Abstract
Exosomes are representative extracellular vesicles (EV) derived from multivesicular endosomes (MVE) and have been described as new particles in the communication of neighborhood and/or distant cells by serving as vehicles for transfer between cells of membrane and cytosolic proteins, lipids, and nucleotides including micro (mi) RNAs. Exosomes from immune cells and tumor cells act in part as a regulator in tumor immunology. CD8+ T cells that show potent cytotoxic activity against tumor cells reside as an inactive naïve form in the T‐cell zone of secondary lymphoid organs. Once receiving tumor‐specific antigenic stimulation by dendritic cells (DC), CD8+ T cells are activated and differentiated into effector CTL. Subsequently, CTL circulate systemically, infiltrate into tumor lesions through the stromal neovasculature where mesenchymal stromal cells, for example, mesenchymal stem cells (MSC) and cancer‐associated fibroblasts (CAF), abundantly exist, destroy mesenchymal tumor stroma in an exosome‐mediated way, go into tumor parenchyma, and attack tumor cells by specific interaction. DC‐derived and regulatory T (Treg) cell‐derived exosomes, respectively, promote and inhibit CTL generation in this setting. In this review, we describe the roles of exosomes from immune cells and tumor cells on the regulation of tumor progression.
Keywords: CD8+ T cell, exosome, extracellular vesicle, tumor immunology, tumor metastasis
Abbreviations
- ADO
adenosine
- CAF
cancer‐associated fibroblast
- CCL
CC chemokine ligand
- cGAS
cyclic GMP‐AMP synthase
- COX
cyclooxygenase
- DC
dendritic cell
- EMT
epithelial‐to‐mesenchymal transition
- ESCRT
endosomal sorting complex required for transport
- EV
extracellular vesicle
- FasL
Fas ligand
- Foxp3
forkhead box protein P3
- GPCR
G protein‐coupled receptor
- GPI
glycosylphosphatidylinositol
- HIF
hypoxia inducible transcription factor
- HSP
heat shock protein
- IFN
interferon
- IL
interleukin
- MDSC
myeloid‐derived suppressor cell
- MIC‐A
MHC class I polypeptide‐related sequence A
- miRNA
micro RNA
- MSC
mesenchymal stem cell
- MV
microvesicle
- MVE
multivesicular endosome
- NK
natural killer
- NKG2D
natural‐killer group 2, member D
- PC‐3
prostate cancer‐3
- PS
phosphatidylserine
- SDF
stem cell‐derived factor
- SOCS
suppressor of cytokine signaling
- STING
stimulator of IFN genes
- TAM
tumor‐associated macrophage
- TGF‐β
transforming growth factor‐beta
- Th
T helper
- TNF
tumor necrosis factor
- Tre
regulatory T
- ULBP
UL16‐binding protein
1. INTRODUCTION
Cells release a diverse type of EV of endosome and plasma membrane origin called exosomes and microvesicles of sizes 40‐250 and 100‐1000 nm, respectively. Various bioactive substances and nucleic acids including mRNAs and miRNAs are found in the exosome surface and lumen; therefore, the present review focuses on exosomes rather than on microvesicles. miRNAs in exosomes can modulate the function of neighboring cells and/or distant recipient cells.1 Immune cell‐derived exosomes seem to partly act in tumor progression or regression.2, 3, 4, 5, 6 Tumor cell exosomes participate in development of the tumor microenvironment by targeting TAM, MDSC, MSC, CAF, and immune suppressive Treg cells.7, 8, 9 Thus, tumor progression seems to be regulated by complex exosome‐mediated actions among tumor cells, tumor stromal cells, and immune cells.
