Abstract
A meta‐analysis published in 2015 noted a marginally increased risk of endometrial and ovarian cancers in non‐smoking women with dietary acrylamide intake, but only a few studies were included, and they were limited to Western countries. The aim of this study was to investigate the association between dietary acrylamide intake and endometrial or ovarian cancer risk in the Japan Public Health Center‐based Prospective Study (JPHC Study). In this prospective cohort study, 47 185 participants aged 45‐74 years at the follow‐up starting point in the JPHC Study were enrolled. Dietary acrylamide intake was assessed using a validated food frequency questionnaire. Cox proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (95%CI). In participants with endometrial and ovarian cancer, the average follow‐up periods were 15.5 and 15.6 years, respectively, and 161 and 122 cases of endometrial and ovarian cancer were diagnosed, respectively. Energy‐adjusted dietary acrylamide intake was negatively associated with endometrial cancer, but the association disappeared after adjusting for coffee consumption with an adjusted HR for the highest vs lowest tertile of 0.85 (95%CI: 0.54‐1.33). No association was observed, however, for ovarian cancer (adjusted HR, 0.77; 95%CI: 0.49‐1.23). Furthermore, after stratifying by smoking status, coffee consumption, alcohol consumption, body mass index, and menopause status, no association was observed. Dietary acrylamide intake was not associated with the risk of endometrial or ovarian cancer in Japanese women with a relatively lower dietary intake of acrylamide compared with Western populations.
Keywords: Asia, dietary acrylamide, endometrial cancer, epidemiology, ovarian cancer
Abbreviations
- CI
confidence interval
- DCO
death certificate only
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FFQ
food frequency questionnaire
- HR
hazard ratio
- ICD‐O‐3
International Classification of Diseases for Oncology, Third Edition
- JPHC Study
Japan Public Health Center‐based Prospective Study
- NHS
Nurses’ Health Study
- NLCS
Netherlands Cohort Study on Diet and Cancer
- SMC
Swedish Mammography Cohort
1. INTRODUCTION
The International Agency for Research on Cancer classified acrylamide as a probable human carcinogen (group 2A) in 1994 given the evidence for the carcinogenicity of acrylamide in animal studies.1 In the general population, smoking was thought to be the main source of acrylamide exposure,2 but in 2002, Swedish researchers also found acrylamide in starchy foods cooked at high temperatures (such as fried potato).3 Therefore, in Western countries, epidemiological studies have been conducted to clarify the risk of dietary acrylamide intake with regard to the incidence of cancers.
A meta‐analysis published in 2015 showed no increased risk for most cancers but, due to the marginally increased risk of endometrial and ovarian cancers identified in non‐smoking women, there is a need for further studies.4 One possible mechanism by which dietary acrylamide exerts its carcinogenic effect is thought to be via the genotoxic pathway of glycidamide, which is an acrylamide metabolite.5, 6, 7 Furthermore, a hormone‐related pathway has also been debated.7, 8, 9 Thus, acrylamide is more likely to cause a non‐negligible increase in the risk of endometrial and ovarian cancers through synergistic genotoxicity effects and hormone changes than with other cancers. The small number of studies included in the meta‐analysis (four studies for each cancer), however, is also considered one of the reasons for this finding. Therefore, accumulation of evidence from further studies is needed.
In the Japan Public Health Center‐based Prospective Study (JPHC Study), the main sources of dietary acrylamide intake based on the dietary records were coffee and green tea, followed by confectionery, vegetables, and potatoes.10 In contrast, the main sources in Western countries were potato‐based foods, wheat‐based products, and coffee.11, 12, 13 These differences might influence the effect of dietary acrylamide on the risk of cancer in Japan. This is because coffee is also known to be a preventive factor for endometrial cancer.14 Thus, in the case of endometrial cancer, it is expected that the carcinogenic effect of acrylamide may be attenuated by the protective effect of coffee. Therefore, in order to evaluate the safety of dietary acrylamide, it is important to examine its influence on cancers in various countries with different dietary sources. Only one study in Asia, however, has assessed the influence of dietary acrylamide intake on the incidence of cancers.15
Therefore, the aim of this study was to investigate the association between dietary acrylamide intake and the incidence of endometrial or ovarian cancers in Japanese women.
2. MATERIALS AND METHODS
2.1. Study participants
The JPHC Study was launched in the 1990s to investigate the associations between lifestyle‐related diseases in two cohorts as a population‐based prospective cohort study. Cohort I areas included Iwate, Akita, Nagano, Okinawa (Chubu), and Tokyo, while cohort II areas included Ibaraki, Niigata, Kochi, Nagasaki, Okinawa (Miyako), and Osaka. The study protocol has been described elsewhere.16, 17 Participants in the JPHC Study aged 40‐69 years in these 11 areas consisted of 140 420 inhabitants (68 722 men and 71 698 women). Dietary surveys were conducted using a self‐administered food frequency questionnaire (FFQ) at baseline and at the 5‐year follow‐up survey. The number of food items and the number of food items with the option of portion size in the FFQ at baseline were limited. In contrast, the FFQ at 5‐year follow‐up contained more detailed dietary information. Thus, we treated the 5‐year follow‐up survey as the starting point of the follow up, and calculated dietary acrylamide intake as the exposure variable using the 5‐year follow‐up FFQ. The study protocol was approved by the institutional review boards of the National Cancer Center, Tokyo, Japan; Osaka University; and Azabu University. All study participants provided informed consent prior to inclusion in the study.
Participants in the Tokyo area were not included in the present study because the incidence data for cancer cases were not available. After excluding the participants who were disqualified, had died, moved out of the study area, or were lost to follow up before the starting point, 62 750 women were eligible for inclusion in this study. Of these, respondents to the 5‐year follow‐up survey consisted of 52 483 women (response rate, 83.6%). Furthermore, the participants who had a past history of endometrial (n = 654) or ovarian (n = 7) cancers identified through the questionnaire and diagnosed during the baseline survey up to the 5‐year follow‐up survey were excluded (endometrial cancer, n = 14; ovarian cancer, n = 11). Participants with missing data or extreme energy intake data (upper and lower 2.5 percentile) were also excluded (n = 4612). Therefore, 47 185 women were included in the present analysis of endometrial and ovarian cancers (Figure 1).
