chain amino acid/fatty acid metabolism that may adversely affect cardiovascular risk in secondary prevention.
Figure 5. Branched-chain amino acid catabolism to produce odd-chain and branched-chain fatty acids.
Starting from the branched-chain amino acids (isoleucine and valine), transamination by a mitochondrial branched-chain aminotransferase followed by decarboxylation with a branched-chain α-ketoacid dehydrogenase complex leads to short branched and odd acyl-CoAs that are subsequently utilized in a similar fashion as acetyl-CoA in de novo lipogenesis.
Predictive model development.
A primary objective of our model development process was to examine whether lipid species could improve upon clinical risk factors in the prediction of cardiovascular outcomes. The model that was developed by Marschner et al. from the LIPID cohort predicted coronary heart disease death and nonfatal MI, a more limited composite end point (28). The 11-covariate base model used in this present study was based on the variables from the Marschner score. The observed improvement in model performance with the addition of 7 lipid species demonstrates their potential as complementary predictors of cardiovascular risk.
The model to predict cardiovascular death showed an improved net reclassification of events [NRIevents = 0.184 (95% CI, 0.180–0.188)], which is equivalent to reclassification of 130 of 708 deaths, but showed no improvement of net reclassification of nonevents [NRInonevents = –0.018 (95% CI, –0.019–0.017)]. In contrast, the model to predict cardiovascular events showed a reclassification of nonevents but no reclassification of events (Table 2). While the continuous NRIs showed a more balanced effect between events and nonevents, the ability to reclassify between risk groups appears greater for cardiovascular death than nonfatal cardiovascular events. In fact, the failure of the cardiovascular events model to reclassify events suggests that the lipid species are not effective in reclassification of nonfatal events, although this model was able to reclassify almost 9% of nonevents (false positives). The same analyses, performed with 22 covariates in the base model, resulted in a more modest improvement with the addition of 7 lipid species. However, this was largely due to the higher C-statistic of the base model, with the final models achieving similar performance. This would suggest that the lipid species are able to replace clinical risk factors where these may not always be available.
We sought to evaluate whether the lipid species utilized in the risk models developed on the LIPID subcohort would also predict in the ADVANCE case cohort, which had a lower history of cardiovascular disease (35% vs. 100%), a higher prevalence of diabetes (100% vs. 8.5%), and 44% participants on lipid-lowering medication at baseline. The association of lipid species with future cardiovascular events and cardiovascular death and multivariate models has been previously published (7). While some covariates used in the analysis of the ADVANCE case cohort were not identical to those available in the LIPID subcohort, most characteristics were included, such that the extent of the clinical phenotype captured by the covariates was similar in each cohort. Interestingly, the performance of the base model in the ADVANCE case cohort was more powerful than in the LIPID subcohort.
While we observed a significant improvement on model performance with the addition of 7 lipid species to a base model of 10 covariates, the improvement was not as distinguished as in the LIPID subcohort. When we examined the association of the individual lipid species in the models with cardiovascular outcomes in the LIPID subcohort and ADVANCE case cohort (Figure 4), we saw that, while most lipid species show similar hazard ratios, two lipid species in the model to predict cardiovascular death showed opposing associations in the LIPID and ADVANCE cohorts [PC(P-40:6) and CE(24:0)]. Interestingly, when we performed interaction analysis of diabetes with the lipid species in the cardiovascular death model in the LIPID subcohort, we observed an interaction with diabetes for PC(P-40:6) showing a stronger positive association with cardiovascular death in the nondiabetic participants (Supplemental Table 18). These results imply that specific lipids/models may be required to optimize risk prediction in diabetes, although we note that most lipids did not show an interaction with diabetes.
