Abstract
While neurofilament light chain (NfL) measurement in serum is a well-established marker of neuroaxonal damage in multiple sclerosis (MS), data on astroglial markers in serum are missing. In our study, glial fibrillary acid protein (GFAP) and NfL were measured in cerebrospinal fluid (CSF) and serum of MS patients and patients with other non-inflammatory neurological diseases (OND) using the Simoa technology. Clinical data like age, gender, expanded disability status scale (EDSS) and MRI findings were correlated to neurochemical markers. We included 80 MS patients: 42 relapsing-remitting MS (RRMS), 38 progressive MS (PMS), as well as 20 OND. Serum GFAP levels were higher in PMS compared to RRMS and OND (p < 0.001, p = 0.02 respectively). Serum GFAP levels correlated with disease severity in the whole MS group and PMS (Spearman-rho = 0.5, p < 0.001 in both groups). Serum GFAP correlated with serum NfL in PMS patients (Spearman-rho = 0.4, p = 0.01). Levels of serum GFAP were higher with increasing MRI-lesion count (p = 0.01). in summary, we report elevated levels of GFAP in the serum of MS patients. Since serum levels of GFAP correlate with the clinical severity scores and MRI lesion count, especially in PMS patients, it might be a suitable disease progression marker.
Subject terms: Multiple sclerosis, Neurodegenerative diseases
Introduction
The role of astrocytes in multiple sclerosis (MS) has been investigated extensively. Beyond their role in scar formation, astrocytes are a potent secretor of different proinflammatory cytokines and cytotoxic factors and contribute to mitochondrial dysfunction1,2. Astrocyte subtype A1 is a potent direct killer of the neurons and oligodendrocytes3. Glial fibrillary acidic protein (GFAP) is a well-established marker of astrogliosis as numerous studies described its use for MS and reported correlations with disease severity, the extent of neuroinflammation and progression4–8. Using a proteomic approach, we previously suggested the use of GFAP in subtyping progressive forms of MS9. However, up to now, data regarding levels of GFAP in blood samples of MS patients are scarce due to the detection limit of available assays10. However, with the introduction of the highly sensitive immunoassay platforms, this now is possible as shown after traumatic brain injury11. In our study, we investigated the diagnostic use of blood levels of GFAP in relation to the neuroaxonal damage marker, neurofilament light chain (NfL), and the clinical subtype of MS.
Results
Patient characteristics
Paired serum and cerebrospinal fluid (CSF) samples of 80 MS patients and 20 other non-inflammatory neurological diseases (OND) patients were analysed. Four relapsing-remitting MS between relapses (RRMS-) patients received disease-modifying therapy (DMT); two received interferon-1-β (INF-β), one received Natalizumab, and another one was on Alemtuzumab. Three secondary progressive MS (SPMS) patients were on DMT (Natalizumab, INF-β and Fingolimod). Teriflunomide was used in one RRMS patient during acute exacerbation (RRMS+) and Natalizumab in another patient. A cranial magnetic resonance imaging (MRI) at the time of lumbar puncture (LP) was available from 64 MS patients. For 59 MS patients, additional MRIs of the cervical and thoracic cord were available. All clinical characteristics of the patients along with serum and cerebrospinal fluid (CSF) levels of GFAP and NfL are summarised in Table 1. All the below mentioned clinical and laboratory parameters did not differ between the treatment-naive patients and patients under DMT.
Table 1.