Figure 1.
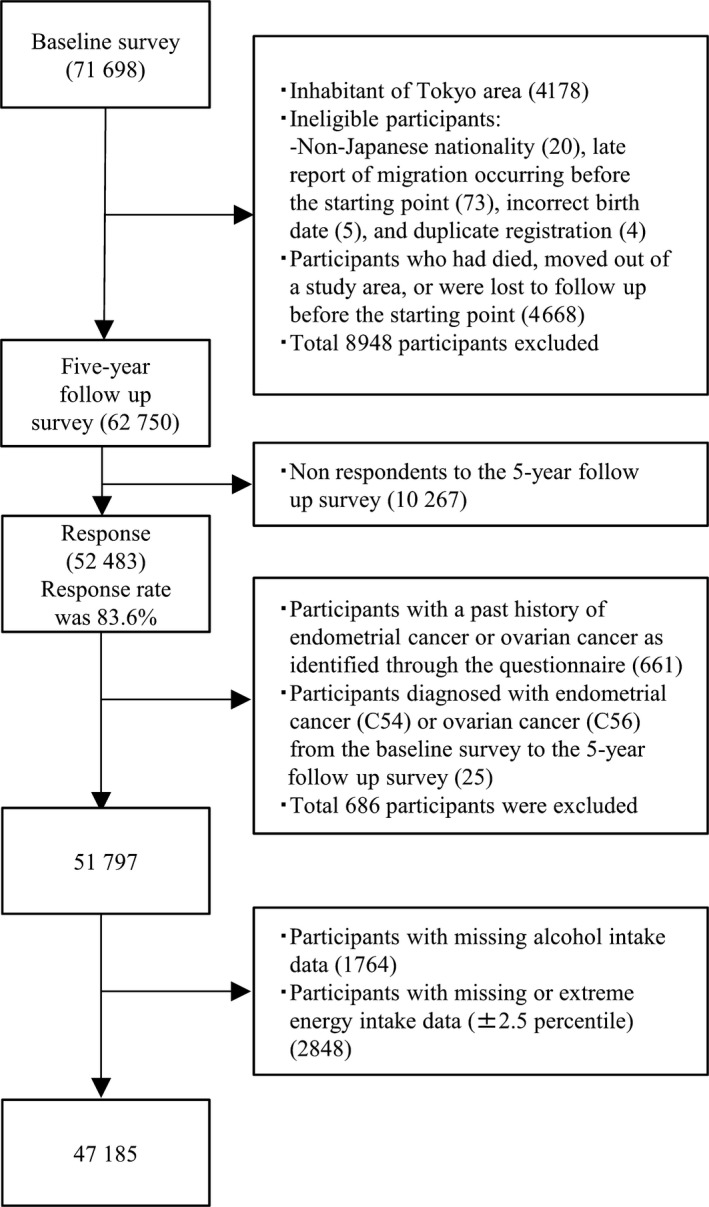
Flowchart of participant selection; Japan Public Health Center‐based Prospective Study
2.2. Assessment of energy and acrylamide intake from FFQ
Overall, 138 food and beverage items were included in the 5‐year follow‐up FFQ. The options for each food item were grouped into 9 categories according to the frequency of eating (never; 1‐3 times/month; 1‐2 times/week; 3‐4 times/week; 5‐6 times/week; once/day; 2‐3 times/day; 4‐6 times/day; or ≥7 times/day) and 3 categories according to portion size (less than half the standard portion size; standard portion size; or >1.5‐fold the standard portion size). The options for each drink were grouped into 9 categories according to the frequency of drinking (<1 cup/week; 1‐2 cups/week; 3‐4 cups/week; 5‐6 cups/week; 1 cup/day; 1‐3 cups/day; 4‐6 cups/day; 7‐9 cups/day; or ≥10 cups/day).
Energy content in each food item was referenced from the fifth revised and enlarged edition of the Standard Tables of Food Composition in Japan.18 The estimated energy intake was calculated as the sum of the product of the eating frequency, portion size, and energy content of each food. From validation studies, on comparison of 28‐day dietary record (DR) in subsamples of two cohorts, the correlation coefficients of energy intake in women were 0.41 and 0.24 in cohort I (n = 113) and cohort II (n = 176), respectively.19, 20, 21
Acrylamide intake was also estimated from the amount of food and beverage intake and the acrylamide content database. The database was developed from published reports of measurements of common Japanese foods.22, 23, 24, 25, 26, 27, 28, 29, 30, 31 In addition to heated processed foods such as bread, biscuit and cookies, or coffee, we considered the influence of home cooking such as stir‐fried vegetables, toast, or fried batter, to estimate the dietary acrylamide more accurately.23 The de‐attenuated correlation coefficients of energy‐adjusted dietary acrylamide intake in women were 0.48 and 0.37 in cohort I and cohort II, respectively.10
2.3. Follow up and identification of endometrial and ovarian cancers
The follow‐up period for all participants was from the starting point of the 5‐year follow‐up survey until 31 December 2013 (until 31 December 2012, only in the Osaka area). Residential status was confirmed annually through the residential registry. During the follow‐up period, 5741 participants (12.2%) died, 3191 (6.8%) moved from the study area, and 31 (0.1%) were lost to follow up.
The cancer incidence was identified through the following data sources: active patient notification from major local hospitals in the study area and data linkage with population‐based cancer registries. Death certificates were used as a supplementary information source. The International Classification of Diseases for Oncology, Third Edition (ICD‐O‐3) was used for coding endometrial (C54) and ovarian (C56) cancer cases. The proportion of cases determined using death certificate only (DCO) was 1.9% and 7.4% for endometrial and ovarian cancers, respectively. Given that these percentages were <10%, they were considered satisfactory for the present study.32 A total of 161 endometrial and 122 ovarian cancers were newly diagnosed by 31 December 2013.
