Abstract
Professional health bodies such as the World Health Organization (WHO), the American Academy of Pediatrics (AAP), and the U.S. Department of Health and Human Services (HHS) recommend breast milk as the sole source of food during the first year of life. This position recognizes human milk as being uniquely suited for infant nutrition. Nonetheless, most neonates in the West are fed alternatives by 6 months of age. Although inferior to human milk in most aspects, infant formulas are able to promote effective growth and development. However, while breast-fed infants feature a microbiota dominated by bifidobacteria, the bacterial flora of formula-fed infants is usually heterogeneous with comparatively lower levels of bifidobacteria. Thus, the objective of any infant food manufacturer is to prepare a product that results in a formula-fed infant developing a breast-fed infant-like microbiota. The goal of this focused review is to discuss the structure, synthesis, and function of carbohydrate additives that play a role in governing the composition of the infant microbiome and have other health benefits.
Keywords: Fructan, Inulin, Levan, Fructooligosaccharide, Galactooligosaccharide, Lactose, Lactitol, Lactulose, Polydextrose, Cellulose, Hemicellulose, Xylooligosacharide, Galactomannans, Carrageenans
Graphical Abstract

1. Introduction
The infant food and formula industry is currently valued at $45 billion per year.[1] While global birth rates are declining, the expansion of the middle class in developing countries is driving the sale of infant food products.[2–4] From a health standpoint, this is a curious development, as the influence of breastfeeding on infant health is well known. Breastfeeding not only provides short- and long-term health benefits to children and women but also provides socioeconomic benefits spanning all income levels.[5–9] Accordingly, exclusive breastfeeding is recommended for the first six months of life with continued breastfeeding, complemented by appropriate foods, for up to two years of age or beyond.[10–14]
While many families hope to follow these recommendations, parents in Western countries usually rely on infant formula and other food products for some portion of their child’s nutritional requirements. In situations where it is not feasible to breastfeed, these products are considered the best alternative. By definition, infant formula is a manufactured food product, usually milk-based, and designed for feeding children less than one year of age. In the United States, the molecular composition of infant formula is highly regulated by the federal government and is considered a safe substitute for breast milk. This is a necessary precaution as 25% of children born each year are fed formula from birth.[15, 16] By the time they reach three months of age, three million infants per year are reliant on formula. Most infant foods are milk-based products fortified with ingredients such as vegetable oils, starches, and vitamins to mirror the molecular composition of human milk.[17–19] Molecular composition is critical as infant formula is the sole source of nutrition for a vulnerable population. The first four to six months of life represent a period of rapid growth and development. Thus, nutritional requirements are greater during this period than at any other time in life. Unlike foods included in a mixed diet, inadequate nutrition in formula cannot be compensated for by other foods. Therefore, it is critical that breast milk substitutes meet the dietary requirements needed during infancy to ensure proper growth and development.
An important area of development is the gut microbiota,[20] which plays a critical role in facilitating normal growth and providing optimal protection against infection during the first months of life.[21–24] Microbial gut colonization in infants is influenced by several factors including location of birth, mode of delivery (vaginal vs. caesarian),[25, 26] gestation period,[21] antibiotic use,[21] and method of feeding.[25, 27–29] Prebiotics and probiotics promote gastrointestinal health by governing the composition of the microbiota.
The breast-fed microbiota is dominated by bifidobacteria[30, 31] and lactobacilli[31], whereas the formula-fed microbiota is highly variable in composition and influenced by the molecular composition of the formula.[32] Several studies have suggested that human milk contains oligosaccharides that play a defensive role against pathogens.[33–40] Evidence suggests that several classes of naturally occurring fibers are prebiotics that select for the growth of symbiotes over pathogens. A number of additional studies have demonstrated further health benefits of these molecules, including reducing the incidence of necrotizing enterocolitis (NEC) and improving immune function.[41–45]
In order to mimic these properties, infant food products can be supplemented with carbohydrate additives marketed as prebiotics.[15–17, 19, 28, 32] Three classes of oligosaccharides are currently recognized as prebiotics: (i) inulin-type fructans, (ii) trans-galactooligosaccharides, and (iii) lactulose. Oligosaccharides studied for use in infant formula are presented in Table 1. Formulas supplemented with galacto-oligosaccharides, fructooligosaccharides, inulin, lactulose, and polydextrose have been marketed in Japan and Europe and have been studied extensively in the clinic in the United States. These molecules generally resist gastric acidity, hydrolysis by mammalian enzymes, and intestinal absorption. They are also fermented by the intestinal microflora into short chain fatty acids (SCFAs), which assists in selectively stimulating the growth of symbiotic bacteria in the colon.[46] While many of these molecules have adult food applications,[47–49] this focused review aims to discuss the structure, production, and function of carbohydrates used to assist in the development of a healthy infant gut flora and promote other health benefits.
Table 1.
Oligosaccharides used in commercial or clinical infant formulas.
| Oligosaccharide | Abbreviation | Degree of Polymerization |
Usage |
|---|---|---|---|
| Galactooligosaccharides | GOS | 2–8 residues | Commercial |
| Inulin | IN | 3–60 residues | Commercial |
| Lactulose | LOS | 2 residues | Clinical Trials |
| Polydextrose | PDX | 3–30 residues | Clinical Trials |
| Fructooligosaccharides | FOS | 2–8 residues | Clinical Trials |
2. Carbohydrate additives
2.1. Fructans
2.1.1. Chemical structure
Fructans are a heterogeneous group of polymers composed of fructose (1) residues.[50–53] The chemical structure of a fructan varies with respect to glycosidic linkage and degree of polymerization.[51–57] In general, fructan structure consists of a linear chain of fructose (1) units terminated by a glucose (2) residue (Figure 1). The simplest example of this structure is sucrose (3), which contains one fructose (1) unit terminated by a glucose (2) residue. Chains contain either β−2,1 or β−2,6 glycosidic bonds suggesting that the polymeric chain is resistant to enzymes in the human digestive track specific for α-glycosidic linkages. Chain lengths are highly variable and incorporate between 2 and 200 residues. Due to the heterogeneity of the family, fructans have been organized into structurally distinct subclasses based on chain length: short chain fructooligosaccharides (scFOS), oligofructose (fructooligosaccharides, FOS), inulin (4), and levan (5).[58, 59]
Figure 1.

Structure of fructans and their common building blocks. Fructan type oligosaccharides are composed of sucrose and fructose residues.
scFOS are defined as mixed, polymerized chains of fructose (1) incorporating between 3 and 5 residues per chain.[60, 61] Oligofructose contain between 6 and 10 residues, with 9 residues being most common. Inulin (4) has a degree of polymerization ranging from below 10 to 200 residues with an average of 12 units per chain. Interestingly, while plant inulin chains incorporate fewer than 200 residues, bacterial inulin chains incorporate 10,000 to 100,000 residues per polymer.[62] Inulin (4) containing a degree of polymerization above 10 is known as long-chain inulin. Finally, levan oligosaccharides (5) are structurally similar to inulin (4), but feature β−2,6 glycosidic bonds.
2.1.2. Production and synthesis
scFOS and oligofructose (fructooligosaccharides) are produced by β-fructofuranosidase, a transfructosylating enzyme that transfers fructose (1) from one molecule of sucrose (3) to another (Figure 2).[63, 64] β-fructofuranosidase first breaks the β−2,1 sucrose glycosidic bond then covalently binds to the freed fructose (1) residue to form intermediate (6). The fructose (1) unit of this covalently linked intermediate (6) is then transferred to a molecule of sucrose (3) or additional fructooligosaccahride.[64] The raw starting material is sugar beet, a plant whose juices contain ca. 60% sucrose (3).[65] Fructans are also directly isolable in varying amounts from onions, garlic, artichoke, wheat, and bananas. During fructan production, three linear oligomers are produced: 1-kestose (40%) (7), nystose (50%) (8), and 1-fructofuranosylnistose (10%) (9). Additionally, scFOS, oligofructose, and inulin (4) can all be produced on industrial scale through the hydrolysis of “long-chain inulin” isolated from chicory, dahlia, or Jerusalem artichoke.[64] Levan (5) is produced by levansucrase, a fructosyltrasferase with both hydrolase and transferase activities that belongs to the glycoside hydrolase (GH) family 68.[66–69] As it is of the same clan of enzymes (GH-J) as β-fructofuranosidase, levansucrase uses sucrose (3) as its source of fructose (1).[69]
Figure 2.
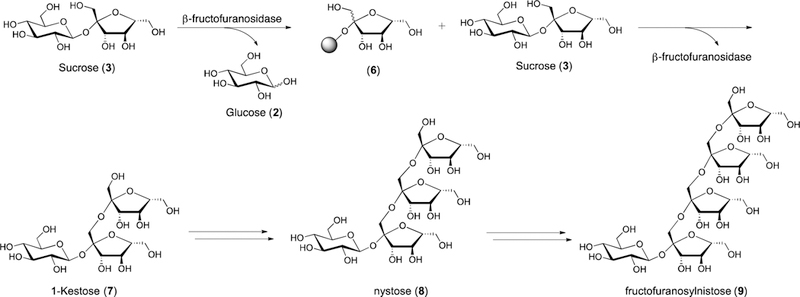
Enzymatic fructan production. The shaded circle in structure (6) represents a covalent linksage between the carbohydrate and the enzyme β-fructofuranosidase.
