Abstract
The Bacillus subtilis GlmR (formerly YvcK) protein is essential for growth on gluconeogenic carbon sources. Mutants lacking GlmR display a variety of phenotypes suggestive of impaired cell wall synthesis including antibiotic sensitivity, aberrant cell morphology and lysis. To define the role of GlmR, we selected suppressor mutations that ameliorate the sensitivity of a glmR null mutant to the beta-lactam antibiotic cefuroxime or restore growth on gluconeogenic carbon sources. Several of the resulting suppressors increase the expression of the GlmS and GlmM proteins that catalyze the first two committed steps in the diversion of carbon from central carbon metabolism into peptidoglycan biosynthesis. Chemical complementation studies indicate that the absence of GlmR can be overcome by provision of cells with N-acetylglucosamine (GlcNAc), even under conditions where GlcNAc cannot re-enter central metabolism and serve as a carbon source for growth. Our results indicate that GlmR facilitates the diversion of carbon from the central metabolite fructose-6-phosphate, which is limiting in cells growing on gluconeogenic carbon sources, into peptidoglycan biosynthesis. Our data suggest that GlmR stimulates GlmS activity, and we propose that this activation is antagonized by the known GlmR ligand and peptidoglycan intermediate UDP-GlcNAc. Thus, GlmR presides over a new mechanism for the regulation of carbon partitioning between central metabolism and peptidoglycan biosynthesis.
Author summary
Bacterial cells are surrounded by a peptidoglycan cell wall that is, under most conditions, required for viability. Synthesis of the cell wall requires a considerable diversion of resources from central carbon metabolism into a lipid-linked precursor (lipid II) that is exported from the cell for wall assembly. Here, we propose that GlmR presides over a new mechanism for the regulation of carbon partitioning between central metabolism and peptidoglycan biosynthesis: GlmR activates the GlmS-dependent diversion of carbon from the glycolytic pathway into peptidoglycan synthesis. This effect is particularly important during gluconeogenesis since the GlmS substrate fructose 6-phosphate is present at a reduced level under these conditions.
Introduction
Bacillus subtilis provides a powerful model system for understanding cell wall homeostasis in Gram positive bacteria. Disruption of pathways for the synthesis of peptidoglycan (PG) and other cell envelope components elicits complex adaptive responses often controlled by alternative σ factors or two-component systems [1, 2]. The ECF σ factor σM regulates numerous operons involved in PG synthesis and mutants are sensitive to PG synthesis inhibitors [3]. Previously, we found that mutation of gdpP, which encodes a cyclic-di-adenosine monophosphate (c-di-AMP) hydrolase, can suppress the sensitivity of B. subtilis sigM null mutants towards beta-lactam antibiotics [4]. This suggests that c-di-AMP plays some role in PG homeostasis. Mutations in the yvcK gene (herein renamed glmR) also exhibit cell envelope defects, as evidenced by cell bulging and lysis when inoculated into non-glycolytic carbon sources [5]. Moreover, a yqfF::Tn insertion suppressed the inability of a glmR mutant to grow on gluconeogenic media [5]. Although unknown at the time, yqfF is now known to encode a second c-di-AMP hydrolase renamed PgpH [6, 7]. These observations encouraged us to investigate possible connections between GlmR, c-di-AMP, and cell envelope homeostasis.
In B. subtilis, GlmR (formerly YvcK) is essential for growth on amino acids and intermediates of the tricarboxylic acid cycle and pentose phosphate pathway, but dispensable for growth on glucose and other glycolytic carbon sources [5]. Previous genetic studies revealed that mutations in genes affecting central carbon metabolism (CCM), including zwf and cggR, allow a glmR null mutant to grow on gluconeogenic carbon sources [5]. These observations suggest that GlmR has a yet undefined role in regulating metabolism. In the absence of GlmR, cells display cell envelope defects and lyse under gluconeogenic growth conditions.
The function of GlmR in CCM, and how this relates to cell envelope integrity, is not yet clear. One model suggests that GlmR may function as a cytoskeletal filament protein analogous to MreB to help coordinate cell wall synthesis [8]. MreB, an actin-like cytoskeletal protein, is important for maintaining a rod shape in B. subtilis and deletion of mreB leads to severe morphological defects and eventual cell lysis, effects attributed to mislocalization of penicillin binding protein 1 (PBP1) [9]. B. subtilis GlmR localizes to the membrane in a helical fashion, and overexpression of GlmR rescues the cell defects seen in an mreB deletion mutant and restores proper localization of PBP1. Conversely, overexpression of MreB rescues the morphological defects of a glmR null mutant when grown on gluconeogenic carbon sources [8].
Recently, GlmR was found to possess a ligand binding site for UDP sugars such as UDP-glucose and UDP-N-acetylglucosamine (UDP-GlcNAc) [10]. Since UDP-GlcNAc is a precursor of PG synthesis, this suggests that GlmR may sense this intermediate to somehow modulate CCM or cell envelope homeostasis. Mutations altering the UDP-sugar binding site did not affect growth on gluconeogenic media in B. subtilis, but did lead to increased sensitivity to bacitracin [10].
Although the biochemical details are unclear, the role of GlmR in metabolism and cell wall homeostasis seems to be widely conserved. Homologs of GlmR are present diverse bacteria and a glmR mutant can be complemented by expression of the Escherichia coli homolog, YbhK [5]. Mutation of glmR homologs in the intracellular pathogens Mycobacterium tuberculosis (cuvA) and Listeria monocytogenes (yvcK) leads to alterations in cell morphology and sensitivity to cell wall acting antibiotics, as well as defects in carbon source utilization and establishment of infection in the host cell [11, 12]. Although these diverse phenotypes, biochemical properties and cell localization studies are all intriguing, a unifying model to account for the role of GlmR in the cell has been elusive.
Here, we show that a B. subtilis strain lacking glmR is susceptible to peptidoglycan (PG) biosynthesis inhibitors such as beta-lactams, vancomycin and moenomycin. Characterization of glmR suppressor mutations indicates that increased expression of genes involved in UDP-GlcNAc biosynthesis is sufficient to increase beta-lactam resistance and restore growth on gluconeogenic carbon sources. Moreover, supplementation with GlcNAc can bypass the requirement for GlmR even in strains where GlcNAc cannot enter into CCM. Our results support a model in which GlmR functions to help divert carbon to PG biosynthesis, likely through direct interaction with GlmS. We propose that this effect is particularly important during gluconeogenesis since the GlmS substrate fructose 6-phosphate is present at a reduced level under these conditions [13].
Results
ΔglmR is sensitive to peptidoglycan synthesis inhibiting antibiotics
To test the role of GlmR in the connection between CCM and PG biosynthesis (Fig 1), we generated a B. subtilis strain with an in-frame, unmarked deletion of glmR (ΔglmR) and characterized its growth properties and sensitivity to cell wall antibiotics. Mueller-Hinton (MH) is a gluconeogenic medium containing amino acids as primary carbon source and is commonly used for antibiotic sensitivity experiments. However, ΔglmR is unable to grow on MH. This phenotype can be complemented by an ectopic, inducible copy of glmR (Fig 2A) or addition of glucose (S1A and S1B Fig), consistent with prior results [5].
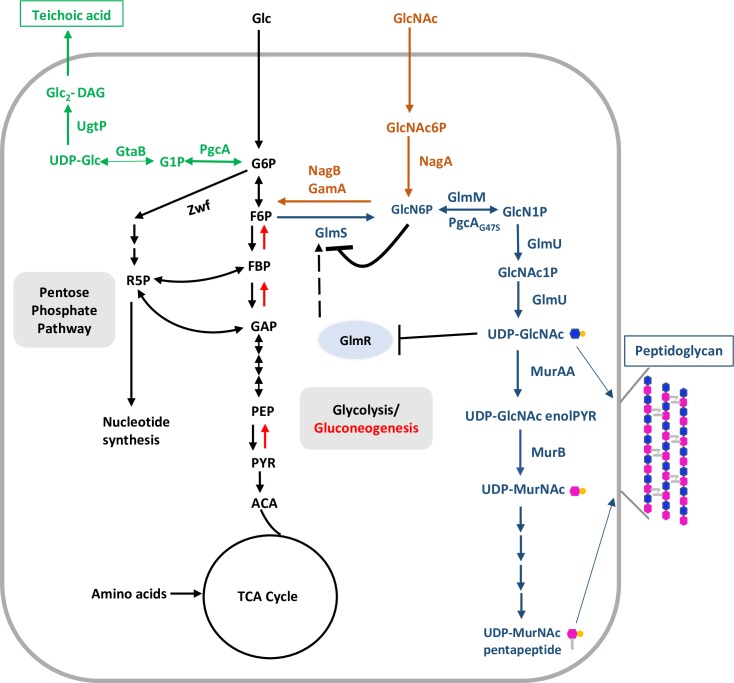
Fig 1. Schematic illustration of central carbon, peptidoglycan, UDP-Glc and UDP-GlcNAc metabolism.
Central carbon metabolism (CCM; glycolysis/gluconeogenesis, pentose phosphate pathway and TCA cycle) is shown with black colored fonts and arrows. Black double headed arrows represent bidirectional enzyme reactions. Single headed black and red arrows represent glycolysis- and gluconeogenesis-specific enzymatic steps, respectively. UDP-Glc biosynthesis and its incorporation in teichoic acids is depicted in green. Steps of peptidoglycan biosynthesis and GlcNAc feeding into central carbon metabolism are shown in blue and orange, respectively. Black dashed arrow indicates activation.
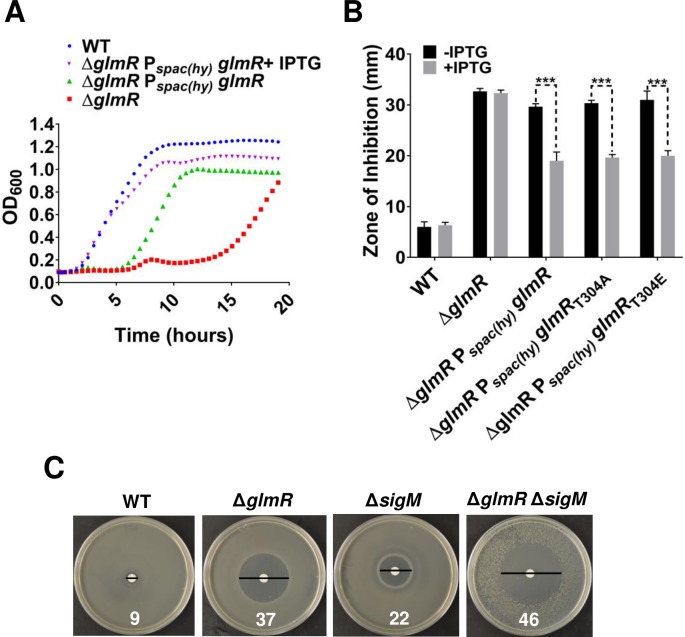
Fig 2. ΔglmR is unable to grow on MH medium and is sensitive to CEF.
(A) Growth curves of WT, ΔglmR and ΔglmR amyE:: Pspac-(hy) glmR on gluconeogenic MH media. (B) CEF susceptibility of ΔglmR and complementation of the phenotype was tested by disc diffusion assay using 6 μg of antibiotic. IPTG was added to 1 mM to induce expression of ectopic copy of glmR. Zone of inhibition (ZOI) was measured after overnight incubation of plates at 37°C. ZOI represents the diameter of clear zone surrounding the disc minus the disc (7 mm). Standard deviation (error bars) is based on at least three biological replicates. Three asterisks indicate significant difference with P<0.001 estimated by comparing IPTG treated samples with untreated samples using Tukey test. (C) Evidence that glmR functions independently of sigM. Epistasis between glmR and sigM was determined by disc diffusion assay with 3 μg of CEF on each filter.
