Abstract
B-lymphocytes are dependent on B-cell receptor (BCR) signaling for the constant maintenance of their physiological function, and in many B-cell malignancies this signaling pathway is prone to aberrant activation. This understanding has led to an ever-increasing interest in the signaling networks activated following ligation of the BCR in both normal and malignant cells, and has been critical in establishing an array of small molecule inhibitors targeting BCR-induced signaling. By dissecting how different malignancies signal through BCR, researchers are contributing to the design of more customized therapeutics which have greater efficacy and lower toxicity than previous therapies. This allows clinicians access to an array of approaches to best treat patients whose malignancies have BCR signaling as a driver of pathogenesis.
KEYWORDS : B-cell receptor signaling, Bruton's tyrosine kinase, chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, Mantle cell lymphoma, phosphoinositide 3-kinase, spleen tyrosine kinase
Practice points.
This review dissects the signaling pathways activated downstream of the B-cell receptor (BCR) in normal and malignant B cells and discusses how a greater understanding of such pathways has led to the discovery of numerous drug targets for the treatment of B-cell lymphomas.
This review discusses the BCR signaling response, describing in detail how this pathway constantly maintains its initiation, amplification and termination through a regulatory mechanism combining protein kinases, protein scaffolds, immuno-receptor tyrosine-based activation/inhibitory motifs, protein phosphatases and ubiquitination.
Mature B-cell lymphoproliferative disorders such as chronic lymphocytic leukemia, diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma and Waldenström's macroglobulinemia show active BCR signaling that contributes to behavior of the malignant clone in these diseases.
This review considers various targets within the BCR signaling pathway. It relates how inhibiting Lyn is controversial because of its positive and negative role in BCR signaling, reviews how Lck is potentially a good target for chronic lymphocytic leukemia treatment because of its unique role in initiating BCR signaling within the malignant cells of this disease, discusses the benefits of targeting Syk, and examines Ibrutinib, a first-in-class BTK inhibitor, and Idelalisib, a first-in-class PI3Kδ inhibitor, as recently approved drugs useful in the treatment of mantle cell lymphoma, chronic lymphocytic leukemia and Waldenström's macroglobulinemia.
BCR signaling pathway inhibitors have proven very successful in the treatment of B-cell malignancies and offer a less toxic alternative to conventional chemotherapeutic approaches. However, some patients have displayed adverse reactions to these inhibitors and therefore research is ongoing to help develop current and new therapeutic targets.
Background
B-lymphocytes are dependent on B-cell receptor (BCR) signaling for the constant maintenance of their physiological function, and in many B-cell malignancies this signaling pathway is prone to aberrant activation. This understanding has led to an ever-increasing interest in the signaling networks activated following ligation of the BCR in both normal and malignant cells, and has been critical in establishing an array of small molecule inhibitors targeting BCR-induced signaling. By dissecting how different malignancies signal through BCR, researchers are contributing to the design of more customized therapeutics which have greater efficacy and lower toxicity than previous therapies. This allows clinicians access to an array of approaches to best treat patients whose malignancies have BCR signaling as a driver of pathogenesis. This review brings together old and new basic research on the pathways activated downstream of the BCR, and reports on how these pathways contribute to both B-cell homeostasis and neoplasia. We also report on how our understanding of BCR signaling has revealed drug targets both proximal and distal to the BCR that have been successfully exploited therapeutically, especially with respect to particular malignancies.
BCR signaling in normal B cells
• The importance of BCR signaling
B-cell development and maturation is dependent on a diverse range of cellular responses relayed by antigen-independent (tonic) and antigen-dependent activation of the BCR [1–4]. This begins at the very earliest stages of B-cell development where tonic signals through the pre-BCR combined with IL-7R activation function together to drive the proliferation and survival of pre-B cells in the bone marrow [5]. Expression of functional BCR helps distinguish immature B cells from pre-B cells during subsequent differentiation, and strong engagement of BCR at this stage enables negative selection (auto deletion) of self-reactive B-cell clones [6–8]. Immature (naive) B cells that survive this selection then egress from the bone marrow, and are able to receive environmental cues in the peripheral lymphoid tissues to differentiate into their various mature B-cell subsets [9]. During this period, and in the absence of antigen encounter, it is thought that tonic BCR signals maintain the survival of B cells as they recirculate in the blood and follicular regions of the lymphoid tissues [2,10] because experiments have shown that failure to express BCR on peripheral immature and mature B cells results in their death [10]. Differentiation of naive B cells does not occur without engagement of the BCR by antigen and concomitant stimulation of cytokine and co-stimulatory receptors within lymphoid tissues. Here, it is the strength of BCR signaling that determines B-cell fate, deciding whether B cells differentiate to follicular B cells, marginal zone B cells or are deleted through neglect [11,12].
• Proximal BCR signaling
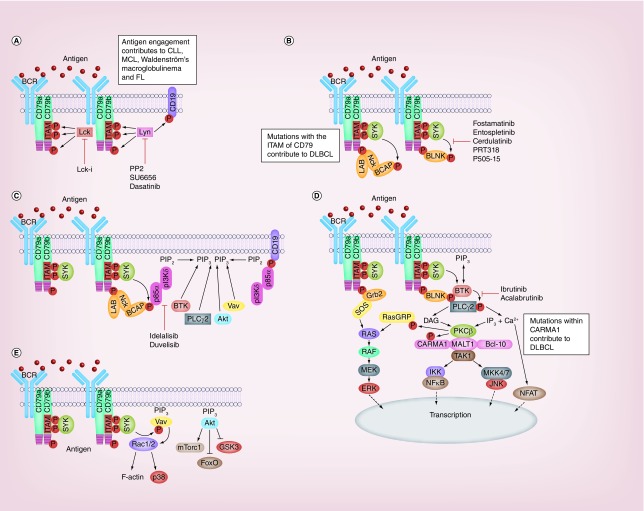
The BCR is comprised of a membrane anchored ligand binding unit formed by immunoglobulin (Ig), and a signaling unit consisting of a heterodimer of Igα (CD79a) and Igβ (CD79b) [13–15]. Antigen binds to hypervariable regions present on the Ig component of the BCR causing receptor clustering and juxtapositioning into areas rich in signaling components [16,17]. Figure 1 illustrates the signaling networks activated following BCR stimulation. Upon antigen binding, two conserved tyrosine residues within the immuno-receptor tyrosine-based activation motifs (ITAMS) of CD79 become phosphorylated by Src-family kinases (SFK) such as Lyn (Figure 1A) [18,19]. Phosphorylated ITAMS act as docking sites for SH2 domains present in Syk, leading to its phosphorylation and subsequent activation (Figure 1B) [20,21]. SFK can also phosphorylate tyrosine residues outside the ITAM motifs of CD79 as well as tyrosine residues within the co-receptor protein CD19 (Figure 1A). Adaptor proteins such as BLNK, LAB and Nck are recruited to these extra-ITAM phosphotyrosine motifs in CD79 (Figure 1B) [22–24]. Active Nck recruits B-cell adapter for PI3K (BCAP) which becomes phosphorylated by Syk and attracts PI3Kδ via its p85α regulatory domain (Figure 1C) [25,26]. Additionally, the SFK-phosphorylated tyrosines within CD19 are additional binding sites for p85α and activation of PI3Kδ (Figure 1C) [27–29]. However, it is suggested that PI3K can be activated independently of BCR engagement because the introduction of exogenous PI3K has been shown to rescue BCR-negative cells from apoptosis [30,31]. A proposed mechanism for this rescue is the recruitment of PI3K to unphosphorylated motifs present on CD79a by the GTPase TC21 [32]. Whatever the mechanism of activation, PI3Kδ converts PIP2 to PIP3 on the plasma membrane, and, in doing so, generates a binding site for the pleckstrin homology (PH) domain of a collection of proteins normally residing in the cytosol of resting B cells. Btk is one such protein, that additionally binds BLNK and becomes phosphorylated by Syk [33], exposing an autophosphorylation site within Btk that culminates in its full activation (Figure 1C & D) [34]. PLCγ2 is also recruited to the plasma membrane via its PH domain and BLNK (Figure 1C & D), and is sequentially activated through phosphorylation by Btk and Syk [35]. Similarly, the Rac GTP-exchange factor Vav is also attracted to the BCR signalosome where it can be phosphorylated and activated by Syk (Figure 1C & E) [36–38]. Finally, PIP3 also attracts the kinases PDK1 and Akt to the plasma membrane (Figure 1C). Once recruited, PDK1 phosphorylates Akt on T308 to increase the kinase activity of the latter. Stabilization of Akt kinase activity then occurs when it is phosphorylated on S473 by mTORC2 through a mechanism involving Akt-mediated phosphorylation of SIN1 [39].
Figure 1. . Signaling pathways of the B-cell receptor, its role in B-cell malignancies and targets of inhibition.
Schematic of the B-cell receptor signaling pathway. (A) Antigen engagement initiates receptor clustering and facilitates Lyn-medated phosphorylation of tyrosines within CD79 and CD19. In chronic lymphocytic leukemia, the SFK Lck mediates phosphorylation of CD79. (B) Syk binds to phospho-tyrosine residues within the ITAM of CD79 and is activated. Adaptor proteins such as BLNK, LAB, NCK, BCAP and Grb2 associate with phospho-tyrosines outside the ITAM on CD79. Proteins such as BLNK and BCAP are substrates of Syk. (C) Phospho-tyrosine residues within BCAP and CD19 attract the regulatory subunit of PI3Kδ leading to the activation of catalytic p110. PI(4,5)P2 is converted to PI(3,4,5)P3 which attracts PH domain containing proteins such as BTK, PLCγ2, Akt and Vav to the plasma membrane. (D) Phospho-tyrosine residues within BLNK act as a scaffold for membrane-associated BTK and PLCγ2, facilitating activation of the former to phosphorate and activate the latter. This catalyzes distal signaling pathway activation leading to NF-κB, JNK and ERK activation. (E) Further distal signals include activation of Vav leading to cytoskeletal rearrangement, and of Akt leading to activation of mTORC1 and inhibition of FoxO.
CLL: Chronic lymphocytic leukemia; DLBCL: Diffuse large B-cell lymphoma; FL: Follicular lymphoma; ITAM: Immuno-receptor tyrosine-based activation motifs; MCL: Mantle cell lymphoma.