2.1.3. Function
Fructans (4–5) are a class of nondigestible oligosaccharides [70–75] that provide both energy and immunoprotective benefits.[73] Because the pancreas and small intestinal mucosa do not secrete enzymes capable of hydrolyzing β−1,2 fructosylfructose glycosyl linkages, fructans are not degraded between the mouth and small intestine. Once they reach the colon, bacteria ferment fructans into SCFAs.[46]
In vitro culture studies have demonstrated scFOS and oligofructose are selectively used by bifidobacteria and lactobacilli.[76–78] Marx and coworkers have studied the growth of various strains of bifidobacteria using fructans as the sole carbon source.[79] The study demonstrated that different strains of bifidobacteria are capable of metabolizing β−2,6 linked FOS when it is supplied as the sole carbon source. B. adolescentis demonstrated the greatest growth increase and was the only strain successful at metabolizing fructans of all lengths. Growth of other strains slowed or stopped once short-chain oligosaccharides were consumed. In a related experiment, Kaplan and Hutkins measured the ability of distinct strains of lactobacilli to ferment scFOS.[80] Of the 16 strains of lactic acid bacteria examined, 12 were able to ferment FOS to SCFAs. Interestingly, some bifidobacteria and lactobacilli are substrate specific and act selectively on fructans based on chain length. For example, some species of lactobacilli are able to use 1-kestose (7) and nystose (8) but not fructosylnystose (9).[81]
Fructan supplementation has been shown to increase the occurrence of beneficial bacteria, such as bifidobacteria and lactobacilli, while decreasing counts of potential pathogens, such as Clostridium perfringens and Escherichia coli.[82, 83] This provides a secondary mechanism by which fructans contribute to a healthy microbiota. Moreover, because the fermentation of fructans produces SCFAs, the pH of the gut is lowered, which inhibits the growth of many pathogenic species.[46, 84]
The benefit of fructans for digestive health extends beyond balancing gut composition to positively impacting various aspects of digestive health spanning regulation of inflammation and immune responses to preventing diarrhea.[85–88] These health effects are likely due to the promotion of select bacteria, which are known to be immunostimulatory (e.g., bifidobacteria and lactobacilli) and/or the increase in concentration of selected SCFAs, which are known to promote healthy colonic tissue and function.[84] Moreover, because the increased presence of fructans increases the amount of carbohydrates in the gut, carbohydrate metabolism will be increased relative to milk protein metabolism. This shift would be accompanied by an increase in the healthy byproducts of carbohydrate metabolism, such as SCFAs, but not the harmful by-products produced through protein metabolism and fermentation, such as urea, uric acid, ammonia, and nitrogenated heterocycles.[89, 90]
Inulin (4) and oligofructose are considered safe inducers of a bifidus flora. The first prebiotics added to infant foods featured a combination of GOS (12–14) and long-chain inulin (4), vide infra.[91] While this formulation has been distributed throughout Europe for close to twenty years, it is not currently available in the American market. Post-marketing reports on this formulation suggest a beneficial effect on the infant gut flora after one month of use as counts of bifidobacteria and lactobaccili increased significantly from baseline levels to levels observed in breast-fed infants.[92, 93] Additionally, the species of bifidobacteria and lactobacilli present paralleled those seen in breast-fed children.[94] A significant reduction in common pathogens was also observed.[95]
Fructans (4–5) also have the potential to modulate immune activity. Various studies have shown that a combination of inulin (4) or GOS (12–14) and FOS (4–5) tends to increase blood IgG levels in infants.[96, 97] IgG is an important antibody in the fight against bacterial and viral infections. Additionally, an inulin (4)/GOS (12–14) mixture was shown to lead to a reduced occurrence of allergic symptoms in infants as shown by increased levels of IgA in fecal matter.[98, 99] Other immunological properties attributed to fructans are detailed in a recent review by Vogt, et. al.[100]
Inulin (4) and oligofructose have also been studied in weaning foods for toddlers. In addition to decreasing the presence of pathogens and increasing the presence of bifidobacteria, inulin (4) and oligofructose supplementation led to fewer incidences of fever and diarrhea in healthy toddlers.[96]
2.2. Galactooligosaccharides (GOS)
2.2.1. Chemical structure
Galactooligosaccharides (GOS) (12–14) are nondigestible oligosaccharides composed of repeating galactose (10) units capped with lactose (11) at the reducing end (Figure 3). The chemical structure of GOS (12–14) varies with respect to chain length, branching, and glycosyl linkage. GOS (12–14) typically have degrees of polymerization ranging from 2 to 10 Glycosyl linkages include β−1,3 (12), β−1,4 (13), and/or β−1,6 (14), and the type of linkage is determined by enzyme source.[101, 102] For example, β-galactosidases from Kluyveromyces lactis and Aspergillus oryzae generate predominantly β−1,6 linked GOS (14).[103, 104] In contrast, β-galactosidase from Bacillus circulans produces predominately β−1,4 linked GOS (13).[103, 104]
Figure 3.
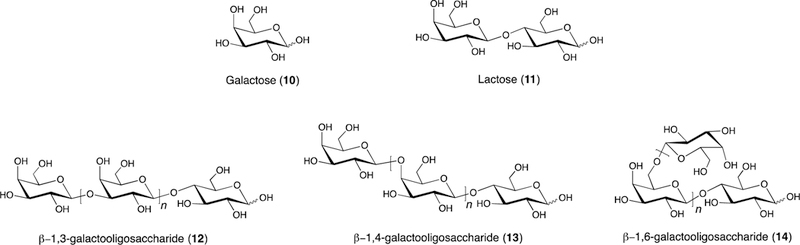
Stucture of galactooligosaccharides (GOS) and GOS building blocks.
2.2.2. Production and synthesis
Galactooligosaccharides (12–14) are produced by the transglycosylation of lactose (11) by microbial β-galactosidases (Figure 4). Although GOS (12–14) can be prepared from purified lactose, it is typically prepared from lactose in the whey by-products of the dairy industry.[105, 106] β-galactosidase is a glycosyl hydrolase with two catalytic activities, hydrolysis and transglycosylation.[107, 108] The rate of hydrolysis to transglycosylation is dependent on enzyme source, but it can be altered by several factors including substrate concentration, temperature, buffer, reaction time, and pH.[109–111] Following cleavage of the glucose-galactose glycosidic bond of lactose (11), a covalent bond is formed between β-galactosidase and galactose (10). Next, the galactose residue from this covalent intermediate (15) is transferred to an acceptor. When the acceptor is lactose (11), the resulting trisaccharide is a β−1,3 galactooligosaccharide (16). This cycle can then be repeated to further elongate the original galactooligosaccahride to yield longer-chain β−1,3 galactooligosaccharides (12). Because any available nucleophile, including water, can serve as the acceptor, the resultant GOS mixture contains substantial amounts of glucose (2) and galactose (10) (products of lactose hydrolysis), along with unreacted lactose (11) and GOS of various lengths. Commercially available GOS products contain a range of concentration of monosaccharide and lactose byproducts but typically contains upwards of 55% GOS content.[101, 105]
Figure 4.

Enzymatic GOS (12–14) production. The shaded circle in compound (15) represents β-galactosidase, the enzyme that facilitates the iterative glycosylation process.
While β-galactosidases are found across nearly all species, microbial β-galactosidases from fungi, yeast, and bacteria are commonly used in the industrial synthesis of GOS (12–14) due to ease of handling and increased oligosaccharide yields.[101] Additionally, β-galactosidases are used in a variety of forms including both crude and purified enzymes, recombinant enzymes, immobilized enzymes, and whole-cell biotransformations.[101] Protein engineering to increase GOS (12–14) production has also been explored. The Jorgensen group found that a truncated β-galactosidase from B. bifidum increased the transgalactosylation activity of the enzyme relative to the parent enzyme.[101, 112] Immobilized enzymes provide a means of “green” prod uction as the enzymes can be reused for several batches and often increase GOS (12–14) production. Systems for β-galactosidase immobilization include microparticle carriers such as ion exchange resins, chitosan, cellulose, agarose or acrylic beads, and silica nanoparticles.[77, 101, 106, 113, 114] Fibrous supports such as cotton cloths are also used.[101, 106]
2.2.3. Function
Similar to fructans, GOS (12–14) are able to withstand the acidic environment of the stomach and the digestive enzymes of the small intestine. Once in the large intestine, they act as prebiotics and are fermented selectively by symbiotic bacteria. The biological effects of human milk versus infant formula supplemented with GOS (12–14) have been studied. A recent review by Macfarlane and coworkers gives an extensive outline of the bacterial metabolism and health-related benefits of GOS (12–14) and other prebiotics.[106] In this review, the authors summarize several studies on the effect of GOS (12–14) on the microbiota of infants. These studies show that infants fed GOS (12–14) have higher counts of certain bifidobacteria and lactobacilli strains in their feces than those on a placebo. Watson and co-workers found that when compared to other carbohydrates, GOS (12–14) and lactulose (24) (described below) have favorable growth characteristics for 29 strains of lactobacilli and 39 strains of bifidobacteria.[31] The authors also suggested that although not all strains of tested bacteria have the ability to use GOS (12–14), it is possible the intestinal community may metabolize GOS (12–14) in a way beneficial to these strains. In a study by Haarman and Knol, it was found that infants fed a combined GOS (12–14)/inulin (4) (9:1 ratio) supplement had increased total bifidobacteria counts dominated by B. infantis, B. longum, and B. breve. This microbiota composition is similar to that of breast-fed infants.[93],[106]
GOS (12–14) supplementation is accompanied by a number of safety concerns. GOS-supplemented formula tends to result in softer stool. Additionally, infant dehydration was observed but was eventually found negligible in a study by Williams et. al.[115]
In a study by Verheijden and coworkers, dietary GOS (12–14) were shown to suppress allergic asthma in a murine model.[116] Similarly, it has been shown that GOS (12–14) enhances response to vaccination and decreases development of allergic responses in murine models.[117] This study was extended to clinical trials of infants with a family history of allergy. Infants fed a GOS-supplemented diet had a reduction of plasma IgE levels and an increase of plasma IgG4 levels, which correlates with reduced allergic response.[117] Using a piglet model, Alizadeh and et. al found that in addition to stimulating the development of a healthy microbiome, GOS (12–14) were able to modulate responses of the innate immune system.[118] Specifically, they saw an increase in pBD-2, a defensin that provides protection against invading pathogens, and sIgA, which binds harmful antigens and neutralizes toxins. These results and others demonstrate the possibility of an immune system enhanced by GOS-supplemented formula.[119]
2.3. Lactose and congeners
2.3.1. Chemical structure, production, and synthesis
Lactose (11) is a disaccharide consisting of glucose (2) at the reducing end linked to galactose (10) through a β−1,4 linkage. In mature human milk, lactose (11) is the largest macronutrient component at a concentration of 70 g/L.[120] Lactitol (22) is a non-naturally occurring sugar alcohol derived from the reduction of lactose (11). Lactulose (24) is an unnatural ketose disaccharide consisting of a reducing end fructose (1) linked to galactose (10) through a β−1,4 glycosydic linkage.
