Summary
Nerve agents are organophosphate (OP) compounds and among the most powerful poisons known to man. A terrorist attack on civilian or military populations causing mass casualties is a real threat. The OP nerve agents include soman, sarin, cyclosarin, tabun and VX. The major mechanism of acute toxicity is the irreversible inhibition of acetylcholinesterase (AChE). AChE inhibition results in the accumulation of excessive acetylcholine levels in synapses leading to progression of toxic signs including hypersecretions, tremors, status epilepticus, respiratory distress and death. Miosis and rhinorrhea are the most common clinical findings in those individuals acutely exposed to OP nerve agents. Prolonged seizures are responsible for the neuropathology. The brain region that shows the most severe damage is the amygdala followed by the piriform cortex, hippocampus, cortex, thalamus, and caudate/putamen. Current medical countermeasures are only modestly effective in attenuating the seizures and neuropathology. Anticonvulsants such as benzodiazepines decrease seizure activity and improve outcome but their efficacy depends upon the administration time post-exposure to the nerve agent. Administration of benzodiazepines may increase the risk for seizure recurrence. Recent studies document long-term neurologic and behavior deficits while technological advances demonstrate structural brain changes on magnetic resonance imaging.
Keywords: organophosphate nerve agents, acetylcholinesterase, human, acute effects, long-term effects
Introduction
Background
Organophosphate (OP) G-series nerve agents were synthesized in the 1930s by German scientists.1 An accidental spill in a laboratory where synthetic production of nerve agents was being conducted highlighted the extreme toxicity of these compounds.2 These compounds quickly and efficiently penetrate the human body via the skin, inhalation, and through the bloodstream. OP nerve agent use during war is particularly advantageous given that they cause mass casualties by inducing status epilepticus and incapacitating organ systems in the body that may result in death. These compounds were deployed during the Iraq-Iran war3 and on Kurdish people in Northern Iraq.4 In 2013, sarin was dropped in Damascus killing over 1400 Syrians,5,6 including 426 children;5,7 sarin gas was suspected to have been deployed again outside of Damascus in 2016 (http://www.telegraph.co.uk/news/2016/05/17/assads-forces-have-used-sarin-nerve-gas-for-the-first-time-since/).
The recent death of a high profile figure by VX, a V-series nerve agent that exerts higher toxicity in comparison with some other G-series nerve agents possibly due to the inability of phosphorylphosphatases to break the phosphorous-sulfur bond in the bloodstream,8 at an international airport (http://www.bbc.com/news/world-asia-39096172) demonstrates facile concealment, accessibility, mobility and immediate deployment of OP nerve agents. Similar but more widespread human intoxication occurred in June of 1994 when sarin gas was surreptitiously released at midnight while people slept in the city of Matsumoto, Japan and again in March 1995 when sarin gas was deployed in the Tokyo subway where thousands of individuals were intoxicated and nineteen died.9 These events illustrate the versatility and destructive capability of chemical warfare agents i.e., killing one person versus incurring mass casualties.
Acetylcholinesterase
Nerve agents have the ability to irreversibly inhibit the enzyme AChE in central and peripheral nervous system synapses. In the mammalian brain, AChE, located in membranes of postsynaptic neurons10, exists mostly as a four subunit enzyme of 70 kDa. The catalytic subunits are linked together by disulfide bonds to the hydrophobic subunit P. Subunit P is required for localization at the cell surface11. The acetylcholinesterase gene is transcribed by alternative splicing at the carboxyl terminal with peptide sequences of R (readthrough), H (hydrophobic). or T (tail) catalytic subunit isoforms that determine post-translational processing, quaternary associations and anchoring12. The soluble and cell membrane-localized acetylcholinesterase forms are generated from the AChER form. AChEH is a glycosylphosphatidylinositol-anchored dimer that is primarily expressed in red blood cells and liver. Perhaps the most interesting, diverse and dynamic isoform is the AChET form because it produces monomers, dimers and the collagen-and hydrophobic-tailed forms and soluble forms13. The “T’ peptide directs the assembly of tetramers of AChE14 and the product of this specific transcript is the synaptic form of AChE that is expressed predominately in the CNS and muscle tissue15. Inducers of the readthrough AChE transcript include stress i.e. forced swim, continued use and toxic concentrations of AChE inhibitors and inflammation16–19. The molecular diversity of AChE leading to three isoforms serves the functional range and location of this enzyme as soluble monomers, dimers, and tetramers that are anchored in membranes, amphiphilic dimers, hydrophobic- and collagen-tailed tetramers13.
