Abstract
BACKGROUND/OBJECTIVES
Benign prostatic hypertrophy (BPH) is a major cause of abnormal overgrowth of the prostate mainly in the elderly. Corni Fructus has been reported to be effective in the prevention and treatment of various diseases because of its strong antioxidant effect, but its efficacy against BPH is not yet known. This study was designed to evaluate the therapeutic efficacy of Corni Fructus water extract (CF) in testosterone-induced BPH rats.
MATERIALS/METHODS
To induce BPH, rats were intraperitoneal injected with testosterone propionate (TP). Rats in the treatment group were orally administered with CF with TP injection, and finasteride, which is a selective inhibitor of 5α-reductase type 2, was used as a positive control.
RESULTS
Our results showed that the increased prostate weight and histopathological changes in BPH model rats were suppressed by CF treatment. CF, similar to the finasteride-treated group, decreased the levels of testosterone and dihydrotestosterone by TP treatment in the serum, and it also reduced 5α-reductase expression and concentration in prostate tissue and serum, respectively. In addition, CF significantly blocked the expression of the androgen receptor (AR), AR co-activators, and proliferating cell nuclear antigen in BPH rats, and this blocking was associated with a decrease in prostate-specific antigen levels in serum and prostate tissue.
CONCLUSIONS
These results suggest that CF may weaken the BPH status through the inactivation of at least 5α-reductase and AR activity and may be useful for the clinical treatment of BPH.
Keywords: Benign prostatic hyperplasia, corni fructus, dihydrotestosterone, androgen receptor, prostate-specific antigen
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a common symptom of the urinary system in old age [1,2]. BPH is characterized by a non-malignant enlargement of the prostate gland due to the uncontrolled proliferation of smooth muscle and epithelial cells located in the periurethral transition zone of the prostate gland surrounding the urethra [3,4]. Although the etiology of BPH is not entirely clear yet, changes in androgens associated with aging are considered to be one of the critical factors; androgens are essential for the development of prostate growth [1,5]. Among the androgens, dihydrotestosterone (DHT) is the most important enzyme. DHT is converted from testosterone in the prostate by 5α-reductases, which are enzymes involved in steroid metabolism, and it interacts with the androgen receptor (AR) with a higher affinity than testosterone [6,7]. Therefore, the excessive production of DHT with decreasing testosterone concentration stimulates the development of BPH by inducing the proliferation of epithelial and stromal cells of the prostate [8,9]. In addition, the serum level of the prostate-specific antigen (PSA), which is widely used for the diagnosis of BPH, is also elevated in BPH patients compared with unaffected men of similar age because PSA production is promoted by binding DHT to the AR [8,10,11].
To date, the transurethral resection of the prostate for BPH patients is the most common surgical procedure worldwide. However, it can cause many complications, such as bleeding, urethral stricture, and urinary incontinence, and it may be limited for the elderly [12,13]. As an alternative to surgical treatment, various medications for the treatment of BPH have been used, and they are largely divided into (α)1-adrenoceptor antagonists (α-blockers) and 5α-reductase inhibitors (5ARIs). However, α-blockers cannot directly reduce the size of the enlarged prostate, and they exhibit adverse effects, such as orthostatic hypotension, nasal congestion, asthenia, sexual dysfunction, dizziness, and headache. 5ARIs also have a variety of side effects on the reproductive system, including erectile and ejaculatory dysfunction and reduced libido [14,15]. Therefore, recently, the application of anti-PBH active herbal medicines as a more effective and safer treatment strategy for patients with BPH has been given much attention.
The Oriental herbal medicine Corni Fructus, which is fruit of Cornus officinalis Sieb. et Zucc. and is known as “Sansuyu” in Korean, has long been considered beneficial not only as tonic agents and for invigorating blood circulation but also for a wide range of medical treatments. Recent studies have demonstrated that extracts of Corni Fructus have been reported to have various pharmacological actions, such as anti-inflammation, antioxidant, immune regulation, anti-neoplasia, anti-diabetic nephropathy, anti-hyperglycemia, hepatoprotection, and anti-sepsis effects [16,17,18,19,20,21,22,23]. In addition, many functional compounds, including a number of glycosides, tannins, and polyphenols, are present in the fruits, and their multiple mechanisms of action are actively under study [24,25,26]. However, the inhibitory effect of Corni Fructus on the development of BPH has not been determined. Therefore, this study aims to evaluate the effectiveness of Corni Fructus water extract (CF) on the testosterone propionate (TP)-induced BPH rat model by evaluating prostate weight, histopathological changes, and the major factors involved in the pathogenesis of BPH.
MATERIALS AND METHODS
Preparation of CF
The dried fruits of C. officinalis, which collected from an area around the city of Gurye (Jeollanam-do Province, Republic of Korea), were provided by Gurye Sansuyu Farming Association Corporation. A voucher specimen of the fruits (WECU-17-2) was deposited in the Department of Biochemistry, Dongeui University College of Korean Medicine (Busan, Republic of Korea). The dried fruits were cut into small pieces and then boiled in distilled water for 2 h. To remove the insoluble materials, the extract was filtered through a 0.45 µm filter, and the filtrate (CF) was lyophilized and then stored at −20℃ until usage.
Animals
Six-week-old male Sprague-Dawley rats (Samtako Bio Korea, Osan, Republic of Korea) with an initial body weight of 340–350 g were used. The animals were housed in a pathogen-free room and maintained under standard laboratory conditions (23 ± 2℃; relative humidity, 70 ± 5%; 12 h light/dark cycle). All animal experiments were conducted in accordance with the Animal Experimental Ethics Committee of Dongeui University (Confirmation number: R2017-004). Water and standard laboratory diet (Samtako Bio Korea) were provided ad libitum.
