Abstract
Mouse models are important and versatile tools to study mechanisms and novel therapies of human disease in vivo. Both, the number and the complexity of murine models are constantly increasing and modification of genes of interest as well as any exogenous challenge may lead to unanticipated biological effects. Laboratory diagnostics of blood samples provide a comprehensive and rapid screening for multiple organ function and are fundamental to detect human disease. Here, we adapt an array of laboratory medicine-based tests commonly used in humans to establish a platform for standardized, multi-parametric, and quality-controlled diagnostics of murine blood samples. We determined sex-dependent reference intervals of 51 commonly used laboratory medicine tests for samples obtained from the C57BL/6J mouse strain. As a proof of principle, we applied these diagnostic tests in a mouse cytomegalovirus (MCMV) infection model to screen for organ damage. Consistent with histopathological findings, plasma concentrations of liver-specific enzymes were elevated, supporting the diagnosis of a virus-induced hepatitis. Plasma activities of aminotransferases correlated with viral loads in livers at various days after MCMV infection and discriminated infected from non-infected animals. This study provides murine blood reference intervals of common laboratory medicine parameters and illustrates the use of these tests for diagnosis of infectious disease in experimental animals.
Introduction
Since the 1980s, the use of mouse models for research in life science has increased continuously. Currently, the number of documented mouse genotypes with phenotype annotation reaches almost 600001. Genetic modification of mice allows for modeling of various human diseases and targeted deletion of a specific gene of interest may help to unravel the underlying molecular mechanisms. However, any genetic modification may cause unpredicted consequences in organ systems that express the gene or are indirectly affected by the presence or absence of the gene product. Similarly, any challenge such as application of pharmacologic agents and microbes, or invasive surgery may lead to unexpected and complex effects. Thus, in addition to histopathology or additional cellular and molecular readout approaches, a comprehensive and rapid organ-specific assessment of newly generated mouse models is needed to identify such unanticipated effects and reduce the risk of assessing epiphenomena rather than the gene or intervention of interest.
Laboratory blood tests are commonly used in clinical pathology to detect organ damage by measurement of surrogate markers in body fluids of human patients. The majority of laboratory tests used for human samples are not applicable for measurement of murine samples. This is mainly due to the fact that most laboratory tests are based on immunoassays using antibodies that are species-specific and recognize a unique peptide sequence within the protein of interest. Thus, non-conserved amino acid sequences of human and mouse proteins as well as posttranslational modifications may interfere with cross-species binding of the antibodies used in the immunoassays. However, some antibodies bind to conserved epitopes, cross-react among various species and these immunoassays may be used to analyze both human and mouse samples. In addition to antibody-based immunoassays, determination of plasma electrolyte concentration using ion-sensitive electrodes and enzymatic activities of plasma proteins using chromogenic substrates are used in clinical chemistry tests with unknown species-specific sensitivities. Another critical issue is the sample volume needed for analysis of a single parameter. Some clinical chemistry analyzers used for human diagnostics require 250 µl of plasma per measurement. Given that a mouse with a body weight of 20 grams has an estimated total blood volume of 1500 µl the number of parameters to be analyzed per animal is restricted due to limited material. Accordingly, a rational selection of laboratory tests is required to detect and monitor pathological conditions in peripheral blood of mice.
Human blood reference intervals are well established for laboratory tests and needed for interpretation of a measured value. Reference intervals may depend on age, sex, ethnical background, pregnancy, medical treatment, or sampling conditions. Some are specific for the various analyzers, tests and reagents used and thus are provided by the manufacturer of the test. In contrast to human samples there is a lack of reference intervals for laboratory tests of murine samples. Thus, to utilize laboratory medicine tests for diagnosis of mouse samples reference intervals based on data from healthy animals need to be determined.
Human cytomegalovirus (HCMV) is a highly prevalent opportunistic pathogen that may cause disease in various organs such as pneumonitis, colitis, and hepatitis. HCMV infection and disease are important causes of morbidity and mortality in transplant recipients2. Moreover, congenital infection with HCMV is the most frequent cause of permanent disabilities in neonates3. However, the majority of immune-competent adults experience only mild or no symptoms after infection4. Cytomegaloviruses exhibit distinct species specificity and thus mice cannot be infected with HCMV. Though, infection of mice with the related mouse cytomegalovirus (MCMV) is a well-established model to study cytomegalovirus pathogenesis and antiviral immune responses in vivo5,6. In both, humans and mice, lymphocytes have been shown to be required for infection control and protection of the host from cytomegalovirus disease7–11. Rag2−/−Il2rg−/− mice lack B, T and natural killer (NK) cells and thus provide a model to further study the role of lymphocytes in antiviral immunity.
Here, we established a panel of 51 laboratory tests including clinical chemistry-based parameters as well as a differential blood count for samples of C57BL/6J mice. We determined sex-dependent reference intervals for each parameter. Applying this panel in an infection model allowed for detection of MCMV-induced liver damage in mice. In line with elevated enzymatic activities of liver proteins in the blood, we found histopathological evidence of severe virus hepatitis. Moreover, all MCMV-infected animals had plasma activity of alanine aminotransferase (ALAT) above the upper limit of the reference interval determined from healthy animals. Accordingly, the use of laboratory medicine-based diagnostics of mouse blood allows for diagnosis of organ disease in experimental animals with versatile possible applications.
Results
Reference intervals and sex differences for laboratory diagnostic parameters of peripheral murine blood
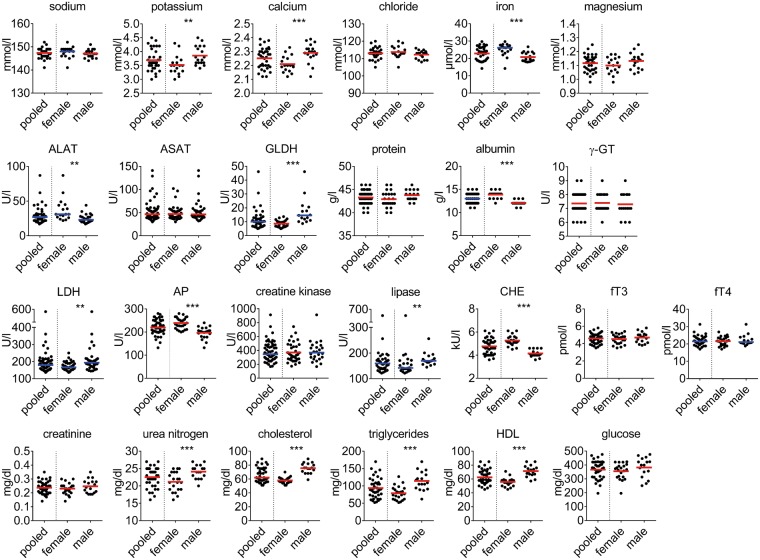
To create a diagnostic screening panel that allows for detection of organ dysfunction we selected for 61 commonly used clinical chemistry parameters including plasma proteins, electrolytes, lipids, hormones, metabolites as well as a differential blood count that comprises additional 26 parameters. We analyzed peripheral blood obtained from inbred C57BL/6J mice. Notably, we found concentrations were below range of linearity of the analyzer or not detectable at all in 30 out of the selected 61 clinical chemistry parameters. Another six parameters required too large plasma volumes to allow for detailed analysis (Supplementary Information Table 1). However, 25 clinical chemistry parameters lead to reproducible signals using the tests and protocols that were designed for the analysis of human blood samples. In addition, 26 differential blood count parameters could be determined reliably utilizing an analyzer designed for veterinary blood samples. Thus, the final panel included a total of 51 diverse parameters that are indicative for functionality of various organs or pathophysiological processes (Tables 1 and 2). One analysis required approximately 300 µl of plasma for all clinical chemistry parameters and 50 µl of whole blood for a differential blood count. Thus, animals had to be exsanguinated to draw sufficient material for measurement of all 51 parameters at once. However, a smaller panel including seven screening clinical chemistry parameters (e.g. ALAT, creatinine, creatine kinase, cholesterol, lactate dehydrogenase (LDH), lipase, and total protein) can be processed with 100 µl of plasma, thus allowing for comprehensive diagnostics without sacrificing of the experimental mouse. Hence, the laboratory tests analyzed here allow for general screening of healthy and diseased mice but also for monitoring of specific organ damage.
