Abstract
Hemorrhagic shock depletes nicotinamide adenine dinucleotide (NAD) and causes metabolic derangements that, in severe cases, cannot be overcome, even after restoration of blood volume and pressure. However, current strategies to treat acute blood loss do not target cellular metabolism. We hypothesized that supplemental nicotinamide mononucleotide (NMN), the immediate biosynthetic precursor to NAD, would support cellular energetics and enhance physiologic resilience to hemorrhagic shock. In a rodent model of decompensated hemorrhagic shock, rats receiving NMN displayed significantly reduced lactic acidosis and serum IL-6 levels, two strong predictors of mortality in human patients. In both livers and kidneys, NMN increased NAD levels and prevented mitochondrial dysfunction. Moreover, NMN preserved mitochondrial function in isolated hepatocytes cocultured with proinflammatory cytokines, indicating a cell-autonomous protective effect that is independent from the reduction in circulating IL-6. In kidneys, but not in livers, NMN was sufficient to prevent ATP loss following shock and resuscitation. Overall, NMN increased the time animals could sustain severe shock before requiring resuscitation by nearly 25% and significantly improved survival after resuscitation (P = 0.018), whether NMN was given as a pretreatment or only as an adjunct during resuscitation. Thus, we demonstrate that NMN substantially mitigates inflammation, improves cellular metabolism, and promotes survival following hemorrhagic shock.
Keywords: Inflammation, Metabolism
Keywords: Bioenergetics, Mitochondria, Surgery
Resuscitation of severe hemorrhagic shock with nicotinamide mononucleotide mitigates inflammation, improves cellular metabolism, and promotes survival.
Introduction
Hemorrhagic shock is a physiologic condition that occurs with rapid blood loss and is characterized by profound vasoconstriction, tissue hypoperfusion, and cellular hypoxia. In response to decreased oxygen tension, there is a dramatic decline in oxidative phosphorylation, with increased anaerobic metabolism in an attempt to preserve cellular energy status (1). Without prompt and adequate resuscitation, hemorrhagic shock progresses from cellular dysfunction to organ failure and, ultimately, to death (2).
Nicotinamide adenine dinucleotide (NAD) is a ubiquitous molecule that plays a key role in cellular metabolism by accepting and donating electrons via interconversion with NADH. NAD is required at the GAPDH-dependent step in glycolysis and provides reducing equivalents to complex I of the electron transport chain to drive oxidative phosphorylation. NAD also serves as an essential cosubstrate for a wide variety of enzymes involved in cellular resilience, including sirtuins and poly (ADP ribose) polymerases (3). During hemorrhagic shock, the tissue concentration of NAD falls rapidly in proportion to the severity of the injury (4). The decreased availability of NAD is further exacerbated by a redox shift in favor of NADH, which is no longer efficiently reoxidized to NAD by the mitochondrial electron transport chain under hypoxic conditions (5). In other contexts, it is well established that NAD depletion leads to mitochondrial dysfunction and cell death (6, 7), and enhancing NAD has been shown to improve tissue function (8–10). Given the central role of NAD in cellular energy metabolism, signaling, and a host of other biochemical reactions, resuscitative strategies that restore NAD could prove therapeutically useful in hemorrhagic shock.
NAD can be synthesized de novo from dietary tryptophan, nicotinic acid (niacin), or intermediates in the synthesis pathways, but the majority of cellular NAD comes from the recycling of liberated nicotinamide. Nicotinamide is converted to nicotinamide mononucleotide (NMN) in a rate-limiting step catalyzed by the enzyme nicotinamide phosphoribosyltransferase (NAMPT). NMN is then converted to NAD by 1 of 3 NMN adenylyltransferase isoforms (NMNAT1–3) (11). Chaudry and colleagues first described using NAD precursors during the resuscitation of hemorrhagic shock in 1976 (12). Although the infusion of NAD, nicotinamide, and niacin each increased NAD concentrations in both liver and kidney tissue, treatment did not restore tissue ATP levels and did not improve survival. As such, these investigators concluded that “these infusions have no salutary effects,” and research investigating the impact of NAD metabolism during hemorrhagic shock remained relatively stagnant in the following decades. However, it is possible that the minimal resuscitation protocol in Chaudry’s pioneering experiments may have limited the potential benefit. Recently, very high-dose oral niacin (1,080 mg/kg) has been shown to improve lung injury following hemorrhagic shock (13). When given at the time of resuscitation, niacin restored lung NAD concentrations to baseline values, mitigated inflammation, and transformed a uniformly lethal model into one with 30% survival. Thus, the use of NAD precursors such as NMN warrants further investigation.
Although the benefit of NMN supplementation has not been investigated in trauma, there is a growing body of literature to suggest that NMN could prove therapeutically useful. Providing exogenous NMN bypasses the need for NAMPT in the salvage pathway and has been shown to increase tissue NAD rapidly (14). This is particularly important, given the fact that NAMPT may be suppressed in the setting of ischemia/reperfusion and depressed NAMPT activity could contribute to the decreased NAD observed in hemorrhagic shock (15). Moreover, the NAMPT-catalyzed reaction is energetically costly and, thus, providing its product directly could be especially helpful when cells are energetically stressed (16). Importantly, there is evidence that NMN can acutely mitigate the effects of ischemia/reperfusion injury. In a murine model of cardiac infarction, pretreatment with NMN 30 minutes before ischemia significantly increased basal levels of NAD, prevented a decline in NAD postischemic insult, and reduced infarct size (17). As such, using NMN as a resuscitative adjunct to support metabolism could improve cellular resilience.
In this study, we investigate the metabolic and mitochondrial effect of exogenous NMN on hemorrhagic shock. Our findings suggest that NMN preserves oxidative phosphorylation, enhances physiologic reserve, and improves survival after severe shock.
Results
Pretreatment and resuscitation with NMN during fixed pressure hemorrhagic shock reduces lactic acidosis.
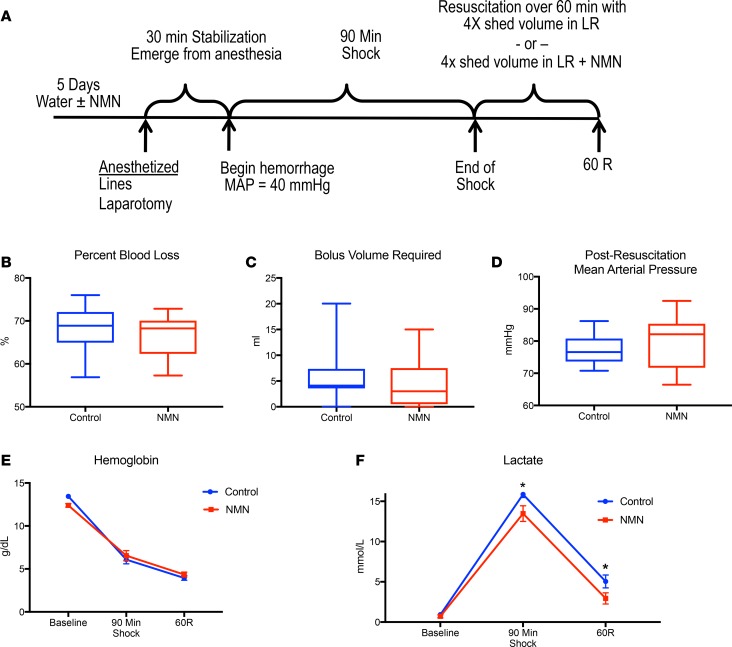
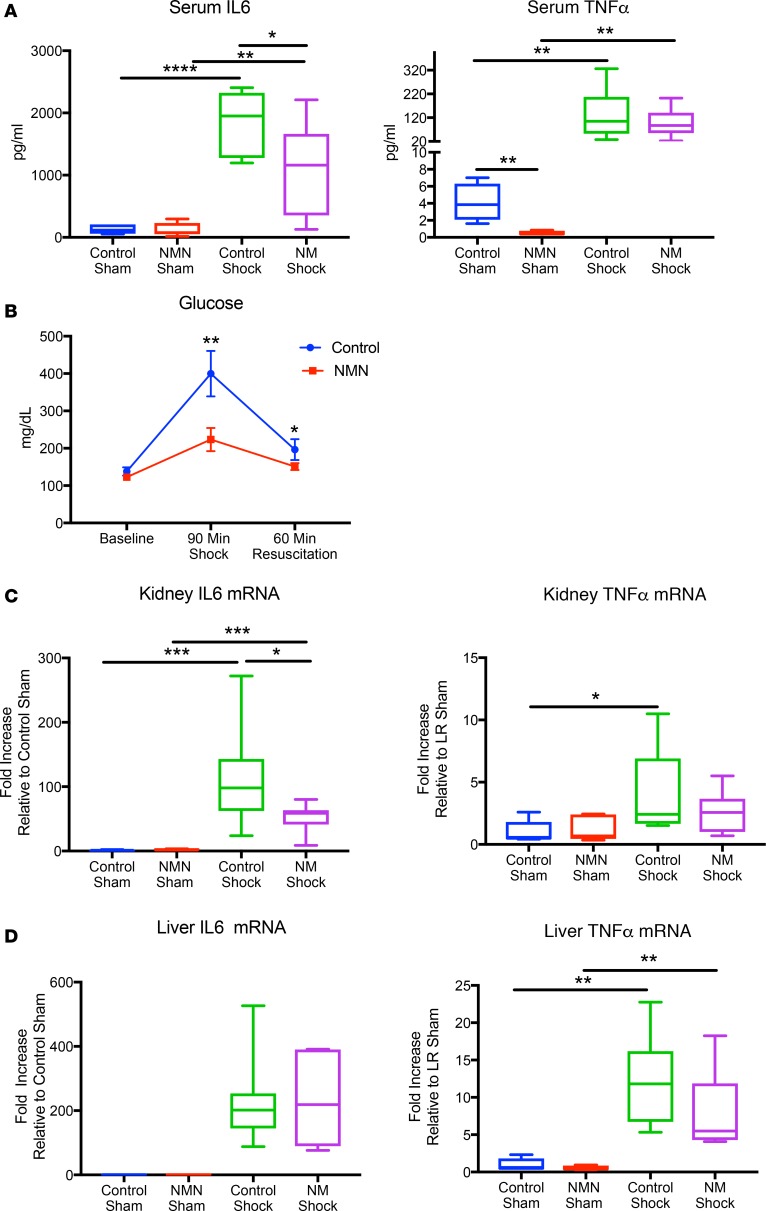
To investigate the physiologic effect of NMN during hemorrhagic shock and resuscitation, we subjected NMN-pretreated rats (400 mg/kg/d for 5 days, oral) and controls to fixed pressure shock followed by resuscitation with and without NMN (400 mg/kg, intravenous) (Figure 1A). The dose of NMN was proportional to body weight, and the volume of resuscitation was 4 times the volume of blood shed during hemorrhagic shock. NMN and control animals were maintained at a mean arterial blood pressure (MAP) of 40 mmHg for 90 minutes. Both groups shed a similar percentage of blood volume and had similar reductions in circulating hemoglobin (Figure 1, B and E). While the volume of fluid required to maintain the shock state for 90 minutes and the blood pressure following resuscitation were statistically indistinguishable between the groups, NMN-treated rats received slightly less volume overall, indicating that they were not resuscitated more completely than controls (Figure 1, C–E). Importantly, despite experiencing the same degree and duration of blood loss, NMN-treated rats had significantly lower serum lactate than did controls during hemorrhagic shock and resuscitation (Figure 1F). As a byproduct of anaerobic metabolism, lactate is used to gauge the success of resuscitation in humans (18). Elevated lactate levels, which reflect ongoing tissue hypoperfusion, correlate with both the severity and survivability of shock in injured patients (19). Even small differences in lactate can translate into significant survival benefits. In fact, for every 1 mM increase in the initial lactate recorded in trauma patients, mortality was found to increase by 15% (20). As such, the 2–3 mM decrease in lactate observed following resuscitation with NMN in our model could be physiologically relevant.