Experimental procedures
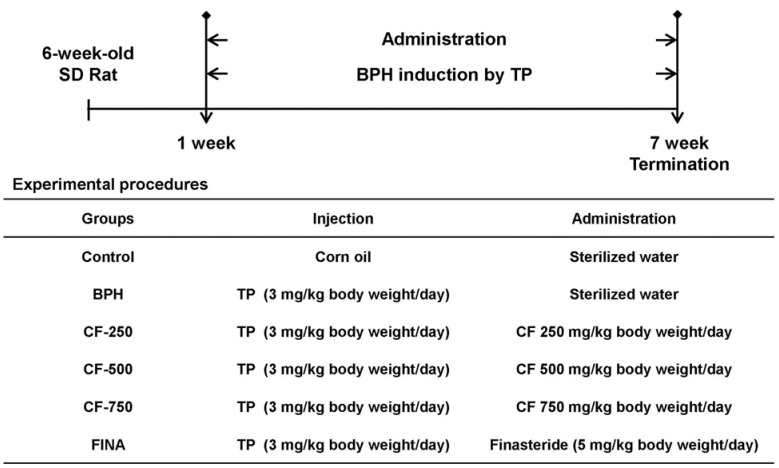
As summarized in Fig. 1, the rats were randomly assigned to the following groups (n = 6 in each group) after one week of acclimation to the laboratory environment: (a) a normal control group that received phosphate-buffered saline (PBS) with corn oil, (b) a BPH group that received PBS with TP (3 mg/kg, Tokyo Chemical Industry Co., Tokyo, Japan), (c) groups that received CF (250, 500, and 750 mg/kg) with TP (3 mg/kg), and (d) a positive control group that received finasteride (5 mg/kg, Sigma-Aldrich Chemical Co., St Louis, MO, USA) with TP (3 mg/kg). The TP-induced BPH groups were administered with TP (3 mg/kg) in a volume of 0.1 mL by intraperitoneal injection and treatment group were oral administration of CF in a volume of 0.1 mL for six weeks. During the experiment, the body weights of experimental animals were measured twice a week. At the end of the experiment, the animals were sacrificed under anesthesia to collect blood samples from the femoral vein for biochemical analysis. The prostate and major organs, such as lung, heart, spleen, liver, and kidney, were also carefully recovered and weighed. The relative prostate weight ratio was calculated as follows: Relative prostate weight ratio = prostate weight of rats/body weight (100 g) of rats. The remainder of each prostate was stored at −80℃ and used for further analyses.
Fig. 1. Flow diagram of the experimental procedure: a rat model of BPH.
Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; TP, testosterone propionate; FINA, finasteride.
Histopathological examination by hematoxylin and eosin (H&E) staining
The prostate glands were fixed in a 10% buffered formaldehyde solution (Sigma-Aldrich Chemical Co.) for 24 h, and then the tissue samples were paraffin-embedded and cut. The sectioned tissues were stained with H&E (Sigma-Aldrich Chemical Co.), and histological changes were evaluated under a light microscope (Carl Zeiss, Oberkochen, Germany) at 200X magnification.
Determination of testosterone and DHT levels in serum
Serum concentrations of testosterone and DHT were determined by using Testosterone enzyme-linked immunosorbent assay (ELISA) kit (BioVision, Inc., Milpitas, CA, USA) and rat DHT ELISA kit (Mybiosource Inc., San Diego, CA, USA), according to the manufacturer's instructions, which are based on the principle of competitive inhibition enzyme immunoassay.
Determination of 5α-reductase and PSA levels in serum
5α-reductase and PSA levels in serum were measured using ELISA kits for Steroid 5α-reductase 2 (Cloud-clone Corp., Katy, TX, USA) and rat KLK/PSA ELISA kit (LifeSpan Biosciences, Inc., Seattle, WA, USA). Standards and samples prepared according to the manufacturer's instructions were added to the precoating with target specific capture antibodies mircotiter plates. The optical density of each sample was determined using a microplate reader at 450 nm.
Immunohistochemical analysis
For immunostaining, tissue sections were incubated with antibodies against 5α-reductase type 2 (1:50 dilution, BioRad Laboratories, Inc., Hercules, CA, USA) and AR (1:50 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4℃. After overnight incubation, the sections were incubated with horseradish peroxidase (HRP)-conjugated AffiniPure goat anti-rabbit IgG or HRP-conjugated AffiniPure goat anti-mouse IgG (1:500 dilution, Jackson Immunoresearch Lab., West Grove, PA, USA) at room temperature for 30 min. The slides were washed with PBS, developed in a diaminobenzidine solution in the presence of hydrogen peroxide (Sigma-Aldrich Chemical Co.), and then visualized using with a fluorescence microscope (Carl Zeiss).
Western blot analysis
The prepared prostate tissue from each rat was cut into small pieces and homogenized using PROPREP lysis buffer (Intron Biotechnology Inc., Seongnam, Republic of Korea). The tissue extracts were centrifuged at 13,000 rpm for 20 min at 4℃ to remove the insoluble materials, and the protein concentration of the supernatant was calculated using a BioRad protein assay (BioRad Laboratories Inc.) according to the manufacturer's protocol. Equivalent amounts of protein (30 µg) per sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Schleicher & Schuell, Keene, NH, USA). The membranes were blocked with TBST (10 mM Tris, 150 mM NaCl, and 0.05% Tween-20, pH 7.6) containing 5% skim milk for 1 h at room temperature, washed with TBST, and then incubated overnight at 4℃ with the appropriate primary antibodies against 5α-reductase type 2 (Thermo Scientific, Waltham, MA, USA), AR (Santa Cruz Biotechnology, Inc.), AR-associated protein 70 (ARA70, Santa Cruz Biotechnology, Inc.), steroid receptor coactivator-1 (SRC1, Santa Cruz Biotechnology, Inc.), proliferating cell nuclear antigen (PCNA, Santa Cruz Biotechnology, Inc.), PSA (Santa Cruz Biotechnology, Inc.), and actin (Santa Cruz Biotechnology, Inc.). The membranes were subsequently reacted with the appropriate HRP-conjugated secondary antibodies (Amersham Biosciences, Westborough, MA, USA) for 2 h at room temperature. The protein bands were detected using an enhanced chemiluminescence kit (Amersham Biosciences) according to the manufacturer's instructions.
Measurement of biochemical markers
The collected blood samples (5 mL per individual) were coagulated by maintenance at room temperature for 30 min and then centrifuged at 3,000 g for 20 min at 4℃ to separate the serum. The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine were determined to assess the liver and kidney functions using the Biochemistry Automatic Analyzer (Hitachi High-Technologies, Tokyo, Japan) according to the protocols presented in the assay kits (Asan Pharm, Seoul, Republic of Korea).
Statistical analysis
All data are expressed as means ± standard errors of the mean (SEM). Statistically significant values were analyzed using one-way ANOVA with Dunnett's test. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) statistical analysis software (Version 19.0, IBM, NY, USA). A P-value below 0.05 was considered significant.
