Abstract
The outcome of adult patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) has improved substantially with the introduction of tyrosine kinase inhibitors (TKIs). TKIs are now integral components of therapy for Ph+ ALL. The current consensus is that they improve patient outcomes compared with historical control patients treated with chemotherapy alone, and increase the number of patients able to receive stem cell transplant. New challenges have emerged with respect to induction of resistance mainly via Abelson tyrosine kinase mutations. Several novel kinase inhibitors with significantly more potent antileukemic activity are currently being developed. Furthermore novel immune therapies, which recruit or modify patient's own T cells to fight leukemic cells, are being developed and could find an important place in Ph+ ALL therapy by few years. In this article, we reviewed treatment approaches in adults with Ph+ ALL with a focus on TKIs and combined chemotherapy regimens.
KEYWORDS : acute lymphoblastic leukemia, allogeneic stem cell transplantation, BCR–ABL, Philadelphia chromosome, treatment, tyrosine kinase inhibitor
Practice points .
Tyrosine kinase inhibitors (TKIs) have become an integral part of front-line therapy for Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Ph+ ALL serves as the first model system for truly targeted treatment.
Although the choice of the most effective TKI is not yet settled, the best results are shown with TKIs incorporated early, daily and continuously with chemotherapy. Current complete remission rates reach 90% and long-term survival rates attain 50–60%.
Real time-PCR breakpoint cluster region–Abelson leukemia viral proto-oncogene quantification is used to monitor minimal residual disease in patients with Ph+ ALL. BCR–ABL transcript levels have been correlated with response.
Development of resistance during treatment remains a major problem in Ph+ ALL, especially with a T315I clone. Novel TKIs as well as monoclonal antibodies and the novel approach based on chimeric antigen receptor T-cells targeting CD19 have already been tested with encouraging first results.
Acute lymphoblastic leukemia (ALL) is a malignant neoplasm of the lymphocyte precursor cells. ALL is characterized by aberrations in proliferation and differentiation of lymphoblasts, leading to failure of normal immune response and decreased production of normal hematopoiesis responsible for anemia, thrombocytopenia and neutropenia. ALL represents a heterogeneous group with distinct morphologic, cytogenetics and molecular groupings, some of which have important clinical implications. The Philadelphia chromosome (Philadelphia chromosome-positive; Ph+), a reciprocal translocation between chromosomes 9 and 22 (t[9;22] [q34;q11]), is the most frequent cytogenetic abnormality in human leukemia [1,2]. It produces a fusion gene on chromosome 22, namely, the BCR–ABL.
How common is Ph+ ALL?
ALL represents <1% of adult cancers, while it represents 25% of all childhood cancers. Incidence of Ph+ ALL increases with age [2]. The Philadelphia chromosome can be detected in a range of 2–5% of children with ALL [3], and 20–40% of younger adults with ALL [4]. The proportion of Ph+ ALL cases increases with age up to 50% with no sex difference [5], but in very old persons the proportion decreases again [4]. The overwhelming majority of patients are diagnosed with de novo Ph+ ALL, although occasional cases of secondary Ph+ ALL have been reported following chemotherapy or radiation therapy.
How do we diagnose Ph+ ALL?
The defining feature of Ph+ ALL is a reciprocal translocation t(9;22) (q34;q11). The t(9;22) translocation leads to a head-to-tail fusion of the ABL proto-oncogene from chromosome 9 with a 5′ half of the BCR sequences on chromosome 22 [6]. By standard cytogenetic analysis this becomes apparent as a shortened chromosome 22 referred to as the Philadelphia chromosome, which can also be visualized by FISH analysis. Transcription of BCR–ABL results either in a 8.5-kb mRNA that codes for a 210-kb protein when ABL moves to the major BCR or in a 7.5-kb RNA encoding a 190-kb protein when it moves to the minor BCR [7]. Both possible chimeric mRNAs (p210 and p190) can be sensitively and specifically detected by the real-time PCR (RT-PCR) [8]. BCR–ABL proteins demonstrate enhanced tyrosine kinase activity compared with the normal ABL gene product. BCR–ABL fusion proteins can alter multiple signaling pathways, contributing to tumor growth and proliferation. P190 exhibits a higher transforming potential than p210 in animal models [9]. The p190 protein is usually found in two out of three adults with de novo Ph+ ALL [10,11]. It has been associated with a significant increase in the risk of relapse [10]. The p210 protein constitutes the rest of the Ph+ ALL population. However, a rare p230 BCR–ABL mutation has also been described and is associated with Ph+ chronic neutrophilic leukemia [12]. BCR–ABL expression in hematopoietic cells is known to induce resistance to apoptosis, growth factor independence, as well as alterations in cell–cell and cell–matrix interactions [13]. Ph+ ALL has an aggressive clinical course. Patients present with a variable white blood cell count, and have an increased risk of developing meningeal leukemia during the course of treatment, although CNS leukemia was not significantly more frequent (5%) at diagnosis [5,14]. Splenomegaly may be present. Ph+ ALL are found almost exclusively among B-cell linage ALL. The most frequent immunologic subtypes are common ALL (78%), and pre-B ALL (20%), whereas only few percentages of patients display the pro-B immunophenotype [15]. Except for few case reports, the Ph chromosome is not found in T-cell lineage ALL. Leukemic cells often present surface expression of CD34 antigen (89%), and frequent expression of myeloid markers (15–20%) [10]. Additional chromosome abnormalities have been observed in 70% of Ph+ ALL patients [16], including mainly 9p abnormalities, monosomy 7 or hyperdiploid karyotypes >50. The main differential diagnosis at diagnostic is chronic myeloid leukemia in lymphoid blast crisis.
What are the treatment options for adult patients with Ph+ ALL?
• Historic regimens (pretyrosine kinase inhibitor era)
Most of adults with Ph+ ALL have an extremely poor prognosis when treated with chemotherapy alone [10,16–22]. Chemotherapy regimens induce complete remissions (CRs) in >70% (moderately lower than the 70–90% achieved in Ph-negative ALL), but most patients relapse within 6–11 months of treatment and die of the disease. During this pre-tyrosine kinase inhibitor (TKI) period, the 5-year overall survival (OS) rates for those treated with intensive chemotherapy alone were <10%. Only, the hyper-CVAD chemotherapy (fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with cycles of high-dose methotrexate and cytarabine) from the MD Anderson Cancer Center reported higher CR rate (91%) with median event-free survival (EFS) and OS of 43 and 42 months, respectively [23]. However, although reported results were significantly higher than those from historical control, survival curves converged after 3 years. Only allogeneic stem cell transplantation (SCT) performed early during remission was potentially curative and has permitted long-term survival (35–65%) [10,24–28]. Nevertheless, approximately 30% of allografted patients experienced relapse, making this the most frequent cause of failure next to treatment-related mortality [29]. Younger age, conditioning with total body irradiation, HLA-identical sibling donor [30], disease status at the time of SCT [30,31] and the occurrence of acute graft-versus-host disease (GvHD) [32] were factors associated with improved survival. Regarding autologous SCT, 3-year survival rates have been shown to be inferior to those of allogeneic SCT as well as to those of continued chemotherapy [33]. Long-term disease-free survival (DFS) was noted in 22% of cases with a median of 52 months when using purged bone marrow or peripheral blood stem cells [34]. However, no long-term surviving patients were observed among those transplanted beyond the first CR. Because the results of SCT correlate with the pretransplant leukemia burden, improved treatment strategies have clearly been warranted to ensure molecular CR at the time of SCT for patients with Ph+ ALL [31]. Chemotherapy in elderly Ph+ ALL patients was also associated with a very poor prognosis. In contrast with younger adults, the presence of Ph chromosome showed here no specific impact on the OS, probably because of the overall poor outcome of the other ALL subtypes in this patient population [35].