2.4. Statistical analysis
Person‐years of follow up were determined from the starting point until the date of diagnosis of endometrial or ovarian cancer, the date of a participant's death, the date of relocation from the study area, or the end of the study period (31 December 2012 for Osaka area and 31 December 2013 for all other areas), whichever occurred first. For participants lost to follow up, the last confirmed date of their presence in the study area was used as the date of censor. The mean follow‐up period was 15.5 and 15.6 years for endometrial and for ovarian cancer analysis, respectively.
Cox proportional hazard modeling was used to estimate the hazard ratio (HR) and 95% confidence intervals (95%CI) to determine the association between tertiles of energy‐adjusted dietary acrylamide intake and endometrial or ovarian cancers. For energy adjustment, the residual method was used. The trend of HR was also assessed using the ordinal values of the tertiles of energy‐adjusted dietary acrylamide intake. HR were adjusted for the following potential confounders in model 1 for endometrial cancer analysis: age (years), area (10 public health center areas), body mass index (BMI; <25, ≥25 kg/m2, or missing), age at menarche (≤13, 14, 15, ≥16 years, or missing), age at first delivery (<26, ≥26 years, or missing), number of deliveries (0, 1‐2, 3, ≥4, or missing), menopause status and age at menopause (premenopause, postmenopause [age <49, 50‐54, ≥55 years], or missing), use of exogenous female hormones (yes, no, or missing), smoking status (current, ever, never, or missing), and alcohol intake (<150 or ≥150 g/week). Furthermore, in the multivariate‐adjusted model 2, energy‐adjusted coffee intake (continuous) was adjusted for in addition to the variables in model 1.
For ovarian cancer analysis, similar confounding factors as those for endometrial cancer were also adjusted for. These variables were identified from the FFQ and are known or suspected risk factors of endometrial or ovarian cancers.33, 34 For sensitivity analysis in model 2, we repeated the same analysis for each cancer but excluded cancer cases diagnosed at ≤3 years of follow‐up. Further, we conducted a stratified analysis using smoking status, frequency of coffee consumption (<1 cup/week, ≥1 cup/week), alcohol consumption, BMI, and menopause status at starting point (pre‐ or postmenopause). All P‐values were two‐sided, and statistical significance level was set at P < .05 using SAS 9.3 (SAS Institute, Cary, NC, USA).
3. RESULTS
3.1. Participant characteristics
Participant characteristics according to acrylamide intake are listed in Table 1. The mean ± SD dietary acrylamide intake was 3.7 ± 1.0, 6.4 ± 0.8, and 11.1 ± 3.3 μg/day in the lowest, middle, and highest tertiles of dietary acrylamide intake, respectively. Overall, the median dietary acrylamide intake was 6.3 μg/day (IQR, 4.5‐8.8 μg/day), and the mean ± SD dietary acrylamide intake was 7.1 ± 3.7 μg/day and 0.14 ± 0.13 μg/kg bodyweight/day in all participants. Foods that highly contributed to the total acrylamide intake were coffee (24%), green tea (22%), biscuit and cookies (13%), potatoes (12%), and vegetables (11%). When the percentages of contributing food were compared between tertiles, coffee (from 14% in the lowest to 29% in the highest), green tea (from 20% in the lowest to 24% in the highest), and biscuit and cookies (from 8% in the lowest to 15% in the highest) increased linearly. The percentages of potatoes (from 16% in the lowest to 10% in the highest) and vegetables (from 17% in the lowest to 8% in the highest), however, decreased linearly.
Table 1.
Subject characteristics for endometrial and ovarian cancer analysis
| Tertiles of acrylamide intake | P‐valuea | |||
|---|---|---|---|---|
| Lowest | Middle | Highest | ||
| T1 Mean ± SD or % | T2 Mean ± SD or % | T3 Mean ± SD or % | ||
| No. participants | 15 728 | 15 729 | 15 728 | |
| Acrylamide intake | ||||
| Median (μg/day)b | 3.9 | 6.3 | 10.2 | |
| Range (μg/day)b | 0.0‐5.1 | 5.1‐7.9 | 7.9‐59.0 | |
| Mean (μg/day)b | 3.7 ± 1.0 | 6.4 ± 0.8 | 11.1 ± 3.3 | |
| Mean (μg/kg bodyweight/day)b | 0.07 ± 0.05 | 0.12 ± 0.11 | 0.22 ± 0.15 | |
| Age at 5‐year follow‐up survey (years) | 58 ± 8 | 57 ± 8 | 55 ± 8 | <.001 |
| Body mass index (kg/m2) | 24 ± 3 | 23 ± 3 | 23 ± 3 | <.001 |
| Smoking status | ||||
| Current | 4.1 | 4.5 | 7.2 | <.001 |
| Past | 0.9 | 1.0 | 1.2 | |
| Never | 89.6 | 89.3 | 86.7 | |
| Missing | 5.5 | 5.3 | 5.0 | |
| Menarche age | ||||
| ≤13 years | 18.8 | 24.1 | 27.7 | <.001 |
| 14 years | 18.9 | 21.7 | 22.2 | |
| 15 years | 19.1 | 18.7 | 17.1 | |
| ≥16 years | 28.3 | 23.4 | 19.6 | |
| Missing | 14.9 | 12.2 | 13.4 | |
| Age at first delivery | ||||
| <26 years | 50.7 | 50.9 | 48.5 | <.001 |
| ≥26 years | 25.9 | 28.8 | 30.0 | |
| Missing | 23.4 | 20.3 | 21.5 | |
| No. deliveries | ||||
| None | 4.8 | 5.5 | 5.7 | <.001 |
| 1‐2 | 33.4 | 36.5 | 37.0 | |
| 3 | 23.7 | 24.8 | 23.7 | |
| ≥4 | 19.4 | 17.9 | 17.5 | |
| Missing | 18.8 | 15.4 | 16.1 | |
| Menopause status | ||||
| Premenopause | 15.7 | 21.7 | 29.0 | <.001 |
| Postmenopause from unknown age | 2.0 | 1.5 | 1.5 | |
| Postmenopause from age <49 years | 36.9 | 34.6 | 32.6 | |
| Postmenopause from age 50‐54 years | 36.2 | 36.2 | 31.8 | |
| Postmenopause from age ≥55 years | 4.6 | 3.7 | 3.2 | |
| Missing | 4.6 | 2.3 | 2.0 | |
| Exogenous hormone use | ||||
| Yes | 2.7 | 2.5 | 2.8 | <.001 |
| No | 89.9 | 93.1 | 93.5 | |
| Missing | 7.4 | 4.4 | 3.7 | |
| Dietary intake | ||||
| Energy (kcal/day) | 1845 ± 570 | 1874 ± 553 | 1858 ± 560 | <.001 |
| Alcohol intake (g/week) | 16 ± 80 | 13 ± 58 | 14 ± 55 | <.001 |
| Coffee (g/day)b | 37 ± 51 | 92 ± 90 | 232 ± 238 | <.001 |
| Green tea (g/day)b | 343 ± 352 | 543 ± 429 | 791 ± 698 | <.001 |
| Biscuit and cookies (g/day)b | 1 ± 1 | 2 ± 3 | 6 ± 8 | <.001 |
| Potato (g/day)b | 12 ± 10 | 19 ± 15 | 23 ± 24 | <.001 |
| Vegetables (g/day)b | 204 ± 123 | 231 ± 124 | 235 ± 135 | <.001 |
Kruskal‐Wallis test for continuous variables and chi‐squared test for categorical variables.
