Abstract
Although the treatment modalities for acute myeloid leukemia (AML) have not changed much over the past 40 years, distinct progress has been made in deciphering the basic biology underlying the pathogenesis of this group of hematological disorders. Studies show that AML development is a multicause, multistep and multipathway process. Accordingly, AMLs constitute a heterogeneous group of diseases. The thorough understanding of the molecular basis of AML is paving the way for better therapeutic approaches. Multiple novel drugs are being introduced and new, more efficient and less toxic formulations of conventional therapeutics are becoming available. Here, we review the recent advances in the comprehension of the molecular processes that lead to the onset of AML and its translation into clinical practice.
Keywords: : AML, leukemogenesis, molecular oncology
Practice points.
Acute myeloid leukemia (AML) is the most common form of acute leukemia in adults.
AML can arise de novo, develop from the progression of myelodysplastic or myeloproliferative disorders or result from previous anticancer therapy.
Leukemogenesis is a multicause, multistep and multipathway process.
The major progress in the understanding of the complex pathogenesis of AML is being translated into therapeutic approaches.
In the future tailor-made treatment schemes may become the clinical standard.
Acute myeloid leukemia (AML) develops as the consequence of a series of genetic and epigenetic changes in hemopoietic stem cells (HSCs) or precursors. The lesions alter normal hemopoietic growth and differentiation, resulting in an accumulation of abnormal, immature myeloid cells in the bone marrow and peripheral blood, while normal hemopoiesis is suppressed. AML blasts proliferate, but do not differentiate. Whereas the features of increased proliferation and maturation defects have been thoroughly studied over the years, research into normal hemopoiesis inhibition has lagged behind. In the past, cytopenias concurrent with AML were attributed to the reduced space in the bone marrow. Current research shows that normal hemopoiesis defects are caused by AML blasts. In fact, in AML patients the number of HSCs appears to be normal or even increased [1]. The expression of myeloproliferative leukemia, the thrombopoietin scavenging receptor, on AML blasts predicts peripheral blood neutropenia and thrombocytopenia [2]. The systemic loss of hemopoietic function is also partially the consequence of AML exosome-directed microRNA trafficking to HSCs [3].
AML is a heterogeneous group of diseases; hence, no single prevalent mutation is present in all or even in the majority of patients. The top ten genes mutated at frequencies > 5% are FLT3, NPM1, DNMT3A, IDH1/2, TET2, AML1, TP53, NRAS, CEBPA and WT1 [4]. The genetic heterogeneity is reflected morphologically: AML blasts exhibit maturation defects corresponding to specific stages of hemopoietic differentiation. The growing knowledge of underlying lesions forms the basis for the current WHO classification of AML [5] that has replaced the morphology-based French–American–British (FAB) classification [6].
AML appears to be maintained by a pool of self-renewing malignant cells denominated leukemic stem cells (LSCs) [7].
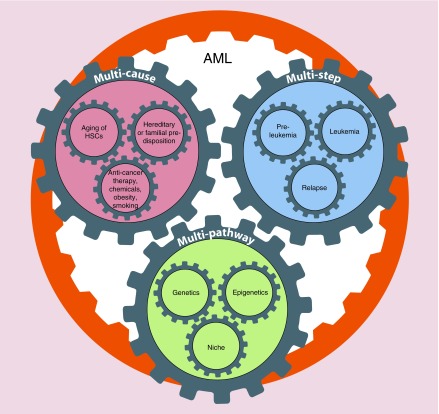
In this review, some aspects of the AML pathogenesis are analyzed (Figure 1). AML is a subject of continuous study with over 2000 articles published in 2016 alone containing the MESH term ‘AML’. Here we discuss the causes, steps and processes necessary for AML generation and conclude with remarks concerning the translation of basic research into clinical practice.
Figure 1. . Acute myeloid leukemia pathogenesis: acute myeloid leukemia development is a multicause, multistep and multipathway process.
AML: Acute myeloid leukemia.
Multicause process
Even though acquired genetic abnormalities are recurrent in leukemic blasts, the direct and exact causes of AML are unknown in the majority of cases. Risk factors are related to both aging of individuals and to some specific genetic and environmental exposures. Several causes play a role in AML onset.
Aging of stem cells, AML & longevity trade-off
HSCs acquire, throughout their lifespan, DNA mutations and micro-environmental alterations that may be responsible for hematological disease development [8,9]. Cellular quiescence of HSCs constitutes a possible mechanism for avoiding DNA damage, chromosomal aberrations and mutations [10]. The expansion and predominance of mutant clones within the pool is a frequent event during aging [8]. There are two main features of aging HSC. First, the alteration of lineage potential in favor of myelopoiesis causing decreased immune competence and increased incidence of myeloid malignancy is observed [11]. Second, loss of proliferative and self-renewal potential occurs and may be due to DNA damage that leads to the reduction of regenerative ability [8]. Different molecular mechanisms such as telomere shortening [12], replication stress [13], chromatin changes, hypermethylation of CpG islands [14], metabolic alterations and defects in DNA repair and chromosome segregations contribute to DNA damage, mutation accumulation and genomic aberrations during aging [10]. A recent study has also shown an association between mutations in various components of the RNA-splicing machinery and age-related malignancies [15].