2. EXOSOMES FROM IMMUNE CELLS
Dendritic cells are indispensable for antigen presentation during T‐cell priming that serve as the center of the acquired immune system. It is reported that antigen protein‐engulfed DC release both MHC‐I‐ and MHC‐II‐expressing exosomes, and exosomes isolated from mature DC culture supernatant have been used for cancer immunotherapy.10, 11 Interestingly, although it is known that tumor cells produce immunosuppressive exosomes, DC that incorporated tumor cell‐derived exosomes release immunostimulatory exosomes expressing tumor antigen peptides in the context of MHC molecules.12 This seems to be related to type‐I IFN secretion mediated by the cGAS/STING pathway in DC by exosomal DNAs.13
Dendritic cells reside in all tissues, including mucous membrane and skin, to prevent intrusion of foreign proteins such as pathogenic microorganisms and development of neoplasms. Epidermal DC, termed Langerhans cells, are in the immature state in normal conditions. Immature DC engulfed antigen proteins rapidly activate and show a mature phenotype with enhancement of MHC‐II molecules; they then migrate into lymph nodes through lymphatic vessels and stimulate specific T cells.14, 15 It is known that immature DC strongly release exosomes, and the amounts are gradually decreased with the maturation process.16 However, the exosomes released by mature DC seem to have stronger antigen‐presenting ability to T cells than do immature DC exosomes.2 The biological significance of DC‐released exosomes other than T‐cell stimulatory efficacy is not well understood, but it must somehow be linked with the above‐mentioned DC dynamics. Interestingly, it has been reported that DC exosomes have a capacity to activate NK cells more vigorously than specific T cells.17, 18
T cells strongly release exosomes with activation.19 Treg cell exosomes have been studied to some extent, all of which are reports regarding immunosuppressive function. CD73 on Treg cells converts extracellular ATP to immunosuppressive ADO and inhibits A2a adenosine receptor‐bearing T cells and NK cells. Treg cell exosomes also express CD73 and seem to participate in the immunosuppression.3, 4 Treg cell exosomal miRNA (Let‐7d) strongly inhibits Th 1 cell activity by inhibition of COX‐2‐mediated IFN‐γ production.20 TGF‐β and suppressive miRNAs in breast milk exosomes are relatively stable against temperature, pH, and freeze‐thaw, and they maintain Treg cells by enhancement of Foxp3 expression by exosomal miR‐155‐mediated inhibition of SOCS 1 and prevent the onset of modern diseases such as atopic dermatitis by reduction of IgE production of B cells.21, 22 Treg cell exosomes may function in tolerance induction of alloreactive CTL caused rejection during organ transplantation in a CD73‐dependent way.23
Similar to the action of CD8+ T cells, NK cells show strong cytotoxicity against tumor cells. FasL expressed on the membrane of NK cell‐released exosomes seems to play a part in killing of Fas+ tumor cells.5 CD8+ T cells express FasL capable of apoptosis of Fas+ tumor cells. However, FasL on CD8+ T‐cell exosomes seems to promote invasion and metastasis of tumor cells, but not tumor cell killing, by MMP‐9‐mediated degradation of extracellular matrix proteins by the Fas/FasL signaling pathway.24
3. TUMOR CELL EXOSOMES AND IMMUNE REGULATION
Immune modulatory effects of tumor cell exosomes are most developed. Tumor cell‐derived exosomes promote activation and accumulation of Treg cells.25, 26, 27 Likewise, tumor cell exosomes enhance production of prostaglandin E2, IL‐6, and TGF‐β of MDSC, resulting in the formation of a strong immunosuppressive environment in tumor lesions.7, 8 NK cells, γδ T cells, and part of CTLs recognize the tumor surface MHC‐I molecule‐like ULBP and MIC‐A by interaction with NKG2D and can lyse tumor cells. However, ULBP‐ and MIC‐A‐bearing exosomes released by tumor cells bind with NKG2D on cytotoxic cells and block cytotoxicity against ULBP‐ and MIC‐A‐expressing tumor cells.28, 29
Tumor cells are always under hypoxic conditions and temperature stress and are also exposed to drug stress during treatment with anticancer agents.30, 31, 32 In malignant tumor lesions, supply of nutrition and oxygen from tumor blood vessels is insufficient, resulting in a hypoxic state. In order to adapt to hypoxic conditions, it is known that tumor cells enhance the expression of HIF‐1α, which promotes angiogenesis and glucose metabolism with formation of the immune suppression environment by Treg cells.30 Tumor growth is greatly affected by outside temperature. A murine study has shown that slightly high temperature during breeding results in reduction of tumor growth with enhancement of heat shock protein expression and antitumor immunity.31 Under these circumstances, it is known that tumor cells release exosomes more aggressively than under normal conditions and show immune‐modulatory effects. In low oxygen, tumor cells release TGF‐β‐bearing exosomes, and promote and inhibit Treg cell activity and NK cell cytotoxicity, respectively.9 Conversely, tumor cell exosomes released under high temperature stress or anticancer drug stress embed HSP‐70 and CCL‐2, ‐4, ‐5, and ‐20 capable of promoting migration and activation of T cells, NK cells, and DC (Table 1).33, 34
Table 1.
