Abstract
BACKGROUND/OBJECTIVES
This prospective study examined gender-specific associations between egg intake and the risk of developing type 2 diabetes using data from a large-scale cohort study.
SUBJECTS/METHODS
A total of 7,002 Korean adults (40–69 years) without type 2 diabetes at baseline were analyzed. Dietary intake was evaluated by a food frequency questionnaire administered at baseline (2001–2002) and the second follow-up examination (2005–2006). Type 2 diabetes was diagnosed as a fasting glucose concentration ≥ 126 mg/dL or current use of glucose-lowering medications or insulin injection. Multivariate Cox proportional hazard models were used to calculate hazard ratios (HRs) with 95% confidence intervals (CIs) for incident type 2 diabetes according to egg consumption or cholesterol intake.
RESULTS
During a 14-year follow up period, 857 subjects developed type 2 diabetes. In men, frequent egg intake (2- < 4 servings/week) was associated with a 40% lower risk of developing type 2 diabetes than infrequent egg intake (0- < 1 serving/week) (HR = 0.60, 95% CI: 0.37–0.97), whereas no association between egg intake and incidence of type 2 diabetes was observed in women (HR = 0.61, 95% CI: 0.27–1.37). There was no association between cholesterol intake and risk of incident type 2 diabetes in either men or women.
CONCLUSIONS
Egg consumption was inversely related to the risk of incident type 2 diabetes in men, but not in women, suggesting gender differences in the relationship between diet and disease risk.
Keywords: Eggs, diabetes mellitus, cholesterol, gender
INTRODUCTION
Type 2 diabetes remains a major health problem around the world [1]. According to the International Diabetes Federation, type 2 diabetes is expected to increase by 54.5% relative to the 2013 level by 2035 [2]. The prevalence of type 2 diabetes increased from 8.6% to 11.0% between 2001 and 2013 in Korean adults aged ≥ 30 years [3].
The risk of type 2 diabetes has been associated with modifiable lifestyle factors including diet [4]. Among dietary factors, high egg intake has been suggested to be associated with increased risk of type 2 diabetes because eggs are high in cholesterol (≈ 200 mg/egg), and cholesterol has been related to an increased glucose level [5]. However, eggs contain various beneficial nutrients such as vitamins, minerals, proteins, and fatty acids [6,7], in addition to nutrients considered detrimental to health such as saturated fats and cholesterol [8,9]. Epidemiologic studies have explored the link between egg consumption and the risk of incident type 2 diabetes, but findings are inconsistent. Studies have found a negative association [10], positive association [11], or no association [12,13,14,15]. Virtanen et al. [10] reported that higher egg intake (> 45 g/d) was associated with a 45% lower risk of incident type 2 diabetes compared with lower egg intake (< 14 g/d) in Finish men aged 42–60 years. In a prospective study, however, daily egg consumption (≥ 7 servings/week) was associated with an increased risk of developing type 2 diabetes compared with no egg consumption in both men and women [11]. In a cohort study using data from the Japan Public Health Center-based Prospective Study, there was no association between egg intake and risk of type 2 diabetes [15].
A recent meta-analysis suggested that the relationship between egg intake and the risk of type 2 diabetes might differ between populations because of different egg intake habits [16]. Most studies have been conducted in populations from Western countries, with only one study conducted in an Asian population [15]. Asian people have different dietary patterns [17], lower body mass, and insulin secretory capacity than Western populations [18,19].
Recently, gender differences with respect to dietary factors and the risk of chronic diseases such as hypertension and metabolic syndrome have been suggested [20,21]. It is well known that men and women had different eating habits. Song et al. [21] reported that men and women have different dietary patterns, and an inverse association between a diet rich in whole grains and legumes and hypertension was found only in women. However, most previous studies have not conducted gender-stratified analyses, or the analyses have been conducted in only one gender group.
Therefore, we explored if there was a gender-specific association between egg consumption and the risk of incident type 2 diabetes in Korean adults using data from a large-scale community-based cohort study. In addition, we examined the effect of dietary cholesterol, which is a major component of eggs, on the risk of type 2 diabetes according to gender.
SUBJECTS AND METHODS
Study subjects
This population-based prospective study used data from the Korean Genome and Epidemiology Study (KoGES). The KoGES was conducted to examine the association among environmental, lifestyle, and dietary factors on the development of chronic diseases such as hypertension and diabetes mellitus in Korean adults. A total of 10,030 Korean adults (40–69 years) were recruited from Ansan and Ansung areas at baseline (2001–2002). Questionnaires on lifestyle factors and demographic information were administered and anthropometric and biochemical measurements were conducted every 2 years. The present study analyzed the data from baseline through the sixth follow-up examination (2013–2014). All participants provided written informed consent. The study procedure was approved by the Institutional Review Boards of Kyung Hee University (KHSIRB-16-022) and the Korea Centers for Disease Control and Prevention.