Table 1.
Reference intervals for clinical chemistry parameters.
| Parameter | Unit | Pooled Reference interval (mean or median, samples) | Female Reference interval (mean or median, samples) | Male Reference interval (mean or median, samples) |
|---|---|---|---|---|
| ALAT | U/l | <54 (27, n = 40) | <71 (31, n = 17) | 10–38 (23, n = 23) |
| albumin | g/l | 11–15 (13, n = 40) | n/a (14, n = 23) | n/a (12, n = 17) |
| AP | U/l | 154–292 (220, n = 49) | 188–290 (239, n = 27) | 135–256 (197, n = 22) |
| ASAT | U/l | 2–90 (47, n = 64) | 12–81 (47, n = 34) | 0–102 (46, n = 30) |
| calcium | mmol/l | 2.10–2.41 (2.25, n = 35) | 2.08–2.33 (2.21, n = 17) | 2.16–2.46 (2.29, n = 18) |
| CHE | U/ml | 3.3–6.2 (4.8, n = 38) | 4.3–6.2 (5.3, n = 21) | 3.4–4.7 (4.1, n = 17) |
| chloride | mmol/l | 107–119 (113, n = 35) | 106–122 (114, n = 17) | 107–118 (112, n = 18) |
| cholesterol | mg/dl | 42–88 (66, n = 38) | 48–67 (58, n = 21) | 60–92 (76, n = 17) |
| creatinine | mg/dl | 0.14–0.33 (0.24, n = 40) | 0.15–0.32 (0.23, n = 23) | 0.14–0.36 (0.25, n = 17) |
| creatine kinase | U/l | 61–650 (344, n = 60) | 55–640 (370, n = 36) | 41–686 (367, n = 24) |
| fT3 | pmol/l | 3.4–5.8 (4.6, n = 40) | 3.3–5.8 (4.5, n = 23) | 3.3–6.0 (4.7, n = 17) |
| fT4 | pmol/l | 15.8–27.2 (21.5, n = 40) | 16.0–27.2 (21.6, n = 23) | 14.3–27.8 (21.1, n = 17) |
| ɣ-GT | U/l | 6–9 (7, n = 40) | n/a (7, n = 23) | 5–9 (7, n = 17) |
| GLDH | U/l | 0–26.2 (10.1, n = 38) | 2.9–14.1 (8.6, n = 23) | 0–36.2 (14.6, n = 15) |
| glucose | mg/dl | 237–507 (367, n = 37) | 235–485 (355, n = 21) | 225–552 (382, n = 16) |
| HDL | mg/dl | 39–84 (62, n = 40) | 43–68 (56, n = 23) | 54–89 (71, n = 17) |
| iron | µmol/l | 14.9–30.9 (22.8, n = 35) | 17.6–34.1 (26.1, n = 17) | 13.9–26.9 (20.8, n = 18) |
| LDH | U/l | 52–304 (180, n = 73) | 114–221 (167, n = 8) | 19–357 (194, n = 35) |
| lipase | U/l | <323 (160, n = 40) | <369 (142, n = 23) | 113–225 (170, n = 17) |
| magnesium | mmol/l | 1.00–1.24 (1.12, n = 35) | 0.97–1.23 (1.10, n = 17) | 1.01–1.26 (1.13, n = 18) |
| potassium | mmol/l | 2.8–4.4 (3.7, n = 35) | 2.7–4.2 (3.5, n = 17) | 3.1–4.6 (3.9, n = 18) |
| protein | g/l | 40–47 (43, n = 40) | 39–46 (43, n = 23) | 41–46 (44, n = 17) |
| sodium | mmol/l | 143–152 (147, n = 35) | 143–153 (148, n = 17) | 143–151 (147, n = 18) |
| triglycerides | mg/dl | 31–151 (94, n = 40) | 36–119 (79, n = 23) | 53–174 (114, n = 17) |
| urea nitrogen | mg/dl | 17–29 (23, n = 38) | 15–27 (21, n = 21) | 20–29 (24, n = 17) |
ALAT, alanine aminotransferase; ASAT, aspartate aminotransferase; ɣ-GT, gamma-glutamyl transpeptidase; GLDH, glutamate dehydrogenase; LDH, lactate dehydrogenase; AP, alkaline phosphatase; CHE, pseudocholinesterase; fT3, free triiodothyronine; fT4, free thyroxine; HDL, high density lipoprotein. n/a = not applicable for albumin and ɣ-GT as plasma levels showed too low variance to perform statistics.
Table 2.
Reference intervals for hematology parameters.
| Parameter | Unit | Pooled Reference interval (mean or median, samples) | Female Reference interval (mean or median, samples) | Male Reference interval (mean or median, samples) |
|---|---|---|---|---|
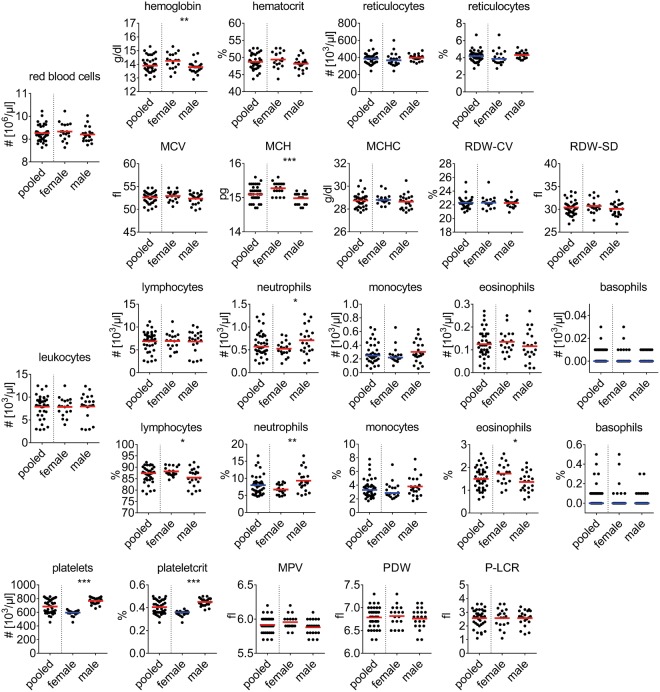
| red blood cells | 106/µl | 8.50–9.95 (9.3, n = 39) | 8.48–10. 16 (9.3, n = 18) | 8.46–9.81 (9.2, n = 21) |
| hemoglobin | g/dl | 12.7–15.1 (14.0, n = 39) | 12.9–15.7 (14.3, n = 18) | 12.8–14.7 (13.8, n = 21) |
| hematocrit | % | 44.3–53.1 (48.7, n = 39) | 44.4–54.7 (49.4, n = 18) | 44.2–51.9 (48.2, n = 21) |
| MCV | fl | 50.2–55.3 (52.6, n = 39) | 50.5–55.5 (52.9, n = 18) | 49.7–55.3 (52.3, n = 21) |
| MCH | pg | 14.7–15.6 (15.1, n = 39) | 14.9–15.7 (15.3, n = 18) | 14.6–15.4 (15.0, n = 21) |
| MCHC | g/dl | 27.5–29.9 (28.8, n = 39) | 27.8–29.8 (28.8, n = 18) | 27.1–30.0 (28.7, n = 21) |
| RDW-CV | % | 20.7–23.8 (22.3, n = 39) | 20.3–24.2 (22.3, n = 18) | 21.0–23.6 (22.3, n = 21) |
| RDW-SD | fl | 27.1–33.5 (30.4, n = 39) | 27.1–33.8 (30.7, n = 18) | 26.7–33.4 (30.1, n = 21) |
| reticulocytes | 103/µl | 256.0–511.1 (386.9, n = 39) | 182.7–540.8 (366.0, n = 18) | 314.2–477.7 (397.2, n = 21) |
| reticulocytes | % | 2.75–5.54 (4.18, n = 39) | 1.88–5.85 (3.87, n = 18) | 3.46–5.14 (4.32, n = 21) |
| leukocytes | 103/µl | 3.23–13.34 (7.90, n = 39) | 3.35–12.32 (7.85, n = 18) | 2.49–14.55 (7.94, n = 21) |
| lymphocytes | 103/µl | 2.73–11.68 (6.86, n = 39) | 2.82–10.92 (6.93, n = 18) | 2.09–12.84 (6.81, n = 21) |
| neutrophils | 103/µl | 0.03–1.12 (0.62, n = 39) | 0.15–0.89 (0.52, n = 18) | 0.03–1.34 (0.71, n = 21) |
| monocytes | 103/µl | 0.00–0.54 (0.25, n = 39) | 0.00–0.49 (0.22, n = 18) | 0.00–0.64 (0.30, n = 21) |