• Distal BCR signaling
The proximal BCR signaling response quickly transcends to activate numerous signaling pathways summarized in Figure 1D. Active PLCγ2 is able to hydrolyze PIP2 to generate two second messengers, IP3 and DAG. IP3 induces the release of Ca2+ from intracellular stores in the endoplasmic reticulum (ER) and, together with DAG, this leads to the activation of PKCβ [40]. PKCβ is then able to mediate the stimulation of multiple downstream signaling pathways. For example, PKCβ together with DAG and Sos can generate active Ras (GTP-Ras), leading to activation of the cRaf-MEK-ERK pathway [41–43]. PKCβ can also phosphorylate CARMA1 (CARD11) [44] to initiate the recruitment of MALT1 and Bcl-10, which forms the CBM complex [45,46]. This complex activates TAK1, a kinase that can catalyze the activation of both NF-κB and JNK [47]. TAK1 activates NF-κB by initially phosphorylating IKKβ [47], which in turn phosphorylates IκBα, flagging it for ubiquitination and degradation by the proteasome [48]. This alleviates its inhibition of NF-κB and allows it to translocate to the nucleus. Calmodulin (CaM) detects increased Ca2+ mobilization from intracellular stores and activates the S/T phosphatase calcineurin (CN). CN dephosphorylates regulatory regions within NFAT causing a conformational change that exposes its nuclear localization sequence and results in its activation [49].
The BCR signal can also be relayed to downstream signaling pathways independently of calcium release from the ER. As mentioned previously, generation of PIP3 by activation of PI3Kδ causes the recruitment of Vav and generation of active GTP-loaded Rac1 and Rac2 (Figure 1E). These small G-proteins can facilitate F-actin reorganization as well as activation of p38MAPK and JNK [24,50–51]. Active Akt plays an important role in stimulating the mTORC1 pathway and in deactivating GSK3 and FoxO to facilitate increased cell growth and survival (Figure 1E) [52–54]. Collectively, these pathways are responsible for the control of a whole host of cellular functions such as; survival, proliferation, oxidative stress response, differentiation and DNA damage repair.
• Negative regulation of BCR signaling
BCR signaling is highly regulated by a complex network of phosphatases, regulatory receptors and negative feedback signals in order to prevent its inappropriate activation. A primary example of this is how the SFK Lyn mediates the BCR signaling response. The protein tyrosine phosphatases (PTP) CD45 and CD148 dephosphorylate Y508 on the C-terminus of Lyn causing a conformational change that allows for its autophosphorylation and activation [55,56]. Then, depending on the context of the BCR signal, active Lyn can moderate the BCR signal by phosphorylating immuno-receptor tyrosine-based inhibitory motifs (ITIM) present on the regulatory receptors CD5, CD22, CD32 and CD72 [57–62]. Phosphorylated ITIMs recruit phosphatases such as SHP-1, SHIP-1, SHIP-2, PTP-PEST, PTPN2, PTPROt, PTEN and PTPN22 which act on proximal targets to downregulate the BCR signaling response. Lyn controls its own inactivation by recruiting the phosphatase PTPN22, which forms a complex with the C-terminal Src kinase (Csk) [63]. Active Lyn binds and phosphorylates the Csk binding protein (Cbp) on Y314 and this serves to recruit Csk. PTPN22 then dephosphorylates the activating tyrosine residue on Lyn and allows Csk access to phosphorylate Lyn on Y508, resulting in the restoration of its closed inactive conformation [64,65]. Additionally, PTPN22 can regulate the stability of SFK and SYK by activating the E3-ubiquitin ligase c-Cbl, which ubiquitinylates these proteins and marks them for degradation [66–68]. Interestingly, SFK and Syk can recruit SOCS-1 which seems to augment their polyubiquitination and degradation by the proteasome [65,69]. However, the role of c-Cbl appears more complex than mere regulation of protein stability because it is reported to downregulate the BCR signal by binding to BLNK and preventing its association with PLCγ2 [70].
Other inhibitory receptors worth mentioning are those regulated by CD22 and CD32. Upon phosphorylation of their ITIMs by Lyn, PTEN and SHIP-1/2, respectively remove the 3′ and 5′ phosphate of PIP3 and attenuate signaling downstream of PI3K [71–73]. This regulatory pathway is activated by prolonged exposure to autoantigens and forms the basis of auto-reactive B-cell elimination [74]. Last, the phosphatase SHP-1 has been reported to downregulate the BCR signal by dephosphorylating targets such as SFK, ITAMs, ZAP70, SYK, BLNK and LAB [75].
Further regulation of the BCR signal is provided by negative feedback mechanisms that are independent of phosphatase activity. Following its activation, Btk can become phosphorylated on S180 by PKCβ activated by Btk-catalyzed stimulation of PLCγ2. This disrupts the interaction of the PH domain of Btk with PIP3 and removes Btk from the plasma membrane [76]. Furthermore, a recent report has demonstrated that Akt can also phosphorylate Btk on S51 and T495 which leads to its sequestration and degradation [77]. Another more distal example of negative feedback inhibition of BCR signaling is found in the regulation of NF-κB by the CBM complex. It is reported that TAK1 can induce both kinase-independent and -dependent degradation of Bcl-10 by respectively coordinating the binding of E3 ubiquitin ligases and activating JNK [78].
This meticulous regulation of the BCR allows it to constantly maintain its initiation, amplification and termination under normal conditions. However, alteration in these pathways has the potential to transform B cells into an array of malignant diseases.
BCR signaling in malignant B cells
• BCR signaling drives B-cell malignancies
It is widely accepted that signals emanating from the BCR play an important role in the initiation and progression of many B-cell malignancies. For example, gene expression profiling of chronic lymphocytic leukemia (CLL) cells isolated from lymphatic tissues found that the BCR pathway was active in these cells to a greater degree than what is found in circulating CLL cells [79]. Furthermore, CLL cells use a restricted repertoire of IGHV genes, with many patients expressing virtually identical BCRs, known as sterotyped BCRs [80–83]. Similarly, mantle cell lymphoma (MCL) cells also express sterotyped BCR [84,85], and display constitutive activation of BCR signaling components such as the PI3K pathway [86,87]. Other examples include diffuse large B-cell lymphoma (DLBCL) [88], follicular lymphoma (FL) [89], hairy cell leukemia (HCL) [90,91], Burkitts lymphoma (BL) [92], Waldenström's macroglobulinemia (WM) [93], marginal zone lymphoma (MZL) [94] and acute lymphoblastic leukemia (ALL) [95] cells which have all been shown to display some sort of defect in BCR signaling. However, the malignant cells in each of these diseases display distinct alterations in the BCR signaling pathway, reflecting defects originating from tonic/autonomous and/or chronic engagement of the BCR. What appears constant in many B-cell lymphomas is that IgM is the typical isotype of their BCR, probably due to the bias of this particular configuration of BCR toward stimulating survival and proliferation of B cells [96]. Below we will summarize some of the major aberrantly activated BCR signaling pathways found in different leukemia and lymphomas, and highlight their importance in the initiation, survival and expansion of these malignant cells.
• Chronic lymphocytic leukemia
CLL is a debilitating disease characterised by the gradual accumulation of mature B cells that are resistant to apoptosis. The disease provides an excellent example of the prominent role BCR signaling plays in the pathogenesis of B-cell malignancies (Figure 1A). This role was determined from early studies of BCR structure in CLL cells which showed the genes coding for variable (antigen binding) regions of BCR heavy chain maintained germline sequences in approximately half of patients diagnosed with this disease. These patients with so-called unmutated CLL (UM-CLL) have disease which has less favorable prognosis than patients where these genes have been somatically hypermutated, so called mutated CLL (M-CLL) [97]. Other studies showed that BCRs on CLL cells from different patients could be virtually identical with respect to IGVH genes and sequences, suggesting a common antigen or feature of the BCR that is involved in the pathology of this disease [82]. Common antigens targeted by BCR on CLL cells are reported to include epitopes associated with apoptosis and oxidation [98], yeast/fungi cell wall components [99], myosin [100] and vimentin [101], and BCR on CLL cells from UM-CLL patients are both polyreactive and responsive to BCR stimulation. In keeping with their ability to strongly respond to BCR engagement, UM-CLL cells generally have high expression and/or activation levels of many of the components of the BCR signaling pathway such as Syk [102], Lyn, Btk, PLCγ2, PI3Kδ, GAB1, PTPN22 [103], PKCβ and NF-κB. Furthermore, UM-CLL cells also generally express ZAP70 [104–106] and conflicting reports argue on one hand that this kinase mediates the phosphorylation of ITAM motifs and subsequent recruitment of Syk [107], while others have shown that kinase dead ZAP70 can still enhance the BCR signaling response by acting as a scaffold protein [108,109]. Work from this Department has demonstrated that another kinase called Lck displays heterogeneous expression in CLL cells and is able to augment the BCR signaling response [110]. An important feature of BCR signaling in CLL cells that distinguishes it from other B-cell malignancies is that it fails to activate the JNK pathway [111], however, why this is the case requires further investigation. Direct engagement of the BCR is not the only way in which this receptor contributes to disease pathogenesis in CLL. Some BCR heavy chain structures are said to be represented stereotypically on CLL cells, and one study has demonstrated that particular regions, namely the FR2 and HCDR3, can interact to allow autonomous BCR signaling, particularly in CLL cells from UM-CLL patients, irrespective of antigen stimulation [112].
In contrast to UM-CLL, CLL cells from patients with M-CLL express low surface IgM and show higher basal levels of Ca2+ and ERK activation consistent with constitutive low level stimulation of BCR [113] resulting in induction of cell anergy [114,115]. Targeting either the ERK or the NF-AT pathway with specific inhibitors is reported to reduce the survival of anergic CLL cells, suggesting that BCR anergy contributes to clonal maintenance in M-CLL anergy [114].
Taken together, these findings in both UM- and M-CLL demonstrate that BCR signaling contributes to proliferation and survival of the malignant clone in CLL. However, the way in which BCR signaling is stimulated, be it through active engagement or through tonic/chronic/autonomous signaling may have impact on disease progression. CLL cells circulate between the peripheral blood (PB) and proliferation centers. Expansion of the malignant clone depends upon its ability to remain within proliferation centers such as the lymph node where there is a microenvironment rich in proliferation and survival signals [113]. BCR engagement on CLL cells induces increased adhesion [116,117] and suppressed expression of the receptor for sphingosine 1-phosphate [118], both which lead to increased lymph node residency. This role of BCR in keeping CLL cells within lymph nodes has recently been exploited therapeutically where drugs targeting Btk (ibrutinib) and PI3Kδ (idelalisib) induce lymphocytosis while reducing both lymphadenopathy and splenomegaly [119,120].