Figure 1. Experimental design and physiologic variables.
Animals were randomized to water with or without NMN (400 mg/kg/d) for 5 days (n = 9–12 per group). Animals were bled to a mean arterial blood pressure (MAP) of 40 mmHg for 90 minutes and then resuscitated with 4 times the shed volume in lactated Ringer’s with or without NMN (400 mg/kg) over 60 minutes (A). Control animals did not differ from NMN-treated animals in terms of percentage of total blood volume shed (B), the volume of LR needed to maintain a MAP of 40 mmHg for 90 minutes (C), or the MAP after 60 minutes of resuscitation (D). Hemoglobin was similar between groups (E). NMN-treated animals had significantly lower lactate levels during shock and following resuscitation (F). Data in B–D are represented using box-and-whiskers plots, with boxes representing the IQR, lines representing the median value, and whiskers representing minimum and maximum values, whereas data in E and F are represented as mean ± SEM. *P < 0.05, 2-tailed Student’s t test.
NMN increases NAD and preserves bioenergetics.
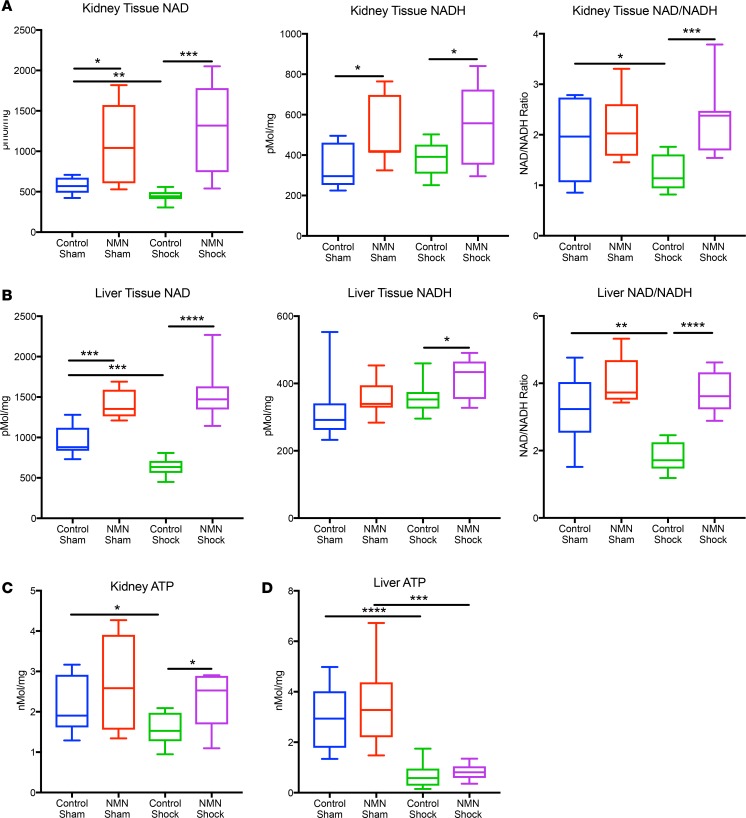
Hemorrhagic shock results in significantly depressed pyridine and adenine nucleotide pools, which can negatively effect the function of vital organs (21). We tested whether exogenous NMN could preserve NAD and ATP levels in kidney and liver tissues following shock. In sham animals, NMN significantly increased NAD levels in both kidneys and livers (Figure 2, A and B, and Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.120182DS1). Following resuscitation, NAD levels were nearly 3-fold higher in NMN-treated animals compared with shock control animals. NADH was also significantly higher in NMN-treated animals following hemorrhagic shock. Interestingly, the decline in NAD induced by hemorrhagic shock (in the absence of supplemental NMN) was less dramatic than in previous reports (12). We speculated that this discrepancy might be secondary to differences in how the animals were resuscitated and when the tissues were harvested. In Chaudry’s original investigation, animals were not pretreated with NAD precursors. Moreover, they were resuscitated with only the shed blood and samples were taken 30 minutes later. It is now recognized that this strategy leaves the animal under resuscitated (22). In contrast, we resuscitated with 4 times the shed volume in lactated Ringer’s (LR) crystalloid and harvested tissues 1 hour later. When we assayed NAD following hemorrhagic shock in unresuscitated animals, we observed a larger decrease (32% in kidney and 51% in liver, Supplemental Figure 1), consistent with the decrease originally described by Chaudry and colleagues (12). Thus, our results suggest that resuscitation per se partially restores NAD but does not reverse the robust depression of the NAD/NADH ratio following shock (Figure 2, A and B, and Supplemental Figure 1). Importantly, NMN completely mitigated the decrease in NAD concentration and preserved the NAD/NADH redox ratio in both tissues.
Figure 2. NMN increases NAD and NADH and preserves renal ATP following resuscitation from hemorrhagic shock.
NAD, NADH, and ATP levels were measured in extracts from snap frozen tissues harvested 1 hour after 90 minutes of hemorrhagic shock, followed by resuscitation with or without NMN (400 mg/kg). In the kidney, NMN increased the NAD and NADH levels in both sham (n = 5–6 per treatment group) and shocked animals (9–12 per treatment group) and prevented the sharp decline in the NAD/NADH ratio observed in shocked animals (A). In the liver, NMN increased NAD levels in both sham and shocked animals but only increased NADH significantly in shocked animals, while again preserving the NAD/NADH ratio (B). ATP levels declined in both tissues following hemorrhagic shock and resuscitation (C and D). Treatment with NMN, however, completely prevented this decline in renal tissue (C). One-way ANOVA was used to compare groups with a post hoc 2-tailed Student’s t or Mann-Whitney test if statistically significant (P < 0.05). Data are represented using box-and-whisper plots, with boxes representing the IQR, lines representing the median value, and whiskers representing minimum and maximum values. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Hemorrhagic shock and resuscitation are also known to deplete ATP reserves, likely as a consequence of mitochondrial dysfunction, which results in increased reliance on anaerobic metabolism. As expected, ATP levels were significantly decreased following shock and resuscitation in both kidneys and livers (Figure 2, C and D). NMN treatment substantially rescued ATP levels in the kidney, whereas liver stores were more depressed and could not be rescued with NMN (Figure 2, C and D).
NMN preserves NAD-dependent mitochondrial respiration.
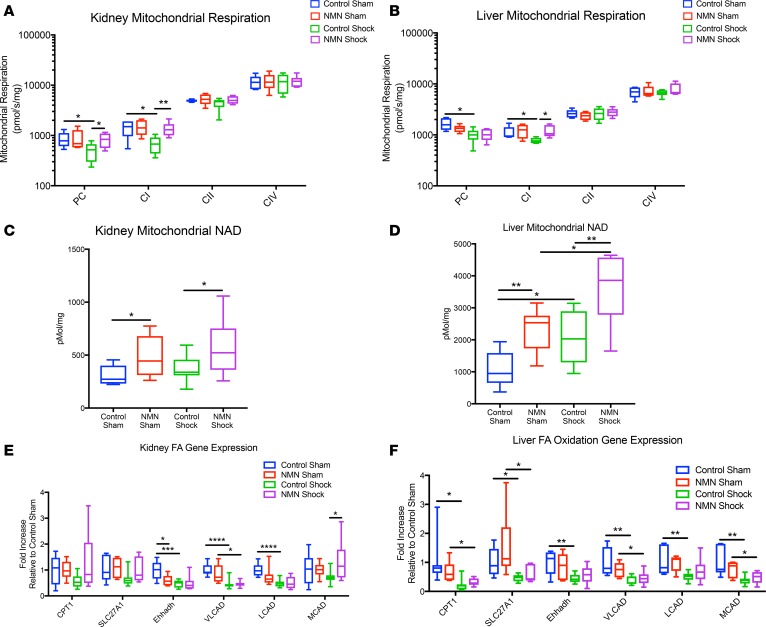
Given the restoration of ATP in the kidney and decrease in lactate accumulation, we wondered if NMN treatment could positively effect mitochondrial respiration. We found that hemorrhagic shock and resuscitation impaired complex I–dependent (CI-dependent) respiration in both tissues when fatty acids or a standard pyruvate/glutamate/malate mixture were provided as substrates (Figure 3, A and B). No defect was apparent when electrons were supplied directly to complex II (CII) via succinate or to complex IV via TMPD/ascorbate. Since CI accepts electrons from NADH, these results suggest a deficiency in the NAD pool, an upstream factor involved in the generation of NADH, or in CI itself. Consistent with the possibility that NAD, and subsequent NADH formation, is the limiting factor, treatment with NMN completely preserved CI-dependent respiration following hemorrhagic shock (Figure 3, A and B).
Figure 3. NMN preserves complex I–dependent mitochondrial respiration and enhances mitochondrial NAD content.
