Abstract
Aim
To determine, among very preterm newborns, if those who are growth restricted are at increased risk of retinopathy of prematurity (ROP), and to explore if the mixed findings of prior studies are the consequence of sampling based upon birth weight instead of gestational age.
Methods
Using data from the ELGAN Study, we created logistic regression models of pre-threshold ROP risk to adjust for confounders and calculate odds ratios and 99% confidence intervals. We created scatter plots to display the gestational age/birth weight relationship in infants enrolled in studies with different selection criteria.
Results
Low gestational age (23–24 weeks, OR 11.6 (2.9, 47); 25–26 weeks, 8.1 (2.1, 32)) and severe growth restriction (birth weight Z-score <−2, OR 9.1 (1.1, 76)) were associated with increased risk of pre-threshold ROP. We documented in scatter plots that a sample defined by birth weight has an excess of gestationally-older, severely growth-restricted newborns.
Conclusion
In this sample, low gestational age and severe growth restriction were associated with increased risk of pre-threshold ROP.
Keywords: ELGAN, growth restriction, prematurity, retinopathy, ROP
INTRODUCTION
Low gestational age and low birth weight due to intrauterine growth restriction (IUGR) are independent risk factors for retinopathy of prematurity (ROP) (1, 2). In moderately to late preterm infants, low birth weight has been associated with abnormal retinal vascularization (3).
Some studies that did not detect a relationship between IUGR and ROP enrolled infants on the basis of low birth weight, or low birth weight or low gestational age (4). Conversely, studies that found growth-restricted infants are at increased risk for ROP enrolled newborns solely based on gestational age (5, 6), or had very generous gestational age and birth-weight criteria (7). Explanations for these apparently disparate findings include study design and sample selection.
We know of no previous report in which ROP risks were evaluated within strata of gestational age and birth weight categories simultaneously, nor has any previous report included multivariable models that adjusted for the interaction between low gestational age and severe growth restriction. Because enrollment on the basis of birth weight can distort the results of studies of immature newborns, the ELGAN (extremely low gestational age newborn) Study provides an opportunity to evaluate the possible influence of growth restriction on the risk of severe ROP (8).
MATERIALS AND METHODS
The ELGAN study was designed to identify characteristics and exposures that increase the risk of neurological disorders in ELGANs (9). During the years 2002–2004, women who delivered before 28 weeks gestation were asked to enroll. The enrollment and consent process was approved by the institutional review boards of participating centers. The 1248 infants who had retinal examinations are the subject of this study.
Newborn variables
The clinical circumstances that led to each maternal admission and preterm delivery were recorded. Gestational age estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on dates of embryo retrieval or intrauterine insemination or fetal ultrasound before the 14th week (62%). Reliance was then placed sequentially on fetal ultrasound at 14 or more weeks (29%), last menstrual period (7%), and gestational age recorded in the unit log (1%).
The birth-weight Z-score is the number of standard deviations the birth weight is above or below the median weight of infants of the same GA in referent samples not delivered for preeclampsia or fetal indications(10). Children whose birth-weight Z-score was < −2 (i.e., more than 2 standard deviations below the median in the standard data set) are identified as severely growth restricted; those whose birth-weight Z-score was ≥ −2 yet < −1 (i.e., between 1 and 2 standard deviations below the median) are identified as moderately growth restricted.
Possible effect modifying factors were analyzed, including blood gas measurements, the number of days on mechanical ventilation, and late bacteremia. We define late bacteremia as evident in weeks 2, 3 or 4. The recovery of a pathogen from blood was reported, but details about the organism were not.
Eye examinations
Participating ophthalmologists prepared a manual and ROP data collection form and participated in efforts to minimize observer variability. The first ophthalmologic examination was within the 31st to 33rd post-menstrual week. Follow-up exams were as clinically indicated until normal vascularization began in zone lll. We focused our analyses on pre-threshold ROP, defined as any ROP in zone 1, or in zone 2, stage 2 with plus disease or stage 3 without plus disease(11). 173 infants had pre-threshold ROP. As in previous analyses (12, 13), the comparison groups always include infants with no or low grade ROP.
Data analysis
We calculated cell-specific risks of pre-threshold ROP in groups defined by gestational age and birth-weight Z-score (Table 1), and further by potential effect modifying postnatal characteristics, such as blood gas derangements, days receiving supplemental oxygen, days ventilated, and whether or not late bacteremia was documented (data not shown).