Mechanism of Action
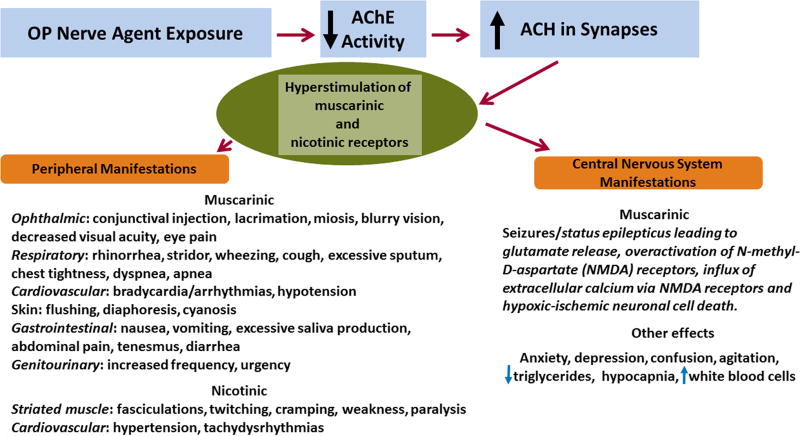
Exposure to nerve agents results in rapid absorption of agent into the bloodstream from every route, including percutaneous, inhalation and oral administration8,20. Nerve agents selectively target and irreversibly inhibit acetylcholinesterase (AChE), the enzyme that breaks down the excitatory neurotransmitter acetylcholine. In the central nervous system, acetylcholinesterase inhibition results in the overactivation of muscarinic receptors leading to the initiation of status epilepticus whereas other types of toxic signs are observed in the peripheral nervous system (Figure 1). Miosis and rhinorrhea are the most common clinical signs of exposure to OP nerve agents.9,21,22 Excessive synaptic acetylcholine levels cause a massive release of glutamate which in turn, sustains and maintains status epilepticus23,24 resulting in hypoxic-ischemic neuronal cell death via N-methyl-D-aspartate (NMDA) receptor-mediated excitotoxicity.23, 25–28
Figure 1. Overview of the mechanism of action and clinical manifestations of the acute exposure to organophosphate nerve agents.
Exposure to organophosphate (OP) nerve agents leads to the irreversible inhibition of acetylcholinesterase (AChE) resulting in the accumulation of the excitatory neurotransmitter acetylcholine (ACH) in synapses and hyperstimulation of muscarinic and nicotinic acetylcholine receptors in the central and peripheral nervous system. The major clinical manifestation in the central nervous system after exposure to OP nerve agents is overactivation of muscarinic receptors resulting in status epilepticus. Prolonged status epilepticus results in hypoxic-ischemic neuronal cell death via an N-methyl-D-aspartate (NMDA) receptor-mediated mechanism. Clinical manifestations in the peripheral nervous system depend upon the type of acetylcholine receptors expressed in the particular organ. Miosis and rhinorrhea are the most common clinical findings of exposure to OP nerve agents whereas excessive bronchial secretions and respiratory depression lead to death.
Status epilepticus is a serious complication of nerve agent exposure and is defined as a prolonged seizure or continuous seizures lasting more than five minutes without regaining consciousness.29 It is well-established that prolonged seizures cause the neuropathology.30–32 Neurodegeneration occurs most frequently in the amygdala, followed by the piriform cortex, hippocampus, cerebral cortex, thalamus, and caudate/putamen.30,33, 34–37
Some characteristics of OP nerve agents
Sarin belongs to the same class of G-series OP nerve agents as tabun, soman and cyclosarin. The OP nerve agents are volatile liquids and persist for a short time in the environment.38 Nerve agents are clear, and odorless liquids at room temperature. The vapor pressures of the nerve agents (2.9 mm Hg (sarin), 0.4 mm Hg (soman), 0.07 mm Hg (tabun) and 0.044 mm Hg (cyclosarin)) are high and the lethal vapor risk follows in descending order with sarin exhibiting the highest lethal vapor risk39. The higher the vapor pressure, the higher the volatility of the organophosphate nerve agent at any given temperature. The vapor/aerosol state enters the body through the respiratory tract and eyes, and the liquid state enters the body through eyes, skin and mouth. While it has been reported that nerve agents that exhibit low volatility do not cause miosis as an initial symptom40, the Center for Disease Control, the Agency for Toxic Substances and Disease Registry (ATSDR) and the National Institutes of Health all list miosis and other symptoms including diarrhea on their websites as symptoms following exposure to organophosphate nerve agents regardless of route of administration (https://www.atsdr.cdc.gov/MMG/MMG.asp?id=523&tid=93; https://emergency.cdc.gov/agent/nerve/tsd.asp; https://chemm.nlm.nih.gov/nerveagents.htm). Clinical examination of any patient exposed to an organophosphate nerve agent regardless of route of administration (oral, systemic, dermal, inhalation) should include evaluation of all signs of acetylcholinesterase inhibition within the sympathetic and parasympathetic system. In particular, evaluation of miosis is rapid and easy to recognize during the examination. A thorough examination of individuals thought or confirmed to be exposed to organophosphate nerve agents will minimize oversight of even an uncommon symptom that may otherwise result in respiratory failure and death.