Energy‐adjusted intake.
The highest acrylamide consumption group were younger; had lower BMI; had a larger proportion of current smokers, higher proportion of younger menarche, lower proportion of older first delivery, lower proportion of none or few deliveries, higher proportion of premenopause status, higher proportion of exogenous female hormone non‐users; and consumed a higher energy diet, less alcohol, more coffee, more green tea, more biscuit and cookies, more potatoes, and more vegetables than the lowest acrylamide consumption group.
3.2. Dietary acrylamide intake and endometrial or ovarian cancers
The association between dietary acrylamide intake and endometrial cancer risk is shown in Table 2. In model 1, dietary acrylamide intake significantly decreased the risk of endometrial cancer. When cancer cases occurring ≤3 years of the starting point were excluded, the risk did not change. In addition to the covariates in model 1, however, we added coffee consumption in model 2; the association attenuated and no significant association was observed. This did not change after excluding cases that occurred ≤3 years of the starting point. Furthermore, although similar associations were observed when stratified according to the confounding factors, there was no significant association in model 2.
Table 2.
Acrylamide intake and the risk of endometrial cancer
| Total | Tertiles of acrylamide intake | P for trend | |||
|---|---|---|---|---|---|
| Lowest (T1) | Middle (T2) | Highest (T3) | |||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |||
| All women | |||||
| No. participants | 47 185 | 15 728 | 15 729 | 15 728 | |
| No. cases | 161 | 67 | 51 | 43 | |
| Person‐years | 733 067 | 246 682 | 244 634 | 241 751 | |
| Age‐ and area‐adjusted | 1.00 (Reference) | 0.77 (0.53‐1.12) | 0.64 (0.43‐0.96) | .03 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.76 (0.53‐1.10) | 0.65 (0.44‐0.97) | .03 | |
| Multivariate‐adjusted (excluding cases ≤ 3 years)a | 1.00 (Reference) | 0.79 (0.53‐1.18) | 0.68 (0.44‐1.05) | .08 | |
| Multivariate‐adjustedb | 1.00 (Reference) | 0.83 (0.57‐1.22) | 0.85 (0.54‐1.33) | .43 | |
| Multivariate‐adjusted (excluding cases ≤ 3 years)b | 1.00 (Reference) | 0.85 (0.56‐1.28) | 0.85 (0.52‐1.38) | .46 | |
| By smoking status | |||||
| Current or past smoker | |||||
| No. cases | 5 | 1 | 0 | 4 | |
| Multivariate‐adjusteda | 1.00 (Reference) | – | 1.68 (0.12‐22.83) | .49 | |
| Never smoker | |||||
| No. cases | 149 | 64 | 48 | 37 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.77 (0.52‐1.12) | 0.62 (0.40‐0.94) | .02 | |
| Multivariate‐adjustedb | 1.00 (Reference) | 0.85 (0.57‐1.25) | 0.82 (0.51‐1.31) | .37 | |
| By coffee consumption | |||||
| <1 cup/week | |||||
| No. cases | 47 | 32 | 7 | 8 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.51 (0.22‐1.18) | 1.14 (0.52‐2.53) | .77 | |
| ≥1 cup/week | |||||
| No. cases | 114 | 35 | 44 | 35 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.83 (0.53‐1.31) | 0.59 (0.36‐0.96) | .03 | |
| Multivariate‐adjustedb | 1.00 (Reference) | 0.91 (0.58‐1.44) | 0.79 (0.46‐1.36) | .40 | |
| By alcohol consumption | |||||
| <150 g/week | |||||
| No. cases | 157 | 64 | 51 | 42 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.80 (0.55‐1.16) | 0.67 (0.44‐1.00) | .05 | |
| Multivariate‐adjustedb | 1.00 (Reference) | 0.88 (0.60‐1.29) | 0.89 (0.57‐1.40) | .58 | |
| ≥150 g/week | |||||
| No. cases | 4 | 3 | 0 | 1 | |
| Multivariate‐adjusteda | 1.00 (Reference) | – | – | – | |
| By body mass index | |||||
| <25 kg/m2 | |||||
| No. cases | 103 | 40 | 35 | 28 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.87 (0.55‐1.39) | 0.72 (0.43‐1.19) | .20 | |
| ≥25 kg/m2 | |||||
| No. cases | 55 | 25 | 16 | 14 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.67 (0.35‐1.26) | 0.57 (0.29‐1.13) | .10 | |
| By menopause status | |||||
| Premenopause | |||||
| No. cases | 49 | 17 | 14 | 18 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.62 (0.30‐1.27) | 0.67 (0.33‐1.35) | .29 | |
| Postmenopause | |||||
| No. cases | 107 | 45 | 37 | 25 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.90 (0.58‐1.40) | 0.69 (0.41‐1.14) | .15 | |
Multivariate‐adjusted model 1, adjusted for age (years), area (10 public health center areas), body mass index (<25, ≥25 kg/m2, or missing), age at menarche (≤13, 14, 15, ≥16 years, or missing), age at first delivery (<26, ≥26 years, or missing), number of deliveries (0, 1‐2, 3, ≥4, or missing), menopause status and age at menopause (premenopause, postmenopause [<49, 50‐54, ≥55 years], or missing), use of exogenous female hormones (yes, no, or missing), smoking status (current or ever, never, or missing), and alcohol intake (<150 or ≥150 g/week).