| eosinophils | 103/µl | 0.00–0.24 (0.12, n = 39) | 0.02–0.24 (0.14, n = 18) | 0.00–0.25 (0.12, n = 21) |
| basophils | 103/µl | n/a (0.0, n = 39) | n/a (0.0, n = 18) | n/a (0.0, n = 21) |
| lymphocytes | % | 70.0–94.6(86.7, n = 39) | 83.5–92.6 (88.2, n = 18) | 77.0–94.3 (85.5, n = 21) |
| neutrophils | % | 1.7–13.4 (8.0, n = 39) | 3.4–10.0 (6.7, n = 18) | 1.9–15.7 (9.3, n = 21) |
| monocytes | % | 0.4–6.1 (3.3, n = 39) | 0.3–5.7 (2.9, n = 18) | 0.3–6.8 (3.8, n = 21) |
| eosinophils | % | 0.5–2.5 (1.5, n = 39) | 0.7–2.8 (1.7, n = 18) | 0.4–2.3 (1.4, n = 21) |
| basophils | % | n/a (0.0, n = 39) | n/a (0.0, n = 18) | n/a (0.0, n = 21) |
| platelets | 103/µl | 476–893 (684, n = 39) | 505–692 (595, n = 18) | 656–881 (767, n = 21) |
| plateletcrit | % | 0.28–0.53 (0.40, n = 39) | 0.30–0.42 (0.36, n = 18) | 0.38–0.53 (0.45, n = 21) |
| MPV | fl | 5.7–6.2 (5.9, n = 39) | 5.7–6.2 (6.0, n = 18) | 5.6–6.1 (5.9, n = 21) |
| PDW | fl | 6.3–7.3 (6.8, n = 39) | 6.3–7.3 (6.8, n = 18) | 6.3–7.3 (6.8, n = 21) |
| P-LCR | % | 1.3–4.0 (2.6, n = 39) | 1.1–4.1 (2.6, n = 18) | 1.4–3.9 (2.6, n = 21) |
MCV, mean corpuscular volume; MCH, mean cellular hemoglobin; MCHC, mean corpuscular/cellular hemoglobin concentration; RDW-CV, red blood cell distribution width - coefficient of variation; RDW-SD, red blood cell distribution width - standard deviation; MPV, mean platelet volume; PDW, platelet distribution width; P-LCR, platelet large cell ratio. n/a = not applicable for basophils as the absolute numbers of events counted was too low to perform statistics.
To determine sex-dependent reference intervals for each of the 51 parameters we sequentially analyzed blood of multiple littermates and pooled the data for further analysis. We utilized the robust reference interval method for calculation as the number of animals examined was below 120, some data sets were non-normally distributed, and/or contained outliers12,13. Results of clinical chemistry parameters are shown in Fig. 1 and Table 1. Plasma concentration of several electrolytes (calcium, iron, and potassium), enzyme activities (ALAT, aspartate aminotransferase (ASAT), glutamate dehydrogenase (GLDH), LDH, lipase, and pseudocholinesterase (CHE)), albumin, cholesterol, triglycerides, high density lipoprotein (HDL), and urea nitrogen were significantly different between female and male mice. Results of differential blood counts are shown in Fig. 2 and Table 2. We found significant sex differences for values of hemoglobin, mean cellular hemoglobin (MCH), platelets, plateletcrit and minor but statistically significant differences for absolute cell counts of neutrophils, and relative distribution of lymphocytes, neutrophils and eosinophils.
Figure 1.
Clinical chemistry parameters. Each data point represents one animal. Lines indicate mean (in red) or median (in blue) for normally distributed or non-normally distributed populations, respectively. Data was pooled from n = 35–73 animals, derived from a total of six independent measurements. Stars indicate statistical significance.
Figure 2.
Hematological parameters. Each data point represents one animal. Lines indicate mean (in red) or median (in blue) for normally distributed or non-normally distributed populations, respectively. MCV, mean corpuscular volume; MCH, mean cellular hemoglobin; MCHC, mean corpuscular/cellular hemoglobin concentration; RDW-CV, red blood cell distribution width - coefficient of variation; RDW-SD, red blood cell distribution width - standard deviation; MPV, mean platelet volume; PDW, platelet distribution width; P-LCR, platelet large cell ratio. Data was pooled from n = 39 animals acquired in four independent measurements. Stars indicate statistical significance.
Next, to compare our results to those published by other authors we focused on reports on blood analyses of eight to twelve week old C57BL/6 mice. Table 3 lists the means or medians of laboratory tests as they have been reported by five different laboratories14–18 (Table 3). We found most of the values to be comparable to the data acquired in the current study. However, our measurements of blood glucose concentration were approximately two to three-fold higher and those of albumin two to three-fold lower than the values reported by all other laboratories. Our dataset did not allow for meaningful analyses of age-related differences in blood. However, to allow a comparative analysis of this study to results obtained by other laboratories we aim to upload our data to the mouse phenome database (phenome.jax.org)14.
Table 3.
Overview of laboratory test values published in comparison to the present study.