Evaluation after OP nerve agent exposure
Exposure to OP nerve agents requires immediate evaluation and treatment because vapor and systemic exposure in particular can result in the rapid development of symptoms leading to death. It is extremely important for personnel caring for individuals exposed to OP nerve agents to keep in mind that these agents can emanate from clothing. Liquid droplets from an OP nerve agent that are absorbed by clothing can cross contaminate the skin of personnel that in turn can result in local signs initially such as sweating and fasciculations but more progressive signs of toxicity will occur as the nerve agent is absorbed and carried systemically throughout the body leading to generalized signs of toxicity including status epilepticus, defecation, miosis, bronchospasm, bronchorrhea, paralysis and respiratory failure40. If dermal exposure is suspected, multiple clinical examinations may be important in detecting delayed systemic signs and symptoms. Dermal exposure via touching contaminated clothing can result in devastating consequences if the contaminated skin like the fingers rub the eyes as ocular contact results in rapid local and systemic toxic effects40. A highly organized and coordinated effort must be in place after dissemination of an OP nerve agent. Tents with showers should be set up in the field and victims must be decontaminated with copious amounts of water. A basic outline of the exposure levels, clinical signs and symptoms and treatments is shown in Table 1.
Table 1.
Preparations and Actions in the field after deployment of an OP nerve agent
| Guidelines for First responders |
Field Equipment and Actions |
Exposure Levels |
Clinical symptoms and signs |
Treatment |
|---|---|---|---|---|
| Personal Protective Equipment, air supplied respirators, butyl rubber gloves and aprons. | HAZMAT teams, experts in chemical warfare agents, rapid decontamination of individuals exposed to organophosphate nerve agents. | Very high | Patient is unconscious, in status epilepticus, having breathing difficulties, muscle paralysis, cardiac dysfunction | Diazepam, 2-PAM and atropine, oxygen mask, intravenous access, watch for deterioration |
| First responders should have ATNAA* autoinjector kits used by the military as this is the most efficacious way to administer life-saving medical countermeasures | If showers are available, all patients need to be divested of their clothes and washed down rapidly and extensively to minimize further absorption, dermal and ocular contact of vapors and liquid | Moderate with caution | Patient is recovering from exposure to organophosphate nerve agent. Patient may need to remain if dermal contact is suspected or confirmed in anticipation of delayed symptoms and signs | 2-PAM, atropine and diazepam should be on stand-by |
| Ensure open airway, breathing and circulation. Start intravenous fluids and monitor heart rate, blood pressure, evidence of seizures. Have 2-PAM and atropine close by in case patient suddenly deteriorates | Run water over eyes extensively to reduce eye injury | Minimal | Patient has a few minor symptoms i.e. miosis, secretions | No treatment necessary |
| Report to hospital approximate number of seriously affected patients. | Showers for victims, personal protective equipment for Emergency personnel | Extremely High | Patient is in prolonged cardiopulmonary arrest | Assisted ventilation, CPR, 2-PAM, atropine and diazepam if seizing |
ATNAA is defined as Antidote treatment-nerve agent autoinjector
At the hospital, a detailed history and examination of individuals suspected of OP nerve agent exposure need to be performed as the rapid development of symptoms could lead to death depending upon the specific agent, route of exposure, and the amount of agent and time of exposure. Taking advantage of easily recognized clinical signs such as sweating, fasciculations and miosis helps to arrive at the correct diagnosis and provide urgent and life-saving treatment.
It is incumbent upon emergency department staff to be properly prepared if victims suspected of OP nerve agent exposure are expected to arrive at the hospital. Medical staff were not wearing the proper equipment in the emergency department while triaging sarin victims after the subway attack in Tokyo and were exposed to sarin vapor leading to signs and symptoms of exposure9. All personnel involved with the care of nerve agent victims need to wear personal protective equipment, and air supplied respirators. Butyl rubber gloves and aprons are protective against dermal exposure. This equipment is required until the patient is decontaminated; air purifying respirators and double latex gloves are not protective41. In addition to protecting staff and caring for victims urgently, it should be emphasized that chemical weapons inflict mass casualties. The deployment and dissemination of OP nerve agents cause chaos in the field as well as in the emergency department due to the overwhelming number of critically ill patients that need urgent treatment. If a significant amount of an OP nerve agent is deployed in a small but crowded area, one can expect that first responders will not be able to administer life-saving countermeasures within minutes of the deployment. Under these circumstances, it is not unreasonable to predict that status epilepticus in some if not many victims may continue for up to an hour before currently approved medical countermeasures can be administered to these victims.
In summary, cognizance of the possibility of OP nerve agent exposure requires an organized, carefully crafted and coordinated plan involving local hazmat teams, police, fire and experts in the field as well as a network of medical centers where well-trained and equipped staff are prepared to further decontaminate and urgently treat the expected large number of victims.
Treatment
Standard-of-care treatment for OP nerve agent acute exposure includes atropine, a muscarinic antagonist, pralidoxime (2-PAM), an oxime that regenerates acetycholinesterase activity in those molecules that are not aged, and diazepam, a benzodiazepine to stop/attenuate seizures.8,25 Repeated administration of atropine five minutes after the first injection may be necessary to reduce secretions, difficulty breathing and improve ventilation. It is critical to thoroughly wash the eyes for 5–10 minutes to limit eye injury after exposure to liquid OP nerve agents; decontamination of eyes is of no use when individuals are exposed to the vapors of OP nerve agents41. Inhibition of AChE activity by OP nerve agents is initially reversible but over time the covalent bond between the active site and the OP nerve agent stabilizes by removal of an alkyl group, a process called aging42, and results in irreversible AChE inhibition.