Table 1.
Cell-specific incidence of pre-threshold ROP§ per 100 infants who, at birth, had both the birth weight Z-score of the column and the gestational age of the row.
| Gestational age (weeks) | Birth weight Z-score | ||
|---|---|---|---|
| < −2 | ≥ −2, < −1 | ≥ −1 | |
| 23–24 | 60 | 37 | 24 |
| 25–26 | 19 | 27 | 13 |
| 27 | 19 | 7 | 1 |
| Column N | 78 | 167 | 1003 |
satisfied ET-ROP criteria for ablative surgery
We created two logistic regression models of pre-threshold ROP risk to calculate odds ratios and 99% confidence intervals for gestational age categories and Z-score categories (Table 2). Both models included potential confounding variables—highest PaO2 and PCO2 in the highest quartile on two of the first three postnatal days, the highest quartile of days ventilated, and late bacteremia. Model #2 included, in addition, variables for interactions between gestational age categories and birth-weight Z-score categories. Interaction terms indicate that the effect of one variable (e.g., birth weight Z-score <−2) depends on another variable (e.g., gestational age: 25–26 weeks).
Table 2.
Odds ratios (and 99% confidence intervals) of pre-threshold ROP associated with low gestational age and low birth weight Z-score in models that adjust for potential mediators. Model 1 does not include interaction terms, whereas Model 2 does.
| Model 1§ | Model 2§ | |
|---|---|---|
| Gestational age 23–24 weeks | 6.8 (2.7, 17) | 11.6 (2.9, 47) |
| Gestational age 25–26 weeks | 4.4 (1.9, 11) | 8.1 (2.1, 32) |
| Birth weight Z-score < −2 | 1.4 (0.6, 3.6) | 9.1 (1.1, 76) |
| Birth weight Z-score ≥ −2, < −1 | 1.7 (0.9, 3.1) | 3.5 (0.5, 23) |
| GA-BW interactions | ||
| GA:23–24 x BWZ < −2 | 0.3 (0.01, 7.1) | |
| GA:23–24 x BWZ ≥ −2, < −1 | 0.4 (0.05, 3.9) | |
| GA:25–26 x BWZ < −2 | 0.1 (0.01, 1.1) | |
| GA:25–26 x BWZ ≥ −2, < −1 | 0.4 (0.06, 3.5) |
Both models are adjusted for highest PaO2, highest PCO2, highest quartile of days ventilated, and “late” bacteremia.
Finally, to demonstrate how defining samples on the basis of low birth weight might selectively include an excess of growth-restricted, older gestational age newborns, we created scatter plots that display the gestational age/birth weight relationship in infants enrolled in two studies. The Developmental Epidemiology Network study recruited infants with birth weights below 1500g (14), while the ELGAN Study recruited infants whose gestational ages were below 28 weeks(9).
RESULTS
Sample description
A comparison of demographics for AGA and SGA/growth restricted babies can be found in a prior ELGAN publication by Steimish, et al(15). Overall, 6% of the 1248 infants in this sample were severely growth restricted, while an additional 13% were moderately growth restricted. Fetal growth restriction varies with the indication for preterm delivery (16) and in our sample, severe growth restriction characterized 2% of newborns delivered for spontaneous indications, 28% delivered for maternal indications (preeclampsia), and 20% delivered for fetal indications.
Cell-specific incidence of pre-threshold ROP among newborns classified by both gestational age and birth-weight Z-score (Table 1)
The incidence of pre-threshold disease was highest among those with very low gestational age and severe growth restriction and tended to decline with increasing gestational age and increasing birth-weight Z-score. Infants born at 25–26 weeks gestation, however, were less likely to be at increasing risk of ROP severity with decreasing birth-weight Z-score than were infants born at younger or older gestational ages.
Odds ratios (Table 2)
We created two logistic regression models of the risk of pre-threshold ROP associated with low gestational age and low birth-weight Z-score. In the model without the interaction terms (left data column), the risk of pre-threshold disease was statistically-significantly increased (p < .01) in both gestational age categories (i.e., 23–24 weeks, and 25–26 weeks), but in neither growth-restriction category (i.e., < −2, and ≥ −2 but < −1).