• Imatinib in the treatment of Ph+ ALL
Imatinib mesylate, a TKI that targets BCR–ABL, is now an integral component of therapy for Ph+ ALL. It partially blocks the ATP-binding site of BCR–ABL [36]. Following initial studies showing that use of imatinib mesylate as a single agent in Ph+ ALL yielded potential responses, but was unlikely to be sufficient for long-term disease control [37], the efficacy of imatinib was explored as front-line treatment combined with chemotherapy, either concurrently (simultaneous administration) or sequentially (alternating administration) [28,38–45]. CRs can be obtained in almost 95% of cases with newly diagnosed Ph+ ALL. In younger adults, imatinib-based regimens used imatinib at 400–800 mg/day. Efficacy analyses based on BCR–ABL transcript levels showed a clear advantage of the simultaneous over the alternating schedule [43]. The current consensus is that imatinib improves patient outcomes compared with historical control patients treated with chemotherapy alone. The number of patients able to receive SCT, as the outcome of SCT, has improved [39–40,43,46–47]. It also appeared important to maintain imatinib dose intensity during the initial phase of treatment [48]. Furthermore, an induction regimen combining reduced-intensity chemotherapy and imatinib was recently validated in a randomized study in which it was compared with a standard imatinib/chemotherapy treatment [47]. The rate of molecular remission increased from 5% to >50%, and the 5-year survival to 50% or more. The number of patients able to receive SCT, as the outcome of SCT, has improved [47]. However, imatinib is ineffective at preventing or treating CNS involvement [49]. Several approaches using imatinib-based induction therapy have been explored for elderly patients. With relatively minimal use of imatinib (600 mg/day for three blocks of 60 days) alternating with chemotherapy, a significant improvement in the 1-year survival was observed compared with historical controls [44]. Similar results were reported with continuous administration of imatinib (800 mg) only combined with prednisone [41]. In a randomized study comparing induction therapy with single-agent imatinib with standard induction chemotherapy [42], response rate was better with single-agent imatinib. In addition to hematologic and cytogenetic monitoring, monitoring of minimal residual disease (MRD) by flow cytometry and/or quantitative RT-PCR may be useful in detecting impending imatinib failure [50]. Achievement of molecular remission was associated with longer DFS. The detection of MRD may allow for targeted therapy before the occurrence of a frank relapse [51]. Unfortunately, imatinib resistance may develop rapidly and lead to disease progression. Multiple mechanisms of resistance have been implicated, of which mainly the acquisition of point mutations within the tyrosine kinase domain [52,53]. The T315I mutation leads to an extreme insensitivity to imatinib. Its presence is associated to shorter survival [54]. Mutations have also been reported to exist before the initiation of imatinib treatment in >40% of patients, and may be responsible for relapse during therapy [14,55]. The other mechanisms of resistance include amplification of the BCR–ABL gene, decreased drug efflux and involvement of secondary downstream pathways such as SRC family kinases [56,57]. One recently identified mechanism of resistance involves the expression of spliced isoforms of IKZF1, a critical regulator of normal lymphocyte development [58]. The presence of genetic deletions affecting PAX5 (a transcription factor expressed throughout B-cell maturation) and the CDKN2A/B (a negative regulator of p53) locus has also been identified in Ph+ ALL patients by genome-wide single nucleotide polymorphism array analysis [58]. Because of the development of potential resistance, the combination of imatimib with chemotherapy has not supplanted allogeneic SCT as treatment of choice in patients with Ph+ ALL.
• Second-generation TKIs in the treatment of Ph+ ALL
Faster and deeper molecular responses can be achieved with second-generation TKIs. Dasatinib is a dual SRC/ABL inhibitor with 30–50-fold more in vivo potency than imatinib against BCR–ABL. It can bind to both the active and inactive conformations of the ABL kinase domain. It also inhibits the c-KIT, PDGFR and ephrin A receptor kinases. Mutations that show a high degree of insensitivity to imatinib are sensitive to dasatinib, with the exception of T315I [59]. In a Phase I trial, a hematologic response rate of 80% has been observed in patients with imatinib-resistant Ph+ ALL [60]. In a Phase II program using single-agent dasatinib (70 mg two-times a day [b.i.d.]) in imatinib-resistant Ph+ ALL patients, a major cytogenetic response was observed in 57% of cases after a median time of 6.9 months [61,62]. The activity of twice daily for dasatinib was confirmed by other studies in this indication [63], but a randomized Phase III dose-optimization study changed the approval dosage to 140 mg once daily (q.d.) [64]. Consequently, safety profile was improved with the once-daily arm. Responses were observed in the presence of ABL tyrosine kinase domain (TKD) mutations other than T315I and E355G.
In the final results of the Gruppo Italiano Malattie Ematologiche dell'Adulto protocol LAL1205, in which dasatinib (70 mg b.i.d.) was combined with steroids in adults with Ph+ ALL, all patients achieved CR with a very marked clearance of blasts already at day 22, irrespective of age, with limited toxicities and no fatalities [65]. A Phase II study combining the hyper-CVAD regimen with dasatinib (50 mg b.i.d.) for the first 14 days of each cycles showed CR achievement in 93% of newly diagnosed Ph+ ALL [66]. Molecular remissions were observed even after the first cycle with 94% of patients achieving MRD negativity assessed by flow cytometry at a median of 3 weeks. A recent long-term follow-up of this study showed that the median DFS and OS were 31 and 47 months, respectively [67]. A high activity of dasatinib as first-line therapy was also demonstrated in patients older than 55 years [68]. After a steroid prephase, dasatinib (100–140 mg q.d.) was combined with four weekly cycles of vincristine and dexamethasone. Induction was followed by alternating blocks of dasatinib, methotrexate plus asparaginase and cytarabine; then maintenance therapy with alternating blocks of dasatinib, mercaptopurine plus methotrexate and dexamethasone plus vincristine. Dasatinib plus chemotherapy gave 96% of CR, and EFS was 41% at 3 years. A third of patients achieved undetectable levels of BCR–ABL transcripts [68]. Dasatinib-based treatment may be used in patient unable to receive SCT. It has also been shown to facilitate SCT, without increasing toxicity [69].
Nilotinib, a second-generation TKI, is an aminopyrimidine derivative of imatinib. While dasatinib has a pan-TKI profile, nilotinib has a profile close to that of imatinib and inhibits BCR–ABL, c-KIT and PDGFR. It is 20–50-fold more potent than imatinib as an ABL kinase inhibitor [70]. Until now, it has not been approved for the treatment of patients with Ph+ ALL. In a Phase I dose-escalation study in imatinib-resistant Ph+ ALL, hematologic responses were observed in 33% of the patients [71]. In a Phase II study, nilotinib, as monotherapy at 400 mg b.i.d. in patients with relapsed or refractory Ph+ ALL, gave CR in 24% of cases [72]. The use of nilotinib in combination with chemotherapy is currently under investigations. First results showed achievement of high cumulative complete molecular remission and hematologic relapse-free survival rates [73]. Mutagenesis-resistance screening indicates the selection of T315I and P-loop mutations Y253H and E255K during nilotinib treatment [52,74].
Bosutinib is a novel dual SRC/ABL inhibitor in early clinical development for Ph+ ALL. Biochemical assays have shown it to be up to 200-fold more potent than imatinib as an inhibitor of BCR–ABL phosphorylation. Bosutinib does not exhibit significant inhibition of c-KIT or PDGFR, which may result in a relatively favorable safety profile. Preliminary data with bosutinib at 500 mg/day in patients who experienced failure on previous imatinib therapy indicate complete hematological response and major molecular response in Ph+ ALL [75].
• Third-generation TKI in the treatment of Ph+ ALL
Ponatinib, a third-generation TKI, which targets the T315I mutation, a common cause of relapse in patients with Ph+ ALL, is currently in development. Ponatinib is a pan-BCR–ABL inhibitor. It also showed potent activity against other kinases including VEGFR, FGFR, ephrin, SRC kinases, KIT (mast/stem-cell growth factor receptor), RET (rearranged during transfection) and FLT3. Ponatinib is 520-times more potent than imatinib for the native BCR–ABL mutation [76]. At a dose of 45 mg orally, responses were observed in patients whose disease was resistant to imatinib or dasatinib [77]. The PACE trial was a pivotal Phase II trial evaluating the efficacy of ponatinib in patients who are resistant or intolerant to dasatinib or nilotinib or had a T315I mutation [78]. Higher response rates were demonstrated in patients previously treated with fewer TKIs. Combinations of chemotherapy regimens and ponatinib may be associated with better response rates and higher likelihood of eradication of MRD. The combination of chemotherapy (hyper-CVAD) with ponatinib is effective in achieving early sustained remissions with major molecular response in 95% [79]. The 2-year EFS rate was 81%. OS was similar with or without censoring for allogeneic SCT. The combination was safe and highly effective in achieving molecular remissions. New strategies, including dosing titration of ponatinib and optimized control of vascular risk factors, might further improve outcomes.
• Monoclonal antibodies & immunotherapy
Monoclonal antibodies represent a new approach for the treatment of B-cell lineage ALL [80]. Ph+ ALL blast cells can express a variety of specific antigens, such as CD19, CD20 and CD22. Hyper-CVAD plus the anti-CD20 monoclonal antibody rituximab combined with TKI gave results better than those observed with historical cohorts [79]. Monoclonal antibodies directed against CD22, such as inotuzumab ozogamicin, are being explored in ALL. In relapsing patients with Ph+ ALL, first results on a small series showed an advantage for inotuzumab compared with standard therapy, but the difference was not significant [81]. Blinatumomab, a bispecific T-cell-engaging antibody binding CD19 and CD3, has showed promising results in patients with high-risk ALL [82]. Activity has been demonstrated in Ph-ALL with T315I mutation [83]. CR was achieved in 36% of case and in 40% of patients with mutation T315I. Preliminary results indicate that treatment with blinatumomab is able to convert MRD-positive ALL into an MRD-negative status. A total of 88% of patients achieved a complete MRD response [83]. Monoclonal antibody development could, however, be challenged by the novel approach based on chimeric antigen receptor (CAR) T cells targeting CD19, which has already been tested with encouraging first results [84].