| Parameter | Unit | Sex | Present study | Jax.org57 | Klempt et al.17 | Champy et al.15 | Boehm et al.18 | Zhou et al.16 |
|---|---|---|---|---|---|---|---|---|
| ALAT | U/l | f | 31 | 43 | 14 | 34 | 22 | 27 |
| m | 23 | 57 | 15 | 33 | 21 | 27 | ||
| albumin | g/l | f | 14 | 39 | 30 | |||
| m | 12 | 37 | 30 | |||||
| AP | U/l | f | 239 | 239 | 154 | 129 | ||
| m | 197 | 178 | 151 | 153 | ||||
| ASAT | U/l | f | 47 | 32 | 91 | 51 | 43 | |
| m | 46 | 32 | 75 | 45 | 32 | |||
| calcium | mmol/l | f | 2.21 | 2.65 | 2.20 | 2.37 | 2.06 | |
| m | 2.29 | 2.60 | 2.10 | 2.45 | 2.04 | |||
| chloride | mmol/l | f | 114 | 116 | ||||
| m | 112 | 114 | ||||||
| cholesterol | mg/dl | f | 58 | 79 | 81 | 70 | 89 | 78 |
| m | 76 | 100 | 93 | 96 | 77 | 112 | ||
| creatinine | mg/dl | f | 0.23 | 0.28 | 0.34 | 0.17 | 0.19 | |
| m | 0.25 | 0.30 | 0.36 | 0.19 | 0.20 | |||
| creatine kinase | U/l | f | 370 | 720 | 77 | 406 | 102 | |
| m | 367 | 799 | 104 | 571 | 139 | |||
| glucose | mg/dl | f | 355 | 176 | 88 | 115 | 178 | 106 |
| m | 382 | 156 | 108 | 124 | 150 | 157 | ||
| HDL | mg/dl | f | 56 | 67 | 56 | 53 | ||
| m | 71 | 90 | 71 | 83 | ||||
| iron | µmol/l | f | 26.1 | 19.0 | ||||
| m | 20.8 | 16.0 | ||||||
| LDH | U/l | f | 167 | 429 | 293 | |||
| m | 194 | 453 | 348 | |||||
| magnesium | mmol/l | f | 1.10 | 1.13 | ||||
| m | 1.13 | 1.25 | ||||||
| potassium | mmol/l | f | 3.5 | 4.8 | 4.6 | 5.8 | ||
| m | 3.9 | 5.1 | 4.3 | 6.4 | ||||
| protein | g/l | f | 43 | 61 | 53 | 51 | 40 | 47 |
| m | 44 | 60 | 53 | 52 | 40 | 49 | ||
| sodium | mmol/l | f | 148 | 152 | 158 | 156 | ||
| m | 147 | 151 | 155 | 151 | ||||
| triglycerides | mg/dl | f | 79 | 93 | 109 | 85 | 97 | 69 |
| m | 114 | 80 | 97 | 110 | 97 | 83 | ||
| urea nitrogen | mg/dl | f | 21 | 27 | 76 | 31 | 24 | |
| 24 | 24 | 72 | 35 | 25 | ||||
| leukocytes | 103/µl | f | 7.9 | 3.5 | 4.0 | 7.2 | ||
| m | 7.9 | 2.6 | 3.4 | 8.2 | ||||
| red blood cells | 106/µl | f | 9.3 | 10.8 | 9.1 | 9.5 | ||
| m | 9.2 | 10.6 | 9.1 | 9.9 | ||||
| hemoglobin | g/dl | f | 14.3 | 17.0 | 13.6 | 14.0 | ||
| m | 13.8 | 16.2 | 13.2 | 15.0 | ||||
| hematocrit | % | f | 49.4 | 51.5 | 43.0 | |||
| m | 48.2 | 52.1 | 45.0 | |||||
| MCV | fl | f | 52.9 | 47.8 | 49.0 | 45.0 | ||
| m | 52.3 | 49.2 | 48.6 | 45.0 | ||||
| MCH | pg | f | 15.3 | 15.8 | 15.0 | |||
| m | 15.0 | 15.4 | 15.0 | |||||
| MCHC | g/dl | f | 28.8 | 33.2 | 34.0 | |||
| m | 28.6 | 31.2 | 33.0 | |||||
| platelets | 103/µl | f | 595 | 1019 | 518 | 1138 | ||
| m | 767 | 1157 | 633 | 1305 |
Laboratory tests for detection of inter-strain differences
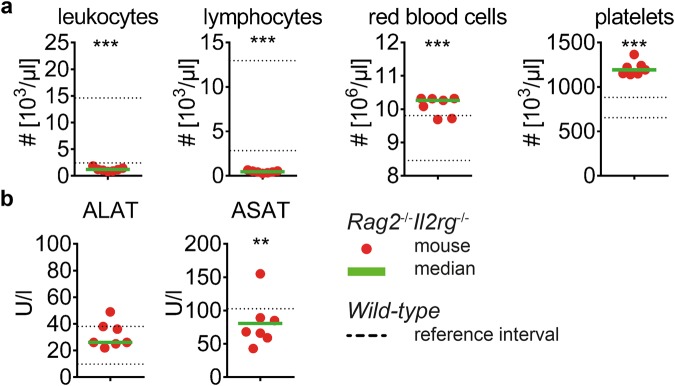
After evaluation of wild-type C57BL/6J mice, we aimed to determine the blood composition of a genetically modified mouse strain. We analyzed peripheral blood of Rag2−/−Il2rg−/− mice in which the targeted mutations interfere with development of T, B and NK cells. Indeed, due to a distinct reduction of lymphocytes the total number of leukocytes in peripheral blood was decreased if compared to wild-type mice (Fig. 3a). The number of monocytes and granulocytes seemed not to be affected by this genetic modification (data not shown) and the number of red blood cells was within or slightly above the reference interval (10.3 vs. 9.2 [×106/µl], Rag2−/−Il2rg−/− median vs. wild-type mean). Notably, Rag2−/−Il2rg−/− exhibited an increased number of platelets in peripheral blood (1192 vs. 767 [×103/µl], Rag2−/−Il2rg−/− median vs. wild-type mean). Enzymatic activity of ALAT and ASAT (68 vs. 46 [U/l], Rag2−/−Il2rg−/− median vs. wild-type mean) was comparable to that in plasma of wild-type mice (Fig. 3b). In summary, Rag2−/−Il2rg−/− mice exhibited a distinct decrease of lymphocytes in peripheral blood whereas concentrations of liver enzymes were similar to wild-type mice.
Figure 3.
Comparative analysis of blood composition of wild-type and Rag2−/−Il2rg−/− mice. Blood composition of Rag2−/−Il2rg−/− male mice was analyzed and depicted in comparison to reference intervals of wild-type mice as defined in this study. (a) Blood cell counts; (b) liver enzymes. Each data point represents one animal, green lines indicate median, dotted lines indicate upper and lower limits of wild-type reference intervals as depicted in Tables 1 and 2 (lower border of ASAT is 0). Data was pooled from n = 7 animals of two independent measurements. The non-parametric Mann-Whitney test was used to test for statistical difference between values obtained from Rag2−/−Il2rg−/− mice and those obtained from wild-type mice for reference interval calculation.
Laboratory tests for diagnosis of MCMV-induced liver damage
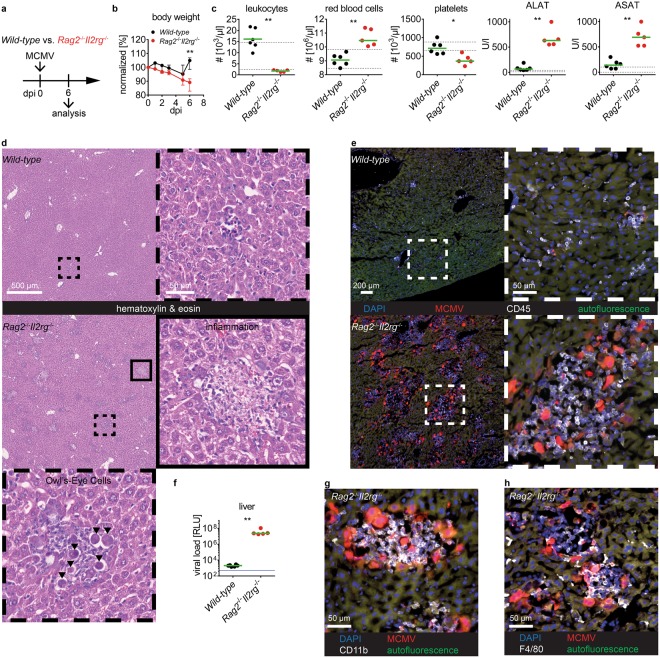
Next, we aimed to evaluate the use of laboratory tests in a murine disease model. For that, we utilized MCMV as a model pathogen to induce infectious disease in mice. We intravenously infected Rag2−/−Il2rg−/− or wild-type mice with a MCMV recombinant that contains the reporter genes mCherry and Gaussia luciferase allowing for visualization of infected cells and quantification of virus load, respectively (Fig. 4a). At six days post infection (dpi) Rag2−/−Il2rg−/− mice had lost significantly more body weight as compared to wild-type mice indicating an illness caused by virus infection in these animals (Fig. 4b). We applied a small panel of clinical chemistry parameters and a differential blood count to examine potential effects of MCMV infection on blood composition (Fig. 4c). The number of leukocytes in MCMV-infected wild-type mice was slightly increased which was due to elevated numbers in monocytes (Fig. 4c and data not shown). However, numbers of red blood cells and platelets were not affected in wild-type mice. Likewise, numbers of red blood cells in Rag2−/−Il2rg−/− mice were not affected by MCMV infection. In contrast, MCMV-infected Rag2−/−Il2rg−/− mice showed an approximately two-fold reduction in platelets as compared to non-infected animals (compare Fig. 4c to Fig. 3a). Indeed, thrombocytopenia is a typical feature of acute cytomegalovirus infection in human patients and here we could observe this phenomenon in Rag2−/−Il2rg−/− mice.
Figure 4.
