Abstract
It has been shown that long noncoding RNAs (lncRNAs) are involved in the carcinogenesis of multiple cancers. However, the roles of lncRNAs in gastroenteropancreatic neuroendocrine neoplasms (GEP‐NENs) remain elusive. In the present study, we found that lncNEN885 was markedly decreased in human gastric NEN samples compared to adjacent normal tissues by transcriptome sequencing. Functionally, silencing or overexpression of lncNEN885 could not obviously affect cell proliferation or apoptosis in BON‐1 or LCC‐18 cells but could affect cell migration and invasion as well as wound‐healing rates. Furthermore, dysregulation of lncNEN885 affected these biological functions by activating epithelial‐mesenchymal transition through increased expression of Snail, vimentin, and N‐cadherin as well as decreased E‐cadherin levels in BON‐1 and LCC‐18 cells. Silencing of lncNEN885 could dramatically increase the phosphorylation of glycogen synthase kinase‐3β and decrease the expression of adenomatous polyposis coli and Axin, with the subsequent accumulation of β‐catenin. Taken together, dysregulation of lncNEN885 can regulate cell migration and invasion by activating epithelial‐mesenchymal transition process partially through canonical Wnt/β‐catenin signaling in GEP‐NEN cells, which may be a novel biomarker for the metastasis of GEP‐NENs.
Keywords: BON‐1 cell, epithelial‐mesenchymal transition, lncNEN885, neuroendocrine neoplasm, Wnt/β‐catenin signaling
1. INTRODUCTION
Neuroendocrine neoplasms (NENs) are a kind of heterogenous solid tumor distributed in the neuroendocrine system. Those common in the digestive tract, such as the stomach, small intestine, rectal, and pancreas, are named gastroenteropancreatic neuroendocrine neoplasms (GEP‐NENs). The incidence and prevalence of GEP‐NENs are rising steeply. These neoplasms represent the second most common gastrointestinal malignancy in the world, with their incidence reportedly rising from 1.09/100 000 to 6.98/100 000 in the last 30 years.1, 2 The survival time of GEP‐NEN patients is relatively long, which indicates more metastasis and poor quality of life. Distant metastasis, especially liver metastasis, is as common as other carcinomas, which results in more difficult treatment and worse prognosis.3, 4 Thus, seeking sensitive and distinctive biomarkers for early diagnosis of metastasis is a key method toward the effective treatment of GEP‐NEN patients.
Long noncoding RNAs (lncRNAs), known as RNAs longer than 200 nt without translated protein products, are vital regulators involved in tumor initiation, progression, and metastasis.5, 6 Previous studies have reported that dysregulated expression of lncRNAs is frequently relevant to tumorigenesis and metastasis as well as novel effective biomarkers for prognosis in other carcinomas. For instance, lncTCF7 regulated transcription factor 7 expression and induced activation of Wnt signaling in human liver cancer stem cells.7 LncRNA‐ATB could foresee metastasis in hepatocellular carcinoma patients and serves as a potential target for antimetastatic therapies.8 However, there is only one published report relating to lncRNAs and GEP‐NENs, which identified a tumor growth and metastasis suppressive modulator named lncMEG3 in pancreatic neuroendocrine tumors.9 Therefore, more potentially effective lncRNAs for early diagnosis of metastasis in GEP‐NEN patients need to be explored.
Epithelial‐mesenchymal transition (EMT) has been known as an important modulator in the tumorigenic process, especially metastasis.10 It has been first described in embryonic development, inducing the transition of epithelial cells to cells with a mesenchymal phenotype. Some prototypical markers, such as Snail, E‐cadherin, and vimentin, are defined as characteristic of EMT.11, 12, 13, 14 Epithelial‐mesenchymal transition is associated with an invasive or metastatic phenotype of several tumors, including colorectal cancer and gastric cancer.15, 16
Wnt/β‐catenin signaling, which functions as a mediator to maintain epithelial cell phenotype, proper cell‐cell junctions, and tissue homeostasis, is involved in the development of cancer and EMT‐related metastasis.17, 18 However, the role of EMT and its mechanism in GEP‐NENs have not been elucidated until now.
In our study, we found a novel lncRNA (Enst00000414885) named lncNEN885 in human gastric neuroendocrine neoplasms by transcriptome sequencing. LncNEN885 was markedly decreased in human gastric neuroendocrine neoplasm samples compared to adjacent normal tissues. Dysregulation of LncNEN885 did not obviously affect cellular proliferation and apoptosis but did influence cell migration, invasion, and wound‐healing rates in BON‐1 and LCC‐18 cells by activating EMT partially through canonical Wnt/β‐catenin signaling. LncNEN885 is a potentially novel biomarker for the metastasis of GEP‐NENs.
2. MATERIALS AND METHOD
2.1. Patients and samples
The GEP‐NEN tissues and adjacent normal gastric tissues were obtained from three patients who underwent surgical resection at the First Affiliated Hospital of Nanjing Medical University (Nanjing, China). None of the patients received any local or systemic treatment before surgery. All experiments were approved by the Research Ethics Committee of Nanjing Medical University. Written informed consent was obtained from all participants.
2.2. Gene expression profiles
Total RNA was extracted and purified according to the protocol described elsewhere. cDNA was prepared according to the standard Affymetrix protocol from 250 ng total RNA by using GeneChip (Genminix Informatics Ltd. Co., Shanghai, China) WT PLUS Reagent. The differentially expressed lncRNAs with statistical significance were identified with volcano plot filtering. The threshold we used to screen upregulated or downregulated lncRNAs is fold change >2 and P‐value < .05.