Clinical characteristics and biomarker levels in CSF and serum of the included subjects. All given values are median values with the interquartile range in brackets. (25–75 percentile).
| median (25–75 percentile) | Multiple sclerosis (n = 80; 46♀ and 34♂) | Controls (n = 20; 13 ♀ and 7 ♂ | |||
|---|---|---|---|---|---|
| RRMS− (n = 24)3) | RRMS+ (n = 18) | SPMS (n = 13) | PPMS (n = 25) | ||
| Age | 36 (27–49) | 28 (24–43) | 50 (49–58) | 53 (45–59) | 44 (27–52) |
| EDSS | 2.25 (1.5–4.75) | 2.0 (1.5–2.0) | 6.5 (6.0–7.5) | 4.5 (3.0–6.5) | n/a |
| MSSS | 5.9 (2.9–7.8) | 5.6 (2.9–6.3) | 7.5 (6.5–8) | 6.8 (5.9–8.6) | n/a |
| ARMSS | 5.9 (2.2–7.4) | 4.6 (3.4–5.9) | 8.7 (7.5–8.9) | 6.8 (3.3–7.9) | n/a |
| Disease duration (in months) | 61 (6–86) | 6 (1–15) | 294 (276–420) | 60 (40–216) | n/a |
| Albumin quotient (Qalb) | 3.5 (0.5–5.5) | 5.0 (3.4–5.8) | 4.2 (3.0–5.7) | 3.8(1.7–6.5) | 1.0 (0.6–1.3) |
| CSF GFAP in pg/ml | 6925 (4365–10600) | 7935.2 (5480.7–11310.4) | 12329.8 (8038.9–16036.1) | 10300 (7100–13680) | 6157.8 (2452.5–7967.5) |
| Serum GFAP in pg/ml | 113.5 (78.3–137.8) | 78.2 (55.0–165.0) | 145.9 (86.5–245.9) | 130.5 (98–167.5) | 92.33 (59–139.1) |
| CSF NfL in pg/ml | 1718.7 (998.9–3347.5) | 1674.0 (824.0–3210.0) | 1570.0 (1450.0–2491.6) | 1241 (898.0–2240.0) | 584.5 (371.5–827.1) |
| Serum NfL in pg/ml | 13.9 (7.9–27.3) | 15.0 (11.0–24.0) | 28.6 (19.7–44.4) | 17.4 (10.8–22.4) | 9.2 (6.0–12.2) |
n = number, ♀ = female, ♂ = male, CSF: cerebrospinal fluid, RRMS-: relapsing-remitting multiple sclerosis between relapse, RRMS + : relapsing-remitting multiple sclerosis during acute exacerbation, SPMS: secondary progressive multiple sclerosis, PPMS: primary progressive multiple sclerosis, n/a: not applicable. EDSS: expanded disability score scale, MSSS: multiple sclerosis severity score, ARMSS: age-related multiple sclerosis severity score.
73.4% (n = 47) of the MS patients had >9 lesions and 25% (n = 16) between 2–9 lesions; only one MS patient had less than two lesions in the cranial MRI. Both supra- and infratentorial lesions were the most common MRI lesion pattern and were found in 57.8% (n = 37) followed by supratentorial lesions alone (40.6%, n = 26). Gd+ and Gd- lesions were equally distributed (50% each, n = 32) and spinal lesions in 66.1% (n = 39) of all available scans.
GFAP
GFAP in CSF and serum showed strong correlation in MS patients (Spearman’s rho = 0.6, p < 0.001) and in controls (Spearman’s rho = 0.7, p < 0.001).
In MS patients CSF and serum GFAP increased with age (Spearman rho = 0.4, p < 0.001 for both). Stronger correlations were found in the controls (Spearman rho = 0.6 and 0.8 p < 0.001 respectively). Moreover, CSF, but not serum GFAP, correlated with albumin quotient (Qalb) in relapsing and progressive MS patients (Spearman rho = 0.3 and 0.4 respectively, p = 0.02 for both). Gender did not affect GFAP levels neither in CSF nor in serum.