Multivariate‐adjusted model 2 was further adjusted for energy‐adjusted coffee intake (continuous) in addition to the variables in model 1.
The associations between dietary acrylamide intake and ovarian cancer risk are given in Table 3. In contrast to the endometrial cancer analysis, no significant association was observed with ovarian cancer. Furthermore, no significant associations were seen on stratification in any of the strata.
Table 3.
Acrylamide intake and the risk of ovarian cancer
| Total | Tertiles of acrylamide intake | P for trend | |||
|---|---|---|---|---|---|
| Lowest (T1) | Middle (T2) | Highest (T3) | |||
| HR (95%CI) | HR (95%CI) | HR (95%CI) | |||
| All women | |||||
| No. participants | 47 185 | 15 728 | 15 729 | 15 728 | |
| No. cases | 122 | 46 | 41 | 35 | |
| Person‐years | 733 572 | 246 889 | 244 758 | 241 925 | |
| Age‐ and area‐adjusted | 1.00 (Reference) | 0.90 (0.59‐1.38) | 0.76 (0.48‐1.21) | .26 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.90 (0.59‐1.38) | 0.77 (0.49‐1.23) | .28 | |
| Multivariate‐adjusted (excluding cases ≤ 3 y)a | 1.00 (Reference) | 0.83 (0.52‐1.33) | 0.69 (0.41‐1.16) | .16 | |
| By smoking status | |||||
| Current or past smoker | |||||
| No. cases | 4 | 2 | 1 | 1 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.42 (0.03‐5.14) | 0.23 (0.02‐3.39) | .27 | |
| Never smoker | |||||
| No. cases | 111 | 41 | 38 | 32 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.94 (0.60‐1.48) | 0.82 (0.50‐1.33) | .43 | |
| By coffee consumption | |||||
| <1 cup/week | |||||
| No. cases | 39 | 25 | 10 | 4 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.90 (0.43‐1.90) | 0.62 (0.21‐1.82) | .40 | |
| ≥1 cup/week | |||||
| No. cases | 83 | 21 | 31 | 31 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 1.02 (0.58‐1.79) | 0.95 (0.53‐1.71) | .86 | |
| By alcohol consumption | |||||
| <150 g/week | |||||
| No. cases | 117 | 42 | 40 | 35 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.95 (0.61‐1.48) | 0.84 (0.52‐1.35) | .47 | |
| ≥150 g/week | |||||
| No. cases | 5 | 4 | 1 | 0 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.74 (0.05‐12.14) | – | .35 | |
| By body mass index | |||||
| <25 kg/m2 | |||||
| No. cases | 89 | 31 | 33 | 25 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 1.09 (0.66‐1.80) | 0.83 (0.48‐1.45) | .53 | |
| ≥25 kg/m2 | |||||
| No. cases | 31 | 14 | 8 | 9 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.54 (0.22‐1.32) | 0.64 (0.26‐1.55) | .29 | |
| By menopause status | |||||
| Premenopause | |||||
| No. cases | 25 | 8 | 9 | 8 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.87 (0.33‐2.27) | 0.70 (0.25‐1.92) | .48 | |
| Postmenopause | |||||
| No. cases | 94 | 35 | 32 | 27 | |
| Multivariate‐adjusteda | 1.00 (Reference) | 0.96 (0.59‐1.57) | 0.86 (0.51‐1.46) | .58 | |
Multivariable Cox proportional hazard models were adjusted for age (years), area (10 public health center areas), body mass index (<25, ≥25 kg/m2, or missing), age at menarche (≤14, ≥15 y, or missing), age at first delivery (<26, ≥26 y, or missing), number of deliveries (0, 1‐2, ≥3, or missing), menopausal status (premenopause, postmenopause, or missing), use of exogenous female hormones (yes, no, or missing), smoking status (current or ever, never, or missing), and alcohol intake (<150 or ≥150 g/week).
Given the wide range of dietary acrylamide intake in the highest group (7.9‐59 μg/day), we divided all participants into 9 quantiles and conducted further analysis to clarify the risk of extremely high consumption (Figure 2). Mean dietary acrylamide intake increased by approximately 1 μg/day between quantiles. No significant association was observed when the highest quantile was compared with the lowest quantile: HR was 0.55 (95%CI: 0.23‐1.33) and P for trend was 0.18 for endometrial cancer risk; with 0.66 (95%CI: 0.25‐1.73) and P for trend = 0.32 for ovarian cancer risk.
Figure 2.