2.3. Cell culture
BON‐1 and LCC‐18, used as human pancreatic and colonic NEN cell lines, were purchased from the Institute of Biochemistry and Cell Biology of the Chinese Academy of Sciences (Shanghai, China). The cells were maintained in DMEM/F12 containing 10% FBS and cultured at a constant temperature of 37°C in humidified air with 5% CO2.
2.4. Overexpression of si‐lncNEN885 in GEP‐NEN cells
A lentivirus overexpression RNA (Lv) and three pairs of siRNAs specifically targeting lncNEN885 were synthesized by Ribobio (Guangzhou, China). The sequences of siRNAs were as follows: si‐lncNEN885‐1, CCAAAGGACACACTTCAAT; si‐lncNEN885‐2, CCCTTGACCATACCAAGAA; and si‐lncNEN885‐3, GCAGCAGAATTAATGACAC. For overexpression of lncNEN885, the cells were transfected with the lentivirus (Lv‐lncNEN885) using Lipofectamine 2000 (Life Technologies). For silencing of lncNEN885 (si‐lncNEN885), the cells were transfected with Lipofectamine RNAiMAX (Life Technologies, New York, NY, USA). Cells transfected with negative control RNAs (Lv‐control or si‐control) were used as control.
2.5. RNA extraction and quantitative real‐time PCR
Total RNA was extracted from cultured cells or tissues with TriPure Isolation Reagent (Roche, Penzberg, Germany) according to the manufacturer's instructions. The first line of cDNA was synthesized using a Reverse Transcription Kit (Takara, Dalian, China). Real‐time PCR analyses were undertaken with Essential DNA Green Master (Roche). The results were normalized to the expression of 18S. The primers were as follows: 18S, 5′‐AGCTCCAATAGCGTATATTAAAG‐3′ (forward) and 5′‐CGGTCCTATTCCATTATTCCTA‐3′ (reverse); lnc‐NEN885, 5′‐TATTGTTCATCCATGTTCTACG‐ 3′ (forward) and 5′‐GTTGTTGTTCTTGTTGTTGTT‐3′ (reverse); E‐cadherin, 5′‐GAACGCATTGCCACATACAC‐3′ (forward) and 5′‐CTCAAATCCTCCCTGTCCA‐3′ (reverse); N‐cadherin, 5′‐AGGATCAACCCCATACACCA‐3′ (forward) and 5′‐GATGATGATGCAGAGCAGGA‐3′ (reverse); Snail, 5′‐CCCAAGCTTAATGCCGCGCTC‐3′ (forward) and 5′‐CGGGATCCTCAGCGGGGACAT‐3′ (reverse); vimentin, 5′‐CCTTGAACGCAAAGTGGAAT‐3′ (forward) and 5′‐TCTTGCGCTCCTGAAAAAT‐3′ (reverse); and GAPDH, 5′‐CGGAGTCAACGGATTTGGTCGTAT‐3′ (forward) and 5′‐AGCCTTCTCCAT GGTGGTGAAGAC‐3′ (reverse).
2.6. Protein extraction and western blotting
The method for protein from cells and tissues was extracted according to the manufacturer's recommended protocol (Vazyme, Nanjing, China). Samples from cell lysates were resolved by SDS‐PAGE and then transferred to PVDF membranes and incubated with specific antibodies (Cell Signaling Technology, Boston, MA, USA). The ECL chromogenic substrate was used to detect specific bands. Actin and GAPDH were used as the inner control. Relative protein levels were quantified by ImageJ (https://imagej.nih.gov/ij/). All antibodies were purchased from Abcam (Cambridge, MA, USA). Vimentin (ab8978), Snail (ab53591), E‐cadherin (ab1416), N‐cadherin (ab18203), β‐catenin (ab16051), phosphorylated glycogen synthase kinase 3β (p‐GSK3β) (ab68476), GSK3β (ab93926), Axin (ab131372), adenomatous polyposis coli (APC) (ab15270), Actin (ab8224), and GAPDH (ab8245).
2.7. Flow cytometry
Fluorescein isothiocyanate‐conjugated annexin V and propidium iodide (AV‐PI; Beyotime Institute of Biotechnology, Jiangsu, China) analysis was carried out according to the manufacturer's protocol. In brief, 1 × 105 pretreated cells were transfected and stained with AV‐PI in the provided binding buffer. After incubation at room temperature for 15 minutes in the dark, cells were analyzed immediately on a FACS Sort Flow Cytometer (Becton Dickinson, San Jose, CA, USA).
2.8. Apoptosis assays
Apoptotic assays were carried out with one‐step TUNEL (Beyotime Institute of Biotechnology) reactions. Briefly, the samples (tissues and pretreated cells) were permeabilized with 0.1% Triton X‐100 for 2 minutes at 4°C, and the TUNEL complex was added for 1 hour at 37°C. Hoechst 33342 (Beyotime Institute of Biotechnology) was used to label nuclei. The TUNEL‐positive cells were imaged with a microscope. The cells with deep green fluorescence were defined as apoptotic cells.
2.9. Cell proliferation assay
Cell proliferation was assessed by CCK‐8 assay (Beyotime Institute of Biotechnology). Cells were trypsinized and plated at a density of 2000 cells/100 μL medium per well in 96‐well culture plates. At different time points after transfection, 10 μL CCK‐8 liquid was added to wells and further incubated for 1 hour at 37°C. The absorbance was measured at 450 nm.