Reported regulatory roles of immune cell‐ and tumor cell‐derived exosomes
| CTL induction | NK cell activation | Th1 cell induction | Treg cell induction | Tumor cell lysis | Tumor progressiona | Ref | |
|---|---|---|---|---|---|---|---|
| Immature DC exo | ↑ | ↑ | 2, 10, 11, 12, 13, 14, 15, 16 | ||||
| Mature DC exo | ↑ | ↑ | 2, 10, 11, 12, 13, 14, 15, 16, 17, 18 | ||||
| Treg cell exo | ↓ | ↓ | ↓ | 3, 4, 20 | |||
| NK cell exo | ↑ | 5 | |||||
| CD8+ T‐cell exo | ↓↑ | 6, 24 | |||||
| Tumor cell exo | ↓ | ↓ | ↑ | 7, 8, 9, 25, 26, 27 |
Tumor progression includes tumor invasion and metastasis. ↑promotion ↓inhibition.
DC, dendritic cell; exo, exosome; NK, natural killer; Th, T helper; Treg, regulatory T.
4. CELL POPULATIONS IN MALIGNANT TUMOR TISSUES
Progressive tumors show development of tumor stroma comprising macrophages, DC, MDSC, endothelial cells (tumor blood vessels), and fibroblastic mesenchymal cells consisting of MSC and CAF in addition to epithelial tumor cells.35, 36, 37 Tumor stroma plays a very important role not only in maintaining tumor morphology and preventing immune attacks but also in malignant formation of tumor cells to acquire invasive and metastatic properties and to promote neovascularization called EMT.38, 39 Soluble form and exosomal TGF‐β or SDF‐1 derived from mesenchymal tumor stroma composed of myofibroblasts such as CAF in hypoxic conditions induce this malignant transition of tumor cells by reduced cell–cell adhesion and enhanced expression of MMP and mesenchymal markers including α‐smooth muscle actin (Figure 1).40, 41
Figure 1.
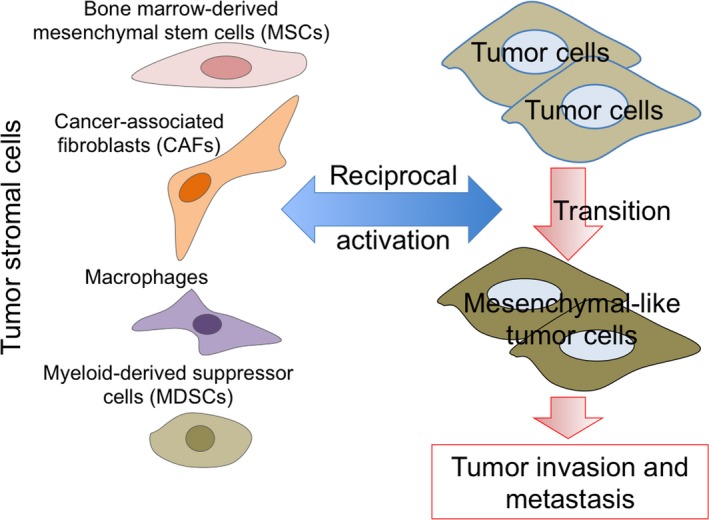
Acquisition of invasive and metastatic properties of tumor cells by tumor stromal cells. Tumor tissues consist of stromal cells such as mesenchymal cells (mesenchymal stem cells and cancer‐associated fibroblasts), macrophages, and myeloid‐derived suppressor cells in addition to tumor cells. Tumor stromal cells are indispensable for acquisition of invasive and metastatic properties of tumor cells
5. PREVENTION OF TUMOR INVASION AND METASTASIS AFTER CD8+ T‐CELL EXOSOME‐MEDIATED DESTRUCTION OF MESENCHYMAL TUMOR STROMA
CD8+ T cells act as a central player in tumor regression.42 CD8+ T‐cell exosomes possibly participate in the prevention of tumor growth, invasion, and metastasis. In studies of intratumoral injection of CD8+ T‐cell exosomes and i.v. transfer of CD8+ T cells in which exosome generation was inhibited by a neutral sphingomyelinase (N‐SMase) inhibitor,43 we found disappearance of mesenchymal tumor stroma with significant reduction of the mesenchymal cell population (MSC and CAF) without MHC restriction compared with no fluctuation in macrophage, MDSC, or DC population. Interestingly, CD8+ T‐cell exosomes from culture supernatants of tumor‐bearing mouse splenocytes had no capacity to deplete mesenchymal tumor stromal cells.6 CD8+ T‐cell exosomes from healthy mice could deplete mesenchymal tumor stromal cells partly in a miRNA (miR‐298‐5p)‐dependent way, but