SCT in the TKI era
• Allogeneic SCT
Allogeneic SCT remains the standard of care for adult Ph+ ALL [85]. All TKIs can increase the feasibility of allogeneic SCT by increasing remission rates and extending remission duration [86–88]. Furthermore, reducing BCR–ABL transcript levels has resulted in a lower pre-SCT leukemia burden [2]. However, the 3-year outcome in children treated with imatinib and chemotherapy compared favorably to those treated with allogeneic SCT, suggesting that patients with Ph+ ALL can be treated successfully without allogeneic SCT [89]. Allogeneic SCT with myeloablative conditioning regimen overcomes MRD prior to SCT in some but not all studies. It is superior to allogeneic SCT after nonmyeloablative conditioning regimen if MRD is not considered, but may be equivalent with complete molecular response. Nonmyeloablative allogeneic SCT approaches are therefore promising in patients with ALL [90]. No particular conditioning regimen was deduced to be optimal. Treatment-related toxicity in Ph+ ALL in first CR has been reported in 20–30% of cases with high rates of chronic graft-versus-host disease [91–95]. Prophylactic TKI given after engraftment may improve outcomes by preventing a resurgence of the leukemic clone [96]. However, the optimal duration of this treatment has not already been established. In MRD-positive patients after SCT, imatinib at 400 mg/day has been shown to prevent relapse and to achieve molecular remission in 52% of cases after 1.5 months of treatment [97]. However, imatinib is sometimes poorly tolerated after allogeneic SCT and many patients require discontinuation or dose reduction [98]. Overall, recent studies showed a trend toward improved outcome in patients who could be treated with imatinib after allogeneic SCT [46,91]. Alternative therapy based on other TKIs or on monoclonal antibody therapy should be proposed to patients who remain positive for BCR–ABL transcripts >2 months after starting imatinib therapy after transplant.
• Haploidentical SCT
Alternative donors may be considered for patients lacking a matched-related or matched-unrelated donor. Haploidentical SCT represents an encouraging treatment option [99]. The incidence of nonrelapse mortality was similar between the patients who received HLA-matched donor cells and those who received haploidentical donor cells. The incidence of cytomegalovirus infection was, however, significantly higher in the last group. Haploidentical SCT reduced the relapse rate.
• Cord blood transplantation
The status of umbilical cord blood transplantation in adults with Ph ALL is not well established. Recent analyses showed that MRD-positivity before umbilical cord blood transplantation was associated with increased relapses [100].
• Autologous SCT
Although autologous SCT has never been considered a standard of care in the setting of Ph+ ALL, it remains a possible therapeutic option when MRD is not present before the procedure [47]. Results of autologous SCT have significantly improved in the era of TKIs [101,102]. There are no data on how best to use TKIs after autologous SCT.
How we currently treat adult Ph+ ALL at Lyon-University Hospital?
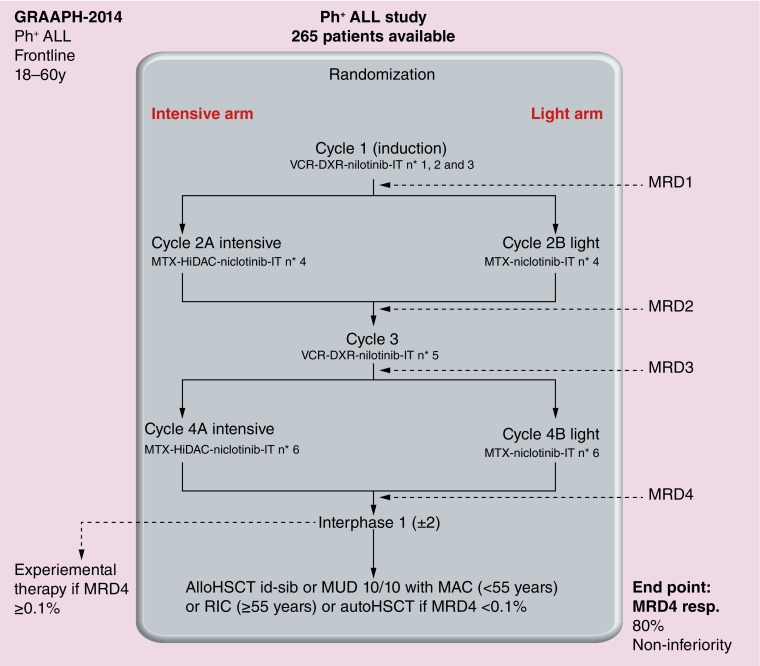
Many trials using TKIs in combination with chemotherapy have been developed for the treatment of adult Ph+ ALL (Table 1). Currently most groups keep testing approved or not approved TKIs in combination with chemotherapy. In our previous study about younger adults with Ph+ ALL, we carried out an initial randomized comparison of imatinib combined with hyper-CVAD against imatinib with dexamethasone and vincristine. Almost all patients in the imatinib-based arm achieved CR versus only 92% in the chemotherapy-based arm, with toxicity being responsible for the difference [47]. However, long-term survival did not differ between the two arms. Molecular response rate was also similar. Our current trial in younger adults is testing nilotinib in combination with chemotherapy (Figure 1). Although not extensively studied in Ph+ ALL, this TKI of second generation is known for a faster efficacy than imatinib [70,71]. Because survival curves after allogeneic SCT (despite improvements since the use of TKIs) did not demonstrate any plateau [42] and encouraging results were observed with autologous SCT [101], patients demonstrating a major molecular response could receive either allogeneic SCT or autologous SCT as continuation therapy. In older adults, dasatinib combined with only vincristine and dexamethasone showed a 90% CR rate. The median OS was 27 months and most relapses were associated with T315I BCR–ABL mutation [68]. Dasatinib, offering inhibition of both tyrosine and SRC kinases, may theoretically hold out more promise of benefit than imatinib. However, studies from the MD Anderson group did not show any significant differences between both TKIs when combined with the hyper-CVAD regimen [38,66]. Our current study in older adults is testing nilotinib at 400 mg b.i.d. from day one throughout induction, consolidation and maintenance according to the backbone schedule of the European Working Group on Adult ALL, in which induction combines vincristine and dexamethasone. This is followed by six cycles of consolidation therapy altering methotrexate-asparaginase and high-dose cytarabine courses, then by a 2-year maintenance therapy with 6-mercaptopurine, methotrexate, dexamethasone and vincristine.
Table 1. . Main clinical trials using tyrosine kinase inhibitors in combination with chemotherapy in the treatment of adult Philadelphia chromosome-positive acute lymphoblastic leukemia.