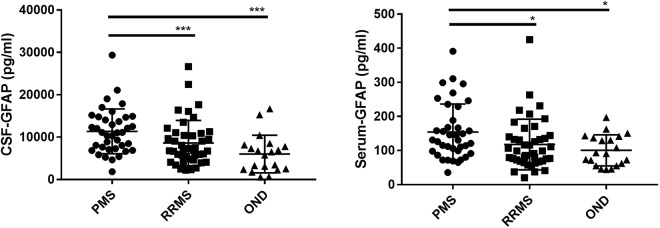
CSF and serum GFAP levels were higher in patients with progressive MS (PMS) compared to RRMS (p = 0.001 and 0.02 respectively) and controls (p = 0.001 and 0.01 respectively) (Fig. 1). However, after correction for age, the difference between PMS and RRMS was no longer statistically significant. Similarly, the correlation between CSF and serum GFAP with disease duration (0.3 and 0.4 respectively, p < 0.01) turned insignificant after correction for age. The concentration of GFAP in CSF and serum in RRMS did not differ significantly from the controls (p = 0.05 and 0.5). Correlations between CSF and serum GFAP and different clinical outcome parameters are reported in Table 2.
Figure 1.
GFAP CSF and serum levels in multiple sclerosis patients and patients with other non-inflammatory neurological diseases (OND). GFAP: glial fibrillary acidic protein. CSF: cerebrospinal fluid, PMS: progressive multiple sclerosis, RRMS: relapsing-remitting multiple sclerosis. P-values were calculated with Kruskal-Wallis test followed by Dunn’s multiple comparison tests. *p < 0.05; ***p < 0.001.
Table 2.
Correlation matrices in different subgroups.
| Whole MS group (n = 80) | RRMS (n = 42) | PMS (n = 38) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EDSS | MSSS | ARMSS | EDSS | MSSS | ARMSS | EDSS | MSSS | ARMSS | |
| GFAP CSF | 0.4** | n.s | n.s | n.s | n.s | n.s | n.s | n.s | n.s |
| GFAP serum | 0.5*** | 0.4** | 0.3* | 0.4* | n.s | n.s | 0.5*** | n.s | 0.4** |
| NfL CSF | n.s | n.s | n.s | n.s | n.s | n.s | n.s | n.s | n.s |
| NfL serum | 0.3 ** | n.s | n.s | n.s | n.s | n.s | 0.3* | n.s | n.s |
RRMS: all relapsing-remitting MS patients (RRMS- and RRMS+); PMS: all patients with progressive MS (SPMS and PPMS); values are Spearman r, *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001. EDSS: expanded disability score scale, MSSS: multiple sclerosis severity score, ARMSS: age-related multiple sclerosis severity score.
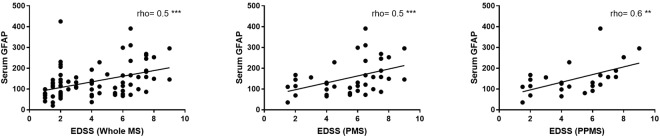
Only in primary progressive MS (PPMS), serum GFAP correlated strongly with expanded disability status scale (EDSS) (Spearman rho = 0.6, p = 0.002) and with age-related multiple sclerosis severity score (ARMSS) (spearman rho = 0.4, p = 0.04) (Fig. 2). In PMS and PPMS but not RRMS patients, the correlation between serum GFAP and EDSS remains significant after correction for age (Spearman rho = 0.4 and 0.5, p = 0.008 and 0.02 respectively).
Figure 2.
Spearman correlation (rho) between serum glial fibrillary acidic protein (GFAP) and expanded disability status scale (EDSS) in the whole multiple sclerosis (MS), progressive (PMS) and primary progressive (PPMS) patients. **p < 0.01, ***p < 0.001.
NfL
CSF and serum levels of NfL showed a high correlation in the MS patients and controls (Spearman rho = 0.7 and 0.6, p < 0.001 and = 0.008, respectively). In contrary to GFAP, NfL levels did not correlate with age or Qalb, except for a weak correlation between serum NfL and age (Spearman rho = 0.2, p = 0.048). Serum NfL correlated with disease duration (Spearman rho = 0.4, p < 0.001), but not after correction for age.