Clinical pathology for diagnosis of MCMV disease. Wild-type or Rag2−/−Il2rg−/− male mice were intravenously infected with 106 PFU MCMV and analyzed 6 dpi. (a) Experimental setup; (b) body weight curves; (c) cell counts and plasma activity of ALAT and ASAT; (d) representative HE- stained liver sections, (e) and detection of MCMV-encoded mCherry in liver cryosections stained for CD45, framed items show higher magnification of the anatomic region as indicated in the overview images, (f) viral loads determined by quantification of MCMV-encoded Gaussia luciferase activity; cryosections of livers stained for g) CD11b and (h) F4/80. (b) Median and interquartile range, (c) and (f) each data point represents one animal, green lines indicate median, dotted lines indicate upper and lower limits of wild-type reference intervals as depicted in Tables 1 and 2, (f) blue line indicates detection limit of the assay. Scale bars: (d) 500 µm and 50 µm for low and high magnification, respectively; (e) 200 µm and 50 µm for low and high magnification, respectively. (g) and (h) 50 µm. Data was pooled from n = 5–6 animals per group, analyzed in two independent experiments, dpi = days post infection, RLU = relative light units. Stars indicate statistical significance.
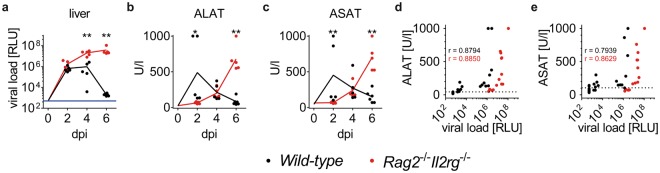
As MCMV is known to show tissue tropism to the liver we analyzed concentrations of ALAT and ASAT in peripheral blood. These aminotransferases are found at high concentrations in liver parenchymal cells and are released into blood circulation during cell damage. Thus, both parameters are sensitive indicators of acute liver cell damage. Indeed, in plasma samples of Rag2−/−Il2rg−/−, but not of wild-type mice, the activities of both enzymes were found to be approximately ten-fold increased to the upper bound of the reference intervals of wild-type mice (Fig. 4c). Hence, we sacrificed the animals and performed in-depth examination of the livers. In wild-type mice we hardly detected any morphological changes in liver anatomy (Fig. 4d). In contrast, in the livers of Rag2−/−Il2rg−/− mice we found multiple areas of focal inflammation, which contained distinctive enlarged cells with intra-nuclear inclusion bodies (Fig. 4d). These cells with owl’s eyes appearance are typically found in CMV-infected patients as a result of the virus-induced cytopathic effect (CPE)19. We confirmed the presence of multiple mCherry+ infected cells in the livers co-localizing with an infiltrate of CD45+ hematopoietic cells (Fig. 4e). Accordingly, the viral loads were remarkably elevated in livers of Rag2−/−Il2rg−/− mice whereas wild-type mice had sufficiently controlled the virus infection at six dpi (Fig. 4f). The immune cells present at the site of infection were positive for CD11b, which is an indicative cell surface marker for cells of the myeloid lineage (Fig. 4g). In addition, we identified F4/80+ macrophages (Fig. 4h). Thus, in Rag2−/−Il2rg−/− mice MCMV infection caused severe liver damage with increased plasma levels of ALAT and ASAT and histopathological findings consistent with virus-induced hepatitis. Hence, we could detect organ damage with tissue-specificity in an experimental infection model by using blood-based laboratory diagnostic tests.
Plasma activities of aminotransferases correlate with viral loads in livers
Finally, we investigated if ALAT and ASAT plasma levels were applicable to detect MCMV-induced hepatitis early after infection. Therefore, we analyzed intravenously infected Rag2−/−Il2rg−/− or wild-type mice already at two and four dpi for viral loads in the liver and concentration of the two enzymes in peripheral blood. Wild-type mice showed similar viral loads at two dpi as observed in Rag2−/−Il2rg−/− mice (Fig. 5a). However, at four dpi the viral loads were significantly higher in Rag2−/−Il2rg−/− mice whereas some wild-type mice already had lower viral loads than observed at two dpi. This difference was more distinctive at six dpi indicating that wild-type mice are capable of controlling the infection within this period whereas viral loads further increased in Rag2−/−Il2rg−/− mice. In line with these data, blood concentrations of ALAT and ASAT increased over time in Rag2−/−Il2rg−/− mice (Fig. 5b+ c). In contrast, blood concentrations of liver enzymes showed a less distinctive pattern in wild-type mice. However, at two dpi, both enzymes showed higher activity in wild-type than in Rag2−/−Il2rg−/− mice indicating that increased cell damage was present in wild-type mice at this time point. Thus, different pathophysiological mechanisms caused the release of aminotransferases from hepatocytes in the two animal models.
Figure 5.
Concentration of plasma aminotransferases and viral loads in acute MCMV infection. Wild-type or Rag2−/−Il2rg−/− male mice were intravenously infected with 106 PFU MCMV and analyzed at various days post infection. (a) Viral loads in livers at dpi as indicated, the value at 0 dpi was set to detection limit of the assay to allow visualization of kinetics, data for six dpi is depicted as in Fig. 4f, blue line indicates detection limit of the assay; plasma activity of (b) ALAT and (c) ASAT at dpi as indicated, the value at 0 dpi was set to median of values measured in untreated mice to allow visualization of kinetics, data for six dpi is depicted as in Fig. 4c; plasma activity of (d) ALAT and (e) ASAT in correlation to viral loads in liver, data from non-infected animals were included and RLU values set to detection limit of the assay. Data was pooled from n = 5–6 animals per group, analyzed in two independent experiments, dpi = days post infection, RLU = relative light units, r = Spearman coefficient. Stars indicate statistical significance.
Plasma concentrations of ALAT as well as ASAT correlated with viral loads in liver of wild-type and Rag2−/−Il2rg−/− mice (Fig. 5d+ e). Moreover, ALAT plasma activity was found in 100% (16/16) of wild-type and 100% (15/15) of Rag2−/−Il2rg−/− mice to be above the upper limit of the reference interval. 87.5% (14/16) of wild-type and 66.7% (10/15) of Rag2−/−Il2rg−/− mice had ASAT levels above the reference interval. Thus, ALAT appeared to be a sensitive parameter to detect MCMV infection of livers in immune-competent and lymphopenic mice.
Discussion
The use of animal models in life sciences is an established means to study pathophysiology of various diseases in vivo. Body weight, food and water intake, fur and skin conditions, physical activity, behavior, and the mouse grimace scale are frequently assessed by scientists to monitor the health status of experimental mice. Although these parameters can be determined via non-invasive procedures some of them are observer-dependent and all of them lack specificity for a certain disease. In contrast, the use of laboratory diagnostics from a single blood withdrawal may provide detection of pathophysiological processes with tissue specificity. In the present study, we generated reference intervals of human laboratory tests for mice that allow for detection of tissue damage in various organs including the hematopoietic system, liver, kidney, pancreas, skeletal muscle, and others. We illustrate how these diagnostic methods can be applied to detect virus-induced hepatitis in MCMV-infected Rag2−/−Il2rg−/− mice indicating that the observed body weight loss in these animals is, at least in part, due to liver inflammation.
Although we are not the first to investigate the use of laboratory diagnostics for murine samples, we here present an elaborate panel of parameters and used a substantial number of mice to determine reference intervals. In this study, we focused on the C57BL/6J mouse, one of the most widely used inbred strain, to allow for adaptation and comparison of the obtained data to genetically modified mice backcrossed to the same strain. Although others have reported measurements of laboratory tests for C57BL/6 mouse blood we could not adapt their confidence intervals to our platform. Comparison of values between different laboratories is limited in general as there are diverging detection methods and reagents for each individual parameter. Indeed, some parameters such as bilirubin that was investigated by others20 could not be measured reliably on our analyzers. Likewise, differential blood count measurements could not be processed on our human hematology analyzers. Instead, we processed the mouse blood with a veterinarian analyzer that provided species-specific cell gating strategies. Furthermore, analysis of animal blood composition may be affected by the various techniques used for blood withdrawal21–23 or the relative volume of anti-coagulant present in the sampling tube. Finally, various housing conditions influence gut microbiota and likely affect the blood composition of experimental animals24. Thus, there are multiple explanations for the observed inter-laboratory differences and we cannot provide a specific explanation for the aberrant values measured for albumin or glucose in this study. However, the measured blood glucose concentrations appeared non-physiological and therefore we do not recommend using this test in future studies.