RESULTS
CF lowers prostate weight in BPH-induced rats
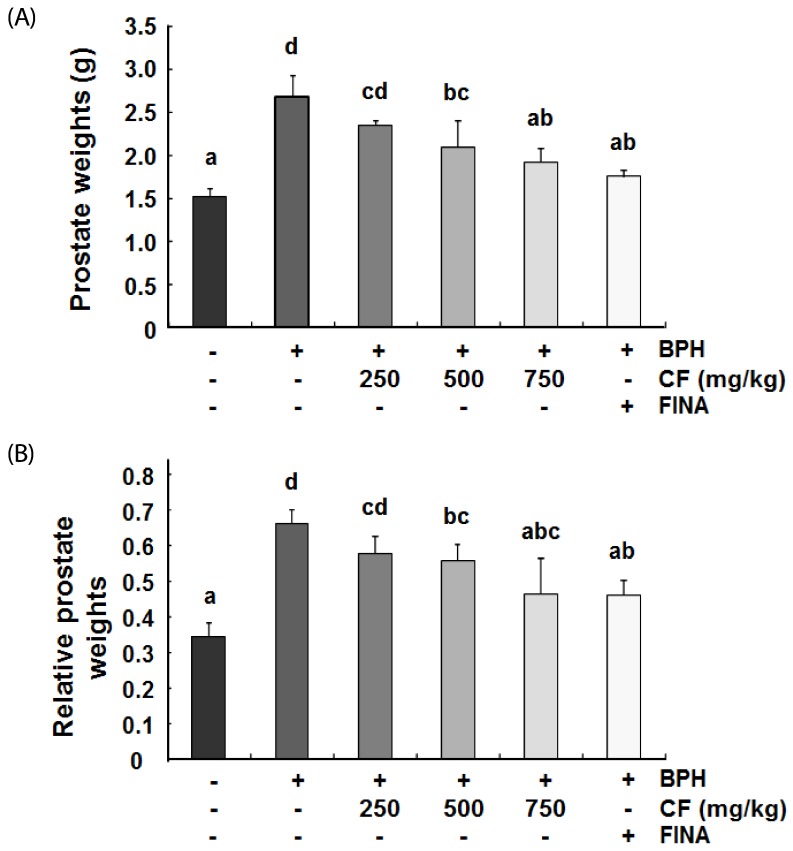
Fig. 2 shows the effects of CF on the increased prostate weight in TP-induced BPH rats. The prostate weight of the BPH group was significantly greater than that of the control group, thus indicating that TP successfully induced BPH. However, the CF-treated group showed a significant tendency to reduce the prostate weight in a concentration dependent manner. The finasteride-treated group, which was used as a positive group, also dramatically decreased prostate weight compared with the BPH group as expected.
Fig. 2. Effects of CF administration on prostate weights in TP-induced BPH rats.
Changes in the prostate total weight (A) and relative prostate weight ratio (B) were assessed for the control, BPH-induced, CF-, and FINA (finasteride)-treated groups. The data shown represent the mean ± SEM of six rats per group (P < 0.05). BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; FINA, finasteride.
CF alleviates prostate histologic changes in BPH-induced rats
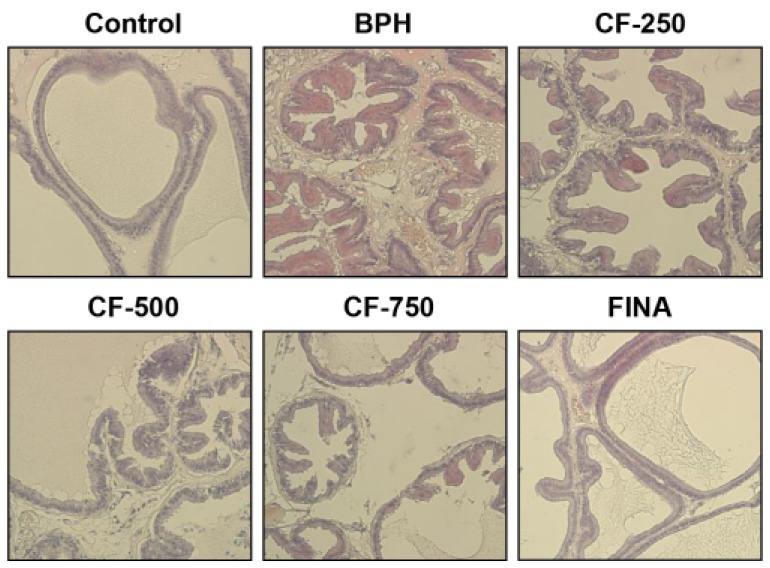
We performed H&E staining on the prostate tissue to compare histologic changes and reaffirmed the effect of CF on mitigating prostate hypertrophy. As shown in Fig. 3, histopathological changes, such as decrease in the cytoplasm and the lumen area by cell proliferation as well as polyp formation, were observed in BPH-induced rats. However, the typical histologic pattern of hyperplasia [3] decreased with increasing concentrations in the CF treatment group. No pathological features were observed in the finasteride treatment group.
Fig. 3. Effects of CF administration on the histological changes in the prostate tissues of TP-induced BPH rats.
Representative photomicrographs of the H&E-stained prostate tissues are presented (magnification, 200X). Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; FINA, finasteride.
CF reduces the elevated levels of testosterone and DHT in the serum of BPH-induced rats
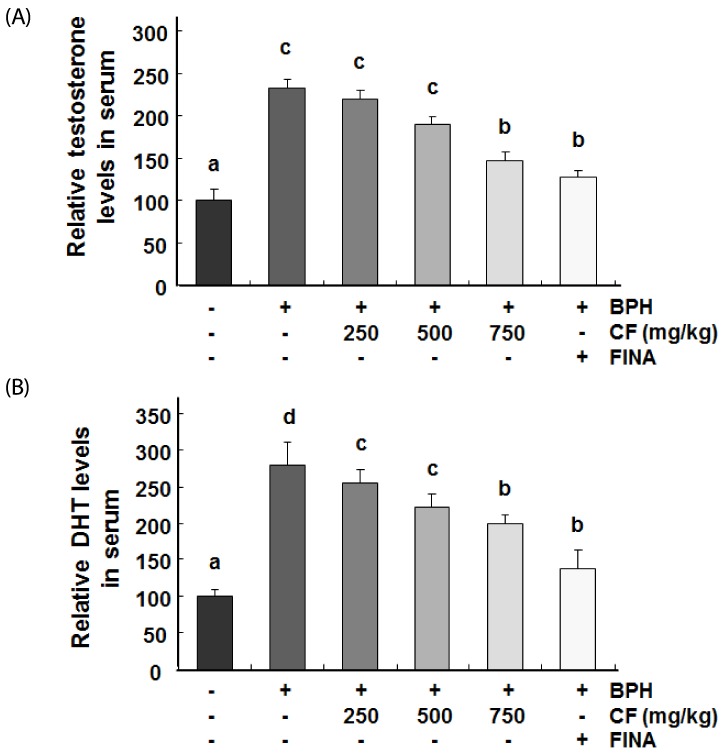
As indicated in Fig. 4, the levels of testosterone and DHT, which are major factors in the pathogenesis of BPH, in serum were significantly higher in the BPH group than in the control group. Conversely, compared with those in the BPH group, these levels were concentration dependently reduced in the CF-treated groups and were markedly decreased down to the control level in the finasteride-treated group.