Therapy-related AML, chemical/radiation exposure & smoking
Chemotherapy, ionizing radiation and chemical exposure have been linked to genetic changes in HSCs and precursor cells. Primary malignancy therapy-related myelodysplastic syndrome/AML (t-MDS/AML) develops after several months or years [16]. The loss of all or parts of chromosomes 5 and/or 7 are the most frequent cytogenetic abnormalities occurring after the exposure to alkylating agents and/or radiation. Otherwise, balanced chromosomal rearrangements involving MLL, AML1 and PML/RARA genes may form after treatment with compounds targeting DNA–topoisomerase II [17]. Individuals carrying a single nucleotide polymorphisms in the NQO1 detoxifying gene [18], in drug-metabolizing enzymes [19] or genes coding for components of DNA repair pathways such as MSH1/2 [20,21], RAD51 [22] or CYP3A4 [22] are more likely to develop treatment-related (t)-AML.
The exposure to vinyl chloride [23] or benzene [24] plays a role in the pathogenesis of AML as well as mutations in EPHX1 enzyme involved in benzene metabolism [25]. Recently, Chakraborty et al. [26] have demonstrated a possible correlation between the onset of t-MDS/AML and telomere length regulation in autologous hemopoetic cell transplantation setting. Cells from patients who underwent autologous hemopoetic cell transplantation and subsequently developed t-AML/t-MDS showed an initial increase in telomere length followed by accelerated telomere shortening [26]. t-AML risk factors are summarized in Table 1.
Table 1. . Therapy-related myelodysplastic syndrome risk factors and their consequences.
| Risk factors | Consequence |
|---|---|
| Alkylating agents/radiation | -5 or 5q- |
| -7 or 7q- | |
| Topoisomerase II inhibitors | MLL-fusion formation |
| AML1 mutations | |
| PML/RARA | |
| Single nucleotide polymorphisms in GST, MSH1/2, RAD51, CYP3A4, NQO1, EPHX1 | Alterations in drug metabolizing enzymes, DNA repair or detoxification pathway members |
| Chemical exposure (vinyl chloride, benzene and formaldehyde) | Hemotoxicity |
In addition, cigarette smoking [27,28] and obesity [29] prove to be significant risk factors for the development of AML in adults.
Familial predisposition & MDS evolution
Familial AMLs are generally rare. Congenital or hereditary disorders such as Down syndrome, ataxia telangiectasia, Bloom syndrome, Fanconi anemia, congenital neutropaenia as well as germline mutations concerning CEBPA, AML1, TP53, ANKRD26, DDX41, ETV6, GATA2, SRP72, TERC and TERT genes have been linked to a higher predisposition to AML [30]. Similarly, patients affected by clonal hematological disorders, including MDS and myeloproliferative neoplasms, may develop AML in the course of disease.
Multistep process
The classical two-hit model of leukemogenesis has been postulated by Gilliland and Griffin [31]. In this model, two lesions, each belonging to a different class, cooperate to cause AML and neither is sufficient to do it alone. Class I mutations (e.g., FLT3-ITD, c-KIT or NRAS mutations) bestow a proliferative advantage without blocking differentiation. Class II mutations (i.e., AML-specific fusion genes in the original model) interfere with hematopoietic differentiation and subsequent apoptosis. The initiating lesions are thought to be class II mutations, in other words, fusion genes, whereas class I mutations are typically later events [31]. For many years this model provided a fitting explanation for the pathogenesis of AML, a disease in which differentiation is blocked and proliferation is increased. However, some of the mutations encountered in AML cannot be classified as type I or type II, thus rendering the two-hit model overly simplistic.
More recently whole-genome and whole-exome sequencing studies [4,32–36] have demonstrated that at least one potential driver mutation exists in nearly all AML patient samples and that a complex interplay of genetic lesions contributes to AML pathogenesis in individual patients [4]. Indeed, it is becoming evident that human AML evolution is a multistep process albeit the timing, intricacy and order of events prior to the diagnosis are much less clear. Clonal evolution within each AML patient appears to be a dynamic process consisting of continuous acquisition and loss of specific mutations often at different times [4,34,37,38]. Such process is subjected to selective pressure exerted by the host microenvironment and, similarly to Darwinian selection, enables the survival of the fittest clones [39]. The end result is simultaneous evolutionary convergence and divergence among clones and subclones during the clinical course of the disease [34,40]. Notably, the genetic composition of subclones has been shown to have a functional significance [41]. Within the same AML patient individual clones may display distinct morphology, surface markers and engraftment potential in immunocompromised mice [41].
Sequencing studies helped to get insight into the clonal evolution of AML from the insurgence of a primary preleukemic clone and its subsequent fate: the onset of overt leukemia and of relapse [34]. In the past, the term ‘preleukemia’ was used to describe conditions with a propensity for progression to AML [42]. A current understanding of preleukemia is the existence of cells carrying a subset of genetic/epigenetic variants ultimately present in AML blasts without displaying any differentiation defect [43]. Preleukeamic lesions have been reviewed by Shlush and Minden [43] and include mutations in genes such as DNMT3A, TET2, IDH1/2, ASXL1 and many others. A recent study has suggested that nonleukemic HSCs and progenitor cells may have a competitive fitness advantage after induction chemotherapy, due to the presence of specific aging-acquired mutations, thus allowing them to expand and endure long the completion of treatment. [44]. Indeed, in patients who achieved complete remission, clonal hemopoesis persists as evidenced by a skewed X-chromosome representation [45]. DNMT3A mutations existing at presentation are the most common lesions remaining during follow-up after achieving complete remission [45]. Moreover, clonal hemopoiesis harboring AML-associated mutations (mainly in DMNT3A and TET2) is ubiquitous in healthy adults [46].