In the LURIC study, a quotient of the sum of the 6 lipids species with the strongest negative associations and the sum of the 6 lipid species with the strongest positive associations had a larger hazard ratio than any of the individual lipid species (9). In this study we analyzed 11 of the 12 lipid species used in the quotient. All 11 showed the same direction of association with cardiovascular death; 7 of these [Cer(d18:1/16:0), SM(34:1), PC(32:0), PC(38:5), PC(38:6), PC(40:6), and PC(O-32:0)] were significant in our data set. We recreated the quotient (using 11 of the 12 reported lipid species), and although this showed a highly significant association with cardiovascular outcomes, the quotient showed a modest improvement in the C-statistic from 0.673 (95% CI, 0.671–0.675) to 0.694 (95% CI, 0.693–0.696), with a categorical NRI of 0.048 (95% CI, 0.045–0.051, relative to the base model with 11 covariates (Supplemental Table 14). Similarly, ceramide ratios, reported to predict cardiovascular death in secondary prevention cohorts (8), showed a strong association with cardiovascular death in this cohort (Supplemental Table 13) but only modest improvement in the performance of multivariate models (Supplemental Table 14). When the quotient and ratios were available for selection, together with lipid species in multivariate models, the quotient was selected, but the overall model was not improved, despite containing 17 lipid species (the 11 species in the quotient and 6 additional species). The disparity in the predictive values of the lipid quotient and ratios likely relates to differences in the cohorts, duration of follow-up, and the extent of risk adjustment (number of covariates) in this study relative to earlier studies.
In a model optimized to predict cardiovascular death in a subcohort from the diabetic ADVANCE study, 4 lipid species [PC(O-36:1), DG(16:0_22:5), SM(34:1), and PC(O-36:5)] were added to 14 covariates to improve the prediction of cardiovascular death, resulting in a 6.0% increase in the C-statistic to 0.701 (95% CI, 0.697–0.705) and a continuous NRI of 0.481 (95% CI, 0.465–0.498) (7). PC(O-36:1) was also used for model development in the LIPID study cohort, while SM(34:1) and DG(16:0_22:5) were both associated with cardiovascular death in the LIPID study cohort, suggesting a degree of redundancy between species but also likely reflecting differences between diabetes and nondiabetes cohorts.
In a large, primary prevention cohort study, the addition of 6 lipid species [TG(54:2), PE(36:5), CE(16:1), SM(34:2), LPC(20:5), and LPC(22:6)] to the Framingham risk score components to predict 10-year risk of cardiovascular events, improved C-statistics and categorical NRI by 3.8% and 14.9%, respectively (6). Three of the six lipids used in this model [SM(34:2), LPC(20:5), and LPC(22:6)] were associated with cardiovascular death in our cohort. These differences likely relate to the primary prevention setting; individuals with established cardiovascular disease (or diabetes) are known to have dysregulated lipid metabolism, affecting multiple lipid species (5, 10). Consequently, lipids identified in secondary prevention studies are likely to represent markers associated with the transition from stable to unstable disease rather than representing the onset or early stages of disease, as may be the case in primary prevention.
We noted that lipid associations were typically stronger for cardiovascular death than cardiovascular events (Supplemental Figure 2) and that this was reflected in the better performance of the model to predict cardiovascular death compared with the model to predict cardiovascular events (Table 2). This suggests that, within this secondary prevention population where the majority of patients are at high risk, those who progress to CV death represent a more severe “metabolic phenotype” than those who suffer a nonfatal stroke or nonfatal MI. Such a metabolic phenotype may also reflect important therapeutic targets that may be amenable to dietary or drug intervention. We have recently demonstrated the potential of this approach by the dietary modulation of plasmalogens in a mouse model of atherosclerosis (29). Further studies will be required to fully evaluate the potential in this area.
Study limitations.
In this very large lipidomic study, we used a single internal standard per lipid class, resulting in a precise approximation of lipid concentrations, rather than accurate quantification. Translation of a select lipid signature with stable isotope internal standards will provide improved precision and accuracy and will likely result in better performance of the models. Similarly, the sum of individual species representing class values will also have reduced accuracy.
A large proportion of patients in the LIPID subcohort were on medication; although randomization to statin treatment was included as a covariate in our analyses, some lipid species at baseline may have been influenced by other medications. Further, we have used the 11 Marschner score covariates as the base model in these analyses; as this score was derived from the LIPID study, this may result in overfitting of the base model and, therefore, an underestimation of the improved performance resulting from the lipid species.