not TNF‐α‐ or Fas‐mediated pathways. Functional CD8+ T‐cell exosomes were transiently released on d‐7 in cultivation after CD3 stimulation,6 indicating that biological investigation of immune cell‐derived exosomes may be necessary to examine various exosomes obtained from different culture periods and conditions. Thus, it appears that exosomes are not a vesicle propagating unexpected functions as described in immunosuppressive roles of Treg cell exosomes and tumoricidal effects of CD8+ T‐cell exosomes excluding one report,24 but inherit the characteristics of the parent immune cells and inform neighboring and distant tissues (Table 1).
Disappearance of mesenchymal tumor stroma by CD8+ T‐cell exosomes should associate with the reduction of invasive and metastatic potential of tumor cells due to loss of EMT. By using metastatic B16F10 (melanoma), 4T1 (mammary carcinoma), and CMS5 m (fibrosarcoma), invasion of subcutaneous tumors on day 18 and lung metastasis on day 40 to day 45 was almost completely suppressed by intratumoral treatment of CD8+ T‐cell exosomes on day 14 (Figure 2).6
Figure 2.
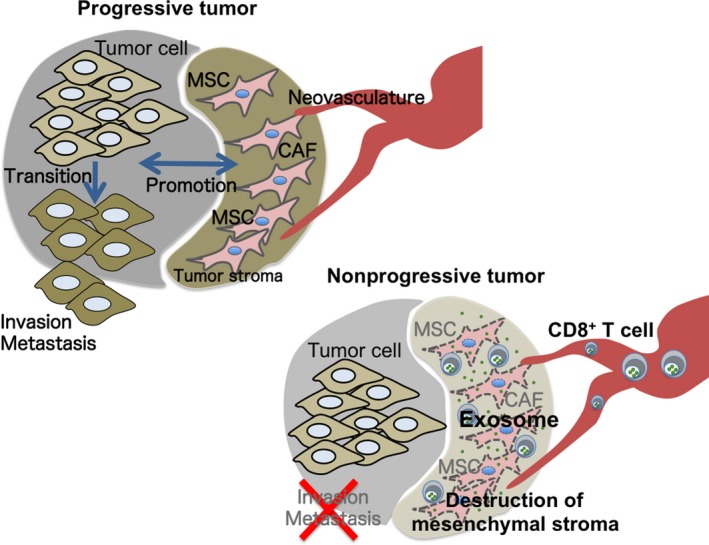
Tumor‐infiltrating CD8+ T cells can deplete mesenchymal tumor stromal cells in an exosome‐dependent method. Progressive tumors develop by reciprocal action between tumor cells and tumor stromal cells, and acquire invasive and metastatic potentials by epithelial‐to‐mesenchymal transition. Tumor depleted mesenchymal tumor stroma by exosomes from CD8+ T cells cannot acquire the invasive and metastatic properties as in progressive tumors6
To clarify the mechanism by which CD8+ T‐cell exosomes show cytotoxicity against mesenchymal tumor stromal cells rather than tumor cells, fluorescent‐labeled CD8+ T‐cell exosomes were injected into subcutaneous tumors or added to tumor cell or MSC culture. Surprisingly, CD8+ T‐cell exosomes were engulfed in mesenchymal tumor stromal cell populations in vivo and cultured MSC immediately, but not other cell populations including tumor cells in vivo or cultured tumor cells. Capacity to engulf CD8+ T‐cell exosomes by tumor cells was also found to be decreased with co‐cultivation with MSC. Preferential engulfment by mesenchymal cell populations seems to be a common feature of T‐cell exosomes because exosomes from murine CD4+ T cells and human T cells were also incorporated by cultured MSC rapidly in vitro.6 Studies on exosome sorting mechanisms show that exosome formation inside MVE can rely on the ESCRT‐dependent and ‐independent pathways.43, 44 The former is characterized by accumulation of ESCRT‐related proteins such as ALG‐2‐interacting protein X (Alix) and tumor susceptibility gene 101 (Tsg101) proteins, and ubiquitinated surface proteins, and the latter is characterized by concentration of sphingolipids including ceramide in combination with tetraspanin molecules. Thus, the unique structure of exosome membrane accumulating various functional molecules may determine the specificity of the target cells.