2.10. Cell migration, invasion, and wound‐healing assays
At 24 hours after transfection, the cells in serum‐free media were seeded into the upper chamber for migration assays (8‐μm pore size; Millipore, Darmstadt, Germany) and invasion assays with the Matrigel‐coated (BD, Franklin Lakes, NJ, USA) filters in 24‐well plates following the protocol described elsewhere.
For wound‐healing assays, cells transfected with Lv‐lncNEN885 or si‐lncNEN885 were cultured in 12‐well plates to approximately 90% confluence, and the monolayer was wounded using a vitreous pin that delivered a precise scratch. Images of the extent of wound healing were captured at 0, 24, 48, 72 hours.
2.11. Statistical analysis
All the experiments were carried out at least three times independently, each in triplicate. Bars represented mean ± SEM. Significance tests were analyzed by Student's t test using SPSS software (IBM SPSS 20.0, Chicago, IL, USA), and a P‐value < .05 was considered significant.
3. RESULTS
3.1. LncNEN885 expression pattern in tissues and dysregulation of LncNEN885
To examine whether lncRNA variation was involved in GEP‐NEN progression and metastasis, we explored the expression profiles of lncRNAs in GEP‐NENs and normal neighboring noncancerous tissues using gene chip analysis. The lncRNAs with a fold change >2 and P‐value < .05 in gene chip data were selected. We found 13 upregulated lncRNAs and 22 downregulated lncRNAs in tumor tissues compared with the adjacent normal tissues (Figure 1A). Of them, the expression of lncNEN885 (Enst00000414885) was remarkably lower in tumor tissues and had not been previously reported, thus it was chosen for further investigation. To further ascertain the gene chip data, we evaluated the expression of lncNEN885 in paired tumor tissues and nontumorous tissues from three patients by quantitative real‐time (qRT)‐PCR and obtained similar results (Figure 1B).
Figure 1.
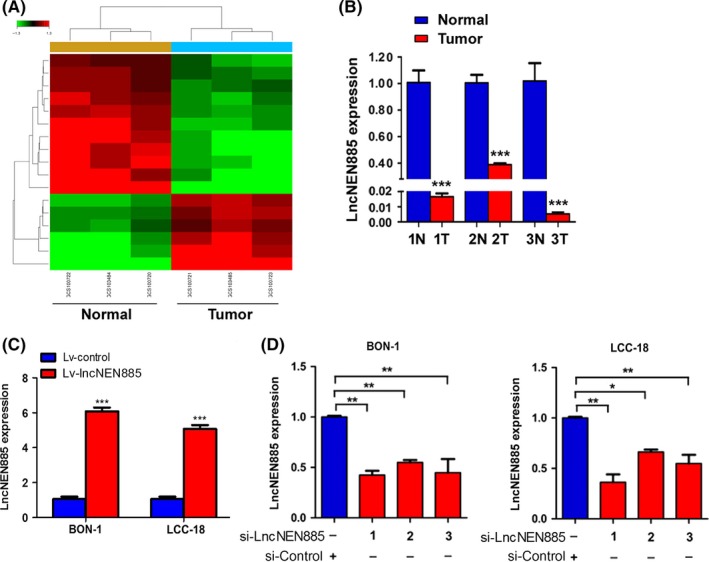
Long non‐coding RNA (lncRNA) expression profiles and efficiency of lentivirus (Lv)‐lncNEN885 or siRNA (si)‐lncNEN885. A, Gene chip analysis of lncRNAs transcripts in gastroenteropancreatic neuroendocrine neoplasms (GEP‐NENs) and adjacent normal tissues. B, Quantitative real‐time PCR analysis of lncNEN885 expression in GEP‐NENs and adjacent normal tissues (N). T, tumor samples. C, Efficiency of Lv‐lncNEN885 and Lv‐control in BON‐1 and LCC‐18 cells (Lv‐, lentivirus, overexpression). D, Efficiency of three pairs of si‐lncNEN885 compared si‐control in BON‐1 and LCC‐18 cells (si‐, siRNA, silencing). LncNEN885 expression was significantly downregulated in the siRNA group compared with the si‐control group, especially the si‐LncNEN885‐1 group. Thus, si‐LncNEN885‐1 was chosen for the following experiments, abbreviated as si‐LncNEN885. **P < .01, ***P < .001
Overexpression or silencing efficiency of lncNEN885 in GEP‐NENs cells was assessed by PCR assay. With Lv‐lncNEN885 transfection, lncNEN885 expression was significantly upregulated compared with the Lv‐control group (Figure 1C). With si‐lncNEN885 transfection, lncNEN885 expression was significantly downregulated compared with the si‐control group, especially si‐LncNEN885‐1 (Figure 1D). Thus, si‐LncNEN885‐1 was chosen for the following experiments.
3.2. Dysregulation of LncNEN885 did not influence cell proliferation
As is well known, cancer results from the imbalance between cell proliferation and apoptosis. To investigate whether dysregulation of lncNEN885 regulated cell proliferation, we undertook CCK‐8 assays in BON‐1 and LCC‐18 cells. Unfortunately, no significant difference was found between Lv‐lncNEN885 and Lv‐control or si‐lncNEN885 and si‐control groups in either BON‐1 cells (Figure 2A) or LCC‐18 cells (Figure 2B).
Figure 2.