The results derived from LIPID subcohort have been evaluated on a case cohort of people with diabetes from the ADVANCE study. There were also some slight differences between the lipid species measured in each study, and the ADVANCE case cohort had fewer cardiovascular outcomes, providing lower power to identify significant associations. The ADVANCE case cohort was not identical to the LIPID subcohort in clinical presentation, lipid-lowering medication at baseline, and clinical covariates, limiting the inferences that can be drawn. However, these differences between cohorts enabled us to identify important limitations of this approach and the need for further validation studies in different clinical settings.
Translation of these biomarkers will require the further development of the technology to provide accurate quantification that is transferable across platforms and laboratories. The exact configuration of any clinical test resulting from this study is not defined, although we note the recent introduction by the Mayo Clinic of “plasma ceramides” as a risk marker for cardiovascular disease (30). It seems likely that signatures containing fewer lipids may be more readily translated, and our results would suggest that relatively few lipid species could provide significant improvement over traditional risk markers.
Methods
Study populations
The design of the LIPID study has been published in detail (31). Briefly, individuals between 31 and 75 years of age, with an MI or hospital admission for unstable angina 3–36 months previously, having plasma total cholesterol levels of between 4.0 and 7.0 mmol/l and fasting triglycerides of <5.0 mmol/l were enrolled in the study. Individuals with heart failure or currently taking lipid-lowering medication were excluded. A single-blind placebo run-in phase was conducted for 8 weeks. After this, 9,014 patients (7,498 men and 1,516 women) were randomly allocated to receive pravastatin (40 mg daily) or matching placebo. The primary outcome was coronary death, with nonfatal MI and stoke included as secondary outcomes. All deaths and acute MI were reviewed by the outcome assessment committee. Primary and secondary outcomes were adjudicated using several criteria and are described in detail in the original publication (31). The median follow-up period was 6 years.
Detailed lipidomic profiling was conducted on 5,991 participants, for whom baseline fasting plasma samples were available, representing 3,002 of those randomized to placebo and 2,989 of those assigned pravastatin. Table 1 summarizes the characteristics of these groups. Within the available cohort, 1,359 patients experienced one or more cardiovascular events (649 nonfatal MI, 308 nonfatal stroke, and 708 cardiovascular death; Figure 1).
The ADVANCE study was a double-blinded, international prospective study; it assessed the effect of intensive glucose control and routine blood pressure lowering on cardiovascular events (cardiovascular death, nonfatal MI, or stroke) and renal outcomes in patients with T2D (32). A total of 11,140 patients (32% with a prior history of major macrovascular disease) with T2D and 1 additional risk factor for cardiovascular disease, aged 55 years or older, were enrolled. A sample (n = 3,154) was selected at random from the 7,376 participants with available blood samples (the unenriched subcohort). This was then enriched with those suffering cardiovascular events or renal or all-cause mortality outcomes (n = 625) from the remaining 4,222 participants, to give a total of 3,779 participants (698 cardiovascular events, 355 of which were fatal [cardiovascular death]) (Figure 1).
Lipidomic profiling
The same lipidomic profiling strategy was used for the LIPID and ADVANCE cohorts. A detailed extraction protocol and analysis method is presented in the Supplemental Methods. Briefly, lipids were extracted from 10 μl human plasma with butanol/methanol (100 μl) as described previously (33). Lipidomic analysis (342 species) was performed by liquid chromatography and electrospray ionization–tandem mass spectrometry using a Agilent 1290 liquid chromatography system combined with an Agilent 6490 triple quadrupole mass spectrometer (7). The experimental conditions are summarized in Supplemental Table 1. The relative concentration of each lipid species was calculated from the area of the resultant chromatograms relative to the corresponding internal standard. Corrections for the batch effect were performed using median centering approach as described previously (7). The relative levels of each lipid class and subclass were calculated as the sum of the species within the class or subclass.
