Abstract
Lymphangiogenesis and increased expression of lymphangiogenic growth factors are associated with high rates of lymph node (LN) metastasis and with poor prognosis in some, but not all, solid tumors. In addition to its involvement in metastasis, lymphangiogenesis has been shown to have other roles in tumor pathogenesis, such as the niche function of tumor stem cells and regulatory functions of antitumor immune responses. In contrast, evidence has accumulated that tumor‐induced lymphangiogenesis displays the heterogeneity in gene signature, structure, cellular origins and functional plasticity. This review summarizes the advances in the research on the heterogeneity of tumor lymphangiogenesis and discusses how it may contribute to functional complexity and multiplicity of lymphangiogenesis in tumor progression.
Keywords: heterogeneity, immunity, lymphatic endothelial cell, metastasis, tumor lymphangiogenesis
1. INTRODUCTION
Most cancer mortalities are due to the metastasis of tumor cells to other organs.1, 2 The lymphatic vasculature is one of major routes for tumor cell dissemination.3 For many carcinomas such as cutaneous melanoma, the dissemination of tumor cells via lymphatic drainage is the most common route.4 Therefore, tumor lymphangiogenesis, the formation of new lymphatic vessels (LV) at primary tumor or distant sites, has been regarded as a key prognostic marker for patients with carcinomas.5 However, lymphangiogenesis in tumors does not always have a significant relationship with LN metastasis.6, 7 In fact, sentinel LN removal does not necessarily extend the overall survival of patients with melanoma.8, 9 Moreover, LV collapse and become dysfunctional during tumor progression.10, 11
Recent studies have revealed the heterogeneity of lymphatic endothelial cells (LEC). In contrast to blood vascular endothelial cells (BEC), LEC have variable extracellular coverage over the lymphatic vasculature. While capillary LEC are attached by filament bundles and then directly anchored to the extracellular matrices, the LEC in precollector vessels are sparsely covered with smooth muscle cells (SMC), but in collector LV they are lined with a basement membrane and a layer of SMC, structurally resembling veins with LEC continuously connected to each other in a “zipper‐like” structure.12 The heterogeneous pattern of gene expression may underlie the distinct structure and specialized function of LV. For example, the lymphatic vessel endothelial hyaluronic acid receptor‐1 (LYVE‐1), an LEC‐specific surface marker, is downregulated in the collecting LV but remains high in lymphatic capillaries,13 likely mediating the trafficking of leukocytes within LV.14 Podoplanin (PDPN), another LEC marker, and is lowly expressed in skin lymphatic precollectors,15 involved in lymphocyte trafficking during skin inflammation.15 In addition, lymphatic capillaries highly express chemokine CCL21, which guides migration of dendritic cells (DC) toward the LV.16 Furthermore, LEC in collecting LV express a high level of endothelial nitric oxide synthase (eNOS),17 which is related to LV contractility and permeability (Figure 1).18 Due to the heterogeneity in LV structure and gene expression pattern, it can be predicted that the LV in different tissues exhibit variable responses to lymphangiogenic factors. Indeed, single subcutaneous injection of vascular endothelial growth factor (VEGF)‐C was enough to induce lymphangiogenesis,19 while in the central nervous system, injection of VEGF‐C into the cisterna magna only caused an increase in the diameter of meningeal LV.20 Notably, significant differences in the lymphangiogenic response were detected in cornea from several different mouse strains,21 suggesting that genetic background significantly influences lymphangiogenic response.
Figure 1.