• Diffuse large B-cell lymphoma
BCR signaling in a subset of DLBCL known as activated B-cell like (ABC)-DLBCL shows traits of active BCR signaling, and gene expression analysis revealed that a large amount of these cells display increased levels of BCR signaling components [121]. Furthermore, studies using internal reflection fluorescence (TIRF) microscopy found that the BCRs on ABC-DLBCL cells form clusters on the cell surface with proteins containing phosphotyrosine localizing underneath [88,122]. Other studies investigating the mechanism of cell survival discovered that these cells depend on anti-apoptotic signals provided by the NF-κB pathway [123], found to be constitutively active in some cases of ABC-DLBCL owing to mutations in CARMA1 [124] (Figure 1D) and loss-of-function mutations in negative regulators of NF-κB [125]. However, malignant cells in the majority of ABC-DLBC contain wild-type CARMA1 but still require this protein to maintain viability [126]. In these cases knockdown of BCR signaling units CD79a/b or any of its proximal signaling components (BLNK, Syk, Btk, PLCγ2, PI3K or PKCβ) induces apoptosis [88]. In particular, the malignant clones from approximately 20% of ABC-DLBCL cases bear mutations within the ITAM motifs of CD79a/b (Figure 1A) leading to reduced receptor endocytosis and increased surface expression of the BCR [88]. These cells have an enhanced BCR signaling response and are able to induce a greater and more prolonged activation of Akt. In particular, CD79b mutants are more resistant to moderation of the BCR signal by Lyn and consequently this may generate an oncogenic addiction to BCR-induced NF-κB activation [88,127]. Another protein that is frequently mutated in ABC-DLBCL is the adapter protein MYD88 [128] which is responsible for coupling toll-like receptors (TLR) to the NF-κB pathway. An L265P mutation in MYD88 was shown to augment autocrine secretion of the cytokines IL-6 and IL-10 resulting in increased ABC-DLBCL survival [128]. The relevance of such mutations to BCR-induced survival signals was highlighted when one group knocked down MYD88 in concert with CD79a/b, and found that this synergized to induce ABC-DLBCL cell killing [128,129]. Interestingly, this same mutation occurs in approximately 3% of CLL patients [130], and MYD88 has recently been shown to work in concert with the BCR of ZAP70 positive CLL cells to stimulate cell survival and proliferation [131].
• Burkitt's lymphoma
BL is an aggressive malignancy derived from germinal center B cells. A distinctive characteristic of this disease is a chromosomal translocation whereby the proto-oncogene MYC is linked to the heavy chain of BCR [132]. However, this alone is insufficient to cause BL, and studies in mice suggest that MYC works with active PI3K to generate the Burkett's lymphoma phenotype [133]. In contrast to the chronically active BCR signal displayed in ABC-DLBCL, BL cells are resistant to BTK and CARMA1 knockdown, but remain sensitive to a reduction in CD79a and Syk expression [127,132]. BCR ligation on BL cells induces activation of the PI3K pathway, and inhibition of this activation either by directly targeting PI3K or by targeting its downstream target mTORC1 kills these cells [132]. Tonic BCR signals are also present, and are the result of mutations within the transcription factor TCF3 (or E2A) or its negative regulator ID3 [132]. TCF3 normally regulates the expression of the BCR and represses the levels of the phosphatase SHiP1. Thus, mutant forms found in BL lead to increased BCR expression and activation of the PI3K pathway.
• Follicular lymphoma
FL cells are malignant germinal center B cells admixed with nonmalignant cells such as T cells, follicular dentritic cells (FDC), macrophages, fibroblasts and endothelial cells [134,135]. A hallmark of this disease is a t(14:18)(q32:q21) translocation resulting in an overexpression of Bcl-2 and resistance to apoptosis [136]. However, these cells also display high basal levels of active Syk, and resistance to apoptosis is at least partially mediated by PI3K activation [137,138]. It is clear that BCR plays a role in FL disease pathology [139,140], autoreactivity is reported to be a feature of some FL clones [141] and a more recent report has suggested BCR signals can be initiated within malignant cells by interaction of mannosyl residues on surface-expressed BCR with lectins expressed on stromal cells in the tumor microenvironment (Figure 1A) [142,143]. Such antigen-independent signaling then drives expansion of the malignant clone. However, this is not the whole story because there also exist subclones that are unresponsive to BCR crosslinking due to elevated phosphatase activity [142]. Further investigation is required to understand the signaling mechanisms present in these latter clones, and how they contribute to disease.
• Mantle cell lymphoma
The malignant clone in MCL, like that in CLL, expresses a restricted repertoire of BCR genes, and it is thought that BCR signaling plays an important role in the survival of MCL cells and progression of disease (Figure 1A) [144]. A recent report found that BTK was constitutively activated in these cells and BCR crosslinking resulted in sustained activation of Syk. These kinases facilitated BCR-induced survival by mediating the secretion of autocrine factors and inducing adhesion to human bone marrow stromal cells (HMSCs) [145]. Like in CLL, drugs targeting the BCR pathway such as ibrutinib and idelalisib have shown remarkable efficacy also in the treatment of MCL [146].
Targeting BCR signaling pathways
• Proximal BCR signaling pathways
Lyn inhibitors
Lyn is found overexpressed and constitutively active in many B-cell malignancies where it contributes to high basal levels of tyrosine phosphorylated proteins. In CLL, this SFK is thought to contribute to malignant cell survival because experiments using PP2 and SU6656 (compounds which inhibit Lyn [Figure 1A]) induce apoptosis of CLL cells [147]. The pro-survival role of Lyn may be mediated by reported ability to deactivate procaspase-8 through phosphorylation-induced dimerization [148], or by downregulating the tumor suppressor activity of PP2A [149]. Another study has suggested that Lyn plays an important role in CLL progression and lymphoid organ infiltration by malignant cells because of its ability to phosphorylate HS1 and regulate cytoskeletal functionality [150]. However, any conclusions drawn from these studies should be approached with caution because the drugs used to inhibit Lyn (PP2, SU6656 and dasatinib) also affect a broad range of SFKs when used at the concentrations reported [147,151]. Attempts to address the potential role of Lyn in CLL cells have used geldanamycin to disrupt Lyn's association with HSP90. Although such treatment results in downregulation of Lyn expression and the induction of CLL cell apoptosis, mechanisms independent of Lyn may be inducing apoptosis because HSP90 regulates the stability of other proteins that may be important to the survival of CLL cells [152]. Nevertheless, a new study by Kim et al. demonstrated that Lyn could be a promising target for treatment of bortezomib-resistant mantle cell lymphoma (BTZ-resistant MCL). In this study, treatment of these resistant cells with dasatinib caused inhibition of proliferation likely by preventing CD19 binding with Lyn and the p85 regulatory subunit of PI3K [153]. Similar observations were made when Lyn was depleted with siRNA, and therapeutic potential is strongly suggested by xenograft studies showing that tumor size in the mice injected with BTZ-resistant MCL cells can be significantly reduced by dasatinib treatment [153]. In humans there is at least one case report showing that a CLL patient with co-existing chronic myeloid leukemia could be successfully treated with dasatinib [154], suggesting that general targeting of SFKs may be an effective approach. In this light, specific inhibition of Lyn may be a controversial target in the therapy of B-cell malignancies [155,156] because of its role as a positive and negative regulator of BCR signaling [157,158] through its ability to phosphorylate both ITAMs and ITIMs [155]. Specific inhibition of Lyn may block negative feedback of BCR signaling and allow strong signals, perhaps mediated by other SFKs, to take precedence.
Lck inhibitors
An example of an SFK mediating BCR signaling in B-cell malignancies is Lck. This SFK is classically expressed in T cells where it plays an important role in proximal antigen receptor (TCR) signaling. However, this kinase is also present in B1 B-lymphocytes and to participate in BCR signaling [159]. In CLL cells our work has shown that Lck is an important mediator of BCR signaling (Figure 1A), treatment of CLL cells with either a specific Lck inhibitor or Lck-specific siRNA resulted in inhibition of BCR-induced phosphorylation of IKK, ERK and Akt, and a reduction in BCR-mediated survival [110]. This same study also noted that constitutive activation of Lyn was not affected by the inhibitor compound used, indicating that Lck could be a potential target for treatment of CLL because of its proximal role in initiating BCR signaling. Work in this laboratory as well as in others has shown that CLL cells display heterogeneous Lck expression [160,161], and it is suggested that Lck induces CLL resistance to glucocorticoid-induced apoptosis [162]. Although the level of Lck expression dictates the strength of BCR signaling, it is yet unclear how this relates to disease outcome.
Syk inhibitors
Syk plays an important role in both autoimmune diseases and hematological malignancies [163]. Initial small molecule inhibitors targeting Syk were designed for the treatment of inflammatory diseases [164]; however, preclinical studies suggested that Syk could also be a promising target for treatment of B-cell malignancies [102,165–167]. For example, Syk has been shown to be activated in 44% of DLBCL patient samples and responsible for regulating pathological BCR signaling [168], making Syk a valid potential target for the treatment of DLBCL. Syk was also shown to be constitutively active in peripheral B cells from a large number of CLL patients, and inhibition of this kinase is resulted in the induction of apoptosis through a mechanism involving downregulation of Mcl-1 protein levels [102].
Fostamatinib is an orally available prodrug of the active drug R406, and is a relatively selective Syk inhibitor [169]. Treatment of CLL cells with fostamatinib resulted in a reduction in cell migration, adhesion and a moderate induction of apoptosis, achieved by an inhibition of both BCR and integrin signaling [102,165,170]. In vivo studies in CLL and non-Hodgkin lymphoma mouse models supported the therapeutic potential of Syk inhibition in B-cell malignancies [167,171]. In the clinic, 55% of CLL patients treated with fostamatinib achieved partial response compared to 22% of DLBCL, 11% of MCL and 10% in FL patients [172].
Cerdulatinib is a dual Syk/JAK kinase inhibitor that showed activity in both ABC- and GCB-types of DLBCL. This compound induced apoptosis in DLBCL as well as G1/S cell cycle arrest [173]. There are more selective Syk inhibitors currently under devolvement, namely entospletinib, PRT318 and P505–15. These compounds have shown encouraging results in preclinical studies in CLL [174] and in DLBCL [168].
BTK inhibitors
BTK is a nonreceptor protein tyrosine kinase that belongs to TEC family kinases and plays a crucial role in BCR signaling [175]. Loss of function mutations within BTK result in X-linked agammaglobulinemia, a disorder characterized by the absence of mature B lymphocytes and immunodeficiency through lack of antibodies [176]. BTK is an important mediator of BCR-induced calcium flux, NF-κB activation and B-cell proliferation, making it a central player in B-cell physiology [175]. With respect to B-cell malignancies, BTK knockdown studies in ABC-DLBCL cell lines have demonstrated this kinase is essential for tumor growth and cell survival [88]. Signaling through BTK is also essential for the survival and proliferation of the malignant B cells in Waldenström macroglobulinemia (WM), a lymphoplasmacytic lymphoma, where mutation of MYD88 leads to induction of these signals [177]. Furthermore, BTK plays a key role in the malignant behavior of CLL and MCL cells where chronic antigen-stimulation of the BCR facilitates disease progression [144,178–179]. Taken collectively, these studies have been used to justify targeting BTK as a valid therapeutic option to treat B-cell malignancies [180].