When we added variables for the interaction between gestational age and birth-weight Z-score categories (right data column), the odds ratios for gestational age categories were increased, but the lower bounds of their 99% confidence interval changed minimally. In contrast, the odds ratio associated with severe growth restriction increased prominently to become statistically significant. Each of the four interaction terms included in the second model had an odds ratio that was less than 0.5 and a 99% confidence interval that included 1.
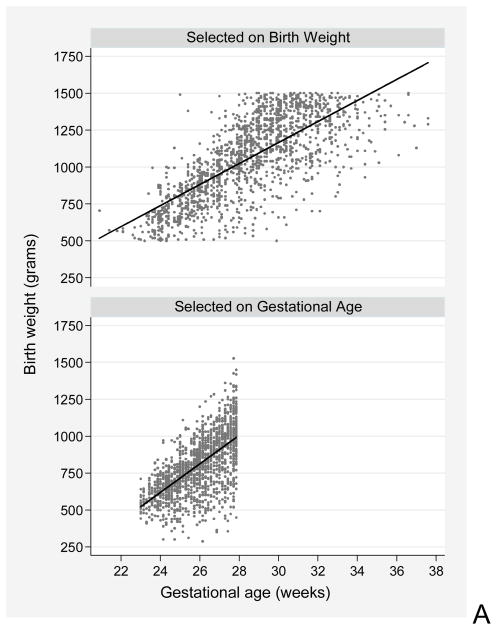
Gestational age and birth-weight distributions in 2 samples (Figure 1)
Figure 1.
These scatterplots demonstrate how birth weight and gestational age distributions of newborns differ depending on enrollment criteria. The top scatterplot is of newborns enrolled on the basis of a birth weight < 1500g(14). All infants whose gestational age at birth is > 33 weeks are in the lowest decile of birth weight for gestational age. The bottom scatterplot is of newborns enrolled on the basis of a gestational age < 28 weeks (9), which avoids the excess of growth-restricted infants seen in the top scatterplot. The lines are regression lines for each sample.
In a birth-weight defined sample (top figure), many severely growth-restricted newborns who were born at older gestational ages than their birth-weight peers are included. A birth weight criterion of 1500g allowed inclusion of babies whose gestational age was as high as 37 weeks. Enrolling preterm newborns on the basis of gestational age (bottom figure) avoids over-inclusion of the most growth restricted, more mature newborns.
DISCUSSION
Our most important finding is that among extremely low gestational age infants, those who were growth restricted were at increased risk of ROP.
Samples of newborns defined by birth weight have an excess of gestationally older, severely growth-restricted newborns. This might distort perceptions of the role of growth restriction in disease processes (8). A recent study found that among moderately to late preterm infants, low birth weight was the strongest predictor of abnormal retinal vascularization(3). Despite the abnormal vascularization, none of these infants had ROP, likely due to the older gestational age of the sample (≥ 32 weeks gestation at birth). It is likely that the interaction of growth restriction and low gestational age at birth is needed for the development of significant ROP. To study the correlates of growth restriction, one must sample based on gestational age (8).
In the ELGAN sample, defined by gestational age less than 28 weeks, we found that in addition to low gestational age, growth restriction was associated with an increased risk of pre-threshold ROP. This was most evident when gestational age- birth-weight Z-score interaction terms were included in the multivariable model. Because all the interaction terms had odds ratios for pre-threshold disease that were appreciably below 1, we infer that the increased risk of ROP associated with growth restriction is independent of gestational age, and that its “contribution” to ROP risk is not additive or multiplicative. Indeed, it might be competitive (17).
Regression models without interaction terms provide information about individual correlates of risk. Adding interaction terms provides information about the influence of combinations of these correlates, and has the potential to clarify the information provided individual correlates of risk. Model 2 in Table 2 indicates that the effect of growth restriction on ROP risk is modified by gestational age. The interaction terms also enhanced the information provided by each of the gestational age categories and SGA.
How growth restriction might influence ROP risk
Growth restriction is a surrogate for low IGF-1 availability
Low blood concentrations of IGF-1 have been associated with ROP(18). A body of literature attests to the close relationship between growth restriction and low concentrations of IGF-1(18–22). Thus, we can raise the possibility that the increased risk of ROP among the most growth restricted reflects sub-optimal availability of IGF-1. Unfortunately, we did not measure IGF-1 in our subjects, and cannot provide a link between growth restriction and ROP risk in our sample.