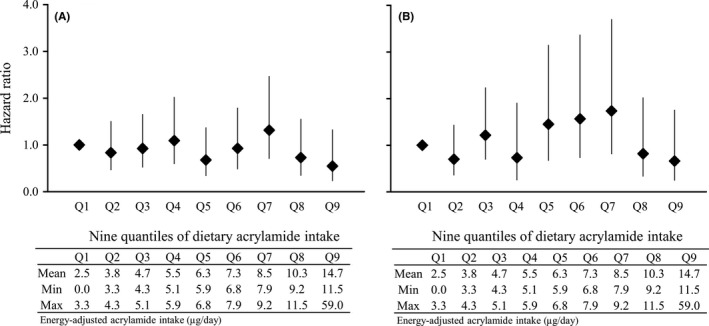
Hazard ratio (HR) for A, endometrial cancer risk and B, ovarian cancer risk vs 9 quantiles of dietary acrylamide intake. The reference group was the lowest ninth quantile of energy‐adjusted dietary acrylamide intake. A, HR and 95%CI were adjusted for age (years), area (10 public health center areas), body mass index (<25, ≥25 kg/m2, or missing), age at menarche (≤13, 14, 15, ≥16 years, or missing), age at first delivery (<26, ≥26 years, or missing), number of deliveries (0, 1‐2, 3, ≥4, or missing), menopause status and age at menopause (premenopause, postmenopause [<49, 50‐54, ≥55 years], or missing), use of exogenous female hormones (yes, no, or missing), smoking status (current, ever, never, or missing), alcohol intake (<150 or ≥150 g/week), and energy‐adjusted coffee intake (continuous). Number of cases from the lowest to the highest of the nine quantiles was 25, 20, 22, 21, 13, 17, 23, 12, and 8, respectively. B, HR and 95%CI were adjusted for age (years), area (10 public health center areas), body mass index (<25, ≥25 kg/m2, or missing), age at menarche (≤14, ≥15 years, or missing), age at first delivery (<26, ≥26 years, or missing), number of deliveries (0, 1‐2, ≥3, or missing), menopause status (premenopause, postmenopause, or missing), use of exogenous female hormones (yes, no, or missing), smoking status (current or ever, never, or missing), and alcohol intake (<150 or ≥150 g/week). Number of cases from the lowest to the highest of the nine quantiles was 16, 11, 19, 8, 16, 17, 19, 9, and 7, respectively
4. DISCUSSION
This study identified no associations between dietary acrylamide intake and endometrial or ovarian cancer risks in Japanese women. Specifically, energy‐adjusted dietary acrylamide intake was inversely associated with endometrial cancer in model 1, but the significant association disappeared after adjustment for coffee consumption. Furthermore, no associations were observed in either cancers after smoking status, coffee consumption, alcohol consumption, BMI, and menopause status stratifications.
In addition to the null association in all women, we did not detect any significant associations between dietary acrylamide intake and endometrial or ovarian cancers in non‐smoking Japanese women. Furthermore, the point estimates showed no increase. In the previous meta‐analysis, a non‐negligible association was observed in non‐smoking women with endometrial cancer (HR, 1.23; 95%CI: 1.00‐1.51) and with ovarian cancer (HR, 1.39; 95%CI: 0.97‐2.00).4 Moreover, the Netherlands Cohort Study on Diet and Cancer (NLCS), the Nurses’ Health Study (NHS), and the European Prospective Investigation into Cancer and Nutrition (EPIC) noted increased HR for endometrial and ovarian cancers.35, 36, 37 In contrast, the Swedish Mammography Cohort (SMC) and the Italian Case‐Control Studies found null associations for endometrial and ovarian cancers.38, 39, 40, 41 Differences in the dietary acrylamide intake in these cohorts may be one of the reasons for the lack of association. The average acrylamide intakes in the lowest and highest quintiles were 9.5 and 36.8 μg/day in the NLCS, and 9 and 26 μg/day in the NHS, respectively.35, 36 Likewise, the EPIC cohort, which included 10 European countries, also had a wide intake range: the lowest was 8.8 μg/day in Italy and the highest was 35.5 μg/day in Denmark.37 In contrast to these cohorts, the range of intake was narrower in the studies that showed no association.38, 39, 40, 41 In the Japanese participants, intake range of quartiles, as well as the average intake, was considerably smaller than in the other studies that showed a significant association.
Differences in the contributing foods also may have had an impact on the results. In this study, specifically, decreased risk was observed with endometrial cancer in model 1, but the association was attenuated after the adjustment for coffee consumption. Coffee intake was reported as a probable preventive factor for endometrial cancer in the World Cancer Research Fund International.14 This was consistent with the decreased risk of endometrial cancer due to coffee consumption in the JPHC Study.34
In the present study, as dietary acrylamide intake increased, the proportion of acrylamide intake from coffee was increased. It was thought that the significantly decreased risk observed in model 1 might be due to the preventive effect of coffee intake. Therefore, the beneficial effect of coffee intake may be greater than the influence of acrylamide intake accompanying coffee consumption, given that coffee was the highest contributing food to total acrylamide intake in this study. In the NHS, coffee was also one of the contributing foods, but, the risk of endometrial cancer increased.36 This difference may be due to the high acrylamide intake and the difference in contributing foods other than coffee.
This study has several limitations. First, the associations might have been attenuated by FFQ measurement errors. Second, the results may be affected by residual confounding factors such as passive smoking. Given, however, that there were no questions regarding passive smoking in the 5‐year follow‐up FFQ, we could not include passive smoking in the adjusted model in the present study. To eliminate these effects, therefore, further studies using biomarkers are needed. Also, of the risk assessments on endometrial cancer, the FFQ result is inconsistent with the results using biomarkers such as hemoglobin adduct concentration in blood.6, 37 Third, the assessment of acrylamide intake was done once. Therefore, we could not consider individual dietary changes during the follow‐up period. There may be differences in the contributing food groups by generation, given that the proportion of acrylamide intake from beverages and from vegetables was lower and higher, respectively, in the Japanese 2012 national dietary survey estimations compared with the present results in the 1990s.22 Individual dietary habits, however, might not have changed dramatically, because the present participants were aged 45‐74 years and their dietary habits were considered to be well established. Fourth, the low cancer incidence might affect the statistical power. The number of endometrial and of ovarian cancer cases was low in this cohort, reflecting the low incidence rate in Japan: age‐standardized rates per 100 000 population in 2012 in Japan were 10.6 for endometrial cancer and 8.4 for ovarian cancer.42
The strengths of this study were that the JPHC Study is one of the largest prospective cohort studies on lifestyle diseases; and recall bias on the exposure was prevented because the data were collected before cancer diagnosis. Participants were selected from the general population and the survey response rate was >80% while the loss to follow‐up rate was considerably small. The proportion of DCO was <10% each for endometrial and ovarian cancers. Therefore, the follow‐up data and the cancer registry in this study population were of sufficient quality.