Current medical countermeasures are only modestly effective when given post-exposure in a mass casualty situation because it is anticipated that large crowds or a crowded areas of limited space would delay administration of life-saving medical countermeasures. Thus, it will take some time for first responders to identify survivors that need urgent treatment with medical countermeasures while victims are being decontaminated. The use of benzodiazepines is relatively ineffective when given thirty minutes or longer after OP nerve agent exposure and may increase seizure recurrence.43–45 Given the expected number of victims and the time it will take for first responders to identify and treat critically ill victims, it is not inconceivable that victims may be in status epilepticus for at least 30 minutes prior to administration of currently approved medical countermeasures.
Long-term effects after exposure to OP nerve agents
Years after exposure to sarin, victims of the Tokyo subway attack presented with significant declines in psychomotor and memory functions,46,47 signifying long-term cognitive impairment. An active duty soldier exposed to low-dose sarin while deployed to Iraq six years prior to evaluation exhibited poor informational processing speed, difficulties with speed-related bilateral manual motor coordination, poor attention, reduced memory and recall.48 In a recent article, 344 adults who were children at the time they were exposed to mustard gas and sarin in the Kurdish city of Halabja and surrounding areas deployed by Iraqi forces in 1988, participated in a study investigating the long-term effects of chemical warfare agents. Eighteen participants were excluded from the study after their forms were lost in transit. Participants exposed to chemical warfare agents were children (10 years or younger) at the time of chemical warfare exposure. The mean age of the group at the time of exposure was 4.9 years and the mean age of subjects when they were examined was 20 years. There were 202 females and 142 males in the study. Only four participants received emergency treatment within 24 hours of exposures; the remainder received treatment between 24 hours and more than one week after chemical warfare exposure. There was no mention whether anyone received currently approved medical countermeasures. Current or previous smokers, anyone who had a medical condition that affected organs relevant to the study or had a history of occupational dust exposure were excluded from the study. Documentation of both acute and long-term effects of chemical warfare agents was conducted in the subjects fourteen to twenty-two years after exposure. Among the maladies at the time of examination by investigators, seventy-four percent of subjects had neurological symptoms. Twenty percent of subjects had convulsions while ninety percent exhibited signs of anxiety/restlessness/muscular cramps. Additional clinical neurologic signs that were found in the subjects included ataxia (31%), paralysis (4%), fasciculations (7%), confusion (38%), dysarthria (22%), headaches (43%) and coma (38%)49.
People exposed to OP pesticides also exhibit long-term effects. Sheep farmers exposed to low-level OP pesticides demonstrated significant cognitive impairment on working, verbal and visual memory testing, response speed, fine motor control, mental flexibility and strategy making. In contrast, other cognitive domains such as visuospatial, verbal abilities and verbal reasoning were not impaired. Two different control groups were used in this study to ensure that there was no selection bias.50 Depression is a major neuropsychiatric disorder found in individuals exposed to either intoxicating or low-dose OP nerve agents, deployed veterans exposed to nerve agents,51,52 individuals of the terrorist attack in the Tokyo subway53 as well as those exposed to OP pesticides,50, 54–58 particularly women.59 Post-traumatic stress disorder (PTSD), an anxiety disorder, was reported in human studies following exposure to OP nerve agents despite administration of standard-of-care drugs to promote survival and stop the nerve agent-induced seizures.60,61 Anxiety in the absence of PTSD has also been found in individuals exposed to OP pesticides.50,57,62
The long-term effects of OP nerve agent exposure in humans are similar to results reported in rodents exposed to OP nerve agents demonstrating neuropathological changes in several brain regions following soman exposure63,64. Neuronal damage continues for days and weeks after OP exposure in rodents despite lifesaving treatment with an oxime, atropine and diazepam.65–67 Learning and memory deficits and depressive-like behavior are also associated with soman-induced brain damage.64–69 In summary, long-term neuropsychiatric deficits have been reported in humans exposed to either low or intoxicating levels of OP nerve agents. A recent report in children exposed to chemical warfare agents suggests that exposure to chemical warfare agents can lead to long-term neurological and neuropsychiatric deficits. Animal models of OP nerve agents replicate many of the adult human cognitive deficits.
Structural brain changes on magnetic resonance imaging after OP nerve agent exposure
Structural brain alterations were observed in the amygdala and left anterior cingulate cortex in those individuals exposed to sarin in the Tokyo subway attack carrying a diagnosis of post-traumatic stress disorder (PTSD).60,70,71 On brain magnetic resonance imaging (MRI), there was a significant reduction in the amygdalar volume on both sides of the brain; a negative correlation existed between the left amygdalar volume and PTSD and the left anterior cingulate cortex was also smaller.60 Exposure to an intoxicating dose of sarin as occurred during the Tokyo subway attack resulted in regional reductions in gray and white matter; the significant decrease in the white matter volume in the left temporal stem near the insula correlated with the serum cholinesterase levels and the severity of the somatic complaints.71 A significant reduction in regional gray matter volume was found in the right insula, temporal cortices and left hippocampus in comparison with controls.71 Structural brain changes occurred in veterans exposed to low-dose sarin and cyclosarin. A significant reduction was found in total gray and white matter volume72,73. Significant reductions in the CA2 and CA3/dentate gyrus subfields of the hippocampus were reported in individuals exposed to low-dose chemical warfare agents74 and remodeling of the white matter is suspected in the temporal stem, corona radiata, superior/inferior cingulum, internal and external capsule, inferior and superior fronto-occipital fasciculus and nine superficial white matter areas located between the cortex and deep white matter.75 Taken together, these results underscore that low-level as well as intoxicating levels of OP nerve agents can result in structural gray and white matter alterations in the brain long after exposure to OP nerve agents.