Growth restriction is an indicator of increased probabilities of epigenetic phenomena
Severely growth-restricted newborns are at increased risk of a wide variety of disorders later in life. This has been attributed to epigenetic phenomena associated with fetal programming(23). In the ELGAN Study, severely growth-restricted newborns did not have a systemic inflammatory signal on the first postnatal day, but two weeks later had a stronger inflammatory response than newborns who were not growth restricted(24). Months later they were at increased risk of bronchopulmonary dysplasia(25). Two years later, they were more likely than their peers to have a low score on a cognition assessment(15). In light of the late consequences of fetal programming, we raise the possibility that the early associations with fetal growth restriction in our extremely preterm sample might be among the earliest consequences of fetal programming.
Limitations and strengths
Our study has three major limitations. First, we are unable to distinguish between causation and association. Second, despite our enrolling a large number of newborns, the small number of children in some of the nine cells defined by three categories of gestational age and three categories of birth weight Z-score limits the power of our study. Third, we did not collect information about IGF-1 concentrations.
Our study also has several strengths. First, our selecting infants based on gestational age minimized confounding due to factors related to fetal growth restriction (8). Second, we collected all of our data prospectively. Third, we adjusted for confounders of the relationships between ROP risk and both gestational age and SGA. Finally, we created a multivariable model that included gestational age and birth-weight Z-score interaction terms.
In summary, in this sample defined by gestational age less than 28 weeks, low gestational age and severe growth restriction were both associated with an increased risk of pre-threshold ROP. We infer that processes associated with fetal growth restriction increase the risk of severe ROP, providing support for antenatal contributions to the occurrence of ROP.
KEY NOTES.
The hypothesis that growth-restricted preterm infants are at increased risk for ROP remains controversial.
In the ELGAN sample, defined by gestational age less than 28 weeks, both low gestational age and severe growth restriction were associated with increased risk of pre-threshold ROP.
We infer that processes associated with fetal growth restriction influence the risk of severe ROP, providing additional support for the claim that ROP has antenatal origins.
Acknowledgments
This research was supported by the National Institute of Neurological Diseases and Stroke (5U01NS040069-05S1 and 2R01NS040069 - 06A2), the National Eye Institute (1R01EY021820), and the National Institute of Child Health and Human Development (NIH-P30-HD-18655).
The authors gratefully acknowledge the contributions of their subjects, and their subjects’ families, as well as those of their colleagues.
List of Abbreviations
- IUGR
intra uterine growth restriction
- ROP
retinopathy of prematurity
- g
grams
- ELGAN
extremely low gestational age newborn
- IGF
insulin like growth factor
Footnotes
None of the authors have any proprietary interests or conflicts of interest related to this submission. This submission has not been published anywhere previously and it is not simultaneously being considered for any other publication.
References
- 1.Holmstrom G, Broberger U, Thomassen P. Neonatal risk factors for retinopathy of prematurity--a population-based study. Acta ophthalmologica Scandinavica. 1998;76:204–7. doi: 10.1034/j.1600-0420.1998.760216.x. [Research Support, Non-U.S Gov’t] [DOI] [PubMed] [Google Scholar]
- 2.Seiberth V, Linderkamp O. Risk factors in retinopathy of prematurity. a multivariate statistical analysis. Ophthalmologica Journal international d’ophtalmologie International journal of ophthalmology Zeitschrift fur Augenheilkunde [Comparative Study] 2000;214:131–5. doi: 10.1159/000027482. [DOI] [PubMed] [Google Scholar]
- 3.Allvin K, Hellstrom A, Dahlgren J, Gronlund MA. Birth weight is the most important predictor of abnormal retinal vascularisation in moderately preterm infants. Acta Paediatr. 2014;103:594–600. doi: 10.1111/apa.12599. [DOI] [PubMed] [Google Scholar]