We excluded 909 individuals who had type 2 diabetes at baseline, 879 individuals who declined to participate in follow-up examinations, 197 individuals who had cardiovascular disease or cancer, 318 individuals who had an extraordinary energy intake (< 500 or > 5,000 kcal), and 725 individuals who did not complete the food frequency questionnaire (FFQ). A total of 7,002 subjects (3,318 men and 3,684 women) were included in the final analysis. A follow-up rate of 71.5% was achieved.
Assessment of dietary intake
Diet was assessed by a 103-item semi-quantitative FFQ [22] at baseline and the second follow-up examination. To assess the validity of this FFQ, 12-day diet records were collected from 124 participants over a period of 1 year and nutrient intake from the diet records was compared with that inferred from the FFQ. The adjusted correlation coefficients between the FFQ and the 12-day diet records in the Korean population ranged between 0.23 and 0.64 (median for all nutrients was 0.39) [22]. Subjects reported the usual frequency and portion sizes of foods consumed during the previous year. Frequency was assessed according to nine categories for each food from “never/almost” to “3 times/day”. Details are provided elsewhere [23]. Portion size choices were as follows: “1/2 serving,” “1 serving (standard),” and “≥ 2 servings.” One serving of eggs was considered to be equivalent to 60 g. For analysis, egg consumption was converted into frequency of egg consumption per week and then multiplied by the portion sizes of eggs. Finally, subjects were classified into four groups according to egg consumption: 0- < 1, 1- < 2, 2- < 4, and ≥ 4 servings/week.
Likewise, intake of meat, vegetables, fruit, and dairy was expressed as food consumption per week and then summed as the total intake of each food group. Egg consumption included whole eggs only, but not mixed egg dishes. Meat included seven types of meat such as beef, pork, chicken, and processed meat (ham, sausage, etc.). Fish referred to 15 types of fish including white fish, omega-3 rich fish, squid, shellfish, fish cake, and salted seafood. Vegetables included 20 types such as green leafy vegetables, dark-yellow vegetables, mushrooms, and vegetable juice. Fruit referred to 12 types of fruit including non-citrus fruits, citrus fruits, and carotene-rich fruits. Dairy included milk, yogurt, and cheese.
Egg consumption was examined from the FFQ at baseline for subjects who developed type 2 diabetes or who were censored between baseline and the second follow-up. Egg consumption was examined based on the average of the two FFQs from baseline and the second follow-up for subjects who developed type 2 diabetes or who were censored after the second follow-up examination. Daily nutrient intake in terms of total energy, dietary cholesterol, and fiber was evaluated using the Korean Food Composition Table [24].
Diagnosis of type 2 diabetes
Type 2 diabetes was diagnosed as a fasting glucose concentration ≥ 126 mg/dL, the current use of glucose-lowering medications, or the use of insulin injection based on the modified WHO criteria [25]. A self-reported physician's diagnosis was also defined as a diagnosis of type 2 diabetes.
Other variables
Data on demographic characteristics, social-economic status, and lifestyle factors were collected using a questionnaire. The study collected data on education level (≤ 6 years (elementary school graduate), 7- ≤ 12 years (middle school or high school graduate), and > 12 years (college graduate or more)), household income level (< 850 US$, 850- < 1,700 US$, 1,700- < 2,500 US$, and ≥ 2,500 US$), alcohol intake (never, former, and current), and smoking status (never, former, and current). Physical activity was evaluated as metabolic equivalent of task (MET) hours per day [26]. Subjects reported hours spent on sleep and four activities categorized according to activity intensity.
Anthropometric data were obtained at baseline. Height and body weight were measured using a stadiometer (Samhwa Instrument, Seoul, Korea) and Inbody 3.0 (Biospace Corp., Seoul, Korea), respectively. Body mass index (BMI) was calculated as kg/m2. Biochemical measurements were conducted every 2 years. Blood samples were collected after 8 hours of fasting. Plasma glucose concentration was measured with an auto analyzer (ADIVA 1650, Bayer HealthCare, Tarrytown, NY, USA).
Statistical analysis
Characteristics of subjects at baseline are shown as means and standard deviations or as numbers and percentages. All data were analyzed according to gender because there was an interaction between gender and egg consumption (P for interaction = 0.0002). Comparison of variables at baseline across egg consumption and dietary cholesterol intake categories was performed using generalized linear models with post-hoc Tukey's HSD test for continuous variables and the χ2 test for categorical data. Hazard ratios (HRs) and 95% confidence intervals (CIs) for incident type 2 diabetes according to egg consumption or cholesterol intake were evaluated by time-dependent Cox proportional hazard models. Average values of egg and cholesterol consumption calculated from two dietary measures were used to minimize within-subject variation in diet [27]. No major violation of the proportional hazard assumption was identified using log-log plots [28] or Schoenfeld's residuals [29].