To monitor the impact of the ΔglmR mutation on antibiotic sensitivity we performed zone-of-inhibition assays using LB (lysogeny broth) medium, a complex medium containing a variety of mono- and disaccharides (a total carbohydrate concentration of ~0.16%; [14]) and abundant amino acids. The ΔglmR mutant is much more sensitive to the beta-lactam antibiotic cefuroxime (CEF) (Fig 2B) as well as to other beta-lactam antibiotics (oxacillin and cefixime), moenomycin, and vancomycin (S2A–S2D Fig), all of which act by affecting the assembly and cross-linking of the peptidoglycan sacculus. However, we did not observe any significant difference in susceptibility between wild-type (WT) and ΔglmR to fosfomycin, bacitracin or nisin (S2E–S2G Fig). The lack of significant effect with these compounds may be due to the presence of inducible resistance mechanisms that might mask the effects of the ΔglmR mutation [15–18].
We selected CEF for further study due to the significantly higher sensitivity of the ΔglmR strain. Induction of an ectopic, IPTG-inducible glmR gene partially complements ΔglmR cefuroxime sensitivity (Fig 2B). Incomplete complementation may indicate that GlmR levels from this construct, while sufficient to restore growth (Fig 2A), are insufficient for robust cell wall synthesis. Consistent with this idea, induction of an N-terminally 3X-FLAG-tagged glmR allele with an optimized ribosome-binding site (AGGAGG-seven base pairs upstream from start codon), complemented CEF resistance to WT levels (S3A Fig). Mutations affecting PG synthesis can often be suppressed by high concentrations of Mg2+ [19, 20]. Indeed, Mg2+ suppresses the growth defect of a glmR deletion mutant on non-glycolytic carbon sources (S1A Fig), as shown previously [5], and also partially suppresses CEF sensitivity (S3B Fig). These results suggest that a ΔglmR strain is impaired in PG synthesis, and therefore more susceptible to antibiotics that interfere directly with PG assembly such as beta-lactams.
Both the ΔglmR and ΔsigM mutants are CEF sensitive, and in both cases mutations known to increase c-di-AMP levels suppress this sensitivity (see below). This suggests that GlmR and σM may function in the same pathway. However, a ΔglmR ΔsigM double mutant is much more sensitive than either single mutant (Fig 2C), suggesting that these are two independent (and additive) pathways for intrinsic CEF resistance.
The role of GlmR in intrinsic CEF resistance is phosphorylation independent
The CEF sensitivity of the ΔglmR strain is suggestive of a defect in PG synthesis. GlmR is also known to be modified on Thr304 by the penicillin binding protein and serine/threonine associated (PASTA) kinase PrkC and phosphatase PrpC [21]. PrkC is activated by muropeptides during spore germination [22] and is regulated by interaction with the cell division protein GpsB during growth [23]. PrkC-dependent phosphorylation of GlmR has been linked to its role in morphogenesis and to resistance to bacitracin, but appears not to be required for growth on gluconeogenic carbon sources [21]. Similarly, this post-translational modification is not required for suppression of CEF sensitivity: both the phosphomimetic GlmRT304E and phosphoablative GlmRT304A mutant proteins complement the null mutant as well as wild-type (Fig 2B).
Many ΔglmR suppressor mutations affect the cdaA-cdaR-glmM-glmS operon
To gain insight into the role of GlmR in B. subtilis, we characterized suppressors (both spontaneous and transposon-generated) that either increased CEF resistance or restored the ability of ΔglmR to grow on MH medium. We isolated CEF resistant ΔglmR suppressors from CEF zone-of-inhibition assays or as colonies on MH medium (S1B Fig). We identified the causative mutations using whole-genome resequencing (spontaneous mutations) or by sequencing of junction fragments (transposon insertions) followed by linkage analysis and/or genetic reconstruction and complementation (Table 1). In general, the selected mutations suppressed both phenotypes associated with ΔglmR. Those suppressors selected for increased CEF resistance also recovered an ability to grow on MH medium. Conversely, for those selected for growth on MH medium, nearly all displayed at least a partial increase in CEF resistance relative to the ΔglmR starting strain (Table 1). In general, in this and previous studies, we find that CEF sensitivity is an excellent reporter for defects in cell wall synthesis. Often, suppressor mutations that fully restore growth may only partially rescue intrinsic CEF resistance. Here, we will focus on those suppressor mutations in the cdaA-cdaR-glmM-glmS region of the chromosome, which encodes the two initial enzymes in the peptidoglycan biosynthesis pathway, a major cyclic-di-AMP synthase (CdaA) and a regulator of CdaA (CdaR). We also recovered mutations in other genes in carbon metabolism, including pgcA and zwf, consistent with prior genetic studies of glmR function [5]. The possible mechanisms of suppression for these and other mutations are considered in the Discussion.
Table 1. ΔglmR suppressor mutations.
| Selection | Mutant | Genomic region changes | Coding region change | Affected gene or non-coding area | Gene annotation | CEFR (ZOI, mm) |
Growth on MH | Linkage |
|---|---|---|---|---|---|---|---|---|
| CEFR | glmS1 | 200068A>T | Inside glmS ribozyme | Senses GlcN6P and controls expression of glmS | 31 | Yes | Yes | |
| rsiW1 | 196049G>A | E208E | Penultimate codon of rsiW (E208E), affects termination loop stability | Anti-sigW | 27 | Yes | Yes | |
| rsiW2 | 196071C>T | Located downstream of rsiW. Affects termination loop stability | Non-coding region | 27 | Yes | Yes | ||
| rsiW3 | Tn insertion downstream of rsiW stop codon | Non-coding region | 35 | ND | Yes | |||
| pgcAG47S | 1006912G>A | G47A | pgcA | Phosphoglucomutase | 30 | Yes | Yes | |
|
yvcJL104H sigAA197V |
3572078T>A 2600750G>A |
L104H A197V |
yvcJ sigA |
GTPase, nucleotide-binding protein; primary σ factor | 31 | ND | ND | |
| tufA1::TnYLB-1 | Tn insertion downstream of tufA after stop codon | Elongation factor Tu | 29 | ND | Yes | |||
| MH | glmS1 | 200068A>T | Inside glmS ribozyme | Senses GlcN6P and controls expression of glmS | 31 | Yes | Yes | |
| zwfD405fs | 2480369delA | D405stop | zwf | Glucose 6-phosphate dehydrogenase | 31 | Yes | Yes | |
| ispAL140P | 2526261A>G | L140P | ispA | Geranyltransferase | 39 | Yes | ND | |
| ispA::TnYLB-1 | Tn insertion in ispA | Geranyltransferase | 35 | Yes | Yes | |||
| clpX::TnYLB-1 | Tn insertion in clpX | ATP-dependent Clp protease | 19 | Yes | Yes | |||
| qoxB::TnYLB-1 | Tn insertion in qoxB | Cytochrome aa3 quinol oxidase | 26 | Yes | Yes |
Table 1: List of ΔglmR suppressors obtained using CEF resistance (CEFR) or growth on MH medium as selection. CEF sensitivity was selected starting with a ΔglmR strain (a zone-of-inhibition, ZOI = 40 mm). For comparison, WT has a ZOI = 12 mm. Genomic region change indicates location of a nucleotide on reference genome of B. subtilis subsp. 168 (NCBI reference sequence NC_000964.3). Coding region changes show predicted amino acid substitutions.
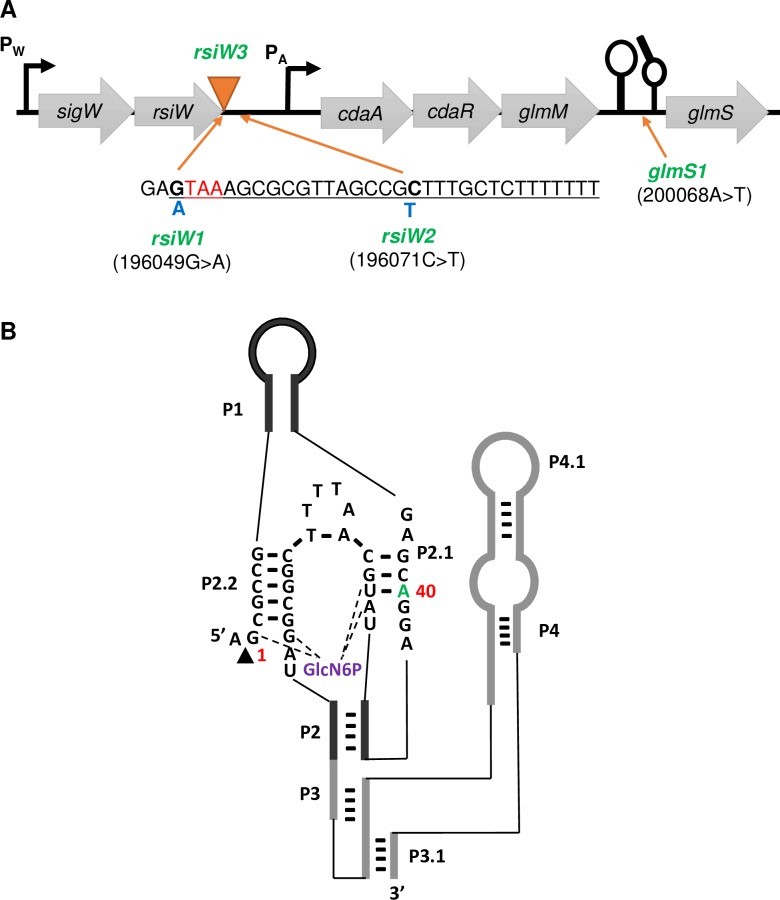
Many of the ΔglmR suppressors (Table 1) contained changes in a chromosomal region around two neighboring operons: sigW-rsiW and cdaA-cdaR-glmM-glmS (Fig 3A). These included a transposon insertion immediately after the rsiW stop codon (rsiW3) and point mutations in the glmS ribozyme (glmS1; 200068A>T), in the penultimate codon of rsiW (rsiW1; 196049G>A), and downstream of rsiW (rsiW2; 196071C>T). Note that the identical glmS mutation (glmS1) was recovered independently in both selection conditions.
Fig 3. Location of glmR suppressor mutations in the sigW-rsiW and cdaA-cdaR-glmM-glmS operons.
(A) The sigW-rsiW transcription termination loop (underlined) is shown with the rsiW stop codon (red). Suppressor mutations included single nucleotide changes (rsiW1 and rsiW2; blue) and a transposon insertion (rsiW3; orange triangle). (B) Secondary structure of glmS ribozyme catalytic domain in B. subtilis. The arrowhead indicates the site of self-cleavage. The guanine at the cleavage site is considered the first residue (G1). The green letter (40A) identifies the site of the glmS1 mutation (40A>T).
Since most of the suppressor mutations did not fully restore CEF resistance to WT levels (Table 1), we selected several with intermediate levels of resistance as a starting point for selection of further increased CEF resistance. The most frequent secondary mutations were in rho (S1 Table). A rho deletion mutant has been associated with beta-lactam resistance in B. subtilis previously [24]. Interestingly, a ΔglmR Δrho double mutant is actually more sensitive to CEF than ΔglmR (S4 Fig), and it is only when a primary suppressor mutation (such as glmS1) is present in ΔglmR that rho mutations confers significant CEF resistance (S4 Fig and S1 Table).