6. PHOSPHATIDYLSERINE‐DERIVED NEGATIVE CHARGES OF EXOSOME SURFACES
Microvesicles and apoptotic bodies are generated as submembrane fragments shed from the plasma membrane of cells during activation and proliferation and in the final step in the process of programmed cell death, respectively.45 Exosomes are generated in MVE under the exact mechanisms resemble with endosomal sorting of HIV and released by fusing MVE with the plasma membrane. Consistent with membranes of dead cells, membranes of extracellular vesicles including MV, apoptotic bodies, and exosomes expose negatively charged PS on the outer leaflet in contrast with localization at the inner leaflet of the plasma membrane of viable cells, which is the main reason that EV are negatively charged.46, 47, 48 When exosomes are given systemically, most of them are engulfed by hepatic macrophages and digested in their lysosomes.47 In the presence of calcium ion, scavenger receptors such as SR‐A (scavenger receptor class A), annexins, and T‐cell immunoglobulin and mucin domain containing (TIM)‐1, ‐3, and ‐4 on hepatic macrophages seem to be ligands for PS‐derived negative surface charge on EV.47, 48 However, in our study regarding intratumoral administration of CD8+ T‐cell exosomes, even though a large number of F4/80+ CD11b+ macrophages were present in tumor lesions, no exosome uptake was seen at 2 hours after intratumoral treatment. Analysis of the membrane components such as lipids and membrane proteins (including glycosylation) of exosomes is important to clarify this contradiction.
7. SPECIALIZED MEMBRANE COMPOSITION OF EXOSOMES
T‐cell exosomes are predominantly engulfed in mesenchymal tumor stromal cells rather than in tumor cells, suggesting utilization of exosomes as carriers of the drug delivery system against tumor invasion and metastasis. Necessity for ceramide on exosome budding in MVE43 results in exosome membranes composed of lipid raft components such as cholesterol and sphingolipids, for example, gangliosides.49, 50 Since HIVs are released from infected cells in a similar manner to exosome formation, sphingolipids are accumulated as HIV membrane components.51 T‐cell receptor (TCR) can transmit strong activation signals into the cytoplasm by accumulating in membrane microdomains termed immune synapse where cholesterol and sphingolipids abundantly exist.52 GPI‐anchored proteins such as Thy‐1 (CD90) molecule and GPCRs as chemokine receptors are known to concentrate in lipid rafts and can transmit signals, suggesting that T‐cell exosomes express bioactive molecules such as transmit immune‐, GPI‐, and GPCR‐mediated signals.6, 53, 54, 55, 56 It has been reported that tetraspanin molecules, for example, CD9, CD63, and CD81, known as exosome surface markers, regulate signal transductions by interacting with GPCR.57 As lipid raft‐related proteins have been reported to concentrate on exosome membranes,58, 59 analysis of lipid structure and lipid raft‐associated proteins on exosomes may resolve preferential engulfment by mesenchymal tumor stromal cells (Table 2).
Table 2.