Ibrutinib, a first-in-class inhibitor of BTK, is an orally available small molecule that covalently binds to Cys-481 and irreversibly blocks the kinase activity of BTK (Figure 1D). The effect of this blockade is observable also in CLL cells isolated from patients being administered this compound, these cells exhibit reduced levels of PLC-γ and ERK phosphorylation, of expression of genes induced by BCR and NF-κB pathways [181]. Studies of in vitro models that resemble the tumor microenvironment and of in vivo xenografts have shown that ibrutinib inhibits cell proliferation, survival and migration of CLL cells [182,183]. Ibrutinib has pleiotropic inhibitory effects allowing it to attenuate signaling induced by the BCR, CD40, BAFF, TLR and chemokine receptors. This indicates that the therapeutic effect of ibrutinib is likely due to regulation of microenvironmental influences rather than to direct induction of apoptosis. Ibrutinib is also shown to inhibit secretion of the chemokines CCL3 and CCL4 from CLL cells, and reduced serum levels of these chemokines is observed in CLL patients treated with this agent [116,182–183]. An interesting feature of most CLL and MCL patients treated with ibrutinib is that they show transient lymphocytosis due to egress of malignant cells from the protective microenvironment in the lymph nodes [119,184–186], and this is likely due to inhibition of chemokine- and BCR-induced integrin α4β1-mediated adhesion [116–117,187–188].
Clinical trials in relapse or refractory CLL patients have demonstrated high frequency of durable remission even in patients with high risk CLL (e.g., mutated TP53, del17p and del11q) [189]. Ibrutinib has been approved by the US FDA for the treatment of relapsed or refractory MCL and CLL [184] and for WM [190]. However, resistance to ibrutinib in MCL and CLL has been described. One of the mechanisms of ibrutinib resistance is cysteine-to-serine mutation at C481 in the binding site of BTK [191,192] as well as gain-of-function mutations within PLCγ2 [193]. Many other reversible and irreversible BTK inhibitors are still under development to overcome such resistance to improve selectivity and tolerability [180]. Moreover, combination studies are ongoing in order to achieve better and more durable responses to ibrutinib [190].
PI3K inhibitors
PI3K plays an important role in mediating signaling through the BCR, adhesion molecules and chemokine receptors and is therefore key to B-cell survival, migration and proliferation [194]. There are four catalytic isoforms of PI3K: p110α, p110β, p110γ and p110δ. With respect to B cells, the δ isoform is particularly important because PI3Kδ deletion or mutation in mice results in lack of B1 lymphocytes, lower numbers of mature B cells and impaired antibody production [195]. Importantly, PI3Kδ expression is limited to lymphocytes and particular subsets of myeloid lineage cells such as mast cells and neutrophils, and this makes compounds that specifically target this isoform, particularly attractive because the profile of toxicities will be less than what could be expected from pan PI3K inhibitors or inhibitors which target the more ubiquitously expressed α and β isoforms of this protein. In CLL, PI3Kδ has been shown to be constitutively active in the malignant cells [196], and its inhibition reduces malignant cell viability, making targeting of this PI3K isoform using small molecular inhibitors a potential treatment approach [197]. Idelalisib (formerly GS-1101 or CAL-101) is a highly selective inhibitor of PI3Kδ [197] which has an efficacy that has allowed fast-track approval for the treatment of relapsed and refractory CLL in USA (Figure 1C) [198]. Idelalisib is reported to inhibit the prosurvival effect of BCR stimulation in CLL cells [199], and its cytotoxic effect is dose- and time-dependent being mediated by induction of the caspase pathway regardless of p53 or IGHV mutation status. Idelalisib treatment also leads to blocking several microenvironmental signals such as CD40L, TNF-α, BAFF, ET1, fibronectin adhesion and nurse-like and stromal cell contact [199–201], likely by also affecting cells such as NK and T cells which produce cytokines known to promote CLL cells survival and proliferation such as TNF-α, CD40L, IL-6 and IFN-γ [200,202–203]. Clinically, CLL patients treated with Idelalisib exhibit lymphocytosis due to redistribution of CLL cells from lymphoid tissues to peripheral blood [204,205]. This redistribution may be caused by reduction in PI3K-mediated cell adhesion and migration [120,199], or by re-expression of the receptor for sphingosine-1 phosphate and induction of lymphocyte egress from lymph nodes/proliferation centers as has been recently suggested [118]. In terms of adverse drug reactions idelalisib has been linked to a characteristic set including, most notably, diarrhea/colitis, increased liver transaminases, skin rash and pneumonitis. Very little is currently known about the biological basis of these adverse drug reactions because they affect only a subset of patients. Thus, more research is needed so that patients at risk of developing these adverse drug reactions can be identified, and methodologies to prevent them introduced.
Future perspective
BCR pathway inhibitors have had remarkable success in the treatment of B-cell malignancies, particularly for patients displaying resistance to conventional chemotherapeutic agents. What is more, these small molecule inhibitors offer a less toxic alternative to conventional chemotherapy and only minor side effects are reported with their use. However, long-term treatment with these molecules may prove more toxic than previously thought. For example, US prescribing information for idelalisib contains a black box risk for serious/fatal diarrhea [198,206]. Moreover, drug resistance of the type observed with the usage of ibrutinib is also a problem [191–193]. To combat the problems associated with toxicity, new-generation drugs against PI3Kδ, such as duvalisib, and BTK, such as acalabrutinib, are either in development or have been introduced into the clinic [207,208]. Furthermore, new targets can be developed within this pathway, and our work has suggested Lck as one target for the treatment of CLL [110]. E3 ubiquitin ligases are also potential targets, recent research has begun to reveal roles for these proteins in B lymphomagenesis [209–211]. Clearly, investigation into BCR signaling and compounds which inhibit this pathway requires ongoing investigation, and it is likely that any inhibitors used will be employed within combinatorial therapeutic approaches in order to increase their efficacy.
Footnotes
Financial & competing interests disclosure
F Talab is an employee of Redx Oncology Plc. JR Slupsky is in receipt of grant support from Bloodwise and the NWCRF. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Monroe JG. ITAM-mediated tonic signaling through pre-BCR and BCR complexes. Nat. Rev. Immunol. 2006;6(4):283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 2.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Cariappa A, Pillai S. Antigen-dependent B-cell development. Curr. Opin. Immunol. 2002;14(2):241–249. doi: 10.1016/s0952-7915(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 4.Pierce SK, Liu W. The tipping points in the initiation of B cell signaling: how small changes make big differences. Nat. Rev. Immunol. 2010;10(11):767–777. doi: 10.1038/nri2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corfe SA, Paige CJ. The many roles of IL-7 in B cell development; mediator of survival, proliferation and differentiation. Semin. Immunol. 2012;24(3):198–208. doi: 10.1016/j.smim.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Goodnow CC, Crosbie J, Adelstein S, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. J. Immunol. 2009;183(9):5442–5448. [PubMed] [Google Scholar]
- 7.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342(6248):385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 8.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353(6346):765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 9.Rickert RC. New insights into pre-BCR and BCR signaling with relevance to B cell malignancies. Nat. Rev. Immunol. 2013;13(8):578–591. doi: 10.1038/nri3487. [DOI] [PubMed] [Google Scholar]
- 10.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 11.Niiro H, Clark EA. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2002;2(12):945–956. doi: 10.1038/nri955. [DOI] [PubMed] [Google Scholar]
- 12.Bojarczuk K, Bobrowicz M, Dwojak M, et al. B-cell receptor signaling in the pathogenesis of lymphoid malignancies. Blood Cells Mol. Dis. 2015;55(3):255–265. doi: 10.1016/j.bcmd.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AC, Mitchell RN, Weaver YK, Campos-Torres J, Abbas AK, Leder P. Mutations of immunoglobulin transmembrane and cytoplasmic domains: effects on intracellular signaling and antigen presentation. Cell. 1990;63(2):381–392. doi: 10.1016/0092-8674(90)90171-a. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez M, Misulovin Z, Burkhardt AL, et al. Signal transduction by immunoglobulin is mediated through Ig alpha and Ig beta. J. Exp. Med. 1993;178(3):1049–1055. doi: 10.1084/jem.178.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams GT, Peaker CJ, Patel KJ, Neuberger MS. The alpha/beta sheath and its cytoplasmic tyrosines are required for signaling by the B-cell antigen receptor but not for capping or for serine/threonine-kinase recruitment. Proc. Natl Acad. Sci. USA. 1994;91(2):474–478. doi: 10.1073/pnas.91.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ketchum C, Miller H, Song W, Upadhyaya A. Ligand mobility regulates B cell receptor clustering and signaling activation. Biophys. J. 2014;106(1):26–36. doi: 10.1016/j.bpj.2013.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng PC, Cherukuri A, Dykstra M, et al. Floating the raft hypothesis: the roles of lipid rafts in B cell antigen receptor function. Semin. Immunol. 2001;13(2):107–114. doi: 10.1006/smim.2000.0302. [DOI] [PubMed] [Google Scholar]
- 18.Stepanek O, Draber P, Drobek A, Horejsi V, Brdicka T. Nonredundant roles of Src-family kinases and Syk in the initiation of B-cell antigen receptor signaling. J. Immunol. 2013;190(4):1807–1818. doi: 10.4049/jimmunol.1202401. [DOI] [PubMed] [Google Scholar]
- 19.Yao XR, Flaswinkel H, Reth M, Scott DW. Immunoreceptor tyrosine-based activation motif is required to signal pathways of receptor-mediated growth arrest and apoptosis in murine B lymphoma cells. J. Immunol. 1995;155(2):652–661. [PubMed] [Google Scholar]
- 20.Kurosaki T, Johnson SA, Pao L, Sada K, Yamamura H, Cambier JC. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J. Exp. Med. 1995;182(6):1815–1823. doi: 10.1084/jem.182.6.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowley RB, Burkhardt AL, Chao HG, Matsueda GR, Bolen JB. Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/Ig beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 1995;270(19):11590–11594. doi: 10.1074/jbc.270.19.11590. [DOI] [PubMed] [Google Scholar]