Statistics
LIPID subcohort.
Prior to association studies, lipid data were log transformed to reduce the skewness of the measurements and scaled by the standard deviation units. After checking the proportional hazard assumptions using scaled Schoenfeld residuals that showed random patterns, Cox proportional hazard models were used to determine the association of each lipid class, subclass, and species with cardiovascular events (nonfatal MI, nonfatal stroke, and cardiovascular death) and cardiovascular death. The models were adjusted for two sets of covariates: 11 covariates derived from the Marschner risk score, previously developed on the LIPID cohort (28) (age, sex, total cholesterol, HDL-C, current smoking, nature of prior acute coronary syndrome, revascularization, diabetes history, stroke history, history of hypertension, and randomized treatment allocation), or 22 covariates that had been shown to be significant predictors of outcomes in a previous analysis of the LIPID study (34) (age, sex, BMI, total cholesterol, HDL-C, triglycerides, current smoking, SBP, fasting glucose, atrial fibrillation, stroke history, diabetes history, hypertension history, nature of prior acute coronary syndrome [no MI or any MI], revascularization, eGFR, dyspnea grade, angina grade, white blood cell count, peripheral vascular disease, aspirin use, and randomized treatment allocation). P values were corrected for multiple comparisons (342 lipid species) using Benjamini-Hochberg approach (35). The resultant hazard ratios, their CIs, and corrected P values were used to determine the strength of association. Interaction analysis of diabetes status or randomized treatment allocation with lipid species was performed using the same 11 covariates in a Cox proportional hazard model. Ranking of the lipid species for each outcome was performed within a 5-fold cross-validation framework (200 repeats) using a forward stepwise Cox regression, adding lipids to the 11 covariates, with Akaike information criterion minimization as the objective. The average rankings of the lipids in these 1,000 iterations were then used to select the lipid species for incorporation into the subsequent risk models.
The addition of lipid species or lipid ratios to the base covariates was evaluated using the C-statistics for 5-year risk (Somers’ D command in STATA), continuous and categorical NRI, IDI, and relative IDI within a 5-fold cross-validation (200 repeats). The calculation of categorical NRI was based on 5-year risk categories of <10%, 10%–15%, and >15%. Using the whole data set, likelihood ratio tests and calibration plots were determined between base models and optimized models containing 7 lipid species.
ADVANCE case cohort.
The lipid species common to the ADVANCE case cohort were also evaluated. Due to an incomplete alignment of covariates between the ADVANCE and LIPID studies, a complementary set of 10 base covariates, consisting of age, sex, current smoking, total cholesterol, HDL-C, diabetes duration, coronary artery bypass graft or percutaneous transluminal coronary angioplasty, history of macrovascular disease, heart failure, current antihypertensive treatment, and statin treatment, was used. Assessment of lipid associations observed in the LIPID cohort was performed by measuring the adjusted hazard ratio of the same lipid species using weighted Cox regression models to accommodate the enriched case cohort from the ADVANCE study.
The predictive ability of the 7 lipid species selected for each of the multivariate models to predict cardiovascular events or cardiovascular death were determined using Cox regression models on the unenriched ADVANCE subcohort and compared with the base model (10 covariates). The improvement in C-statistics, NRI, and IDI resulting from the addition of the 7 lipid species, identified in the LIPID subcohort, to the base model (10 covariates) was determined.
Study approval
The analysis of archived plasma samples from the LIPID and ADVANCE studies was approved by the Alfred Hospital Ethics Committee, Melbourne, Victoria, Australia. The subsequent analysis of archived plasma samples was approved by the Alfred Hospital Ethics Committee.
Author contributions
PAM, EHB, AK, KJS, CG, MW, GW, BAK, and PJM developed the statistical analysis protocols and performed the analysis. CKB, ZHA, NAM, KH, MJM, and PJM developed the mass spectroscopy protocols, performed the experiments, and generated the data. PJN, EHB, AK, PT, DRS, JS, and AMT oversaw the LIPID trial and provided key inputs on interpretation of the data. SZ, GSH, JC, and MW oversaw the ADVANCE trial and provided key inputs on interpretation of the data. PAM, CKB, BAK, and PJM wrote the manuscript.