The glmS1 ribozyme mutation abolishes negative feedback regulation of glmS
GlmS is an amidotransferase that catalyzes the first step in PG synthesis (Fig 1) by conversion of the glycolysis intermediate fructose-6-phosphate (F6P) into glucosamine-6-phosphate (GlcN6P) using glutamine as an amino group donor [25]. Expression of GlmS is under negative feedback control mediated by a ribozyme structure encoded in the 5'-untranslated region (5’-UTR) of the glmS mRNA. Upon binding to the GlmS product, GlcN6P, the ribozyme promotes site specific self-cleavage of glmS mRNA and consequently reduces glmS expression [26].
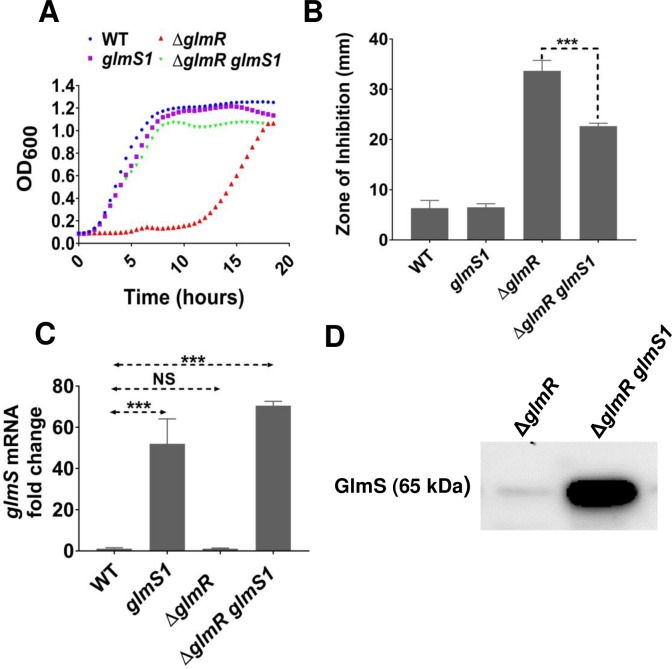
The glmS1 suppressor mutation is a base change in the catalytic domain of the glmS ribozyme (Fig 3B) [27]. After moving the glms1 mutation into a ΔglmR strain, the reconstructed ΔglmR glmS1 strain regains the ability to grow on gluconeogenic carbon sources (Fig 4A) and has increased resistance to CEF (Fig 4B). We hypothesized that glmS1 might interfere with the catalytic activity of the glmS ribozyme. Consistent with this idea, the glmS1 mutation caused a >50-fold increase in glmS mRNA compared to WT (Fig 4C) and a corresponding increase in GlmS protein levels (Fig 4D). We did not see any significant difference in glmS mRNA level between WT and ΔglmR.
Fig 4. glmS1 suppresses ΔglmR by abolishing negative feedback regulation of glmS expression.
(A) Representative growth curves in MH medium (n>3) showing the effect of point mutation glmS1 in ΔglmR compared to WT, glmS1 and ΔglmR. (B) Disc diffusion assay showing the effect of glmS1 on CEF sensitivity phenotype of ΔglmR. 6 μg CEF was used in the assay. Standard deviation (error bars) is based on at least three biological replicates. Three asterisks indicate significant difference with P <0.001 using Tukey test. (C) qRT-PCR results showing glmS mRNA level in glmS1, ΔglmR and ΔglmR glmS1 relative to WT from cells harvested at OD600 of 0.5 grown in LB. Standard deviation (error bars) is based on at least three biological replicates. Statistical significance is determined by Tukey test where three asterisks indicate P <0.001 and NS is non-significant (P >0.05). (D) Western blot analysis of GlmS protein in ΔglmR and ΔglmR glmS1 using anti-GlmS antibodies. 5 μg of total protein was loaded in each lane.
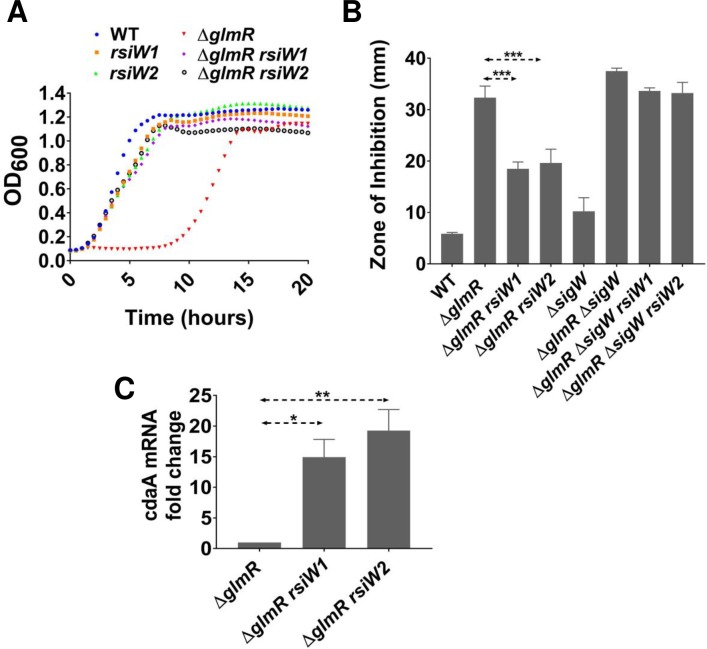
Point mutations in the sigW-rsiW transcription termination loop suppress ΔglmR
Reconstruction of ΔglmR strains with mutations rsiW1 or rsiW2 confirmed that these changes allow growth of ΔglmR on gluconeogenic growth medium (Fig 5A) as well as increased resistance to CEF (Fig 5B). The rsiW1 mutation is silent with respect to the sequence of RsiW and rsiW2 is downstream of the rsiW coding region (Fig 3A). We hypothesized that these point mutations might affect the intrinsic transcription terminator of the sigW-rsiW operon. In silico analysis indicated that each mutation generates a mismatch in the stem of the transcription terminator that is predicted to decrease stability and therefore increase readthrough from the sigW-rsiW operon into the downstream cdaA-cdaR-glmS-glmM operon (S5 Fig). Indeed, the rsiW1 or rsiW2 suppressor mutations led to a >10-fold increase in the mRNA level for the first gene of this operon, cdaA (Fig 5C).
Fig 5. Point mutations in the sigW-rsiW operon transcription termination loop suppress ΔglmR phenotypes.
(A) Growth curves showing the effect of rsiW1 and rsiW2 on the ability of ΔglmR to grow on gluconeogenic MH medium. (B) Disc diffusion assays carried out with 6 μg CEF. Standard deviation (error bars) is based on at least three biological replicates. Three asterisks represent statistical significance with P <0.001 with the Tukey test. (C) qRT-PCR analysis of cdaA mRNA fold change relative to glmR. One and two asterisks represent statistical significance with P value less than 0.05 and 0.01 respectively.
Expression of the sigW-rsiW operon is dependent on an autoregulatory σW-dependent promoter. An in-frame deletion mutation of sigW abolished the ability of the rsiW1 and rsiW2 mutations to suppress the ΔglmR phenotype (Fig 5B). However, in a strain with a sigW::erm disruption mutation the rsiW1 and rsiW2 mutations still conferred increased CEF resistance since the erm σA promoter now reads into the cdaA operon (S5B Fig). These observations support our hypothesis that rsiW1 and rsiW2 increase expression of cdaA-cdaR-glmM-glmS. A similar increase in transcription may explain the phenotype of the rsiW3 Tn insertion (Table 1).
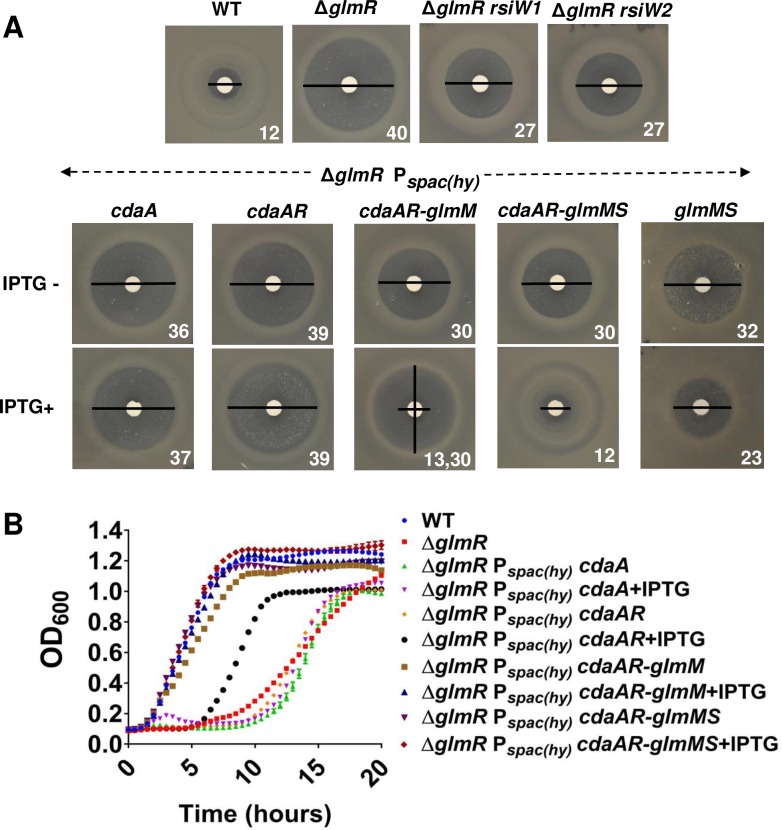
Increased expression of genes from the cdaA-cdaR-glmM-glmS operon suppresses ΔglmR growth phenotypes
We reasoned that the rsiW1, rsiW2 and rsiW3 mutations likely lead to elevated expression of the cdaA-cdaR-glmM-glmS operon. The first two genes encode the major synthase (CdaA) for c-di-AMP and an activator protein (CdaR) [6, 7]. The final two genes encode enzymes for the initial steps of PG biosynthesis that (together with GlmU; also known as GcaD; [28]) convert F6P to UDP-GlcNAc (Fig 1). To determine which gene(s) in this operon are involved in suppression of the ΔglmR phenotypes we integrated IPTG-inducible copies of various portions of this operon (including cdaA, cdaA-cdaR, cdaA-cdaR-glmM, cdaA-cdaR-glmM-glmS, glmM-glmS) at the amyE locus in the ΔglmR strain. These strains were tested for CEF sensitivity and growth on MH medium. Overexpression of cdaA or cdaA-cdaR was not sufficient to increase CEF resistance of ΔglmR (Fig 6A), although we did note an increased frequency of spontaneous suppressors. Overexpression of cdaA-cdaR-glmM or glmM-glmS partially restored CEF resistance (Fig 6A). However, when the whole operon (cdaA-cdaR-glmM-glmS) was induced CEF resistance was restored to essentially WT levels (Fig 6A). Increased expression of cdaA-cdaR-glmM or cdaA-cdaR-glmM-glmS also suppressed the essentiality of ΔglmR on gluconeogenic MH medium (Fig 6B). In contrast, induction of cdaA-cdaR alone has a comparatively weak and variable effect on growth, which may reflect the rapid emergence of suppressors in this strain (Fig 6B). From these results we conclude that the key factor in increased fitness of the ΔglmR strain is elevated expression of GlmS and/or GlmM, but that c-di-AMP may also play a role.
Fig 6. Suppression of glmR by overexpression of glmS and glmM.
(A) Disc diffusion assays (representative images; n>3) illustrating effects of overexpression of cdaA, cdaA-cdaR (cdaAR), glmM-glmS, cdaAR-glmM or cdaAR-glmM-glmS on the CEF sensitivity of the ΔglmR strain. Numbers represents diameter of ZOI (mm). Note that for cdaAR-glmM there is a small clear inner zone (13 mm), and a larger zone of greatly reduced growth (30 mm). (B) Growth curves in MH media for the strains shown in panel (A).