| Study (year) | Regimen | Patients/age (years) | Response to therapy | Survival | Ref. |
|---|---|---|---|---|---|
| Lee et al. (2005) | Ima + chemo | 20/≥15 | 95% CR | 2-year DFS: 62% 2-year OS: 59% |
[40] |
| Delannoy et al. (2006) | Ima + chemo (GRAALL-AFR09) |
30/≥55 | 90% CR | 1-year RFS: 58% 1-year OS: 66% |
[44] |
| Wassmann et al. (2006) | Ima + chemo (GMALL) |
45/≥18 | 96% CR 52% CMR |
2-year OS: 43% | [43] |
| De Labarthe et al. (2007) | Ima + chemo (GRAAPH) |
45/15–59 | 96% CR 38% CMR |
1.5-year DFS: 51% 1.5-year OS: 65% |
[45] |
| Ottmann et al. (2007) | Ima + chemo (GMALL) |
28/≥55 | 96% CR | 1.5-year DFS: 30% 1.5-year OS: 57% |
[42] |
| Vignetti et al. (2007) | Ima + steroids | 30/>60 | 100% CR 4% CMR |
1-year OS: 74% | [41] |
| Thomas et al. (2010) | Ima + chemo (Hyper-CVAD) |
54/≥15 | 93% CR 52% CMR |
3-year DFS: 62% 3-year OS: 55% |
[88] |
| Yanada et al. (2008) | Ima + chemo (JALSG ALL202) |
80/15–64 | 96% CR 71% CMR |
2-year EFS: 49% 2-year OS: 58% |
[103] |
| Bassan et al. (2010) | Ima + chemo (NILG) |
59/19–66 | 92% CR | 5-year DFS: 39% 5-year OS: 38% |
[104] |
| Foa et al. (2011) | Dasa (70 mg daily) | 53/>18 | 100% CR | 1.7-year DFS: 51% 1.7-year OS: 69% |
[65] |
| Fielding et al. (2014) | Ima + chemo (MRC UKALL XII/ECOG 2993) |
175/16–64 | 92% CR | 4-year OS: 38% | [105] |
| Jabbour et al. (2015) | Pona + chemo (Hyper-CVAD) |
37/≥18 | 94% CCR | 2-year EFS: 81% 2-year OS: 80% |
[79] |
| Kim et al. (2015) | Nilo + chemo | 90/17–71 | 91% CR | 2-year RFS: 72% 2-year OS: 72% |
[73] |
| Ravandi et al. (2015) | Dasa + chemo | 72/21–80 | 96% CR 83% CCR |
Median OS: 47 months | [67] |
| Sartor et al. (2015) | Nilo (400 mg) and Ima (300 mg) alternating every 6 weeks | 34/>65 | 94% CR | 2-year OS: 64% | [106] |
| Chalandon et al. (2015) | Ima + chemo (low intensity) or chemo (Hyper-CVAD) | 268/18–59 | 99 vs 91% CR | 5-year EFS: 42 vs 32% 5-year OS: 48 vs 43% |
[47] |
| Rousselot et al. (2016) | Dasa + chemo (EWALL-Ph-01) |
71/>55 | 96% CR | 5-year OS: 36% | [68] |
CCR: Complete cytogenetic remission; Chemo: Chemotherapy; CMR: Complete molecular remission; CR: Complete remission; Dasa: Dasatinib; DFS: Disease-free survival; ECOG: Eastern Cooperative Oncology Group; EFS: Event-free survival; EWALL: European Working Group on Adult Acute Lymphoblastic Leukemia; GMALL: German Multicenter study group for adult Acute Lymphoblastic Leukemia; GRAALL: Group for Research in Adult Acute Lymphoblastic Leukemia; GRAAPH: Group for Research in Adult Philadelphia chromosome-positive Acute Lymphoblastic Leukemia; Hyper-CVAD: Fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with cycles of high-dose methotrexate and cytarabine; Ima: Imatinib; JALSG: Japan Adult Leukemia Study Group; MRC: Medical Research Council leukemia trial; NILG: Northern Italy Leukemia Group; Nilo: Nilotinib; OS: Overall survival; Pona: Ponatinib; RFS: Relapse-free survival; UKALL XII: UK Acute Lymphoblastic Leukemia XII.
Figure 1. . Schema of the Group for Research in Adult Philadelphia chromosome-positive Acute Lymphoblastic Leukemia-2014: treatment from the French Group for Research on Adult Acute Lymphoblastic Leukemia for young adults with Philadelphia chromosome-positive acute lymphoblastic leukemia.
ALL: Acute lymphoblastic leukemia; AlloHSCT id-sib: Allogeneic hematopoietic stem cell transplantation from identical sibling; AutoHSCT: Autologous hematopoietic stem cell transplantation; DXR: Doxorubicin; HiDAC: High-dose cytarabine; IT: Intrathecal; MAC: Myeloablative conditioning; MRD: Minimal residual disease; MTX: Methotrexate; MUD: Matched unrelated donor; Ph+: Philadelphia chromosome-positive; RIC: Reduced intensity conditioning; VCR: Vincristine.
In relapsed Ph+ ALL and in patients with T315I mutation, ponatinib could be given at 45 mg per os (p.o.) daily for the first 14 days of cycle one of hyper-CVAD [79].
‘Ph-like’ ALL: a new entity with specific therapeutic implications
Last decade has been marked by a dramatic improvement in molecular and cytogenetic characterization in ALL, thus providing robust surrogate markers for risk assessment and therapeutic intensification. However, leukemic cells from many patients with B-cell lineage-ALL lack known chromosomal alterations [1]. A new entity of high-risk B-cell precursor ALL has been recently described, namely ‘Ph-like’ ALL or ‘BCR–ABL1-like’ ALL, defined by a similar gene signature than Ph+ ALL without BCR–ABL translocation [107]. This subtype represents up to 15 and 25% in pediatrics and adolescent/young adult ALL respectively and is associated with a poor outcome [108]. Comparative genomic hybridization arrays and molecular cytogenetics are necessary for the diagnosis. However, validation of a robust gene expression classifier is still warranted for a routine clinical use [109]. More than 80% of ‘Ph-like’ ALL cases have abnormalities in genes involved in B-cell development (i.e., IKZF1 deletions in about 40% of cases), which facilitate leukemia transformation by inducing constitutive kinase activation and signaling through the activation of ABL1 and/or JAK/STAT pathways, but also CLRF2 overexpression and tyrosine kinase-activating rearrangements involving ABL1, JAK2, PDGFRB and several other genes [110]. Preclinical results of ‘Ph-like’ ALL pointed out potent role of a targeted therapeutic strategy according to the molecular profile of leukemia cells [111,112]. Recently, several cases have been reported with responses to TKIs (mostly imatinib) [113–115]. 50% of ‘Ph-like’ ALL show activation of JAK-STAT and PI3K/mTOR pathways and should also be sensitive to JAK and mTOR inhibitors [100,112].
Conclusion: what is the prognosis of adult Ph+ALL? Future perspectives?
The outcome of adult patients with Ph+ ALL has substantially improved since the introduction of TKIs. The best results are shown with TKIs incorporated early, daily and continuously with chemotherapy. Current CR rates reach 90% and long-term survival rates attain 50–60%. Now, key challenges are the selection of appropriate pretransplantation therapy, the minimization of transplantation toxicity, the use of TKIs after transplantation and the appropriate use of BCR–ABL monitoring. Clinical trials with TKIs currently recruiting in adult Ph+ ALL are summarized in Table 2. Although recent data suggest that addition of chemotherapy to dasatinib might help to prevent the emergence of dasatinib-resistant mutation [116], this is now logical to ask whether there is a rationale for reducing cytotoxic agents from initial induction [41,47,65]. Less chemotherapy intensity in induction prior to SCT was shown not inferior, while initial dose intensity of TKI was demonstrated as important. Second and third generation TKIs improved results in terms of MRD, but there are no prospective randomized trials on outcome. Furthermore, there is little evidence to date that allogeneic SCT is a dispensable part of therapy. In a Phase II trial combining dasatinib with chemotherapy, there was no difference in outcomes between patients in CR who did and did not undergo allogeneic SCT [117]. MRD monitoring by RT-PCR and multiparameter flow cytometry may identify patients in first CR for whom further consolidation with allogeneic SCT may not be needed [117]. After molecular response achievement, autologous SCT or chemotherapy plus TKI yielded similar results than allogeneic SCT. However, the long-term outcome of chemotherapy-free induction strategies is currently not assessable.
Table 2. . Recruiting clinical trials with tyrosine kinase inhibitors in adult Philadelphia chromosome-positive acute lymphoblastic leukemia.
| Study | Age (years)/status | Treatment | Identifier |
|---|---|---|---|
| Phase II NCI | ≥65/newly diagnosed | Dasatinib + blinatumomab + prednisone | NCT02143414 |
| Phase II MDACC | ≥10/Ph-like ALL | Dasatinib + chemo (hyper-CVAD) | NCT02420717 |
| Phase I MSKCC | ≥40/newly diagnosed | Dasatinib + ruxolitinib + Dex | NCT02494882 |
| Phase II University of California, San Diego | 18–60/newly diagnosed | Dasatinib + rituximab† + chemo | NCT02043587 |
| Phase I Novartis Pharmaceuticals | ≥18/R/R to TKIs | ABL001 + dasatinib or imatinib or nilotinib | NCT02081378 |
| Phase III PETHEMA | ≥55/newly diagnosed | Dasatinib or imatinib + chemo | NCT01376427 |
| Phase II MDACC | ≥55/newly diagnosed | Dasatinib or imatinib + rituximab† + chemo (hyper-CVAD) | NCT01319981 |
| Phase I and II University of Toronto | ≥18/R/R to TKIs | Nilotinib + ruxolitinib | NCT01914484 |
| Phase III GRAALL (GRAAPH 2014) | 18–59/newly diagnosed | Nilotinib + chemo | NCT02611492 |
| Phase II OHSU Knight Cancer Institute | 21–70/relapsed | Nilotinib or ponatinib or dasatinib | NCT01620216 |
| Phase I and II Novartis Pharmaceuticals | ≥18/R/R | Nilotinib + ruxolitinib | NCT02253277 |
| Phase II MDACC | ≥18/R/R | Bosutinib + inotuzumab ozogamicin | NCT02311998 |
| Phase II GIMEMA | ≥18/newly diagnosed | Ponatinib | NCT01641107 |
| Phase II MDACC | ≥18/newly diagnosed | Ponatinib + chemo (hyper-CVAD) | NCT01424982 |
| Phase IV PETHEMA | <55/newly diagnosed | Imatinib + chemo | NCT01491763 |
| Phase II Asan Medical Center (RADICAL) | ≥15/newly diagnosed | Imatinib + rituximab† + chemo | NCT01429610 |
†In patients expressing CD20.