Specialized exosome membrane composition resembling lipid raft
| Exosome membrane | Reference | Exosome source | Characteristics |
|---|---|---|---|
| Exposure of PS on outer leaflet | 46 | Human amniotic fluid | Diversity of negative charge |
| 47 | Murine B16BL6 culture sup. | Engulfment by macrophages by scavenger receptors | |
|
Sphingolipids Cholesterol |
49 | PC‐3 culture sup. | Sphingomyelin concentration compared with parent cells |
| 50 | LIM1215 culture sup. | Identification of many sphingolipids by lipidome analysis | |
| GPI‐anchored proteins | 54 | NKG2D ligands as GPI‐anchored proteins for immune activation | |
| GPCR | 56 | Human cell culture sup. Human saliva | Detection of several exosomal GPCR |
GPCR, G protein‐coupled receptor; GPI, glycosylphosphatidylinositol; NKG2D, natural‐killer group 2, member D; PC‐3, prostate cancer‐3; PS, phosphatidylserine; sup., supernatant.
8. CONCLUSION REMARKS
As a result of the many studies on exosomes in tumor biology, there is no doubt that exosomes act as a central player in the regulation of tumor progression including tumor invasion and metastasis.6, 60, 61 In addition, it is also being shown that exosomes have strong affinity with mesenchymal cells including fibroblasts, MSC, and CAF, vascular endothelial cells, pericytes and macrophages rather than tumor cells.6, 48, 60, 61 However, in order to understand the binding affinity between these cells and exosomes, we must improve our poor knowledge of molecular structures focusing on the exosome membrane. Dissolving the exosome membrane structures on a molecular basis seems to be indispensable for elucidating exosome‐mediated modification of tumor progression.
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.
ACKNOWLEDGMENT
This work was supported in part by a grant of CREST Japan Science and Technology Agency (JST) (JPMJCR17H2).
Seo N, Akiyoshi K, Shiku H. Exosome‐mediated regulation of tumor immunology. Cancer Sci. 2018;109:2998–3004. 10.1111/cas.13735
REFERENCES
- 1. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226‐1232. [DOI] [PubMed] [Google Scholar]
- 2. Quah BJ, O'Neill HC. Maturation of function in dendritic cells for tolerance and immunity. J Cell Mol Med. 2005;9:643‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schuler PJ, Saze Z, Hong CS, et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co‐expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smyth LA, Ratnasothy K, Tsang JYS, et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430‐2440. [DOI] [PubMed] [Google Scholar]
- 5. Lugini L, Cecchetti S, Huber V, et al. Immune surveillance properties of human NK cell‐derived exosomes. J Immunol. 2012;189:2833‐2842. [DOI] [PubMed] [Google Scholar]
- 6. Seo N, Shirakura Y, Tahara Y, et al. Activated CD8+ T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat Commun. 2018;9:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chalmin F, Ladoire S, Mignot G, et al. Membrane‐associated Hsp72 from tumor‐derived exosomes mediates STAT3‐dependent immunosuppressive function of mouse and human myeloid‐derived suppressor cells. J Clin Invest. 2010;120:457‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiang X, Poliakov A, Liu C, et al. Induction of myeloid‐derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621‐2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berchem G, Noman MZ, Bosseler M, et al. Hypoxic tumor‐derived microvesicles negatively regulate NK cell function by a mechanism involving TGF‐β and miR23a transfer. Oncoimmunology. 2015;5:e1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W. Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol. 2015;40:72‐81. [DOI] [PubMed] [Google Scholar]
- 11. Pitt JM, Charrier M, Viaud S, et al. Dendritic cell‐derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193:1006‐1011. [DOI] [PubMed] [Google Scholar]
- 12. Gu X, Erb U, Büchler MW, Zöller M. Improved vaccine efficacy of tumor exosome compared to tumor lysate loaded dendritic cells in mice. Int J Cancer. 2015;136:E74‐E84. [DOI] [PubMed] [Google Scholar]
- 13. Zhang H, Tang K, Zhang Y, et al. Cell‐free tumor microparticle vaccines stimulate dendritic cells via cGAS/STING signaling. Cancer Immunol Res. 2015;3:196‐205. [DOI] [PubMed] [Google Scholar]