- 22.Kabak S, Skaggs BJ, Gold MR, et al. The direct recruitment of BLNK to immunoglobulin alpha couples the B-cell antigen receptor to distal signaling pathways. Mol. Cell. Biol. 2002;22(8):2524–2535. doi: 10.1128/MCB.22.8.2524-2535.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castello A, Gaya M, Tucholski J, et al. Nck-mediated recruitment of BCAP to the BCR regulates the PI(3)K-Akt pathway in B cells. Nat. Immunol. 2013;14(9):966–975. doi: 10.1038/ni.2685. [DOI] [PubMed] [Google Scholar]
- 24.Malhotra S, Kovats S, Zhang W, Coggeshall KM. Vav and Rac activation in B cell antigen receptor endocytosis involves Vav recruitment to the adapter protein LAB. J. Biol. Chem. 2009;284(52):36202–36212. doi: 10.1074/jbc.M109.040089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leu CM. Nck, a missing adaptor between the B-cell receptor complex and the BCAP/PI3K/Akt pathway. Cell. Mol. Immunol. 2014;11(2):120–122. doi: 10.1038/cmi.2013.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okada T, Maeda A, Iwamatsu A, Gotoh K, Kurosaki T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity. 2000;13(6):817–827. doi: 10.1016/s1074-7613(00)00079-0. [DOI] [PubMed] [Google Scholar]
- 27.Carter RH, Doody GM, Bolen JB, Fearon DT. Membrane IgM-induced tyrosine phosphorylation of CD19 requires a CD19 domain that mediates association with components of the B cell antigen receptor complex. J. Immunol. 1997;158(7):3062–3069. [PubMed] [Google Scholar]
- 28.Tuveson DA, Carter RH, Soltoff SP, Fearon DT. CD19 of B cells as a surrogate kinase insert region to bind phosphatidylinositol 3-kinase. Science. 1993;260(5110):986–989. doi: 10.1126/science.7684160. [DOI] [PubMed] [Google Scholar]
- 29.Buhl AM, Pleiman CM, Rickert RC, Cambier JC. Qualitative regulation of B cell antigen receptor signaling by CD19: selective requirement for PI3-kinase activation, inositol-1,4,5-trisphosphate production and Ca2+ mobilization. J. Exp. Med. 1997;186(11):1897–1910. doi: 10.1084/jem.186.11.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srinivasan L, Sasaki Y, Calado DP, et al. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139(3):573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambier JC, Johnson SA. Differential binding activity of ARH1/TAM motifs. Immunol. Lett. 1995;44(2–3):77–80. doi: 10.1016/0165-2478(94)00196-x. [DOI] [PubMed] [Google Scholar]
- 32.Delgado P, Cubelos B, Calleja E, et al. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nat. Immunol. 2009;10(8):880–888. doi: 10.1038/ni.1749. [DOI] [PubMed] [Google Scholar]
- 33.Baba Y, Hashimoto S, Matsushita M, et al. BLNK mediates Syk-dependent Btk activation. Proc. Natl Acad. Sci. USA. 2001;98(5):2582–2586. doi: 10.1073/pnas.051626198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park H, Wahl MI, Afar DE, et al. Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity. 1996;4(5):515–525. doi: 10.1016/s1074-7613(00)80417-3. [DOI] [PubMed] [Google Scholar]
- 35.Kim YJ, Sekiya F, Poulin B, Bae YS, Rhee SG. Mechanism of B-cell receptor-induced phosphorylation and activation of phospholipase C-gamma2. Mol. Cell. Biol. 2004;24(22):9986–9999. doi: 10.1128/MCB.24.22.9986-9999.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johmura S, Oh-hora M, Inabe K, et al. Regulation of Vav localization in membrane rafts by adaptor molecules Grb2 and BLNK. Immunity. 2003;18(6):777–787. doi: 10.1016/s1074-7613(03)00139-0. [DOI] [PubMed] [Google Scholar]
- 37.Fu C, Turck CW, Kurosaki T, Chan AC. BLNK: a central linker protein in B cell activation. Immunity. 1998;9(1):93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 38.Deckert M, Tartare-Deckert S, Couture C, Mustelin T, Altman A. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity. 1996;5(6):591–604. doi: 10.1016/s1074-7613(00)80273-3. [DOI] [PubMed] [Google Scholar]
- 39.Yang G, Murashige DS, Humphrey SJ, James DE. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Rep. 2015;12(6):937–943. doi: 10.1016/j.celrep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Newton AC. Protein kinase C: poised to signal. Am. J. Physiol. Endocrinol. Metab. 2010;298(3):E395–E402. doi: 10.1152/ajpendo.00477.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coughlin JJ, Stang SL, Dower NA, Stone JC. RasGRP1 and RasGRP3 regulate B cell proliferation by facilitating B cell receptor-Ras signaling. J. Immunol. 2005;175(11):7179–7184. doi: 10.4049/jimmunol.175.11.7179. [DOI] [PubMed] [Google Scholar]
- 42.Stone JC. Regulation and function of the RasGRP family of Ras activators in blood cells. Genes Cancer. 2011;2(3):320–334. doi: 10.1177/1947601911408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roose JP, Mollenauer M, Ho M, Kurosaki T, Weiss A. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol. Cell. Biol. 2007;27(7):2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sommer K, Guo B, Pomerantz JL, et al. Phosphorylation of the CARMA1 linker controls NF-kappaB activation. Immunity. 2005;23(6):561–574. doi: 10.1016/j.immuni.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Shinohara H, Yasuda T, Aiba Y, et al. PKC beta regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med. 2005;202(10):1423–1431. doi: 10.1084/jem.20051591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosebeck S, Rehman AO, Lucas PC, McAllister-Lucas LM. From MALT lymphoma to the CBM signalosome: three decades of discovery. Cell Cycle. 2011;10(15):2485–2496. doi: 10.4161/cc.10.15.16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen ZJ. TAK1 is a ubiquitin-dependent kinase of MKK and IKK. Nature. 2001;412(6844):346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki H, Chiba T, Kobayashi M, Takeuchi M, Furuichi K, Tanaka K. In vivo and in vitro recruitment of an IkappaBalpha-ubiquitin ligase to IkappaBalpha phosphorylated by IKK, leading to ubiquitination. Biochem. Biophys. Res. Commun. 1999;256(1):121–126. doi: 10.1006/bbrc.1999.0296. [DOI] [PubMed] [Google Scholar]
- 49.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl.):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Zhang F, Chen F, et al. MEKK3 regulates IFN-gamma production in T cells through the Rac1/2-dependent MAPK cascades. J. Immunol. 2011;186(10):5791–5800. doi: 10.4049/jimmunol.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81(7):1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 52.Gold MR, Scheid MP, Santos L, et al. The B cell antigen receptor activates the Akt (protein kinase B)/glycogen synthase kinase-3 signaling pathway via phosphatidylinositol 3-kinase. J. Immunol. 1999;163(4):1894–1905. [PubMed] [Google Scholar]
- 53.Limon JJ, Fruman DA. Akt and mTOR in B cell activation and differentiation. Front. Immunol. 2012;3:228. doi: 10.3389/fimmu.2012.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim. Biophys. Acta. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 55.Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A. Structurally distinct phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity. 2008;28(2):183–196. doi: 10.1016/j.immuni.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi H, Hendrickson WA. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature. 1996;384(6608):484–489. doi: 10.1038/384484a0. [DOI] [PubMed] [Google Scholar]
- 57.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 58.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383(6597):263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Koizumi T, Watanabe T. Altered antigen receptor signaling and impaired Fas-mediated apoptosis of B cells in Lyn-deficient mice. J. Exp. Med. 1996;184(3):831–838. doi: 10.1084/jem.184.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J. Exp. Med. 1998;187(5):807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)-mediated inhibitory signal. J. Exp. Med. 1998;187(8):1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adachi T, Flaswinkel H, Yakura H, Reth M, Tsubata T. The B cell surface protein CD72 recruits the tyrosine phosphatase SHP-1 upon tyrosine phosphorylation. J. Immunol. 1998;160(10):4662–4665. [PubMed] [Google Scholar]
- 63.Cloutier JF, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15(18):4909–4918. [PMC free article] [PubMed] [Google Scholar]
- 64.Cloutier JF, Veillette A. Cooperative inhibition of T-cell antigen receptor signaling by a complex between a kinase and a phosphatase. J. Exp. Med. 1999;189(1):111–121. doi: 10.1084/jem.189.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ingley E. Src family kinases: regulation of their activities, levels and identification of new pathways. Biochim. Biophys. Acta. 2008;1784(1):56–65. doi: 10.1016/j.bbapap.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Cohen S, Dadi H, Shaoul E, Sharfe N, Roifman CM. Cloning and characterization of a lymphoid-specific, inducible human protein tyrosine phosphatase, Lyp. Blood. 1999;93(6):2013–2024. [PubMed] [Google Scholar]
- 67.Sohn HW, Gu H, Pierce SK. Cbl-b negatively regulates B cell antigen receptor signaling in mature B cells through ubiquitination of the tyrosine kinase Syk. J. Exp. Med. 2003;197(11):1511–1524. doi: 10.1084/jem.20021686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shao Y, Yang C, Elly C, Liu YC. Differential regulation of the B cell receptor-mediated signaling by the E3 ubiquitin ligase Cbl. J. Biol. Chem. 2004;279(42):43646–43653. doi: 10.1074/jbc.M404082200. [DOI] [PubMed] [Google Scholar]
- 69.Katkere B, Rosa S, Drake JR. The Syk-binding ubiquitin ligase c-Cbl mediates signaling-dependent B cell receptor ubiquitination and B cell receptor-mediated antigen processing and presentation. J. Biol. Chem. 2012;287(20):16636–16644. doi: 10.1074/jbc.M112.357640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yasuda T, Maeda A, Kurosaki M, et al. Cbl suppresses B cell receptor-mediated phospholipase C (PLC)-γ2 activation by regulating B cell linker protein-PLC-γ2 binding. J. Exp. Med. 2000;191(4):641–650. doi: 10.1084/jem.191.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem. Sci. 2002;27(9):462–467. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 72.Brauweiler A, Tamir I, Dal Porto J, et al. Differential regulation of B cell development, activation, and death by the src homology 2 domain-containing 5′ inositol phosphatase (SHIP) J. Exp. Med. 2000;191(9):1545–1554. doi: 10.1084/jem.191.9.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vivier E, Daeron M. Immunoreceptor tyrosine-based inhibition motifs. Immunol. Today. 1997;18(6):286–291. doi: 10.1016/s0167-5699(97)80025-4. [DOI] [PubMed] [Google Scholar]