Multivariable adjusted models were used. Age was adjusted in model 1. BMI, residential location, education, income, smoking status, alcohol consumption, and physical activity were further adjusted in model 2. Confounders such as intake of energy, cholesterol (or eggs), fiber, meat, fish, vegetables, fruit, and dairy were further adjusted in model 3. Residual analysis was used to adjust for the intake of nutrients such as cholesterol and fiber. To select variables for adjustment in the multivariable model, potential confounders from the scientific literature were taken into account using stepwise procedures or by comparing adjusted and unadjusted effect estimates [30]. A sex interaction was identified using both a likelihood ratio test and stratified analyses. Statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC, USA). A P value < 0.05 was considered a significant difference.
RESULTS
Characteristics of subjects according to egg consumption at baseline
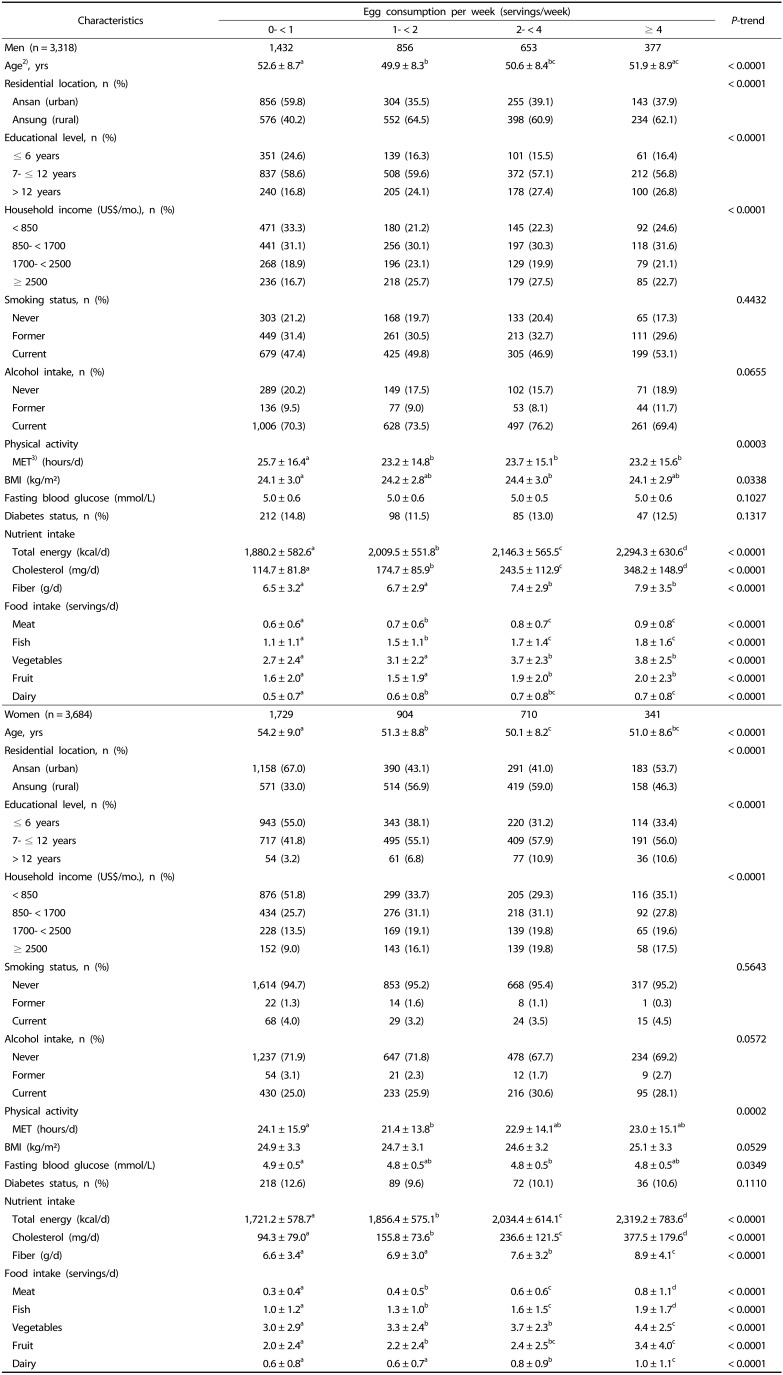
A total of 857 subjects (442 men and 415 women, 12.2%) developed type 2 diabetes during the average follow-up of 110.2 months (range 15~152 months). Characteristics of subjects at baseline according to egg consumption by gender are described in Table 1. Regardless of gender, frequent egg consumers (≥ 4 servings/week) were younger, more likely to live in a rural area, to be educated, and have higher income than infrequent egg consumers (< 1 serving/week). Also, frequent egg consumers had a higher intake of total energy, cholesterol, fiber, meat, fish, vegetables, fruit, and dairy. Baseline characteristics of 7,002 subjects who were included in the analysis were compared with those of 3,028 participants who were excluded from the analysis (data not shown). Participants who were excluded from the analysis were older, more likely to live in a rural area, and have a lower income than those who were included in the analysis. Furthermore, participants who were excluded had a higher intake of total energy, cholesterol, fiber, meat, fish, vegetables, and dairy.
Table 1. Baseline characteristics of participants according to egg consumption (n = 7,002)1).