Following 90 minutes of hemorrhagic shock, tissues were harvested 1 hour after resuscitation (with or without NMN 400 mg/kg). Mitochondria from kidney and liver tissue were freshly isolated and evaluated using high-resolution respirometry (log10 scale). In both tissues, a defect in respiration was noted when NAD-dependent substrates, such as palmitoylcarnitine (PC) and pyruvate/glutamate/malate (CI), were used. Pretreatment, and subsequent resuscitation, with NMN completely restored mitochondrial respiration in the kidney (A) and preserved respiration with CI substrates in the liver (B). Without NMN treatment, NAD content was preserved following resuscitation in the kidney (C) and increased in the liver (D). NMN treatment augmented mitochondrial NAD levels in both the sham and shocked states (C and D). n = 5–6 per treatment group. Data are represented using box-and-whisper plots, with boxes representing the IQR, lines representing the median value, and whiskers representing minimum and maximum values. We measured the mRNA expression of key enzymes in fatty acid metabolism, including carnitine palmitoyltransferase 1 (CPT1), long-chain fatty acid transport protein 1 (SLC27A1), enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase (Ehhadh), very-long-chain acyl-CoA dehydrogenase (VLCAD), long-chain acyl-CoA dehydrogenase, (LCAD), and medium-chain acyl-CoA dehydrogenase (MACD). The expression of key transcripts related to fatty acid oxidation was decreased by hemorrhagic shock in both the liver and kidney, but NMN tended to restore a subset of these only in the kidney (E and F). One-way ANOVA was used to compare groups with a post hoc 2-tailed Student’s t or Mann-Whitney test if statistically significant.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
To more directly test the hypothesis that mitochondrial NAD depletion causes the defect in CI-dependent respiratory capacity, we next sought to determine NAD levels in isolated mitochondria following shock. To our knowledge, the mitochondrial NAD pool, which is separate from the nuclear and cytosolic pool (23), has not previously been examined in hemorrhagic shock. Although NMN significantly increased mitochondrial NAD in both the kidney and liver, we were surprised to discover that hemorrhagic shock did not decrease mitochondrial NAD. In fact, in contrast to whole tissue NAD, mitochondrial levels were maintained in kidneys (Figure 3C) and significantly increased in the livers of control animals following shock (Figure 3D). NMN treatment further augmented mitochondrial NAD concentrations (Figure 3, C and D). Thus, the size of the mitochondrial NAD pool per se cannot account for the changes in mitochondrial respiration. NADH was not reproducibly detected in isolated mitochondria, most likely due to collapse of the redox potential during isolation.
Interestingly, mitochondria exhibited a reduced capacity for fatty acid oxidation after hemorrhagic shock, and this effect was reversed by NMN in organelles from the kidney but not the liver. Similarly, we found that the expression of key transcripts related to fatty acid oxidation was decreased by hemorrhagic shock in both the liver and kidney but that NMN tended to restore a subset of these only in the kidney (Figure 3, E and F). Thus, the mRNA expression of transcripts related to fatty acid oxidation correlates with the ability of isolated mitochondria to oxidize palmitoyl carnitine as well as whole-tissue ATP levels.
NMN and hemorrhagic shock influence the expression of enzymes in the NAD salvage pathway.
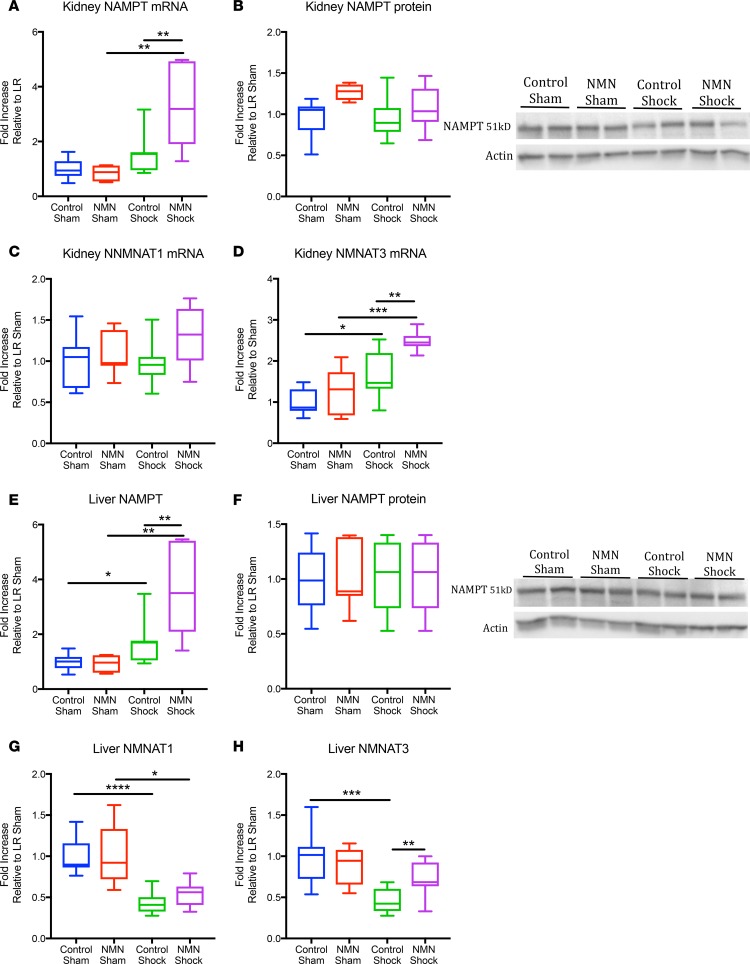
To better understand the effect of hemorrhagic shock on total and mitochondrial NAD levels, we examined the expression patterns of enzymes in the NAD salvage pathway. Metabolic stress influences the expression of NAMPT, and increasing NAMPT can mitigate ischemic injury in a variety of models (15, 17, 24, 25). The expression of NAMPT and the downstream enzymes NMNAT1–3, however, have not previously been examined in hemorrhagic shock. Following shock, there was a trend toward increased NAMPT gene expression in both kidneys and livers (Figure 4, A and E). Of the 3 NMNAT isoforms, NMNAT1 (nuclear) and NMNAT3 (mitochondrial) were readily detected, while NMNAT2 (which has been reported to bind the cytosolic face of the Golgi complex and be present in axons) was not (11). Hemorrhagic shock and resuscitation had minimal effect on the renal expression of NMNAT1 but modestly increased expression of NMNAT3. In contrast, the expression of both enzymes was reduced in liver tissue (Figure 4, C, D, G, and H). Although NMN had no effect on NAMPT or NMNAT gene expression in sham-operated rats, it enhanced the gene expression of both NAMPT and NMNAT3 relative to controls following hemorrhagic shock (Figure 4, A, D, E, and H). Shock, however, was not associated with any clear change in NAMPT protein expression at the end of resuscitation (Figure 4, B and F). These results indicate that NMN can influence NAD metabolism in part via enzyme expression independently from its direct contribution to synthesis.
Figure 4. NMN increases NAMPT and NMNAT3 expression following hemorrhagic shock.
Following hemorrhagic shock, there was a trend toward increased NAMPT mRNA expression that was further enhanced with NMN treatment in the kidney (A) and the liver (E). NAMPT protein expression was not affected by shock in either tissue (B and F). NMNAT1 expression was not affected in the kidney (C) but declined following shock and resuscitation in the liver and was not affected by NMN (G). NMNAT3 expression was increased in the kidney and decreased in the liver following shock but was enhanced in both tissues by NMN treatment only in animals that had been shocked (D and H). n = 5–12 per treatment group. Data are represented using box-and-whisper plots, with boxes representing the IQR, lines representing the median value, and whiskers representing minimum and maximum values. One-way ANOVA was used to compare groups with a post hoc 2 tailed Student’s t or Mann-Whitney test if statistically significant.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
NMN mitigates inflammation following hemorrhagic shock and resuscitation.
Hemorrhagic shock and resuscitation results in a proinflammatory state characterized by elevated cytokine levels, oxidative stress, and insulin-resistant hyperglycemia. Circulating levels of the cytokine IL-6 are a strong predictor of mortality, and directly targeting IL-6 is beneficial, suggesting that inflammation mediated by this pathway is highly relevant to the pathological effects of hemorrhagic shock (26, 27). Given the key role that NAD plays in modulating sirtuin-dependent inflammatory pathways (28), we hypothesized that NMN might dampen the inflammatory response following hemorrhagic shock and resuscitation. Indeed, pretreatment and resuscitation with NMN significantly reduced systemic IL-6 cytokine levels and tended to decrease TNF-α, while substantially ameliorating shock-induced hyperglycemia (Figure 5, A and B). Interestingly, basal TNF-α was significantly reduced in sham-treated animals, suggesting that NMN can have a potent effect on this pathway, although the repression was largely overcome during shock. At the tissue level, NMN also significantly improved the cytokine profile after resuscitation in the kidney (Figure 5C). Whether these changes in gene expression reflect immune cell infiltration or the activation of inflammatory pathways in resident cells is not yet clear. In contrast, NMN had no effect on the induction of inflammatory genes in the liver (Figure 5D).
Figure 5. NMN mitigates inflammation following hemorrhagic shock.
Serum IL-6 and TNF-α were determined by ELISA. As expected, serum levels of both proinflammatory cytokines increased with hemorrhagic shock, whereas NMN significantly lowered IL-6 with a trend toward improved TNF-α levels (A). A decrease in proinflammatory cytokines corresponded with a reduction in hyperglycemia following shock (B). Inflammatory cytokine expression was measured by qPCR in kidney and liver tissues. NMN partially mitigated IL-6 mRNA expression in the kidney but not in the liver (C and D). n = 5–12 per treatment group. Data are represented using box-and-whisper plots, with boxes representing the IQR, lines representing the median value, and whiskers representing minimum and maximum values. One-way ANOVA was used to compare groups with a post hoc 2-tailed Student’s t or Mann-Whitney test if statistically significant.*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
NMN improves mitochondrial function in isolated hepatocytes.