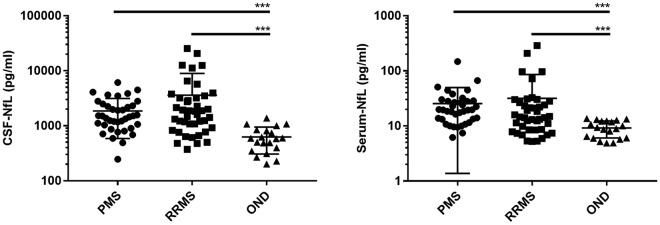
Concentrations of NfL in CSF and serum were higher in PMS and RRMS (p < 0.001 for all comparisons) compared to controls. However, NfL in CSF and serum did not differ between progressive and relapsing MS course (Fig. 3). Correlations with the different clinical parameters are listed in Table 2.
Figure 3.
NfL CSF and serum levels in multiple sclerosis patients and patients with other non-inflammatory neurological diseases (OND). NfL: neurofilament light chain, CSF: cerebrospinal fluid, PMS: progressive multiple sclerosis, RRMS: relapsing-remitting multiple sclerosis. OND: other non-inflammatory neurological diseases. P-values were calculated with Kruskal-Wallis test followed by Dunn’s multiple comparison test. ***p < 0.001.
GFAP in correlation to NfL
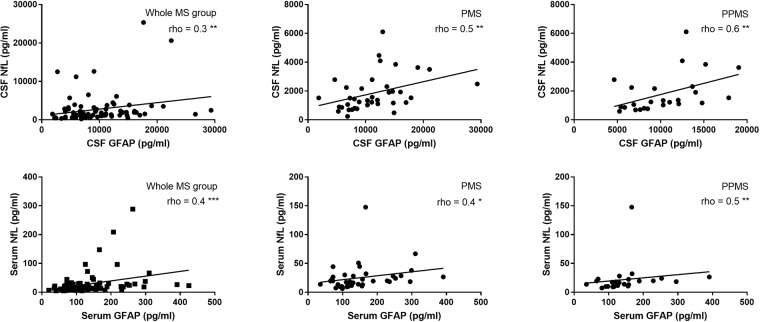
In the whole MS group and in PMS patients, GFAP correlated with NfL in CSF (Spearman rho = 0.3 and 0.5, p = 0.007 and 0.003, respectively) and serum (Spearman rho = 0.4 for both, p < 0.001 and = 0.01, respectively). The correlation was even stronger in PPMS (Spearman rho = 0.6, p = 0.003 for CSF and 0.5, p = 0.007 for serum values) (Fig. 4). In RRMS patients, only serum GFAP and NfL showed a significant correlation (Spearman rho = 0.4, p = 0.01).
Figure 4.
Spearman correlation (rho) between glial fibrillary acidic protein (GFAP) and neurofilaments light (NfL) in cerebrospinal fluid (CSF) and serum in the whole multiple sclerosis (MS), progressive (PMS) and primary progressive (PPMS) patients. *p < 0.05, **p < 0.01, ***p < 0.001.
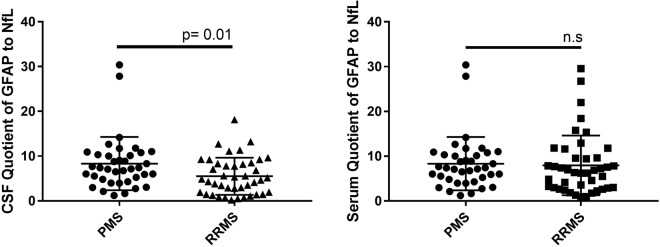
To describe the relation of astroglial impact versus axonal impairment, we introduced a quotient of GFAP to NfL in CSF. This ratio (Q-GFAP/NfL-CSF) yielded significantly higher values for PMS patients (median: 7.3, 25–75 percentile: 4.8–10.3) compared to RRMS patients (median: 4.8, 25–75 percentile: 1.9–8.3) (p < 0.01) (Fig. 5).
Figure 5.