Lactose (11) is synthesized in the mammory gland by the lactose synthase complex via the transfer of uridine 5’-diphosphate galactose (UDP-galactose) (19) to glucose (2) (Figure 5B). The generation of UDP-galactose (19) can occur through two pathways. In the first of these pathways, glucose 1-phosphate (17) is converted to UDP-glucose (18) by UDP-glucose pyrophosphorylase (Figure 5A). Next, UDP-glucose 4-epimerase epimerizes the C4 stereocenter to generate UDP-galactose (19). Presumably, epimerization occurs through selective oxidization of the alcohol to its corresponding ketone followed by reduction back to the alcohol. In the second possible pathway, galactose (10) is phosphorylated by galactokinase to yield galactose 1-phosphate (20) (Figure 5A). Galctose-1-phosphate uridylyltransferase then transfers a uridine 5’-monophosphate (UMP) from UDP-glucose (18) to galactose-1-phosphate (20) to generate glucose 1-phosphate (17) and UDP-galactose (19).[121]
Figure 5.

Uridine 5’-diphosphate galactose (UDP-galactose, 19) generation and lactose (11) biosynthesis.
The lactose synthase complex consists of two proteins, galactosyltransferase and α-lactalbumin.[122] While galactosyltransferase is found outside the mammory gland, α-lactalbumin is expressed only in the mammory gland. α-lactalbumin is vital to lactose production as it promotes the preferential use of glucose (2) by galactosyltransferase. In the absence of α-lactalbumin, glucose (2) is a poor substrate and galactosyltransferase will catalyze the transfer of UDP-galactose (19) to N-acetylglucosamine. In the presence of α-lactalbumin, however, the Km of galactosyltransferase for glucose (2) is reduced by three orders of magnitude, allowing for lactose (11) synthesis at physiological glucose concentrations.[123]
Lactitol (22) was first described by J.B. Senderens in 1920. Senderens, along with Paul Sabatier, also founded the modern process for lactitol (22) production via lactose (11) hydrogenation using a nickel catalyst.[124] Following this initial preparation, additional preparations were described by Karrier and Buchi and Wolfram, et. al.[125] Currently, lactitol (22) is prepared industrially via catalytic hydrogenation (Figure 6). Once heated to 100°C, lactose ( 11) in its open chain form (21) is subjected to hydrogenolysis at 40 bar. The solution is then filtered, purified using ion-exchange resins and activated carbon, concentrated, and crystallized to yield lactitol (22).[125] Lactose (11) hydrogenation can, however, often result in numerous undesired side products.[126] Hydrogenation is generally performed with a Raney nickel or sponge nickel catalyst.[125] Ruthenium over carbon catalysts have also been used. While sponge nickel and Ru/C catalysts have been shown to be highly selective for lactitol (22) production, these catalysts can suffer from severe catalyst deactivation.[126]
Figure 6.

Lactitol (22) is produced through hydrogenation of Lactose (10).
Lactulose (24) is an isomeric form of lactose (11) produced through Lobry de Bruyn-Alberda Ekenstein rearrangement of lactose (11) in basic solution (Figure 7A). The initial preparation of lactulose (24) was reported by Montgomery and Hudson in 1930 via the heating of a lactose solution in limewater.[127] Since its initial preparation, a variety of catalysts for lactulose production have been reported including various alkaline hydroxides, sulphites, phosphates, and tertiary amines.[128] A second method of production relies on the Amadori rearrangement (Figure 7B). Under this method, reaction with an amine and subsequent hydrolysis results in lactulose formation. To reduce side products, aluminates or borates are added as complexation reagents to shift the reaction equilibrium toward lactulose production.[129] Lactulose (24) is also produced by heating milk. As such, lactulose (24) may be present in heat-processed dairy products even though it is not present in nature.[130],[131] Finally, lactulose (24) can be synthesized via enzymatic synthesis using β-galactosidases from various sources, such as Kluyveromyces lactis and Aspergillus oryzae, in a transglycosylation reaction of lactose (11) and fructose (1).[132], [133]
Figure 7.

Lactulose (24) is synthesized from lactose (11) using either the Lobry de Bruyn-Alberda Ekenstein or Amadori rearrangements.
2.3.2. Function
Lactose (11) cannot be directly absorbed in the small intestine. Rather, when lactose (11) reaches the small intestine, it is hydrolyzed into galactose (10) and glucose (2) by the intestinal lactase β-galactosidase.[134] Glucose (2) then enters circulation where it is used as an energy source. Galactose (10), however, must be converted to glucose (2) in the liver before it can be used as an energy source.[135] When lactose (11) is digested in the small intestine, it provides about 4 kcal/g of energy. Although lactase deficiency causing lactose intolerance is rare in full-term infants, if lactose (11) escapes digestion it is fermented by saccharolytic colonic bacteria into SCFAs providing approximately 2 kcal/g of energy.[136] This anomaly has led some to consider lactose (11) as a conditional prebiotic.[137]
While simple sugars such as glucose (2), sucrose (3), or fructose (1) are also potential energy sources, their increased sweetness relative to lactose (11) makes them less appealing as carbohydrate infant formula additives. An increased sweetness of milk has been thought to potentially encourage overeating and lead to an increased preference for sweet foods. In addition to lower sweetness levels, lactose (11) has been found to be less cariogenic than simple sugars. This is likely due to the relatively slow fermentation of lactose (11) in the oral cavity.[136]
Interestingly, lactose (11) enhances mineral absorption. In a study by Ziegler and Fomon, infants fed formula featuring lactose (11) as the sole carbohydrate source showed significantly greater net absorption and retention of calcium, magnesium, and manganese than infants fed formula containing sucrose (3) and corn starch hydrolysate.[138] In a similar study by Abrams, et. al. infant formula containing lactose (11) was found to significantly increase calcium absorption compared to formula containging glucose polymers.[139] Moya et. al. also found lactose (11) to enhance calcium absorption compared to glucose polymers.[140]
Unlike lactose (11), lactulose (24) cannot be broken down by lactase and is not digested and absorbed in the small intestine.[141] Instead, lactulose (24) is fermented predominately by bifidobacteria and lactobacilli. The lack of digestion and the selective fermentation by symbiotic bacteria qualifies lactulose (24) as a prebiotic. Bacterial fermentation of lactulose (24) results in about 2 kcal/g of energy.[136] Petuely first reported lactulose’s ability to serve as a bifidus factor in 1957.[142] Since this initial report, lactulose’s prebiotic effects have been well documented and it is often cited as the original prebiotic.[143]
In a report by Nagendra et. al., supplementation of infant formula with 0.5–1.0% lactulose (24) was sufficient to stimulate bifidobacteria growth comparable to that found in breast-fed infants. Lactulose incorporation of 1%, however, also resulted in a partial laxative effect. In addition, formula with 0.5% lactulose (24) was shown to have similar pH lowering effects as that of breast milk.[144] Numerous additional studies have illustrated the ability of lactulose (24) to stimulate the growth of bifidobacteria and lactobacilli.[31, 145–147] In a study by Watson and coworkers, 29 human-derived lactobacilli and 39 bifidobacteria strains were tested for their ability to metabolize ten different carbohydrates. Of the ten tested, lactulose (24) (along with GOS) supported the most favorable growth for both lactobacilli and bifidobacteria.[31] In an additional study by Rastall et. al. comparing in vitro fermenation of various disaccharides by lactobacilli and bifidobacertia, lactulose (24) was again found to support one of the highest levels of growth for both bacterial types.[148]
In addition to increasing counts of healthy bacteria like bifidobacteria and lactobacilli, lactulose (24) has also been shown to inhibit the growth of several gram-negative bacteria. In a nine week study by Ballongue et. al., the effect of lactulose (24) on human intestinal flora composition was determined via microbial analysis of feces.[149] Their analysis showed a decrease in the populations of Bacterosides, Clostridium, Eubacterium, and coliforms. This decrease was accompanied by an increase in bifidobacteria and lactobacilli populations and a drop in fecal pH. The ability of bifidobacteria and lactobacilli to better use lactulose (24) was also shown by Rastall et. al.[148] In this study, while lactulose (24) was found to increase bacterial populations of Bacterosides, Clostridium, and Eubacterium after 12 h of fermentation, the increase in bacterial counts for bifidobacteria and lactobacilli were much larger. These selective growth patterns gave lactulose (24) the second highest prebiotic score of the fructose-containing disaccharides tested.
Much like lactose (11), lactulose (24) does not cause tooth decay do to its low cariogenicity.[136] Lactulose (24) is also similar to lactose (11) in its lowered sweetness compared to sucrose (3). It is, however, sweeter than lactose (11).[150]
Lactitol (22) is not digested in the upper gastrointestinal tract due to the lack of a suitable β-galactosidase. A study by Jägerstad and Nilsson fo und hydrolytic activities of β-galactosidase towards lactitol (22) to be less than 2% of that towards lactose (11).[151] Lactitol (22) thus reaches the colon where it can be metabolized by saccharolytic bacteria to provide 2 kcal/g.[136] As with lactulose (24), higher lactitol (22) intakes can produce laxative effects. Several studies have shown lactitol (22) to act as a prebiotic by promoting the growth of bifidobacteria and lactobacilli.[125, 145, 146] Kontula et. al. tested nine strains of lactic acid bacteria and human fecal and biopsy isolates.[152] While various lactobacilli strains were found to grow well on lactitol (22), lactulose (24) was generally able to stimulate greater bacterial growth. Similar results were found in an in vitro study by Kneifel et. al. investigating the influence of various reported prebiotic carbohydrates on the growth of several bifidobacteria and lactobacilli strains.[147] Again, while lactitol (22) was shown to promote growth for several of the tested strains, the growth was not as high or as consistent as was seen with lactulose (24).