1) Data were missing for the following variables: educational level, n = 38; household income, n = 103; smoking status, n = 58; alcohol intake, n = 23; body mass index, n = 4.
2) Values are means ± SD or numbers (percentages).
3) MET: metabolic equivalent of task
a,b,c,d Difference in variables among categories were evaluated by a generalized linear model (Tukey's test of multiple comparisons).
Baseline characteristics of subjects according to cholesterol intake
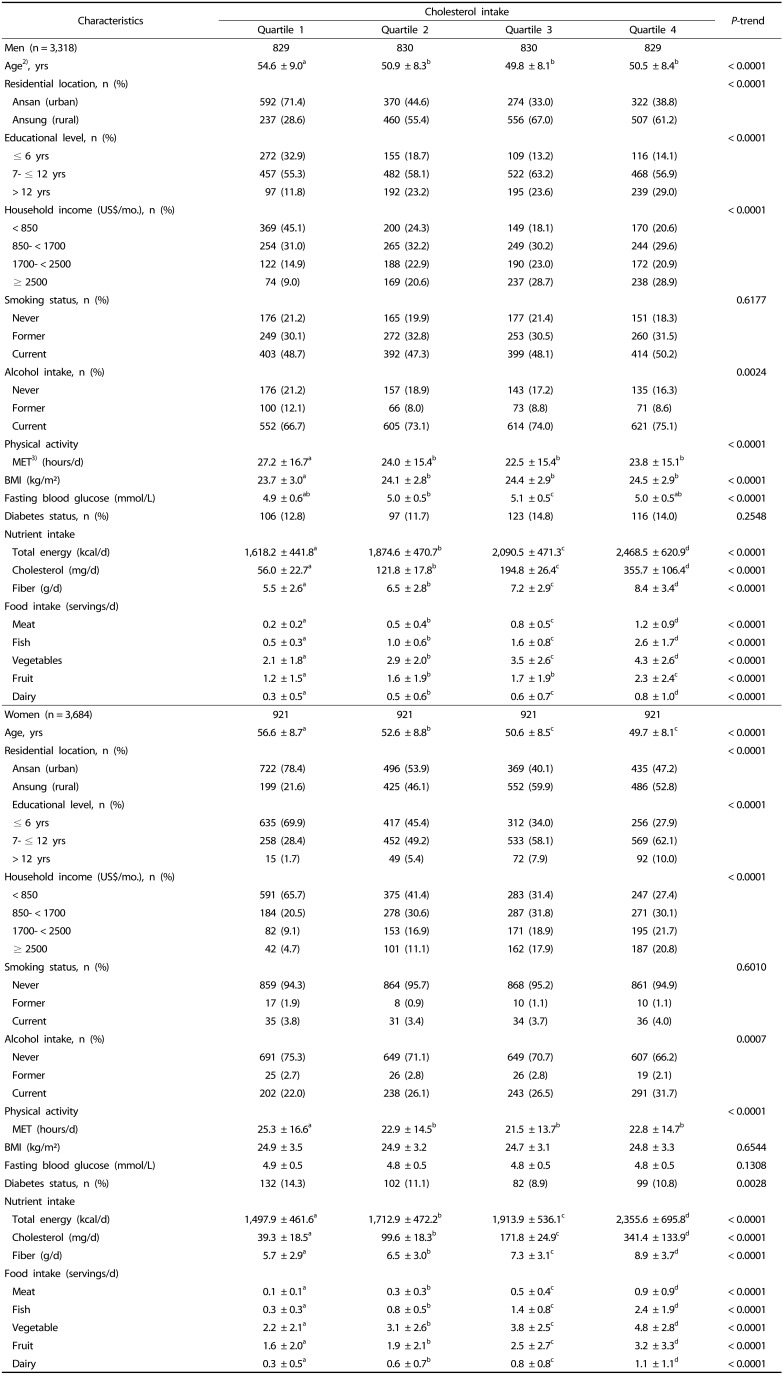
The characteristics of subjects at baseline according to cholesterol intake by gender are shown in Table 2. Regardless of gender, subjects in the highest quartile of cholesterol intake were younger, more likely to live in a rural area, to be educated, have a higher income, and to be inactive than those in the lowest quartile of cholesterol intake. Also, subjects who were in the highest quartile had a greater intake of total energy, cholesterol, fiber, meat, fish, vegetables, fruit, and dairy than those in the lowest quartile.
Table 2. Baseline characteristics of participants according to quartile categories of cholesterol intake (n = 7,002)1).
1) Data were missing for the following variables: educational level, n = 38; household income, n = 103; smoking status, n = 58; alcohol intake, n = 23; body mass index, n = 4.
2) Values are means ± SD or numbers (percentages).
3) MET: metabolic equivalent of task
a,b,c,d Differences in variables among categories were examined by a generalized linear model (Tukey's test of multiple comparisons).