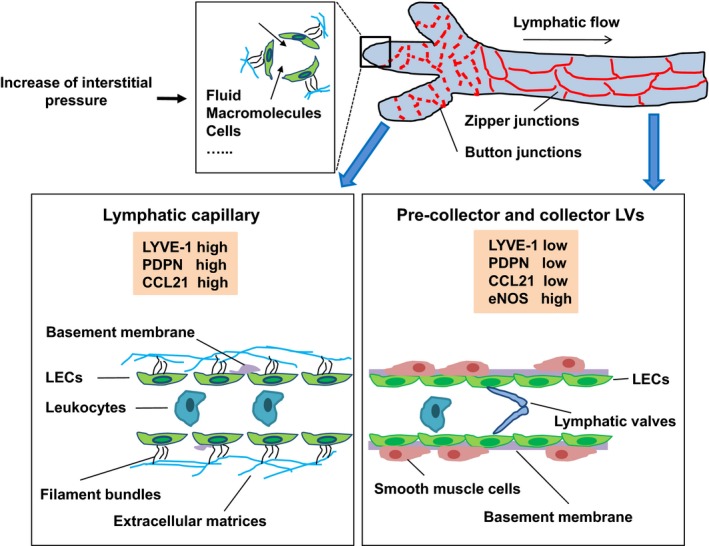
Structure and molecular features of lymphatic capillaries and collectors. The blind‐ended lymphatic capillaries are characterized by button‐like intracellular junctions, discontinuous basement membrane, attached by filament bundles, and anchored to the extracellular matrices. Lymphatic capillaries highly expressed lymphatic vessel endothelial hyaluronic acid receptor‐1 (LYVE‐1), podoplanin and CCL21 to mediate the recruitment of leukocytes. When interstitial pressure increases, the lymph drains from the lymphatic capillaries to precollector and collector lymphatic vessels (LV). Collecting LV have zipper‐like intracellular junctions, continuous basement membrane, smooth muscle cell (SMC) coverage and valves that prevent backflow of the lymph. Collecting LV highly express eNOS, which is related to LV contractility and permeability
Recent studies have shown that the heterogeneity of lymphatic vasculature with organ‐specific structural and functional features may be due to the diverse developmental origins of LEC and specializations of lymphatic vasculature to adapt to organ‐specific environments and to meet functional requirements during development and physiological processes. The advances of these findings have been covered by excellent recent reviews.22, 23, 24 In this review, we examine what we know so far about the heterogeneity of tumor lymphangiogenesis in an attempt to understand new functions of lymphangiogenesis in tumor progression. Elucidating the mechanism that causes lymphangiogenesis heterogeneity in solid tumors may provide better insight into the role of lymphangiogenesis in tumor progression and potential implications for tumor treatment.
2. STRUCTURAL HETEROGENEITY OF TUMOR LYMPHANGIOGENESIS
Under pathophysiological conditions such as inflammation and tumors, the structural heterogeneity in lymphatic vasculature renders LEC highly multiplex and plastic in response to various injury stimuli. Consequently, the heterogeneity of tumor lymphangiogenesis appears to be even more severe.25, 26 As a complex tissue, tumors grow within an intricate network of epithelial cells, vessels, cytokines and chemokines, and infiltrating immune cells. Such multiple types of cells are participating in heterotypic interactions with one another.27 Not unexpectedly, although the molecular signaling pathways responsible for LV growth are similar in development and tumors, there are distinct characteristics of lymphangiogenesis that frequently undergo even more extensive remodeling during tumor progression (Table 1).28, 29
Table 1.
Heterogeneity of tumor lymphangiogenesis
| Features | Descriptions | References |
|---|---|---|
| Gene | Tumor LEC show different gene expression profiles vs normal LEC | 30 |
| LEC/BEC markers are heterogeneously expressed in different parts of LV | 31, 32, 33 | |
| Highly metastatic tumors show different gene expression profiles vs non‐metastatic tumors | 30, 33, 34 | |
| Structure | Peritumoral LV are dilated with large open lumina, while intratumor are small, flattened, compressed and collapsed | 10 |
| Collecting LV display variable alteration, such as lymphangiogenesis and enlargement of diameter | 29, 34, 37 | |
| LV density is variable among different tumors and even in the same tumor | 10, 35 | |
| Cellular origins | Pre‐existing LEC | 4, 35, 40 |
| LEPC | 41, 42 | |
| Roles in regulating tumor metastasis | Tumor LV density positively correlates with metastasis and survival | 2, 3, 43, 49, 62, 63 |
| Tumor cells utilize peritumoral LV to spread rather than intratumoral LV | 2, 10, 45, 46, 47 | |
| Roles in regulating tumor immunity | Lymphangiogenesis promote tumor development by immune suppression | 61, 66, 67, 68 |
| Lymphangiogenesis prevent tumor progression by immune surveillance | 43, 60, 69, 70, 71 |
2.1. Lymphatic endothelial gene signature
Tumor LV display unique expression profiles and transcriptional programs that potentially enhance tumor progression and metastasis compared with normal tissues. LEC isolated from solid tumors showed significant differences in expression of some 792 genes compared with LEC from nontumor tissues.30 For example, the upregulated expression of lymphangiogenic factors have been linked to tumor metastasis by enhancing lymphangiogenesis in the peritumoral area and enlarging the collecting LV as well as forming new lymphatic networks in LN. Similar to physiological conditions, the expression level of LEC markers varies within tumors, such as LYVE‐1, which is usually highly expressed in tumors with high metastasis ability.31 In addition, CD34, a BEC marker, was preferentially expressed in intratumoral LEC of colon, breast, lung and skin tumors.32 Notably, LEC intercellular junction molecules show heterogeneous expression in various tumors.30 Disruption of lymphatic endothelial barrier integrity by upregulating the VE‐cadherin phosphorylation expression level accelerated tumor cell migration into LV and promoted tumor metastasis.33 In addition, collecting LV alter their gene signature during VEGF‐D‐driven metastasis tumors compared to nonmetastasis tumors.34 These studies suggest diverse and dynamic gene signature in different types of tumors.