Quotient of glial fibrillary acidic protein (GFAP) and neurofilaments light (NfL) in cerebrospinal fluid (CSF) and serum in progressive multiple sclerosis patients (PMS) compared to relapsing-remitting multiple sclerosis (RRMS). n.s.: not significant.
Imaging correlations
CSF and serum concentration of GFAP was higher in patients with >9 lesions compared to patients with 2–9 lesions (2028 vs 1520 and 133 vs 80.5, p = 0.01 for both). GFAP in CSF and serum were not influenced by the location of the lesions, the presence of gadolinium enhancement (Gd+) or spinal lesions. We found no correlation between NfL in CSF and serum with the MRI parameters.
Discussion
In contrast to the well-established neuroaxonal marker NfL12, data regarding the concentration of astroglial markers in serum are still scarce. This is especially important as there is mounting evidence of an altered astroglial response in MS9,13. In our study, we report elevated levels of GFAP in CSF and serum in MS patients using the SIMOA assay.
Our results from CSF are in agreement with earlier studies using the traditional ELISA4–7,13–19. We report a strong correlation between GFAP in CSF and serum in MS patients as well as controls, as described previously for NfL in CSF and serum using the same method20. The positive correlation between GFAP and age may be explained by the well-described increasing GFAP expression from astrocytes with ageing, which may be mediated through hormonal mechanisms21.
We describe higher levels of serum and CSF GFAP in progressive MS types, but not necessarily in RRMS patients, compared to controls, and their correlation with disease severity. This may emphasise an increasing role of astrogliosis in advanced stages of the disease9,22,23 but not in the early phase. The observed strong correlation between the astroglial marker GFAP with a neuroaxonal damage marker like NfL in CSF and serum in PMS substantiate this assumption. Moreover, the chronic active and inactive lesions dominate in the brain of PMS patients24 with abundant scare forming astrocytes, which are known to inhibit remyelination and axonal regeneration1,25. On the other hand, the reactive astrocytes (RA) found in the predominantly active lesions in RRMS play a dual role; they may increase inflammatory activity by secreting proinflammatory cytokines, cytotoxic factors and recruiting various subtypes of immune cells. However, they may terminate the immune response, support differentiation of regulatory T-Helper cells and promote remyelination1. This dual role of RA may explain the absence of correlation between GFAP and EDSS in RRMS patients.
Beyond the degree of astrogliosis, the different lesion patterns in MS brains vary in the extent of neuroaxonal demise; active lesions exhibit more axonal damage than chronic active or inactive lesions. Hence, a ratio of GFAP to NfL might reflect, at least partially, the predominant lesion pattern and differentiate between PMS to RRMS. The higher values found in PMS patients may be explained by the predominance of the chronic lesions in comparison to RRMS patients.
Interestingly, the correlation of GFAP with the different clinical parameters seems particularly strong and constant in PPMS. Thereupon, it would be interesting to explore a possible role of serum GFAP as a marker of disease severity in patients with PPMS.
Moreover, in our study, the correlation of GFAP with the clinical severity scores was most prominent in serum. GFAP expression occurs mainly on the branches of astrocytes, which also participate in the formation of blood-brain barriers and therefore are in direct contact with the blood vessels26,27. Sofroniew and Vinters et al. reported increased GFAP expression on the astrocyte processes and endfeet in response to inflammation in mice with experimental autoimmune encephalomyelitis26. Taking into consideration the perivascular distribution of most of MS lesions28,29, GFAP might partially drain directly into the blood and not into the CSF.
Nevertheless, caution is warranted while interpreting the meaning of GFAP levels as both neurotoxic A1 and the possibly neuroprotective A2 reactive astrocytes overexpress GFAP30. To the best of our knowledge, while the role of A1 astrocytes is established in neuroinflammation, the presence and role of A2 astrocytes are still to be defined3.
Beyond that, the observed higher CSF and serum NfL concentrations in PMS and RRMS underscore their value as a marker of neuroaxonal demise as reported in various studies18,19,31.