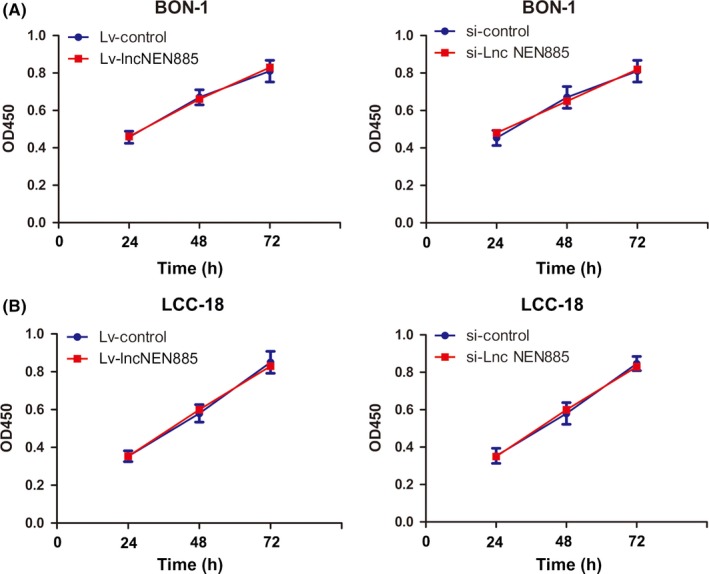
Dysregulation of long non‐coding RNA lncNEN885 did not affect proliferation or apoptosis of gastroenteropancreatic neuroendocrine tumor cells. A, Cell proliferation rates under lentivirus (Lv)‐lncNEN885 or siRNA (si)‐lncNEN885 conditions and control groups were measured by CCK‐8 assay in BON‐1 cells at 24, 48, and 72 h. B, Cell proliferation rates under Lv‐lncNEN885 or si‐lncNEN885 conditions and control groups were detected in LCC‐18 cells at 24, 48, and 72 h. OD, optical density
3.3. Dysregulation of LncNEN885 did not influence cell apoptosis
In view of the effect of lncNEN885 on the growth of cancers, we still considered that dysregulation of lncNEN885 could affect cell apoptosis in GEP‐NENs. Thus, we detected the influence of lncNEN885 dysregulation on annexin V‐positive cells by observing apoptotic rates with flow cytometry (Figure 3A,B) and TUNEL assay (Figure 3C,D). The results showed that Lv‐lncNEN885 or si‐lncNEN885 treatment did not significantly affect cell apoptosis compared to the control group. Therefore, we concluded that dysregulation had no effect on cell proliferation or apoptosis in GEP‐NENs.
Figure 3.
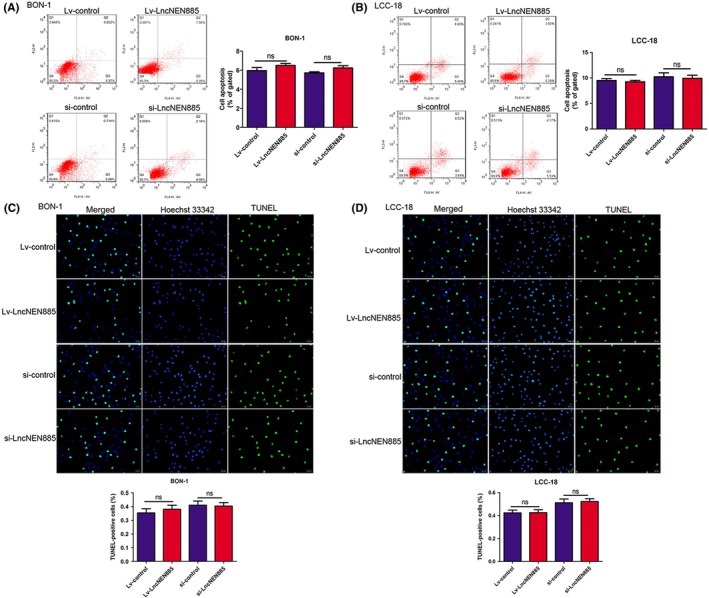
Dysregulation of long non‐coding RNA lncNEN885 did not affect apoptosis in gastroenteropancreatic neuroendocrine tumor cells. A,B, Cell apoptosis rates under lentivirus (Lv)‐lncNEN885 or siRNA (si)‐lncNEN885 conditions and control groups were measured by flow cytometry assay in BON‐1 and LCC‐18 cells. C,D, Cell apoptosis rates under si‐lncNEN885 or si‐control conditions were reconfirmed by TUNEL with Hoechst 33342 assay in BON‐1 and LCC‐18 cells. ns, no significance
3.4. Dysregulation of LncNEN885 mediated cell migration, invasion, and wound‐healing rate
Migration and invasion are crucial for tumor progression and result in poor prognosis in GEP‐NENs. We undertook a series of experiments to explore whether dysregulation of lncNEN885 could mediate GEP‐NEN cell migration and invasion. To our surprise, overexpression of lncNEN885 dramatically attenuated the migration and invasion, and knockdown of lncNEN885 obviously enhanced the migration and invasion abilities both in BON‐1 and LCC‐18 cells (Figure 4A,B).
Figure 4.