An increase of c-di-AMP has been previously associated with CEF resistance since mutations in gdpP, encoding the major c-di-AMP hydrolase, suppress the CEF sensitivity of a sigM mutant [4]. Moreover, a yqfF::Tn insertion, affecting a second c-di-AMP hydrolase renamed PgpH [6, 7], suppresses the inability of a glmR(yvcK) mutant to grow on gluconeogenic media [5]. We have confirmed these findings and here demonstrate that inactivation of gdpP increases CEF resistance of ΔglmR, although pgpH does not have a significant effect under our conditions (S6A and S6B Fig). It is interesting to note that a gdpP pgpH double mutant, which has greatly elevated c-di-AMP levels and is growth impaired [7], is also highly sensitive to CEF. This effect is not additive with ΔglmR, suggesting that excess c-di-AMP may affect the same pathway as GlmR (S6A and S6B Fig). Consistently, the ability of CdaA and CdaR to increase CEF resistance in a ΔglmR mutant seems to be contingent on the additional expression of GlmM and GlmS, as noted above (Fig 6A). CdaA forms a complex with both CdaR and GlmM [7, 29], suggesting that c-di-AMP may modulate GlmM activity.
Increasing expression of UDP-GlcNAc biosynthetic enzymes suppresses ΔglmR phenotypes
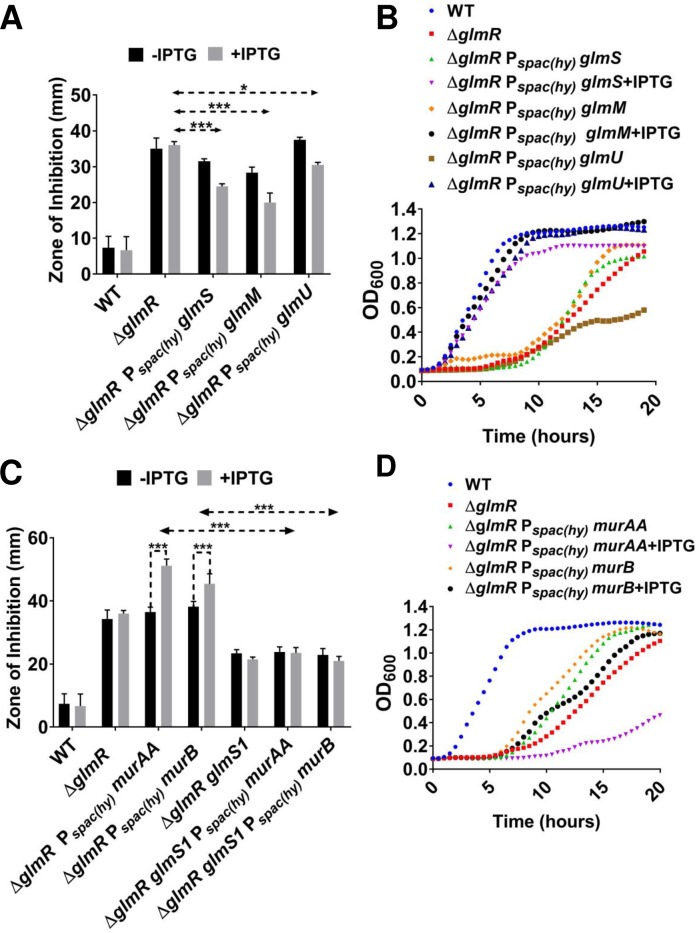
We next considered whether a ΔglmR strain might be phenotypically suppressed by over-expression of other individual enzymes upstream and downstream of UDP-GlcNAc. Induction of glmS, glmM or glmU (Fig 1), partially restored CEF resistance (Fig 7A) and restored the ability of ΔglmR to grow on gluconeogenic medium (Fig 7B). We suggest that these enzymes increase the forward reaction catalyzed by GlmS by consumption of the product, GlcN6P. GlcN6P is potent inhibitor of GlmS (product inhibition) [30], a property shared with the human ortholog [31].
Fig 7. Increasing UDP-GlcNAc suppresses ΔglmR.
(A) Disc diffusion showing the change in CEF susceptibility of ΔglmR when glmM, glmS and glmU were overexpressed. Standard deviation (error bars) is based on at least three biological replicates. One and three asterisks indicate significant value with P <0.05 and P <0.001 respectively as determined by Tukey test. (B) Growth curves in MH media with ΔglmR overexpressing glmM, glmS and glmU in comparison to WT and ΔglmR. (C) Disc diffusion assay showing CEF susceptibility of ΔglmR when murAA and murB are overexpressed. The figure also shows the effect on CEF sensitivity when glmS1 is introduced in ΔglmR amyE:: Pspac(hy) murAA and ΔglmR amyE::Pspac(hy) murB respectively. Three asterisks indicate significance (P <0.001) as determined by Tukey test. (D) Growth curve experiment done in MH medium showing the consequence of murAA and murB overexpression on ΔglmR.
A portion of cellular UDP-GlcNAc is converted to UDP-MurNAc, the second building block of PG, by MurA and MurB (Fig 1). B. subtilis has two MurA paralogs, MurAA and MurAB, but only MurA is essential. UDP-MurNAc is then modified by addition of a pentapeptide side-chain and transferred to the undecaprenylphosphate carrier lipid to ultimately generate lipid II (Fig 1), a lipid-linked GlcNAc-MurNAc-pentapeptide that is the substrate for extracellular PG synthesis [32]. Overexpression of murAA or murB increased the sensitivity of the ΔglmR strain to CEF (Fig 7C), and neither rescued the growth defect of ΔglmR on MH medium (Fig 7D).
We reasoned that the effects of MurAA and MurB overproduction might be relieved in cells that have increased capacity to synthesize UDP-GlcNAc. To test this hypothesis, we introduced the glmS1 mutation (which abolishes negative feedback regulation of glmS) into the ΔglmR amyE::Pspac(hy) murAA and ΔglmR amyE::Pspac(hy) murB strains. In these glmS1 strains, induction of murAA or murB no longer increases sensitivity to CEF (Fig 7C). Based on these observations we hypothesize that B. subtilis lacking GlmR is impaired specifically in UDP-GlcNAc biosynthesis. The resulting inability to efficiently synthesize PG is a likely reason for the essentiality of glmR on gluconeogenic media.
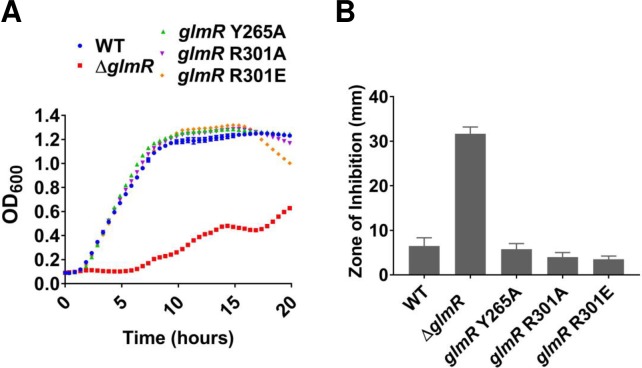
Mutations of the GlmR UDP-GlcNAc binding site do not significantly affect CEF resistance
GlmR was recently found to bind UDP-sugars such as UDP-glucose and UDP-GlcNAc [10]. UDP-GlcNAc bound with five times higher affinity that UDP-Glc, suggesting that the former may be a regulatory ligand for GlmR. We used CRISPR-gene editing to introduce single amino acid substitutions in the UDP-GlcNAc binding site of GlmR that were previously shown to abolish ligand binding (Y265A, R301A and R301E). Consistent with prior results [10], none of these three mutations affected the ability of GlmR to support growth on gluconeogenic MH medium (Fig 8A), nor did they have a significant impact on CEF resistance (Fig 8B). We therefore suggest that ligand binding serves as a feedback mechanism to down-regulate GlmR activity when UDP-GlcNAc levels are high. Under gluconeogenic conditions, when GlmR is required for redirecting carbon from CCM into PG synthesis, this binding site would be vacant, and therefore these mutations would not affect the stimulatory function of GlmR (Fig 1).
Fig 8. Mutations of the GlmR UDP-GlcNAc binding site do not affect gluconeogenic growth.
(A) Growth curve with UDP-GlcNAc binding site mutants of GlmR in gluconeogenic MH medium and (B) CEF sensitivity of GlcNAc binding site mutants of GlmR tested on LB medium with 6 μg of antibiotic.
Addition of GlcNAc bypasses the essentiality of glmR on gluconeogenic media
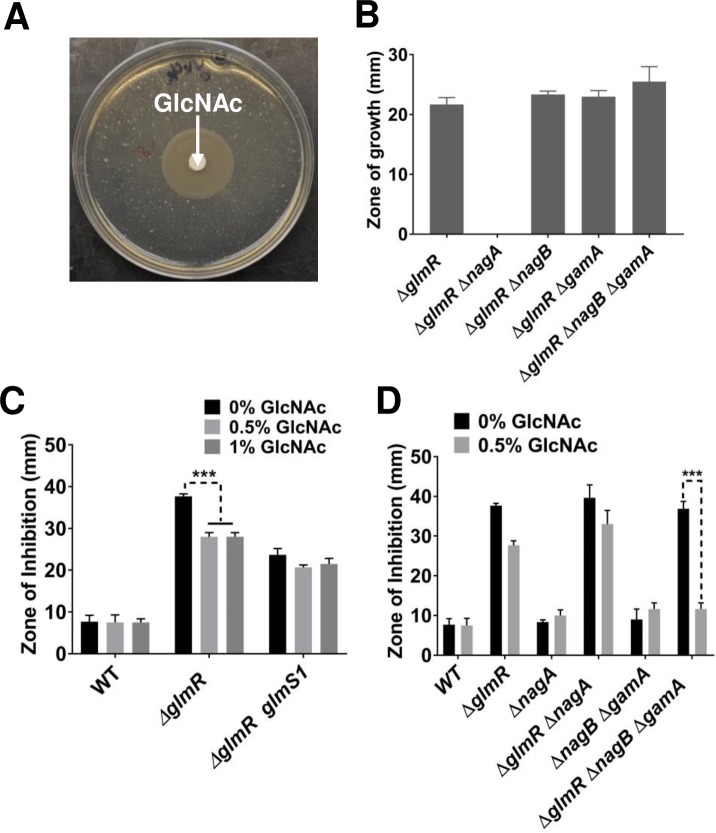
Since ΔglmR suppressor mutations lead to increased glmS expression (Fig 4C and 4D), we reasoned that the ΔglmR strain may be specifically defective in GlmS activity. If this is the case, we hypothesized that provision of cells with GlcNAc would chemically complement the ΔglmR growth defect. Indeed, when a disc containing GlcNAc was placed on a MH medium plate strong growth of the ΔglmR strain was observed (Fig 9A).
Fig 9. Addition of GlcNAc bypasses the essentiality of glmR on gluconeogenic media.
(A) An MH agar plate with ΔglmR showing the zone of growth around a GlcNAc disc. (B) Bar graphs representing the zone of growth for ΔglmR, ΔglmR ΔnagA, ΔglmR ΔnagB, ΔglmR ΔgamA and ΔglmR ΔnagB ΔgamA. (C) Disc diffusion assay showing the effect of GlcNAc (0.5 or 1%) on CEF sensitivity of WT, ΔglmR and ΔglmR glmS1 and (D) Disc diffusion assay comparing the effect of 0.5% GlcNAc on CEF sensitivity of WT, ΔglmR, ΔnagA, ΔglmR ΔnagA, ΔgamA ΔnagB and ΔglmR ΔgamA ΔnagA. Three asterisks indicate significance with P <0.001 as determined by Tukey test.