Key Point Box.
Organophosphate (OP) nerve agents are deadly poisons that inflict mass casualties in human populations.
Irreversible inhibition of acetylcholinesterase activity by OP nerve agents leads to accumulation of acetylcholine in synapses and hyperstimulation of muscarinic and nicotinic receptors in the central and peripheral nervous systems.
Hyperstimulation of muscarinic receptors in brain results in status epilepticus
A plethora of clinical manifestations in the central and peripheral nervous system results from exposure to OP nerve agents; miosis and rhinorrhea are the most common clinical signs of OP nerve agent exposure.
Long-term effects occur after low or intoxicating levels of exposure to OP nerve agents.
Acknowledgments
Our research has been supported by the CounterACT Program, National Institutes of Health, Office of the Director and the National Institute of Neurologic Disorders and Stroke [Grant Number 5U01NS058162-07 to MFB].
Footnotes
Disclosure of Conflicts of Interest
The authors have no conflicts of interest.
Ethical Publication
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.López-Muñoz F, García-García P, Alamo C. The pharmaceutical industry and the German National Socialist Regime: I.G. Farben and pharmacological research. J Clin Pharm Ther. 2009;34:67–77. doi: 10.1111/j.1365-2710.2008.00972.x. [DOI] [PubMed] [Google Scholar]
- 2.López-Muñoz F, Alamo C, Guerra JA, et al. The development of neurotoxic agents as chemical weapons during the National Socialist period in Germany. Rev Neurol. 2008;47:99–106. [PubMed] [Google Scholar]
- 3.Balali-Mood M, Saber H. Recent advances in the treatment of organophosphorous poisonings. Iran J Med Sci. 2012;37:74–91. [PMC free article] [PubMed] [Google Scholar]
- 4.Haines DD, Fox SC. Acute and Long-Term Impact of Chemical Weapons: Lessons from the Iran-Iraq War. Forensic Sci Rev. 2014;26:97–114. [PubMed] [Google Scholar]
- 5.Dolgin E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat Med. 2013;19:1194–1195. doi: 10.1038/nm1013-1194. [DOI] [PubMed] [Google Scholar]
- 6.Rosman Y, Eisenkraft A, Milk N, et al. Lessons learned from the Syrian sarin attack: evaluation of a clinical syndrome through social media. Ann Intern Med. 2014;160:644–648. doi: 10.7326/M13-2799. [DOI] [PubMed] [Google Scholar]
- 7.Thiermann H, Worek F, Kehe K. Limitations and challenges in treatment of acute chemical warfare agent poisoning. Chem Biol Interact. 2013;206:435–443. doi: 10.1016/j.cbi.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Bajgar J. Complex view on poisoning with nerve agents and organophosphates. Acta Medica (Hradec Kralove) 2005;48:3–21. [PubMed] [Google Scholar]
- 9.Okumura T, Takasu N, Ishimatsu S, et al. Report on 640 victims of the Tokyo subway sarin attack. Ann Emerg Med. 1996;28:129–135. doi: 10.1016/s0196-0644(96)70052-5. [DOI] [PubMed] [Google Scholar]
- 10.Teravainen H. Histochemical localization of acetylcholinesterase in isolated brain synaptosomes. Histochemie. 1969;18:191–194. doi: 10.1007/BF00281001. [DOI] [PubMed] [Google Scholar]
- 11.Massoulié J, Sussman J, Bon S, Silman I. Structure and functions of acetylcholinesterase and butyrylcholinesterase. Prog Brain Res. 1993;98:139–246. doi: 10.1016/s0079-6123(08)62391-2. [DOI] [PubMed] [Google Scholar]
- 12.Massoulié J. The origin of the molecular diversity and functional anchoring of cholinesterases. Neurosignals. 2002;11:130–143. doi: 10.1159/000065054. [DOI] [PubMed] [Google Scholar]
- 13.Massoulié, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S. The polymorphism of acetycholinesterase: post-translational processing, quaternary associations and localization. Chemico-Biological Interactions. 1999;119–120:20–42. doi: 10.1016/s0009-2797(99)00011-3. [DOI] [PubMed] [Google Scholar]
- 14.Bon S, Massoulié J Quaternary associations of acetylcholinesterase. I. Oligomeric associations of T subunits with and without the amino-terminal domain of the collagen tail. J Biol Chem. 1997;272:3007–3015. doi: 10.1074/jbc.272.5.3007. [DOI] [PubMed] [Google Scholar]
- 15.Massoulie J, Millard CB. Cholinesterases and the basal lamina a vertebrate neuromuscular junctions. Curr Opin Pharmacol. 2009;9:316–325. doi: 10.1016/j.coph.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Kaufer D, Friedman A, Seidman S, Soreq H. Acute stress facilitates long-lasting changes in cholinergic gene expression. Nature. 1998;393:349–360. doi: 10.1038/30741. [DOI] [PubMed] [Google Scholar]