In the context of such a background, two scenarios may underlie relapse. It may either constitute the regrowth of the original leukemia, if the treatment were unsuccessful, or its further clonal evolution following the selective pressure of chemotherapy. Clearly, de novo generation of AML through accumulation of additional novel mutations in the preceding preleukemic clone is also possible though technically it cannot be described as relapse. Mutational analysis on relapsed disease shows that clonal structure of AMLs changes markedly post therapy, with alterations occurring at both genetic and epigenetic levels [34,47].
Finally, massively parallel sequencing studies enabled the discovery of hitherto unknown mutations in DNMT3A and IDH1 genes as well as in cohesin complex and spliceosome machinery genes [4].
Common AML mutations grouped by functional categories are listed in Table 2.
Table 2. . Functional categories of genes recurrently mutated in acute myeloid leukemia.
| Functional category | Genes |
|---|---|
| Signal transduction genes | FLT3, NRAS, KRAS, c-KIT, PTPN11 |
| DNA modification genes | DNMT3, IDH1/2, TET2 |
| Chromatin modifiers | MLL-fusions, ASXL1, EZH2, MLL-partial tandem duplication |
| Multi-function | NPM1 |
| Chimeric or mutated transcription factors | PML/RARA, AML1/ETO, CBFB/MYH11, CEBPA, AML1 |
| Tumor-suppressor genes | TP53, PHF6, WT1 |
| Spliceosome genes | SF3B1, SRSF2, U2AF1 |
| Cohesin complex genes | SMC1A, SMC3, RAD21, STAG2 |
Multipathway process
AML is not a single disease, but a group of disorders, underscored by alterations in multiple intracellular processes and pathways. AML-associated mutations often involve epigenetic regulators (frequently present in the preleukemic clone) such as DMNT3A, TET2, WT1, IDH1/2, while chromosomal abnormalities target numerous transcription factors (TFs) and transcriptional activators [4]. Mutations in the cohesion complex seem to contribute to leukemogenesis through the modulation of the chromatin accessibility in HSCs and progenitor cells [48]. The deregulation of long noncoding RNAs adds a further level of gene expression deregulation [49]. As a consequence, changes in chromatin status and altered transcription resulting in inhibition of some and activation of other signaling pathways are observed (Figure 2). Numerous networks that control normal hemopoesis, including RAF/MEK/ERK, JAK/STAT or PI3K/AKT/NFKB, are deregulated [50]. Moreover, LSCs reactivate evolutionary conserved signaling pathways, such as Notch, Wnt or Hedgehog [51]. Last, but not least, LSCs interact with the hemopoietic niche in several ways [52]. On the one hand, LSCs induce microenvironmental reprogramming of the niche that may act as a sanctuary in which LSCs acquire a therapy-resistant phenotype [52]. On the other hand, mutations occurring in stromal cells may by themselves cause AML [53,54].
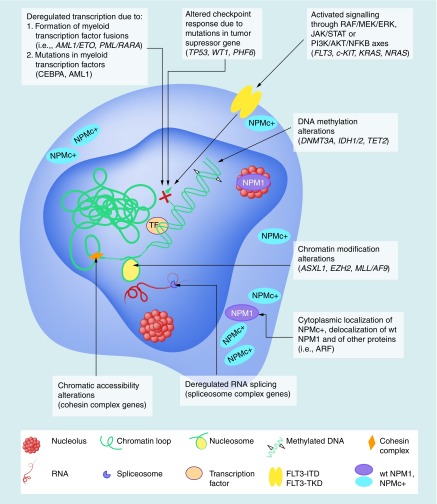
Figure 2. . The molecular consequences of common acute myeloid leukemia mutations.
Mutations in myeloid transcription factor (TF) and TF fusions caused by chromosomal rearrangements such as translocations lead to transcriptional deregulation and impaired hematopoietic differentiation (top left). Mutations in tumor suppressor genes influence the transcription and checkpoint responses of the cell (top center). Mutations in signaling genes (e.g., FLT3 receptor) confer a proliferative advantage through the RAF/MEK/ERK, JAK/STAT and PI3K/AKT/NFKB signaling axes (top right). DNMT3A, IDH1/2 and TET2 mutations acting through the 2-hydroxyglutarate oncometabolite production deregulate DNA methylation (top middle). Mutations in genes responsible for the cellular epigenetic regulation (e.g., ASXL1 and EZH2) lead to alterations in chromatin modification (H3 and H2A histone methylation on K79, K27 and K119 lysine residues, respectively), while MLL/AF9 fusion gene through aberrant methylation upregulate HOX gene expression and thus expands stem cells and blocks differentiation (bottom middle). Mutations of NPM1 gene, encoding a multifunctional shuttling protein, result in the formation of cytoplasmic mutant of NPM1 known as NPMc+ and cause delocalization of NPM1-interacting proteins, influence ribosome biogenesis and TP53 stability (bottom right). Mutations in spliceosome complex genes (SRSF2, SF3B1 and U2AF1) are involved in deregulated RNA processing including intron retention (bottom center). Cohesin complex gene mutations, in other words, SMC1A, SMC3, STAG2 and RAD21, trigger increased chromatin accessibility and enhanced binding of AML1 and GATA2 TFs enforcing stem cell programs (bottom left).