Association of egg consumption with type 2 diabetes
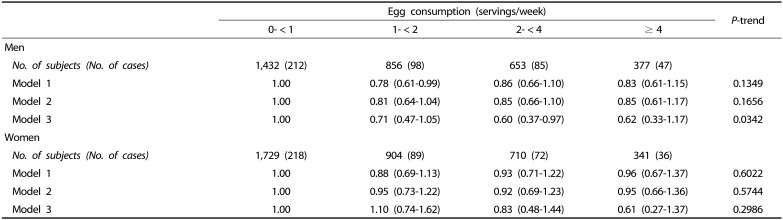
The risks of developing type 2 diabetes according to egg consumption by gender are shown in Table 3. In men, the risk for developing type 2 diabetes was 40% lower in frequent egg consumers (2- < 4 servings/week) than infrequent egg consumers (0- < 1 serving/week) (HR = 0.60, 95% CI = 0.37–0.97). The risk for type 2 diabetes tended to decrease in egg consumers who had ≥ 4 servings/week compared with infrequent egg consumers (0- < 1 serving/week), but this difference was not significant. No association was observed between egg consumption and the incidence of type 2 diabetes in women (HR = 0.61, 95% CI = 0.27–1.37, P = 0.2986).
Table 3. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of incident type 2 diabetes according to egg consumption.
Model 1 was adjusted for age.
Model 2 was adjusted for age, BMI, residential location, education level, household income, smoking status, alcohol intake, and physical activity.
Model 3 was adjusted for covariates included in model 2 plus intake of total energy, cholesterol, fiber, meat, fish, vegetables, fruit, and dairy.
Association of cholesterol intake with type 2 diabetes
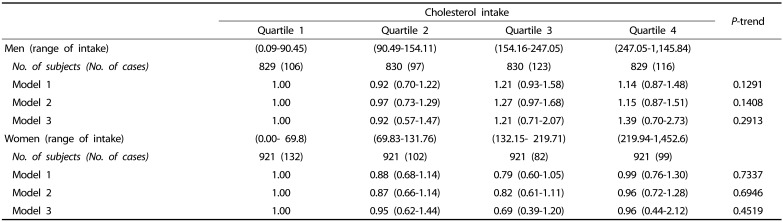
The risk of developing type 2 diabetes according to quartile categories of cholesterol intake by gender are shown in Table 4. There was no association between cholesterol intake and risk of incident type 2 diabetes in either men or women.
Table 4. Hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of incident type 2 diabetes according to quartile categories of cholesterol intake.
Model 1 was adjusted for age.
Model 2 was adjusted for age, BMI, residential location, education level, household income, smoking status, alcohol intake, and physical activity.
Model 3 was adjusted for covariates included in model 3 plus intake of total energy, fiber, meat, fish, vegetables, fruit, dairy, and eggs.
DISCUSSION
Frequent egg consumption (2- < 4 servings/week) was associated with a 40% lower risk of developing type 2 diabetes than infrequent egg consumption (0- < 1 serving/week) in middle-aged and older Korean men after adjusting for potential confounders, whereas no significant association was observed between egg consumption and the risk of incident type 2 diabetes in Korean women. Cholesterol intake was not significantly associated with the risk of incident type 2 diabetes in either sex. These results suggest that high egg consumption may be an independent risk factor that protects against the development of type 2 diabetes, and that there may be gender differences between dietary factors and the risk of type 2 diabetes in Korean adults.
These findings are consistent with the results from one previous study. In a cohort study, higher egg intake (> 45 g/d) was associated with a 45% lower risk of incident type 2 diabetes than low egg intake (< 14 g/d) after adjusting for confounding factors including dietary cholesterol in middle-aged and older Finish men aged 42–60 years during 19 years of follow-up [10]. Results from other studies are, however, inconsistent with our findings. In a prospective study using data from the Physician's Health Study with a follow-up of 20 years and the Women's Health Study with follow up of 11.7 years, men aged ≥ 40 years and women aged ≥ 45 years old who were daily egg consumers (≥ 7 servings/week) had a 58% and 77% increased risk of developing type 2 diabetes, respectively, compared with no-egg consumers after adjustment for potential confounding factors [11]. Other cohort studies have reported no relationship between egg intake and type 2 diabetes [14,31]. These studies cannot be compared directly because there are differences in study design, egg consumption level, and age, gender, and ethnicity of subjects among studies. Different results may be due to different egg intake levels among ethnic groups. Egg intake in Asian populations may be lower than intake in populations from Western countries. In a study of a Western population, the risk of type 2 diabetes increased with egg consumption of ≥ 7 servings per week [11], but only a few subjects in our study ate this quantity of eggs per week; thus, the relatively lower egg consumption in our study (≥ 4 servings per week) may have had a favorable effect on type 2 diabetes. Average egg intake was 16.5 g/day in our study, whereas egg intake averaged 30 g/day in a Western population [12]. In addition, differences in dietary habits related to egg consumption, which may be related to the risk of type 2 diabetes, could affect the results. In many countries, eggs are generally consumed as part of a mixed dish [10]. In Western countries, eggs are commonly eaten with processed meats such as sausage, bacon, or burgers, which are themselves associated with a higher risk of type 2 diabetes [32]. However, in Korea, middle-aged and older Korean men do not commonly eat processed meats with eggs. In Korea, eggs are often consumed with ‘banchan,’ which refers to various side dishes usually consumed with rice; eggs are also often consumed in isolation as a boiled or baked egg. Thus, differences in dietary habits among different ethnic groups could affect the relationship between egg intake and incident type 2 diabetes. It would be interesting to explore Korean dietary patterns associated with the risk of type 2 diabetes in a prospective study.