The sex-dependent differences for blood cells and some clinical chemistry parameters observed in the present study have not consistently been reported by others. Accordingly, we could not find any reports explaining the observed higher LDH plasma concentrations in male mice. Differences in cholesterol and HDL levels between female and male mice have been described previously25,26.
MCMV has a broad tissue tropism and MCMV-associated hepatitis has been described in various mouse strains via histological analysis and plasma activity of aminotransferases27–34. Viral loads are high in livers of MCMV-infected mice within the first four dpi and then decline within one week. In line with the high viral loads plasma activity of liver enzymes is increased early after infection and reduction of enzyme activity is paralleled by the drop of viral titers after intraperitoneal application of the virus29,30. As immune control of MCMV infection relies on T and NK cells5,6, higher viral loads can be found in organs at six dpi in Rag2−/−Il2rg−/− as compared to wild-type mice. Likely, control of MCMV infection by lymphocytes in immune-competent mice leads to early liver cell damage and release of aminotransferases into peripheral blood. Thus, plasma activity of ALAT and ASAT are increased in wild-type mice and drop until six dpi when most of infected cells are controlled by the immune system. In contrast, lymphopenic mice allow ongoing replication of MCMV and the release of liver enzymes is rather due to virus-mediated CPE than by an immune cell-mediated mechanism. Virus-mediated CPE occurs later after infection as plasma concentrations of ALAT and ASAT were higher in wild-type than in Rag2−/−Il2rg−/− mice at two dpi. Similar findings were observed in other immune-compromised (SCID) or T cell-depleted mice where immunopathology was less pronounced than in wild-type controls at four dpi35. A progressive MCMV hepatitis has also been observed in T and NK cell-deficient E26 mice29. In general, mice lacking T, B and/or NK cells or irradiated mice show higher viral loads, morbidity and mortality than immune-competent animals36–39. However, due to the various antiviral mechanisms mediated by different immune cell types these phenotypes may occur early or late after infection. To our knowledge the present study is the first to report MCMV-associated hepatitis in Rag2−/−Il2rg−/− mice. As NK and T cells are crucial to control the acute MCMV infection phase5,6,39,40 this animal model is equivalent to other models with impaired innate and adaptive immunity. The presence of multiple juxtapositioned MCMV-infected cells and a local immune cell infiltrate show morphological similarities to nodular inflammatory foci (NIFs) which are found in immunocompromised patients infected with human cytomegalovirus41, have previously been described in lungs of neonatal and adult wild-type mice42, and similar structures have been also observed in various organs and mouse models27,28,35,43–45. Interestingly, lymphocytes are not a prerequisite for the formation of NIFs as myeloid cells accumulate at the site of infection independently from the presence of T and NK cells39. In contrast, plaque-like formation of infected cells without immune cell infiltrates have been observed in gamma-irradiated mice which lack both myeloid and lymphoid cells36,39,46,47.
As Rag2−/−Il2rg−/− mice exhibit a distinctive lymphopenia we stained liver sections for CD11b and F4/80 which are typically expressed by myeloid cells. Interestingly, absolute numbers of monocytes were decreased in peripheral blood of MCMV-infected Rag2−/−Il2rg−/− mice (data not shown) indicating that either many of the cells were captured in the liver or MCMV interfered with hematopoiesis. Since also the number of platelets was also decreased after MCMV infection impaired hematopoiesis appears to be more likely48–50. In the absence of T and NK cells, MCMV leads to severe liver cell damage at six dpi as plasma activities of aminotransferases were considerably increased in Rag2−/−Il2rg−/− mice. This data argues that the observed tissue lesions can occur without the presence of lymphocytes. Thus, potential roles of (i) direct CPE of MCMV and (ii) myeloid cells present in NIFs in tissue damage of MCMV-infected mice need to be considered in future studies.
The use of reference intervals allowed for discrimination of MCMV-infected from non-infected wild-type and Rag2−/−Il2rg−/− mice as ALAT plasma concentrations were above the upper limit of the reference values in all infected animals tested. Thus, ALAT is a sensitive marker for detection of MCMV-mediated liver damage in experimental animals. Moreover, viral loads in livers as well as plasma levels of ALAT and ASAT showed comparable kinetics in Rag2−/−Il2rg−/− mice. Accordingly, enzyme activities >300 U/l likely correlate with viral disease that may indicate suffering of the animals as values >500 U/l were only found in animals with very high viral loads and pronounced liver pathology at six dpi. Thus, aminotransferases are suitable parameters to monitor the health status of MCMV-infected Rag2−/−Il2rg−/− mice.
Taken together, the use of laboratory diagnostics allows for rapid detection and monitoring of infection-induced liver damage. Moreover, utilizing laboratory diagnostics for early detection of MCMV disease will allow us to promptly terminate an experiment and minimize pain and suffering of the animal to improve animal welfare (refinement). Moreover, the use of highly quality-controlled methods reduces the variance of values and therefore the number of mice needed to characterize robust phenotypes in mouse models (reduction). Hence, application of the methods described here are in line with principles of the 3 R’s (Replacement, Reduction and Refinement) of animal welfare. We offer the screeing service to others (www.hheld.net).
In summary, we established reference intervals for important clinical pathology laboratory tests and illustrated several sex differences for C57BL/6J mice. Application of laboratory diagnostics in peripheral blood of experimental mice allowed for organ-specific detection of disease. This diagnostic platform complements other methods to monitor the health status of experimental animals.
Methods
Animals
C57BL/6J mice were purchased from Charles River Laboratories (Sulzfeld, Germany) to generate a colony at the local animal facility of the University Medical Center Hamburg-Eppendorf. Mice were bread in individually ventilated cages under specific pathogen free conditions according to the recommendations of the Federation of European Laboratory Animal Science Associations (FELASA)51. Pasteurella pneumotropica, Helicobacter species and murine norovirus 1 were detected in sentinel animals tested in this breeding barrier. Food and water was provided ad libitum. Animals were analyzed at the age of 6–10 weeks, Rag2−/−Il2rg−/−52,53 were on C57BL/6 background and maintained similarly.
Ethics statement
All animal experiments were performed according to the recommendations and guidelines of the FELASA and Society of Laboratory Animals (GV-SOLAS) and approved by the institutional review board and local authorities (Behörde für Gesundheit und Verbraucherschutz, Amt für Verbraucherschutz, Freie und Hansestadt Hamburg, reference numbers 85/15 and 39/17).
Blood withdrawal
Blood was obtained from the retro-orbital plexus with the use of heparinized micro-haematocrit tubes (Vitrex, Product No. 161813) in deep anesthesia (ketamine and xylazine). Blood was subsequently collected in tubes prefilled with EDTA (Kabe Labortechnik GmbH, Product No. 077011) for determination of differential blood counts or heparin (Sarstedt AG & Co, Product No. 41.1393.005) for determination of clinical chemistry parameters, respectively.
Clinical Chemistry
Heparin-anticoagulated plasma was obtained following 10 min of centrifugation at 2000 rcf and analyzed for clinical chemistry parameters on Dimension Vista 1500 Lab Systems (Siemens Healthcare) at the Central Laboratory of the University Medical Center Hamburg-Eppendorf within four hours after blood withdrawal. Sodium, potassium and chloride were quantified by indirect potentiometry; calcium, iron, magnesium, total protein, albumin, creatinine, urea-nitrogen, cholesterol, triglycerides, HDL and glucose as well as enzymatic activity of ALAT, ASAT, LDH, gamma-glutamyl transpeptidase (ɣ-GT), alkaline phosphatase (AP), creatine kinase, lipase, CHE were quantified photometrically with reagents provided by Siemens Healthcare. The hormones free triiodothyronine (fT3) and free tyroxine (fT4) were quantified using a Luminescent Oxygen Channeling Immunoassay. GLDH was assessed using the reagents from Roche Diagnostics. All procedures were performed according to the manufacturers’ protocols.
Hematology
Differential blood counts were analyzed from EDTA-anticoagulated whole blood samples using a ProCyte Dx Hematology Analyzer (IDEXX Laboratories) within four hours after blood withdrawal. Briefly, this system uses a combination of optical fluorescence, laminar flow impedance, and cyanide-free sodium lauryl sulphate (SLS) - hemoglobin method to determine all presented parameters in one run.