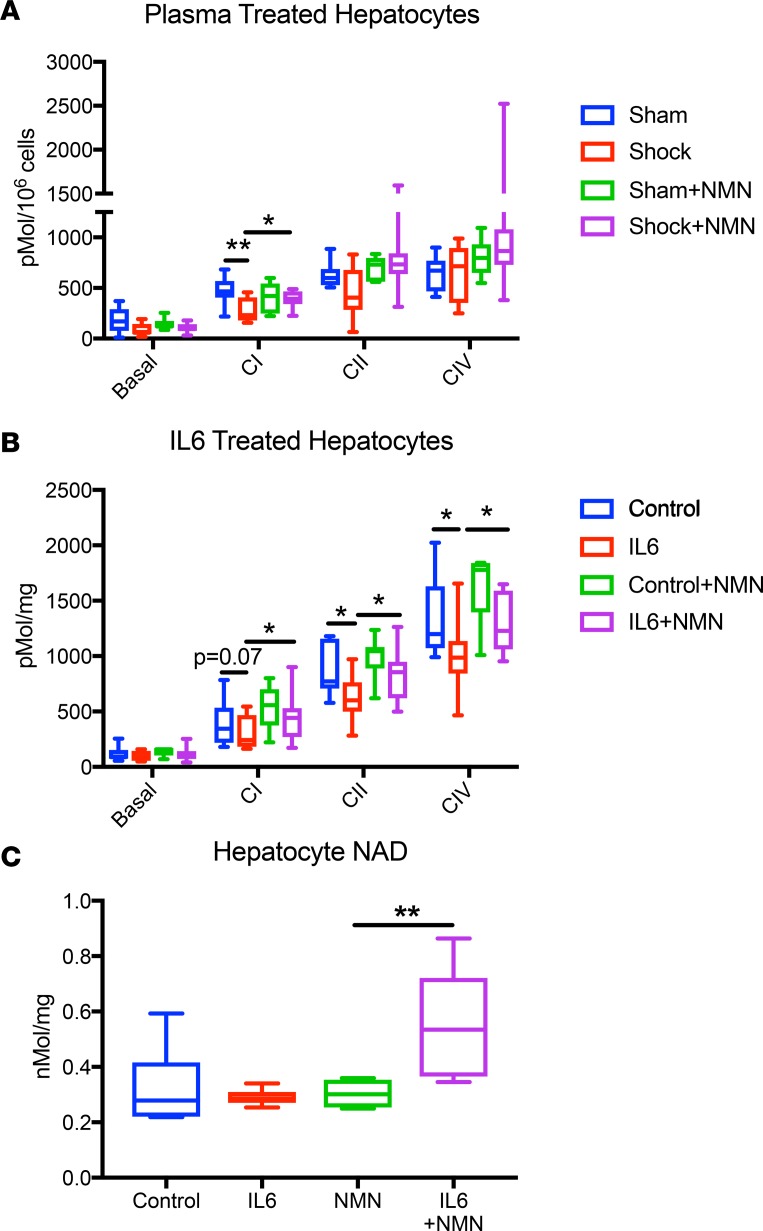
Because NMN improves the inflammatory status, its beneficial effects on mitochondrial function may be secondary to either decreased inflammation or direct protective effects in targets tissues. In order to determine whether NMN directly affects cellular mitochondrial function, we exposed primary hepatocytes to plasma harvested from both sham and shocked rats. Consistent with the hypothesis that circulating inflammatory factors can induce mitochondrial dysfunction, primary hepatocytes treated with shocked plasma had significantly depressed CI-dependent respiration. When hepatocytes were cocultured with NMN, however, this CI defect was completely prevented (Figure 6A).
Figure 6. NMN rescues mitochondrial function and increases NAD in isolated hepatocytes after cytokine exposure.
After coculture for 24 hours with plasma harvested from animals in hemorrhagic shock, digitonin-permeabilized isolated hepatocytes exhibited a defect in complex I–dependent respiration that was mitigated by concurrent treatment with NMN (100 μM) (A). When treated with IL-6 (20 ng/ml) for 1 hour, isolated hepatocytes developed global mitochondrial dysfunction, with decreased respiratory capacity at every complex that was mitigated by cotreatment with NMN (100 μM) (B). NMN increased NAD levels only in cells that were cotreated with IL-6 (C). n = 6–8 per group. Data are represented using box-and-whisper plots, with boxes representing the IQR, lines representing the median value, and whiskers representing minimum and maximum values. One-way ANOVA was used to compare groups with a post hoc 2-tailed Student’s t or Mann-Whitney test if statistically significant.*P < 0.05, **P < 0.01.
Given that plasma from shocked and sham animals may have varying concentrations of many different proinflammatory cytokines, we also treated primary hepatocytes with a fixed concentration of IL-6. In these experiments, cotreatment with NMN again completely prevented mitochondrial dysfunction (Figure 6B). Thus, NMN can preserve mitochondrial function in a cell-autonomous manner, although the reduction in circulating IL-6 also likely contributes to its net benefit in vivo.
Interestingly, treatment with IL-6 did not significantly effect NAD levels in our primary hepatocytes. However, with the addition of NMN, cytokine-exposed cells nearly doubled their NAD over baseline, suggesting that inflammation enhances either NMN uptake or the enzymatic activity of NAD biosynthesis pathways or decreases NAD turnover (Figure 6C).
NMN treatment does not lead to major changes in mitochondrial oxidative damage or global acetylation profiles following hemorrhagic shock.
Given the relationship between SIRT1 activation and enhanced antioxidant expression, we examined the expression of key antioxidant enzymes as well as oxidative damage in mitochondria. Although NMN significantly increased the mRNA expression of GPX1 in the liver, its effects on the mRNA expression of antioxidant genes were small and inconsistent across tissues (Supplemental Figure 2). Consistently, NMN did not have a measurable effect on mitochondrial lipid peroxidation 1 hour after resuscitation (Supplemental Figure 2C). Because mitochondrial NAD levels could affect the activity of the major mitochondrial deacetylase, SIRT3, we also investigated the acetylation status of mitochondrial proteins. Contrary to expectation, pretreatment with NMN led to a modest increase in mitochondrial protein acetylation in sham animals, whereas no differences were apparent after resuscitation (Supplemental Figure 2B). Thus, neither oxidative damage nor changes in acetylation can easily explain the functional differences observed in CI following NMN treatment.
The effect of NMN on organ function.
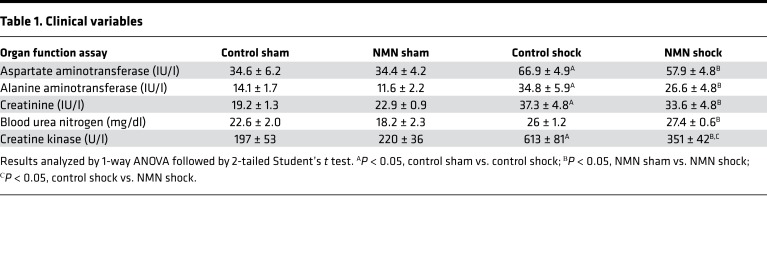
In order to determine if NMN preserved organ function, blood samples were taken from sham and shocked animals at the end of resuscitation and assayed for markers of liver, kidney, and cardiac dysfunction. Although hemorrhagic shock and resuscitation resulted in a significant increase in markers of liver and kidney damage, values remained within the normal range, with only a trend toward decreased injury in NMN-treated animals (Table 1). The fact that these injury markers remained relatively low is likely a reflection of the early time point examined. Even at this early time point, however, hemorrhagic shock and resuscitation were associated with an increased in creatine kinase activity that was mitigated by NMN. Creatine kinase levels can result from either skeletal or cardiac muscle damage. Acute blood loss does not generally lead to skeletal muscle damage in the absence of crush injury and is associated more strongly with cardiac injury (29). Thus, NMN may have cardioprotective effects that warrant further investigation in this model.
Table 1. Clinical variables.
NMN enhances physiologic reserve and decreases mortality.
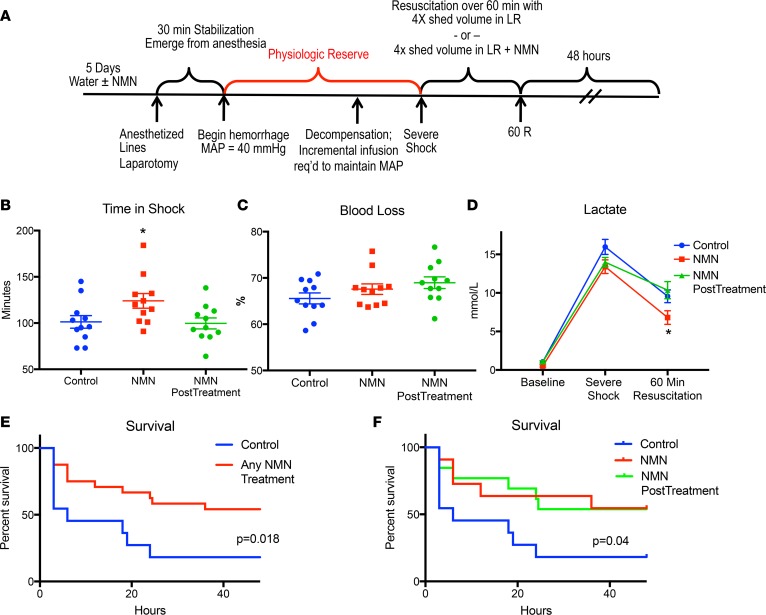
Based on improvements in inflammatory status, lactate metabolism, mitochondrial function, and trends toward decreased injury biomarkers, we hypothesized that NMN would enhance physiologic reserve and improve survival following hemorrhagic shock. Using a decompensated shock model, we performed a second set of experiments to evaluate physiologic reserve, as indicated by the overall time animals could be maintained in severe shock without resuscitation (Figure 7A). Pretreatment with NMN increased the time animals could sustain severe shock by nearly 25%, despite equal or greater blood loss when compared with controls (Figure 7, B and C). In spite of enduring this more strenuous injury, animals treated with NMN maintained lower lactate levels and exhibited improved survival (Figure 7, D and E). As such, pretreatment and subsequent resuscitation with NMN substantially improved the ability of rats to tolerate hemorrhagic shock.
Figure 7. NMN enhances tolerance to hemorrhagic shock.
Prior to the induction of hemorrhagic shock, animals received regular water or water containing 400 mg/kg/d NMN for 5 days. All rats (n = 11/group) were then bled to a mean arterial blood pressure (MAP) of 40 mmHg and then maintained at 40 mmHg, with incremental fluid boluses until 40% of the shed blood volume had been returned. Animals pretreated with water were then resuscitated with 4 times the shed volume in lactated Ringer’s (Control) or lactated Ringer’s with 400 mg/kg NMN over 60 minutes (NMN PostTreatment). Animals pretreated with NMN in their drinking water were also resuscitated with 4 times the shed volume in lactated Ringer’s plus 400 mg/kg NMN over 60 minutes (NMN). All animals (Control, NMN, NMN PostTreatment) were then observed for 48 hours (A). Animals pretreated with NMN were able to sustain a shock state longer than controls before requiring resuscitation (B), despite a similar percentage of blood loss (C). As expected control animals and animals that received NMN only during resuscitation (NMN PostTreatment) were similar in terms of shock time and blood loss. NMN-pretreated animals had lower blood lactate concentrations following resuscitation than control animals (D). Whether NMN was given as both a pretreatment and a resuscitation adjunct (NMN) or only an adjunct during resuscitation (NMN PostTreatment), animals treated with NMN were significantly more likely to survive than were controls (E). When both NMN pretreatment and resuscitation-only strategies were combined, animals treated with NMN had a clear survival advantage when compared with control animals (F). Data are represented as mean ± SEM. One-way ANOVA was used to compare groups with a post hoc 2-tailed Student’s t or Mann-Whitney test if statistically significant. Survival was analyzed using Kaplan-Meier curves with a log-rank Mantel-Cox test. *P < 0.05.