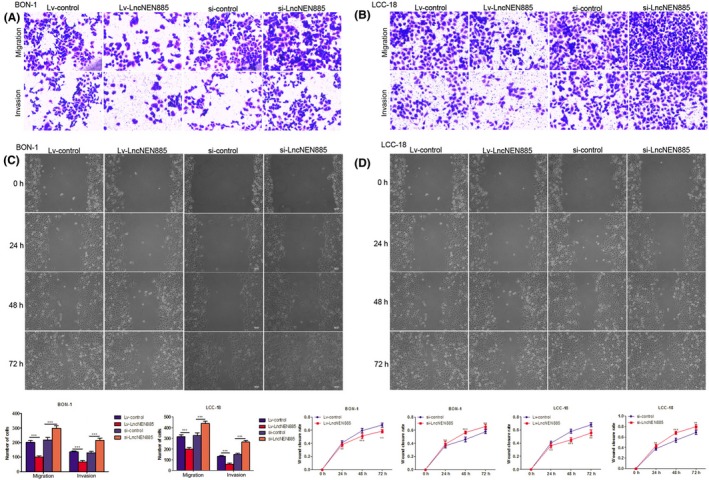
Dysregulation of long non‐coding RNA lncNEN885 could significantly affect cell metastasis in gastroenteropancreatic neuroendocrine tumor cells. A,B, Cell migration and invasion rates under lentivirus (Lv)‐lncNEN885 or Lv‐control and si‐RNA (si)‐LvNEN885 or si‐control conditions were detected with Transwell assays in BON‐1 and LCC‐18 cells transfected for 24 h. C,D, Cell wound‐healing rates under Lv‐lncNEN885 or Lv‐control and si‐LvNEN885 or si‐control conditions in BON‐1 and LCC‐18 cells were detected at 24, 48, and 72 h after transfection. **P < .01, ***P < .001
To further confirm this function of lncNEN885 in metastasis of GEP‐NENs, we undertook wound‐healing experiments using overexpression or silencing of lncNEN885 conditions in BON‐1 cells and LCC‐18 cells. The wound‐healing rate in the Lv‐lncNEN885 group was markedly decreased compared to the Lv‐control group, from 24 to 72 hours, in BON‐1 and LCC‐18 cells. The wound‐healing rate in the si‐lncNEN885 group was markedly increased compared to the si‐control group in BON‐1 and LCC‐18 cells, from 24 to 72 hours (Figure 4C,D). These results indicated that dysregulation of lncNEN885 might affect metastasis of GEP‐NENs.
3.5. Dysregulation of LncNEN885‐mediated EMT
Epithelial‐mesenchymal transition had been reported as a pivotal factor for invasion and metastasis in several cancers. Thus, we deduced that the influence of lncNEN885 dysregulation on metastasis of GEP‐NENs might be relevant to EMT. Thus, we detected the expressions of EMT‐related markers with PCR assays. The epithelial marker E‐cadherin was increased, whereas the mesenchymal markers N‐cadherin, vimentin, and Snail at protein and mRNA levels were decreased further by Lv‐lncNEN885 than by Lv‐control in BON‐1 cells. When transfected with si‐lncNEN885 or si‐control, the effects were reversed (Figure 5A,C). The same phenomena were confirmed in LCC‐18 cells (Figure 5B,D). These results indicated that dysregulation of lncNEN885 might affect metastasis of GEP‐NENs by activating EMT.
Figure 5.
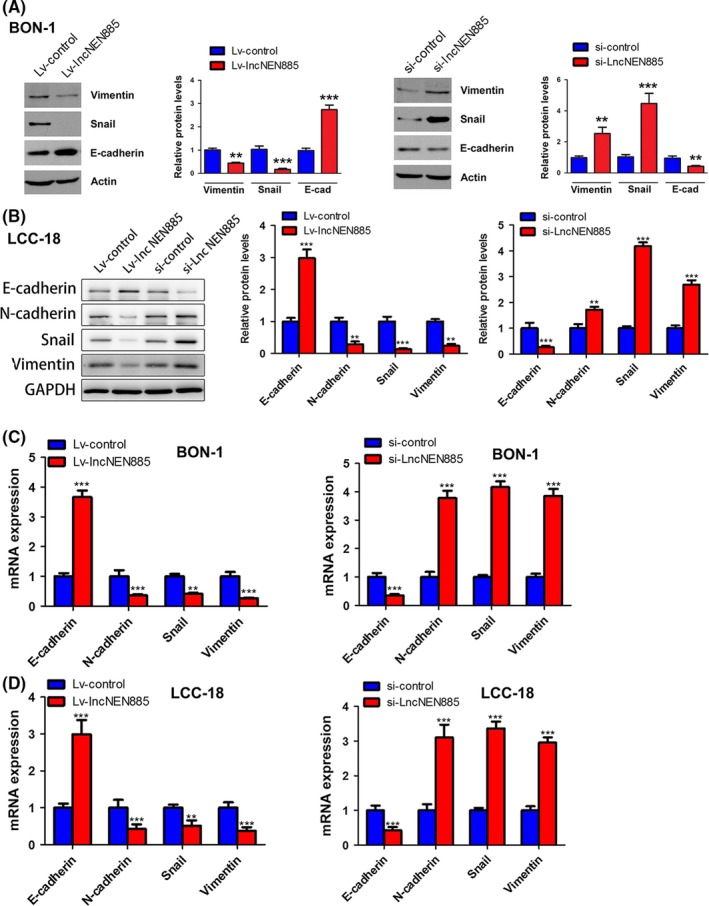
Dysregulation of long non‐coding RNA lncNEN885 could significantly affect epithelial‐mesenchymal transition (EMT) in gastroenteropancreatic neuroendocrine tumor cells. A,B, Classical EMT markers E‐cadherin, N‐cadherin, Snail, and vimentin levels under lncNEN885 overexpression or silencing conditions in BON‐1 and LCC‐18 cells were evaluated with western blot analysis. C,D, Expressions of E‐cadherin, N‐cadherin, Snail, and vimentin under lncNEN885 overexpression or silencing conditions in BON‐1 and LCC‐18 cells were detected with quantitative real‐time PCR. **P < .01, ***P < .001
3.6. LncNEN885‐mediated EMT partially relied on Wnt/β‐catenin signaling
How did dysregulation of lncNEN885 activate EMT? The canonical Wnt/β‐catenin pathway is a family of extracellular signaling involved in several cancers. The related GSK3β level could affect the phosphorylation of β‐catenin, which initiated several cellular behaviors, such as EMT. Thus, we detected the levels of Wnt/β‐catenin signaling with qRT‐PCR and western blot assays. In the Lv‐lncNEN885 group, phosphorylation of GSK3β was significantly decreased, whereas Axin and APC were significantly increased, which induced the degradation and lower level of β‐catenin than in the Lv‐control group. Conversely, β‐catenin was accumulated following with high level of GSK3β phosphorylation and lower levels of Axin and APC expression in BON‐1 cells (Figure 6A,C). The same phenomena were confirmed in LCC‐18 cells (Figure 6B,C). Moreover, to further confirm that the role of lncNEN885 in EMT might be through the regulation of Wnt/β‐catenin signaling, we transfected si‐β‐catenin in BON‐1 and LCC‐18 cells, and qRT‐PCR and western blot assays were undertaken to examine the levels of EMT. As shown in Figure 7, we found that after transfection with si‐β‐catenin, the expressions of N‐cadherin and vimentin were observed to be significantly downregulated, whereas the expression of E‐cadherin was obviously upregulated. Furthermore, cotransfection with si‐catenin and Lv‐lncNEN885 could enhance this phenomenon, and si‐lncNEN885 could reverse the effect of β‐catenin on the expression of EMT. These results showed that dysregulation of lncNEN885‐related EMT partially relied on Wnt/β‐catenin signaling.