Statistical Analysis
We performed a d’Agostino and Pearson omnibus normality test for each analyte to test for Gaussian distribution. Accordingly, the parametric unpaired Student’s t-test was used for normally distributed whereas the non-parametric Mann-Whitney test was used for non-normally distributed populations to compare results obtained from female or male mice. The non-parametric (Spearman) test was performed to analyze for correlation of viral loads and liver enzymes. The mean or median is given for normally distributed or non-normally distributed populations, respectively. Statistical significance was depicted as follows: *p < 0.05; **p < 0.01; and ***p < 0.001. Data was processed with GraphPad Prism (version 6) software.
Reference intervals were determined by the two-sided 95% robust reference interval according to Horn et al.13 with the following constants: tuning constant 1 = 3.700, tuning constant 2 = 205.408, MAD scale factor = 0.674500, and bootstrap samples = 10000. Data was processed with NCSS statistical software (version 11.0.13).
MCMV infection
The MCMV-3DR recombinant has been described previously54–56 and was produced on 10.1 immortalized mouse embryonic fibroblasts45 and titrated on M2-10B4 mouse bone marrow stromal cells (ATCC CRL-1972). MCMV-3DR was generated from the pSM3fr bacterial artificial chromosome. It encodes Gaussia luciferase, mCherry, contains a sequence within the m164 ORF encoding the SIINFEKL peptide, and contains the complete Mck2 ORF but its m157 ORF is replaced by the sequences for the reporter proteins. Mice were anaesthetized (isoflurane) and received a single intravenous injection of 106 PFU MCMV-3DR.
Histology
Organs were fixed in either PBS-buffered 2% paraformaldehyde with 30% sucrose for cryosections and immune fluorescence histology, or 4% formalin for paraffin sections and hematoxylin & eosin (HE) staining. For immune fluorescence histology 7 µm-thick organ slices were stained with antibodies (clone) after serum blocking as follows: CD45-APC (30-F11), CD11b-APC (M1/70), or F4/80-APC (BM8). Images were taken with an Axiovert 200 M fluorescence microscope (Carl Zeiss) and processed with AxioVision 4.9 software. Images of HE stained sections were generated by the local mouse pathology facility.
Luciferase assay
Explanted livers were kept on ice-cooled saline, homogenized with TissueLyser II (Qiagen), centrifuged, and supernatants were measured for luciferase expression by quantification of luminescence after the addition of native coelenterazine (Synchem) with Centro XS³ LB 960 (Berthold Technologies).
Electronic supplementary material
Acknowledgements
We thank the local mouse pathology facility for paraffin embedment, HE staining, and visualization of livers, the Institute of Clinical Chemistry and Laboratory Medicine members for technical assistance, and Phillip Stahl for help with histopathological analysis. FRS acknowledges support from the German Center of Infection Research (DZIF 07.001‐Stahl), the German Research Foundation (DFG) (STA 1549/1–1), the German Society for Clinical Chemistry and Laboratory Medicine, and the Faculty of Medicine of the University of Hamburg (NWF-17/11). TR acknowledges support from the DFG (SFB877, TP A11 and SFB841, TP B8), and a European Research Council grant (ERC-StG-2012–311575_F-12). SRJ was supported by the DFG (SFB877, TP A01 and SFB841, TP C1) and by the Cluster of Excellence ‘Inflammation at Interfaces’. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author Contributions
F.R.S. and T.R. conceived the study. F.R.S. designed the experiments and performed them together with S.T. F.R.S., T.R., R.J., V.J., S.H., A.K. and W.B. analyzed the data. E.O., R.B., W.B., M.M., P.C.A., S.R.J. contributed reagents/materials/analysis tools. F.R.S. wrote and all authors reviewed the manuscript.
Data Availability Statement
Data obtained for calculation of reference intervals will be uploaded to the mouse phenome database (phenome.jax.org)14.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33167-7.
References
- 1.Blake JA, et al. Mouse Genome Database (MGD)-2017: community knowledge resource for the laboratory mouse. Nucleic acids research. 2017;45:D723–D729. doi: 10.1093/nar/gkw1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P, et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clin Infect Dis. 2017;64:87–91. doi: 10.1093/cid/ciw668. [DOI] [PubMed] [Google Scholar]
- 3.Rawlinson William D, Boppana Suresh B, Fowler Karen B, Kimberlin David W, Lazzarotto Tiziana, Alain Sophie, Daly Kate, Doutré Sara, Gibson Laura, Giles Michelle L, Greenlee Janelle, Hamilton Stuart T, Harrison Gail J, Hui Lisa, Jones Cheryl A, Palasanthiran Pamela, Schleiss Mark R, Shand Antonia W, van Zuylen Wendy J. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. The Lancet Infectious Diseases. 2017;17(6):e177–e188. doi: 10.1016/S1473-3099(17)30143-3. [DOI] [PubMed] [Google Scholar]
- 4.Lancini D, Faddy HM, Flower R, Hogan C. Cytomegalovirus disease in immunocompetent adults. Med J Aust. 2014;201:578–580. doi: 10.5694/mja14.00183. [DOI] [PubMed] [Google Scholar]
- 5.Reddehase MJ. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat Rev Immunol. 2002;2:831–844. doi: 10.1038/nri932. [DOI] [PubMed] [Google Scholar]
- 6.Lisnic B, Lisnic VJ, Jonjic S. NK cell interplay with cytomegaloviruses. Curr Opin Virol. 2015;15:9–18. doi: 10.1016/j.coviro.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Henson D, Smith RD, Gehrke J. Non-fatal mouse cytomegalovirus hepatitis. Combined morphologic, virologic and immunologic observations. Am J Pathol. 1966;49:871–888. [PMC free article] [PubMed] [Google Scholar]
- 8.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. J Immunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 9.Reddehase MJ, et al. Interstitial murine cytomegalovirus pneumonia after irradiation: characterization of cells that limit viral replication during established infection of the lungs. J Virol. 1985;55:264–273. doi: 10.1128/jvi.55.2.264-273.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddehase MJ, Mutter W, Munch K, Buhring HJ, Koszinowski UH. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J Virol. 1987;61:3102–3108. doi: 10.1128/jvi.61.10.3102-3108.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonjic S, Pavic I, Lucin P, Rukavina D, Koszinowski UH. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J Virol. 1990;64:5457–5464. doi: 10.1128/jvi.64.11.5457-5464.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reed AH, Henry RJ, Mason WB. Influence of statistical method used on the resulting estimate of normal range. Clin Chem. 1971;17:275–284. [PubMed] [Google Scholar]
- 13.Horn PS, Pesce AJ, Copeland BE. A robust approach to reference interval estimation and evaluation. Clin Chem. 1998;44:622–631. [PubMed] [Google Scholar]
- 14.Bogue MA, Grubb SC. The Mouse Phenome Project. Genetica. 2004;122:71–74. doi: 10.1007/s10709-004-1438-4. [DOI] [PubMed] [Google Scholar]
- 15.Champy M-F, et al. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm Genome. 2004;15:768–783. doi: 10.1007/s00335-004-2393-1. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Hansson GK. Effect of sex and age on serum biochemical reference ranges in C57BL/6J mice. Comp Med. 2004;54:176–178. [PubMed] [Google Scholar]
- 17.Klempt M, et al. Genotype-specific environmental impact on the variance of blood values in inbred and F1 hybrid mice. Mamm Genome. 2006;17:93–102. doi: 10.1007/s00335-005-0119-7. [DOI] [PubMed] [Google Scholar]
- 18.Boehm O, et al. Clinical chemistry reference database for Wistar rats and C57/BL6 mice. Biol Chem. 2007;388:547–554. doi: 10.1515/BC.2007.061. [DOI] [PubMed] [Google Scholar]
- 19.Herriot R, Gray ES. Images in clinical medicine. Owl’s-eye cells. N Engl J Med. 1994;331:649. doi: 10.1056/NEJM199409083311005. [DOI] [PubMed] [Google Scholar]