The potential protective mechanisms of egg consumption on lowering the risk of type 2 diabetes may be explained by various components in eggs. Eggs, especially egg yolks, contain a lot of nutrients that have a favorable effect on health such as high quality proteins, PUFAs, minerals, and vitamins [33]. In addition to these nutrients, eggs may also contain many bioactive compounds such as phosphatidylcholine and carotenoids such as zeaxanthin and lutein, which have anti-inflammatory characteristics [34,35,36]. An inverse association between egg intake and the risk of type 2 diabetes could be explained by the beneficial effects of these nutrients and food components on glucose metabolism. In a clinical trial, daily consumption of two eggs/day in a high protein and high cholesterol diet (containing 400 mg cholesterol) for 12 weeks reduced fasting glucose concentrations by 0.32 mmol/L compared to a similar amount of animal protein (100 g of chicken, meat, or fish) in a diet that was low in both saturated fat and cholesterol in individuals with type 2 diabetes [37].
Interestingly, there was a negative association between egg consumption and the risk of diabetes in men only, suggesting that gender is an important factor that affects the relationship between diet and the risk of type 2 diabetes. It is not clear yet why the relationship between diet and disease risk differs between men and women. One possible explanation is that sex steroid hormones may lead to the gender differences. For instance, androgens such as testosterone and dihydrotestosterone have been shown to be inversely related to the risk of developing diabetes in middle-aged or older men in prospective cohort studies [38,39]. High levels of androgens may protect against insulin resistance and reduce the risk of diabetes by reducing fat mass [40,41] or by influencing insulin resistance independent of adiposity [42]. Another explanation is that gender differences may be due to sex-specific food preferences and dietary habits in relation to type 2 diabetes. Generally, men prefer animal foods such as meat and fish (sources of fat including cholesterol) to vegetables, fruit, or dairy and thus consume higher quantities of fat and cholesterol and less fiber than women. Furthermore, men consume less of a variety of food groups and thus have lower quality diets than women [43,44]. In our study, women had higher scores for healthy dietary patterns i.e. they consumed various food groups such as vegetables, mushrooms, fish, potatoes, and seaweed compared with men, whereas men had higher scores for westernized dietary patterns including consumption of bread, pizza, hamburgers, cereals, snacks, and meat compared with women (data not shown). The healthier diet of women could attenuate the favorable impact of egg intake on the risk of type 2 diabetes. Vitale et al. [43] reported that women consumed more vegetables, fruits, legumes, milk, eggs, and vegetable oils, whereas men consumed more soft drinks, starchy foods, and alcoholic beverages, suggesting gender differences in dietary habits in middle-aged and older adults.
A major strength of the study is that this is the first study to report a cause-effect relationship between egg consumption and the risk of developing type 2 diabetes in an Asian population and to reveal gender differences in this association based on analyses of data from a large population-based cohort with a long follow up period (≈14 y). By using average egg intake from two FFQs (baseline and follow-up), random error due to dietary changes during follow-up and within-person variation were minimized. In addition, standardized protocols were used to obtain data on exposure and outcome and the sample size was large.
The study also had several limitations. First, egg consumption included whole eggs only, but egg consumption from egg mixed dishes such as breads and cakes was not measured. Second, information on how the eggs were cooked was not collected, so we could not determine if the relationships we found differed according to cooking method (e.g. boiled eggs vs. fried eggs). Finally, residual confounders or unmeasured confounders such as saturated fatty acids, trans fatty acids, and family history of diabetes that could affect the risk of type 2 diabetes might have affected the results.
In conclusion, low egg intake was related to increased risk of developing type 2 diabetes in middle-aged and older Korean men but not women, suggesting gender differences in the association between diet and the risk of type 2 diabetes. Dietary recommendations to limit egg intake should be re-considered in the general population. In addition, gender differences should be considered when making dietary recommendations for the prevention and management of chronic diseases.
ACKNOWLEDGMENTS
Epidemiologic data used in this study were obtained from the Korean Genome and Epidemiology Study (KoGES; 4851-302), Korea Centers for Disease Control and Prevention, Republic of Korea.
Footnotes
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF2016 R1D1A1B03931307).
CONFLICT OF INTEREST: The authors declare no potential conflicts of interests.