- 74.Seda V, Mraz M. B-cell receptor signaling and its crosstalk with other pathways in normal and malignant cells. Eur. J. Haematol. 2015;94(3):193–205. doi: 10.1111/ejh.12427. [DOI] [PubMed] [Google Scholar]
- 75.Pao LI, Badour K, Siminovitch KA, Neel BG. Nonreceptor protein-tyrosine phosphatases in immune cell signaling. Annu. Rev. Immunol. 2007;25:473–523. doi: 10.1146/annurev.immunol.23.021704.115647. [DOI] [PubMed] [Google Scholar]
- 76.Kang SW, Wahl MI, Chu J, et al. PKCβ modulates antigen receptor signaling via regulation of Btk membrane localization. EMBO J. 2001;20(20):5692–5702. doi: 10.1093/emboj/20.20.5692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohammad DK, Nore BF, Hussain A, Gustafsson MO, Mohamed AJ, Smith CI. Dual phosphorylation of Btk by Akt/protein kinase b provides docking for 14-13-3zeta, regulates shuttling, and attenuates both tonic and induced signaling in B cells. Mol. Cell. Biol. 2013;33(16):3214–3226. doi: 10.1128/MCB.00247-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moreno-Garcia ME, Sommer K, Rincon-Arano H, et al. Kinase-independent feedback of the TAK1/TAB1 complex on BCL10 turnover and NF-kappaB activation. Mol. Cell. Biol. 2013;33(6):1149–1163. doi: 10.1128/MCB.06407-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Herishanu Y, Perez-Galan P, Liu D, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117(2):563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fais F, Ghiotto F, Hashimoto S, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J. Clin. Invest. 1998;102(8):1515–1525. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tobin G, Thunberg U, Karlsson K, et al. Subsets with restricted immunoglobulin gene rearrangement features indicate a role for antigen selection in the development of chronic lymphocytic leukemia. Blood. 2004;104(9):2879–2885. doi: 10.1182/blood-2004-01-0132. [DOI] [PubMed] [Google Scholar]
- 82.Agathangelidis A, Darzentas N, Hadzidimitriou A, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119(19):4467–4475. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Messmer BT, Albesiano E, Efremov DG, et al. Multiple distinct sets of stereotyped antigen receptors indicate a role for antigen in promoting chronic lymphocytic leukemia. J. Exp. Med. 2004;200(4):519–525. doi: 10.1084/jem.20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hadzidimitriou A, Agathangelidis A, Darzentas N, et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood. 2011;118(11):3088–3095. doi: 10.1182/blood-2011-03-343434. [DOI] [PubMed] [Google Scholar]
- 85.Thelander EF, Rosenquist R. Molecular genetic characterization reveals new subsets of mantle cell lymphoma. Leuk. Lymphoma. 2008;49(6):1042–1049. doi: 10.1080/10428190801947559. [DOI] [PubMed] [Google Scholar]
- 86.Martinez N, Camacho FI, Algara P, et al. The molecular signature of mantle cell lymphoma reveals multiple signals favoring cell survival. Cancer Res. 2003;63(23):8226–8232. [PubMed] [Google Scholar]
- 87.Rizzatti EG, Falcao RP, Panepucci RA, et al. Gene expression profiling of mantle cell lymphoma cells reveals aberrant expression of genes from the PI3K-AKT, WNT and TGFbeta signaling pathways. Br. J. Haematol. 2005;130(4):516–526. doi: 10.1111/j.1365-2141.2005.05630.x. [DOI] [PubMed] [Google Scholar]
- 88.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signaling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sachen KL, Strohman MJ, Singletary J, et al. Self-antigen recognition by follicular lymphoma B-cell receptors. Blood. 2012;120(20):4182–4190. doi: 10.1182/blood-2012-05-427534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arons E, Roth L, Sapolsky J, Suntum T, Stetler-Stevenson M, Kreitman RJ. Evidence of canonical somatic hypermutation in hairy cell leukemia. Blood. 2011;117(18):4844–4851. doi: 10.1182/blood-2010-11-316737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forconi F, Cencini E, Sicuranza A, Sozzi E, Lauria F. Molecular insight into the biology and clinical course of hairy cell leukemia utilizing immunoglobulin gene analysis. Leuk. Lymphoma. 2011;52(1):15–23. doi: 10.3109/10428194.2010.530362. [DOI] [PubMed] [Google Scholar]
- 92.Schmitz R, Ceribelli M, Pittaluga S, Wright G, Staudt LM. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb. Perspect. Med. 2014;4(2) doi: 10.1101/cshperspect.a014282. pii:a014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varettoni M, Zibellini S, Capello D, et al. Clues to pathogenesis of Waldenstrom macroglobulinemia and immunoglobulin M monoclonal gammopathy of undetermined significance provided by analysis of immunoglobulin heavy chain gene rearrangement and clustering of B-cell receptors. Leuk. Lymphoma. 2013;54(11):2485–2489. doi: 10.3109/10428194.2013.779689. [DOI] [PubMed] [Google Scholar]
- 94.Yan Q, Huang Y, Watkins AJ, et al. BCR and TLR signaling pathways are recurrently targeted by genetic changes in splenic marginal zone lymphomas. Haematologica. 2012;97(4):595–598. doi: 10.3324/haematol.2011.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melo JV. The diversity of BCR–ABL fusion proteins and their relationship to leukemia phenotype. Blood. 1996;88(7):2375–2384. [PubMed] [Google Scholar]
- 96.Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat. Immunol. 2009;10(12):1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 97.Damle RN, Wasil T, Fais F, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–1847. [PubMed] [Google Scholar]
- 98.Catera R, Silverman GJ, Hatzi K, et al. Chronic lymphocytic leukemia cells recognize conserved epitopes associated with apoptosis and oxidation. Mol. Med. 2008;14(11–12):665–674. doi: 10.2119/2008-00102.Catera. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoogeboom R, van Kessel KP, Hochstenbach F, et al. A mutated B cell chronic lymphocytic leukemia subset that recognizes and responds to fungi. J. Exp. Med. 2013;210(1):59–70. doi: 10.1084/jem.20121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chu CC, Catera R, Hatzi K, et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood. 2008;112(13):5122–5129. doi: 10.1182/blood-2008-06-162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Binder M, Lechenne B, Ummanni R, et al. Stereotypical chronic lymphocytic leukemia B-cell receptors recognize survival promoting antigens on stromal cells. PLoS ONE. 2010;5(12):e15992. doi: 10.1371/journal.pone.0015992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gobessi S, Laurenti L, Longo PG, et al. Inhibition of constitutive and BCR-induced Syk activation downregulates Mcl-1 and induces apoptosis in chronic lymphocytic leukemia B cells. Leukemia. 2009;23(4):686–697. doi: 10.1038/leu.2008.346. [DOI] [PubMed] [Google Scholar]
- 103.Negro R, Gobessi S, Longo PG, et al. Overexpression of the autoimmunity-associated phosphatase PTPN22 promotes survival of antigen-stimulated CLL cells by selectively activating AKT. Blood. 2012;119(26):6278–6287. doi: 10.1182/blood-2012-01-403162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lanham S, Hamblin T, Oscier D, Ibbotson R, Stevenson F, Packham G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood. 2003;101(3):1087–1093. doi: 10.1182/blood-2002-06-1822. [DOI] [PubMed] [Google Scholar]
- 105.Allsup DJ, Kamiguti AS, Lin K, et al. B-cell receptor translocation to lipid rafts and associated signaling differ between prognostically important subgroups of chronic lymphocytic leukemia. Cancer Res. 2005;65(16):7328–7337. doi: 10.1158/0008-5472.CAN-03-1563. [DOI] [PubMed] [Google Scholar]
- 106.Wiestner A, Rosenwald A, Barry TS, et al. ZAP-70 expression identifies a chronic lymphocytic leukemia subtype with unmutated immunoglobulin genes, inferior clinical outcome, and distinct gene expression profile. Blood. 2003;101(12):4944–4951. doi: 10.1182/blood-2002-10-3306. [DOI] [PubMed] [Google Scholar]
- 107.Chen L, Widhopf G, Huynh L, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002;100(13):4609–4614. doi: 10.1182/blood-2002-06-1683. [DOI] [PubMed] [Google Scholar]
- 108.Chen L, Huynh L, Apgar J, et al. ZAP-70 enhances IgM signaling independent of its kinase activity in chronic lymphocytic leukemia. Blood. 2008;111(5):2685–2692. doi: 10.1182/blood-2006-12-062265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gobessi S, Laurenti L, Longo PG, Sica S, Leone G, Efremov DG. ZAP-70 enhances B-cell-receptor signaling despite absent or inefficient tyrosine kinase activation in chronic lymphocytic leukemia and lymphoma B cells. Blood. 2007;109(5):2032–2039. doi: 10.1182/blood-2006-03-011759. [DOI] [PubMed] [Google Scholar]
- 110.Talab F, Allen JC, Thompson V, Lin K, Slupsky JR. LCK is an important mediator of B-cell receptor signaling in chronic lymphocytic leukemia cells. Mol. Cancer Res. 2013;11(5):541–554. doi: 10.1158/1541-7786.MCR-12-0415-T. [DOI] [PubMed] [Google Scholar]; •• It is the first paper to discuss and confirm the importance of Lck in B-cell receptor (BCR) signaling in chronic lymphocytic leukemia.
- 111.Petlickovski A, Laurenti L, Li X, et al. Sustained signaling through the B-cell receptor induces Mcl-1 and promotes survival of chronic lymphocytic leukemia B cells. Blood. 2005;105(12):4820–4827. doi: 10.1182/blood-2004-07-2669. [DOI] [PubMed] [Google Scholar]
- 112.Duhren-von Minden M, Ubelhart R, Schneider D, et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signaling. Nature. 2012;489(7415):309–312. doi: 10.1038/nature11309. [DOI] [PubMed] [Google Scholar]; •• The first to describe a potential role of cell-autonomous signaling in driving disease pathogenesis in chronic lymphocytic leukemia.
- 113.Packham G, Krysov S, Allen A, et al. The outcome of B-cell receptor signaling in chronic lymphocytic leukemia: proliferation or anergy. Haematologica. 2014;99(7):1138–1148. doi: 10.3324/haematol.2013.098384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Apollonio B, Scielzo C, Bertilaccio MT, et al. Targeting B-cell anergy in chronic lymphocytic leukemia. Blood. 2013;121(19):3879–3888. doi: 10.1182/blood-2012-12-474718. S3871–S3878. [DOI] [PubMed] [Google Scholar]
- 115.Muzio M, Apollonio B, Scielzo C, et al. Constitutive activation of distinct BCR-signaling pathways in a subset of CLL patients: a molecular signature of anergy. Blood. 2008;112(1):188–195. doi: 10.1182/blood-2007-09-111344. [DOI] [PubMed] [Google Scholar]
- 116.de Rooij MFM, Kuil A, Geest CR, et al. The clinically active BTK inhibitor PCI-32765 targets B-cell receptor- and chemokine-controlled adhesion and migration in chronic lymphocytic leukemia. Blood. 2012;119(11):2590–2594. doi: 10.1182/blood-2011-11-390989. [DOI] [PubMed] [Google Scholar]
- 117.Spaargaren M, Beuling EA, Rurup ML, et al. The B cell antigen receptor controls integrin activity through Btk and PLCγ2. J. Exp. Med. 2003;198(10):1539–1550. doi: 10.1084/jem.20011866. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Reveals a novel function for the BCR and Btk in regulating integrin α4β1 activity.