- 4.Fortes Filho JB, Valiatti FB, Eckert GU, Costa MC, Silveira RC, Procianoy RS. Is being small for gestational age a risk factor for retinopathy of prematurity? A study with 345 very low birth weight preterm infants. J Pediatr (Rio J) 2009;85:48–54. doi: 10.2223/JPED.1870. [Comparative Study] [DOI] [PubMed] [Google Scholar]
- 5.Bardin C, Zelkowitz P, Papageorgiou A. Outcome of small-for-gestational age and appropriate-for-gestational age infants born before 27 weeks of gestation. Pediatrics. 1997;100(2):E4. doi: 10.1542/peds.100.2.e4. [DOI] [PubMed] [Google Scholar]
- 6.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics. 2003;111(6 Pt 1):1273–7. doi: 10.1542/peds.111.6.1273. [DOI] [PubMed] [Google Scholar]
- 7.Allegaert K, Vanhole C, Casteels I, Naulaers G, Debeer A, Cossey V, et al. Perinatal growth characteristics and associated risk of developing threshold retinopathy of prematurity. J AAPOS. 2003;7:34–7. doi: 10.1067/mpa.2003.S1091853102420150. [Research Support, Non-U.S Gov’t] [DOI] [PubMed] [Google Scholar]
- 8.Arnold CC, Kramer MS, Hobbs CA, McLean FH, Usher RH. Very low birth weight: a problematic cohort for epidemiologic studies of very small or immature neonates. Am J Epidemiol. 1991;15(134):604–13. doi: 10.1093/oxfordjournals.aje.a116133. [DOI] [PubMed] [Google Scholar]
- 9.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 11.Early Treatment For Retinopathy Of Prematurity Cooperative G. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 12.Hauspurg AK, Allred EN, Vanderveen DK, Chen M, Bednarek FJ, Cole C, et al. Blood Gases and Retinopathy of Prematurity. The ELGAN Study. Neonatology. 2010;99:104–11. doi: 10.1159/000308454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolsma KW, Allred EN, Chen ML, Duker J, Leviton A, Dammann O, et al. Neonatal Bacteremia and Retinopathy of Prematurity: The ELGAN Study. Arch Ophthalmol. 2011;129:1555–63. doi: 10.1001/archophthalmol.2011.319. [DOI] [PubMed] [Google Scholar]
- 14.Van Marter LJ, Allred EN, Pagano M, Sanocka U, Parad R, Moore M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics. 2000;105:1194–201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 15.Streimish IG, Ehrenkranz RA, Allred EN, O’Shea TM, Kuban KC, Paneth N, et al. Birth weight- and fetal weight-growth restriction: impact on neurodevelopment. Early Hum Dev. 2012;88:765–71. doi: 10.1016/j.earlhumdev.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElrath TF, Allred EN, Kuban KCK, Hecht JL, Onderdonk A, O’Shea TM, et al. Factors associated with small head circumference at birth among infants born before the 28th week. Am J Obstet Gynecol. 2010;203:138.e1–.e8. doi: 10.1016/j.ajog.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–70. doi: 10.1093/ije/dyr213. [Research Support, N.I.H., Extramural Research Support, Non-U.S Gov’t Review] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lofqvist C, Andersson E, Sigurdsson J, Engstrom E, Hard AL, Niklasson A, et al. Longitudinal postnatal weight and insulin-like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol. 2006;124:1711–8. doi: 10.1001/archopht.124.12.1711. [DOI] [PubMed] [Google Scholar]
- 19.Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29:219–25. doi: 10.1203/00006450-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Eremia SC, de Boo HA, Bloomfield FH, Oliver MH, Harding JE. Fetal and amniotic insulin-like growth factor-I supplements improve growth rate in intrauterine growth restriction fetal sheep. Endocrinology. 2007;148:2963–72. doi: 10.1210/en.2006-1701. [DOI] [PubMed] [Google Scholar]
- 21.Engstrom E, Niklasson A, Wikland KA, Ewald U, Hellstrom A. The role of maternal factors, postnatal nutrition, weight gain, and gender in regulation of serum IGF-I among preterm infants. Pediatr Res. 2005;57:605–10. doi: 10.1203/01.PDR.0000155950.67503.BC. [DOI] [PubMed] [Google Scholar]
- 22.Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123:e638–45. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 23.Thorn SR, Rozance PJ, Brown LD, Hay WW., Jr The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med. 2011;29:225–36. doi: 10.1055/s-0031-1275516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McElrath TF, Allred EN, Van Marter L, Fichorova RN, Leviton A. Perinatal systemic inflammatory responses of growth-restricted preterm newborns. Acta Paediatr. 2013;102:e439–42. doi: 10.1111/apa.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bose C, Van Marter LJ, Laughon M, O’Shea TM, Allred EN, Karna P, et al. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124:e450–8. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]