2.2. Lymphatic vessel structure
Compared to LV in normal tissues, tumor LV, which are patchy and not homogenously distributed within tumors, display distinct morphological features such as being disorganized without a hierarchical vascular pattern. Morphological differences of LV were even observed in the same tumor lesions. Peritumoral LV, which have relatively high density,10, 35 are dilated with large open lumina, and are functional channels for transportation of interstitial fluid, tumor cells and immune cells. In contrast, intratumoral LV are usually small, flattened, compressed and collapsed, and areAuto nonfunctional channels,10 although they display a proliferating feature (Figure 2).36 Besides the newly formed LV within the primary tumor, larger collecting LV, which connect the LV in the primary tumor with LN, also undergo substantial remodeling,29 such as lymphangiogenesis and enlargement of the diameter.28 This enlargement, which involves proliferation of LEC, nonproliferative mechanisms, and structural remodeling of SMC,34 is closely associated with enhanced drainage function of LV and increased sentinel LN metastasis.34, 37
Figure 2.
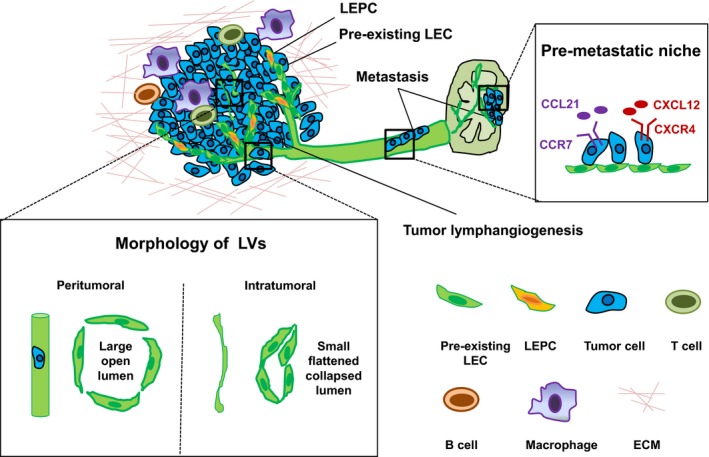
Heterogeneity of tumor‐associated lymphatic vessels (LV). Tumor‐induced LV, which are heterogeneously distributed within tumor tissues, are dictated by the complicated interactions among tumor cells, macrophage, B cells, T cells and extracellular matrix (ECM). Peritumoral LV with open lumina are responsible for tumor cell invasion and facilitate further metastasis, while intratumoral LV, which appear to be compressed and collapsed, are regarded as nonfunctional for tumor cell metastasis. Tumor lymphangiogenesis occurs mostly via sprouting from pre‐existing lymphatic endothelial cells (LEC), and, in some cases, lymphatic endothelial progenitor cells (LEPC) play a role. Besides, tumor‐induced enlargement of collector LV and lymphangiogenesis in LN (serves as a “pre‐metastatic” niche) promote tumor metastasis
2.3. Lymphatic endothelial cells cellular origins
De novo origins have been shown to contribute to pathological lymphangiogenesis. For example, recipient‐derived lymphatic endothelial progenitor cells (LEPC) contribute to lymphangiogenesis in human renal transplants.38 In a mouse inflammatory model, a subpopulation of CD11b+ macrophages were involved in pathological lymphangiogenesis in the diaphragm.39 Studies indicate that tumor lymphangiogenesis is also composed of heterogeneous cell subpopulations, derived from pre‐existing LEC and LEPC (Figure 2). Several lines of evidence indicate that locally pre‐existing LEC mainly contribute to tumor lymphangiogenesis: (i) in a subcutaneous implantation model, tumor‐induced LV were predominantly on the epidermal side but not on the side facing the abdominal muscle layer, suggesting that pre‐existing derma LV might play an important role in mediating tumor lymphangiogenesis;4 (ii) a bone marrow (BM) transplantation assay showed that newly formed LV in LLC tumor models were mainly derived from pre‐existing LEC; and4 (iii) in a mouse model that lacks dermal LV (K14‐VEGFR3‐Ig mice), subcutaneous tumors failed to induce lymphangiogenesis, indicating that pre‐existing LV are necessary for tumor‐induced LV.40 Such pre‐existing LV‐dependent tumor lymphangiogensis was validated in a prox1 genetic lineage tracing study.35 However, several studies showed that de novo lymphangiogenesis also contributes to tumor lymphangiogenesis. For example, VEGFR‐2+ or VEGFR‐3+ BM cells were involved in lymphangiogenesis in subcutaneously inoculated fibrosarcoma.41 In addition, BM‐derived podoplanin+ cells were shown as LEPC to participate in lymphangiogenesis in subcutaneously inoculated melanoma.42 Currently, the precise contribution of various LEC sources in tumor lymphangiogenesis remains incompletely understood.