This is the first study to assess the effect of dietary acrylamide intake on endometrial or ovarian cancer risks in Asian countries. We found no association between dietary acrylamide intake and endometrial or ovarian cancer risks regardless of smoking status, coffee consumption, alcohol consumption, body size, or menopause status in this large prospective cohort study of Japanese women with a relatively lower dietary intake of acrylamide compared with Western populations.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
J.I. and T.S. designed the research; S.T., T.S., J.I., N.S., and M.I. conducted the research; A.K. contributed to the calculation of dietary acrylamide intake; A.K., L.Z., and R.L. performed the statistical analysis; A.K. interpreted the results and wrote the paper; and J.I. was primarily responsible for the final content. All authors reviewed the manuscript and contributed to the discussion.
ACKNOWLEDGMENTS
The members of the JPHC study are listed at the following site (as of April 2017): https://epi.ncc.go.jp/en/jphc/781/7951.html. This study was supported by a grant from the Food Safety Commission, Cabinet Office, Government of Japan (Research Program for Risk Assessment Study on Food Safety, No. 1503, the principal investigator is T.S.), the National Cancer Center Research and Development Fund (since 2011, the principal investigator is S.T.), and a Grant‐in‐Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (from 1989 to 2010; principal investigator from 1997 to 2010 is S.T.).
Kotemori A, Ishihara J, Zha L, et al. Dietary acrylamide intake and the risk of endometrial or ovarian cancers in Japanese women. Cancer Sci. 2018;109:3316–3325. 10.1111/cas.13757
REFERENCES
- 1. International Agency for Research on Cancer . IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. IARC Monogr Eval Carcinog Risks Hum. 1994;60:1‐560.7869568 [Google Scholar]
- 2. Food Safety Commission of Japan . Evaluation document of dietary acrylamide produced by heating. [Food Safety Commission of Japan.] 2016. https://www.fsc.go.jp/osirase/acrylamide1.data/acrylamide_hyokasyo1.pdf. Accessed January 17, 2017.
- 3. Tareke E, Rydberg P, Karlsson P, Eriksson S, Törnqvist M. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J Agric Food Chem. 2002;50:4998‐5006. [DOI] [PubMed] [Google Scholar]
- 4. Pelucchi C, Bosetti C, Galeone C, La Vecchia C. Dietary acrylamide and cancer risk: an updated meta‐analysis. Int J Cancer. 2015;136:2912‐2922. [DOI] [PubMed] [Google Scholar]
- 5. Shipp A, Lawrence G, Gentry R, et al. Acrylamide: review of toxicity data and dose‐response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481‐608. [DOI] [PubMed] [Google Scholar]
- 6. Obón‐Santacana M, Freisling H, Peeters PH, et al. Acrylamide and glycidamide hemoglobin adduct levels and endometrial cancer risk: a nested case‐control study in nonsmoking postmenopausal women from the EPIC cohort. Int J Cancer. 2016;138:1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obón‐Santacana M, Lujan‐Barroso L, Travis RC, et al. Acrylamide and glycidamide hemoglobin adducts and epithelial ovarian cancer: a nested case‐control study in nonsmoking postmenopausal women from the EPIC cohort. Cancer Epidemiol Biomarkers Prev. 2016;25:127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hogervorst JG, Fortner RT, Mucci LA, et al. Associations between dietary acrylamide intake and plasma sex hormone levels. Cancer Epidemiol Biomarkers Prev. 2013;22:2024‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagata C, Konishi K, Tamura T, et al. Associations of acrylamide intake with circulating levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Epidemiol Biomarkers Prev. 2015;24:249‐254. [DOI] [PubMed] [Google Scholar]
- 11. Granby K, Nielsen NJ, Hedegaard RV, Christensen T, Kann M, Skibsted LH. Acrylamide‐asparagine relationship in baked/toasted wheat and rye breads. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2008;25:921‐929. [DOI] [PubMed] [Google Scholar]
- 12. Konings EJ, Hogervorst JG, van Rooij L, et al. Validation of a database on acrylamide for use in epidemiological studies. Eur J Clin Nutr. 2010;64:534‐540. [DOI] [PubMed] [Google Scholar]
- 13. Freisling H, Moskal A, Ferrari P, et al. Dietary acrylamide intake of adults in the European Prospective Investigation into Cancer and Nutrition differs greatly according to geographical region. Eur J Nutr. 2013;52:1369‐1380. [DOI] [PubMed] [Google Scholar]
- 14. World Cancer Research Fund International . Summary of strong evidence on diet, nutrition, physical activity and the prevention of cancer. [World Cancer Research Fund International.] https://www.wcrf.org/int/research-we-fund/continuous-update-project-findings-reports/endometrial-womb-cancer. Accessed August 15, 2017.