ABL001: A potent allosteric BCR–ABL inhibitor; Chemo: Chemotherapy; Dex: Dexamethasone; GIMEMA: Gruppo Italiano Malattie Ematologiche dell'Adulto; GRAALL: Group for Research in Adult Acute Lymphoblastic Leukemia; GRAAPH: Group for Research in Adult Philadelphia chromosome-positive Acute Lymphoblastic Leukemia; Hyper-CVAD: Fractionated cyclophosphamide, vincristine, doxorubicin and dexamethasone alternating with cycles of high-dose methotrexate and cytarabine; MDACC: MD Anderson Cancer Center; MSKCC: Memorial Sloan Kettering Cancer Center; NCI: National Cancer Institute; PETHEMA: Programa para el Tratamiento de Hemopatias Malignas; Ph+ ALL: Philadelphia chromosome-positive acute lymphoblastic leukemia; R/R: Relapsed/refractory; TKI: Tyrosine kinase inhibitor.
RT-PCR BCR–ABL quantification is used to monitor MRD in patients with Ph+ ALL. BCR–ABL transcript levels have been correlated with response [61]. However, no consensus has been drawn on what represents an optimal response. A 3-log reduction in BCR–ABL transcripts after 1 month of imatinib therapy has been shown to predict a reduced relapse risk [118], as no rapid achievement of BCR–ABL negativity was described for achieving a long-term outcome [119]. However, MRD at 3 months has better discrimination for OS and relapse-free survival than did MRD status at CR. Patients who achieve complete molecular response at 3 months have superior survival compared with those with lesser molecular responses and have excellent long-term outcomes even when SCT is not performed [120]. However, currently BCR–ABL should be monitored after allogeneic SCT and re-emergence of BCR–ABL positivity should be a formal indication for intervention.
Many patients with Ph+ ALL relapse with a T315I clone, which is resistant to imatinib and second-generation TKIs. Novel molecules have shown interesting first results in Ph+ leukemias. Among them, ponatinib is a more potent third-generation BCR–ABL1 TKI that also suppresses the T315I clones. More recently, danusertib, a pan-aurora kinase inhibitor with potent activity against ABL kinase including the gatekeeper T315I mutant, has demonstrated an acceptable toxicity profile and is active in patients with BCR–ABL-associated advanced hematologic malignancies [121]. Axitinib is a VEGFR TKI that has also been shown as a selective and effective inhibitor for T315I mutant BCR–ABL1-driven leukemia [122]. New monoclonal antibodies are also going to change the landscape of ALL therapy. Monoclonal antibody development could, however, be challenged by the novel approach based on CAR T cells targeting CD19, which has already been tested with encouraging first results. CAR T cells targeting CD22 or CD19/CD22 are also under investigation. Further challenges should include: how to modulate immunotoxicity without losing efficacy; defining the optimal placement of monoclonal antibodies and CAR therapy either as bridge to SCT or alternative to SCT; defining the optimal therapeutic strategy with administration in relapse or after remission achievement; and define when it should be given in relation to chemotherapy.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Faderl S, Jeha S, Kantarjian HM. The biology and therapy of adult acute lymphoblastic leukemia. Cancer. 2003;98:1337–1354. doi: 10.1002/cncr.11664. [DOI] [PubMed] [Google Scholar]
- 2.Thomas DA. Philadelphia chromosome-positive acute lymphocytic leukemia: a new era of challenges. Hematology Am. Soc. Hematol. Educ. Program. 2007;2007(1):435–443. doi: 10.1182/asheducation-2007.1.435. [DOI] [PubMed] [Google Scholar]
- 3.Schlieben S, Borkhardt A, Reinisch I, et al. Incidence and clinical outcome of children with BCR/ABL-positive acute lymphoblastic leukemia (ALL): a prospective RT-PCR study based on 673 patients enrolled in the German pediatric multicenter therapy trials ALL-BFM 90 and CoALL-05–92. Leukemia. 1996;10:957–963. [PubMed] [Google Scholar]
- 4.Groupe Français de Cytogénétique Hématologique. Cytogenetic abnormalities in adult acute lymphoblastic leukemia: correlations with hematologic findings and outcome. Blood. 1996;87:3135–3142. [PubMed] [Google Scholar]
- 5.Radich JP. Philadelphia chromosome-positive acute lymphocytic leukemia. Hematol. Oncol. Clin. North Am. 2001;15:21–36. doi: 10.1016/s0889-8588(05)70198-2. [DOI] [PubMed] [Google Scholar]
- 6.Bartram CR, de Klein A, Hagemeijer A, et al. Translocation of c-abl oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983;306:277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- 7.Chan LC, Karhi KK, Rayter SI, et al. A novel abl protein expressed in Philadelphia chromosome positive acute lymphoblastic leukaemia. Nature. 1987;325:635–637. doi: 10.1038/325635a0. [DOI] [PubMed] [Google Scholar]
- 8.Maurer J, Janssen JWG, Thiel E, et al. Detection of chimeric BCR–ABL genes in acute lymphoblastic leukaemia by the polymerase chain reaction. Lancet. 1991;337:1055–1058. doi: 10.1016/0140-6736(91)91706-z. [DOI] [PubMed] [Google Scholar]
- 9.Lugo TG, Pendergast AM, Muler AJ, et al. Tyrosine kinase activity and transformation potency of BCR–ABL oncogene products. Science. 1990;247:1079–1083. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 10.Dombret H, Gabert J, Boiron JM, et al. Outcome of treatment in adults with Philadelphia chromosome-positive leukemia – results of the prospective multicenter LALA-94 trial. Blood. 2002;100:2357–2366. doi: 10.1182/blood-2002-03-0704. [DOI] [PubMed] [Google Scholar]
- 11.Gleissner B, Gökbuget N, Bartram CR, et al. Leading prognostic relevance of the BCR–ABL translocation in adult acute B-lineage lymphoblastic leukemia: a prospective study of the German Multicenter Trial Group and confirmed polymerase chain reaction analysis. Blood. 2002;99:1536–1543. doi: 10.1182/blood.v99.5.1536. [DOI] [PubMed] [Google Scholar]
- 12.Melo JV. The diversity of BCR–ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88:2375–2384. [PubMed] [Google Scholar]
- 13.Faderl S, Talpaz M, Estrov Z, et al. The biology of chronic myeloid leukemia. N Engl. J. Med. 1999;341:164–172. doi: 10.1056/NEJM199907153410306. [DOI] [PubMed] [Google Scholar]
- 14.Pfeifer H, Wassmann B, Pavlova A, et al. Kinase domain mutations of BCR–ABL frequently precede imatinib-based therapy and give rise to relapse in patients with de novo Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2007;110:727–734. doi: 10.1182/blood-2006-11-052373. [DOI] [PubMed] [Google Scholar]
- 15.Bene MC, Castoldi G, Knapp W, et al. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;10:1783–1786. [PubMed] [Google Scholar]
- 16.Rieder H, Ludwig WD, Gassmann W, et al. Prognostic significance of additional chromosome abnormalities in adult patients with Philadelphia chromosome positive acute lymphoblastic leukaemia. Br. J. Haematol. 1996;95:678–691. doi: 10.1046/j.1365-2141.1996.d01-1968.x. [DOI] [PubMed] [Google Scholar]
- 17.Secker-Walker LM, Prentice HG, Durrant J, et al. Cytogenetics adds independent prognostic information in adults with acute lymphoblastic leukaemia on MRC trial UKALL XA. Br. J. Haematol. 1997;96:601–610. doi: 10.1046/j.1365-2141.1997.d01-2053.x. [DOI] [PubMed] [Google Scholar]