Although pretreatment and subsequent resuscitation with NMN could be useful when significant surgical blood loss (e.g., cardiac or vascular surgery) or traumatic injury is anticipated (e.g., combat), we wanted to determine if NMN could be used after injury to treat unexpected bleeding events or trauma. In order to address this issue, animals were subjected to our decompensated hemorrhagic shock model and then resuscitated with LR plus NMN (400 mg/kg). Although NMN given during resuscitation did not effect lactate levels in this model, it significantly improved 48-hour survival (Figure 7, D–F). These data suggest that NMN treatment is effective even after injury and lend support to the growing body of literature suggesting that NAD precursors can mitigate ischemia/reperfusion injury (15, 17, 30, 31).
Discussion
In the United States, traumatic injury is the leading cause of death in those under the age of 45, with nearly 40% of patients who develop hemorrhagic shock dying within the first 24 hours (32, 33). Because both the severity of blood loss and duration of hypoperfusion correlate directly with organ failure and mortality, current resuscitative strategies focus on rapidly repairing the injury, restoring blood volume, and improving perfusion pressure. If the shock state is too severe or prolonged, however, cellular metabolism remains depressed and resuscitation efforts fail to improve clinical outcomes. Developing therapies to restore and support cellular metabolism following hemorrhagic shock could potentially mitigate organ dysfunction and improve survival.
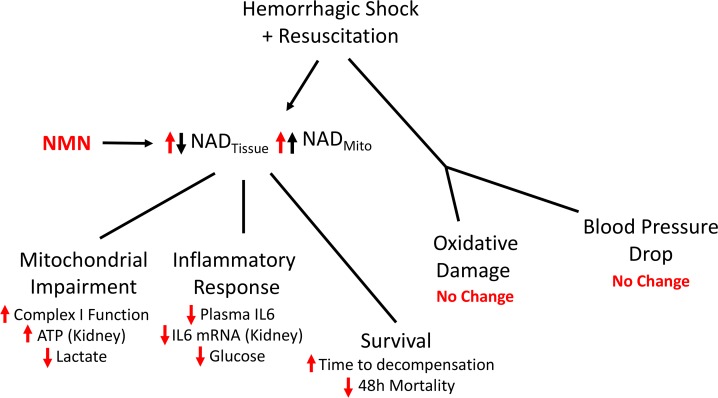
During prolonged hypoperfusion there is a dramatic decline in NAD availability due to both a reduction in the size of the NAD pool and a shift toward the reduced form (NADH). Given the essential roles that NAD plays in glycolysis, oxidative phosphorylation, and pathways that promote cellular resilience, we investigated the possible benefit of using NMN to restore NAD levels as a treatment for hemorrhagic shock (14, 34, 35). Although NMN could positively affect many tissues in our model (13, 36, 37), we specifically focused on the effect on bioenergetics in the kidney and liver, because these organs are at a high risk of failure following acute blood loss (38, 39). In the kidney and liver, pretreatment and subsequent resuscitation with NMN increased NAD levels, restored the NAD/NADH ratio, improved mitochondrial function, and mitigated inflammation (Figure 8). Importantly, NMN also enhanced whole organism physiologic resilience during hemorrhagic shock and improved survival following resuscitation. As such, NMN holds promise as a therapeutic adjunct and warrants further clinical investigation.
Figure 8. NMN improves the pathophysiology of hemorrhagic shock.
Hemorrhagic shock leads to decreased tissue perfusion followed by reperfusion injury that is characterized by decreased tissue NAD levels, oxidative damage, mitochondrial impairment, and inflammation. Interestingly, mitochondrial NAD levels do not fall but actually increase during hemorrhagic shock. Pretreatment and resuscitation with NMN augments both tissue and mitochondrial NAD stores and is associated with improved mitochondrial function, decreased inflammation, improved physiologic reserve, and decreased mortality, despite having no major effect on blood pressure or oxidative damage.
One of the most prominent metabolic effects of using NMN to restore NAD was the improvement in mitochondrial function. During hemorrhagic shock, there is a progressive defect in mitochondrial respiration when NAD-linked substrates, such as isocitrate, α-ketoglutarate, and β-hydroxybutyrate, are used, suggesting that severe blood loss preferentially inhibits CI (1, 40). Although NMN has been shown to improve overall mitochondrial respiratory capacity in chronic models of aging and Alzheimer’s disease (41, 42), our research provides the first data to our knowledge demonstrating that NMN completely preserves CI-dependent mitochondrial respiration following hemorrhagic shock. We initially hypothesized that this rescue was secondary to NMN’s ability to mitigate a decline in mitochondrial NAD. Our data, however, do not support this hypothesis. In fact, in contrast to the decline in total tissue NAD that we observed, mitochondria harvested from control animals actually had preserved or increased levels of NAD following hemorrhagic shock. Mitochondrial NAD levels were further enhanced in NMN-treated animals. Although little is known about the concentration of mitochondrial NAD following hemorrhagic shock, our findings do support Hift and Strawitz’s description of increased “light absorbing material” in isolated liver mitochondria harvested from dogs in “irreversible hemorrhagic shock.” Writing in 1961, these investigators attributed the increased spectrophotometric absorption peak noted at 265 to 270 mμ to “nucleotide material”(43). In retrospect, this likely represented NADH, with a UV absorption peak of 259 nm. Although the precise mechanisms accounting for the generation and maintenance of the mitochondrial NAD pool remain a subject of debate, the finding of increased mitochondrial NAD suggests either augmented cytoplasmic-to-mitochondrial transfer or enhanced mitochondrial NAD recycling (3). In either case, the ability to increase mitochondrial NAD following hemorrhagic shock may reflect an adaptive response to cellular stress and suggests that the depletion of cytosolic NAD may be even greater than indicated by bulk tissue measurements.
Given the fact that mitochondrial NAD levels do not decrease, and are in fact preserved during hemorrhagic shock, there must be another mechanism by which NMN benefits mitochondrial function. CI is prone to oxidant damage and dysfunction that can be rescued with the induction of antioxidant defenses (44–48). Increasing nuclear/cytosolic NAD has been shown to modulate the response to cellular stress by upregulating antiinflammatory and antioxidant pathways (49). In particular, SIRT1 uses NAD as an obligatory cosubstrate to deacetylate the RelA/p65 subunit of NF-κB in macrophages, leading to decreased expression of proinflammatory cytokines, such as IL-6 and TNF-α (50). SIRT1 may also decrease the damage associated with ischemia/reperfusion by deacetylating and activating PGC1α. In turn, PGC1α increases the expression of antioxidant enzymes as well as SIRT3, a mitochondrial NAD-dependent deacetylase that mitigates mitochondrial oxidative stress by directly activating isocitrate dehydrogenase 2 and manganese superoxide dismutase (51, 52). In support of a potential role for SIRT1, we and others have shown that resuscitation with resveratrol, a SIRT1 activator, improves mitochondrial function, attenuates inflammation, mitigates oxidant damage, and improves organ function following hemorrhagic shock (53–56). Similarly, Jeong et al. demonstrated that high-dose oral niacin (another NAD precursor and SIRT1 activator) attenuates systemic inflammation and is associated with decreased pulmonary NF-κB expression and oxidative stress in a rodent model of hemorrhagic shock (13). In this study, NMN mitigated both the systemic and tissue-specific inflammatory response. Importantly, changes in systemic inflammation were not purely responsible for improved mitochondrial function because NMN also preserved CI-dependent mitochondrial respiration in permeabilized hepatocytes exposed to a controlled dose of inflammatory cytokines. Thus, NMN also appears to have a cell-autonomous protective effect against damage induced by inflammatory stress.
Interestingly, while NMN rescued NAD levels and mitochondrial function in both the kidney and liver, it only preserved ATP in the kidney. The total amount of ATP, however, is influenced by both production and consumption. During hemorrhagic shock, ATP consumption is significantly decreased in the kidney because hypotension leads to decreased glomerular filtration and thus a decreased need for ATP dependent resorption in the distal nephron. In contrast, ATP consumption in the liver actually escalates during hemorrhagic shock because increased sodium influx requires increased Na+-K+ ATPase activity (57). Thus, failure to rescue ATP in the liver may not reflect a tissue difference in how NMN affects mitochondrial function but rather a difference in the degree of energetic stress experienced by these two tissues during shock.
In contrast to our promising results with NMN, prior studies using infusion of NAD, nicotinamide, or niacin as NAD precursors during the resuscitation of hemorrhagic shock failed to improve tissue energetics or overall survival, despite improving NAD concentration (12). One advantage of NMN over nicotinamide or niacin is that it can be incorporated into the cellular NAD pool without an energetically costly phosphoribosyltransferase step (14), which may be a crucial distinction in energetically stressed cells. In this regard, nicotinamide riboside may also be interesting to test, as it can be converted to NMN intracellularly by the action of nicotinamide riboside kinases (58). Experiments in cells suggest that NAD itself is not readily taken up intact but must instead be broken down to nicotinamide riboside or nicotinamide before crossing the plasma membrane (59). Thus, NMN has some advantages over the NAD precursors that were used by Chaudry et al., although other factors, such as the resuscitation protocol, may have also contributed to the better outcomes in our study. Indeed, we provided substantially more fluid during resuscitation than in the original Chaudry method and speculate that continued hypoperfusion might have masked any protective effect of restored NAD in those experiments. Moreover, Jeong et al. recently used oral niacin in combination with shed blood resuscitation to ameliorate lung injury following hemorrhagic shock (13). Thus, the rejection of NAD precursors as therapeutic agents in hemorrhagic shock may have been premature.
Very little is known about the NAD salvage pathway in hemorrhagic shock. We wondered if decreased expression of either NAMPT or NMNAT contributed to the NAD depletion during shock or, alternatively, whether any compensatory response would be present. Although ischemia/reperfusion injury decreases NAMPT expression in cardiomyocytes (22), hemorrhagic shock appears to increase the expression of NAMPT mRNA in the liver and tended to increase it in the kidney. Surprisingly, NMN supplementation further enhanced NAMPT mRNA expression in both tissues following resuscitation but not in sham NMN-treated controls. We were also interested in determining if the expression of NMNAT1–3 changed following hemorrhagic shock. NMNAT2 was not reliably detected, but we were able to quantify transcripts for NMNAT1 and NMNAT3, which convert NMN to NAD in the nucleus/cytoplasm and mitochondria, respectively. Both NMNAT1 and NMNAT3 were suppressed due to shock in the liver, suggesting that NAD biosynthetic capacity may be decreased in this organ during acute blood loss. In contrast, NMNAT1 was not affected and NMNAT3 was increased in the kidney. Intriguingly, NMN enhanced NMNAT3 expression following shock in both organs. The induction of transcripts related to NAD biosynthesis, particularly when precursors are available, likely reflects an adaptive response to cellular stress and may explain why our isolated hepatocytes only dramatically increased NAD in the presence of both IL-6 and NMN (23). Thus, hemorrhagic shock leads to organ-specific changes in the expression of genes related to NAD biosynthesis, and we reveal that, in the setting of severe injury, the direct contribution of NMN to the NAD pool may be augmented by the induction of biosynthetic enzymes.