- 14. Seo N, Tokura Y, Nishijima T, et al. Percutaneous peptide immunization via corneum barrier‐disrupted murine skin for experimental tumor immunoprophylaxis. Proc Natl Acad Sci USA. 2000;97:371‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo N, Furukawa F, Tokura Y, Takigawa M. Vaccine therapy for cutaneous T cell lymphoma. Hematol Oncol Clin North Am. 2003;17:1467‐1474. [DOI] [PubMed] [Google Scholar]
- 16. Quah BJ, O'Neill HC. The immunogenicity of dendritic cell‐derived exosomes. Blood Cells Mol Dis. 2005;35:94‐110. [DOI] [PubMed] [Google Scholar]
- 17. Pitt JM, André F, Amigorena S, et al. Dendritic cell‐derived exosomes for cancer therapy. J Clin Invest. 2016;126:1224‐1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gehrmann U, Näslund TI, Hiltbrunner S, Larssen P, Gabrielsson S. Harnessing the exosome‐induced immune response for cancer immunotherapy. Semin Cancer Biol. 2014;28:58‐67. [DOI] [PubMed] [Google Scholar]
- 19. Momose F, Seo N, Akahori Y, et al. Guanine‐rich sequences are a dominant feature of exosomal microRNAs across the mammalian species and cell types. PLoS ONE. 2016;11:e0154134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okoye IS, Coomes SM, Pelly VS, et al. MicroRNA‐containing T‐regulatory‐cell‐derived exosomes suppress pathogenic T helper 1 cells. Immunity. 2014;41:89‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pieters BC, Arntz OJ, Bennink MB, et al. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF‐β. PLoS ONE. 2015;10:e0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Melnik BC, John SM, Schmitz G. Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med. 2014;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu X, Huang C, Song B, et al. CD4+CD25+ regulatory T cells‐derived exosomes prolonged kidney allograft survival in a rat model. Cell Immunol. 2013;285:62‐68. [DOI] [PubMed] [Google Scholar]
- 24. Cai Z, Yang F, Yu L, et al. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188:5954‐5961. [DOI] [PubMed] [Google Scholar]
- 25. Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL. Tumor‐derived exosomes regulate expression of immune function‐related genes in human T cell subsets. Sci Rep. 2016;6:20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mrizak D, Martin N, Barjon C, et al. Effect of nasopharyngeal carcinoma‐derived exosomes on human regulatory T cells. J Natl Cancer Inst. 2014;107:363. [DOI] [PubMed] [Google Scholar]
- 27. Szajnik M, Czystowska M, Szczepanski MJ, Mandapathil M, Whiteside TL. Tumor‐derived microvesicles induce, expand and up‐regulate biological activities of human regulatory T cells (Treg). PLoS ONE. 2010;5:e11469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernández‐Messina L, Ashiru O, Boutet P, et al. Differential mechanisms of shedding of the glycosylphosphatidylinositol (GPI)‐anchored NKG2D ligands. J Biol Chem. 2010;285:8543‐8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ashiru O, Boutet P, Fernández‐Messina L, et al. Natural killer cell cytotoxicity is suppressed by exposure to the human NKG2D ligand MICA*008 that is shed by tumor cells in exosomes. Cancer Res. 2010;70:481‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petrova V, Annicchiarico‐Petruzzelli M, Melino G, Amelio I. The hypoxic tumour microenvironment. Oncogenesis. 2018;7:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eng JW, Reed CB, Kokolus KM, et al. Housing temperature‐induced stress drives therapeutic resistance in murine tumour models through β2‐adrenergic receptor activation. Nat Commun. 2015;6:6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brown JM, Woulters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391‐1399. [PubMed] [Google Scholar]
- 33. Chen T, Guo J, Yang M, Zhu X, Cao X. Chemokine‐containing exosomes are released from heat‐stressed tumor cells via lipid raft‐dependent pathway and act as efficient tumor vaccine. J Immunol. 2011;186:2219‐2228. [DOI] [PubMed] [Google Scholar]
- 34. Lv LH, Wan YL, Lin Y, et al. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J Biol Chem. 2012;287:15874‐15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ugel S, De Sanctis F, Mandruzzato S, Bronte V. Tumor‐induced myeloid deviation: when myeloid‐derived suppressor cells meet tumor‐associated macrophages. J Clin Invest. 2015;125:3365‐3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cortez E, Roswall P, Pietras K. Functional subsets of mesenchymal cell types in the tumor microenvironment. Semin Cancer Biol. 2014;25:3‐9. [DOI] [PubMed] [Google Scholar]