GlcNAc is taken up by the GlcNAc-specific phosphoenolpyruvate phosphotransferase system (PTS) protein NagP and enters the cell as GlcNAc-6-phosphate [33]. Deacetylation by NagA then generates GlcN6P (Fig 1), which is also the product generated by GlmS [34]. GlcN6P can either feed into peptidoglycan biosynthesis (GlmM and GlmU) or feed CCM by conversion to F6P by either of two inducible deaminases (NagB and GamA) [33, 35] (Fig 1). The ability of GlcNAc to support growth of the ΔglmR strain requires NagA, but is independent of the GamA and NagB deaminases (Fig 9B). This indicates that the limiting step in metabolism during growth of the ΔglmR strain on largely gluconeogenic carbon sources is the GlmS-catalyzed conversion of F6P to GlcN6P. This limitation can be by-passed by up-regulation of GlmS (e.g. by overexpression, Fig 7B, or in the glmS1 mutant strain, Fig 4) or by provision of cells with GlcNAc. The ability of overproduced GlmM or GlmU to support growth (Fig 7B) may therefore seem surprising, but may be explained by more rapid consumption of GlcN6P, which would prevent product inhibition of GlmS and also increase translation of GlmS by inhibiting glmS ribozyme cleavage.
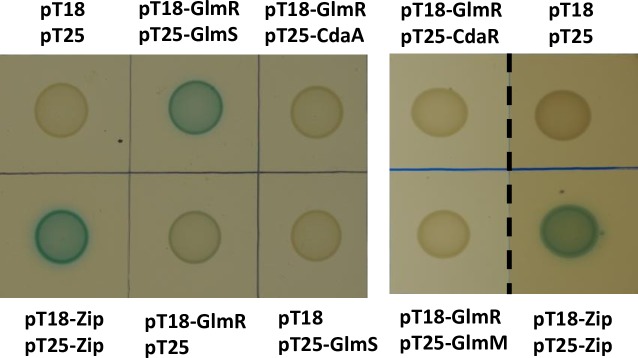
To test if GlcNAc addition also suppresses the increased CEF sensitivity, we tested WT and ΔglmR strains on LB agar supplemented with 0.5% and 1% GlcNAc. Addition of GlcNAc partially suppressed the CEF sensitivity of ΔglmR, but had no significant effect on a strain in which GlmS was up-regulated by the glmS1 suppressor mutation (Fig 9C). In a ΔglmR ΔnagB ΔgamA strain in which added GlcNAc cannot re-enter CCM, CEF resistance is restored to near WT levels (Fig 9D). The greater suppression seen in this strain may result from the inability of this strain to catabolize incoming GlcNAc, which thereby further increases the flux into PG synthesis. This supports the notion that a major contributor to CEF sensitivity is a metabolic defect that limits the ability of the cell to synthesize PG, apparently due to a limitation in the ability of GlmS to redirect carbon from CCM to cell wall synthesis. We hypothesize that GlmR may directly stimulate GlmS enzyme activity. This is supported by evidence of a GlmR-GlmS protein interaction in bacterial two-hybrid assays (Fig 10). The observed interaction is robust, as compared to the positive control, and GlmR did not interact with other proteins tested including CdaA, GlmM or CdaR (Fig 10).
Fig 10. Bacterial two hybrid assay.
pT18-containing glmR and the compatible plasmid pT25 containing glmS or cdaA were transformed into E. coli strain BTH101. When co-expressed protein fusions interact, the Bordetella pertussis adenylate cyclase is active as assessed by blue color in the presence of X-gal. Negative controls include cells containing one or both empty vectors, and the positive control is the pT18-ZIP and pT25-ZIP plasmids [60]. Left panel is a single plate, and the right panel is images from a separate experiment showing a lack of signal for interaction of GlmR with CdaR or GlmM and the corresponding positive control.
Discussion
Here we present a forward genetic analysis that indicates that GlmR regulates the redirection of carbon from CCM into PG biosynthesis, likely by stimulation of GlmS activity. The regulation of CCM as cells adapt to nutrient availability is exceptionally complex and involves numerous transcriptional regulators and post-transcriptional regulatory mechanisms [36, 37]. The carbon catabolite control protein CcpA plays a central role in this process and represses genes for the utilization of non-preferred carbon sources when glucose is available [38], as well as the operon encoding glmR: yvcI-yvcJ-glmR-yvcL-crh-yvcN [39]. As a result, GlmR should be most abundant when CcpA activity is low. CcpA repressor activity is indirectly stimulated by elevated levels of fructose-1,6-bisphosphate present during growth on preferred carbon sources [40–42]. During growth on non-preferred, gluconeogenic carbon sources GlmR will be more abundant, consistent with its role in diverting carbon to PG synthesis under these conditions.
The GlmR (formerly YvcK) protein is conditionally essential and plays a poorly defined role in cell morphology and antibiotic resistance [10–12, 21]. Homologs in M. tuberculosis (CuvA) and L. monocytogenes (YvcK) appear to also play a role in helping maintain cell shape [11, 12]. GlmR was suggested to lead to a dysregulation of carbon metabolism since mutations affecting metabolic enzymes (e.g. Zwf) and CCM regulatory proteins (e.g. CggR) suppress the glmR null mutant and allow growth on gluconeogenic carbon sources [5]. Cytological evidence suggests that GlmR and CuvA localize to membrane sites associated with PG synthesis, and it has been noted that GlmR and MreB appear to functionally substitute for one another, perhaps in coordinating the assembly of PG biosynthetic complexes [8, 11]. Despite intensive study, the connection between these disparate phenotypes has been elusive. Here, we propose that several of these phenotypes can be explained by GlmR-dependent stimulation of the key branchpoint enzyme, GlmS.
It remains possible that, in addition to stimulation of GlmS activity, GlmR may have other functions. This is suggested by the observation that the role of GlmR in intrinsic CEF resistance is independent of protein phosphorylation as judged by the analysis of phosphomimetic and phosphablative mutants (Fig 2). In contrast, phosphorylation of GlmR was shown to affect bacitracin sensitivity and cell morphogenesis in an mreB mutant background [21]. Although the M. tuberculosis GlmR ortholog CuvA is also modified by phosphorylation by Ser/Thr PASTA kinases, this modification is not important for complementation of carbon source specific growth defects or for localization to sites of PG synthesis [11, 12], and perhaps regulates other functions. Analysis of phosphosite mutants of the L. monocytogenes GlmR ortholog suggests that a phosphomimetic variant is unaffected in metabolism and cell wall homeostasis, but is impaired in virulence [11, 12]. Further studies are needed to clarify how GlmR phosphorylation affects some, but not all, activities of this protein.
A model for GlmR as a feedback inhibited activator of GlmS
Our genetic analysis supports a model in which GlmR activates GlmS, and we suggest that this activity is inhibited when GlmR is bound to the downstream metabolite, UDP-GlcNAc (Fig 1). This model is supported by several key observations. First, overproduction of GlmS, in either the glmS1 mutant or by induction from an ectopic glmS gene, is sufficient to restore growth of the glmR null mutant on MH medium (Figs 4 and 7). Second, a glmR mutant can be chemically complemented by GlcNAc, even under conditions where GlcNAc cannot be routed into CCM (Fig 9). Since metabolism of GlcNAc generates GlcN6P, this addition specifically bypasses the GlmS reaction (Fig 1). Therefore, we suggest that GlmS (rather than GlmM or GlmU) is limiting the flux of carbon into PG in the ΔglmR strain. Third, GlmR and GlmS interact in vivo as judged by a bacterial two-hybrid assay (Fig 10). Fourth, previous metabolomics measurements indicate that F6P levels are ~16-fold lower during growth on gluconeogenic carbon sources when compared to glucose [13], consistent with the requirement for GlmR under these conditions (Fig 1). Fifth, GlmR was recently found to bind UDP-GlcNAc [10]. However, mutations that abolish binding do not affect the ability of GlmR to stimulate growth under gluconeogenic conditions [10] or to provide intrinsic CEF resistance (Fig 8), as predicted by the hypothesis that UDP-GlcNAc antagonizes GlmR function (Fig 1). GlmS is recognized as the key branch-point enzyme in bacteria for diverting carbon from CCM into PG synthesis, and in eukaryotes the GlmS ortholog diverts carbon into hexosamine synthesis. Both classes of enzyme are in some cases feedback regulated by UDP-GlcNAc [43–47]. Here, UDP-GlcNAc binding is proposed to antagonize GlmR function, and therefore reduce stimulation of GlmS.
In addition to GlmS, we also demonstrate that overproduction of either GlmM or GlmU, but not by enzymes downstream of the key intermediate UDP-GlcNAc, can suppress the glmR growth defect under gluconeogenic conditions. GlmS catalyzes a reversible reaction, and its product (GlcN6P) is a potent inhibitor of the forward reaction [30]. Moreover, GlcN6P binds to the glmS ribozyme to cleave the mRNA and suppress translation [26]. Therefore, we suggest that increasing the level of GlmM and/or GlmU likely helps pull the reaction in the forward direction and may also stimulate GlmS translation.
GlmR activation of GlmS as a framework for understanding other suppressor mutations
With a defined model in hand, we can revisit the other suppressor mutations recovered both in our selection conditions (Table 1) and the studies of Görke et al. [5]. As noted previously, many of the mutations that suppress glmR affect CCM. We recovered a frameshift mutation in zwf, a gene also recovered in the previous transposon-based selection for glmR suppressors [5]. Normally, Zwf diverts a substantial fraction of glucose-6-phosphate from glycolysis into the pentose phosphate pathway [48]. We speculate that in the absence of Zwf there is increased flux leading to F6P, the GlmS substrate. We also recovered a mutation in pgcA, which encodes another branch point enzyme that uses glucose-6-phosphate. Previously, it was reported that a mutation in cggR, encoding the central glycolytic genes regulator, also suppresses glmR [5]. Since a cggR null mutant will have increased levels of several key enzymes that function in both glycolysis and gluconeogenesis [49], we speculate that this mutation alleviates the metabolic restriction in the glmR strain by increasing gluconeogenesis and therefore F6P levels.
A second class of mutations that increase the fitness of the ΔglmR strain are those that lead to elevated c-di-AMP levels. This was foreshadowed by the finding that a pgpH (formerly yqfF) mutation suppresses glmR [5]. In our studies, we find that gdpP suppresses glmR both for growth on MH medium and for CEF resistance, whereas pgpH has a lesser effect (S6 Fig). CdaA is regulated by interaction with the CdaR protein and also forms a complex with GlmM [7, 29]. Indeed, the cdaA-cdaR-glmM genes are co-transcribed in a wide variety of species, suggesting a functional connection. This has led to the suggestion that GlmM may regulate c-di-AMP synthesis [7, 29]. Conversely, CdaA may regulate GlmM. In this scenario, conditions that lead to elevated c-di-AMP may alter the CdaA-CdaR complex to favor a stimulatory interaction of CdaA with GlmM. Indeed, it is striking that induction of the entire cdaARglmMS operon fully restores CEF resistance to a glmR mutant (Fig 6), whereas this is not the case for the glmR glmS1 strain (Fig 4) or for induction of glmS alone (Fig 7). Alternatively, c-di-AMP is also known to regulate potassium homeostasis by interaction with both protein and RNA (riboswitch) targets [50–53]. This c-di-AMP dependent osmolyte transport is important for maintaining turgor pressure in the cell and it has been proposed that perturbations of c-di-AMP metabolism can affect cell envelope integrity by increasing resistance against osmotic stresses [54].