Taken together, such a wide range of heterogeneities collectively points to the functional complexity of lymphangiogenesis in tumor progression.
3. FUNCTIONAL HETEROGENEITY OF TUMOR LYMPHANGIOGENESIS
Although it is well known that lymphangiogenesis both in primary tumors and draining LN promotes the spread of tumors,43 the tumor context and the extent to which lymphangiogenesis contributes are unclear. Strikingly, a recent study using phylogenetic reconstruction methods showed that most colon cancer metastases in distant organs bypass LN in colon cancer.44 Therefore, the findings on the functional heterogeneity and plasticity of tumor lymphangiogenesis should provide important insight into the role of lymphangiogenesis in tumor progression.
3.1. Different functions of peritumoral and intratumoral lymphatic vessels
The function of LV in mediating tumor cell metastasis varies among LV in different locations within tumors. Strikingly, tumor cells prefer to utilize LV in the peritumoral area to metastasize rather than those in the deep area of tumor.45 Thus, it is not difficult to infer that LV with normal open lumina in the tumor margin serve as the functional channels for tumor cell drainage, while the disorganized, destructed and collapsed LV in the deep area of tumor fail to facilitate tumor cell invasion and metastasis (Figure 2).10 Consistently, inhibition of peritumoral LV successfully reduced tumor metastasis,46 while inhibition of intratumoral LV did not have significant effects on metastasis.47 These studies suggest that peritumoral LV play an essential role in mediating the migration of tumor cells to LN and distant organs, and functional LV in the tumor margin should be targeted therapeutically. However, intratumor LV were reported to be associated with gastric tumor invasion,48 suggesting that the intratumoral LV might be functional, even though this has not been commonly observed in other types of tumors.
3.2. Active role in tumor metastasis
Lymphatic metastasis was thought to be a passive process in the past; however, accumulated evidence suggests that tumor LEC play active roles in mediating tumor cell invasion into LV and successful penetration into LN.49A study showed that LEC extensively formed filopodia toward VEGF‐C producing tumor cells, facilitating tumor cell entry into the lymphatic vasculature.3, 46 Another study demonstrated that LYVE‐1‐expressing LEC attracted hyaluronan‐expressing cancer cells to invade into LV.31 Notably, chemokines mediate LEC‐induced promotion of tumor cells homing to lymphatics.50 For example, CXCL12‐expressing LV promoted the invasiveness of various types of tumor cells in which CXCR4 was expressed.51 Similarly, CCL21 production by LEC was reported to guide the CCR7+ cancer cells’ migration to Niche roles in tumor progression to LV.52
3.3. Niche roles in tumor progression
Previous studies suggest that newly induced LV in LN form a premetastasis lymphovascular niche in the LN even before the presence of tumor cells, which is regarded as a potential mediator of tumor metastasis to distant organs.53, 54, 55 In addition, blood vessels in the LN also serve as an exit route for metastatic tumor cell dissemination.56, 57 These findings suggest that premetastasis niches formed in the LN are important for tumor cell metastasis to LN or distant organs (Figure 2). Interestingly, stem‐like tumor cells (STC) were found in the vicinity of LV, suggesting that newly formed LV within tumors may serve as a niche for STC.58, 59 Because the half‐time of STC is long and are able to travel over long distances, which might be related to metastasis, it is tempting to speculate that the stem‐like tumor cells trapped within the “lymphovascular” niche might be partly responsible for the tumor recurrence. So far, the molecular cues are not clear, but evidence shows that there is considerable crosstalk between the LEC and cancer stem cell. For example, in the B16F10 melanoma model, activated LEC secrete CXCL12 to attract a subpopulation of CXCR4‐expressing stem‐like melanoma cells.58 In addition, another study indicated that expression of CCR7 promoted breast cancer progression by amplifying breast cancer STC.59
3.4. Paradoxical roles in antitumor immune response
Although LV are essential in controlling the immune‐cell trafficking to prevent the development of tumors and inhibit tumor progression,43, 60 lymphangiogenesis has been reported to help tumors escape from immune response to facilitate tumor cell metastasis.61 As mentioned previously, many tumors associated with metastasis, such as melanoma and breast cancer, express high levels of VEGF‐C and contain a dense network of LV.62, 63 An increase in VEGF‐C expression in tumors is highly correlated with LN metastasis and poor prognosis in individuals with different tumor types, including skin, breast and lung cancers.2 Furthermore, LEC not only present antigens and modulate immune cell activation in physiology conditions64, 65 but also promote tumor progression and metastasis by inhibiting the function of immune cells (Figure 3).40, 66 Mechanistically, VEGF‐C plays an immunosuppressive role for LEC to scavenge and cross‐present antigens for direct suppression of cytotoxic T lymphocytes.61 LEC can also express immunosuppressive factors such as TGF‐β and nitric oxide to maintain peripheral tolerance to self‐antigens in lymph nodes by directly or indirectly regulating T‐cell fate and function in immunity.67 In addition, LEC can directly dampen DC maturation, in turn reducing their ability to activate effector T cells, and promote tumor tolerance.68
Figure 3.