Figure 6.
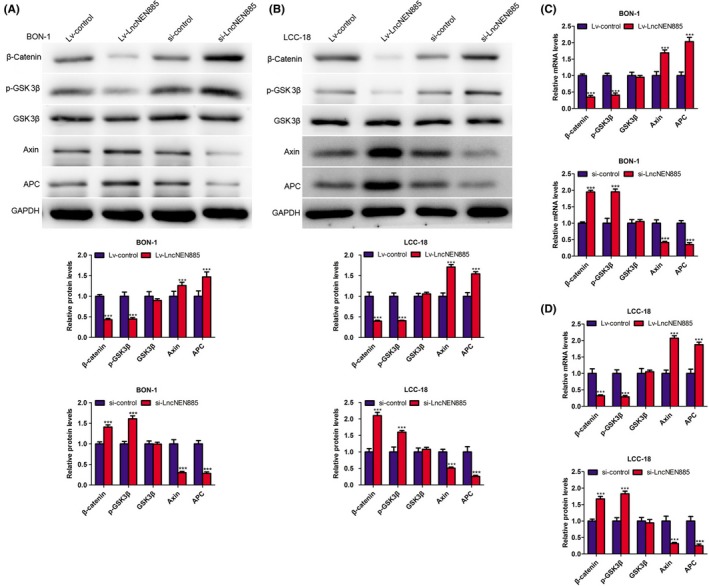
Long non‐coding RNA lncNEN885 could significantly inhibit Wnt/β‐catenin signaling. A, Markers of classical Wnt/β‐catenin signaling glycogen synthase kinase 3β (GSK3β), phosphorylated (p‐)GSK3β, Axin, adenomatous polyposis coli (APC), and β‐catenin levels under lncNEN885 overexpression or silencing conditions in BON‐1 cells were detected with western blotting. B, Expressions of GSK3β, p‐GSK3β, Axin, APC, and β‐catenin under lncNEN885 overexpression or silencing conditions in LCC‐18 cells were detected with western blotting. C,D, Expressions of GSK3β, p‐GSK3β, Axin, APC, and β‐catenin under lncNEN885 overexpression or silencing conditions in BON‐1 and LCC‐18 cells were detected with quantitative real‐time PCR assay. **P < .01, ***P < .001
Figure 7.
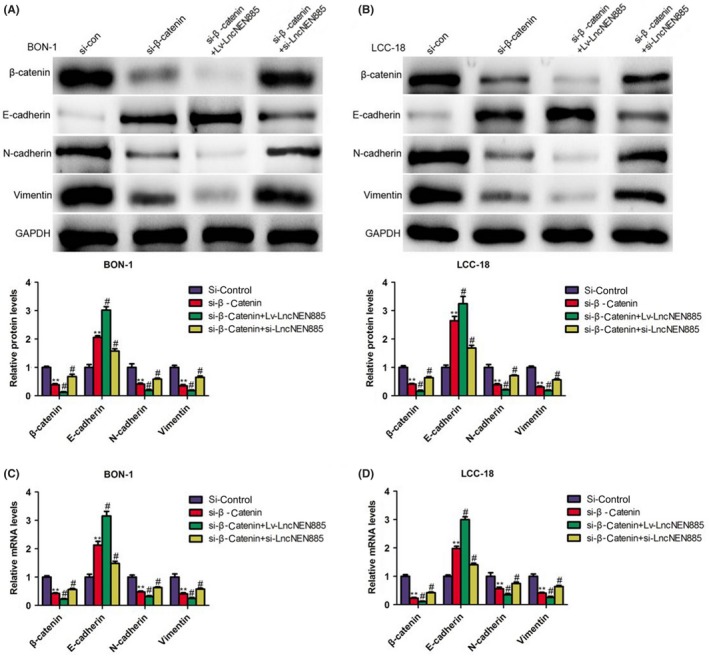
Long non‐coding RNA lncNEN885‐related epithelial‐mesenchymal transition (EMT) might partially rely on Wnt/β‐catenin signaling. A,B, Classical EMT markers E‐cadherin, N‐cadherin, Snail, and vimentin levels under siRNA (si)‐β‐catenin or cotransfection with si‐β‐catenin and lentivirus (Lv)‐LncNEN885 or si‐LncNEN885 conditions in BON‐1 and LCC‐18 cells were evaluated with western blotting. C,D, Expressions of classical EMT markers E‐cadherin, N‐cadherin, Snail, and vimentin levels under si‐β‐catenin or cotransfection with si‐β‐catenin and Lv‐LncNEN885 or si‐LncNEN885 conditions in BON‐1 and LCC‐18 cells were detected with quantitative real‐time PCR assay. **P < .01, #P < .001
4. DISCUSSION
In recent years, lncRNAs have been a hot point in the research of developmental processes and disease states as a potentially novel and crucial layer of biological regulation.19 Several studies have reported that dysregulation of lncRNAs might induce multiple diseases, such as diabetes, kidney dysfunction, cardiovascular diseases, and malignant tumors.20, 21, 22, 23 The role of lncRNAs in cancer has been the focus of this research.24 However, little is known about the role of lncRNAs in GEP‐NENs.