It is out of the scope and possibility of this review to discuss every facet of AML pathogenesis. Instead, we touch upon the limited and in our arbitrary opinion, most interesting and surprising findings concerning the processes and pathways involved in AML.
Epithelial-to-mesenchymal transition program may play a role in leukemogenesis
Epithelial-to-mesenchymal transition (EMT) is not a single pathway, but a process through which epithelial cells lose cell-to-cell attachment and acquire a motile phenotype that allows them to move to and colonize distant sites. The loss of E-cadherin is considered to be fundamental for EMT. TFs that repress E-cadherin directly or indirectly are considered EMT inducing. SNAI1, SNAI2, ZEB1, ZEB2, TCF3 and KLF8 transcriptionally repress E-cadherin by binding to its promoter, whereas factors such as Twist, GSC, TCF4, SIX1 homeobox protein and FOXC2 TF repress E-cadherin indirectly [55]. EMT is physiologically relevant during organogenesis and to the progression of solid tumors [56]. Until now, it seemed counterintuitive to study EMT in the context of AML cells that are intrinsically endowed with migratory properties. However, induction of EMT also generates cells with stem cell properties [57]. To date, a possible involvement of the EMT program in hematological tumor generation has not been sought directly. Nonetheless, altered expression of some of its modulators has been described [58]. For example, Li et al. identified ZEB2 EMT-inducing TF as a novel AML dependency through an RNAi screen [59]. ZEB2 depletion impaired proliferation of human and mouse AML cells, and led to aberrant differentiation of human AML cells through transcriptional repression of myeloid differentiation and cell adhesion and migration genes [59]. Another study showed that knockdown of ZEB1, a further EMT-inducing TF, dramatically reduced leukemic blast invasion in an MLL/AF9-driven leukemia model [60]. The authors identified several EMT-related genes significantly associated with poor overall survival in AML patients [60]. In our hands, the expression of AML1/ETO fusion protein in EML C1 murine hematopoietic stem/progenitor cell line and in primary murine stem/progenitor cells, led to an upregulation of three different EMT signatures. In particular, AML1/ETO downregulated E-cadherin and upregulated Zeb2 causing a phenotype of decreased adhesion and increased motility reminiscent of EMT [61].
Taken together, the findings may suggest that the machinery involved in EMT is deployed to achieve a more mobile and less adherent phenotype of leukemic cells. Data obtained by Stavropoulou et al. suggest an instinctive connection between EMT induction and aggressive forms of AML with extensive liver and lung infiltration [60,62].
Wnt activation in AML
Wnt pathway is an evolutionary conserved signaling pathway. It plays a pivotal role in HSC maintenance [63], in differentiation of blood cells and its constitutive activation is a common feature of AML [64–66]. In particular, canonical Wnt signaling is known to be active in AML expressing specific oncogenes such as MLL/AF9, AML1/ETO and PML/RARA [66–68]. In mouse models of AML induced either by co-expression of the Hoxa9 and Meis1a oncogenes or by the MLL/AF9 fusion oncoprotein it has been shown that Wnt signaling is specifically required for the self-renewal of LSCs [68]. Curiously, AML can be induced by an activating mutation in the β-catenin gene, the major Wnt signal transducer, in osteoblasts [53].
Two recent studies extend the findings regarding Wnt signaling activation in AML. We have recently reported that canonical Wnt signaling is active in the patient-derived OCI-AML3 cell line expressing a leukemogenic cytoplasmic mutant of the NPM1 (NPMc+) protein and in primary AML-NPMc+ patients’ blasts. Furthermore, ectopic expression of NPMc+ activates canonical Wnt signaling during zebrafish development [69].
Lazzaroni et al. [70] reported a novel mechanism for Wnt activation in AML via a genetic rearrangement in blast cells involving intron 1 (IVS1) of the WNT10B locus flanked at the 5′ end by nonhuman DNA. The resulting WNT10BIVS1 transcript is expressed mainly in intermediate/unfavorable risk AML patients. Blasts from another patient carried a genomic transposable short form of human WNT10B (ht-WNT10B), possibly involved in a nonrandom microhomology-mediated recombination generating WNT10BIVS1. Interestingly, the expression of WNT10B in zebrafish embryos promotes the accumulation of hemapoietic precursors [70]. Incidentally, Wnt signaling is one of the multiple pathways involved in the execution of the EMT program [71].
The issue of differentiation block
Lack of appropriate myeloid maturation is a key feature of AML. A recent study proposes that myeloid differentiation constitutes a requirement for AML initiation. LSCs often share features of lineage-restricted progenitors and the relative contribution of differentiation status to LSC transformation is unclear. Using MLL/AF9 and MOZ/TIF2 murine AML models, Ye et al. [72] showed that myeloid differentiation to granulocyte macrophage progenitors is crucial for LSC generation. Disrupting granulocyte macrophage progenitor formation by deleting the CEBPA lineage-restricted TF blocked normal granulocyte production and prevented AML formation. However, restoring myeloid differentiation in CEBPA mutants using inflammatory cytokines re-established AML transformation capacity [72].