Fig. 4. Effects of CF administration on testosterone and DHT in serum of TP-induced BPH rats.
The serum concentrations of testosterone (A) and DHT (B) were examined by ELISA. The data shown represent the mean ± SEM of six rats per group (P < 0.05). BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; FINA, finasteride; DHT, dihydrotestosterone.
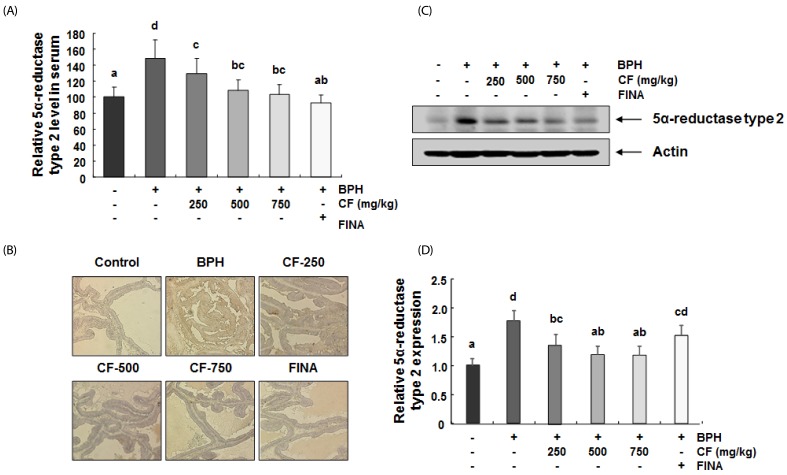
CF inhibits the elevated concentration and expression of 5α-reductase type 2 in serum and prostate tissue of BPH-induced rats
As shown in Fig. 5A, a significant increase was found in the serum 5α-reductase type 2 concentration in the BPH-induced group in comparison with the control group. Conversely, its concentration gradually decreased with increasing CF treatment concentration in the CF-treated groups and was significantly lower than that observed in the BPH-induced group in the finasteride treatment group. In the microscopic examination of prostate tissues, the expression of 5α-reductase type 2 was enhanced in the BPH-induced group, but its expression was suppressed in the CF- and finasteride-treated groups (Fig. 5B). We also compared the expression level of 5α-reductase type 2 protein in prostate tissue to investigate whether the effect of CF was related to a change in the 5α-reductase type 2 expression. As indicated in the immunoblotting results in Fig. 5C, the 5α-reductase type 2 level from BPH-induced prostate tissue was significantly reduced by finasteride and CF treatments, consistent with the immunohistochemistry staining.
Fig. 5. Effects of CF administration on 5α-reductase type 2 in TP-induced BPH rats.
(A) The serum concentrations of 5α-reductase type 2 were examined by ELISA. The data shown represent the mean ± SEM of six rats per group (P < 0.05). (B) Representative photomicrographs of prostate tissues immunostained with an anti-5α-reductase type 2 are presented (magnification, 200X). Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. (C and D) The expression levels of 5α-reductase type 2 in prostate tissues were determined by Western blotting. The experiment was repeated three times among different experimental groups, and all the results were similar (C). Actin was used as an internal control. Values are mean ± SD of data from three separate experiments (P < 0.05) (D). BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; FINA, finasteride.
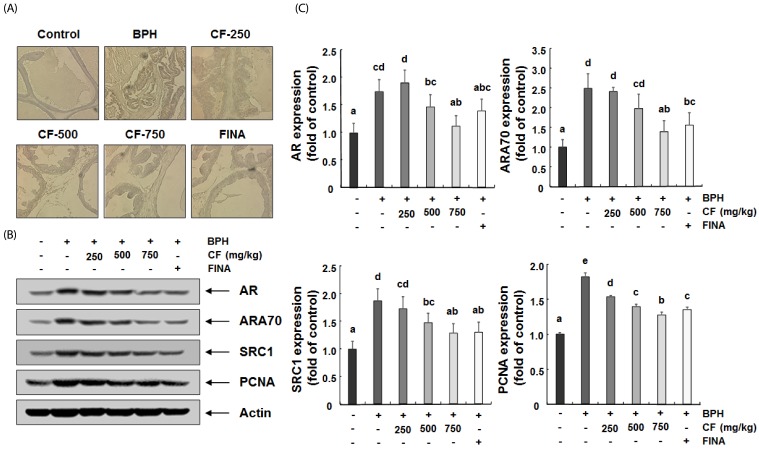
CF attenuates the expression of AR and its co-activators in the prostate tissue of BPH-induced rats
According to the results of prostate immunostaining, the AR expression increased in the TP-induced BPH group, but CF administration, as in the finasteride-treated group, significantly reduced the increased expression of AR in comparison with the TP-treated group (Fig. 6A). In the Western blot analysis performed to confirm the results of immunostaining at the protein level, the expression of AR protein was enhanced in the BPH group. Conversely, the increased the AR expression was markedly inhibited by CF treatment, and the finasteride treatment also reduced the AR expression (Fig. 6B). In addition, the induced protein levels of AR co-activators, such as ARA70 and SRC1, and PCNA in the BPH group were down-regulated by CF and finasteride treatments (Fig. 6B).
Fig. 6. Effects of CF administration on AR expression in TP-induced BPH rats.
(A) Representative photomicrographs of prostate tissues immunostained with an anti-AR are presented (magnification, 200X). Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. (B and C) The expression levels of AR, AR co-activators (ARA70 and SRC1) and PCNA were determined by Western blotting. The experiment was repeated three times among different experimental groups, and all the results were similar (B). Actin was used as an internal control. Values are mean ± SD of data from three separate experiments (P < 0.05) (C). BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; FINA, finasteride; DHT, dihydrotestosterone; AR, androgen receptor; ARA70, AR-associated protein 70; SRC1, steroid receptor coactivator-1; PCNA, proliferating cell nuclear antigen.