- 37. Casazza A, Di Conza G, Wenes M, et al. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33:1743‐1754. [DOI] [PubMed] [Google Scholar]
- 38. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Becker JC, Andersen MH, Schrama D, Thor Straten P. Immune‐suppressive properties of the tumor microenvironment. Cancer Immunol Immunother. 2013;62:1137‐1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katsuno Y, Lamouille S, Derynck R. TGF‐β signaling and epithelial‐mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76‐84. [DOI] [PubMed] [Google Scholar]
- 41. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761‐1767. [DOI] [PubMed] [Google Scholar]
- 42. Seo N, Hayakawa S, Tokura Y. Mechanisms of immune privilege for tumor cells by regulatory cytokines produced by innate and acquired immune cells. Semin Cancer Biol. 2002;12:291‐300. [DOI] [PubMed] [Google Scholar]
- 43. Trajkovic K, Hsu C, Chiantia S, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244‐1247. [DOI] [PubMed] [Google Scholar]
- 44. van Niel G, Charrin S, Simoes S, et al. The tetraspanin CD63 regulates ESCRT‐independent and ‐dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21:708‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yáñez‐Mó M, Siljander PR, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kosanović M, Milutinović B, Goč S, Mitić N, Janković M. Ion‐exchange chromatography purification of extracellular vesicles. Biotechniques. 2017;63:65‐71. [DOI] [PubMed] [Google Scholar]
- 47. Matsumoto A, Takahashi Y, Nishikawa M, et al. Role of phosphatidylserine‐derived negative surface charges in the recognition and uptake of intravenously injected B16BL6‐derived exosomes by macrophages. J Pharm Sci. 2017;106:168‐175. [DOI] [PubMed] [Google Scholar]
- 48. Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer‐associated fibroblasts. Blood. 2015;126:1106‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30‐41. [DOI] [PubMed] [Google Scholar]
- 50. Lydic TA, Townsend S, Adda CG, et al. Rapid and comprehensive ‘shotgun’ lipidome profiling of colorectal cancer cell derived exosomes. Methods. 2015;87:83‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Campbell SM, Crowe SM, Mak J. Lipid rafts and HIV‐1: from viral entry to assembly of progeny virions. J Clin Virol. 2001;22:217‐227. [DOI] [PubMed] [Google Scholar]
- 52. Yang W, Bai Y, Xiong Y, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyagawa‐Yamaguchi A, Kotani N, Honke K. Each GPI‐anchored protein species forms a specific lipid raft depending on its GPI attachment signal. Glycoconj J. 2015;32:531. [DOI] [PubMed] [Google Scholar]
- 54. López‐Cobo S, Campos‐Silva C, Valés‐Gómez M. Glycosyl‐phosphatidyl‐inositol (GPI)‐anchors and metalloproteases: their roles in the regulation of exosome composition and NKG2D‐mediated immune recognition. Front Cell Dev Biol. 2016;4:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barnett‐Norris J, Lynch D, Reggio PH. Lipids, lipid rafts and caveolae: their importance for GPCR signaling and their centrality to the endocannabinoid system. Life Sci. 2005;77:1625‐1639. [DOI] [PubMed] [Google Scholar]
- 56. Medapati MR, Singh A, Korupally RR. Characterization of GPCRs in extracellular vesicle (EV). Methods Cell Biol. 2017;142:119‐132. [DOI] [PubMed] [Google Scholar]
- 57. Little KD, Hemler ME, Stipp CS. Dynamic regulation of a GPCR‐tetraspanin‐G protein complex on intact cells: central role of CD81 in facilitating GPR56‐Galpha q/11 association. Mol Biol Cell. 2004;15:2375‐2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Gassart A, Geminard C, Fevrier B, Raposo G, Vidal M. Lipid raft‐associated protein sorting in exosomes. Blood. 2003;102:4336‐4344. [DOI] [PubMed] [Google Scholar]
- 59. Tan SS, Yin Y, Lee T, et al. Therapeutic MSC exosomes are derived from lipid raft microdomains in the plasma membrane. J Extracell Vesicles. 2013;23:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hoshino A, Costa‐Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tominaga N, Kosaka N, Ono M, et al. Brain metastatic cancer cells release microRNA‐181c‐containing extracellular vesicles capable of destructing blood‐brain barrier. Nat Commun. 2015;6:6716. [DOI] [PMC free article] [PubMed] [Google Scholar]