A third class of suppressor mutations is in genes important for energy generation by the electron transport chain. These include mutations in qoxB, encoding cytochrome aa3 quinol oxidase, and yqiD(ispA), encoding a geranyltransferase that is involved in synthesis of isoprenoid compounds including menaquinone, an electron carrier important for respiration (Table 1). Mutations in both of these loci have been previously associated with an increased ability of cells to survive the transition to L-forms that lack a peptidoglycan cell wall [55]. This observation led to a model in which a lethal consequence of cell wall defects is oxidative damage triggered by increased flux through the electron transport chain when carbon flux into peptidoglycan is eliminated [55]. Regardless of the precise mechanism, it is intriguing that mutations in these same genes were recovered as suppressors of ΔglmR.
Finally, we recovered one strain containing a missense mutation in yvcJ (Table 1), the gene immediately upstream of glmR. The role of YvcJ is unknown, but it has GTPase activity, affects phosphorylation of an uncharacterized cell component, and has an apparent role in natural competence [56, 57]. Since this strain contained an additional mutation in sigA (Table 1), further work is needed to determine the effect of the yvcJ mutation on CEF resistance. Curiously, mutants of the E. coli YvcJ homolog (RapZ; formerly YhbJ) lead to overproduction of GlmS [58]. RapZ appears to sense GlcN6P and regulates the processing and stability of a small RNA, GlmZ, that activates GlmS synthesis [46, 58, 59]. It is presently unknown whether YvcJ plays a related role in B. subtilis, perhaps by interacting either with GlmR or the glmS ribozyme.
In conclusion, the results presented here highlight the importance of the GlmS branch point in regulating the flow of carbon from CCM into PG synthesis. In eukaryotes, GlmS orthologs serve as the initiating enzyme for hexosamine biosynthesis, and are sensitive to both GlcN6P product inhibition [31] and feedback regulation by UDP-GlcNAc, which binds to the isomerase domain [43, 44]. In bacteria, GlmS is also subject to complex regulation at the level of both synthesis and activity [45–47]. In B. subtilis, GlmS is feedback inhibited by its immediate product, GlcN6P [30], which also activates the glmS ribozyme [26]. GlmR provides another layer of regulation. Our results support a model in which GlmR stimulates GlmS activity, and we propose that binding of UDP-GlcNAc may attenuate this stimulation.
Methods
Bacterial strains and growth conditions
B. subtilis strains used are derived from strain 168 (trpC2) (S2 Table). E. coli strain DH5α was used for cloning and strain BTH101 [60] for bacterial two hybrid experiments. Bacteria were cultured in LB broth. Strains with a glmR deletion mutation were cultured on LB with 20 mM MgSO4 unless specified otherwise. Antibiotics were added to growth media when required at the following concentrations: 100 μg/ml ampicillin, 30 μg/ml chloramphenicol for E. coli, 10 μg/ml kanamycin, 10 μg/ml chloramphenicol, 5 μg/ml tetracycline, 100 μg/ml spectinomycin and 1 μg/ml erythromycin with 25 μg/ml lincomycin (erm; macrolide-lincomycin-streptogramin B resistance).
Cloning, transformation and strain construction
For cloning procedures, restriction digestion and ligation with T4 ligase was done as per manufacturer's instructions (NEB, USA). Plasmids were then transformed into competent DH5α cells [61]. Cloning was confirmed by polymerase chain reaction (PCR) followed by Sanger sequencing. B. subtilis transformation was carried out in minimal competence media with 12 mM MgSO4. DNA was added when cells reached OD600 of ~0.7–0.8. Generation of B. subtilis strains overexpressing gene(s) at amyE was achieved using pPL82 [62] carrying gene(s) of interest followed by transformation into the indicated B. subtilis recipient strain.
Bacillus knockout erythromycin (BKE) strains with various gene deletion mutations of B. subtilis were obtained from the Bacillus Genetic Stock Center (BGSC) [63]. Chromosomal DNA from each BKE strain was transformed into our lab strain B. subtilis 168. The erythromycin resistance cassette was removed using pDR244 [63], which produces Cre recombinase at the permissive temperature of 30°C, to generate in-frame deletions. pDR244 was transformed into B. subtilis strain at 30°C and plated on LB plates with spectinomycin. Colonies were picked after two overnight incubations and patched three successive times on LB plates incubated at the non-permissive temperature 42°C overnight. Strains were then patched on spectinomycin- and erythromycin-containing plates to confirm the absence of both markers. All the deletion mutants used in study are markerless deletions except Δrho (rho::erm).
Single nucleotide mutations glmS1, rsiW1 and rsiW2 were reconstructed using the integration vector pMutin4 that has an erm resistance marker and lacZ [64]. A fragment of DNA with the mutation of interest was cloned into pMutin4 and confirmed with PCR and Sanger sequencing. The vector was transformed into B. subtilis where it integrated at locus by single crossover homologous recombination. Transformants were selected on plates with Erm and 40 μg/ml X-gal. After overnight incubation, a few blue color colonies were picked. Since pMutin4 integration is unstable, cells were grown without antibiotic selection three consecutive times with each time adding 1:100 dilution of cells from previous culture. Cells were then plated on LB plates with X-gal and white colonies were picked and sequenced to find those strains that retained the single nucleotide mutation of interest.
Mariner transposon mutagenesis
Mariner transposon mutagenesis procedure was carried out in ΔglmR as described previously [65]. In brief, ΔglmR was transformed with the pMarA vector. The strain with pMarA was grown in 5 ml LB broth until mid-exponential phase and various dilutions of cells were plated on selection medium. In independent experiments CEF resistance and ability to grow on MH media were used as a selection.
Spontaneous suppressor analysis
Spontaneous suppressors of ΔglmR were picked from the clear zone of CEF disc diffusion plates and independently from MH plates after overnight incubation at 37°C. Chromosomal DNA extracted from these suppressors was sequenced using an Illumina machine. The sequencing data were analyzed using CLC genomics workbench.
Antibiotic sensitivity assays
Antibiotic sensitivity was tested using disc diffusion assays, which were carried out on LB medium. Strains to be tested were grown in 5 ml LB broth at 37°C with vigorous shaking to an OD600 of ~0.4. 100 μl of cells were added to 4 ml top LB agar (0.7% agar) kept at 50°C. 1 mM IPTG was added to top agar when indicated. Top agar with cells was poured over 15 ml LB bottom agar (1.5%) plate. A Whatman paper disc (7mm dia) with 6 μg CEF was put on the plate unless specified otherwise. Plates were incubated at 37°C overnight and the clear zone of inhibition was measured the next day. Values for CEF resistance (Table 1) report the diameter of the zone of growth inhibition. For all histograms, the values shown have the diameter of the filter disk (7 mm) subtracted from the average diameter.
Growth Assay on MH
To test the ability of B. subtilis mutants to grow under gluconeogenic conditions we used MH medium (Sigma-Aldrich, USA) prepared per the manufacturer's instruction. Growth was monitored using a Bioscreen growth analyzer with 200 μl of MH broth in 100 well Bioscreen plates inoculated with 2 μl of B. subtilis strains pre-grown in LB broth at 37°C to an OD600 of ~0.4. When required, glucose, MgSO4 and IPTG were added to the final concentrations of 1%, 20 mM and 1 mM respectively.
qRT-PCR
Strains of interest were grown to an OD600 of ~0.5. 1.5 ml of culture was used for RNA extraction. RNA isolation (Qiagen, USA) and cDNA preparation (Thermofisher, USA) was carried out as suggested by the manufacturer. qRT-PCR was carried out using a Bio-Rad iTaq universal SYBR green super mix. 23S rRNA was used to normalize the cycle threshold (Ct) value.
Cell lysate preparation and western blot
For GlmS measurements, ΔglmR and ΔglmR glmS1 strains were grown in LB medium to an OD600 of ~0.3 at 37°C with shaking. 30 ml of culture was withdrawn and centrifuged at 5000 rpm for 10 minutes. Cell pellets were frozen at -20°C. Pellets were washed once with 1X phosphate buffer saline (pH 7.4). 150 μl of lysis buffer (20 mM tris-HCl, 100 mM NaCl, 1 mM EDTA, 1 mM DTT, 10% glycerol and protease inhibitor cocktail) was used to re-suspend the cell pellets. One tablet of protease inhibitor cocktail from Roche diagnostics was added to 10 ml of lysis buffer. Cells were lysed by sonication. After centrifugation cell lysates were transferred to fresh tubes. Protein concentration was measured by Bradford assay (Bio-Rad). 5 μg of protein was run on a 4–15% gradient gel from Bio-Rad. Protein was transferred onto a PVDF membrane using a Bio-Rad transblot turbo transfer system. The membrane was blocked with 5% milk powder for one hour followed by overnight incubation with primary anti-GlmS polyclonal antibodies [66] added to 1:3000 dilution in 1X tris buffer saline with 0.1% tween 20 and 0.5% milk powder. After three washes, the membrane was incubated with a 1:3000 dilution of HRP conjugated anti-Rabbit antibodies (Sigma). Bands were visualized on a Bio-Rad Chemidoc MP imaging system.
GlcNAc disc diffusion assay
Strains of interest were grown in 5 ml LB medium to an OD600 of ~0.4. 100 μl of cells were added to 4 ml top MH agar (0.7% agar) preheated at 50°C and was laid on a 15 ml MH agar (1.5%) plate. A disc with 0.5 mg GlcNAc (Sigma, USA) was put on the plate. After overnight incubation at 37°C, the zone of growth surrounding the disc was measured.
CRISPR editing
DNA changed encoding single amino acid substitutions (GlmRY255A, GlmRR301A and GlmRR301E) were generated at the native glmR locus using CRISPR editing as described [67]. In brief, oligonucleotides encoding a 20 nucleotide gRNA with flanking BsaI sites and a repair fragment carrying mutations of interest with flanking SfiI restrictions sites were cloned sequentially into vector pJOE8999 followed by transformation into E. coli DH5α cells. The resultant plasmid was transformed into recipient B. subtilis strain and cells were plated on 15 μg/ml kanamycin plates with 0.2% mannose. Transformation was carried out at 30°C as pJOE8999 cannot replicate at higher temperatures. The transformants were patched on LB agar plates and incubated at the non-permissive temperature of 42°C. The loss of vector was confirmed by the inability of selected isolates on kanamycin plates. The presence of the desired mutations was confirmed by Sanger sequencing.
Bacterial two hybrid
Vectors pT18 and pT25 and strains for bacterial two hybrid were prepared as described [60]. E. coli BTH101 strains carrying pT18 and pT25 with genes of interest were grown in LB broth overnight at 30°C with 100 μg/ml ampicillin, 50 μg/ml chloramphenicol and 0.5 mM IPTG. 10 μl of cells were spotted on LB plate with 100 μg/ml ampicillin, 50 μg/ml chloramphenicol, 0.5 mM IPTG and 40 μg/ml X-gal. Plates were incubated overnight at 30°C.
RNA structure analysis
In silico analysis was carried out using NUPACK web application [68].
Supporting information
(DOCX)
(DOCX)
(A) Growth curves showing the effect of addition of glucose and MgSO4 on growth of ΔglmR in MH medium compared to WT. (B) Growth stimulation on MH medium by glucose. Top MH agar (4 ml) was plated with 100 μl of ΔglmR cells and filter discs containing 2.5 mg and 5 mg of glucose were put on the plate followed by overnight incubation at 37°C.