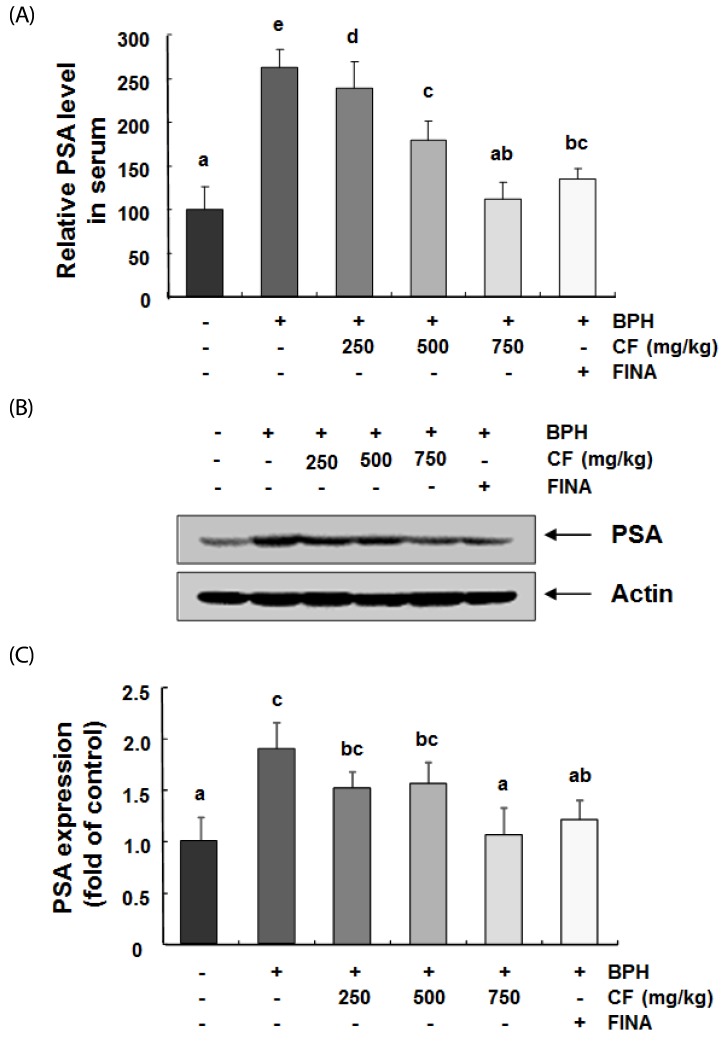
CF down-regulates the level and expression of PAS by CF treatment in BPH-induced rats
We further investigated the effect of CF treatment on the PSA level and confirmed that the amount of PSA in the serum was greatly increased by TP treatment, as shown by the immunohistochemistry results in Fig. 7A. Conversely, CF or finasteride treatment significantly lowered this effect in comparison with the BPH group. This tendency was similarly observed in the PSA protein expression patterns in prostate tissue (Fig. 7B).
Fig. 7. Effects of CF administration on PSA in TP-induced BPH rats.
(A) The serum concentrations of PSA were examined by ELISA. The data shown represent the mean ± SEM of six rats per group. The ***P < 0.001 vs. Control group; ##P < 0.005, ###P < 0.001 vs. BPH group. (B and C) The expression levels of PSA in prostate tissues were determined by Western blotting. The experiment was repeated three times among different experimental groups, and all the results were similar. Actin was used as an internal control. Values are mean ± SD of data from three separate experiments (P < 0.05) (C). BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; FINA, finasteride; PSA, prostate-specific antigen.
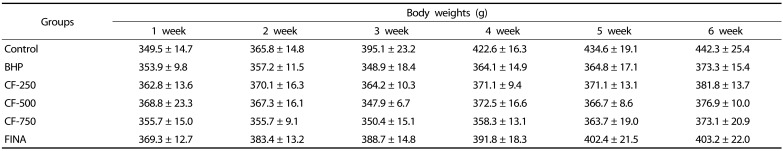
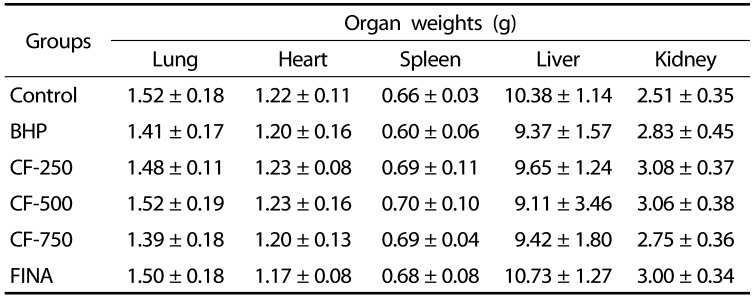
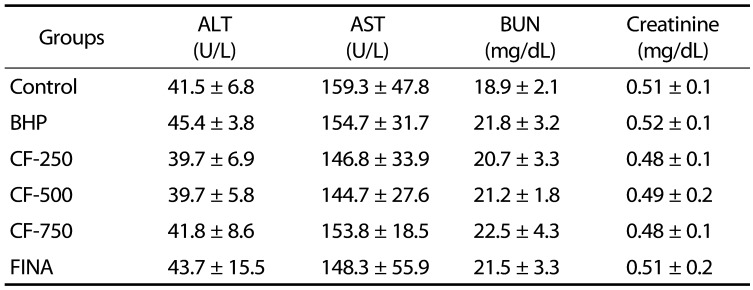
Evaluation of the toxicity of CF in BPH-induced rats
To assess the toxicity of CF, the body weights, weights of the major organs and the levels of serum hormones were examined. As indicated in Table 1, The initial body weights ranged from 346.0–358.5 g. The final weight of the normal group was 442.3 g after six weeks of experiment which was higher than those in the testosterone-treated groups. After six weeks, the body weight of the CF- and finasteride-treated rats was slightly lower than that of the normal group, but there was no significant difference between the groups. Food intakes were also similar among the groups (Data not shown). As shown in Table 2, the relative weights of vital organs, including lung, heart, spleen, liver, and kidney, of BHP-induced rats and CF- and finasteride-treated rats were not significantly different from those of normal controls. As summarized in Table 3, the amounts of ALT, AST, BUN, and creatinine corresponding to the serum biochemical parameters for the liver and kidney functions were also not much different in all experimental groups.
Table 1. Effects of CF treatment on the body weights of experimental rats.
Values are represented as the mean ± SEM of six rats.
Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. CF, Corni Fructus water extract; BHP, benign prostatic hyperplasia; TP, testosterone propionate. FINA, finasteride
Table 2. Effects of CF treatment on the relative organ weights of BHP rats.
Values are represented as the mean ± SEM of six rats.
Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. CF, Corni Fructus water extract; BHP, benign prostatic hyperplasia; TP, testosterone propionate. FINA, finasteride.
Table 3. Effects of CF treatment on the serum biochemical parameters for the liver and kidney functions of BHP rats.
Values are represented as the mean ± SEM of six rats.
Control, corn oil-injected and PBS-treated rats; BHP, TP (3 mg/kg)- and PBS-treated rats; CF-250, TP (3 mg/kg)- and CF (250 mg/kg)-treated rats; CF-500, TP (3 mg/kg)- and CF (500 mg/kg)-treated rats; CF-750, TP (3 mg/kg)- and CF (750 mg/kg)-treated rats; FINA, TP (3 mg/kg)- and finasteride (5 mg/kg)-treated rats. CF, Corni Fructus water extract; BHP, benign prostatic hyperplasia; TP, testosterone propionate. FINA, finasteride; ALT, alanine aminotransferase; AST, aspartate aminotransferase, BUN, blood urea nitrogen
DISCUSSION
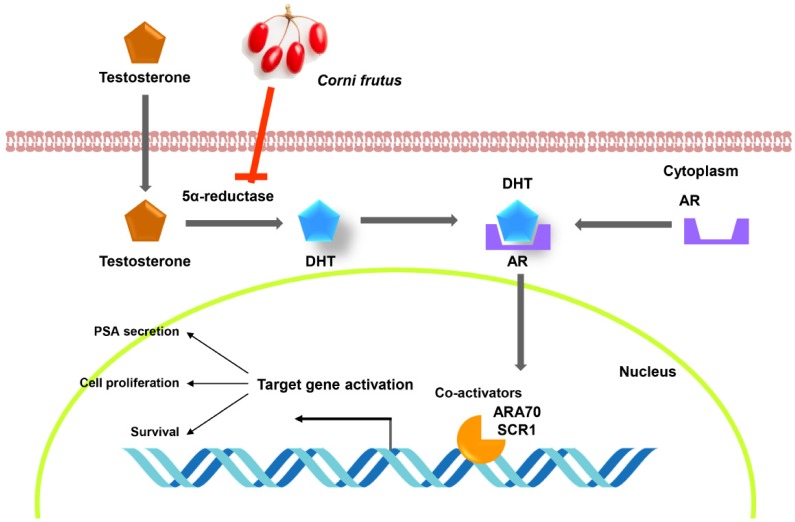
Recently, interest in the utilization of natural products has increased to overcome the adverse effects of drugs used for the treatment of BPH, which is rapidly increasing with the aging of the population. Not only do the onset and the progression of BPH change the activity of hormones related to prostate function regulation, but inflammation and oxidative stresses also play an important role [27,28]. Therefore, natural products with anti-inflammatory and antioxidant properties may be useful in the treatment of BPH. In this study, we investigated the effects of CF, which is known to have high anti-inflammatory and antioxidant activities [17,19,23,25,26], as part of the search for natural products with high potential for the treatment of BPH from traditional medicinal resources. Our main findings demonstrated that CF greatly reduced prostatic enlargement and histopathological changes in TP-induced BPH rats; this reduction is associated with the inhibition of the 5α-reductase type 2 expression in prostate tissue and the concentration of this enzymes in serum. In addition, CF significantly blocked the levels of testosterone and DHT in serum in BPH rats and suppressed the expression of AR and PCNA, and the production of PSA. These results suggest that CF can effectively block the progression of BPH and can be developed as a treatment for BPH. A proposed schematic model of the effects of CF on BPH is shown in Fig. 8.
Fig. 8. A suggested schematic model for the inhibitory effect of CF on pathogenesis of BPH.
BPH, benign prostatic hyperplasia; CF, Corni Fructus water extract; DHT, dihydrotestosterone; AR, androgen receptor; ARA70, AR-associated protein 70; SRC1, steroid receptor coactivator-1;PAS, prostate-specific antigen.
Although the pathogenesis of BPH is not yet well understood, 5α-reductases are recognized as a major target for the control of BPH because they are the most important enzymes for DHT biosynthesis from testosterone [6,7]. Of the three currently known 5α-reductase isoenzymes, types 1 and 2 are the most clinically important. Type 2, located on the prostatic nuclear membrane for both the stroma and the epithelium, is reported to be more involved in the production of DHT associated with BPH than type 1 [29,30]. Although both testosterone and DHT play an important role in the development of male reproductive organs, DHT, as a principal androgen in the prostate, has a higher affinity for AR than testosterone. This high affinity of DHT to AR can strongly stimulate the transcriptional activity of the androgen-dependent growth factors, which are needed to promote the proliferation of prostate epithelial and stromal cells rather than testosterone [8,9]. This process requires the recruitment of specific AR co-activators to bind to the DNA sequence of the androgen response elements (AREs) resulting from the translocation of the AR to the nucleus [31,32]. Among AR co-activators, ARA70 has been reported to enhance the transactivational potential of ARs. And, SRC1 interacts with and activates ARs in the presence of hormones [32,33]. Ultimately, the overproduction of DHT, accompanied by aging, leads to the development and deterioration of BPH [6,7]. In addition, PCNA is a major protein that controls cell division progression and is considered a reliable indicator of cell division. Previous studies have shown that PCNA expression is significantly enhanced in animal models with BPH [34,35]. According to our study, CF significantly down-regulated the levels of 5α-reductase type 2 as well as serum testosterone and DHT in BPH rats. In agreement with the reduction in serum 5α-reductase type 2 levels, the protein expression of this enzyme in prostate tissue also diminished in rats fed with CF, and this reduction was associated with the decreased expression of AR, AR co-activators including ARA70 and SRC1, and PCNA. These phenomena can also be observed in the finasteride group; finasteride is a selective inhibitor of 5α-reductase type 2 isoenzyme [9,30]. Therefore, the present results suggest that the blocking effect of CF on the increased prostate weight and the histological changes in BPH-induced animals is probably due to the reduction of 5α-reductase and AR activities.