In the present study, we aimed to search anomalous lncRNAs in GEP‐NEN patients for early diagnosis and therapeutic targets. With transcriptome sequencing analysis, we found that the level of lncNEN885 was downregulated in human gastric neuroendocrine neoplasm tissues. LncNEN885 is a 567‐bp lncRNA that is expressed at low levels in cancer tissues. However, there have been no published reports into lncNEN885 and human disease until now. Cancer is often considered a disease of deregulated cell proliferation and apoptosis.25 Thus, we explored the role of lncNEN885 in GEP‐NENs. Regrettably, silencing or overexpression of lncNEN885 showed no significant effect on cellular proliferation or apoptosis in BON‐1 or LCC‐18 cells. We concluded that dysregulation of lncNEN885 might not affect cell death or growth in GEP‐NENs.
In view of the metastatic features of GEP‐NENs, especially the liver metastasis,26 we detected the influence of lncNEN885 on metastatic ability and found that overexpression of lncNEN885 could inhibit cellular migration and invasion in BON‐1 and LCC‐18 cells. Moreover, overexpression of lncNEN885 could inhibit the healing rate, but silencing of lncNEN885 could promote the healing rate. We concluded that dysregulation of lncNEN885 could affect the metastasis of GEP‐NENs. We then wondered how lncNEN885 variation affected metastasis. Previous studies have suggested that EMT contributes to dissemination of cancer cells and is pivotal for invasion and metastasis.27, 28 The assay on the level of EMT‐related protein revealed that high levels of lncNEN885 could increase the expression of Snail, vimentin, and N‐cadherin and decrease E‐cadherin levels. Thus, we believed that dysregulation of lncNEN885 could mediate metastasis by activating EMT in GEP‐NENs.
The canonical Wnt/β‐catenin pathway is a family of extracellular signaling that can influence cellular differentiation, division, and survival in several cancers and other diseases.29, 30, 31, 32 A named “destruction complex” composed of β‐catenin, APC, Axin, casein kinase 1, and GSK3β proteins often results in the phosphorylation, polyubiquitination, and proteasomal degradation of β‐catenin in the absence of Wnt ligand.33 Phosphorylation of GSK3β can break this complex and inhibit its ability to phosphorylate β‐catenin, which initiates several cellular behaviors such as EMT.34, 35, 36 In our study, we found that silencing of lncNEN885 could dramatically increase the phosphorylation of GSK3β and decrease the expression of APC and Axin, with the subsequent accumulation of β‐catenin. Furthermore, si‐β‐catenin could decrease the expressions of N‐cadherin and vimentin and increase the expression of E‐cadherin. Cotransfection with si‐catenin and Lv‐lncNEN885 could enhance this phenomenon, and si‐lncNEN885 could reverse the effect of β‐catenin on the expressions of EMT. That is, lncNEN885 induces EMT partially through canonical Wnt/β‐catenin signaling.
Taken together, lncNEN885 regulates cellular migration and invasion by activating the EMT process partially through canonical Wnt/β‐catenin signaling in GEP‐NENs, but does not influence cellular apoptosis or proliferation. LncNEN885 could be a novel diagnostic and therapeutic biomarker for the metastasis of GEP‐NENs.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
ACKNOWLEDGMENTS
This study was supported by the Medical Key Talents Project of Jiangsu Province (Grant No. ZDRCA2016008) and the “333” Project of Jiangsu Province (Grant No. BRA2017535).
Wei Y‐L, Hua J, Liu X‐Y, et al. LncNEN885 inhibits epithelial‐mesenchymal transition by partially regulation of Wnt/β‐catenin signalling in gastroenteropancreatic neuroendocrine neoplasms. Cancer Sci. 2018;109:3139–3148. 10.1111/cas.13747
Funding information
The “333” Project of Jiangsu Province, Grant/Award Number: BRA2017535 Medical Key Talents Project of Jiangsu Province, Grant/Award Number: ZDRCA2016008
Wei and Hua contributed equally to this work.
Contributor Information
Jian‐An Bai, Email: baijianan031@163.com.
Qi‐Yun Tang, Email: tqy831@163.com.