Supplementary Material
Acknowledgments
We would like to thank the investigators of the LIPID and ADVANCE studies and the patients who participated in these studies. We acknowledge Michelle Cinel and Ricardo Tan for their technical support in the lipidomic analyses. The LIPID study was supported by a research grant from Bristol-Myers Squibb and was conducted independently of the sponsor, under the auspices of the National Heart Foundation of Australia. The ADVANCE study was funded by the National Health and Medical Research Council of Australia (grants 211086 and 358395). This work was funded by the National Health and Medical Research Council of Australia (grant 1029754) and the Operational Infrastructure Support Program of the Victorian Government of Australia. See the Supplemental Acknowledgments for The LIPID study Investigators details.
Version 1. 09/06/2018
Electronic publication
Footnotes
Conflict of interest: PJM has licensed biomarker intellectual property to Zora Biosciences.
Reference information: JCI Insight. 2018;3(17):e121326. https://doi.org/10.1172/jci.insight.121326.
Contributor Information
Adrienne Kirby, Email: Adrienne.Kirby@ctc.usyd.edu.au.
Kevin Huynh, Email: Kevin.Huynh@baker.edu.au.
Corey Giles, Email: Corey.Giles@baker.edu.au.
Sophia Zoungas, Email: sophia.zoungas@monash.edu.
Graham S. Hillis, Email: Graham.Hillis@health.wa.gov.au.
John Chalmers, Email: jchalmers@george.org.au.
Mark Woodward, Email: mwoodward@george.org.au.
Gerard Wong, Email: gerard.wong@zoho.com.
John Simes, Email: John@ctc.usyd.edu.au.
References
- 1.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Karalis DG. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clin Proc. 2009;84(4):345–352. doi: 10.1016/S0025-6196(11)60544-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 4.Puri R, et al. C-reactive protein, but not low-density lipoprotein cholesterol levels, associate with coronary atheroma regression and cardiovascular events after maximally intensive statin therapy. Circulation. 2013;128(22):2395–2403. doi: 10.1161/CIRCULATIONAHA.113.004243. [DOI] [PubMed] [Google Scholar]
- 5.Meikle PJ, et al. Plasma lipidomic analysis of stable and unstable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31(11):2723–2732. doi: 10.1161/ATVBAHA.111.234096. [DOI] [PubMed] [Google Scholar]
- 6.Stegemann C, et al. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation. 2014;129(18):1821–1831. doi: 10.1161/CIRCULATIONAHA.113.002500. [DOI] [PubMed] [Google Scholar]
- 7.Alshehry ZH, et al. Plasma lipidomic profiles improve on traditional risk factors for the prediction of cardiovascular events in type 2 diabetes mellitus. Circulation. 2016;134(21):1637–1650. doi: 10.1161/CIRCULATIONAHA.116.023233. [DOI] [PubMed] [Google Scholar]
- 8.Laaksonen R, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. 2016;37(25):1967–1976. doi: 10.1093/eurheartj/ehw148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigruener A, Kleber ME, Heimerl S, Liebisch G, Schmitz G, Maerz W. Glycerophospholipid and sphingolipid species and mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. PLoS ONE. 2014;9(1):e85724. doi: 10.1371/journal.pone.0085724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am J Epidemiol. 2006;163(10):903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 11.Bellis C, et al. Human plasma lipidome is pleiotropically associated with cardiovascular risk factors and death. Circ Cardiovasc Genet. 2014;7(6):854–863. doi: 10.1161/CIRCGENETICS.114.000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel O, Jesel L, Freyssinet JM, Toti F. Cellular mechanisms underlying the formation of circulating microparticles. Arterioscler Thromb Vasc Biol. 2011;31(1):15–26. doi: 10.1161/ATVBAHA.109.200956. [DOI] [PubMed] [Google Scholar]