While encouraging, our study has a number of limitations. First, the mechanisms by which NAD improves mitochondrial function and survival remain to be fully elucidated. Given that mitochondrial NAD levels do not decrease following hemorrhagic shock, NMN’s ability to restore mitochondrial function in the kidney and liver must be occurring through a pathway that is distinct from NAD content. It is possible that nuclear or cytosolic NAD deficiency following hemorrhagic shock might cause mitochondrial dysfunction indirectly by altering the activity of NAD-dependent enzymes, including sirtuins, PARPs, or CD38 (7, 60, 61). Second, we did not investigate the optimal timing or duration of therapy. This is particularly important because the duration of increased NAD and the timing of SIRT1 activation may influence how acute inflammation progresses to a more chronic hypoinflammatory state (28). Finally, although we did see a trend toward improved organ function following hemorrhagic shock in NMN-treated animals, studying early changes in mitochondrial function required sacrificing rats at a time point that was not optimal for these assays; future studies over an extended time course may strengthen these findings.
Together, our findings suggest that NMN holds significant promise as an adjunct to resuscitation. In our severe hemorrhagic shock model, NMN supported cellular metabolism by increasing tissue NAD levels, preserving the cellular redox ratio, and enhancing CI-dependent mitochondrial respiration. NMN also appeared to have antiinflammatory properties, blunting both the systemic inflammatory response as well as the cellular effects of cytokine exposure. Importantly, NMN’s biochemical and immunologic benefits translated to improved physiologic resilience. NMN-pretreated animals were able to tolerate longer periods of hypoperfusion, and animals resuscitated with NMN exhibited improved survival. Given these encouraging preclinical findings, future research investigating NMN’s therapeutic potential in injured patients is warranted.
Methods
All chemicals and cell culture solutions were obtained from MilliporeSigma or Thermo Fisher Scientific, unless otherwise stated.
Animals and experimental procedures.
Male Long-Evans rats (250–300 g, Charles River Laboratories) were housed with constant temperature, humidity, and a timed 12-hour-light/dark cycle. Animals were allowed to acclimate for at least 3 days before randomization to either plain drinking water or water with NMN (400 mg/kg/d, a gift from Metro International Biotech LLC). Animals were given access to standard rat chow (PMI Rodent Diet, LabDiet) and water (with or without NMN) ad libitum for 5 days prior to surgery.
Spontaneously breathing rats were anesthetized using vaporized isoflurane by mask (2%–4%, MilliporeSigma) and underwent sterile placement of femoral vascular catheters (PE50, Braintree Scientific Inc.). MAP was recorded throughout the experimental protocol (Digi-Med Signal Analyzers). A sterile prepped 5-cm midline laparotomy was performed to simulate soft tissue trauma. All surgical sites were bathed in 1% lidocaine (APP Fresenius Kabi) and closed in layers. Animals received 0.25% buprenorphine (0.05 mg/kg, subcutaneously, Reckitt Benkiser Healthcare Ltd.). Animals were then placed in a plexiglass restraining apparatus and allowed to fully emerge from anesthesia (~30 minutes).
Animals were randomized to either fixed pressure hemorrhagic shock (n = 9–12 per treatment group) or sham controls (n = 5 per treatment group). Shocked animals were passively bled via the femoral artery and maintained at a MAP of 40 mmHg for 90 minutes. If the MAP fell below 40 mmHg, small boluses (0.2 ml) of intravenous LR were provided to maintain the MAP. At 90 minutes, animals were intravenously resuscitated with 4 times the shed blood volume in LR with or without sterile filtered NMN (400 mg/kg) over 60 minutes (60R). Sham animals were gently restrained for 90 minutes and then were given 5 ml of LR with or without sterile filtered NMN (400 mg/kg) over 60 minutes). Blood samples were taken at Baseline, 90 minutes and 60R (Figure 1). Following resuscitation, animals were again anesthetized with isoflurane (2%–4%, MilliporeSigma). Liver and kidney tissues were rapidly harvested after 90 minutes of shock or at 60R. Animals were euthanized under anesthesia.
For survival experiments, animals were randomized to water with or without NMN (400 mg/kg/d) for 5 days prior to surgery. Animals underwent general anesthesia with vascular access, laparotomy, and full reversal as described. Animals were then randomized to either decompensated hemorrhagic shock (n = 11 per treatment) or served as sham controls (n = 5 per treatment) (62). Shocked animals were passively bled via the femoral artery and maintained at a MAP of 40 mmHg. When the blood pressure could no longer be maintained without fluid infusion (Decompensation), a MAP of 40 mmHg was sustained by incrementally infusing 0.2-ml boluses of LR until 40% of the total shed volume had been returned in the form of boluses (Severe Shock). Animals were then resuscitated with 4 times the shed volume in LR with or without NMN (400 mg/kg) over 60 minutes and followed for an additional 48 hours (Figure 7). The total time from initiation of bleeding to the start of resuscitation represents each animal’s ability to tolerate the shock and reflects individual physiologic resilience. Animals received buprenorphine (0.05 mg/kg, every 8 hours) and a subcutaneous LR bolus (50 ml/kg) with or without NMN (400 mg/kg) at 24 hours. Blood samples were taken at baseline, severe shock, 60R, and 48 hours. At 48 hours, any surviving animals were anesthetized using isoflurane (2%–4%) by mask followed by tissue harvest and euthanasia.
In a separate set of experiments, animals pretreated with water only were subjected to decompensated hemorrhagic shock as previously described and resuscitated with 4 times the shed volume in LR plus NMN (400 mg/kg) over 60 minutes and followed for an additional 48 hours (Figure 8). Animals received buprenorphine (0.05 mg/kg, every 8 hours) and a subcutaneous LR bolus (50 ml/kg) with or without NMN (400 mg/kg) at 24 hours. Blood samples were taken at baseline, severe shock, 60R, and 48 hours. At 48 hours, any surviving animals were anesthetized using isoflurane (2%–4%) by mask followed by tissue harvest and euthanasia.
Primary hepatocytes were isolated as previously described using a modified 2-step perfusion method (63). Animals (n = 4) were anesthetized with isoflurane (2%–4%). The abdomen was prepped and draped in sterile fashion. The inferior vena cava was cannulated with a 24-g angiocath, and the portal vein was cut. The liver was then infused with 300 ml liver perfusion media (Invitrogen, 17701-038) at 17 ml/kg/min to desanguinate, followed by 400 ml liver digestion media (Krebs Ringer bicarbonate buffer [KRBB] + 20 mM HEPES [pH 7.4] [MilliporeSigma, Thermo Fisher Scientific], 500 μM CaCl2, collagenase [≥20,000 units]/elastase [30 units] [LK002066, Worthington], and DNAse I [200 units, LK003170, Worthington]). After the perfusion, the liver was removed and disrupted to release cells using cell scrapers. The cell suspension was then filtered through a 70-μm filter and centrifuged at 50 g for 5 minutes at 4°C, washed once in KRBB, and precipitated in 25% Percoll gradient at 120 g for 5 minutes at 4°C. Cells were resuspended in hepatocyte media (M199, NaHCO3 [2.2 g/l]), glutamine 0.1 g/l, 0.25% BSA, 10% Fetal Bovine Serum (Hyclone), and 1% penicillin-streptomycin. Using precoated collagen 6-well plates, cells were plated at 1 × 106 cells/well and incubated for 12 hours (37°C, 5% CO2).
In order to obtain sham and shock plasma for hepatocyte coculture experiments, animals were anesthetized as described, underwent vessel cannulation, and were allowed to awaken and stabilize prior to bleeding. Sham animals (n = 9) were rapidly bled into heparinized collection tubes (BD Vacutainer), and lactate was confirmed to be ≤2 mmol/l. The shock animals (n = 9) were bled to a MAP of 40 mmHg for 90 minutes and then exsanguinated into heparinized tubes. Collected blood was immediately centrifuged (1,300 rcf, 10 min, 4°C), and plasma was immediately stored at –80°C.
Blood chemistries and inflammatory cytokines.
Blood was assayed for arterial blood gases, lactate, hemoglobin, glucose, and blood urea nitrogen using point-of-care veterinary cartridges (i-STAT, Abbot Point of Care Inc.). Serum creatinine, aspartate aminotransferase, alanine aminotransferase, and creatinine kinase were analyzed spectrophotometrically using commercially available kits (Pointe Scientific). IL-6 and TNF-α were assayed by ELISA (Invitrogen).
NAD/NADH assays.
NAD was extracted from frozen tissue samples (50 mg), isolated mitochondria (100 μg), or primary hepatocytes (1 × 106 cells) in ice-cold 0.6 M perchloric acid (Thermo Fisher Scientific). Tissues were homogenized at 20 Hz for 1 minute by tissue lyser (Qiagen). Mitochondria and cells were vortexed hard for 30–45 seconds. After centrifugation for 10 minutes (15,000 g, 4°C), the clear supernatant was removed and diluted 1:100 in ice-cold 100 mM sodium phosphate buffer, pH 8 (Thermo Fisher Scientific). Using a modified Graeff and Lee enzymatic cycling assay (64), NAD was measured in a 96-well format. In brief, 5 μl NAD standards and diluted tissue extracts were mixed with 95 μl cycling mixture (0.2% ethanol, 0.11/ml alcohol dehydrogenase, 1.1 mg/ml diaphorase, 20 μM resazurin, 10 μM flavin mononucleotide, 10 mM nicotinamide, and 0.1 mg/ml BSA in 100 mM phosphate buffer, pH 8.0, all from Thermo Fisher Scientific). NAD concentration was determined based on the rate of resorufin accumulation using spectrophotometry (excitation at 544nm, emission at 590 nm, Synergy H1, Biotek).
NADH was extracted from frozen tissue (50 mg) and isolated mitochondria (100 μg) in ice-cold extraction buffer (0.25 N KOH in 50% ETOH, Thermo Fisher Scientific). Samples were homogenized and centrifuged as described. The supernatant was removed and heated at 55°C for 10 minutes to hydrolyze free NAD. After dilution in ice-cold 100 mM sodium phosphate buffer (1:50), samples were assayed using the described enzymatic cycling assay. The protein concentrations from the NAD and NADH extraction pellets were determined by using a Pierce BCA Protein Assay kit (Thermo Fisher Scientific).
Primary hepatocytes.
Primary hepatocytes were washed with PBS 12 hours after plating. Cells were treated with sterile filtered plasma (0.22 μm, Thermo Fisher Scientific) harvested under shock and sham conditions, with and without NMN (100 nM) for 24 hours. Cell survival was assessed by trypan blue exclusion (89%–96% survival) and did not differ between groups.