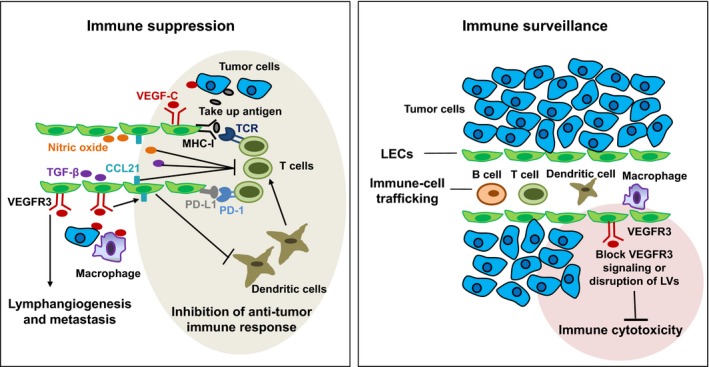
Paradoxical roles of tumor lymphatic vessels (LV) in antitumor immune response. Tumor‐induced LV help tumor escape from immune response by promoting tumor metastasis and inhibiting the function of immune cells, such as T cells and dendritic cells (DC). LV suppress T‐cell responses by presenting tumor antigens, upregulating PD‐L1 and secreting immunosuppressive factors such as TGF‐β, nitric oxide and CCL21. In addition, LV are essential in controlling the immune‐cell trafficking to prevent tumor development, and inhibition of LV impairs immune cytotoxicity (density and function of tumor‐infiltrating cytotoxic immune cells)
Unexpectedly, however, in a recent study using mouse melanoma models, VEGFR3‐blocking antibodies suppressed the therapeutic effects, accompanied by decreased tumor infiltration of naïve T cells. Importantly, in human metastatic melanoma, gene expression of VEGF‐C strongly correlated with CCL21 and T‐cell inflammation, and serum VEGF‐C concentrations were associated with both T‐cell activation and expansion after peptide vaccination and clinical response to checkpoint blockade.69 These findings suggest that VEGF‐C‐mediated lymphangiogenesis potentiates the effects of immunotherapy despite promoting an immunosuppressive microenvironment. In fact, several studies have revealed an immune protective role of LV against tumor. LEC are able to take up antigen from the lymph, process it, and cross‐present peptides on MHC‐I molecules to CD8+ T cells.70 In addition, LV may play an important role in the regulation of immune cytotoxicity, as evidenced by the observation that decreased presence of LV correlate with reduced immune cytotoxicity (Figure 3). Consistently, increased marginal LV are negatively correlated with distant metastases in human colorectal carcinoma.71 Furthermore, melanomas implanted in K14‐VEGFR3‐Ig mice exhibited drastically decreased immune cytotoxicity.40 Along this line, the inhibition of LV regeneration by photodynamic therapy impaired the development of antitumor immunity.72
Thus, LV may have paradoxical roles in tumor progression, not only allowing metastatic escape but also enhancing the immune recognition and critical checkpoints in antitumor responses. Therefore, it is the balance between protumor and antitumor immune responses that likely determines tumor progression.
The LV are not just passive channels but can actively participate in the regulation of tumor cell behaviors and modulation of antitumor immune responses. To understand the precise role of lymphangiogenesis in tumor progression, it is necessary to investigate the molecular mechanism underlying functional heterogeneity and plasticity of tumor lymphangiogenesis.