Using Hox9a AML model system, Sykes et al. [73] performed high-throughput phenotypic screen and identified dihydroorotate dehydrogenase enzyme as a target in AML. The inhibition of this metabolic enzyme involved in pyrimidine synthesis caused differentiation of leukemic cells. Importantly, inhibitors of dihydroorotate dehydrogenase showed therapeutic potential in a range of murine and xenotransplant AML models, independently of oncogenic driver [73]. Clearly, both findings have a potential of being exploited in new therapeutic approaches.
Where are we now: major lines of research
Despite major progress in understanding the biology of AML, a high relapse rate is still a foremost problem in clinical hematology. The progress has been made both in the fields of the basic biology and the translation of molecular findings into better patient care [74,75]. For example, the discovery of mutations in genes such as IDH and FLT3 gave rise to the development and implementation of specific inhibitors [76,77]. Indeed, CALGB 10603/RATIFY study provided the first evidence for a survival advantage attributable to therapy with the midostaurin FLT3-inhibitor [78]. Still, more work has to be done to improve the survival of AML patients.
High-throughput identification of novel so-called ‘druggable’ targets in AML represents a possible way of approaching the quest for innovative treatment modalities. Until recently, RNA interference loss-of-function screens in combination or not with chemotherapeutic agents were commonly used to study tumor vulnerabilities [79–82]. These studies identified Brd4 [79], WEE1 [80], GSK-3a [81] and ROCK1 [82] as potential targets. More recently, Tzelepis et al. [83] optimized a genome-wide clustered regularly interspaced short palindromic repeats screening platform. A total of 492 AML-specific cell-essential genes were identified including several established therapeutic targets such as DOT1L, BCL2 and MEN1 [83]. Accordingly, early development of inhibitors targeting DOT1L, BET, WEE1, CDK and others has been undertaken [84].
Evidently, the success of targeted treatments depends on the presence of the very target in any individual AML. Mutational profiling has to be implemented on a large scale into clinical practice allowing for assigning tailored therapeutic schemes for any given patient. Integrated genomic analysis is also needed for more precise evaluation of prognosis.
Current AML research focuses on elderly patients, since AML incidence peaks at around 65 years of age. There are fewer therapeutic options for this group of frequently frail patients with comorbidities. At the same time, age should not be regarded as an obstacle for treating fit elderly patients with the modalities reserved for the group of young patients. Novel agents alone or in combination with low-dose standard cytotoxic drugs are being tested [85].
Conclusion
Although objectively, the overall outcome of AML treatment is still poor, identification/design of new, smart agents and clinical trials of the ones already identified are under way and should drastically change the prognosis of the disease in the near future. Continued research into the biology and therapy of AML is essential.
Future perspective
In the years to come the diagnosis of AML will have to be comprehensive of detailed molecular analysis. Any patient will be assigned to a genetic subgroup and consequently to an appropriate risk group and will receive personalized treatment to better match the mutations present in the AML blasts.
Acknowledgements
The authors thank M Mazza, M Saia, G Frigé and P Massa for the critical reading of the manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Miraki-Moud F, Anjos-Afonso F, Hodby KA, et al. Acute myeloid leukemia does not deplete normal hematopoietic stem cells but induces cytopenias by impeding their differentiation. Proc. Natl Acad. Sci. USA. 2013;110(33):13576–13581. doi: 10.1073/pnas.1301891110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauch PJ, Ellegast JM, Widmer CC, et al. MPL expression on AML blasts predicts peripheral blood neutropenia and thrombocytopenia. Blood. 2016;128(18):2253–2257. doi: 10.1182/blood-2016-04-711986. [DOI] [PubMed] [Google Scholar]
- 3.Hornick NI, Doron B, Abdelhamed S, et al. AML suppresses hematopoiesis by releasing exosomes that contain microRNAs targeting c-MYB. Sci. Signal. 2016;9(444):ra88. doi: 10.1126/scisignal.aaf2797. [DOI] [PubMed] [Google Scholar]
- 4.Cancer Genome Atlas Research Network. Ley TJ, Miller C, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A seminal paper that identified at least one potential driver mutation in nearly all of 200 acute myeloid leukemia samples analyzed and found that a complex interplay of genetic events contributes to acute myeloid leukemia pathogenesis in individual patients. The databases have been of crucial importance to AML research since its publication.
- 5.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the world health organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114(5):937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br. J. Haematol. 1976;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 7.Reinisch A, Chan SM, Thomas D, Majeti R. Biology and clinical relevance of acute myeloid leukemia stem cells. Semin. Hematol. 2015;52(3):150–164. doi: 10.1053/j.seminhematol.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132(4):681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 9.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl. J. Med. 2014;371(26):2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams PD, Jasper H, Rudolph KL. Aging-induced stem cell mutations as drivers for disease and cancer. Cell Stem Cell. 2015;16(6):601–612. doi: 10.1016/j.stem.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Discusses potential explanations for age-associated increase in the incidence of mutant stem/progenitor clones highlighting the roles of quiescence, replication-associated DNA damage, telomere shortening, epigenetic and metabolic alterations as determinants of clonal dominance in aging.