- 15. Kotemori A, Ishihara J, Zha L, et al. Dietary acrylamide intake and risk of breast cancer: the Japan Public Health Center‐based Prospective Study. Cancer Sci. 2018;109:843‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Watanabe S, Tsugane S, Sobue T, Konishi M, Baba S. Study design and organization of the JPHC study. Japan Public Health Center‐based Prospective Study on Cancer and Cardiovascular Diseases. J Epidemiol. 2001;11:S3‐S7. [DOI] [PubMed] [Google Scholar]
- 17. Tsugane S, Sawada N. The JPHC study: design and some findings on the typical Japanese diet. Jpn J Clin Oncol. 2014;44:777‐782. [DOI] [PubMed] [Google Scholar]
- 18. Resources Council, Science and Technology Agency, Government of Japan . Standard Tables of Food Composition in Japan, 5th edn Tokyo: Printing Bureau, Ministry of Finance; 2002. [Google Scholar]
- 19. Ishihara J, Inoue M, Kobayashi M, et al. Impact of the revision of a nutrient database on the validity of a self‐administered food frequency questionnaire (FFQ). J Epidemiol. 2006;16:107‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tsugane S, Sasaki S, Kobayashi M, Tsubono Y, Akabane M; JPHC . Validity and reproducibility of the self‐administered food frequency questionnaire in the JPHC Study cohort I: study design, conduct and participant profiles. J Epidemiol. 2003;13(1 Suppl):S2‐S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ishihara J, Sobue T, Yamamoto S, et al. Validity and reproducibility of a self‐administered food frequency questionnaire in the JPHC study cohort II: study design, participant profile and results in comparison with cohort I. J Epidemiol. 2003;13(1 Suppl):S134‐S147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Food Safety Commission of Japan . Study on estimate of acrylamide intake from food; interim report. 2016. https://www.fsc.go.jp/fsciis/technicalResearch/show/cho99920151507. Accessed January 17, 2017.
- 23. National Institute for Environmental Studies, Japan . Study on statistical estimate of acrylamide intake from foods. [National Institute for Environmental Studies, Japan.] http://www.fsc.go.jp/fsciis/technicalResearch/show/cho99920141408. Accessed January 17, 2017.
- 24. Ministry of Agriculture, Forestry and Fisheries . Risk profile sheet relating to the food safety; for acrylamide. [Ministry of Agriculture, Forestry and Fisheries.] http://www.maff.go.jp/j/syouan/seisaku/risk_analysis/priority/pdf/150807_rp_aa.pdf. Accessed January 17, 2017.
- 25. National Institute of Health Sciences . Acrylamide analysis in food. [National Institute of Health Sciences.] http://www.mhlw.go.jp/topics/2002/11/tp1101-1a.html. Accessed January 17, 2017.
- 26. Mizukami Y, Kohata K, Yamaguchi Y, et al. Analysis of acrylamide in green tea by gas chromatography‐mass spectrometry. J Agric Food Chem. 2006;54:7370‐7377. [DOI] [PubMed] [Google Scholar]
- 27. Takatsuki S, Nemoto S, Sasaki K, Maitani T. [Production of acrylamide in agricultural products by cooking.] Shokuhin Eiseigaku Zasshi. 2004;45:44‐48 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 28. Yoshida M, Ono H, Ohnishi‐Kameyama M, et al. [Determination of acrylamide in processed foodstuffs in Japan.] Nippon Shokuhin Kagaku Kogaku Kaishi. 2002;49:822‐825 (in Japanese). [Google Scholar]
- 29. Yoshida M, Miyoshi K, Horibata K, Mizukami Y, Takenaka M, Yasui A. [Estimation of acrylamide intake from cooked rice in Japan.] Nippon Shokuhin Kagaku Kogaku Kaishi. 2011;58:525‐530 (in Japanese). [Google Scholar]
- 30. Food Safety Commission of Japan . Information clearing sheet for acrylamide. [Food Safety Commission of Japan.] https://www.fsc.go.jp/fsciis/attachedFile/download?retrievalId=kai20111222sfc&fileId=520. Accessed January 17, 2017.
- 31. Food and Agriculture Organization/World Health Organization . Health implications of acrylamide in food. 2002. http://www.who.int/foodsafety/publications/acrylamide-food/en/ Accessed January 17, 2017.
- 32. International Agency for Research on Cancer . Cancer Incidence in Five Continents. Vol 9 Lyon, France: IARC Scientific Publications; 2008. [Google Scholar]
- 33. Weiderpass E, Sandin S, Inoue M, et al. Risk factors for epithelial ovarian cancer in Japan: results from the Japan Public Health Center‐based Prospective Study cohort. Int J Oncol. 2012;40:21‐30. [DOI] [PubMed] [Google Scholar]
- 34. Shimazu T, Inoue M, Sasazuki S, et al. Coffee consumption and risk of endometrial cancer: a prospective study in Japan. Int J Cancer. 2008;123:2406‐2410. [DOI] [PubMed] [Google Scholar]
- 35. Hogervorst JG, Schouten LJ, Konings EJ, Goldbohm RA, van den Brandt PA. A prospective study of dietary acrylamide intake and the risk of endometrial, ovarian, and breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2304‐2313. [DOI] [PubMed] [Google Scholar]
- 36. Wilson KM, Mucci LA, Rosner BA, Willett WC. A prospective study on dietary acrylamide intake and the risk for breast, endometrial, and ovarian cancers. Cancer Epidemiol Biomarkers Prev. 2010;19:2503‐2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Obon‐Santacana M, Kaaks R, Slimani N, et al. Dietary intake of acrylamide and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition cohort. Br J Cancer. 2014;111:987‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larsson SC, Hakansson N, Akesson A, Wolk A. Long‐term dietary acrylamide intake and risk of endometrial cancer in a prospective cohort of Swedish women. Int J Cancer. 2009;124:1196‐1199. [DOI] [PubMed] [Google Scholar]
- 39. Larsson SC, Akesson A, Wolk A. Long‐term dietary acrylamide intake and risk of epithelial ovarian cancer in a prospective cohort of Swedish women. Cancer Epidemiol Biomarkers Prev. 2009;18:994‐997. [DOI] [PubMed] [Google Scholar]
- 40. Pelucchi C, Galeone C, Negri E, et al. Dietary acrylamide and the risk of endometrial cancer: an Italian case‐control. Nutr Cancer. 2016;68:187‐192. [DOI] [PubMed] [Google Scholar]
- 41. Pelucchi C, Galeone C, Levi F, et al. Dietary acrylamide and human cancer. Int J Cancer. 2006;118:467‐471. [DOI] [PubMed] [Google Scholar]
- 42. Ervik M, Lam F, Ferlay J, Mery L, Soerjomataram I, Bray F. Cancer Today. Lyon, France: International Agency for Research on Cancer; 2016. [Google Scholar]