- 13.Tarasov K, et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab. 2014;99(1):E45–E52. doi: 10.1210/jc.2013-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frangioudakis G, Garrard J, Raddatz K, Nadler JL, Mitchell TW, Schmitz-Peiffer C. Saturated- and n-6 polyunsaturated-fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology. 2010;151(9):4187–4196. doi: 10.1210/en.2010-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meikle PJ, et al. Plasma lipid profiling shows similar associations with prediabetes and type 2 diabetes. PLoS One. 2013;8(9):e74341. doi: 10.1371/journal.pone.0074341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiban J, Tidhar R, Futerman AH. Ceramide synthases: roles in cell physiology and signaling. Adv Exp Med Biol. 2010;688:60–71. doi: 10.1007/978-1-4419-6741-1_4. [DOI] [PubMed] [Google Scholar]
- 17.Bietrix F, et al. Inhibition of glycosphingolipid synthesis induces a profound reduction of plasma cholesterol and inhibits atherosclerosis development in APOE*3 Leiden and low-density lipoprotein receptor-/- mice. Arterioscler Thromb Vasc Biol. 2010;30(5):931–937. doi: 10.1161/ATVBAHA.109.201673. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez C, et al. Plasma lipid composition and risk of developing cardiovascular disease. PLoS One. 2013;8(8):e71846. doi: 10.1371/journal.pone.0071846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris WS, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902–907. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 20.Shin SY, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543–550. doi: 10.1038/ng.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr. 1998;68(1):72–81. doi: 10.1093/ajcn/68.1.72. [DOI] [PubMed] [Google Scholar]
- 22.Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285(15):11348–11356. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green CR, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12(1):15–21. doi: 10.1038/nchembio.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang TJ, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee CC, et al. Branched-chain amino acids and insulin metabolism: The Insulin Resistance Atherosclerosis Study (IRAS) Diabetes Care. 2016;39(4):582–588. doi: 10.2337/dc15-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lotta LA, et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a mendelian randomisation analysis. PLoS Med. 2016;13(11):e1002179. doi: 10.1371/journal.pmed.1002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnusson M, et al. A diabetes-predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34(26):1982–1989. doi: 10.1093/eurheartj/ehs424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marschner IC, et al. Long-term risk stratification for survivors of acute coronary syndromes. Results from the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Study. LIPID Study Investigators. J Am Coll Cardiol. 2001;38(1):56–63. doi: 10.1016/S0735-1097(01)01360-2. [DOI] [PubMed] [Google Scholar]
- 29.Rasmiena AA, et al. Plasmalogen modulation attenuates atherosclerosis in ApoE- and ApoE/GPx1-deficient mice. Atherosclerosis. 2015;243(2):598–608. doi: 10.1016/j.atherosclerosis.2015.10.096. [DOI] [PubMed] [Google Scholar]
- 30. Test ID: CERAM Ceramides, Plasma. Mayo Clinic. https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/65054 Accessed August 28, 2018.
- 31.Design features baseline characteristics of the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) Study: a randomized trial in patients with previous acute myocardial infarction and/or unstable angina pectoris Am J Cardiol. 1995;76(7):474–479. doi: 10.1016/S0002-9149(99)80133-7. [DOI] [PubMed] [Google Scholar]
- 32.ADVANCE Collaborative Group. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 33.Alshehry ZH, Barlow CK, Weir JM, Zhou Y, McConville MJ, Meikle PJ. An efficient single phase method for the extraction of plasma lipids. Metabolites. 2015;5(2):389–403. doi: 10.3390/metabo5020389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White HD, et al. Association of contemporary sensitive troponin I levels at baseline and change at 1 year with long-term coronary events following myocardial infarction or unstable angina: results from the LIPID Study (Long-Term Intervention With Pravastatin in Ischaemic Disease) J Am Coll Cardiol. 2014;63(4):345–354. doi: 10.1016/j.jacc.2013.08.1643. [DOI] [PubMed] [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.