Fermentation of lactitol (22) by saccharolytic bacteria has also been shown to decrease counts of proteolytic bacteria such as Bacteroides, coliforms, Enterobacteria, and Enterococci.[125, 153] In the aforementioned study by Ballongue et. al., lactitol treatment over nine weeks was shown to decrease bacterial populations of Bacteroides, Clostridium, coliforms, and Eubacterium. The population decreases were not as large as those seen with lactulose treatment. Additionally, the tempered population decreases were accompanied by a smaller drop in fecal pH with lactitol (22) as compared to lactulose (24). This led to the conclusion that the total concentration of undissociated acids may not have been large enough to inhibit the gram-negative flora sufficiently.[149]
As a polyol, lactitol (22) is not fermented, or is fermented poorly, by oral bacteria. As the acid fermentation products would dematerialize enamel and cause cavities, lactitol (22) is considered non-cariogenic.[125, 136] Lactitol (22) is also considered to be of mild sweetness and has been found to have a lower sweetness than lactulose (24). For many, the lowered sweetness of lactitol (22) serves as an advantage of lactitol-supplementation compared to lactulose-supplementation.[125, 150]
2.4. Glucose Polymers
2.4.1. Chemical structure
Glucose polymers exist in several forms and can be categorized as starch, glycogen, or cellulose (27), each of which has subclasses (Figure 9). Cellulose (27) is the most abundant glucose polymer found in nature and consists of hundreds to thousands of glucose (2) residues connected through β−1,4 linkages.[154] Largely found in plant cell walls, cellulose (27) is a linear polysaccharide with no branching.
Figure 9.

Structures of the different subclasses of hemicellulose polymers.
While animals can store energy as glycogen, plants store energy in the form of starch. Starch consist of α−1,4 and/or α−1,6 linked glucose (2) residues. Amylose (28), a subcategory of starch, consist of linear polymers containing only α−1,4 linkages.[154] Amylopectin (29) are branched polysaccharides containing both α−1,4 and α−1,6 linkages.[154] Although starch consists of α-linkages, which have the potential to be broken down by human enzymes in the small intestine, resistant starch, named for their ability to resist hydrolysis, are not hydrolyzed in the small intestine due their complex branching motifs. Instead, they are often fermented by bacteria in the colon.[154]
Polydextrose (30) is an artificial glucose polymer synthesized by heating glucose (2) in the presence of acid and sorbitol.[155] Due to its method of synthesis, polydextrose (30) is randomly branched with both α and β linkages. Many feature β−1,6 glycosidic linkages and contain sorbitol or citric acid residues.[156]
2.4.2. Production, synthesis, and function.
While the cellulose (27) and starch found in plants are synthesized by cellulose and starch synthases,[157] glucose polymers found in infant formula are typically produced through the hydrolysis of starch into smaller oligosaccharides with chain lengths ranging from 5 to 10 glucose (2) units.[158] Handling methods and source of glucose polymer additives alter the biological effect of these polymers.[158–160]
Fortification of infant foods is most important for infants who are born preterm and/or with low-birth weights as well as those with short bowel syndrome, metabolic disorders, or lactose intolerance.[158, 160] As fortifiers, glucose polymers provide carbohydrate and energy sources to meet some of the nutritional requirements of fast-growing or diet-restricted infants.[159, 160] Lactose (11) and various monosaccharides are often substituted with glucose polymers to lower the osmotic concentration of infant formula.[158, 161] A decrease in osmotic concentration is thought to be advantageous because hyperosmolarity is associated with diarrhea and necrotizing enterocolitis.[158, 161, 162] Conversely, fortifying human milk with glucose polymers has been shown to increase the osmotic load due to the action of endogenous breast milk amylase that breaks down polysaccharides into smaller oligosaccharides and monosaccharides.[18, 159, 160]
Reducing the amount of lactose (11) in formula is also beneficial for low-birth weight and premature infants because they have increased chances of lactose intolerance.[162] A study by Fagundes-Neto and coworkers demonstrated that symptoms of infants who had diarrhea due to lactose intolerance were improved by replacing cow milk-based formula with a lactose-free soy protein formula supplemented with glucose polymers.[162]
Not surprisingly, glucose polymers added to infant formula vary in length due to the aggressive method of production (acidic hydrolysis). Consequently, polymer lengths affect break down and absorption of glucose polymers in the body. In a study monitoring the absorption and oxidation of glucose polymers by young infants, it was shown that long-chain glucose polymers were absorbed at a slower rate than short-chain glucose polymers.[164] While it is thought that a lack of pancreatic amylase in infants less than four months old accounts for the lack of absorption of glucose polymers, other mechanisms such as salivary amylase and colonic flora may also aid in the absorption of glucose polymers.[164] In one Canadian study, an infant diagnosed with glycogen storage disease type 1b was successfully treated with a diet including soy-based infant formula enriched with glucose polymers from cornstarch.[163]
Glucose polymers are also used as thickening agents. Infant formula thickened with modified starch from rice increased the availability of calcium, iron, and zinc from formula when compared to a standard infant formula control.[165, 166] In the same study, when maltodrextrin was used as the thickener there was an increase in calcium availability but a decrease in both iron and zinc availability.
Dumping syndrome is a common complication in children and infants. The need to delay gastric emptying time in these patients has led to treatments that replace shorter saccharides with complex carbohydrates such as uncooked starch.[167]
While polydextrose (30) is an FDA-approved food additive for adults and children, it has not yet been approved for use in infant foods. Herfel et. al. sought to investigate the safety of polydextrose (30) in infant formula by studying its effect on neonatal pigs.[156] It was found that polydextrose (30) was non-toxic and displayed prebiotic properties similar to known prebiotics.
Unlike starch, cellulose (27) and its derivatives are typically banned from use in infant formula. One derivative of cellulose that can be used for special medical purposes in infants and toddlers is carboxymethyl cellulose (CMC).[168] The European Union allows a low dosage (10 g/L) of CMC for infants who have disorders in fatty acid metabolism in order to help maintain a stable blood-glucose level.[168]
2.5. Hemicellulose
2.5.1. Chemical Structure
Hemicelluloses are heteropolymers found in plant cell walls. Incorporating several different compositional motifs, their structure and abundance vary among different species and cell types (Figure 9).[169] Xylans (31) are polysaccharides with a backbone featuring repeating xylose residues connected via β−1,4 linkages.[169] Modifications of the xylose backbone vary, but some common modifications include glucuronosylation, arabinosylation, and acetylation. Xylooligosaccharides (XOS) (31) are composed of varying numbers of β−1,4 linked xylose residues.[170] Glucuronoxylans (32) are composed of a polymeric β−1,4 linked xylose backbone with glucuronic acid branches and acetyl groups.[171] Arabinoxylans (33) are polysaccharides consisting of a polymeric β−1,4 linked xylose backbone with arabinose substituents of varying linkages.[172] Glucomannans (34) are a component of secondary cell walls that aid in structural stability.[173] They are branched polysaccharides isolated from the tubers and stems of plants containing D-mannose and D-glucose in a molar ratio of 1.6:1. Xyloglucans (35) contain a polymeric β−1,4 linked glucose backbone with various xylose side chains.[169] The xylose side chains can include other carbohydrate moieties such as galactose (10), arabinose, and fucose.[169]
2.5.2. Production and Synthesis
Hemicellulose biosynthesis occurs in the Golgi and uses a number of glycosyltransferases. Due to the varying compositions of hemicelluloses, there are a number of unknowns concerning the specific enzymes involved in their production.[169] Xylan biosynthesis features several membrane-bound transferases that elongate the xylose backbone and install various modifications.[174] While XOS (31) are present in small quantities in bamboo shoots, fruits, vegetables, milk, and honey, commercial XOS sources consist of the hydrolysis products of xylan containing lignocellulosic materials.[170] Glucuronoxylan biosynthesis uses a number of enzymes including glycosyltransferases, acetyl- and methyl transferases.[171] Arabinoxylan biosynthesis is believed to occur by the action of a xylopyranose synthase and arabinose synthase.[175] Glucomannan (34) is produced by a glucomannan synthase complex.[176] Finally, the backbone of xyloglucans is synthesized by cellulose synthase-like proteins, and the side chains are added with the aid of the corresponding glycosyltransferases.[169]
2.5.3. Function
There are few studies to date detailing the function of hemicelluloses in infant formula. Nevertheless, several hemicellulose mixtures intended for use in infant formula have been patented. In one recent example, xylans (31), arabinoxylans, (33) glucomannans, (34), and xyloglucans (35) were added to infant formula due to their symbiotic effects on Bifidobacterium breve and their potential prevention of immune disorders.[177]
XOS (31) are believed to be fermented slower than other prebiotic oligosaccharides resulting in an increased production of butyric acid, an important SCFA.[46, 178] Arabinoxylans (33) are considered to be prebiotics and to improve the function of the intestinal epithelium. Unfortunately, there exist no extensive studies on their efficacy in infant foods.[179]
Glucomannans (34) form gels and are used as thickening agents in foods. Clinical trials have assessed the effects of glucomannan (34) on children with constipation.[180] However, no studies have included infants due to concerns regarding undesired effects on growth rate and/or vitamin status. In a mouse model, the prebiotic effects of glucomannan (34), acid-hydrolyzed glucomannan, and cellulose (27) were compared.[181] While the acid-hydrolyzed glucomannan had the greatest prebiotic effect, it was found that both types of glucommanans was more capable of promoting the growth of intestinal bifidobacteria and suppressed the growth of C. perfringens, a pathogenic bacterium, when compared to cellulose.