- 11.Henry CJ, Marusyk A, Degregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what's the connection? Aging. 2011;3(6):643–656. doi: 10.18632/aging.100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holohan B, Wright WE, Shay JW. Cell biology of disease: telomeropathies: an emerging spectrum disorder. J. Cell Biol. 2014;205(3):289–299. doi: 10.1083/jcb.201401012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter D, Lier A, Geiselhart A, et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature. 2015;520(7548):549–552. doi: 10.1038/nature14131. [DOI] [PubMed] [Google Scholar]
- 14.Greer EL, Maures TJ, Hauswirth AG, et al. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature. 2010;466(7304):383–387. doi: 10.1038/nature09195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crews LA, Balaian L, Delos Santos NP, et al. RNA splicing modulation selectively impairs leukemia stem cell maintenance in secondary human AML. Cell Stem Cell. 2016;19(5):599–612. doi: 10.1016/j.stem.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godley LA, Larson RA. Therapy-related myeloid leukemia. Semin. Oncol. 2008;35(4):418–429. doi: 10.1053/j.seminoncol.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayser S, Dohner K, Krauter J, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117(7):2137–2145. doi: 10.1182/blood-2010-08-301713. [DOI] [PubMed] [Google Scholar]
- 18.Smith MT, Wang Y, Skibola CF, et al. Low NAD(P)H:quinone oxidoreductase activity is associated with increased risk of leukemia with MLL translocations in infants and children. Blood. 2002;100(13):4590–4593. doi: 10.1182/blood-2001-12-0264. [DOI] [PubMed] [Google Scholar]
- 19.Allan JM, Wild CP, Rollinson S, et al. Polymorphism in glutathione S-transferase P1 is associated with susceptibility to chemotherapy-induced leukemia. Proc. Natl Acad. Sci. USA. 2001;98(20):11592–11597. doi: 10.1073/pnas.191211198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worrillow LJ, Travis LB, Smith AG, et al. An intron splice acceptor polymorphism in hMSH2 and risk of leukemia after treatment with chemotherapeutic alkylating agents. Clin. Cancer Res. 2003;9(8):3012–3020. [PubMed] [Google Scholar]
- 21.Worrillow LJ, Smith AG, Scott K, et al. Polymorphic MLH1 and risk of cancer after methylating chemotherapy for Hodgkin lymphoma. J. Med. Genet. 2008;45(3):142–146. doi: 10.1136/jmg.2007.053850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voso MT, Fabiani E, D'alo F, et al. Increased risk of acute myeloid leukaemia due to polymorphisms in detoxification and DNA repair enzymes. Ann. Oncol. 2007;18(9):1523–1528. doi: 10.1093/annonc/mdm191. [DOI] [PubMed] [Google Scholar]
- 23.Poynter JN, Richardson M, Roesler M, et al. Chemical exposures and risk of acute myeloid leukemia and myelodysplastic syndromes in a population-based study. Int. J. Cancer. 2017;140(1):23–33. doi: 10.1002/ijc.30420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt L, Nilsson PG, Mitelman F. Occupational exposure to petroleum products in men with acute non-lymphocytic leukaemia. Br. Med. J. 1978;1(6112):553. doi: 10.1136/bmj.1.6112.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebailly P, Willett EV, Moorman AV, et al. Genetic polymorphisms in microsomal epoxide hydrolase and susceptibility to adult acute myeloid leukaemia with defined cytogenetic abnormalities. Br. J. Haematol. 2002;116(3):587–594. doi: 10.1046/j.0007-1048.2001.03320.x. [DOI] [PubMed] [Google Scholar]
- 26.Chakraborty S, Sun CL, Francisco L, et al. Accelerated telomere shortening precedes development of therapy-related myelodysplasia or acute myelogenous leukemia after autologous transplantation for lymphoma. J. Clin. Oncol. 2009;27(5):791–798. doi: 10.1200/JCO.2008.17.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fircanis S, Merriam P, Khan N, Castillo JJ. The relation between cigarette smoking and risk of acute myeloid leukemia: an updated meta-analysis of epidemiological studies. Am. J. Hematol. 2014;89(8):E125–E132. doi: 10.1002/ajh.23744. [DOI] [PubMed] [Google Scholar]
- 28.Colamesta V, D'aguanno S, Breccia M, Bruffa S, Cartoni C, La Torre G. Do the smoking intensity and duration, the years since quitting, the methodological quality and the year of publication of the studies affect the results of the meta-analysis on cigarette smoking and acute myeloid leukemia (AML) in adults? Crit. Rev. Oncol. Hematol. 2016;99:376–388. doi: 10.1016/j.critrevonc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Poynter JN, Richardson M, Blair CK, et al. Obesity over the life course and risk of acute myeloid leukemia and myelodysplastic syndromes. Cancer Epidemiol. 2016;40:134–140. doi: 10.1016/j.canep.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Godley LA. Inherited predisposition to acute myeloid leukemia. Semin. Hematol. 2014;51(4):306–321. doi: 10.1053/j.seminhematol.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100(5):1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 32.Ley TJ, Mardis ER, Ding L, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456(7218):66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361(11):1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481(7382):506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter MJ, Shen D, Ding L, et al. Clonal architecture of secondary acute myeloid leukemia. N. Engl. J. Med. 2012;366(12):1090–1098. doi: 10.1056/NEJMoa1106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shiba N, Yoshida K, Shiraishi Y, et al. Whole-exome sequencing reveals the spectrum of gene mutations and the clonal evolution patterns in paediatric acute myeloid leukaemia. Br. J. Haematol. 2016;175(3):476–489. doi: 10.1111/bjh.14247. [DOI] [PubMed] [Google Scholar]
- 37.Anderson K, Lutz C, Van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356–361. doi: 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 38.Kim T, Yoshida K, Kim YK, et al. Clonal dynamics in a single AML case tracked for 9 years reveals the complexity of leukemia progression. Leukemia. 2016;30(2):295–302. doi: 10.1038/leu.2015.264. [DOI] [PubMed] [Google Scholar]; • Presents a 9 years, whole–exome sequencing study of a single case thus covering the longest time span of any similar dataset. Data show that the development and extinction of subclones, as well as their anticorrelated expansion occurs via varying drug responses.