In a separate experiment, hepatocytes were washed with PBS after 12 hours after initial plating and the media were changed to Dulbecco’s Modified Eagle’s Medium and 10% FBS with 1% penicillin-streptomycin (MilliporeSigma). Cells were treated with and without recombinant rat IL-6 (20 ng/ml, SRP4145, MilliporeSigma) with or without 100 μM NMN for 1 hour. Survival ranged from 96%–98% and did not differ between treatment groups. Cells were immediately assessed using high-resolution respirometry or were treated with 0.6 M perchloric acid and stored at –80°C until assayed for NAD.
ATP determination.
ATP from frozen tissues (50 mg) was measured using a commercially available ATP determination kit (Life Technologies) according to the manufacturer’s instructions.
Isolation of mitochondria.
Liver and kidney tissues (4–6 g) were immediately immersed in ice-cold mitochondrial isolation buffer (MIB) (210 mM mannitol, 70 mM sucrose, 10 mM HEPES, 1 mM EDTA, final pH adjusted to 7.2 using KOH, and freshly supplemented with 0.5% fatty acid-free BSA, all from Thermo Fisher Scientific). Tissue was homogenized in MIB with 5% BSA, and mitochondria were isolated using differential centrifugation as previously described (53, 55). The final mitochondrial pellet was resuspended in MIB or treated with 0.6 M perchloric acid for NAD measurements. Protein concentration was measured by Pierce BCA Protein Assay (Thermo Fisher Scientific).
Mitochondrial respiratory capacity using high-resolution respirometry.
A standard substrate/inhibitor titration protocol was used for functional analysis of mitochondrial respiratory function (53, 55). Freshly isolated mitochondria (0.15 mg) were resuspended in respiration medium (110 mM mannitol, 0.5 mM EGTA, 3 mM MgCl2, 20 mM taurine, 10 mM KH2PO4, 60 mM K lactobionate, 0.3 mM DTT, and 0.1% BSA [fatty acid free], adjusted to pH of 7.1 with KOH, all from Thermo Fisher Scientific) (65). Oxygen consumption was measured using high-resolution respirometry at 37°C with constant stirring (Oxygraph-2k, Oroboros Instruments). Following stabilization (3–5 minutes), real-time oxygen concentration and flux data were continuously collected (DatLab software 4.3, Oroboros Instruments). After the basal respiration rate was recorded, CI-dependent respiration was induced by adding 10 mM glutamate, 5 mM malate, and 1 mM ADP to the respiration chamber. In order to determine CII-dependent respiration, rotenone (0.5 μM), a selective inhibitor of CI, was added followed by 10 mM of succinate. Antimycin A (5 μM) was then added to inhibit complex III; followed by TMPD (0.5 mM) and ascorbate (2 mM) as artificial substrates for complex IV. This protocol was completed within 60 minutes. (All agents were purchased from Thermo Fisher Scientific.)
Similarly, isolated hepatocytes (2.5 × 105 cells) were resuspended in respiration buffer and analyzed using high-resolution respirometry. After stabilization, baseline oxygen consumption was recorded, and cells were permeabilized digitonin (3 μg) and allowed to stabilize for 10 minutes. Cells were then subjected to the substrate/inhibitor titration protocol used for isolated mitochondria.
Immunoblotting.
For Western blot analysis, frozen liver and kidney tissue was lysed in RIPA buffer supplemented with phosphatase inhibitors (PhosSTOP, Roche), protease inhibitors (Complete, Roche), nicotinamide (1 mM), and trichostatin A (1 μM) using a tissue lyser (Qiagen). Lysates were centrifuged for 15 minutes (15,000 g, 4°C). Lysates were denatured in 25% laemmli buffer + BME at 95°C for 5 minutes and were resolved on 4%–15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels. Gels were transferred to polyvinylidene fluoride membranes (MilliporeSigma) blocked with nonfat soy milk (66) and probed using anti-NAMPT (1:5,000, SC67020, Santa Cruz), anti-hydroxynonenal (1:1,000, Abcam), or anti-acetylated lysine (1:1,000, Cell Signaling), followed by anti-actin (1:10,000, Abcam) or anti-VDAC antibodies (1:1,000, Affinity Bio) after membranes where stripped, in Tris-buffered saline with 0.1% Tween20. Proteins of interest were detected by chemiluminescence using horseradish peroxidase–conjugated secondary antibodies and Western Lightening Plus ECL (Perkin Elmer). Images were captured using a Bio-Rad imaging station and quantified using Image J (National Institutes of Health).
Gene expression.
Liver and kidney RNA was extracted from frozen tissue using Trizol (MilliporeSigma) with ethanol precipitation. RNA (1 μg) was used to create cDNA using a High-Capacity cDNA Reverse Transcriptase Kit (Applied Biosystems) according to the manufacturer’s recommendation. Real-time PCR was performed using an Applied Biosystems 7900HT system with SYBR green (Applied Biosystems). Two technical replicates per sample were obtained, and relative mRNA expression levels were calculated using the ΔΔCT method normalized to actin as a housekeeping gene. The following primers were used: β-actin (forward, GGCTGTATTCCCCTCCATCG; reverse, CCAGTTGGTAACAATGCCATGT), IL-6 (forward, TAGTCCTTCCTACCCCAACTTCC; reverse, TTGGTCCTTAGCCACTCCTTC), TNF-α (forward, CTGTGCCTCAGCCTCTTCTC; reverse, ACTGATGAGAGGGAGCCCAT), NAMPT (forward, TCTGGAAATCCGCTCGACAC; reverse, TATCCACTCCGTCCCCTTGA), NMNAT1 (forward, CAGAGCATCCGCTACTTGGT; reverse, ATCGGGTGGAATGGTTGTGT), NMNAT2 (forward, TCTGACTGGATCAGGGTGGA; reverse, ATGGTGCTCTAACACACTGC), and NMNAT3 (forward, CCTGCGTTTGTTTGAGGTGG; reverse, ATGATGCCGTTTCCATCCACT).
Statistics.
Results are expressed as mean ± SEM. Comparison between 2 groups was performed using 2-tailed Student’s t or Mann-Whitney test, depending on normality of data distribution. One-way ANOVA was used to compare 3 or more groups with a post hoc 2-tailed Student’s t or Mann-Whitney test if statistically significant (P < 0.05). Survival was analyzed using Kaplan-Meier curves with a log-rank Mantel-Cox test. A χ2 test was used to evaluate survival at 24 hours. All statistical analysis were performed using Prism 7 (GraphPad Software Inc.), with P < 0.05 considered statistically significant.
Study approval.
All experiments were approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania and were conducted in accordance with the guidelines established by the National Institutes of Health.
Author contributions
CAS designed research studies, conducted experiments, acquired data, analyzed data, and wrote the manuscript. YG conducted experiments, acquired data, and analyzed data. SM conducted experiments, acquired data, and analyzed data. KS conducted experiments, acquired data, and analyzed data. PB conducted experiments, acquired data, and analyzed data. AD conducted experiments, acquired data, analyzed data, and wrote manuscript. JAB designed research studies, conducted experiments, acquired data, analyzed data, and wrote the manuscript.
Supplementary Material
Acknowledgments
We wish to thank James G. Davies, Karthikeyani Chellappa, and Qingwei Chu for their technical assistance. This work was supported in part by grants from the National Institutes of Health (K08 GM97614, R01 DK098656, and R01 AG043483). NMN was provided by Metro International Biotech, and the work was supported in part by a gift from E. Schulak. Neither participated in data analysis, interpretation, or preparation of the manuscript, which is entirely the work of the listed authors.
Version 1. 09/06/2018
Electronic publication
Footnotes
Conflict of interest: SM and JAB disclose that they have intellectual property related to the use of NAD precursors and liver regeneration.
Reference information: JCI Insight. 2018;3(16):e120182. https://doi.org/10.1172/jci.insight.120182.
Contributor Information
Yuxia Guan, Email: yuxia.guan@uphs.upenn.edu.
Sarmistha Mukherjee, Email: sarmuk@mail.med.upenn.edu.
Khushboo Singh, Email: khushsingh1886@gmail.com.
Paul Botolin, Email: paulbotolin@gwmail.gwu.edu.
Antonio Davila, Jr., Email: antonio.davila@uphs.upenn.edu.