2.6. Other Plant Polymers
2.6.1. Chemical stucture
Pectins (36) are a family of structurally complex polysaccharides composed mainly of galacturonic acid in an α−1,4 linked backbone.[182] Pectins (36) also incorporate other monosaccharides into both the backbone and side chains. These polysaccharides are diversified further via methyl esterification and acetylation.[182]
Galactomannans (37) consist of β−1,4 linked mannose residues with varying amounts of α−1,6 linked galactose (10) residues.[183] Carrageenans (38) are a family of sulfated galactans isolated from red seaweeds of the Rhodophyceae class.[184] They are linear hydrophilic saccharides containing varying numbers of α and β linkages as well as varying degrees of sulfonation.[184]
2.6.2. Production and synthesis
Due to the diverse structure of pectins (36), there are over 67 biosynthetic transferases used during biosynthesis. Research continues to elucidate how these transferases work together to make this class of complex polysaccharides.[182]
Galactomannan synthesis requires three enzymes: mannan synthase, galactosyltransferase, and α-galactosidase.[185] The mannan backbone is constructed by mannan synthase, while the galactosyl side chains are added by the action of galactosyltransferases. Finally, α-galactosidase is responsible for the degree of galactose substituion.
Recent genome sequencing of several seaweeds has given insight into the enzymes involved in carrageenan biosynthesis.[186] Accordingly, glycosyl transferases are believed to be involved in the biosynthesis. Carbohydrate sulfotransferases are responsible for modifying the carbohydrate moieties with sulfo groups.
2.6.3. Function
Prebiotic mixtures containing acidic pectic oligosaccharides have been used recently to mimic the beneficial effects of the acidic fraction of human milk oligosaccharides. The immune modulating effects of these prebiotic mixtures show their ability to influence the development of an infant’s immune response. A study by Stam and coworkers demonstrated that a combination of GOS (12–14), long chain FOS, and pectin-derived acidic oligosaccharides had no adverse effects on vaccine specific antibody levels in healthy term infants.[187] Additionally, it was postulated that this mixture could promote TH1 and Treg dependent immune responses and induce a downstream regulation of IgE allergic responses. In two mice studies it was shown that adding a similar oligosaccharide mixture containing acidic pectic oligosaccharides had similar immunologic benefits. In one of these mice studies a combination of GOS/FOS mixture and arabino-oligosaccharides gave an optimal TH1 dependent DTH response and reduced TH2 cytokine production in young adult mice compared to GOS/FOS alone .[188] In the second mouse study, the same combination of oligosaccharides decreased symptoms of allergic asthma, such as airway hyperreactivity and inflammatory cells.[189]
Bean gum (galactomannan) (37), sodium carboxymethylcellulose (Gelilact), pectin (36), and cellulose (27) are used as milk-thickening agents, which help to reduce regurgitation and improve sleep.[190] Carrageenans (38) can also be added to infant formulas as thickening agents.[168]
Some pectins (36) and hemicelluloses exhibit undesired effects. In an in vitro model, supplementation of infant formulas with either esterified pectin or locust bean gum resulted in a decrease in the availability of calcium, iron, and zinc when compared to standard infant formula and mature human milk.[165] The decrease in the availability of these nutrients has the potential to correlate to a change in the bioavailability of nutrients in vivo.
Fabiani and coworkers found no significant effect of galactomannans (37) on gastric emptying time when fiber amounts ranged from 0.4–0.6 g per feeding, but they did caution against potential prolonged gastric emptying times when using greater amounts or different forms of fiber. [191]
Diagnosis of invasive aspergillosis is often done through detection of galactomannans (37), which are present in the cell wall of Aspergillus spp.[192] As such, there has been some concern about false-positive testing for invasive aspergillosis in infants who are fed formula thickened with galactomannans (37).[193]
3. Future Perspectives
While carbohydrate polymers have been extensively studied and used in a select number of infant formulas, the future of infant prebiotics will revolve around the human milk oligosaccharides (HMOs). Over the past two decades, we have learned that these molecules are of great importance to infant health and development.[34, 44, 120, 194] While major advances have been made in producing authentic samples of HMOs, these compounds are currently not used as additives to infant food products. Their production, using both chemical and chemoenzymatic strategies is a frontier topic in synthetic carbohydrate chemistry. Presently, a number of research teams are focused on bridging the gap between HMOs and carbohydrate polymers, by synthesizing so called “HMO mimics” that simulate the size, tertiary structure, and functional groups (i.e. sialylation and fucosylation) present in HMOs.[195, 196] In addition to being easier to synthesize than native HMOs, these unnatural glycoconjugates have been shown to reduce necrotizing enterocolitis in neonatal rats.[44] Ultimately, the field of carbohydrate prebiotics will continue to pursue products that assist in allowing the formula-fed infant to develop a microbiome mirroring the breast fed infant.
Figure 8.

Structure of glucose-based polymers. Each molecule differs significantly in terms of branching and the location and configuration of glycosidic linkages.
Figure 10.

Structure of additional carbohydrate polymers that have been evaluated for prebiotic properties.
Acknowledgements
The authors would like to acknowledge Vanderbilt University and the Institute of Chemical Biology for financial support. DLA acknowledges a financial award from Amgen. KMC acknowledges support from the Vanderbilt Chemical Biology Interface (CBI) training program (T32 GM065086). We are grateful to Drs. David Aronoff, Jennifer Gaddy and Ryan Doster for helpful discussions. We acknowledge the reviewers for their thoughtful comments on an earlier version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Rollins NC, Bhandari N, Hajeebhoy N, Horton S, Lutter CK, Martines JC, Piwoz EG, Richter LM, Victora CG, Lancet Breastfeeding Series G, Lancet, 387 (2016) 491–504. [DOI] [PubMed] [Google Scholar]
- [2].Tang L, Lee AH, Binns CW, Yang Y, Wu Y, Li Y, Qiu L, Birth, 41 (2014) 339–343. [DOI] [PubMed] [Google Scholar]
- [3].Binns CW, Lee MK, Asia Pac. J. Clin. Nutr, 23 (2014) 344–350. [DOI] [PubMed] [Google Scholar]
- [4].Allen LH, Ann. Nutr. Metab, 61 Suppl 1 (2012) 29–37. [DOI] [PubMed] [Google Scholar]
- [5].Hackett KM, Mukta US, Jalal CS, Sellen DW, Matern. Child Nutr, 11 (2015) 173–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mellor K, Skouteris H, Nagle C, Women Birth, 26 (2013) e94–96. [DOI] [PubMed] [Google Scholar]
- [7].Jiang M, Foster EM, Health Serv. Res, 48 (2013) 628–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Section on B, Pediatrics, 129 (2012) e827–841.22371471 [Google Scholar]
- [9].Renfrew MJ, McCormick FM, Wade A, Quinn B, Dowswell T, Cochrane Database Syst. Rev, (2012) CD001141. [DOI] [PMC free article] [PubMed]
- [10].Qasem W, Fenton T, Friel J, BMC Pediatr, 15 (2015) 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kimura LJ, McGee A, Baird S, Viloria J, Nagatsuka M, Hawaii J Med. Public Health, 74 (2015) 101–111. [PMC free article] [PubMed] [Google Scholar]