- 118.Till KJ, Pettitt AR, Slupsky JR. Expression of functional sphingosine-1-phosphate receptor 1 is increased by idelalisib but not fostamatinib or ibrutinib in chronic lymphocytic leukaemia cells. J. Immunol. 2015;194(5):2439–2446. doi: 10.4049/jimmunol.1402304. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Explains the different causes of lymphocytosis in patient treated with idelalisib, ibrutinib and fostamatinib.
- 119.Woyach JA, Smucker K, Smith LL, et al. Prolonged lymphocytosis during ibrutinib therapy is associated with distinct molecular characteristics and does not indicate a suboptimal response to therapy. Blood. 2014;123(12):1810–1817. doi: 10.1182/blood-2013-09-527853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fiorcari S, Brown WS, McIntyre BW, et al. The PI3-kinase delta inhibitor idelalisib (GS-1101) targets integrin-mediated adhesion of chronic lymphocytic leukemia (CLL) cell to endothelial and marrow stromal cells. PLoS ONE. 2013;8(12):e83830. doi: 10.1371/journal.pone.0083830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Monti S, Savage KJ, Kutok JL, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105(5):1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 122.Tolar P, Sohn HW, Liu W, Pierce SK. The molecular assembly and organization of signaling active B-cell receptor oligomers. Immunol. Rev. 2009;232(1):34–41. doi: 10.1111/j.1600-065X.2009.00833.x. [DOI] [PubMed] [Google Scholar]
- 123.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 2001;194(12):1861–1874. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lenz G, Davis RE, Ngo VN, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008;319(5870):1676–1679. doi: 10.1126/science.1153629. [DOI] [PubMed] [Google Scholar]
- 125.Thomas RK, Wickenhauser C, Tawadros S, et al. Mutational analysis of the IkappaBalpha gene in activated B cell-like diffuse large B-cell lymphoma. Br. J. Haematol. 2004;126(1):50–54. doi: 10.1111/j.1365-2141.2004.05000.x. [DOI] [PubMed] [Google Scholar]
- 126.Ngo VN, Davis RE, Lamy L, et al. A loss-of-function RNA interference screen for molecular targets in cancer. Nature. 2006;441(7089):106–110. doi: 10.1038/nature04687. [DOI] [PubMed] [Google Scholar]
- 127.Young RM, Staudt LM. Targeting pathological B cell receptor signaling in lymphoid malignancies. Nat. Rev. Drug Discov. 2013;12(3):229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470(7332):115–119. doi: 10.1038/nature09671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lim KH, Yang Y, Staudt LM. Pathogenetic importance and therapeutic implications of NF-kappaB in lymphoid malignancies. Immunol. Rev. 2012;246(1):359–378. doi: 10.1111/j.1600-065X.2012.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Puente XS, Pinyol M, Quesada V, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–105. doi: 10.1038/nature10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wagner M, Oelsner M, Moore A, et al. Integration of innate into adaptive immune responses in ZAP-70 positive chronic lymphocytic leukaemia. Blood. 2016;127(4):436–448. doi: 10.1182/blood-2015-05-646935. [DOI] [PubMed] [Google Scholar]
- 132.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature. 2012;490(7418):116–120. doi: 10.1038/nature11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell. 2012;22(2):167–179. doi: 10.1016/j.ccr.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kridel R, Sehn LH, Gascoyne RD. Pathogenesis of follicular lymphoma. J. Clin. Invest. 2012;122(10):3424–3431. doi: 10.1172/JCI63186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stevenson FK, Stevenson GT. Follicular lymphoma and the immune system: from pathogenesis to antibody therapy. Blood. 2012;119(16):3659–3667. doi: 10.1182/blood-2011-11-367730. [DOI] [PubMed] [Google Scholar]
- 136.Hiddemann W, Cheson BD. How we manage follicular lymphoma. Leukemia. 2014;28(7):1388–1395. doi: 10.1038/leu.2014.91. [DOI] [PubMed] [Google Scholar]
- 137.Leseux L, Hamdi SM, Al Saati T, et al. Syk-dependent mTOR activation in follicular lymphoma cells. Blood. 2006;108(13):4156–4162. doi: 10.1182/blood-2006-05-026203. [DOI] [PubMed] [Google Scholar]
- 138.Gulmann C, Espina V, Petricoin E, et al. Proteomic analysis of apoptotic pathways reveals prognostic factors in follicular lymphoma. Clin. Cancer Res. 2005;11(16):5847–5855. doi: 10.1158/1078-0432.CCR-05-0637. [DOI] [PubMed] [Google Scholar]
- 139.Zuckerman NS, McCann KJ, Ottensmeier CH, et al. Ig gene diversification and selection in follicular lymphoma, diffuse large B cell lymphoma and primary central nervous system lymphoma revealed by lineage tree and mutation analyses. Int. Immunol. 2010;22(11):875–887. doi: 10.1093/intimm/dxq441. [DOI] [PubMed] [Google Scholar]
- 140.Bahler DW, Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc. Natl Acad. Sci. USA. 1992;89(15):6770–6774. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuppers R. Mechanisms of B-cell lymphoma pathogenesis. Nat. Rev. Cancer. 2005;5(4):251–262. doi: 10.1038/nrc1589. [DOI] [PubMed] [Google Scholar]
- 142.Amin R, Mourcin F, Uhel F, et al. DC-SIGN-expressing macrophages trigger activation of mannosylated IgM B-cell receptor in follicular lymphoma. Blood. 2015;126(16):1911–1920. doi: 10.1182/blood-2015-04-640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Linley A, Krysov S, Ponzoni M, Johnson PW, Packham G, Stevenson FK. Lectin binding to surface Ig variable regions provides a universal persistent activating signal for follicular lymphoma cells. Blood. 2015;126(16):1902–1910. doi: 10.1182/blood-2015-04-640805. [DOI] [PubMed] [Google Scholar]; • Describes the mechanism responsible for constitutive antigen receptor signaling in follicular lymphoma.
- 144.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J. Clin. Invest. 2012;122(10):3416–3423. doi: 10.1172/JCI61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bernard S, Danglade D, Gardano L, et al. Inhibitors of BCR signaling interrupt the survival signal mediated by the micro-environment in mantle cell lymphoma. Int. J. Cancer. 2015;136(12):2761–2774. doi: 10.1002/ijc.29326. [DOI] [PubMed] [Google Scholar]
- 146.Campo E, Rule S. Mantle cell lymphoma: evolving management strategies. Blood. 2015;125(1):48–55. doi: 10.1182/blood-2014-05-521898. [DOI] [PubMed] [Google Scholar]
- 147.Contri A, Brunati AM, Trentin L, et al. Chronic lymphocytic leukemia B cells contain anomalous Lyn tyrosine kinase, a putative contribution to defective apoptosis. J. Clin. Invest. 2005;115(2):369–378. doi: 10.1172/JCI22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zonta F, Pagano MA, Trentin L, et al. Lyn-mediated procaspase 8 dimerization blocks apoptotic signaling in B-cell chronic lymphocytic leukemia. Blood. 2014;123(6):875–883. doi: 10.1182/blood-2013-02-485540. [DOI] [PubMed] [Google Scholar]
- 149.Zonta F, Pagano MA, Trentin L, et al. Lyn sustains oncogenic signaling in chronic lymphocytic leukemia by strengthening SET-mediated inhibition of PP2A. Blood. 2015;125(24):3747–3755. doi: 10.1182/blood-2014-12-619155. [DOI] [PubMed] [Google Scholar]
- 150.ten Hacken E, Scielzo C, Bertilaccio MTS, et al. Targeting the LYN/HS1 signaling axis in chronic lymphocytic leukemia. Blood. 2013;121(12):2264–2273. doi: 10.1182/blood-2012-09-457119. [DOI] [PubMed] [Google Scholar]
- 151.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 2007;408(3):297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Trentin L, Frasson M, Donella-Deana A, et al. Geldanamycin-induced Lyn dissociation from aberrant Hsp90-stabilized cytosolic complex is an early event in apoptotic mechanisms in B-chronic lymphocytic leukemia. Blood. 2008;112(12):4665–4674. doi: 10.1182/blood-2008-02-139139. [DOI] [PubMed] [Google Scholar]
- 153.Kim A, Seong KM, Kang HJ, Park S, Lee SS. Inhibition of Lyn is a promising treatment for mantle cell lymphoma with bortezomib resistance. Oncotarget. 2015;6(35):38225–38238. doi: 10.18632/oncotarget.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nagao T, Takahashi N, Kameoka Y, et al. Dasatinib-responsive chronic lymphocytic leukemia in a patient treated for coexisting chronic myeloid leukemia. Intern. Med. 2013;52(22):2567–2571. doi: 10.2169/internalmedicine.52.0392. [DOI] [PubMed] [Google Scholar]
- 155.Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22(1):9–18. doi: 10.1016/j.immuni.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 156.Ingley E. Functions of the Lyn tyrosine kinase in health and disease. Cell Commun. Signal. 2012;10(1):21. doi: 10.1186/1478-811X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hibbs ML, Harder KW, Armes J, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J. Exp. Med. 2002;196(12):1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hibbs ML, Tarlinton DM, Armes J, et al. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83(2):301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 159.Ulivieri C, Valensin S, Majolini MB, Matthews RJ, Baldari CT. Normal B-1 cell development but defective BCR signaling in Lck-/- mice. Eur. J. Immunol. 2003;33(2):441–445. doi: 10.1002/immu.200310019. [DOI] [PubMed] [Google Scholar]
- 160.Longo NS, Wang X, Wildin RS, Abraham KM. Regulation of Src-family protein tyrosine kinase transcription during lymphocyte ontogeny. Mol. Immunol. 1999;36(15–16):979–992. doi: 10.1016/s0161-5890(99)00134-0. [DOI] [PubMed] [Google Scholar]
- 161.Majolini MB, D'Elios MM, Galieni P, et al. Expression of the T-cell-specific tyrosine kinase Lck in normal B-1 cells and in chronic lymphocytic leukemia B cells. Blood. 1998;91(9):3390–3396. [PubMed] [Google Scholar]
- 162.Harr MW, Caimi PF, McColl KS, et al. Inhibition of Lck enhances glucocorticoid sensitivity and apoptosis in lymphoid cell lines and in chronic lymphocytic leukemia. Cell Death Differ. 2010;17(9):1381–1391. doi: 10.1038/cdd.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat. Rev. Immunol. 2010;10(6):387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Braselmann S, Taylor V, Zhao H, et al. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J. Pharmacol. Exp. Ther. 2006;319(3):998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 165.Quiroga MP, Balakrishnan K, Kurtova AV, et al. B-cell antigen receptor signaling enhances chronic lymphocytic leukemia cell migration and survival: specific targeting with a novel spleen tyrosine kinase inhibitor, R406. Blood. 2009;114(5):1029–1037. doi: 10.1182/blood-2009-03-212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen L, Monti S, Juszczynski P, et al. SYK-dependent tonic B-cell receptor signaling is a rational treatment target in diffuse large B-cell lymphoma. Blood. 2008;111(4):2230–2237. doi: 10.1182/blood-2007-07-100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Young RM, Hardy IR, Clarke RL, et al. Mouse models of non-Hodgkin lymphoma reveal Syk as an important therapeutic target. Blood. 2009;113(11):2508–2516. doi: 10.1182/blood-2008-05-158618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cheng S, Coffey G, Zhang XH, et al. SYK inhibition and response prediction in diffuse large B-cell lymphoma. Blood. 2011;118(24):6342–6352. doi: 10.1182/blood-2011-02-333773. [DOI] [PubMed] [Google Scholar]