References
- 1.Kretowski A, Ruperez FJ, Ciborowski M. Genomics and metabolomics in obesity and type 2 diabetes. J Diabetes Res. 2016;2016:9415645. doi: 10.1155/2016/9415645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Ha KH, Kim DJ. Trends in the diabetes epidemic in Korea. Endocrinol Metab. 2015;30:142–146. doi: 10.3803/EnM.2015.30.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Djoussé L, Driver JA, Gaziano JM, Buring JE, Lee IM. Association between modifiable lifestyle factors and residual lifetime risk of diabetes. Nutr Metab Cardiovasc Dis. 2013;23:17–22. doi: 10.1016/j.numecd.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamopoulos PN, Papamichael CM, Zampelas A, Moulopoulos SD. Cholesterol and unsaturated fat diets influence lipid and glucose concentrations in rats. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:659–663. doi: 10.1016/0305-0491(95)02078-0. [DOI] [PubMed] [Google Scholar]
- 6.Herron KL, McGrane MM, Waters D, Lofgren IE, Clark RM, Ordovas JM, Fernandez ML. The ABCG5 polymorphism contributes to individual responses to dietary cholesterol and carotenoids in eggs. J Nutr. 2006;136:1161–1165. doi: 10.1093/jn/136.5.1161. [DOI] [PubMed] [Google Scholar]
- 7.Song WO, Kerver JM. Nutritional contribution of eggs to American diets. J Am Coll Nutr. 2000;19:556S–562S. doi: 10.1080/07315724.2000.10718980. [DOI] [PubMed] [Google Scholar]
- 8.Salmerón J, Hu FB, Manson JE, Stampfer MJ, Colditz GA, Rimm EB, Willett WC. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73:1019–1026. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 9.Song Y, Manson JE, Buring JE, Liu S. A prospective study of red meat consumption and type 2 diabetes in middle-aged and elderly women: the women's health study. Diabetes Care. 2004;27:2108–2115. doi: 10.2337/diacare.27.9.2108. [DOI] [PubMed] [Google Scholar]
- 10.Virtanen JK, Mursu J, Tuomainen TP, Virtanen HE, Voutilainen S. Egg consumption and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2015;101:1088–1096. doi: 10.3945/ajcn.114.104109. [DOI] [PubMed] [Google Scholar]
- 11.Djoussé L, Gaziano JM, Buring JE, Lee IM. Egg consumption and risk of type 2 diabetes in men and women. Diabetes Care. 2009;32:295–300. doi: 10.2337/dc08-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59:441–448. doi: 10.1038/sj.ejcn.1602094. [DOI] [PubMed] [Google Scholar]
- 13.Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab. 2008;52:96–104. doi: 10.1159/000121365. [DOI] [PubMed] [Google Scholar]
- 14.Djoussé L, Kamineni A, Nelson TL, Carnethon M, Mozaffarian D, Siscovick D, Mukamal KJ. Egg consumption and risk of type 2 diabetes in older adults. Am J Clin Nutr. 2010;92:422–427. doi: 10.3945/ajcn.2010.29406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurotani K, Nanri A, Goto A, Mizoue T, Noda M, Oba S, Sawada N, Tsugane S Japan Public Health Center-based Prospective Study Group. Cholesterol and egg intakes and the risk of type 2 diabetes: the Japan Public Health Center-based Prospective Study. Br J Nutr. 2014;112:1636–1643. doi: 10.1017/S000711451400258X. [DOI] [PubMed] [Google Scholar]
- 16.Wallin A, Forouhi NG, Wolk A, Larsson SC. Egg consumption and risk of type 2 diabetes: a prospective study and dose-response meta-analysis. Diabetologia. 2016;59:1204–1213. doi: 10.1007/s00125-016-3923-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kong A, Odoms-Young AM, Schiffer LA, Berbaum ML, Porter SJ, Blumstein L, Fitzgibbon ML. Racial/ethnic differences in dietary intake among WIC families prior to food package revisions. J Nutr Educ Behav. 2013;45:39–46. doi: 10.1016/j.jneb.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torréns JI, Skurnick J, Davidow AL, Korenman SG, Santoro N, Soto-Greene M, Lasser N, Weiss G Study of Women's Health Across the Nation (SWAN) Ethnic differences in insulin sensitivity and beta-cell function in premenopausal or early perimenopausal women without diabetes: the Study of Women's Health Across the Nation (SWAN) Diabetes Care. 2004;27:354–361. doi: 10.2337/diacare.27.2.354. [DOI] [PubMed] [Google Scholar]
- 19.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian X, Xu X, Zhang K, Wang H. Gender difference of metabolic syndrome and its association with dietary diversity at different ages. Oncotarget. 2017;8:73568–73578. doi: 10.18632/oncotarget.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song S, Kim J, Kim J. Gender differences in the association between dietary pattern and the incidence of hypertension in middle-aged and older adults. Nutrients. 2018;10:pii: E252. doi: 10.3390/nu10020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahn Y, Kwon E, Shim JE, Park MK, Joo Y, Kimm K, Park C, Kim DH. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur J Clin Nutr. 2007;61:1435–1441. doi: 10.1038/sj.ejcn.1602657. [DOI] [PubMed] [Google Scholar]