- [12].Eisenberg SR, Bair-Merritt MH, Colson ER, Heeren TC, Geller NL, Corwin MJ, Pediatrics, 136 (2015) e315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sharma AJ, Dee DL, Harden SM, Pediatrics, 134 Suppl 1 (2014) S42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fewtrell MS, Morgan JB, Duggan C, Gunnlaugsson G, Hibberd PL, Lucas A, Kleinman RE, Am. J. Clin. Nutr, 85 (2007) 635S–638S. [DOI] [PubMed] [Google Scholar]
- [15].Kent G, Int. Breastfeed J, 1 (2006) 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fein SB, Falci CD, J. Am. Diet. Assoc, 99 (1999) 1234–1240. [DOI] [PubMed] [Google Scholar]
- [17].Quinn EA, Am. J. Hum. Biol, 26 (2014) 10–17. [DOI] [PubMed] [Google Scholar]
- [18].Rochow N, Fusch G, Choi A, Chessell L, Elliott L, McDonald K, Kuiper E, Purcha M, Turner S, Chan E, Xia MY, Fusch C, J. Pediatr, 163 (2013) 1001–1007. [DOI] [PubMed] [Google Scholar]
- [19].Belamarich PF, Bochner RE, Racine AD, Clin. Pediatr. (Phila), 55 (2016) 437–442. [DOI] [PubMed] [Google Scholar]
- [20].Wall R, Ross RP, Ryan CA, Hussey S, Murphy B, Fitzgerald GF, Stanton C, Clin. Med. Pediatr, 3 (2009) 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eggesbo M, Mandal S, Midtvedt T, Microb. Ecol. Health Dis, 26 (2015) 28062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Azad MB, Konya T, Guttman DS, Field CJ, Sears MR, HayGlass KT, Mandhane PJ, Turvey SE, Subbarao P, Becker AB, Scott JA, Kozyrskyj AL, Investigators CS, Clin. Exp. Allergy, 45 (2015) 632–643. [DOI] [PubMed] [Google Scholar]
- [23].Pham VT, Lacroix C, Braegger CP, Chassard C, Environ. Microbiol, (2016). [DOI] [PubMed]
- [24].Avershina E, Lundgard K, Sekelja M, Dotterud C, Storro O, Oien T, Johnsen R, Rudi K, Environ. Microbiol, (2016). [DOI] [PubMed]
- [25].Fooladi AA, Khani S, Hosseini HM, Mousavi SF, Aghdam EM, Nourani MR, Inflamm. Allergy Drug Targets, 12 (2013) 410–418. [DOI] [PubMed] [Google Scholar]
- [26].Frese SA, Mills DA, Cell Host Microbe, 17 (2015) 543–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C, Curr. Opin. Biotechnol, 21 (2010) 149–156. [DOI] [PubMed] [Google Scholar]
- [28].Civardi E, Garofoli F, Longo S, Mongini ME, Grenci B, Mazzucchelli I, Angelini M, Castellazzi A, Fasano F, Grinzato A, Fanos V, Budelli A, Stronati M, Clin. Nutr, (2015). [DOI] [PubMed]
- [29].Goldsmith F, O’Sullivan A, Smilowitz JT, Freeman SL, J. Mammary Gland Biol. Neoplasia, 20 (2015) 149–158. [DOI] [PubMed] [Google Scholar]
- [30].Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O’Toole PW, van Sinderen D, Marchesi JR, Ventura M, PLoS One, 7 (2012) e36957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Watson D, O’Connell Motherway M, Schoterman MH, van Neerven RJ, Nauta A, van Sinderen D, J. Appl. Microbiol, 114 (2013) 1132–1146. [DOI] [PubMed] [Google Scholar]
- [32].Hascoet JM, Hubert C, Rochat F, Legagneur H, Gaga S, Emady-Azar S, Steenhout PG, J. Pediatr. Gastroenterol. Nutr, 52 (2011) 756–762. [DOI] [PubMed] [Google Scholar]
- [33].Yu ZT, Chen C, Kling DE, Liu B, McCoy JM, Merighi M, Heidtman M, Newburg DS, Glycobiology, 23 (2013) 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Newburg DS, Biochemistry (Mosc), 78 (2013) 771–785. [DOI] [PubMed] [Google Scholar]
- [35].Liu B, Newburg DS, Breastfeed Med, 8 (2013) 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Newburg DS, Ruiz-Palacios GM, Morrow AL, Annu. Rev. Nutr, 25 (2005) 37–58. [DOI] [PubMed] [Google Scholar]
- [37].Newburg DS, Ruiz-Palacios GM, Altaye M, Chaturvedi P, Meinzen-Derr J, Guerrero Mde L, Morrow AL, Glycobiology, 14 (2004) 253–263. [DOI] [PubMed] [Google Scholar]
- [38].Morrow AL, Ruiz-Palacios GM, Altaye M, Jiang X, Guerrero ML, Meinzen-Derr JK, Farkas T, Chaturvedi P, Pickering LK, Newburg DS, J. Pediatr, 145 (2004) 297–303. [DOI] [PubMed] [Google Scholar]
- [39].Newburg DS, Curr. Med. Chem, 6 (1999) 117–127. [PubMed] [Google Scholar]
- [40].Newburg DS, Pickering LK, McCluer RH, Cleary TG, J. Infect. Dis, 162 (1990)1075–1080. [DOI] [PubMed] [Google Scholar]
- [41].Newburg DS, J. Nutr, 135 (2005) 1308–1312. [DOI] [PubMed] [Google Scholar]
- [42].Newburg DS, Adv. Exp. Med. Biol, 501 (2001) 3–10. [DOI] [PubMed] [Google Scholar]
- [43].Newburg DS, J. Pediatr. Gastroenterol. Nutr, 30 Suppl 2 (2000) S8–17. [PubMed] [Google Scholar]
- [44].Yu H, Lau K, Thon V, Autran CA, Jantscher-Krenn E, Xue M, Li Y, Sugiarto G, Qu J, Mu S, Ding L, Bode L, Chen X, Angew. Chem. Int. Ed, 53 (2014) 6687–6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Autran CA, Schoterman MH, Jantscher-Krenn E, Kamerling JP, Bode L, Br. J. Nutr, (2016) 1–6. [DOI] [PubMed]
- [46].den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM, J Lipid Res, 54 (2013) 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Voragen AGJ, Trends Food Sci. Technol, 9 (1998) 328–335. [Google Scholar]
- [48].Stephen AM, Food Polysaccharides and Their Applications, Marcel Dekker, Inc, New York, 1995. [Google Scholar]
- [49].Crittenden RG, Playne MJ, Trends Food Sci. Technol, 7 (1996) 353–361. [Google Scholar]
- [50].Roberfroid MB, Delzenne NM, Annu. Rev. Nutr, 18 (1998) 117–143. [DOI] [PubMed] [Google Scholar]
- [51].Satyanarayana MN, Indian J Biochem. Biophys, 13 (1976) 408–412. [PubMed] [Google Scholar]
- [52].Satyanarayana MN, Indian J Biochem. Biophys, 13 (1976) 398–407. [PubMed] [Google Scholar]
- [53].Satyanarayana MN, Indian J Biochem. Biophys, 13 (1976) 261–266. [PubMed] [Google Scholar]
- [54].Finke B, Stahl B, Pritschet M, Facius D, Wolfgang J, Boehm G, J. Agric. Food Chem, 50 (2002) 4743–4748. [DOI] [PubMed] [Google Scholar]
- [55].L’Homme C, Peschet JL, Puigserver A, Biagini A, J. Chromatogr. A, 920 (2001) 291– 297. [DOI] [PubMed] [Google Scholar]
- [56].Stahl B, Linos A, Karas M, Hillenkamp F, Steup M, Anal. Biochem, 246 (1997) 195–204. [DOI] [PubMed] [Google Scholar]
- [57].Ebisu S, Kato K, Kotani S, Misaki A, J. Biochem, 78 (1975) 879–887. [DOI] [PubMed] [Google Scholar]
- [58].Cho S, Finocchiaro ET, Handbook of Prebiotics and Probiotics Ingredients: Health Benefits and Food Applications, CRC Press, Boca Raton, 2010. [Google Scholar]
- [59].Anwar MA, Kralj S, Pique AV, Leemhuis H, van der Maarel MJ, Dijkhuizen L, Microbiology, 156 (2010) 1264–1274. [DOI] [PubMed] [Google Scholar]
- [60].Heyer AG, Schroeer B, Radosta S, Wolff D, Czapla S, Springer J, Carbohydr. Res, 313 (1998) 165–174. [DOI] [PubMed] [Google Scholar]
- [61].Bancal P, Henson CA, Gaudillere JP, Carpita NC, Carbohydr. Res, 217 (1991) 137– 151. [DOI] [PubMed] [Google Scholar]
- [62].Rahul R, Kumar S, Jha U, Sen G, Int. J. Biol. Macromol, 72 (2015) 868–874. [DOI] [PubMed] [Google Scholar]
- [63].Itaya NM, Figueiredo-Ribeiro RC, Buckeridge MS, Braz. J. Med. Biol. Res, 32 (1999) 435–442. [DOI] [PubMed] [Google Scholar]
- [64].Singh RS, Singh RP, Kennedy JF, Int. J. Biol. Macromol, 85 (2016) 565–572. [DOI] [PubMed] [Google Scholar]
- [65].Smeekens S, Nat. Biotechnol, 16 (1998) 822–823. [DOI] [PubMed] [Google Scholar]
- [66].Zhang T, Li RJ, Qian HB, Mu WM, Miao M, Jiang B, Carbohydr. Polym, 101 (2014) 975–981. [DOI] [PubMed] [Google Scholar]
- [67].Franken J, Brandt BA, Tai SL, Bauer FF, PloS One, 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Oner ET, Hernandez L, Combie J, Biotechnol. Adv, 34 (2016) 827–844. [DOI] [PubMed] [Google Scholar]
- [69].Lombard V, Golaconda Ramulu H, Drula E, Coutinho PM, Henrissat B (2014) The Carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–D495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Falony G, Calmeyn T, Leroy F, De Vuyst L, Appl. Environ. Microbiol, 75 (2009) 2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kyazze G, Dinsdale R, Hawkes FR, Guwy AJ, Premier GC, Donnison IS, Bioresour. Technol, 99 (2008) 8833–8839. [DOI] [PubMed] [Google Scholar]
- [72].Ritsema T, Smeekens S, Curr. Opin. Plant Biol, 6 (2003) 223–230. [DOI] [PubMed] [Google Scholar]
- [73].Di Bartolomeo F, Van den Ende W, Plant Foods Hum. Nutr, 70 (2015) 227–237. [DOI] [PubMed] [Google Scholar]