PSA is one of the representative androgen-responsive genes expressed in the prostate gland [8,11]. Although a small amount of PSA is generally detected in the blood of healthy men, the PSA level is widely used as an indicator of the progression of prostate cancer [10,11]. However, in BPH patients with high DHT activity, DHT binds to AR, which in turn causes it to interact with ARE in the promoter region of PSA, thereby enhancing the transcriptional activity of PSA [32,33]. Therefore, the level of PSA is elevated not only in patients with prostate cancer but also in the serum of BPH patients, and a reduction in PSA levels may represent the therapeutic effect of BPH. We also investigated whether the level of PSA could be decreased by CF treatment in BPH-induced animals. Our results showed that the amount of PSA in serum and its expression in the prostate tissue of BPH rats were significantly suppressed in CF-fed rats. These results suggest that CF attenuates the androgen signal transduction system in prostate cells, which can be attributed to the reduction of PSA expression and production by CF treatment. In this experiment, we compared the weights of the major organs for toxicity testing related to the efficacy of CF on BHP. No significant changes were found in the weights of lung, heart, spleen, liver, and kidney among the control, finasteride-, and CF-treated groups. Additionally, the serum levels of AST, AST, BUN, and creatinine in rats fed with CF were not significantly different in the control group. Although further studies on the potential chronic toxicity and genotoxicity are required to support CF safety, the results of the present study show that CF did not have significant toxic effects on the liver and kidney. The major active ingredients of CF are organic acids and iridoids, of which morroniside, loganin, and cornin have been extensively studied [36,37]. Although there has been no study on BPH to date, previous studies have shown that they exhibit a variety of pharmacological effects including potent antioxidant and anti-inflammatory effects [37,38,39,40,41], suggesting that they may have suppressive effect on BPH. Therefore, in order to obtain additional data of CF for the prevention and treatment of BPH, studies on the active ingredients of CF including these should be continued.
In conclusion, the results suggest that CF has a protective effect on TP-induced BPH in rats as evidenced by its ability to restrain prostate hyperplasia and histologic damage. These effects are at least due to the decrease in serum DHT and PSA levels with the inhibition of 5α-reductase and AR expression, thus suggesting that CF can effectively reduce the development and progression of BPH.
Footnotes
This work was supported by “Food Functionality Evaluation program (G0090200-09)” under the Ministry of Agriculture, Food and Rural Affairs and partly Korea Food Research Institute and Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (2018R1A2B2005705).
CONFLICT OF INTEREST: The authors declare no potential conflicts of interest.
References
- 1.Cornu JN, Ahyai S, Bachmann A, de la Rosette J, Gilling P, Gratzke C, McVary K, Novara G, Woo H, Madersbacher S. A systematic review and meta-analysis of functional outcomes and complications following transurethral procedures for lower urinary tract symptoms resulting from benign prostatic obstruction: an update. Eur Urol. 2015;67:1066–1096. doi: 10.1016/j.eururo.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 2.Pyo JS, Cho WJ. Systematic review and meta-analysis of prostatic artery embolisation for lower urinary tract symptoms related to benign prostatic hyperplasia. Clin Radiol. 2017;72:16–22. doi: 10.1016/j.crad.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Aaron L, Franco OE, Hayward SW. Review of prostate anatomy and embryology and the etiology of benign prostatic hyperplasia. Urol Clin North Am. 2016;43:279–288. doi: 10.1016/j.ucl.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strand DW, Costa DN, Francis F, Ricke WA, Roehrborn CG. Targeting phenotypic heterogeneity in benign prostatic hyperplasia. Differentiation. 2017;96:49–61. doi: 10.1016/j.diff.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vignozzi L, Rastrelli G, Corona G, Gacci M, Forti G, Maggi M. Benign prostatic hyperplasia: a new metabolic disease? J Endocrinol Invest. 2014;37:313–322. doi: 10.1007/s40618-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 6.Steers WD. 5alpha-reductase activity in the prostate. Urology. 2001;58:17–24. doi: 10.1016/s0090-4295(01)01299-7. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson TM, Ricke WA. Androgens and estrogens in benign prostatic hyperplasia: past, present and future. Differentiation. 2011;82:184–199. doi: 10.1016/j.diff.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andriole G, Bruchovsky N, Chung LW, Matsumoto AM, Rittmaster R, Roehrborn C, Russell D, Tindall D. Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol. 2004;172:1399–1403. doi: 10.1097/01.ju.0000139539.94828.29. [DOI] [PubMed] [Google Scholar]
- 9.Azzouni F, Mohler J. Role of 5α-reductase inhibitors in benign prostatic diseases. Prostate Cancer Prostatic Dis. 2012;15:222–230. doi: 10.1038/pcan.2012.1. [DOI] [PubMed] [Google Scholar]
- 10.Gharaee-Kermani M, Macoska JA. Promising molecular targets and biomarkers for male BPH and LUTS. Curr Urol Rep. 2013;14:628–637. doi: 10.1007/s11934-013-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho CK, Habib FK. Estrogen and androgen signaling in the pathogenesis of BPH. Nat Rev Urol. 2011;8:29–41. doi: 10.1038/nrurol.2010.207. [DOI] [PubMed] [Google Scholar]
- 12.Thorner DA, Weiss JP. Benign prostatic hyperplasia: symptoms, symptom scores, and outcome measures. Urol Clin North Am. 2009;36:417–429. doi: 10.1016/j.ucl.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Smith RD, Patel A. Transurethral resection of the prostate revisited and updated. Curr Opin Urol. 2011;21:36–41. doi: 10.1097/MOU.0b013e3283411455. [DOI] [PubMed] [Google Scholar]
- 14.Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M. Adverse effects of 5α-reductase inhibitors: what do we know, don't know, and need to know? Rev Endocr Metab Disord. 2015;16:177–198. doi: 10.1007/s11154-015-9319-y. [DOI] [PubMed] [Google Scholar]