4. CONCLUSION AND PROSPECTS
The research on the heterogeneity of tumor lymphangiogenesis discussed in this review does not provide a complete list of biological aspects and associations with tumor progression. Although lymphangiogenesis has been proposed as a possible target to block cancer metastasis, there are still no antilymphangiogenic drugs approved for clinical trials. To translate the potential bench findings into the clinical setting, understanding the mechanisms underlying the precise role of heterogeneous lymphangiogenesis in tumor progression is essential. Methodologically, the isolation methods for pure LEC and sophisticated deep and single‐cell RNA sequencing technologies should enable researchers to reveal the cellular origins of tumor LEC and identify the origin‐specific LEC markers. Genetic lineage tracing tools in combination with advanced whole mount imaging techniques will enable research into the dynamics and pathological significance of lymphangiogenesis in the context of hierarchical lymphatic networks in tumor progression.
As we continue to elucidate the molecular mechanism for the structural heterogeneity and functional plasticity of tumor lymphangiogenesis, we hope to become increasingly capable of understanding the phenotypes of tumor lymphangiogenesis to inhibit tumor progression. Antilymphangiogenesis therapy may be more precise and effective when the factors involved in the structural and functional heterogeneity of dynamic lymphangiogenesis in tumor progression are sufficiently considered.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Hu X, Luo J. Heterogeneity of tumor lymphangiogenesis: Progress and prospects. Cancer Sci. 2018;109:3005‐3012. 10.1111/cas.13738
REFERENCES
- 1. Ma Q, Dieterich LC, Detmar M. Multiple roles of lymphatic vessels in tumor progression. Curr Opin Immunol. 2018;53:7‐12. [DOI] [PubMed] [Google Scholar]
- 2. Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573‐583. [DOI] [PubMed] [Google Scholar]
- 3. Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946‐953. [DOI] [PubMed] [Google Scholar]
- 4. He Y, Rajantie I, Ilmonen M, et al. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737‐3740. [DOI] [PubMed] [Google Scholar]
- 5. Raica M, Jitariu AA, Cimpean AM. Lymphangiogenesis and anti‐lymphangiogenesis in cutaneous melanoma. Anticancer Res. 2016;36:4427‐4435. [DOI] [PubMed] [Google Scholar]
- 6. Williams CS, Leek RD, Robson AM, et al. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol. 2003;200:195‐206. [DOI] [PubMed] [Google Scholar]
- 7. Sipos B, Kojima M, Tiemann K, et al. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. J Pathol. 2005;207:301‐312. [DOI] [PubMed] [Google Scholar]
- 8. Morton DL, Thompson JF, Cochran AJ, et al. Final trial report of sentinel‐node biopsy versus nodal observation in melanoma. N Engl J Med. 2014;370:599‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van Akkooi AC, Eggermont AM. Melanoma: MSLT‐1‐SNB is a biomarker, not a therapeutic intervention. Nat Rev Clin Oncol. 2014;11:248‐249. [DOI] [PubMed] [Google Scholar]
- 10. Padera TP, Kadambi A, di Tomaso E, et al. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883‐1886. [DOI] [PubMed] [Google Scholar]
- 11. Proulx ST, Luciani P, Christiansen A, et al. Use of a PEG‐conjugated bright near‐infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials. 2013;34:5128‐5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349‐2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mäkinen T, Adams RH, Bailey J, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson LA, Banerji S, Lawrance W, et al. Dendritic cells enter lymph vessels by hyaluronan‐mediated docking to the endothelial receptor LYVE‐1. Nat Immunol. 2017;18:762‐770. [DOI] [PubMed] [Google Scholar]
- 15. Wick N, Haluza D, Gurnhofer E, et al. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27‐expressing lymphatic endothelial cells. Am J Pathol. 2008;173:1202‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weber M, Hauschild R, Schwarz J, et al. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science. 2013;339:328‐332. [DOI] [PubMed] [Google Scholar]
- 17. Kawai Y, Hosaka K, Kaidoh M, Minami T, Kodama T, Ohhashi T. Heterogeneity in immunohistochemical, genomic, and biological properties of human lymphatic endothelial cells between initial and collecting lymph vessels. Lymphat Res Biol. 2008;6:15‐27. [DOI] [PubMed] [Google Scholar]
- 18. Liao S, Cheng G, Conner DA, et al. Impaired lymphatic contraction associated with immunosuppression. Proc Natl Acad Sci USA. 2011;108:18784‐18789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Enholm B, Karpanen T, Jeltsch M, et al. Adenoviral expression of vascular endothelial growth factor‐C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623‐629. [DOI] [PubMed] [Google Scholar]