(PDF)
Disc diffusion assay for WT and ΔglmR done with (A) oxacillin (1 μg), (B) cefixime (40 μg), (C) moenomycin (10 μg), (D) vancomycin (10 μg), (E) fosfomycin (50 μg), (F) bacitracin (400 μg) and (G) nisin (50 μg). One asterisk and three asterisks represent significance with P <0.05 and P <0.001 respectively. NS indicates that differences were not significant.
(PDF)
(A) CEF susceptibility of ΔglmR and complementation of phenotype by IPTG inducible ectopic expression of 3X-FLAG glmR. RBS was optimized to AGGAGG that is seven base pair apart from start codon. (B) Disc diffusion assay showing suppression of CEF sensitivity of ΔglmR by addition of 20 mM MgSO4. In both figures, statistical significance is indicated by asterisks with P <0.001.
(PDF)
CEF disc diffusion assay showing effect of rho mutation. Statistical significance with P <0.001 is indicated with three asterisks.
(PDF)
(A) Transcription termination loop secondary structure prediction for WT and with point mutations (rsiW1 and rsiW2) are shown with their relative free energy value prediction. (B) CEF disc diffusion assays performed on strains with sigW::erm mutation in WT, ΔglmR, ΔglmR rsiW1 and ΔglmR rsiW2 backgrounds.
(PDF)
(A) CEF susceptibility and (B) growth on MH medium for ΔglmR in combination with gdpP and pgpH deletions and the gdpP pgpH double deletion.
(PDF)
Acknowledgments
We thank Dr Ulf Gerth (Institute of Microbiology, Ernst-Moritz-Arndt-Universität Greifswald, Greifswald, Germany) for providing us with anti-GlmS antibodies and Emma Roszkowski for her contribution in the transposon mutagenesis screen. We thank Tobias Doerr and Kyu Rhee for helpful comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by a grant from the National Institutes of Health (to JDH) under award number R35GM122461. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Helmann JD. Bacillus subtilis extracytoplasmic function (ECF) sigma factors and defense of the cell envelope. Current Opinion in Microbiology. 2016;30:122–32. 10.1016/j.mib.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mascher T, Helmann JD, Unden G. Stimulus Perception in Bacterial Signal-Transducing Histidine Kinases. Microbiology and Molecular Biology Reviews. 2006;70(4):910–38. 10.1128/MMBR.00020-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eiamphungporn W, Helmann JD. The Bacillus subtilis σM Regulon and its Contribution to Cell Envelope Stress Responses. Molecular microbiology. 2008;67(4):830–48. 10.1111/j.1365-2958.2007.06090.x PubMed PMID: PMC3025603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo Y, Helmann JD. Analysis of the role of Bacillus subtilis σM in β-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Molecular Microbiology. 2012;83(3):623–39. 10.1111/j.1365-2958.2011.07953.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Görke B, Foulquier E, Galinier A. YvcK of Bacillus subtilis is required for a normal cell shape and for growth on Krebs cycle intermediates and substrates of the pentose phosphate pathway. Microbiology. 2005;151(11):3777–91. [DOI] [PubMed] [Google Scholar]
- 6.Huynh TN, Luo S, Pensinger D, Sauer J-D, Tong L, Woodward JJ. An HD-domain phosphodiesterase mediates cooperative hydrolysis of c-di-AMP to affect bacterial growth and virulence. Proceedings of the National Academy of Sciences. 2015;112(7):E747–E56. 10.1073/pnas.1416485112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gundlach J, Mehne FMP, Herzberg C, Kampf J, Valerius O, Kaever V, et al. An Essential Poison: Synthesis and Degradation of Cyclic Di-AMP in Bacillus subtilis. Journal of Bacteriology. 2015;197(20):3265–74. 10.1128/JB.00564-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulquier E, Pompeo F, Bernadac A, Espinosa L, Galinier A. The YvcK protein is required for morphogenesis via localization of PBP1 under gluconeogenic growth conditions in Bacillus subtilis. Molecular Microbiology. 2011;80(2):309–18. 10.1111/j.1365-2958.2011.07587.x [DOI] [PubMed] [Google Scholar]
- 9.Kawai Y, Daniel RA, Errington J. Regulation of cell wall morphogenesis in Bacillus subtilis by recruitment of PBP1 to the MreB helix. Molecular Microbiology. 2009;71(5):1131–44. 10.1111/j.1365-2958.2009.06601.x [DOI] [PubMed] [Google Scholar]
- 10.Foulquier E, Galinier A. YvcK, a protein required for cell wall integrity and optimal carbon source utilization, binds uridine diphosphate-sugars. Scientific Reports. 2017;7(1):4139 10.1038/s41598-017-04064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mir M, Prisic S, Kang C-M, Lun S, Guo H, Murry JP, et al. Mycobacterial Gene cuvA Is Required for Optimal Nutrient Utilization and Virulence. Infection and Immunity. 2014;82(10):4104–17. 10.1128/IAI.02207-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pensinger DA, Boldon KM, Chen GY, Vincent WJB, Sherman K, Xiong M, et al. The Listeria monocytogenes PASTA Kinase PrkA and Its Substrate YvcK Are Required for Cell Wall Homeostasis, Metabolism, and Virulence. PLOS Pathogens. 2016;12(11):e1006001 10.1371/journal.ppat.1006001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleijn RJ, Buescher JM, Le Chat L, Jules M, Aymerich S, Sauer U. Metabolic Fluxes during Strong Carbon Catabolite Repression by Malate in Bacillus subtilis. Journal of Biological Chemistry. 2010;285(3):1587–96. 10.1074/jbc.M109.061747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baev MV, Baev D, Radek AJ, Campbell JW. Growth of Escherichia coli MG1655 on LB medium: monitoring utilization of sugars, alcohols, and organic acids with transcriptional microarrays. Applied Microbiology and Biotechnology. 2006;71(3):310–6. 10.1007/s00253-006-0317-6 [DOI] [PubMed] [Google Scholar]
- 15.Cao M, Bernat BA, Wang Z, Armstrong RN, Helmann JD. FosB, a cysteine-dependent fosfomycin resistance protein under the control of sigma(W), an extracytoplasmic-function sigma factor in Bacillus subtilis. J Bacteriol. 2001;183(7):2380–3. 10.1128/JB.183.7.2380-2383.2001 ; PubMed Central PMCID: PMCPMC95149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kingston AW, Liao X, Helmann JD. Contributions of the sigma(W), sigma(M) and sigma(X) regulons to the lantibiotic resistome of Bacillus subtilis. Mol Microbiol. 2013;90(3):502–18. 10.1111/mmi.12380 ; PubMed Central PMCID: PMCPMC4067139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radeck J, Gebhard S, Orchard PS, Kirchner M, Bauer S, Mascher T, et al. Anatomy of the bacitracin resistance network in Bacillus subtilis. Mol Microbiol. 2016;100(4):607–20. 10.1111/mmi.13336 . [DOI] [PubMed] [Google Scholar]
- 18.Thompson MK, Keithly ME, Harp J, Cook PD, Jagessar KL, Sulikowski GA, et al. Structural and chemical aspects of resistance to the antibiotic fosfomycin conferred by FosB from Bacillus cereus. Biochemistry. 2013;52(41):7350–62. 10.1021/bi4009648 ; PubMed Central PMCID: PMCPMC4108214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Formstone A, Errington J. A magnesium-dependent mreB null mutant: implications for the role of mreB in Bacillus subtilis. Molecular Microbiology. 2005;55(6):1646–57. 10.1111/j.1365-2958.2005.04506.x [DOI] [PubMed] [Google Scholar]
- 20.Chastanet A1 C-LR. The actin-like MreB proteins in Bacillus subtilis: a new turn. Front Biosci (Schol Ed). 2012:1582–606. [DOI] [PubMed] [Google Scholar]
- 21.Foulquier E, Pompeo F, Freton C, Cordier B, Grangeasse C, Galinier A. PrkC-mediated Phosphorylation of Overexpressed YvcK Protein Regulates PBP1 Protein Localization in Bacillus subtilis mreB Mutant Cells. Journal of Biological Chemistry. 2014;289(34):23662–9. 10.1074/jbc.M114.562496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah IM, Laaberki M-H, Popham DL, Dworkin J. A Eukaryotic-like Ser/Thr Kinase Signals Bacteria to Exit Dormancy in Response to Peptidoglycan Fragments. Cell. 2008;135(3):486–96. 10.1016/j.cell.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pompeo F, Foulquier E, Serrano B, Grangeasse C, Galinier A. Phosphorylation of the cell division protein GpsB regulates PrkC kinase activity through a negative feedback loop in Bacillus subtilis. Molecular Microbiology. 2015;97(1):139–50. 10.1111/mmi.13015 [DOI] [PubMed] [Google Scholar]
- 24.Lee YH, Helmann JD. Mutations in the Primary Sigma Factor σA and Termination Factor Rho That Reduce Susceptibility to Cell Wall Antibiotics. Journal of Bacteriology. 2014;196(21):3700–11. 10.1128/JB.02022-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milewski S. Glucosamine-6-phosphate synthase—the multi-facets enzyme. Biochimica et Biophysica Acta (BBA)—Protein Structure and Molecular Enzymology. 2002;1597(2):173–92. 10.1016/S0167-4838(02)00318-7. [DOI] [PubMed] [Google Scholar]
- 26.Winkler WC, Nahvi A, Roth A, Collins JA, Breaker RR. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281 10.1038/nature02362 [DOI] [PubMed] [Google Scholar]
- 27.Cochrane JC, Lipchock SV, Strobel SA. Structural Investigation of the GlmS Ribozyme Bound to Its Catalytic Cofactor. Chemistry & Biology. 2007;14(1):97–105. 10.1016/j.chembiol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hove-Jensen B. Identification of tms-26 as an allele of the gcaD gene, which encodes N-acetylglucosamine 1-phosphate uridyltransferase in Bacillus subtilis. J Bacteriol. 1992;174(21):6852–6. ; PubMed Central PMCID: PMCPMC207362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Y, Pham TH, Nhiep THN, Vu NMT, Marcellin E, Chakrabortti A, et al. Cyclic-di-AMP synthesis by the diadenylate cyclase CdaA is modulated by the peptidoglycan biosynthesis enzyme GlmM in Lactococcus lactis. Molecular Microbiology. 2016;99(6):1015–27. 10.1111/mmi.13281 [DOI] [PubMed] [Google Scholar]
- 30.Deng MD, Grund AD, Wassink SL, Peng SS, Nielsen KL, Huckins BD, et al. Directed evolution and characterization of Escherichia coli glucosamine synthase. Biochimie. 2006;88(5):419–29. . [DOI] [PubMed] [Google Scholar]
- 31.Broschat KO, Gorka C, Page JD, Martin-Berger CL, Davies MS, Huang Hc HC, et al. Kinetic characterization of human glutamine-fructose-6-phosphate amidotransferase I: potent feedback inhibition by glucosamine 6-phosphate. J Biol Chem. 2002;277(17):14764–70. 10.1074/jbc.M201056200 . [DOI] [PubMed] [Google Scholar]
- 32.van Heijenoort J. Lipid Intermediates in the Biosynthesis of Bacterial Peptidoglycan. Microbiology and Molecular Biology Reviews. 2007;71(4):620–35. 10.1128/MMBR.00016-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaugué I, Oberto J, Putzer H, Plumbridge J. The Use of Amino Sugars by Bacillus subtilis: Presence of a Unique Operon for the Catabolism of Glucosamine. PLOS ONE. 2013;8(5):e63025 10.1371/journal.pone.0063025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Souza JM, Plumbridge JA, Calcagno ML. N-Acetylglucosamine-6-phosphate Deacetylase from Escherichia coli: Purification and Molecular and Kinetic Characterization. Archives of Biochemistry and Biophysics. 1997;340(2):338–46. 10.1006/abbi.1997.9780. [DOI] [PubMed] [Google Scholar]