- 20.Mazzaccara C, et al. Age-Related Reference Intervals of the Main Biochemical and Hematological Parameters in C57BL/6J, 129SV/EV and C3H/HeJ Mouse Strains. PLoS One. 2008;3:e3772. doi: 10.1371/journal.pone.0003772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM. Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther. 2002;13:155–161. doi: 10.1089/10430340152712700. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez I, Pena A, Del Teso N, Perez V, Rodriguez-Cuesta J. Clinical biochemistry parameters in C57BL/6J mice after blood collection from the submandibular vein and retroorbital plexus. J Am Assoc Lab Anim Sci. 2010;49:202–206. [PMC free article] [PubMed] [Google Scholar]
- 23.Chan YK, et al. Influence of tail versus cardiac sampling on blood glucose and lipid profiles in mice. Lab Anim. 2012;46:142–147. doi: 10.1258/la.2011.011136. [DOI] [PubMed] [Google Scholar]
- 24.Franklin CL, Ericsson AC. Microbiota and reproducibility of rodent models. Lab Anim (NY) 2017;46:114–122. doi: 10.1038/laban.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruell JH, Daroczy AF, Hellerstein HK. Strain and sex differences in serum cholesterol levels of mice. Science. 1962;135:1071–1072. doi: 10.1126/science.135.3508.1071. [DOI] [PubMed] [Google Scholar]
- 26.Link JC, et al. Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes. Arterioscler Thromb Vasc Biol. 2015;35:1778–1786. doi: 10.1161/ATVBAHA.115.305460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadimitriou JM, Shellam GR, Allan JE. The effect of the beige mutation on infection with murine cytomegalovirus: histopathologic studies. Am J Pathol. 1982;108:299–309. [PMC free article] [PubMed] [Google Scholar]
- 28.Olver SD, Price P, Shellam GR. Cytomegalovirus hepatitis: characterization of the inflammatory infiltrate in resistant and susceptible mice. Clin Exp Immunol. 1994;98:375–381. doi: 10.1111/j.1365-2249.1994.tb05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orange JS, Salazar-Mather TP, Opal SM, Biron CA. Mechanisms for virus-induced liver disease: tumor necrosis factor-mediated pathology independent of natural killer and T cells during murine cytomegalovirus infection. Journal of virology. 1997;71:9248–9258. doi: 10.1128/jvi.71.12.9248-9258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolger G, et al. Acute murine cytomegalovirus infection: a model for determining antiviral activity against CMV induced hepatitis. Antiviral Res. 1999;44:155–165. doi: 10.1016/S0166-3542(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 31.Trgovcich J, et al. Immune responses and cytokine induction in the development of severe hepatitis during acute infections with murine cytomegalovirus. Archives of virology. 2000;145:2601–2618. doi: 10.1007/s007050070010. [DOI] [PubMed] [Google Scholar]
- 32.Gaddi PJ, et al. IL-10 mediated regulation of liver inflammation during acute murine cytomegalovirus infection. PLoS One. 2012;7:e42850. doi: 10.1371/journal.pone.0042850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khairallah C, et al. gammadelta T cells confer protection against murine cytomegalovirus (MCMV) PLoS Pathog. 2015;11:e1004702. doi: 10.1371/journal.ppat.1004702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popovic B, et al. IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLoS Pathog. 2017;13:e1006345. doi: 10.1371/journal.ppat.1006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livingston-Rosanoff D, et al. Antiviral T cell response triggers cytomegalovirus hepatitis in mice. Journal of virology. 2012;86:12879–12890. doi: 10.1128/JVI.01752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas S, et al. Evaluating Human T-Cell Therapy of Cytomegalovirus Organ Disease in HLA-Transgenic Mice. PLoS Pathog. 2015;11:e1005049. doi: 10.1371/journal.ppat.1005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klenovsek K, et al. Protection from CMV infection in immunodeficient hosts by adoptive transfer of memory B cells. Blood. 2007;110:3472–3479. doi: 10.1182/blood-2007-06-095414. [DOI] [PubMed] [Google Scholar]
- 38.Sell S, et al. Control of murine cytomegalovirus infection by gammadelta T cells. PLoS Pathog. 2015;11:e1004481. doi: 10.1371/journal.ppat.1004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lueder Yvonne, Heller Katrin, Ritter Christiane, Keyser Kirsten A., Wagner Karen, Liu Xiaokun, Messerle Martin, Stahl Felix R., Halle Stephan, Förster Reinhold. Control of primary mouse cytomegalovirus infection in lung nodular inflammatory foci by cooperation of interferon-gamma expressing CD4 and CD8 T cells. PLOS Pathogens. 2018;14(8):e1007252. doi: 10.1371/journal.ppat.1007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16:367–377. doi: 10.1038/nri.2016.38. [DOI] [PubMed] [Google Scholar]
- 41.Travis, W. D. et al. In Non-Neoplastic Disorders of the Lower Respiratory Tract (Atlas of Nontumor Pathology) (ed Armed Forces Institute of Pathology) 639–641 (2002).
- 42.Stahl FR, et al. Nodular inflammatory foci are sites of T cell priming and control of murine cytomegalovirus infection in the neonatal lung. PLoS Pathog. 2013;9:e1003828. doi: 10.1371/journal.ppat.1003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein 1alpha (MIP-1alpha)-dependent pathways. J Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krmpotic A, Bubic I, Polic B, Lucin P, Jonjic S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003;5:1263–1277. doi: 10.1016/j.micinf.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Harvey D M, Levine A J. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes & Development. 1991;5(12b):2375–2385. doi: 10.1101/gad.5.12b.2375. [DOI] [PubMed] [Google Scholar]
- 46.Bohm V, et al. Epitope-specific in vivo protection against cytomegalovirus disease by CD8 T cells in the murine model of preemptive immunotherapy. Med Microbiol Immunol. 2008;197:135–144. doi: 10.1007/s00430-008-0092-3. [DOI] [PubMed] [Google Scholar]
- 47.Alterio De Goss M, et al. Control of cytomegalovirus in bone marrow transplantation chimeras lacking the prevailing antigen-presenting molecule in recipient tissues rests primarily on recipient-derived CD8 T cells. Journal of virology. 1998;72:7733–7744. doi: 10.1128/jvi.72.10.7733-7744.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer A, et al. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. Journal of virology. 1997;71:4589–4598. doi: 10.1128/jvi.71.6.4589-4598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jordan S, et al. Natural killer cells are required for extramedullary hematopoiesis following murine cytomegalovirus infection. Cell Host Microbe. 2013;13:535–545. doi: 10.1016/j.chom.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Hirche C, et al. Systemic Virus Infections Differentially Modulate Cell Cycle State and Functionality of Long-Term Hematopoietic Stem Cells InVivo. Cell Rep. 2017;19:2345–2356. doi: 10.1016/j.celrep.2017.05.063. [DOI] [PubMed] [Google Scholar]
- 51.Mahler Convenor M, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim. 2014;48:178–192. doi: 10.1177/0023677213516312. [DOI] [PubMed] [Google Scholar]
- 52.Shinkai Y, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-C. [DOI] [PubMed] [Google Scholar]
- 53.Cao X, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 54.Lemmermann NAW, et al. Immune evasion proteins of murine cytomegalovirus preferentially affect cell surface display of recently generated peptide presentation complexes. Journal of virology. 2010;84:1221–1236. doi: 10.1128/JVI.02087-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marquardt A, et al. Single cell detection of latent cytomegalovirus reactivation in host tissue. J Gen Virol. 2011;92:1279–1291. doi: 10.1099/vir.0.029827-0. [DOI] [PubMed] [Google Scholar]
- 56.Stahl F R, Keyser K A, Heller K, Bischoff Y, Halle S, Wagner K, Messerle M, Förster R. Mck2-dependent infection of alveolar macrophages promotes replication of MCMV in nodular inflammatory foci of the neonatal lung. Mucosal Immunology. 2014;8(1):57–67. doi: 10.1038/mi.2014.42. [DOI] [PubMed] [Google Scholar]
- 57.Laboratory, J. Hematological survey of 11 strains of mice. MPD:Jaxpheno4., https://www.jax.org/strain/000664 (2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data obtained for calculation of reference intervals will be uploaded to the mouse phenome database (phenome.jax.org)14.