References
- 1.Chaudry IH. Cellular mechanisms in shock and ischemia and their correction. Am J Physiol. 1983;245(2):R117–R134. doi: 10.1152/ajpregu.1983.245.2.R117. [DOI] [PubMed] [Google Scholar]
- 2.Peitzman AB, Billiar TR, Harbrecht BG, Kelly E, Udekwu AO, Simmons RL. Hemorrhagic shock. Curr Probl Surg. 1995;32(11):925–1002. doi: 10.1016/S0011-3840(05)80008-5. [DOI] [PubMed] [Google Scholar]
- 3.Cantó C, Menzies KJ, Auwerx J. NAD(+) Metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22(1):31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wurth MA, Sayeed MM, Baue AE. Nicotinamide adenine dinucleotide (NAD) content of liver with hemorrhagic shock. Proc Soc Exp Biol Med. 1973;144(2):654–658. doi: 10.3181/00379727-144-37656. [DOI] [PubMed] [Google Scholar]
- 5.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol, Cell Physiol. 2011;300(3):C385–C393. doi: 10.1152/ajpcell.00485.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Wijk SJ, Hageman GJ. Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radic Biol Med. 2005;39(1):81–90. doi: 10.1016/j.freeradbiomed.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 7.Alano CC, Garnier P, Ying W, Higashi Y, Kauppinen TM, Swanson RA. NAD+ depletion is necessary and sufficient for poly(ADP-ribose) polymerase-1-mediated neuronal death. J Neurosci. 2010;30(8):2967–2978. doi: 10.1523/JNEUROSCI.5552-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantó C, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15(6):838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomes AP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155(7):1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gero D, Szabo C. Salvage of nicotinamide adenine dinucleotide plays a critical role in the bioenergetic recovery of post-hypoxic cardiomyocytes. Br J Pharmacol. 2015;172(20):4817–4832. doi: 10.1111/bph.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J Biol Chem. 2005;280(43):36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- 12.Chaudry IH, Zweig S, Sayeed MM, Baue AE. Failure of nicotinamide in the treatment of hemorrhagic shock. J Surg Res. 1976;21(1):27–32. doi: 10.1016/0022-4804(76)90006-8. [DOI] [PubMed] [Google Scholar]
- 13.Jeong KY, Suh GJ, Kwon WY, Kim KS, Jung YS, Kye YC. The therapeutic effect and mechanism of niacin on acute lung injury in a rat model of hemorrhagic shock: Down-regulation of the reactive oxygen species-dependent nuclear factor κB pathway. J Trauma Acute Care Surg. 2015;79(2):247–255. doi: 10.1097/TA.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 14.Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14(4):528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu CP, Oka S, Shao D, Hariharan N, Sadoshima J. Nicotinamide phosphoribosyltransferase regulates cell survival through NAD+ synthesis in cardiac myocytes. Circ Res. 2009;105(5):481–491. doi: 10.1161/CIRCRESAHA.109.203703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgos ES, Schramm VL. Weak coupling of ATP hydrolysis to the chemical equilibrium of human nicotinamide phosphoribosyltransferase. Biochemistry. 2008;47(42):11086–11096. doi: 10.1021/bi801198m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto T, Byun J, Zhai P, Ikeda Y, Oka S, Sadoshima J. Nicotinamide mononucleotide, an intermediate of NAD+ synthesis, protects the heart from ischemia and reperfusion. PLoS ONE. 2014;9(6):e98972. doi: 10.1371/journal.pone.0098972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broder G, Weil MH. Excess lactate: An index of reversibility of shock in human patients. Science. 1964;143(3613):1457–1459. doi: 10.1126/science.143.3613.1457. [DOI] [PubMed] [Google Scholar]
- 19.Rixen D, Siegel JH. Bench-to-bedside review: oxygen debt and its metabolic correlates as quantifiers of the severity of hemorrhagic and post-traumatic shock. Crit Care. 2005;9(5):441–453. doi: 10.1186/cc3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gale SC, Kocik JF, Creath R, Crystal JS, Dombrovskiy VY. A comparison of initial lactate and initial base deficit as predictors of mortality after severe blunt trauma. J Surg Res. 2016;205(2):446–455. doi: 10.1016/j.jss.2016.06.103. [DOI] [PubMed] [Google Scholar]
- 21.Chaudry IH, Sayeed MM, Baue AE. Differences in the altered energy metabolism of hemorrhagic shock and hypoxemia. Can J Physiol Pharmacol. 1976;54(5):750–756. doi: 10.1139/y76-104. [DOI] [PubMed] [Google Scholar]
- 22.Bacter CR, Canizaro PC, Carrico CJ, Shires GT. Fluid resuscitation of hemorrhagic shock. Postgrad Med. 1970;48(3):95–99. doi: 10.1080/00325481.1970.11693553. [DOI] [PubMed] [Google Scholar]
- 23.Yang H, et al. Nutrient-sensitive mitochondrial NAD+ levels dictate cell survival. Cell. 2007;130(6):1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rongvaux A, et al. Nicotinamide phosphoribosyl transferase/pre-B cell colony-enhancing factor/visfatin is required for lymphocyte development and cellular resistance to genotoxic stress. J Immunol. 2008;181(7):4685–4695. doi: 10.4049/jimmunol.181.7.4685. [DOI] [PubMed] [Google Scholar]
- 25.Tran MT, et al. PGC1α drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature. 2016;531(7595):528–532. doi: 10.1038/nature17184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stensballe J, Christiansen M, Tønnesen E, Espersen K, Lippert FK, Rasmussen LS. The early IL-6 and IL-10 response in trauma is correlated with injury severity and mortality. Acta Anaesthesiol Scand. 2009;53(4):515–521. doi: 10.1111/j.1399-6576.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 27.Meng ZH, Dyer K, Billiar TR, Tweardy DJ. Essential role for IL-6 in postresuscitation inflammation in hemorrhagic shock. Am J Physiol, Cell Physiol. 2001;280(2):C343–C351. doi: 10.1152/ajpcell.2001.280.2.C343. [DOI] [PubMed] [Google Scholar]
- 28.Liu TF, Vachharajani VT, Yoza BK, McCall CE. NAD+-dependent sirtuin 1 and 6 proteins coordinate a switch from glucose to fatty acid oxidation during the acute inflammatory response. J Biol Chem. 2012;287(31):25758–25769. doi: 10.1074/jbc.M112.362343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapoor R, Kalra J, Prasad K. Cardiac depression and cellular injury in hemorrhagic shock and reinfusion: role of free radicals. Mol Cell Biochem. 1997;176(1-2):291–301. [PubMed] [Google Scholar]
- 30.Klaidman L, Morales M, Kem S, Yang J, Chang ML, Adams JD. Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69(3):150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- 31.Trueblood NA, Ramasamy R, Wang LF, Schaefer S. Niacin protects the isolated heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;279(2):H764–H771. doi: 10.1152/ajpheart.2000.279.2.H764. [DOI] [PubMed] [Google Scholar]
- 32.Heron M. Deaths: leading causes for 2014. Natl Vital Stat Rep. 2016;65(5):1–96. [PubMed] [Google Scholar]
- 33.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 34.Cerutti R, et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014;19(6):1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouchiroud L, Houtkooper RH, Auwerx J. NAD+ metabolism: a therapeutic target for age-related metabolic disease. Crit Rev Biochem Mol Biol. 2013;48(4):397–408. doi: 10.3109/10409238.2013.789479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skarda DE, Putt KS, Hergenrother PJ, Mulier KE, Beilman GJ. Increased poly(ADP-ribose) polymerase activity during porcine hemorrhagic shock is transient and predictive of mortality. Resuscitation. 2007;75(1):135–144. doi: 10.1016/j.resuscitation.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki K, et al. Characterization of energy metabolism and blood flow distribution in myocardial ischemia in hemorrhagic shock. Am J Physiol. 1997;273(2 Pt 2):H600–H607. doi: 10.1152/ajpheart.1997.273.2.H600. [DOI] [PubMed] [Google Scholar]
- 38.Heckbert SR, et al. Outcome after hemorrhagic shock in trauma patients. J Trauma. 1998;45(3):545–549. doi: 10.1097/00005373-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Benns M, Carr B, Kallan MJ, Sims CA. Benchmarking the incidence of organ failure after injury at trauma centers and nontrauma centers in the United States. J Trauma Acute Care Surg. 2013;75(3):426–431. doi: 10.1097/TA.0b013e31829cfa19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhodes RS, DePalma RG. Mitochondrial dysfunction of the liver and hypoglycemia in hemorrhagic shock. Surg Gynecol Obstet. 1980;150(3):347–352. [PubMed] [Google Scholar]
- 41.Mills KF, et al. Long-term administration of nicotinamide mononucleotide mitigates age-associated physiological decline in mice. Cell Metab. 2016;24(6):795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Long AN, Owens K, Schlappal AE, Kristian T, Fishman PS, Schuh RA. Effect of nicotinamide mononucleotide on brain mitochondrial respiratory deficits in an Alzheimer’s disease-relevant murine model. BMC Neurol. 2015;15:19. doi: 10.1186/s12883-015-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hift H, Strawitz JG. Irreversible hemorrhagic shock in dogs: structure and function of liver mitochondria. Am J Physiol. 1961;200:264–268. doi: 10.1152/ajplegacy.1961.200.2.264. [DOI] [PubMed] [Google Scholar]
- 44.Carreras MC, et al. Mitochondrial nitric oxide synthase drives redox signals for proliferation and quiescence in rat liver development. Hepatology. 2004;40(1):157–166. doi: 10.1002/hep.20255. [DOI] [PubMed] [Google Scholar]
- 45.Svistunenko DA, Davies N, Brealey D, Singer M, Cooper CE. Mitochondrial dysfunction in patients with severe sepsis: an EPR interrogation of individual respiratory chain components. Biochim Biophys Acta. 2006;1757(4):262–272. doi: 10.1016/j.bbabio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Patil NK, Parajuli N, MacMillan-Crow LA, Mayeux PR. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: mitochondria-targeted antioxidant mitigates injury. Am J Physiol Renal Physiol. 2014;306(7):F734–F743. doi: 10.1152/ajprenal.00643.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González R, et al. N-acetylcysteine, coenzyme Q10 and superoxide dismutase mimetic prevent mitochondrial cell dysfunction and cell death induced by d-galactosamine in primary culture of human hepatocytes. Chem Biol Interact. 2009;181(1):95–106. doi: 10.1016/j.cbi.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Mukhopadhyay P, et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med. 2012;53(5):1123–1138. doi: 10.1016/j.freeradbiomed.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sebastián C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012;287(51):42444–42452. doi: 10.1074/jbc.R112.402768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schug TT, et al. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol Cell Biol. 2010;30(19):4712–4721. doi: 10.1128/MCB.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu W, Dittenhafer-Reed KE, Denu JM. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 2012;287(17):14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Wang H, et al. Resveratrol rescues kidney mitochondrial function following hemorrhagic shock. Shock. 2015;44(2):173–180. doi: 10.1097/SHK.0000000000000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayub A, Poulose N, Raju R. Resveratrol improves survival and prolongs life following hemorrhagic shock. Mol Med. 2015;21:305–312. doi: 10.2119/molmed.2015.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, et al. Resveratrol ameliorates mitochondrial dysfunction but increases the risk of hypoglycemia following hemorrhagic shock. J Trauma Acute Care Surg. 2014;77(6):926–933. doi: 10.1097/TA.0000000000000358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell RD, Swet JH, Kennedy KL, Huynh TT, McKillop IH, Evans SL. Resveratrol attenuates hypoxic injury in a primary hepatocyte model of hemorrhagic shock and resuscitation. J Trauma Acute Care Surg. 2014;76(2):409–417. doi: 10.1097/TA.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 57.Baue AE, Chaudry IH, Wurth MA, Sayeed MM. Cellular alterations with shock and ischemia. Angiology. 1974;25(1):31–42. doi: 10.1177/000331977402500106. [DOI] [PubMed] [Google Scholar]
- 58.Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117(4):495–502. doi: 10.1016/S0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 59.Nikiforov A, Dölle C, Niere M, Ziegler M. Pathways and subcellular compartmentation of NAD biosynthesis in human cells: from entry of extracellular precursors to mitochondrial NAD generation. J Biol Chem. 2011;286(24):21767–21778. doi: 10.1074/jbc.M110.213298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Camacho-Pereira J, et al. CD38 dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016;23(6):1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem Biophys Res Commun. 2006;345(4):1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 62. Ayala A, Wang P, Chaudry IH. Shock Models: Hemorrhage. In: Souba W, Wilmore D, ed. Surgical Research. San Diego, CA: Academic Press; 2001:317–330. [Google Scholar]
- 63.Miller RA, et al. Adiponectin suppresses gluconeogenic gene expression in mouse hepatocytes independent of LKB1-AMPK signaling. J Clin Invest. 2011;121(6):2518–2528. doi: 10.1172/JCI45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Graeff R, Lee HC. A novel cycling assay for nicotinic acid-adenine dinucleotide phosphate with nanomolar sensitivity. Biochem J. 2002;367(Pt 1):163–168. doi: 10.1042/BJ20020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3(6):965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 66.Galva C, Gatto C, Milanick M. Soymilk: an effective and inexpensive blocking agent for immunoblotting. Anal Biochem. 2012;426(1):22–23. doi: 10.1016/j.ab.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.