- 18.Westbrook CA, Hooberman AL, Spino C, et al. Clinical significance of the BCR–ABL fusion gene in adult acute lymphoblastic leukemia: a Cancer and Leukemia Group B Study (8762) Blood. 1992;80:2983–2990. [PubMed] [Google Scholar]
- 19.Götz G, Weh HJ, Walter TA, et al. Clinical and prognostic significance of the Philadelphia chromosome in adult patients with acute lymphoblastic leukemia. Ann. Hematol. 1992;64:97–100. doi: 10.1007/BF01715353. [DOI] [PubMed] [Google Scholar]
- 20.Preti HA, O'Brien S, Giralt S, et al. Philadelphia-chromosome-positive adult acute lymphocytic leukemia: characteristics, treatment results, and prognosis in 41 patients. Am. J. Med. 1994;97:60–65. doi: 10.1016/0002-9343(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 21.Annino L, Vegna ML, Camera A, et al. Treatment of adult acute lymphoblastic leukemia (ALL): long-term follow-up of the GIMEMA ALL 0288 randomized study. Blood. 2002;99:863–871. doi: 10.1182/blood.v99.3.863. [DOI] [PubMed] [Google Scholar]
- 22.Thomas X, Thiebaut A, Olteanu N, et al. Philadelphia chromosome positive adult acute lymphoblastic leukemia: characteristics, prognostic factors and treament outcome. Hematol. Cell Ther. 1998;40:119–128. [PubMed] [Google Scholar]
- 23.Kantarjian HM, O'Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J. Clin. Oncol. 2000;18:547–561. doi: 10.1200/JCO.2000.18.3.547. [DOI] [PubMed] [Google Scholar]
- 24.Forman SJ, O'Donnell MR, Nademanee AP, et al. Bone marrow transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1987;70:587–588. [PubMed] [Google Scholar]
- 25.Barrett AJ, Horowitz MM, Ash RC, et al. Bone marrow transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1992;79:3067–3070. [PubMed] [Google Scholar]
- 26.Chao NJ, Blume KG, Forman SJ, et al. Long-term follow-up of allogeneic bone marrow recipients for Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 1995;85:3353–3354. [PubMed] [Google Scholar]
- 27.Snyder DS, Nademanee AP, O'Donnell MR, et al. Long-term follow-up of 23 patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with allogeneic bone marrow transplant in first complete remission. Leukemia. 1999;13:2053–2058. doi: 10.1038/sj.leu.2401589. [DOI] [PubMed] [Google Scholar]
- 28.Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia-chromosome positive acute lymphoblastic leukaemia confirms superiority of allogeneic transplant over chemotherapy in the pre-imatinib era: results from the international ALL trial MRC UKALLXII/ECOG E2993) Blood. 2009;113:4489–4496. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin TG, Gajewski JL. Allogeneic stem cell transplantation for acute lymphocytic leukemia in adults. Hematol. Oncol. Clin. North Am. 2001;15:97–120. doi: 10.1016/s0889-8588(05)70201-x. [DOI] [PubMed] [Google Scholar]
- 30.Yanada M, Naoe T, Iida H, et al. Myeloablative allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia in adults: significant roles of total body irradiation and chronic graft-versus-host disease. Bone Marrow Transplant. 2005;36:867–872. doi: 10.1038/sj.bmt.1705148. [DOI] [PubMed] [Google Scholar]
- 31.Laport GG, Alvarnas JC, Palmer JM, et al. Long-term remission of Philadelphia chromosome-positive acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation from matched sibling donors: a 20 year experience with the fractionated total body irradiation-etoposide regimen. Blood. 2008;112:903–909. doi: 10.1182/blood-2008-03-143115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esperou H, Boiron JM, Cayuela JM, et al. A potential graft-versus-leukemia effect after allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: results from the French Bone Marrow Transplantation Society. Bone Marrow Transplant. 2003;31:909–918. doi: 10.1038/sj.bmt.1703951. [DOI] [PubMed] [Google Scholar]
- 33.Thomas X, Boiron JM, Huguet F, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J. Clin. Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 34.Martin H, Atta J, Klein SA, et al. Autologous BMT/PBSCT in 40 patients with BCR–ABL positive acute lymphoblastic leukemia: long-term follow-up of a GMALL study. Hematology. 2001;1 Abstract 187. [Google Scholar]
- 35.Houot R, Tavernier E, Le QH, et al. Philadelphia chromosome-positive acute lymphoblastic leukemia in the elderly: prognostic factors and treatment outcome. Hematology. 2004;9:369–376. doi: 10.1080/10245330400001983. [DOI] [PubMed] [Google Scholar]
- 36.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of BCR–ABL positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 37.Ottmann OG, Druker BJ, Sawyers CL, et al. A Phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 38.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 39.Yanada M, Takeuchi J, Sugiura I, et al. High complete remission rate and promising outcome by combination of imatinib and chemotherapy for newly diagnosed BCR–ABL-positive acute lymphoblastic leukemia: a Phase II study by the Japan Adult Leukemia Study Group. J. Clin. Oncol. 2006;24:460–466. doi: 10.1200/JCO.2005.03.2177. [DOI] [PubMed] [Google Scholar]
- 40.Lee KH, Lee JH, Choi SJ, et al. Clinical effect of imatinib added to intensive combination chemotherapy for newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2005;19:1509–1516. doi: 10.1038/sj.leu.2403886. [DOI] [PubMed] [Google Scholar]
- 41.Vignetti M, Fazi P, Cimino G, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell’ Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109:3676–3678. doi: 10.1182/blood-2006-10-052746. [DOI] [PubMed] [Google Scholar]
- 42.Ottmann OG, Wassmann B, Pfeifer H, et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) Cancer. 2007;109:2068–2076. doi: 10.1002/cncr.22631. [DOI] [PubMed] [Google Scholar]
- 43.Wassmann B, Pfeifer H, Goekbuget N, et al. Alternating versus concurrent schedules of imatinib and chemotherapy as front-line therapy for Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2006;108:1469–1477. doi: 10.1182/blood-2005-11-4386. [DOI] [PubMed] [Google Scholar]
- 44.Delannoy A, Delabesse E, Lhéritier V, et al. Imatinib and methylprednisolone alternated with chemotherapy improve the outcome of elderly patients with Philadelphia-positive acute lymphoblastic leukemia: results of the GRAALL AFR09 study. Leukemia. 2006;20:1526–1532. doi: 10.1038/sj.leu.2404320. [DOI] [PubMed] [Google Scholar]
- 45.De Labarthe A, Rousselot P, Huguet-Rigal F, et al. Imatinib combined with induction or consolidation chemotherapy in patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the GRAAPH-2003 study. Blood. 2007;109:1408–1413. doi: 10.1182/blood-2006-03-011908. [DOI] [PubMed] [Google Scholar]
- 46.Burke MJ, Trotz B, Luo X, et al. Allo-hematopoietic cell transplantation for Ph chromosome-positive ALL: impact of imatinib on relapse and survival. Bone Marrow Transplant. 2009;43:107–113. doi: 10.1038/bmt.2008.296. [DOI] [PubMed] [Google Scholar]
- 47.Chalandon Y, Thomas X, Hayette S, et al. Randomized study of reduced-intensity chemotherapy combined with imatinib in adults with Ph-positive acute lymphoblastic leukemia. Blood. 2015;125:3711–3719. doi: 10.1182/blood-2015-02-627935. [DOI] [PubMed] [Google Scholar]; •• A randomized study validating an induction regimen combining reduced-intensity chemotherapy and imatinib.