- [74].Franco-Robles E, Lopez MG, ScientificWorldJournal, 2015 (2015) 289267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Falony G, Lazidou K, Verschaeren A, Weckx S, Maes D, De Vuyst L, Appl. Environ. Microbiol, 75 (2009) 454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Paineau D, Respondek F, Menet V, Sauvage R, Bornet F, Wagner A, J. Nutr. Sci. Vitaminol. (Tokyo), 60 (2014) 167–175. [DOI] [PubMed] [Google Scholar]
- [77].Bartosch S, Woodmansey EJ, Paterson JC, McMurdo ME, Macfarlane GT, Clin. Infect. Dis, 40 (2005) 28–37. [DOI] [PubMed] [Google Scholar]
- [78].Kolida S, Tuohy K, Gibson GR, Br. J. Nutr, 87 Suppl 2 (2002) S193–197. [DOI] [PubMed] [Google Scholar]
- [79].Marx SP, Winkler S, Hartmeier W, FEMS Microbiol. Lett, 182 (2000) 163–169. [DOI] [PubMed] [Google Scholar]
- [80].Kaplan H, Hutkins RW, Appl. Environ. Microbiol, 66 (2000) 2682–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Mckellar RC, Modler HW, Appl. Microbiol. Biotechnol, 31 (1989) 537–541. [Google Scholar]
- [82].Swanson KS, Grieshop CM, Flickinger EA, Bauer LL, Wolf BW, Chow J, Garleb KA, Williams JA, Fahey GC, J. Nutr, 132 (2002) 3042–3050. [DOI] [PubMed] [Google Scholar]
- [83].Swanson KS, Grieshop CM, Flickinger EA, Bauer LL, Chow JM, Wolf BW, Garleb KA, Fahey GC, J. Nutr, 132 (2002) 3721–3731. [DOI] [PubMed] [Google Scholar]
- [84].Elsen RJ, Bistrian BR, Nutrition, 7 (1991) 7–10; discussion 10–11. [PubMed] [Google Scholar]
- [85].Oli MW, Petschow BW, Buddington RK, Dig. Dis. Sci, 43 (1998) 138–147. [DOI] [PubMed] [Google Scholar]
- [86].Hachul AC, Mennitti LV, de Oliveira JL, Moreno MF, Okuda MH, Dos Santos B, Oyama LM, Ribeiro EB, do Nascimento CM, Pisani LP, Lipids Health Dis, 12 (2013) 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Li J, Kim IH, J. Anim. Sci, 91 (2013) 5336–5343. [DOI] [PubMed] [Google Scholar]
- [88].Clarke ST, Green-Johnson JM, Brooks SPJ, Ramdath DD, Bercik P, Avila C, Inglis GD, Green J, Yanke LJ, Selinger LB, Kalmokoff M, Brit. J. Nutr, 115 (2016) 1748–1759. [DOI] [PubMed] [Google Scholar]
- [89].Hughes R, Magee EA, Bingham S, Curr. Issues Intest. Microbiol, 1 (2000) 51–58. [PubMed] [Google Scholar]
- [90].Smith EA, Macfarlane GT, Anaerobe, 3 (1997) 327–337. [DOI] [PubMed] [Google Scholar]
- [91].Veereman G, J. Nutr, 137 (2007) 2585S–2589S. [DOI] [PubMed] [Google Scholar]
- [92].Haarman M, Knol J, Appl. Environ. Microbiol, 72 (2006) 2359–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Haarman M, Knol J, Appl. Environ. Microbiol, 71 (2005) 2318–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Knol J, Scholtens P, Kafka C, Steenbakkers J, Gross S, Helm K, Klarczyk M, Schopfer H, Bockler HM, Wells J, J. Pediatr. Gastroenterol. Nutr, 40 (2005) 36–42. [DOI] [PubMed] [Google Scholar]
- [95].Knol J, Boehm G, Lidestri M, Negretti F, Jelinek J, Agosti M, Stahl B, Marini A, Mosca F, Acta. Paediatr, 94 (2005) 31–33. [DOI] [PubMed] [Google Scholar]
- [96].Saavedra JM, Tschernia A, Brit. J. Nutr, 87 (2002) S241–S246. [DOI] [PubMed] [Google Scholar]
- [97].Raes M, Scholtens PA, Alliet P, Hensen K, Jongen H, Boehm G, Vandenplas Y, Rummens JL, Pediatr. Allergy Immunol, 21 (2010) e377–385. [DOI] [PubMed] [Google Scholar]
- [98].Scholtens PA, Alliet P, Raes M, Alles MS, Kroes H, Boehm G, Knippels LM, Knol J, Vandenplas Y, J. Nutr, 138 (2008) 1141–1147. [DOI] [PubMed] [Google Scholar]
- [99].Bakker-Zierikzee AM, Tol EA, Kroes H, Alles MS, Kok FJ, Bindels JG, Pediatr. Allergy Immunol, 17 (2006) 134–140. [DOI] [PubMed] [Google Scholar]
- [100].Vogt L, Meyer D, Pullens G, Faas M, Smelt M, Venema K, Ramasamy U, Schols HA, De Vos P, Crit. Rev. Food Sci. Nutr, 55 (2015) 414–436. [DOI] [PubMed] [Google Scholar]
- [101].Intanon M, Arreola SL, Pham NH, Kneifel W, Haltrich D, Nguyen TH, FEMS Microbiol. Lett, 353 (2014) 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].van Leeuwen SS, Kuipers BJH, Dijkhuizen L, Kamerling JP, Carbohydr. Res, 425 (2016) 48–58. [DOI] [PubMed] [Google Scholar]
- [103].Gosling A, Stevens GW, Barber AR, Kentish SE, Gras SL, Food Chem, 121 (2010) 307–318. [Google Scholar]
- [104].Otieno DO, Compr. Rev. Food Sci. Food Saf, 9 (2010) 471–482. [DOI] [PubMed] [Google Scholar]
- [105].Angus F, Smart S, Shortt C, in: Probiotic Dairy Products, Blackwell Science Ltd, 2007, pp. 120–137. [Google Scholar]
- [106].Macfarlane GT, Steed H, Macfarlane S, J. Appl. Microbiol, 104 (2008) 305–344. [DOI] [PubMed] [Google Scholar]
- [107].Matthews BW, C. R. Biol, 328 (2005) 549–556. [DOI] [PubMed] [Google Scholar]
- [108].Ganzle MG, Int. Dairy J, 22 (2012) 116–122. [Google Scholar]
- [109].Boon MA, Janssen AE, van ‘t Riet K, Enzyme Microb. Technol, 26 (2000) 271–281. [DOI] [PubMed] [Google Scholar]
- [110].Huber RE, Kurz G, Wallenfels K, Biochemistry, 15 (1976) 1994–2001. [DOI] [PubMed] [Google Scholar]
- [111].Huber RE, Wallenfels K, Kurz G, Can. J. Biochem, 53 (1975) 1035–1038. [DOI] [PubMed] [Google Scholar]
- [112].Jorgensen F, Hansen OC, Stougaard P, Appl. Microbiol. Biotechnol, 57 (2001) 647– 652. [DOI] [PubMed] [Google Scholar]
- [113].Warmerdam A, Benjamins E, de Leeuw TF, Broekhuis TA, Boom RM, Janssen AEM, Food Bioprod. Process, 92 (2014) 383–392. [Google Scholar]
- [114].Benjamins E, Boxem L, KleinJan-Noeverman J, Broekhuis TA, Int. Dairy J, 38 (2014) 160–168. [Google Scholar]
- [115].Stiverson J, Williams T, Chen J, Adams S, Hustead D, Price P, Guerrieri J, Deacon J, Yu Z, Appl. Environ. Microbiol, 80 (2014) 7388–7397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Verheijden KA, Willemsen LE, Braber S, Leusink-Muis T, Jeurink PV, Garssen J, Kraneveld AD, Folkerts G, Eur. J. Nutr, 55 (2016) 1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Fanaro S, Boehm G, Garssen J, Knol J, Mosca F, Stahl B, Vigi V, Acta Paediatr, 94 (2005) 22–26. [DOI] [PubMed] [Google Scholar]
- [118].Alizadeh A, Akbari P, Difilippo E, Schols HA, Ulfman LH, Schoterman MH, Garssen J, Fink-Gremmels J, Braber S, Br. J. Nutr, 115 (2016) 605–618. [DOI] [PubMed] [Google Scholar]
- [119].Arslanoglu S, Moro GE, Boehm G, J. Nutr, 138 (2008) 1521–1521. [DOI] [PubMed] [Google Scholar]
- [120].Bode L, Glycobiology, 22 (2012) 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Holden HM, Rayment I, Thoden JB, J. Biol. Chem, 278 (2003) 43885–43888. [DOI] [PubMed] [Google Scholar]
- [122].Willi E PDK. Heine, and Peter J. Reeds, J. Nutr, 121 (1990) 277–283. [Google Scholar]
- [123].Brew R.H.R.a.K., J. Biol. Chem, 255 (1980) 3377–3385. [PubMed] [Google Scholar]
- [124].Senderens JB, Compt. Rend, 170 (1920). [Google Scholar]
- [125].O’Donnell K and Kearsley M Sweeteners and Sugar Alternatives in Food Technology, 2nd ed.; John Wiley & Sons, Ltd. 2012. [Google Scholar]
- [126].Kuusisto J, Tokarev AV, Murzina EV, Roslund MU, Mikkola J-P, Murzin DY, Salmi T, Catal. Today, 121 (2007) 92–99. [Google Scholar]
- [127].Hudson E.M.M.a.C.S., J. Am. Chem. Soc, 52 (1930) 2101–2106. [Google Scholar]
- [128].Aider M, Halleux D.d., Trends Food Sci. Technol, 18 (2007) 356–364. [Google Scholar]
- [129].Panesar PS, Kumari S, Biotechnol. Adv, 29 (2011) 940–948. [DOI] [PubMed] [Google Scholar]
- [130].Olano A, Corzo N, Sci J. Food Agric, 89 (2009) 1987–1990. [Google Scholar]
- [131].Adachi S, Nature, 181 (1958) 840–841. [DOI] [PubMed] [Google Scholar]
- [132].Lee YJ, Kim CS, Oh DK, Appl. Microbiol. Biotechnol, 64 (2004) 787–793. [DOI] [PubMed] [Google Scholar]
- [133].Guerrero C, Vera C, Plou F, Illanes A, J. Mol. Catal. B: Enzym, 72 (2011) 206–212. [Google Scholar]
- [134].Skovbjerg H, Sjostrom H, Noren O, Eur. J. Biochem, 114 (1981) 653–661. [DOI] [PubMed] [Google Scholar]
- [135].Urashima T, Fukuda K, Messer M, Animal, 6 (2012) 369–374. [DOI] [PubMed] [Google Scholar]
- [136].Schaafsma G, Int. Dairy J, 18 (2008) 458–465. [Google Scholar]
- [137].Szilagyi A, Can. J. Gastroenterol. Hepatol, 18 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Ziegler EE, Fomon SJ, J. Pediatr. Gastroenterol. Nutr, 2 (1983) 288–294. [PubMed] [Google Scholar]