- 23.Kim D, Kim J. Dairy consumption is associated with a lower incidence of the metabolic syndrome in middle-aged and older Korean adults: the Korean Genome and Epidemiology Study (KoGES) Br J Nutr. 2017;117:148–160. doi: 10.1017/S000711451600444X. [DOI] [PubMed] [Google Scholar]
- 24.The Korean Nutrition Society. Recommended Dietary Allowances for Koreans. 7th ed. Seoul: The Korean Nutrition Society; 2000. Food composition table. [Google Scholar]
- 25.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, Jacobs DR, Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 28.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 29.Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 30.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–184. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 31.Zazpe I, Beunza JJ, Bes-Rastrollo M, Basterra-Gortari FJ, Mari-Sanchis A, Martínez-González MA SUN Project Investigators. Egg consumption and risk of type 2 diabetes in a Mediterranean cohort; the sun project. Nutr Hosp. 2013;28:105–111. doi: 10.3305/nh.2013.28.1.6124. [DOI] [PubMed] [Google Scholar]
- 32.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Willett WC, Hu FB. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94:1088–1096. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kovacs-Nolan J, Phillips M, Mine Y. Advances in the value of eggs and egg components for human health. J Agric Food Chem. 2005;53:8421–8431. doi: 10.1021/jf050964f. [DOI] [PubMed] [Google Scholar]
- 34.Kim JE, Leite JO, DeOgburn R, Smyth JA, Clark RM, Fernandez ML. A lutein-enriched diet prevents cholesterol accumulation and decreases oxidized LDL and inflammatory cytokines in the aorta of guinea pigs. J Nutr. 2011;141:1458–1463. doi: 10.3945/jn.111.141630. [DOI] [PubMed] [Google Scholar]
- 35.Chung HY, Rasmussen HM, Johnson EJ. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J Nutr. 2004;134:1887–1893. doi: 10.1093/jn/134.8.1887. [DOI] [PubMed] [Google Scholar]
- 36.Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C. Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study. Am J Clin Nutr. 2008;87:424–430. doi: 10.1093/ajcn/87.2.424. [DOI] [PubMed] [Google Scholar]
- 37.Pearce KL, Clifton PM, Noakes M. Egg consumption as part of an energy-restricted high-protein diet improves blood lipid and blood glucose profiles in individuals with type 2 diabetes. Br J Nutr. 2011;105:584–592. doi: 10.1017/S0007114510003983. [DOI] [PubMed] [Google Scholar]
- 38.Joyce KE, Biggs ML, Djoussé L, Ix JH, Kizer JR, Siscovick DS, Shores MM, Matsumoto AM, Mukamal KJ. Testosterone, dihydrotestosterone, sex hormone-binding globulin, and incident diabetes among older men: the cardiovascular health study. J Clin Endocrinol Metab. 2017;102:33–39. doi: 10.1210/jc.2016-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- 40.Vandenput L, Mellström D, Lorentzon M, Swanson C, Karlsson MK, Brandberg J, Lönn L, Orwoll E, Smith U, Labrie F, Ljunggren O, Tivesten A, Ohlsson C. Androgens and glucuronidated androgen metabolites are associated with metabolic risk factors in men. J Clin Endocrinol Metab. 2007;92:4130–4137. doi: 10.1210/jc.2007-0252. [DOI] [PubMed] [Google Scholar]
- 41.Fan W, Yanase T, Nomura M, Okabe T, Goto K, Sato T, Kawano H, Kato S, Nawata H. Androgen receptor null male mice develop late-onset obesity caused by decreased energy expenditure and lipolytic activity but show normal insulin sensitivity with high adiponectin secretion. Diabetes. 2005;54:1000–1008. doi: 10.2337/diabetes.54.4.1000. [DOI] [PubMed] [Google Scholar]
- 42.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, Dobs A, Basaria S, Golden SH, Platz EA. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 43.Hiza HA, Casavale KO, Guenther PM, Davis CA. Diet quality of Americans differs by age, sex, race/ethnicity, income, and education level. J Acad Nutr Diet. 2013;113:297–306. doi: 10.1016/j.jand.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 44.Vitale M, Masulli M, Cocozza S, Anichini R, Babini AC, Boemi M, Bonora E, Buzzetti R, Carpinteri R, Caselli C, Ceccarelli E, Cignarelli M, Citro G, Clemente G, Consoli A, Corsi L, De Gregorio A, Di Bartolo P, Di Cianni G, Fontana L, Garofolo M, Giorda CB, Giordano C, Grioni S, Iovine C, Longhitano S, Mancastroppa G, Mazzucchelli C, Montani V, Mori M, Perriello G, Rinaldi ME, Ruffo MC, Salvi L, Sartore G, Scaranna C, Tonutti L, Zamboni C, Zogheri A, Krogh V, Cappellini F, Signorini S, Riccardi G, Vaccaro O TOSCA.IT Study Group. Sex differences in food choices, adherence to dietary recommendations and plasma lipid profile in type 2 diabetes-The TOSCA.IT study. Nutr Metab Cardiovasc Dis. 2016;26:879–885. doi: 10.1016/j.numecd.2016.04.006. [DOI] [PubMed] [Google Scholar]