- 169.McAdoo SP, Tam FW. Fostamatinib disodium. Drugs Future. 2011;36(4):273. doi: 10.1358/dof.2011.036.04.1588554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Buchner M, Baer C, Prinz G, et al. Spleen tyrosine kinase inhibition prevents chemokine- and integrin-mediated stromal protective effects in chronic lymphocytic leukemia. Blood. 2010;115(22):4497–4506. doi: 10.1182/blood-2009-07-233692. [DOI] [PubMed] [Google Scholar]
- 171.Suljagic M, Longo PG, Bennardo S, et al. The Syk inhibitor fostamatinib disodium (R788) inhibits tumor growth in the Emu- TCL1 transgenic mouse model of CLL by blocking antigen-dependent B-cell receptor signaling. Blood. 2010;116(23):4894–4905. doi: 10.1182/blood-2010-03-275180. [DOI] [PubMed] [Google Scholar]
- 172.Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood. 2010;115(13):2578–2585. doi: 10.1182/blood-2009-08-236471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma J, Xing W, Coffey G, et al. Cerdulatinib, a novel dual SYK/JAK kinase inhibitor, has broad anti-tumor activity in both ABC and GCB types of diffuse large B cell lymphoma. Oncotarget. 2015;6(41):43881–43896. doi: 10.18632/oncotarget.6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hoellenriegel J, Coffey GP, Sinha U, et al. Selective, novel spleen tyrosine kinase (Syk) inhibitors suppress chronic lymphocytic leukemia B-cell activation and migration. Leukemia. 2012;26(7):1576–1583. doi: 10.1038/leu.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int. Rev. Immunol. 2012;31(2):119–132. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 176.Hendriks RW, Bredius RG, Pike-Overzet K, Staal FJ. Biology and novel treatment options for XLA, the most common monogenetic immunodeficiency in man. Expert Opin. Ther. Targets. 2011;15(8):1003–1021. doi: 10.1517/14728222.2011.585971. [DOI] [PubMed] [Google Scholar]
- 177.Yang G, Zhou Y, Liu X, et al. A mutation in MYD88 (L265P) supports the survival of lymphoplasmacytic cells by activation of Bruton tyrosine kinase in Waldenstrom macroglobulinemia. Blood. 2013;122(7):1222–1232. doi: 10.1182/blood-2012-12-475111. [DOI] [PubMed] [Google Scholar]
- 178.Zhang S, Kipps TJ. The pathogenesis of chronic lymphocytic leukemia. Ann. Rev. Pathol. 2014;9(1):103–118. doi: 10.1146/annurev-pathol-020712-163955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pighi C, Gu T-L, Dalai I, et al. Phospho-proteomic analysis of mantle cell lymphoma cells suggests a pro-survival role of B-cell receptor signaling. Cellular Oncology. 2011;34(2):141–153. doi: 10.1007/s13402-011-0019-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hendriks RW, Yuvaraj S, Kil LP. Targeting Bruton's tyrosine kinase in B cell malignancies. Nat. Rev. Cancer. 2014;14(4):219–232. doi: 10.1038/nrc3702. [DOI] [PubMed] [Google Scholar]
- 181.Herman SE, Mustafa RZ, Gyamfi JA, et al. Ibrutinib inhibits BCR and NF-kappaB signaling and reduces tumor proliferation in tissue-resident cells of patients with CLL. Blood. 2014;123(21):3286–3295. doi: 10.1182/blood-2014-02-548610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Herman SE, Gordon AL, Hertlein E, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood. 2011;117(23):6287–6296. doi: 10.1182/blood-2011-01-328484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ponader S, Chen SS, Buggy JJ, et al. The Bruton tyrosine kinase inhibitor PCI-32765 thwarts chronic lymphocytic leukemia cell survival and tissue homing in vitro and in vivo . Blood. 2012;119(5):1182–1189. doi: 10.1182/blood-2011-10-386417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Furtado M, Wang ML, Munneke B, McGreivy J, Beaupre DM, Rule S. Ibrutinib-associated lymphocytosis corresponds to bone marrow involvement in mantle cell lymphoma. Br. J. Haematol. 2015;170(1):131–134. doi: 10.1111/bjh.13275. [DOI] [PubMed] [Google Scholar]
- 186.Chang BY, Francesco M, De Rooij MFM, et al. Egress of CD19+CD5+ cells into peripheral blood following treatment with the Bruton tyrosine kinase inhibitor ibrutinib in mantle cell lymphoma patients. Blood. 2013;122(14):2412–2424. doi: 10.1182/blood-2013-02-482125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.de Gorter DJJ, Beuling EA, Kersseboom R, et al. Bruton's tyrosine kinase and phospholipase Cγ2 mediate chemokine-controlled B cell migration and homing. Immunity. 2007;26(1):93–104. doi: 10.1016/j.immuni.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 188.de Rooij MFM, Kuil A, Kraan W, et al. Ibrutinib and idelalisib target B cell receptor- but not CXCL12/CXCR4-controlled integrin-mediated adhesion in Waldenström macroglobulinemia. Haematologica. 2015 doi: 10.3324/haematol.2015.137265. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013;369(1):32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Alinari L, Quinion C, Blum KA. Bruton's tyrosine kinase inhibitors in B-cell non-Hodgkin's lymphomas. Clin. Pharmacol. Ther. 2015;97(5):469–477. doi: 10.1002/cpt.65. [DOI] [PubMed] [Google Scholar]
- 191.Woyach JA, Furman RR, Liu TM, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 2014;370(24):2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Discusses the different mechanisms of ibrutinib resistance, which is a step toward overcoming this phenomenon.
- 192.Cheng S, Guo A, Lu P, Ma J, Coleman M, Wang YL. Functional characterization of BTK(C481S) mutation that confers ibrutinib resistance: exploration of alternative kinase inhibitors. Leukemia. 2015;29(4):895–900. doi: 10.1038/leu.2014.263. [DOI] [PubMed] [Google Scholar]
- 193.Liu T-M, Woyach JA, Zhong Y, et al. Hypermorphic mutation of phospholipase C, γ2 acquired in ibrutinib-resistant CLL confers BTK independency upon B-cell receptor activation. Blood. 2015;126(1):61–68. doi: 10.1182/blood-2015-02-626846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Okkenhaug K, Vanhaesebroeck B. PI3K in lymphocyte development, differentiation and activation. Nat. Rev. Immunol. 2003;3(4):317–330. doi: 10.1038/nri1056. [DOI] [PubMed] [Google Scholar]
- 195.Vanhaesebroeck B, Ali K, Bilancio A, Geering B, Foukas LC. signaling by PI3K isoforms: insights from gene-targeted mice. Trends Biochem. Sci. 2005;30(4):194–204. doi: 10.1016/j.tibs.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 196.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cδ. Blood. 2002;100(10):3741–3748. doi: 10.1182/blood-2002-02-0539. [DOI] [PubMed] [Google Scholar]
- 197.Lannutti BJ, Meadows SA, Herman SE, et al. CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood. 2011;117(2):591–594. doi: 10.1182/blood-2010-03-275305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Miller BW, Przepiorka D, de Claro RA, et al. FDA approval: Idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin. Cancer Res. 2015;21(7):1525–1529. doi: 10.1158/1078-0432.CCR-14-2522. [DOI] [PubMed] [Google Scholar]
- 199.Hoellenriegel J, Meadows SA, Sivina M, et al. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood. 2011;118(13):3603–3612. doi: 10.1182/blood-2011-05-352492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Herman SE, Gordon AL, Wagner AJ, et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood. 2010;116(12):2078–2088. doi: 10.1182/blood-2010-02-271171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Maffei R, Bulgarelli J, Fiorcari S, et al. Endothelin-1 promotes survival and chemoresistance in chronic lymphocytic leukemia B cells through ETA receptor. PLoS ONE. 2014;9(6):e98818. doi: 10.1371/journal.pone.0098818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ghia P, Chiorazzi N, Stamatopoulos K. Microenvironmental influences in chronic lymphocytic leukaemia: the role of antigen stimulation. J. Intern. Med. 2008;264(6):549–562. doi: 10.1111/j.1365-2796.2008.02030.x. [DOI] [PubMed] [Google Scholar]
- 203.Buschle M, Campana D, Carding SR, Richard C, Hoffbrand AV, Brenner MK. Interferon gamma inhibits apoptotic cell death in B cell chronic lymphocytic leukemia. J. Exp. Med. 1993;177(1):213–218. doi: 10.1084/jem.177.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Brown JR, Byrd JC, Coutre SE, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Furman RR, Sharman JP, Coutre SE, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Coutré SE, Barrientos JC, Brown JR, et al. Management of adverse events associated with idelalisib treatment: expert panel opinion. Leuk. Lymphoma. 2015;56(10):2779–2786. doi: 10.3109/10428194.2015.1022770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Byrd JC, Harrington B, O'Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2016;374(4):323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (Duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015;29(9):1811–1822. doi: 10.1038/leu.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang Y, Kelly P, Shaffer AL, et al. Targeting non-proteolytic protein ubiquitination for the treatment of diffuse large B cell lymphoma. Cancer Cell. 2016;29(4):494–507. doi: 10.1016/j.ccell.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Satpathy S, Wagner SA, Beli P, et al. Systems-wide analysis of BCR signalosomes and downstream phosphorylation and ubiquitylation. Mol. Syst. Biol. 2015;11(6):810. doi: 10.15252/msb.20145880. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The first to use mass spectrometry approaches to analyze protein phosphorylation and ubiquitination in B cells responding to antigen receptor engagement.
- 211.Park H-Y, Go H, Song HR, et al. Pellino 1 promotes lymphomagenesis by deregulating BCL6 polyubiquitination. J. Clin. Invest. 2014;124(11):4976–4988. doi: 10.1172/JCI75667. [DOI] [PMC free article] [PubMed] [Google Scholar]