- [139].Abrams SA, Griffin IJ, Davila PM, Am. J. Clin. Nutr, 76 (2002) 442–446. [DOI] [PubMed] [Google Scholar]
- [140].Moya M.e.a, J. Pediatr. Gastroenterol. Nutr, 14 (1992) 57–61. [DOI] [PubMed] [Google Scholar]
- [141].Brand-Miller JC, McVeagh P, McNeil Y, Messer M, J. Pediatr, 133 (1998) 95–98. [DOI] [PubMed] [Google Scholar]
- [142].Petuely FZK, Kinderheilkd Z, 79 (1957). [Google Scholar]
- [143].Schumann C, Eur. J. Nutr, 41 Suppl 1 (2002) I17–25. [DOI] [PubMed] [Google Scholar]
- [144].Nagendra SVR, Arun Kumar S, Nutr. Res, 15 (1995) 15–24. [Google Scholar]
- [145].Finney M, Smullen J, Foster HA, Brokx S, Storey DM, Eur. J. Nutr, 46 (2007) 307–314. [DOI] [PubMed] [Google Scholar]
- [146].Drakoularakou A, Hasselwander O, Edinburgh M, Ouwehand AC, Food Science & Technology Bulletin: Functional Foods, 3 (2007) 71–80. [Google Scholar]
- [147].Kneifel W.e.a., Microb. Ecol. Health Dis, 12 (2000). [Google Scholar]
- [148].Sanz ML, Gibson GR, Rastall RA, Agric J. Food Chem, 53 (2005) 5192–5199. [DOI] [PubMed] [Google Scholar]
- [149].Ballongue J, Schumann C, Quignon P, Scand J Gastroenterol, 32 Suppl 222 (1997) 41–44. [DOI] [PubMed] [Google Scholar]
- [150].Parrish F.W.e.a., J. Food Sci , 44 (1979). [Google Scholar]
- [151].Nilsson U, Jagerstad M, Br. J. Nutr, 58 (1987) 199–206. [DOI] [PubMed] [Google Scholar]
- [152].Kontula P, Suihko ML, Von Wright A, Mattila-Sandholm T, J. Dairy Sci, 82 (1999) 249–256. [DOI] [PubMed] [Google Scholar]
- [153].Grembecka M, Eur. Food Res. Technol, 241 (2015) 1–14. [Google Scholar]
- [154].Brody T, Nutritional biochemistry, 2nd ed., Academic Press, San Diego, 1999. [Google Scholar]
- [155].Wang H, Shi Y, Le G, Carbohydr. Polym, 113 (2014) 225–230. [DOI] [PubMed] [Google Scholar]
- [156].Herfel TM, Jacobi SK, Lin X, Walker DC, Jouni ZE, Odle J, Food. Chem. Toxicol, 47 (2009) 1530–1537. [DOI] [PubMed] [Google Scholar]
- [157].Li S, Bashline L, Lei L, Gu Y, Arabidopsis, 12 (2014) e0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Koo WW, Poh D, Leong M, Tam YK, Succop P, Checkland EG, Parenter J. Enteral Nutr, 15 (1991) 144–147. [DOI] [PubMed] [Google Scholar]
- [159].Fenton TR, Belik J, J. Pediatr. Gastroenterol. Nutr, 35 (2002) 298–302. [DOI] [PubMed] [Google Scholar]
- [160].Choi A, Fusch G, Rochow N, Fusch C, PLoS One, 11 (2016) e0148941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Cicco R, Holzman IR, Brown DR, Becker DJ, Pediatrics, 67 (1981) 498–501. [PubMed] [Google Scholar]
- [162].Fagundes-Neto U, Viaro T, Lifshitz F, Am. J. Clin. Nutr, 41 (1985) 228–234. [DOI] [PubMed] [Google Scholar]
- [163].Bock DE, Rupar CA, Prasad C, Acta Paediatr, 100 (2011) e130–132. [DOI] [PubMed] [Google Scholar]
- [164].Shulman RJ, Kerzner B, Sloan HR, Boutton TW, Wong WW, Nichols BL, Klein PD, Pediatr. Res, 20 (1986) 740–743. [DOI] [PubMed] [Google Scholar]
- [165].Bosscher D, Van Caillie-Bertrand M, Van Cauwenbergh R, Deelstra H, Nutrition, 19 (2003) 641–645. [DOI] [PubMed] [Google Scholar]
- [166].Bosscher D, Van Caillie-Bertrand M, Van Dyck K, Robberecht H, Van Cauwenbergh R, Deelstra H, J. Pediatr. Gastroenterol. Nutr, 30 (2000) 373–378. [DOI] [PubMed] [Google Scholar]
- [167].Bufler P, Ehringhaus C, Koletzko S, Pediatr. Surg. Int, 17 (2001) 351–355. [DOI] [PubMed] [Google Scholar]
- [168].Wüstenberg T, Cellulose and Cellulose Deriva tives in the Food Industry: Fundamentals and Applications, Wiley VCH Verlag GmbH, 2014. [Google Scholar]
- [169].Scheller HV, Ulvskov P, Annu. Rev. Plant. Biol, 61 (2010) 263–289. [DOI] [PubMed] [Google Scholar]
- [170].Aachary AA, Prapulla SG, Compr. Rev. Food Sci. Food Saf, 10 (2011) 2–16. [Google Scholar]
- [171].Urbanowicz BR, Pena MJ, Ratnaparkhe S, Avci U, Backe J, Steet HF, Foston M, Li H, O’Neill MA, Ragauskas AJ, Darvill AG, Wyman C, Gilbert HJ, York WS, Proc. Natl. Acad. Sci. U.S.A, 109 (2012) 14253–14258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Verbruggen MA, Spronk BA, Schols HA, Beldman G, Voragen AG, Thomas JR, Kamerling JP, Vliegenthart JF, Carbohydr. Res, 306 (1998) 265–274. [DOI] [PubMed] [Google Scholar]
- [173].Maeda M, Shimahara H, Sugiyama N, Agr. Biol. Chem. Tokyo, 44 (1980) 245–252. [Google Scholar]
- [174].Rennie EA, Scheller HV, Curr. Opin. Biotechnol, 26 (2014) 100–107. [DOI] [PubMed] [Google Scholar]
- [175].Zhang Q, Shirley NJ, Burton RA, Lahnstein J, Hrmova M, Fincher GB, Plant Physiol, 153 (2010) 555–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Goubet F, Barton CJ, Mortimer JC, Yu X, Zhang Z, Miles GP, Richens J, Liepman AH, Seffen K, Dupree P, Plant J, 60 (2009) 527–538. [DOI] [PubMed] [Google Scholar]
- [177].Speelmans G, Knol J, Haarman M, Garssen J, in, Google Patents, 2016.
- [178].Gullon P, Moura P, Esteves MP, Girio FM, Dominguez H, Parajo JC, J. Agric. Food Chem, 56 (2008) 7482–7487. [DOI] [PubMed] [Google Scholar]
- [179].Ekhart PF, Van Der Saag H, Possemiers S, Van Den Abbeele P, Van De Wiele T, Neyrinck AM, Delzenne NMN, Cani PD, in, Google Patents, 2013.
- [180].Salvatore S, Nutrition, 23 (2007) 615–616. [DOI] [PubMed] [Google Scholar]
- [181].Chen HL, Fan YH, Chen ME, Chan Y, Nutrition, 21 (2005) 1059–1064. [DOI] [PubMed] [Google Scholar]
- [182].Mohnen D, Curr. Opin. Plant Biol, 11 (2008) 266–277. [DOI] [PubMed] [Google Scholar]
- [183].Stephen AM, Phillips GO, Food Polysaccharides and Their Applications, CRC Press, 2016. [Google Scholar]
- [184].Campo VL, Kawano DF, da Silva DB, Carvalho I, Carbohyd. Polym, 77 (2009) 167–180. [Google Scholar]
- [185].Joet T, Laffargue A, Salmona J, Doulbeau S, Descroix F, Bertrand B, Lashermes P, Dussert S, J. Exp. Bot, 65 (2014) 323–337. [DOI] [PubMed] [Google Scholar]
- [186].Collen J, Porcel B, Carre W, Ball SG, Chaparro C, Tonon T, Barbeyron T, Michel G, Noel B, Valentin K, Elias M, Artiguenave F, Arun A, Aury JM, Barbosa-Neto JF, Bothwell JH, Bouget FY, Brillet L, Cabello-Hurtado F, Capella-Gutierrez S, Charrier B, Cladiere L, Cock JM, Coelho SM, Colleoni C, Czjzek M, Da Silva C, Delage L, Denoeud F, Deschamps P, Dittami SM, Gabaldon T, Gachon CM, Groisillier A, Herve C, Jabbari K, Katinka M, Kloareg B, Kowalczyk N, Labadie K, Leblanc C, Lopez PJ, McLachlan DH, Meslet-Cladiere L, Moustafa A, Nehr Z, Nyvall Collen P, Panaud O, Partensky F, Poulain J, Rensing SA, Rousvoal S, Samson G, Symeonidi A, Weissenbach J, Zambounis A, Wincker P, Boyen C, Proc. Natl. Acad. Sci. U.S.A, 110 (2013) 5247–5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Stam J, van Stuijvenberg M, Garssen J, Knipping K, Sauer PJ, Vaccine, 29 (2011) 7766–7772. [DOI] [PubMed] [Google Scholar]
- [188].Vos AP, Haarman M, van Ginkel JWH, Knol J, Garssen J, Stahl B, Boehm G, M’Rabet L, Pediatr, Allergy Immunol, 18 (2007) 304–312. [DOI] [PubMed] [Google Scholar]
- [189].Vos AP, van Esch BC, Stahl B, M’Rabet L, Folkerts G, Nijkamp FP, Garssen J, Int. J. Immunopharmacol, 7 (2007) 1582–1587. [DOI] [PubMed] [Google Scholar]
- [190].Vandenplas Y, Belli D, Benhamou P, Cadranel S, Cezard JP, Cucchiara S, Dupont C, Faure C, Gottrand F, Hassall E, Heymans H, Kneepkens CM, Sandhu B, Eur. J. Pediatr, 156 (1997) 343–357. [DOI] [PubMed] [Google Scholar]
- [191].Fabiani E, Bolli V, Pieroni G, Corrado G, Carlucci A, De Giacomo C, Catassi C, J. Pediatr. Gastroenterol. Nutr, 31 (2000) 248–250. [DOI] [PubMed] [Google Scholar]
- [192].Verdaguer V, Walsh TJ, Hope W, Cortez KJ, Expert. Rev. Mol. Diagn, 7 (2007) 21–32. [DOI] [PubMed] [Google Scholar]
- [193].Aceti A, Corvaglia L, Faldella G, Pediatr. Infect. Dis. J, 27 (2008) 769. [DOI] [PubMed] [Google Scholar]
- [194].Newburg DS, Grave G, Pediatr. Res, 75 (2014) 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Autran CA, Schoterman MH, Jantscher-Krenn E, Kamerling JP, Bode L, Br. J. Nutr, 116 (2016) 294–299. [DOI] [PubMed] [Google Scholar]
- [196].Zeuner B, Holck J, Perna V, Mikkelsen JD, Meyer AS, Enzyme Microb. Technol, 82 (2016) 42–50. [DOI] [PubMed] [Google Scholar]