REFERENCES
- 1. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turaga KK, Kvols LK. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J Clin. 2011;61:113‐132. [DOI] [PubMed] [Google Scholar]
- 3. Frilling A, Modlin IM, Kidd M, et al. Recommendations for management of patients with neuroendocrine liver metastases. Lancet Oncol. 2014;15:e8‐e21. [DOI] [PubMed] [Google Scholar]
- 4. Zhang M, Zhao P, Shi X, et al. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: a large, retrospective single‐centre study. BMC Endocr Disord. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Luo X, Qiu Y, Jiang Y, et al. Long non‐coding RNA implicated in the invasion and metastasis of head and neck cancer: possible function and mechanisms. Mol Cancer. 2018;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang Y, He L, Du Y, et al. The long noncoding RNA lncTCF7 promotes self‐renewal of human liver cancer stem cells through activation of Wnt signaling. Cell Stem Cell. 2015;16:413‐425. [DOI] [PubMed] [Google Scholar]
- 8. Yuan JH, Yang F, Wang F, et al. A long noncoding RNA activated by TGF‐beta promotes the invasion‐metastasis cascade in hepatocellular carcinoma. Cancer Cell. 2014;25:666‐681. [DOI] [PubMed] [Google Scholar]
- 9. Zhang YY, Feng HM. MEG3 suppresses human pancreatic neuroendocrine tumor cells growth and metastasis by down‐regulation of Mir‐183. Cell Physiol Biochem. 2017;44:345‐356. [DOI] [PubMed] [Google Scholar]
- 10. Brabletz T, Kalluri R, Nieto MA, et al. EMT in cancer. Nat Rev Cancer. 2018;18:128‐134. [DOI] [PubMed] [Google Scholar]
- 11. Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu R, Liu Y, Zhou H, et al. Deubiquitinating enzyme PSMD14 promotes tumor metastasis through stabilizing SNAIL in human esophageal squamous cell carcinoma. Cancer Lett. 2018;418:125‐134. [DOI] [PubMed] [Google Scholar]
- 13. Wang Y, Shi J, Chai K, et al. The role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets. 2013;13:963‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo X, Zhao L, Cheng D, et al. AKIP1 promoted epithelial‐mesenchymal transition of non‐small‐cell lung cancer via transactivating ZEB1. Am J Cancer Res. 2017;7:2234‐2244. [PMC free article] [PubMed] [Google Scholar]
- 15. Vu T, Datta PK. Regulation of EMT in colorectal cancer: a culprit in metastasis. Cancers (Basel). 2017;9:E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao QH, Liu F, Li CZ, et al. Testes‐specific protease 50 (TSP50) promotes invasion and metastasis by inducing EMT in gastric cancer. BMC Cancer. 2018;18:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/beta‐catenin signalling pathway regulates epithelial‐mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638‐1649. [DOI] [PubMed] [Google Scholar]
- 18. Santiago L, Daniels G, Wang D, et al. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7:1389‐1406. [PMC free article] [PubMed] [Google Scholar]
- 19. Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193:651‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737‐750. [DOI] [PubMed] [Google Scholar]
- 21. Leung A, Natarajan R. Long noncoding RNAs in diabetes and diabetic complications. Antioxid Redox Signal. 2017. 10.1089/ars.2017.7315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang C, Yuan J, Hu H, et al. Long non‐coding RNA CHCHD4P4 promotes epithelial‐mesenchymal transition and inhibits cell proliferation in calcium oxalate‐induced kidney damage. Braz J Med Biol Res. 2017;51:e6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Zhu G, Ma Y, et al. LncRNA CCAT1 contributes to the growth and invasion of gastric cancer via targeting miR‐219‐1. J Cell Biochem. 2017. 10.1002/cb.26560 [DOI] [PubMed] [Google Scholar]
- 24. He Q, Liu Y, Sun W. Statistical analysis of non‐coding RNA data. Cancer Lett. 2018;417:161‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342‐348. [DOI] [PubMed] [Google Scholar]
- 26. Sherman SK, Maxwell JE, Carr JC, et al. Gene expression accurately distinguishes liver metastases of small bowel and pancreas neuroendocrine tumors. Clin Exp Metastasis. 2014;31:935‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zheng X, Carstens JL, Kim J, et al. Epithelial‐to‐mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525‐530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bill R, Christofori G. The relevance of EMT in breast cancer metastasis: correlation or causality? FEBS Lett. 2015;589:1577‐1587. [DOI] [PubMed] [Google Scholar]
- 29. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781‐810. [DOI] [PubMed] [Google Scholar]
- 30. Willert K, Nusse R. Wnt proteins. Cold Spring Harb Perspect Biol. 2012;4:a007864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ashihara E, Takada T, Maekawa T. Targeting the canonical Wnt/beta‐catenin pathway in hematological malignancies. Cancer Sci. 2015;106:665‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bastakoty D, Young PP. Wnt/beta‐catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB J. 2016;30:3271‐3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cadigan KM. Wnt‐beta‐catenin signaling. Curr Biol. 2008;18:R943‐R947. [DOI] [PubMed] [Google Scholar]
- 34. Douchi D, Ohtsuka H, Ariake K, et al. Silencing of LRRFIP1 reverses the epithelial‐mesenchymal transition via inhibition of the Wnt/beta‐catenin signaling pathway. Cancer Lett. 2015;365:132‐140. [DOI] [PubMed] [Google Scholar]
- 35. Shan S, Lv Q, Zhao Y, et al. Wnt/beta‐catenin pathway is required for epithelial to mesenchymal transition in CXCL12 over expressed breast cancer cells. Int J Clin Exp Pathol. 2015;8:12357‐12367. [PMC free article] [PubMed] [Google Scholar]
- 36. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial‐mesenchymal transition. Sci Signal. 2014;7:re8. [DOI] [PMC free article] [PubMed] [Google Scholar]