- 39.Greaves M, Maley CC. Clonal evolution in cancer. Nature. 2012;481(7381):306–313. doi: 10.1038/nature10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paguirigan AL, Smith J, Meshinchi S, Carroll M, Maley C, Radich JP. Single-cell genotyping demonstrates complex clonal diversity in acute myeloid leukemia. Sci. Transl. Med. 2015;7(281):281re282. doi: 10.1126/scitranslmed.aaa0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klco JM, Spencer DH, Miller CA, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell. 2014;25(3):379–392. doi: 10.1016/j.ccr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koeffler HP, Leong G. Preleukemia: one name, many meanings. Leukemia. 2017;31(3):534–542. doi: 10.1038/leu.2016.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shlush LI, Minden MD. Preleukemia: the normal side of cancer. Curr. Opin. Hematol. 2015;22(2):77–84. doi: 10.1097/MOH.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 44.Wong TN, Miller CA, Klco JM, et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood. 2016;127(7):893–897. doi: 10.1182/blood-2015-10-677021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otto A, Lilly M, Herold S, et al. Clonal hematopoiesis in AML patients in hematological CR is present in many patients with intermediate risk AML and is associated with a high prevalence of DNMT3A gene mutations. Blood. 2014;124(21):121–121. [Google Scholar]
- 46.Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat. Commun. 2016;7:12484. doi: 10.1038/ncomms12484. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Clonal hematopoiesis was thought to be a rare albeit increasing in frequency with age and predisposing to hematological malignancy. The authors show that clonal hematopoiesis bearing mutations in DNMT3A and TET2 is instead present in 95% of individuals studied.
- 47.Li S, Garrett-Bakelman F, Perl AE, et al. Dynamic evolution of clonal epialleles revealed by methclone. Genome Biol. 2014;15(9):472. doi: 10.1186/s13059-014-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazumdar C, Majeti R. The role of mutations in the cohesin complex in acute myeloid leukemia. Int. J. Hematol. 2017;105(1):31–36. doi: 10.1007/s12185-016-2119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei S, Wang K. Long noncoding RNAs: pivotal regulators in acute myeloid leukemia. Exp. Hematol. Oncol. 2015;5:30. doi: 10.1186/s40164-016-0059-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scholl C, Gilliland DG, Frohling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin. Oncol. 2008;35(4):336–345. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Heidel FH, Arreba-Tutusaus P, Armstrong SA, Fischer T. Evolutionarily conserved signaling pathways: acting in the shadows of acute myelogenous leukemia's genetic diversity. Clin. Cancer Res. 2015;21(2):240–248. doi: 10.1158/1078-0432.CCR-14-1436. [DOI] [PubMed] [Google Scholar]
- 52.Zhou HS, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol. Med. 2016;13(2):248–259. doi: 10.20892/j.issn.2095-3941.2016.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kode A, Manavalan JS, Mosialou I, et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature. 2014;506(7487):240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raaijmakers MH, Mukherjee S, Guo S, et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464(7290):852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell. 2008;14(6):818–829. doi: 10.1016/j.devcel.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 56.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 57.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goossens S, Haigh JJ. The role of EMT modulators in hematopoiesis and leukemic transformation. In: Lawrie C, editor. Hematology - Science and Practice. InTech; 2012. pp. 101–120. [Google Scholar]
- 59.Li H, Mar BG, Zhang H, et al. The EMT regulator ZEB2 is a novel dependency of human and murine acute myeloid leukemia. Blood. 2016;129(4):497–508. doi: 10.1182/blood-2016-05-714493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stavropoulou V, Kaspar S, Brault L, et al. MLL-AF9 expression in hematopoietic stem cells drives a highly invasive AML expressing EMT-related genes linked to poor outcome. Cancer Cell. 2016;30(1):43–58. doi: 10.1016/j.ccell.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 61.Saia M, Termanini A, Rizzi N, et al. AML1/ETO accelerates cell migration and impairs cell-to-cell adhesion and homing of hematopoietic stem/progenitor cells. Sci. Rep. 2016;6:34957. doi: 10.1038/srep34957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Watanabe T, Ernst P. Context, context, context: new gene programs linked to bad behavior in MLL-AF9-initiated leukemia. Cancer Cell. 2016;30(1):3–5. doi: 10.1016/j.ccell.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 63.Reya T, Duncan AW, Ailles L, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 64.Luis TC, Weerkamp F, Naber BA, et al. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113(3):546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 65.Luis TC, Naber BA, Roozen PP, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9(4):345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 66.Luis TC, Ichii M, Brugman MH, Kincade P, Staal FJ. Wnt signaling strength regulates normal hematopoiesis and its deregulation is involved in leukemia development. Leukemia. 2012;26(3):414–421. doi: 10.1038/leu.2011.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mikesch JH, Steffen B, Berdel WE, Serve H, Muller-Tidow C. The emerging role of Wnt signaling in the pathogenesis of acute myeloid leukemia. Leukemia. 2007;21(8):1638–1647. doi: 10.1038/sj.leu.2404732. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Krivtsov AV, Sinha AU, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbieri E, Deflorian G, Pezzimenti F, et al. Nucleophosmin leukemogenic mutant activates Wnt signaling during zebrafish development. Oncotarget. 2016;7(34):55302–55312. doi: 10.18632/oncotarget.10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lazzaroni F, Del Giacco L, Biasci D, et al. Intronless WNT10B-short variant underlies new recurrent allele-specific rearrangement in acute myeloid leukaemia. Sci. Rep. 2016;6:37201. doi: 10.1038/srep37201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye M, Zhang H, Yang H, et al. Hematopoietic differentiation is required for initiation of acute myeloid leukemia. Cell Stem Cell. 2015;17(5):611–623. doi: 10.1016/j.stem.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sykes DB, Kfoury YS, Mercier FE, et al. Inhibition of dihydroorotate dehydrogenase overcomes differentiation blockade in acute myeloid leukemia. Cell. 2016;167(1):171e115–186e115. doi: 10.1016/j.cell.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shafer D, Grant S. Update on rational targeted therapy in AML. Blood Rev. 2016;30(4):275–283. doi: 10.1016/j.blre.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood. 2016;127(1):71–78. doi: 10.1182/blood-2015-07-604538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Kouchkovsky I, Abdul-Hay M. ‘Acute myeloid leukemia: a comprehensive review and 2016 update’. Blood Cancer J. 2016;6(7):e441. doi: 10.1038/bcj.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medeiros BC, Fathi AT, Dinardo CD, Pollyea DA, Chan SM, Swords R. Isocitrate dehydrogenase mutations in myeloid malignancies. Leukemia. 2017;31(2):272–281. doi: 10.1038/leu.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stone RM, Mandrekar S, Sanford BL, et al. The Multi-Kinase Inhibitor Midostaurin (M) Prolongs Survival Compared with Placebo (P) in Combination with Daunorubicin (D)/Cytarabine (C) Induction (ind), High-Dose C Consolidation (consol), and As Maintenance (maint) Therapy in Newly Diagnosed Acute Myeloid Leukemia (AML) Patients (pts) Age 18–60 with FLT3 Mutations (muts): An International Prospective Randomized (rand) P-Controlled Double-Blind Trial (CALGB 10603/RATIFY [Alliance]) Blood. 2015;126(23):6–6. [Google Scholar]
- 79.Zuber J, Shi J, Wang E, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tibes R, Bogenberger JM, Chaudhuri L, et al. RNAi screening of the kinome with cytarabine in leukemias. Blood. 2012;119(12):2863–2872. doi: 10.1182/blood-2011-07-367557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banerji V, Frumm SM, Ross KN, et al. The intersection of genetic and chemical genomic screens identifies GSK-3alpha as a target in human acute myeloid leukemia. J. Clin. Invest. 2012;122(3):935–947. doi: 10.1172/JCI46465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wermke M, Camgoz A, Paszkowski-Rogacz M, et al. RNAi profiling of primary human AML cells identifies ROCK1 as a therapeutic target and nominates fasudil as an antileukemic drug. Blood. 2015;125(24):3760–3768. doi: 10.1182/blood-2014-07-590646. [DOI] [PubMed] [Google Scholar]
- 83.Tzelepis K, Koike-Yusa H, De Braekeleer E, et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in acute myeloid leukemia. Cell Rep. 2016;17(4):1193–1205. doi: 10.1016/j.celrep.2016.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sallman DA, Lancet JE. What are the most promising new agents in acute myeloid leukemia? Curr. Opin. Hematol. 2017;24(2):99–107. doi: 10.1097/MOH.0000000000000319. [DOI] [PubMed] [Google Scholar]; • Summarizes the current status of novel agents which are shaping the clinical management of AML patients highlighting the fact that personalized therapy is becoming possible through growing understanding of the molecular architecture and survival pathways of any individual disease.
- 85.Podoltsev NA, Stahl M, Zeidan AM, Gore SD. Selecting initial treatment of acute myeloid leukaemia in older adults. Blood Rev. 2017;31(2):43–62. doi: 10.1016/j.blre.2016.09.005. [DOI] [PubMed] [Google Scholar]