- 35.Vincent F, Yates D, Garman E, Davies GJ, Brannigan JA. The Three-dimensional Structure of the N-Acetylglucosamine-6-phosphate Deacetylase, NagA, from Bacillus subtilis: A MEMBER OF THE UREASE SUPERFAMILY. Journal of Biological Chemistry. 2004;279(4):2809–16. 10.1074/jbc.M310165200 [DOI] [PubMed] [Google Scholar]
- 36.Chubukov V, Gerosa L, Kochanowski K, Sauer U. Coordination of microbial metabolism. Nature Reviews Microbiology. 2014;12:327 10.1038/nrmicro3238 [DOI] [PubMed] [Google Scholar]
- 37.Fujita Y. Carbon Catabolite Control of the Metabolic Network in Bacillus subtilis. Bioscience, Biotechnology, and Biochemistry. 2009;73(2):245–59. 10.1271/bbb.80479 [DOI] [PubMed] [Google Scholar]
- 38.Warner JB, Lolkema JS. CcpA-Dependent Carbon Catabolite Repression in Bacteria. Microbiology and Molecular Biology Reviews. 2003;67(4):475–90. 10.1128/MMBR.67.4.475-490.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blencke H-M, Homuth G, Ludwig H, Mäder U, Hecker M, Stülke J. Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: regulation of the central metabolic pathways. Metabolic Engineering. 2003;5(2):133–49. 10.1016/S1096-7176(03)00009-0. [DOI] [PubMed] [Google Scholar]
- 40.Meyer FM, Jules M, Mehne FM, Le Coq D, Landmann JJ, Gorke B, et al. Malate-mediated carbon catabolite repression in Bacillus subtilis involves the HPrK/CcpA pathway. J Bacteriol. 2011;193(24):6939–49. 10.1128/JB.06197-11 ; PubMed Central PMCID: PMCPMC3232832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schumacher MA, Sprehe M, Bartholomae M, Hillen W, Brennan RG. Structures of carbon catabolite protein A-(HPr-Ser46-P) bound to diverse catabolite response element sites reveal the basis for high-affinity binding to degenerate DNA operators. Nucleic Acids Res. 2011;39(7):2931–42. 10.1093/nar/gkq1177 ; PubMed Central PMCID: PMCPMC3074128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujita Y. Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci Biotechnol Biochem. 2009;73(2):245–59. 10.1271/bbb.80479 [DOI] [PubMed] [Google Scholar]
- 43.Kornfeld R. Studies on L-glutamine D-fructose 6-phosphate amidotransferase. I. Feedback inhibition by uridine diphosphate-N-acetylglucosamine. J Biol Chem. 1967;242(13):3135–41. [PubMed] [Google Scholar]
- 44.Assrir N, Richez C, Durand P, Guittet E, Badet B, Lescop E, et al. Mapping the UDP-N-acetylglucosamine regulatory site of human glucosamine-6P synthase by saturation-transfer difference NMR and site-directed mutagenesis. Biochimie. 2014;97:39–48. 10.1016/j.biochi.2013.09.011 [DOI] [PubMed] [Google Scholar]
- 45.Ferre-D'Amare AR. The glmS ribozyme: use of a small molecule coenzyme by a gene-regulatory RNA. Q Rev Biophys. 2010;43(4):423–47. 10.1017/S0033583510000144 ; PubMed Central PMCID: PMCPMC3409577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gopel Y, Khan MA, Gorke B. Menage a trois: post-transcriptional control of the key enzyme for cell envelope synthesis by a base-pairing small RNA, an RNase adaptor protein, and a small RNA mimic. RNA Biol. 2014;11(5):433–42. 10.4161/rna.28301 ; PubMed Central PMCID: PMCPMC4152352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kawada-Matsuo M, Oogai Y, Komatsuzawa H. Sugar Allocation to Metabolic Pathways is Tightly Regulated and Affects the Virulence of Streptococcus mutans. Genes (Basel). 2016;8(1). 10.3390/genes8010011 ; PubMed Central PMCID: PMCPMC5295006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Opheim D, Bernlohr RW. Purification and Regulation of Glucose-6-Phosphate Dehydrogenase from Bacillus licheniformis. Journal of Bacteriology. 1973;116(3):1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ludwig H, Homuth G, Schmalisch M, Dyka FM, Hecker M, Stülke J. Transcription of glycolytic genes and operons in Bacillus subtilis: evidence for the presence of multiple levels of control of the gapA operon. Molecular Microbiology. 2001;41(2):409–22. 10.1046/j.1365-2958.2001.02523.x [DOI] [PubMed] [Google Scholar]
- 50.Commichau FM, Dickmanns A, Gundlach J, Ficner R, Stülke J. A jack of all trades: the multiple roles of the unique essential second messenger cyclic di-AMP. Molecular Microbiology. 2015;97(2):189–204. 10.1111/mmi.13026 [DOI] [PubMed] [Google Scholar]
- 51.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Gründling A. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proceedings of the National Academy of Sciences. 2013;110(22):9084–9. 10.1073/pnas.1300595110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nelson JW, Sudarsan N, Furukawa K, Weinberg Z, Wang JX, Breaker RR. Riboswitches in eubacteria sense the second messenger c-di-AMP. Nature Chemical Biology. 2013;9:834 10.1038/nchembio.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gundlach J, Herzberg C, Kaever V, Gunka K, Hoffmann T, Weiß M, et al. Control of potassium homeostasis is an essential function of the second messenger cyclic di-AMP in Bacillus subtilis. Science Signaling. 2017;10(475). 10.1126/scisignal.aal3011 [DOI] [PubMed] [Google Scholar]
- 54.Commichau FM, Gibhardt J, Halbedel S, Gundlach J, Stülke J. A Delicate Connection: c-di-AMP Affects Cell Integrity by Controlling Osmolyte Transport. Trends in Microbiology. 26(3):175–85. 10.1016/j.tim.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 55.Kawai Y, Mercier R, Wu Ling J, Domínguez-Cuevas P, Oshima T, Errington J. Cell Growth of Wall-Free L-Form Bacteria Is Limited by Oxidative Damage. Current Biology. 2015;25(12):1613–8. 10.1016/j.cub.2015.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luciano J, Foulquier E, Fantino J-R, Galinier A, Pompeo F. Characterization of YvcJ, a Conserved P-Loop-Containing Protein, and Its Implication in Competence in Bacillus subtilis. Journal of Bacteriology. 2009;191(5):1556–64. 10.1128/JB.01493-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pompeo F, Luciano J, Brochier-Armanet C, Galinier A. The GTPase Function of YvcJ and Its Subcellular Relocalization Are Dependent on Growth Conditions in Bacillus subtilis. Journal of Molecular Microbiology and Biotechnology. 2011;20(3):156–67. 10.1159/000329298 [DOI] [PubMed] [Google Scholar]
- 58.Kalamorz F, Reichenbach B, März W, Rak B, Görke B. Feedback control of glucosamine-6-phosphate synthase GlmS expression depends on the small RNA GlmZ and involves the novel protein YhbJ in Escherichia coli. Molecular Microbiology. 2007;65(6):1518–33. 10.1111/j.1365-2958.2007.05888.x [DOI] [PubMed] [Google Scholar]
- 59.Gopel Y, Papenfort K, Reichenbach B, Vogel J, Gorke B. Targeted decay of a regulatory small RNA by an adaptor protein for RNase E and counteraction by an anti-adaptor RNA. Genes Dev. 2013;27(5):552–64. 10.1101/gad.210112.112 ; PubMed Central PMCID: PMCPMC3605468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proceedings of the National Academy of Sciences. 1998;95(10):5752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen SN, Chang ACY, Hsu L. Nonchromosomal Antibiotic Resistance in Bacteria: Genetic Transformation of Escherichia coli by R-Factor DNA. Proceedings of the National Academy of Sciences of the United States of America. 1972;69(8):2110–4. PubMed PMID: PMC426879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Quisel JD, Burkholder WF, Grossman AD. In Vivo Effects of Sporulation Kinases on Mutant Spo0A Proteins in Bacillus subtilis. Journal of Bacteriology. 2001;183(22):6573–8. 10.1128/JB.183.22.6573-6578.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koo B-M, Kritikos G, Farelli JD, Todor H, Tong K, Kimsey H, et al. Construction and Analysis of Two Genome-Scale Deletion Libraries for Bacillus subtilis. Cell Systems. 2017;4(3):291–305.e7. 10.1016/j.cels.2016.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vagner V, Dervyn E, Ehrlich SD. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology. 1998;144(11):3097–104. 10.1099/00221287-144-11-3097 [DOI] [PubMed] [Google Scholar]
- 65.Le Breton Y, Mohapatra NP, Haldenwang WG. In Vivo Random Mutagenesis of Bacillus subtilis by Use of TnYLB-1, a mariner-Based Transposon. Applied and Environmental Microbiology. 2006;72(1):327–33. 10.1128/AEM.72.1.327-333.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gerth U, Kock H, Kusters I, Michalik S, Switzer RL, Hecker M. Clp-Dependent Proteolysis Down-Regulates Central Metabolic Pathways in Glucose-Starved Bacillus subtilis. Journal of Bacteriology. 2008;190(1):321–31. 10.1128/JB.01233-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altenbuchner J. Editing of the Bacillus subtilis Genome by the CRISPR-Cas9 System. Applied and Environmental Microbiology. 2016;82(17):5421–7. 10.1128/AEM.01453-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zadeh JN, Steenberg CD, Bois JS, Wolfe BR, Pierce MB, Khan AR, et al. NUPACK: Analysis and design of nucleic acid systems. Journal of Computational Chemistry. 2011;32(1):170–3. 10.1002/jcc.21596 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(A) Growth curves showing the effect of addition of glucose and MgSO4 on growth of ΔglmR in MH medium compared to WT. (B) Growth stimulation on MH medium by glucose. Top MH agar (4 ml) was plated with 100 μl of ΔglmR cells and filter discs containing 2.5 mg and 5 mg of glucose were put on the plate followed by overnight incubation at 37°C.
(PDF)
Disc diffusion assay for WT and ΔglmR done with (A) oxacillin (1 μg), (B) cefixime (40 μg), (C) moenomycin (10 μg), (D) vancomycin (10 μg), (E) fosfomycin (50 μg), (F) bacitracin (400 μg) and (G) nisin (50 μg). One asterisk and three asterisks represent significance with P <0.05 and P <0.001 respectively. NS indicates that differences were not significant.
(PDF)
(A) CEF susceptibility of ΔglmR and complementation of phenotype by IPTG inducible ectopic expression of 3X-FLAG glmR. RBS was optimized to AGGAGG that is seven base pair apart from start codon. (B) Disc diffusion assay showing suppression of CEF sensitivity of ΔglmR by addition of 20 mM MgSO4. In both figures, statistical significance is indicated by asterisks with P <0.001.
(PDF)
CEF disc diffusion assay showing effect of rho mutation. Statistical significance with P <0.001 is indicated with three asterisks.
(PDF)
(A) Transcription termination loop secondary structure prediction for WT and with point mutations (rsiW1 and rsiW2) are shown with their relative free energy value prediction. (B) CEF disc diffusion assays performed on strains with sigW::erm mutation in WT, ΔglmR, ΔglmR rsiW1 and ΔglmR rsiW2 backgrounds.
(PDF)
(A) CEF susceptibility and (B) growth on MH medium for ΔglmR in combination with gdpP and pgpH deletions and the gdpP pgpH double deletion.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.