- 20. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337‐341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Regenfuss B, Onderka J, Bock F, Hos D, Maruyama K, Cursiefen C. Genetic heterogeneity of lymphangiogenesis in different mouse strains. Am J Pathol. 2010;177:501‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ulvmar MH, Makinen T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc Res. 2016;111:310‐321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrova TV, Koh GY. Organ‐specific lymphatic vasculature: from development to pathophysiology. J Exp Med. 2018;215:35‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Potente M, Mäkinen T. Vascular heterogeneity and specialization in development and disease. Nat Rev Mol Cell Biol. 2017;18:477‐494. [DOI] [PubMed] [Google Scholar]
- 25. Lee S, Choi I, Hong YK. Heterogeneity and plasticity of lymphatic endothelial cells. Semin Thromb Hemost. 2010;36:352‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma W, Oliver G. Lymphatic endothelial cell plasticity in development and disease. Physiology. 2017;32:444‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell Metab. 2000;100:57‐70. [DOI] [PubMed] [Google Scholar]
- 28. Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer. 2014;14:159‐172. [DOI] [PubMed] [Google Scholar]
- 29. Karaman S, Detmar M. Mechanisms of lymphatic metastasis. J Clin Invest. 2014;124:922‐928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Clasper S, Royston D, Baban D, et al. A novel gene expression profile in lymphatics associated with tumor growth and nodal metastasis. Cancer Res. 2008;68:7293‐7303. [DOI] [PubMed] [Google Scholar]
- 31. Du Y, Cao M, Liu Y, et al. Low‐molecular‐weight hyaluronan (LMW‐HA) accelerates lymph node metastasis of melanoma cells by inducing disruption of lymphatic intercellular adhesion. Oncoimmunology. 2016;5:e1232235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fiedler U, Christian S, Koidl S, et al. The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol. 2006;168:1045‐1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng W, Nurmi H, Appak S, et al. Angiopoietin 2 regulates the transformation and integrity of lymphatic endothelial cell junctions. Genes Dev. 2014;28:1592‐1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karnezis T, Shayan R, Caesar C, et al. VEGF‐D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012;21:181‐195. [DOI] [PubMed] [Google Scholar]
- 35. Hong M, Jung E, Yang S, et al. Efficient assessment of developmental, surgical and pathological lymphangiogenesis using a lymphatic reporter mouse and its embryonic stem cells. PLoS ONE. 2016;11:e0157126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beasley NJ, Prevo R, Banerji S, et al. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315‐1320. [PubMed] [Google Scholar]
- 37. Hoshida T, Isaka N, Hagendoorn J, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor‐C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065‐8075. [DOI] [PubMed] [Google Scholar]
- 38. Kerjaschki D, Huttary N, Raab I, et al. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230‐234. [DOI] [PubMed] [Google Scholar]
- 39. Kim KE, Koh YJ, Jeon BH, et al. Role of CD11b+ macrophages in intraperitoneal lipopolysaccharide‐induced aberrant lymphangiogenesis and lymphatic function in the diaphragm. Am J Pathol. 2009;175:1733‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lund AW, Wagner M, Fankhauser M, et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest. 2016;126:3389‐3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao R. Presence of bone marrow‐derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184‐4190. [DOI] [PubMed] [Google Scholar]
- 42. Lee JY, Park C, Cho YP, et al. Podoplanin‐expressing cells derived from bone marrow play a crucial role in postnatal lymphatic neovascularization. Circulation. 2010;122:1413‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107‐1111. [DOI] [PubMed] [Google Scholar]
- 44. Naxerova K, Reiter JG, Brachtel E, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ohno F, Nakanishi H, Abe A, et al. Regional difference in intratumoral lymphangiogenesis of oral squamous cell carcinomas evaluated by immunohistochemistry using D2‐40 and podoplanin antibody: an analysis in comparison with angiogenesis. J Oral Pathol Med. 2007;36:281‐289. [DOI] [PubMed] [Google Scholar]
- 46. He Y, Rajantie I, Pajusola K, et al. Vascular endothelial cell growth factor receptor 3‐mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739‐4746. [DOI] [PubMed] [Google Scholar]