- 17.Nijholt I1, Farchi N, Kye M, Sklan EH, Shoham S, Verbeure B, Owen D, Hochner B, Spiess J, Soreq H, Blank T. Stress-induced alternative splicing of acetylcholinesterase results in enhanced fear memory and long-term potentiation. Mol Psychiatry. 2004;9:174–183. doi: 10.1038/sj.mp.4001446. [DOI] [PubMed] [Google Scholar]
- 18.Dori A, Ifergane G, Saar-Levy T, Bersudsky M, Mor I, Soreq H, Wirguin I. Readthrough acetylcholinesterase in inflammation-associated neuropathies. Life Sci. 2007;80:2369–2374. doi: 10.1016/j.lfs.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Evron T, Greenberg D, Mor TS, Soreq H. Adaptive changes in acetylcholinesterase gene expression as mediators of recovery from chemical and biological insults. Toxicology. 2007;233:97–107. doi: 10.1016/j.tox.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Nozaki H, Aikawa N, Shinozawa Y, et al. Sarin poisoning in Tokyo subway. Lancet. 1995;345:980–981. [PubMed] [Google Scholar]
- 21.Brown MA, Brix KA. Review of health consequences from high-, intermediate- and low-level exposure to organophosphorus nerve agents. J Appl Toxicol. 1998;18:393–408. doi: 10.1002/(sici)1099-1263(199811/12)18:6<393::aid-jat528>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Okudera H. Clinical features on nerve gas terrorism in Matsumoto. J Clin Neurosci. 2002;9:17–21. doi: 10.1054/jocn.2001.1020. [DOI] [PubMed] [Google Scholar]
- 23.Lallement G, Carpentier P, Collet A, et al. Effects of soman-induced seizures on different extracellular amino acid levels and on glutamate uptake in rat hippocampus. Brain Res. 1991;563:234–240. doi: 10.1016/0006-8993(91)91539-d. [DOI] [PubMed] [Google Scholar]
- 24.Wade JV, Samson FE, Nelson SR, et al. Changes in extracellular amino acids during soman- and kainic acid-induced seizures. J Neurochem. 1987;49:645–650. doi: 10.1111/j.1471-4159.1987.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 25.Lallement G, Dorandeu F, Filliat P, et al. Medical management of organophosphate-induced seizures. J Physiol Paris. 1998;92:369–373. doi: 10.1016/S0928-4257(99)80007-2. [DOI] [PubMed] [Google Scholar]
- 26.McDonough JH, Jr, Shih TM. Neuropharmacological mechanisms of nerve agent-induced seizure and neuropathology. Neurosci Biobehav Rev. 1997;21:559–579. doi: 10.1016/s0149-7634(96)00050-4. [DOI] [PubMed] [Google Scholar]
- 27.Raveh L, Chapman S, Cohen G, et al. The involvement of the NMDA receptor complex in the protective effect of anticholinergic drugs against soman poisoning. Neurotoxicology. 1999;20:551–559. [PubMed] [Google Scholar]
- 28.Solberg Y, Belkin M. The role of excitotoxicity in organophosphorous nerve agents central poisoning. Trends Pharmacol Sci. 1997;18:183–185. doi: 10.1016/s0165-6147(97)89540-5. [DOI] [PubMed] [Google Scholar]
- 29.Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus--Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia. 2015;56:1515–1523. doi: 10.1111/epi.13121. [DOI] [PubMed] [Google Scholar]
- 30.Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003;188:69–80. doi: 10.1016/s0041-008x(03)00019-x. [DOI] [PubMed] [Google Scholar]
- 31.McDonough JH, Jr, McMonagle J, Copeland T, et al. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999;73:473–478. doi: 10.1007/s002040050637. [DOI] [PubMed] [Google Scholar]
- 32.Shih TM, McDonough JH., Jr Neurochemical mechanisms in soman-induced seizures. J Appl Toxicol. 1997;17:255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Carpentier P, Lambrinidis M, Blandchet G. Early dendritic changes in hippocampal pyriamidal neurons (field CA1) of rats subjected to acute soman intoxication: a light microscopic study. Brain Res. 1991;541:293–299. doi: 10.1016/0006-8993(91)91030-5. [DOI] [PubMed] [Google Scholar]
- 34.Aroniadou-Anderjaska V, Figueiredo TH, Apland JP, et al. Primary brain targets of nerve agents: the role of the amygdala in comparison to the hippocampus. Neurotoxicology. 2009;30:772–776. doi: 10.1016/j.neuro.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baze WB. Soman-induced morphological changes: an overview in the non-human primate. J Appl Toxicol. 1993;13:173–177. doi: 10.1002/jat.2550130306. [DOI] [PubMed] [Google Scholar]
- 36.McDonough JH, Jr, Zoeffel LD, McMonagle J, et al. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000;38:1–14. doi: 10.1016/s0920-1211(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 37.Prager EM, Aroniadou-Anderjaska V, Almeida-Suhett CP, et al. Acetylcholinesterase inhibition in the basolateral amygdala plays a key role in the induction of status epilepticus after soman exposure. Neurotoxicology. 2013;38:84–90. doi: 10.1016/j.neuro.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Bajgar J. Organophosphates/nerve agent poisoning: mechanism of action, diagnosis, prophylaxis. Adv Clin Chem. 2004;38:151–216. doi: 10.1016/s0065-2423(04)38006-6. [DOI] [PubMed] [Google Scholar]