- 48.Lim SN, Joo YD, Lee KH, et al. Long-term follow-up of imatinib plus combination chemotherapy in patients with newly diagnosed Philadelphia chromosome-positive acute lymphoblastic leukemia. Am. J. Hematol. 2015;90:1013–1020. doi: 10.1002/ajh.24137. [DOI] [PubMed] [Google Scholar]
- 49.Leis JF, Stepan DE, Curtin PT, et al. Central nervous system failure in patients with chronic myelogenous leukemia lymphoid blast crisis and Philadelphia chromosome positive acute lymphoblastic leukemia treated with imatinib (STI-571) Leuk. Lymphoma. 2004;45:695–698. doi: 10.1080/10428190310001625728. [DOI] [PubMed] [Google Scholar]
- 50.Wassmann B, Pfeifer H, Scheuring UJ, et al. Early prediction of response in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukaemia (Ph+ ALL) treated with imatinib. Blood. 2004;103:1495–1498. doi: 10.1182/blood-2003-01-0154. [DOI] [PubMed] [Google Scholar]
- 51.Radich JP. Molecular measurement of minimal residual disease in Philadelphia-positive acute lymphoblastic leukaemia. Best Pract. Res. Clin. Haematol. 2002;15:91–103. doi: 10.1053/beha.2002.0187. [DOI] [PubMed] [Google Scholar]
- 52.Von Bubnoff N, Peschel C, Duyster J. Resistance of Philadelphia-chromosome positive leukemia towards the kinase inhibitor imatinib (STI571, Glivec): a targeted oncoprotein strikes back. Leukemia. 2003;17:829–838. doi: 10.1038/sj.leu.2402889. [DOI] [PubMed] [Google Scholar]
- 53.Hofmann WK, Komor M, Hoelzer D, et al. Mechanisms of resistance to STI571 (imatinib) in Philadelphia-chromosome positive acute lymphoblastic leukemia. Leuk. Lymphoma. 2004;45:655–660. doi: 10.1080/10428190310001625755. [DOI] [PubMed] [Google Scholar]
- 54.Nicolini FE, Mauro MJ, Martinelli G, et al. Epidemiologic study on survival of chronic myeloid leukemia and Ph(+) acute lymphoblastic leukemia patients with BCR–ABL T315I mutation. Blood. 2009;114:5271–5278. doi: 10.1182/blood-2009-04-219410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hofmann WK, Komor M, Wassmann B, et al. Presence of the BCR–ABL mutation Glu255Lys prior to STI571 (imatinib) treatment in patients with Ph+ acute lymphoblastic leukemia. Blood. 2003;102:659–661. doi: 10.1182/blood-2002-06-1756. [DOI] [PubMed] [Google Scholar]
- 56.Mauro MJ. Defining and managing imatinib resistance. Hematology Am. Soc. Hematol. Educ. Program. 2006;2006(1):219–225. doi: 10.1182/asheducation-2006.1.219. [DOI] [PubMed] [Google Scholar]
- 57.Li S. Src-family kinases in the development and therapy of Philadelphia chromosome-positive chronic myeloid leukemia and acute lymphoblastic leukemia. Leuk. Lymphoma. 2008;49:19–26. doi: 10.1080/10428190701713689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iacobucci I, Lonetti A, Messa F, et al. Expression of spliced oncogenic Ikaros isoforms in Philadelphia-positive acute lymphoblastic leukemia patients treated with tyrosine kinase inhibitors: implications for a new mechanism of resistance. Blood. 2008;112:3847–3855. doi: 10.1182/blood-2007-09-112631. [DOI] [PubMed] [Google Scholar]
- 59.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of BCR–ABL inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 60.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 61.Ottmann O, Dombret H, Martinelli G, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a Phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 62.Porkka K, Simonsson B, Dombret H, et al. Efficacy of dasatinib in patients with Philadelphia-chromosome-positive acute lymphoblastic leukemia who are resistant or intolerant to imatinib: 2 year follow-up data from START-L (CA180–015) Blood. 2007;110 Abstract 826A-827A. [Google Scholar]
- 63.Sakamaki H, Ishizawa K, Taniwaki M, et al. Phase 1/2 clinical study of dasatinib in Japanese patients with chronic myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia. Int. J. Hematol. 2009;89:332–341. doi: 10.1007/s12185-009-0260-2. [DOI] [PubMed] [Google Scholar]
- 64.Lilly MB, Ottmann OG, Shah NP, et al. Dasatinib 140 mg once daily versus 70 mg twice daily in patients with Ph-positive acute lymphoblastic leukemia who failed imatinib: results from a Phase 3 study. Am. J. Hematol. 2010;85:164–170. doi: 10.1002/ajh.21615. [DOI] [PubMed] [Google Scholar]
- 65.Foa R, Vitale A, Vignetti M, et al. Dasatinib as first line treatment for adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;118:6521–6528. doi: 10.1182/blood-2011-05-351403. [DOI] [PubMed] [Google Scholar]
- 66.Ravandi F, O'Brien S, Thomas D, et al. First report of Phase II study of dasatinib with hyperCVAD for the frontline treatment of patients with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravandi F, O'Brien S, Cortes JE, et al. Long-term follow-up of a Phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121:4158–4164. doi: 10.1002/cncr.29646. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes long-term results achieved in a Phase II trial combining dasatinib with chemotherapy.
- 68.Rousselot P, Coudé MM, Gokbuget N, et al. Dasatinib and low-intensity chemotherapy in elderly patients with Philadelphia chromosome-positive ALL. Blood. 2016;128(6):774–782. doi: 10.1182/blood-2016-02-700153. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Describes results achieved with dasatinib plus chemotherapy in elderly patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL).
- 69.Shimoni A, Leiba M, Schleuning M, et al. Prior treatment with the tyrosine kinase inhibitors dasatinib and nilotinib allows stem cell transplantation (SCT) in a less advanced disease phase and does not increase SCT toxicity in patients with chronic myelogenous leukemia and philadelphia positive acute lymphoblastic leukemia. Leukemia. 2009;23:190–194. doi: 10.1038/leu.2008.160. [DOI] [PubMed] [Google Scholar]
- 70.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N. Engl. J. Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 71.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N. Engl. J. Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 72.Ottmann OG, Larson RA, Kantarjian HM, et al. Phase II study of nilotinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2013;27:1411–1413. doi: 10.1038/leu.2012.324. [DOI] [PubMed] [Google Scholar]
- 73.Kim DY, Joo YD, Lim SN, et al. Nilotinib combined with multiagent chemotherapy for newly diagnosed Philadelphia-positive acute lymphoblastic leukemia. Blood. 2015;126:746–756. doi: 10.1182/blood-2015-03-636548. [DOI] [PubMed] [Google Scholar]
- 74.La Rosee P, Holm-Eriksen S, Konig H, et al. Phospho-CRKL monitoring for the assessment of BCR–ABL activity in imatinib-resistant chronic myeloid leukemia or Ph+ acute lymphoblastic leukemia patients with nilotinib. Haematologica. 2008;93:765–769. doi: 10.3324/haematol.12186. [DOI] [PubMed] [Google Scholar]
- 75.Gambacorti-Passerini C, Cortes JE, Khoury HJ, et al. Safety and efficacy of bosutinib in patients AP and BP CML and Ph+ ALL following resistance/intolerance to imatinib and other TKIs: update from study SKI-200. J. Clin. Oncol. 2010;28 Abstract 15s. [Google Scholar]
- 76.Zhou T, Commodore L, Huang W, et al. Strutural mechanism of the pan-BCR–ABL inhibitor ponatinib (AP24534): lessons for overcoming kinase inhibitor resistance. Chem. Biol. Drug Des. 2011;77:1–11. doi: 10.1111/j.1747-0285.2010.01054.x. [DOI] [PubMed] [Google Scholar]
- 77.Cortes J, Kantarjian H, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A Phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 2013;369:1783–1796. doi: 10.1056/NEJMoa1306494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: a single-centre, Phase 2 study. Lancet Oncol. 2015;16:1547–1555. doi: 10.1016/S1470-2045(15)00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important paper showing interesting results with the combination of chemotherapy with ponatinib.
- 80.Le Jeune C, Thomas X. Antibody-based therapies in B-cell lineage acute lymphoblastic leukaemia. Eur. J. Haematol. 2015;94:99–108. doi: 10.1111/ejh.12408. [DOI] [PubMed] [Google Scholar]
- 81.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N. Engl. J. Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An important report about the use of anti-CD22 inotuzumab in refractory/relapsed ALL.
- 82.Topp MS, Kufer P, Gokbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J. Clin. Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 83.Martinelli G, Dombret H, Chevallier P, et al. Complete molecular and hematologic response in adult patients with relapsed/refractory (R/R) Philadelphia chromosome-positive B-precursor acute lymphoblastic leukemia (ALL) following treatment with blinatumomab: results from a Phase 2 single-arm, multicenter study (ALCANTARA) Blood. 2015;126(23):679. [Google Scholar]; •• An important study showing the efficacy of blinatumomab in refractory/relapsed Ph+ ALL.
- 84.Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]; • One of the first studies about CD19-targeted T cells in ALL.