- 15.Lepor H. Alpha-blockers for the treatment of benign prostatic hyperplasia. Urol Clin North Am. 2016;43:311–323. doi: 10.1016/j.ucl.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Chang JS, Chiang LC, Hsu FF, Lin CC. Chemoprevention against hepatocellular carcinoma of Cornus officinalis in vitro. Am J Chin Med. 2004;32:717–725. doi: 10.1142/S0192415X04002296. [DOI] [PubMed] [Google Scholar]
- 17.Kang DG, Moon MK, Lee AS, Kwon TO, Kim JS, Lee HS. Cornuside suppresses cytokine-induced proinflammatory and adhesion molecules in the human umbilical vein endothelial cells. Biol Pharm Bull. 2007;30:1796–1799. doi: 10.1248/bpb.30.1796. [DOI] [PubMed] [Google Scholar]
- 18.Jiang WL, Chen XG, Zhu HB, Hou J, Tian JW. Cornuside attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Pharmacology. 2009;84:162–170. doi: 10.1159/000235621. [DOI] [PubMed] [Google Scholar]
- 19.Jeong EJ, Kim TB, Yang H, Kang SY, Kim SY, Sung SH, Kim YC. Neuroprotective iridoid glycosides from Cornus officinalis fruits against glutamate-induced toxicity in HT22 hippocampal cells. Phytomedicine. 2012;19:317–321. doi: 10.1016/j.phymed.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Telang NT, Li G, Sepkovic DW, Bradlow HL, Wong GY. Antiproliferative effects of Chinese herb Cornus officinalis in a cell culture model for estrogen receptor-positive clinical breast cancer. Mol Med Rep. 2012;5:22–28. doi: 10.3892/mmr.2011.617. [DOI] [PubMed] [Google Scholar]
- 21.Zhang QC, Zhao Y, Bian HM. Antiplatelet activity of a novel formula composed of malic acid, succinic acid and citric acid from Cornus officinalis fruit. Phytother Res. 2013;27:1894–1896. doi: 10.1002/ptr.4934. [DOI] [PubMed] [Google Scholar]
- 22.Sun X, Kong L, Zhou L. Protective effect of Fructus corni polysaccharide on hippocampal tissues and its relevant mechanism in epileptic rats induced by lithium chloride-pilocarpine. Exp Ther Med. 2018;16:445–451. doi: 10.3892/etm.2018.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang KA, Hwang YJ, Song J. Antioxidant activities and oxidative stress inhibitory effects of ethanol extracts from Cornus officinalis on raw 264.7 cells. BMC Complement Altern Med. 2016;16:196. doi: 10.1186/s12906-016-1172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JY, Han AR, Kil YS, Kang U, Kim SH, Nam SJ, Seo EK. A new secoiridoid glycoside from the fruits of Cornus officinalis (Cornaceae) Nat Prod Res. 2016;30:1504–1510. doi: 10.1080/14786419.2015.1115996. [DOI] [PubMed] [Google Scholar]
- 25.Yoon JH, Youn K, Ho CT, Karwe MV, Jeong WS, Jun M. p-Coumaric acid and ursolic acid from Corni fructus attenuated β-amyloid(25-35)-induced toxicity through regulation of the NF-κB signaling pathway in PC12 cells. J Agric Food Chem. 2014;62:4911–4916. doi: 10.1021/jf501314g. [DOI] [PubMed] [Google Scholar]
- 26.Ma W, Wang KJ, Cheng CS, Yan GQ, Lu WL, Ge JF, Cheng YX, Li N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J Ethnopharmacol. 2014;153:840–845. doi: 10.1016/j.jep.2014.03.051. [DOI] [PubMed] [Google Scholar]
- 27.Minciullo PL, Inferrera A, Navarra M, Calapai G, Magno C, Gangemi S. Oxidative stress in benign prostatic hyperplasia: a systematic review. Urol Int. 2015;94:249–254. doi: 10.1159/000366210. [DOI] [PubMed] [Google Scholar]
- 28.Bostanci Y, Kazzazi A, Momtahen S, Laze J, Djavan B. Correlation between benign prostatic hyperplasia and inflammation. Curr Opin Urol. 2013;23:5–10. doi: 10.1097/MOU.0b013e32835abd4a. [DOI] [PubMed] [Google Scholar]
- 29.Keam SJ, Scott LJ. Dutasteride: a review of its use in the management of prostate disorders. Drugs. 2008;68:463–485. doi: 10.2165/00003495-200868040-00008. [DOI] [PubMed] [Google Scholar]
- 30.Traish AM. 5α-reductases in human physiology: an unfolding story. Endocr Pract. 2012;18:965–975. doi: 10.4158/EP12108.RA. [DOI] [PubMed] [Google Scholar]
- 31.Pihlajamaa P, Sahu B, Jänne OA. Determinants of receptor- and tissue-specific actions in androgen signaling. Endocr Rev. 2015;36:357–384. doi: 10.1210/er.2015-1034. [DOI] [PubMed] [Google Scholar]
- 32.Robins DM. Androgen receptor and molecular mechanisms of male-specific gene expression. Novartis Found Symp. 2005;268:42–52. [PubMed] [Google Scholar]
- 33.Prins GS. Molecular biology of the androgen receptor. Mayo Clin Proc. 2000;75(Suppl):S32–S35. [PubMed] [Google Scholar]
- 34.Watanabe M, Yamada Y, Kato H, Imai H, Nakano H, Araki T, Shiraishi T. Malignant phyllodes tumor of the prostate: retrospective review of specimens obtained by sequential transurethral resection. Pathol Int. 2002;52:777–783. doi: 10.1046/j.1440-1827.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- 35.Cunha GR, Wang YZ, Hayward SW, Risbridger GP. Estrogenic effects on prostatic differentiation and carcinogenesis. Reprod Fertil Dev. 2001;13:285–296. doi: 10.1071/rd01010. [DOI] [PubMed] [Google Scholar]
- 36.Huang J, Zhang Y, Dong L, Gao Q, Yin L, Quan H, Chen R, Fu X, Lin D. Ethnopharmacology, phytochemistry, and pharmacology of Cornus officinalis Sieb. et Zucc. J Ethnopharmacol. 2018;213:280–301. doi: 10.1016/j.jep.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Park CH, Noh JS, Park JC, Yokozawa T. Beneficial effect of 7-O-galloyl-D-sedoheptulose, a polyphenol isolated from Corni fructus, against diabetes-induced alterations in kidney and adipose tissue of type 2 diabetic db/db mice. Evid Based Complement Alternat Med. 2013;2013:736856. doi: 10.1155/2013/736856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vareed SK, Schutzki RE, Nair MG. Lipid peroxidation, cyclooxygenase enzyme and tumor cell proliferation inhibitory compounds in Cornus kousa fruits. Phytomedicine. 2007;14:706–709. doi: 10.1016/j.phymed.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Sun F, An Y, Ai H, Zhang L, Huang W, Li L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur J Pharmacol. 2009;613:19–23. doi: 10.1016/j.ejphar.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Shen J, Liu H, Shi Y, Li L, Wei M. Morroniside and loganin extracted from Cornus officinalis have protective effects on rat mesangial cell proliferation exposed to advanced glycation end products by preventing oxidative stress. Can J Physiol Pharmacol. 2006;84:1267–1273. doi: 10.1139/y06-075. [DOI] [PubMed] [Google Scholar]
- 41.Xu YD, Cui C, Sun MF, Zhu YL, Chu M, Shi YW, Lin SL, Yang XS, Shen YQ. Neuroprotective effects of loganin on MPTP-induced Parkinson's disease mice: neurochemistry, glial reaction and autophagy studies. J Cell Biochem. 2017;118:3495–3510. doi: 10.1002/jcb.26010. [DOI] [PubMed] [Google Scholar]