- 39.Ramesh C, Gupta, editors. Handbook of Toxicology of Chemical Warfare Agents. 2009. pp. 88–100. [Google Scholar]
- 40.Holstege CP, Kirk M, Sidell FR. Chemical Warfare. Medical Toxicology. 1997;13:923–942. doi: 10.1016/s0749-0704(05)70374-2. [DOI] [PubMed] [Google Scholar]
- 41.Medical Management Guidelines for Acute Chemical Exposures. Washington, DC: ATSDR, US Department of Health and Human Services; 1994. Registry of Agency for Toxic Substances and Disease: Managing Hazardous Materials Incidents, vol 3. [Google Scholar]
- 42.Millard CB, Kryger G, Ordentlich A, et al. Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level. Biochemistry. 1999;38:7032–7039. doi: 10.1021/bi982678l. [DOI] [PubMed] [Google Scholar]
- 43.Reddy SD, Reddy DS. Midazolam as an anticonvulsant antidote for organophosphate intoxication--A pharmacotherapeutic appraisal. Epilepsia. 2015;56:813–821. doi: 10.1111/epi.12989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Araujo Furtado M, Lumley LA, Robison C, et al. Spontaneous recurrent seizures after status epilepticus induced by soman in Sprague-Dawley rats. Epilepsia. 2010;51:1503–1510. doi: 10.1111/j.1528-1167.2009.02478.x. [DOI] [PubMed] [Google Scholar]
- 45.Apland JP, Aroniadou-Anderjaska V, Figueiredo TH, Rossetti F, Miller SL, Braga MF. The limitations of diazepam as a treatment for nerve agent-induced seizures and neuropathology in rats: comparison with UBP302. J Pharmacol Exp Ther. 2014;351:359–372. doi: 10.1124/jpet.114.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyaki K, Nishiwaki Y, Maekawa K, et al. Effects of sarin on the nervous system of subway workers seven years after the Tokyo subway sarin attack. Journal of occupational health. 2005;47:299–304. doi: 10.1539/joh.47.299. [DOI] [PubMed] [Google Scholar]
- 47.Nishiwaki Y, Maekawa K, Ogawa Y, et al. Effects of sarin on the nervous system in rescue team staff members and police officers 3 years after the Tokyo subway sarin attack. Environmental health perspectives. 2001;109:1169–1173. doi: 10.1289/ehp.011091169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loh Y, Swanberg MM, Ingram MV, et al. Case report: Long-term cognitive sequelae of sarin exposure. Neurotoxicology. 2010;31:244–246. doi: 10.1016/j.neuro.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Talabani JM, Ali AL, Kadir AM, Rashid R, Samin F, Greenwood D, Hay AWM. Long-term health effects of chemical warfare agents on children following a single heavy exposure. Human and Experimental Toxicology. 2017 Jan 1; doi: 10.1177/0960327117734620. 960327117734620. [DOI] [PubMed] [Google Scholar]
- 50.Mackenzie Ross SJ, Brewin CR, Curran HV, et al. Neuropsychological and psychiatric functioning in sheep farmers exposed to low levels of organophosphate pesticides. Neurotoxicol Teratol. 2010;32:452–459. doi: 10.1016/j.ntt.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toomey R, Alpern R, Vasterling JJ, Baker DG, Reda DJ, et al. Neuropsychological functioning of U.S. Gulf War veterans 10 years after the war. Journal of the International Neuropsychological Society: JINS. 2009;15:717–729. doi: 10.1017/S1355617709990294. [DOI] [PubMed] [Google Scholar]
- 52.Seidel F. Soman and sarin: Clinical manifestations and treatment of accidental poisoning by organophosphates. Clinical Toxicology. 1974;7:1–17. doi: 10.3109/15563657408987971. [DOI] [PubMed] [Google Scholar]
- 53.Yokoyama K, Araki S, Murata K, et al. Chronic neurobehavioral effects of Tokyo subway sarin poisoning in relation to posttraumatic stress disorder. Arch Environ Health. 1998;53:249–256. doi: 10.1080/00039899809605705. [DOI] [PubMed] [Google Scholar]
- 54.West I. Sequelae of poisoning from phosphate ester pesticides. Ind Med Surg. 1968;37:832–836. [PubMed] [Google Scholar]
- 55.Gershon S, Shaw FH. Psychiatric sequelae of chronic exposure to organophosphorus insecticides. Lancet. 1961;1:1371–1374. doi: 10.1016/s0140-6736(61)92004-9. [DOI] [PubMed] [Google Scholar]
- 56.Beard JD, Umbach DM, Hoppin JA, et al. Pesticide exposure and depression among male private pesticide applicators in the agricultural health study. Environ Health Perspect. 2014;122:984–991. doi: 10.1289/ehp.1307450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malekirad AA, Faghih M, Mirabdollahi M, et al. Neurocognitive, mental health, and glucose disorders in farmers exposed to organophosphorus pesticides. Arh Hig Rada Toksikol. 2013;64:1–8. doi: 10.2478/10004-1254-64-2013-2296. [DOI] [PubMed] [Google Scholar]