- 85.Fielding AK, Goldstone AH. Allogeneic haematopoietic stem cell transplant in Philadelphia-positive acute lymphoblastic leukaemia. Bone Marrow Transplant. 2008;41:447–453. doi: 10.1038/sj.bmt.1705904. [DOI] [PubMed] [Google Scholar]
- 86.Hatta Y, Mizuta S, Ohtake S, et al. Promising outcome of imatinib-combined chemotherapy followed by allogeneic hematopoietic stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia: results of the Japan Adult leukemia Study Group (JALSG) Ph+ALL202 regimen. Blood. 2009;114 Abstract 3090. [Google Scholar]
- 87.Tanguy-Schmidt A, Rousselot P, Chalandon Y, et al. Long-term follow-up of the imatinib GRAAPH-2003 study in newly diagnosed patients with de novo Philadelphia chromosome-positive acute lymphoblastic leukemia: a GRAALL study. Biol. Blood Marrow Transplant. 2013;19:150–155. doi: 10.1016/j.bbmt.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 88.Thomas DA, O'Brien S, Faderl S, et al. Long-term outcome after hyper-CVAD and imatinib (IM) for de novo or minimally treated Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph-ALL) J. Clin. Oncol. 2010;28 doi: 10.1200/JCO.2009.26.9456. Abstract 6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pui CH, Yang JJ, Hunger SP, et al. Childhood acute lymphoblastic leukemia: progress through collaboration. J. Clin. Oncol. 2015;33:2938–2948. doi: 10.1200/JCO.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mohty M, Labopin M, Volin L, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116:4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 91.Ram R, Storb R, Sandmaier BM, et al. Nonmyeloablative conditioning with allogeneic hematopoietic cell transplantation for the treatment of high risk acute lymphoblastic leukemia. Haematologica. 2011;96:1113–1120. doi: 10.3324/haematol.2011.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stein A, O'Donnell M, Snyder DS, et al. Reduced-intensity stem cell transplantation for high-risk acute lymphoblastic leukaemia. Biol. Blood Marrow Transplant. 2009;15:1407–1414. doi: 10.1016/j.bbmt.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arnold R, Massenkiel G, Bornhauser M, et al. Nonmyeloablative stem cell transplantation in adults with high-risk ALL may be effective in early but not in advanced disease. Leukemia. 2002;16:2423–2428. doi: 10.1038/sj.leu.2402712. [DOI] [PubMed] [Google Scholar]
- 94.Martino R, Giralt S, Caballero MD, et al. Allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning in acute lymphoblastic leukemia: a feasibility study. Haematologica. 2003;88:555–560. [PubMed] [Google Scholar]
- 95.Bachanova V, Verneris MR, DeFor T, Brunstein CG, Weisdorf DJ. Prolonged survival in adults with acute lymphoblastic leukemia after reduced-intensity conditioning with cord blood or sibling donor transplantation. Blood. 2009;113:2902–2905. doi: 10.1182/blood-2008-10-184093. [DOI] [PubMed] [Google Scholar]
- 96.Carpenter PA, Snyder DS, Flowers ME, et al. Prophylactic administration of imatinib after hematopoietic cell transplantation for high-risk Philadelphia chromosome-positive leukemia. Blood. 2007;109:2791–2793. doi: 10.1182/blood-2006-04-019836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wassmann B, Pfeifer H, Stadler M, et al. Early molecular response to post-transplantation imatinib determines outcome in MRD+ Philadelphia-positive acute lymphoblastic leukemia (Ph+ ALL) Blood. 2005;106:458–463. doi: 10.1182/blood-2004-05-1746. [DOI] [PubMed] [Google Scholar]
- 98.Wassmann B, Pfeifer H, Bethge W, et al. Up-front versus minimal residual disease triggered imatinib after stem cell transplantation for Philadelphia chromosome-positive acute lymphoblastic leukaemia: interim results of a randomized Phase III GMALL study. Bone Marrow Transplant. 2009;43 Abstract S48. [Google Scholar]
- 99.Gao L, Zhang C, Gao L, et al. Favorable outcome of haploidentical hematopoietic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia: a multicentre study in Southwest China. J. Hematol. Oncol. 2015;8:90. doi: 10.1186/s13045-015-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tucunduva L, Ruggeri A, Sanz G, et al. Impact of minimal residual disease on outcomes after umbilical cord blood transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia: an analysis on behalf of Eurocord, Cord Blood Committee and the Acute Leukaemia working party of the European group for Blood and Marrow Transplantation. Br. J. Haematol. 2014;166:749–757. doi: 10.1111/bjh.12970. [DOI] [PubMed] [Google Scholar]
- 101.Giebel S, Labopin M, Gorin NC, et al. Improving results of autologous stem cell transplantation for Philadelphia-positive acute lymphoblastic leukaemia in the era of tyrosine kinase inhibitors: a report from the Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation. Eur. J. Cancer. 2014;50:411–417. doi: 10.1016/j.ejca.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 102.Böhm A, Herrmann H, Mitterbauer-Hohendanner G, et al. Stable non-transforming minimal residual disease in Philadelphia chromosome positive acute lymphoblastic leukemia after autologous transplantation: origin from neoplastic yet ‘pre-leukemic’ stem cells? Leuk. Lymphoma. 2011;52:842–848. doi: 10.3109/10428194.2011.557168. [DOI] [PubMed] [Google Scholar]
- 103.Yanada M, Takeuchi J, Sugiura I, et al. Karyotype at diagnosis is the major prognostic factor predicting relapse-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib-combined chemotherapy. Haematologica. 2008;93:287–290. doi: 10.3324/haematol.11891. [DOI] [PubMed] [Google Scholar]
- 104.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J. Clin. Oncol. 2010;28:3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 105.Fielding AK, Foroni L, Gerrard G, et al. UKALLXII/ECOG2993: addition of imatinib to a standard treatment regimen enhances long-term outcomes in Philadelphia positive acute lymphoblastic leukemia. Blood. 2014;123:843–850. doi: 10.1182/blood-2013-09-529008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sartor C, Papayannidis C, Piciocchi A, et al. Sequential use of first and second generation TKIs are effective on prolonged overall survival in elderly population affected by Ph+ acute lymphoblastic leukemia: the GIMEMA experience. Proc. Annual AACR Meeting. 2015;7(15) Abstract 5491. [Google Scholar]
- 107.Den Boer JM, Marchante RM, Horstmann MA, et al. BCR–ABL1-like cases in pediatric acute lymphoblastic leukemia: a comparison between COG/St Jude and Dutch DCOG signatures. Blood. 2013;122 doi: 10.3324/haematol.2015.124941. Abstract 2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Den Boer ML, van Slegtenhorst M, De Menezes RX, et al. A subtype of childhood acute lymphoblastic leukaemia with poor treatment outcome: a genome-wide classification study. Lancet Oncol. 2009;10:125–134. doi: 10.1016/S1470-2045(08)70339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harvey RC, Kang H, Roberts KG, et al. Development and validation of a highly sensitive and specific gene expression classifier to prospectively screen and identify B-precursor acute lymphoblastic leukemia (ALL) patients with a Philadelphia chromosome-like (“Ph-like” or “BCR–ABL1-like”) signature for therapeutic targeting and clinical intervention. Blood. 2013;122 Abstract 826. [Google Scholar]
- 110.Roberts KG, Morin RD, Zhang J, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22:153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maude SL, Tasian SK, Vincent T, et al. Targeting JAK1/2 and mTOR in murine xenograft models of Ph-like acute lymphoblastic leukemia. Blood. 2012;120:3510–3518. doi: 10.1182/blood-2012-03-415448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Eyre T, Schwab CJ, Kinstrie R, et al. Episomal amplification of NUP214–ABL1 fusion gene in B-cell acute lymphoblastic leukemia. Blood. 2012;120:4441–4443. doi: 10.1182/blood-2012-09-456517. [DOI] [PubMed] [Google Scholar]
- 113.Soler G, Radford-Weiss I, Ben-Abdelali R, et al. Fusion of ZMIZ1 to ABL1 in a B-cell acute lymphoblastic leukaemia with a t(9;10)(q34;q22.3) translocation. Leukemia. 2008;22:1278–1280. doi: 10.1038/sj.leu.2405033. [DOI] [PubMed] [Google Scholar]
- 114.Lengline E, Beldjord K, Dombret H, et al. Successful tyrosine kinase inhibitor therapy in a refractory B-cell precursor acute lymphoblastic leukemia with EBF1-PDGFRB fusion. Haematologica. 2013;98:e146–e148. doi: 10.3324/haematol.2013.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Masuzawa A, Kiyotani C, Osumi T, et al. Poor responses to tyrosine kinase inhibitors in a child with precursor B-cell acute lymphoblastic leukemia with SNX2–ABL1 chimeric transcript. Eur. J. Haematol. 2014;92:263–267. doi: 10.1111/ejh.12234. [DOI] [PubMed] [Google Scholar]
- 116.Boulos N, Mulder HL, Calabrese CR, et al. Chemotherapeutic agents circumvent emergence of dasatinib-resistant BCR–ABL kinase mutations in a precise mouse model of Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2011;117:3585–3595. doi: 10.1182/blood-2010-08-301267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ravandi F, Jorgensen JL, Thomas DA, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–1221. doi: 10.1182/blood-2012-11-466482. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• An important study identifying patients with Ph+ ALL who should benefit from intensification by allogeneic stem cell transplantation.
- 118.Lee S, Kim DW, Cho B, et al. Risk factors for adults with Philadelphia-chromosome-positive acute lymphoblastic leukaemia in remission treated with allogeneic bone marrow transplantation: the potential of real-time quantitative reverse-transcription polymerase chain reaction. Br. J. Haematol. 2003;120:145–153. doi: 10.1046/j.1365-2141.2003.03988.x. [DOI] [PubMed] [Google Scholar]
- 119.Yanada M, Sugiera I, Takeuchi J, et al. Prospective monitoring of BCR–ABL transcript levels in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia undergoing imatinib-combined chemotherapy. Br. J. Haematol. 2008;143:503–510. doi: 10.1111/j.1365-2141.2008.07377.x. [DOI] [PubMed] [Google Scholar]
- 120.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128(4):504–507. doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A study showing patients with earlier complete molecular response have superior survival compared with those with lesser molecular responses.
- 121.Borthakur G, Dombret H, Schafhausen P, et al. A Phase I study of danusertib (PHA-739358) in adult patients with accelerated or blastic phase myeloid leukemia and Philadelphia chromosome-positive acute lymphoblastic leukemia resistant or intolerant to imatinib and/or other second generation c-ABL therapy. Haematologica. 2015;100:898–904. doi: 10.3324/haematol.2014.115279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pemovska T, Johnson E, Kontro M, et al. Axitinib effectively inhibits BCR–ABL1 (T315I) with a distinct binding conformation. Nature. 2015;519:102–105. doi: 10.1038/nature14119. [DOI] [PubMed] [Google Scholar]