- 47. Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor‐secreted vascular endothelial growth factor‐C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789‐9798. [DOI] [PubMed] [Google Scholar]
- 48. Pak KH, Jo A, Choi HJ, Choi Y, Kim H, Cheong JH. The different role of intratumoral and peritumoral lymphangiogenesis in gastric cancer progression and prognosis. BMC Cancer. 2015;15:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Podgrabinska S, Skobe M. Role of lymphatic vasculature in regional and distant metastases. Microvasc Res. 2014;95:46‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Müller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50‐56. [DOI] [PubMed] [Google Scholar]
- 51. Schimanski CC, Bahre R, Gockel I, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Issa A, Le TX, Shoushtari AN, Shields JD, Swartz MA. Vascular endothelial growth factor‐C and C‐C chemokine receptor 7 in tumor cell‐lymphatic cross‐talk promote invasive phenotype. Cancer Res. 2009;69:349‐357. [DOI] [PubMed] [Google Scholar]
- 53. Hirakawa S. From tumor lymphangiogenesis to lymphvascular niche. Cancer Sci. 2009;100:983‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wakisaka N, Hasegawa Y, Yoshimoto S, et al. Primary tumor‐secreted lymphangiogenic factors induce pre‐metastatic lymphvascular niche formation at sentinel lymph nodes in oral squamous cell carcinoma. PLoS ONE. 2015;10:e0144056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qian CN, Berghuis B, Tsarfaty G, et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006;66:10365‐10376. [DOI] [PubMed] [Google Scholar]
- 56. Brown M, Assen FP, Leithner A, et al. Lymph node blood vessels provide exit routes for metastatic tumor cell dissemination in mice. Science. 2018;359:1408‐1411. [DOI] [PubMed] [Google Scholar]
- 57. Pereira ER, Kedrin D, Seano G, et al. Lymph node metastases can invade local blood vessels, exit the node, and colonize distant organs in mice. Science. 2018;359:1403‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kim M, Koh YJ, Kim KE, et al. CXCR4 signaling regulates metastasis of chemoresistant melanoma cells by a lymphatic metastatic niche. Cancer Res. 2010;70:10411‐10421. [DOI] [PubMed] [Google Scholar]
- 59. Boyle ST, Ingman WV, Poltavets V, et al. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem‐like cells. Oncogene. 2016;35:105‐115. [DOI] [PubMed] [Google Scholar]
- 60. Galon J, Costes A, Sanchez‐Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960‐1964. [DOI] [PubMed] [Google Scholar]
- 61. Lund AW, Duraes FV, Hirosue S, et al. VEGF‐C promotes immune tolerance in B16 melanomas and cross‐presentation of tumor antigen by lymph node lymphatics. Cell Rep. 2012;1:191‐199. [DOI] [PubMed] [Google Scholar]
- 62. Skobe M, Hawighorst T, Jackson DG, et al. Induction of tumor lymphangiogenesis by VEGF‐C promotes breast cancer metastasis. Nat Med. 2001;7:192‐198. [DOI] [PubMed] [Google Scholar]
- 63. Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Card CM, Yu SS, Swartz MA. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J Clin Invest. 2014;124:943‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aebischer D, Iolyeva M, Halin C. The inflammatory response of lymphatic endothelium. Angiogenesis. 2014;17:383‐393. [DOI] [PubMed] [Google Scholar]
- 66. Dieterich LC, Ikenberg K, Cetintas T, Kapaklikaya K, Hutmacher C, Detmar M. Tumor‐associated lymphatic vessels upregulate PDL1 to inhibit T‐cell activation. Front Immunol. 2017;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lukacs‐Kornek V, Malhotra D, Fletcher AL, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096‐1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Podgrabinska S, Kamalu O, Mayer L, et al. Inflamed lymphatic endothelium suppresses dendritic cell maturation and function via Mac‐1/ICAM‐1‐dependent mechanism. J Immunol. 2009;183:1767‐1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fankhauser M, Broggi MAS, Potin L, et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci Transl Med. 2017;9:pii: eaal4712. [DOI] [PubMed] [Google Scholar]
- 70. Hirosue S, Vokali E, Raghavan VR, et al. Steady‐state antigen scavenging, cross‐presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol. 2014;192:5002‐5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mlecnik B, Bindea G, Kirilovsky A, et al. The tumor microenvironment and Immunoscore are critical determinants of dissemination to distant metastasis. Sci Transl Med. 2016;8:327ra26. [DOI] [PubMed] [Google Scholar]
- 72. Muchowicz A, Wachowska M, Stachura J, et al. Inhibition of lymphangiogenesis impairs antitumour effects of photodynamic therapy and checkpoint inhibitors in mice. Eur J Cancer. 2017;83:19‐27. [DOI] [PubMed] [Google Scholar]