- 58.Beseler CL, Stallones L, Hoppin JA, et al. Depression and pesticide exposures among private pesticide applicators enrolled in the Agricultural Health Study Environ Health Perspect. 2008:1161713–1719/1719. doi: 10.1289/ehp.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bazylewicz-Walczak B, Majczakowa W, Szymczak M. Behavioral effects of occupational exposure to organophosphorous pesticides in female greenhouse planting workers. Neurotoxicology. 1999;20:819–826. [PubMed] [Google Scholar]
- 60.Rogers MA, Yamasue H, Abe O, et al. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiatry Res. 2009;174:210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Yamasue H, Kasai K, Iwanami A, et al. Voxel-based analysis of MRI reveals anterior cingulate gray-matter volume reduction in posttraumatic stress disorder due to terrorism. Proc Natl Acad Sci U S A. 2003;100:9039–9043. doi: 10.1073/pnas.1530467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamasue H, Abe O, Kasai K, et al. Human brain structural change related to acute single exposure to sarin. Ann Neurol. 2007;61:37–46. doi: 10.1002/ana.21024. [DOI] [PubMed] [Google Scholar]
- 63.Salvi RM, Lara DR, Ghisolfi ES, et al. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- 64.Schultz MK, Wright LK, de Araujo Furtado M, et al. 2014 Caramiphen edisylate as adjunct to standard therapy attenuates soman-induced seizures and cognitive deficits in rats. Neurotoxicol Teratol. 2014;44:89–104. doi: 10.1016/j.ntt.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Pan H, Piermartiri TC, Chen J, et al. Repeated systemic administration of the nutraceutical alpha-linolenic acid exerts neuroprotective efficacy, an antidepressant effect and improves cognitive performance when given after soman exposure. Neurotoxicology. 2015;51:38–50. doi: 10.1016/j.neuro.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 66.Philippens IH, Melchers BP, de Groot DM, Wolthuis OL, et al. Behavioral performance, brain histology, and EEG sequela after immediate combined atropine/diazepam treatment of soman-intoxicated rats. Pharmacol Biochem Behav. 1992;42:711–719. doi: 10.1016/0091-3057(92)90019-c. [DOI] [PubMed] [Google Scholar]
- 67.Piermartiri TCB, Pan H, Chen J, et al. Alpha-linolenic acid-induced increase of neurogenesis is a key factor in the improvement in the passive avoidance task after soman exposure. NeuroMolecular Medicine. 2015;17:251–269. doi: 10.1007/s12017-015-8353-y. [DOI] [PubMed] [Google Scholar]
- 68.Moffett MC, Schultz MK, Schwartz JE, et al. Impaired auditory and contextual fear conditioning in soman-exposed rats. Pharmacol Biochem Behav. 2011;98:120–129. doi: 10.1016/j.pbb.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 69.Piermartiri T, Pan H, Figueiredo TH, et al. α-Linolenic Acid, A Nutraceutical with Pleiotropic Properties That Targets Endogenous Neuroprotective Pathways to Protect against Organophosphate Nerve Agent-Induced Neuropathology. Molecules. 2015;20(11):20355–80. doi: 10.3390/molecules201119698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riekkinen P, Jr, Riekkinen M, Sirviö J. Cholinergic drugs regulate passive avoidance performance via the amygdala. J Pharmacol Exp Ther. 1993;267:1484–1492. [PubMed] [Google Scholar]
- 71.Abe O, Yamasue H, Kasai K, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. 2006;146:231–242. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Chao LL, Rothlind JC, Cardenas VA, et al. Effects of low-level exposure to sarin and cyclosarin during the 1991 Gulf War on brain function and brain structure in US veterans. Neurotoxicology. 2010;31:493–501. doi: 10.1016/j.neuro.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chao LL, Kriger S, Buckley S, et al. Effects of low-level sarin and cyclosarin exposure and Gulf War illness on brain structure and function: a study at 4T. Neurotoxicology. 2011;32:814–822. doi: 10.1016/j.neuro.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 74.Chao LL, Kriger S, Buckley S, et al. Effects of low-level sarin and cyclosarin exposure on hippocampal subfields in Gulf War Veterans. Neurotoxicology. 2014;44:263–269. doi: 10.1016/j.neuro.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chao LL, Zhang Y, Buckley S. Effects of low-level sarin and cyclosarin exposure on white matter integrity in Gulf War Veterans. Neurotoxicology. 2015;48:239–248. doi: 10.1016/j.neuro.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]