Our findings still have to be confirmed in further prospective studies with larger sample size, follow up samples to explore the temporal evolution of the mentioned biomarkers during and after acute exacerbations, and more detailed MRI protocols addressing further aspects beyond inflammatory lesion load/volumentry such as white and grey matter atrophy and cortical pathology. However, the novelty of the method and the present results might give new insights into the pathophysiology of the progressive MS forms.
Conclusions
In summary, we report elevated levels of GFAP in the serum of MS patients. Correlations between GFAP and NfL, as well as disease severity, might indicate a possible role of astrocytes in the neuroaxonal demise in MS. Since serum levels of GFAP correlate with the EDSS, especially in PPMS patients, it might be a suitable disease severity marker that is easily accessible for follow-up observations. Certainly, further studies are recommended to validate these findings.
Methods
Patient selection
In a prospective study, CSF and serum samples were collected from eighty MS patients attending the department of neurology at the University of Ulm between 2012 and 2017 as a part of the routine diagnostic workup and were included into the biomaterial bank using rigid standard operating procedures (SOPs). In all patients, the diagnosis was revised according to the 2017 revision of the McDonald criteria32. Patients were divided into relapsing-remitting MS, with the LP and serum sampling performed during acute exacerbation (RRMS+), or afterword (RRMS-), PPMS and SPMS. Exacerbations were defined as focal neurological disturbance lasting more than 24 h, without an alternate explanation33. In RRMS+ patients, CSF and serum samples collection were performed before the initiation of methylprednisolone treatment. The clinical severity was measured by assessing the EDSS, multiple sclerosis severity score (MSSS) as well as the ARMSS as reported recently34. The control group consisted of eighteen patients with other non-inflammatory neurological diseases (OND) with normal CSF and magnetic resonance imaging MRI findings (e.g., hypesthesia, dissociative disorder and tension headache).
CSF and serum sampling
CSF and serum samples were taken on the day of presentation and stored according to consensus protocol for the standardisation of CSF collection and biobanking35. Haemolytic CSF specimens were excluded. LP in RRMS+ patients was performed within the first three months after the onset of the exacerbation. Only in three out of 18 RRMS+ -patients, the treatment was initiated after a long latency (42, 60 and 90 days), while the majority of these patients was treated in less than 2 Weeks. We used the CSF-serum quotient for albumin (Qalb)36 as an indicator for the blood-CSF barrier (BCB) function.
GFAP and NfL measurements
Both, GFAP and NfL were measured in CSF and serum using the Simoa technology and GFAP Discovery and NfL Early Access assays (Quanterix Corporation, Lexington, MA, USA). Samples were diluted, as recommended by the manufacturer, and concentrations were calculated using the corresponding standard curve. The intra-assay coefficient of variation (CV) was assessed by measuring a QC serum, and CSF sample in 5 replicates and a CV below 10% was obtained. Only samples that showed a higher signal than the lowest point of the standard curve (1.37 pg/ml for GFAP and 0.69 pg/ml for NfL) were taken into account for the analysis (this was true for all CSF and serum samples).
Magnetic resonance imaging (MRI) scans
MRI scans of the brain and spinal cord were performed on a 1.5 Tesla MRI scanner (Symphony, Siemens, Erlangen, Germany) according to a standard protocol including T1-weighted spin-echo (SE) axial scans with and without application of gadolinium-DTPA (Gd) as well as T2-weighted turbo inversion recovery with frequency-selective fat saturation (FLAIR) coronal scans, where the hyperintense lesions larger than three mm2 were quantified. Scan results were divided into subgroups according to the number of lesions (≤1, 2–9, and >9 lesions), localisation (supra- vs infratentorial) and Gadolinium (Gd) enhancement (Gd+ vs Gd−). The scans were analysed by a rater blinded to the CSF and serum analysis results.
Statistical methods
All statistical tests were performed using SPSS® Statistics version 25 (IBM Corporation, Armonk, NY, USA). The Shapiro-Wilk test was used to examine the distribution of the data. Mann-Whitney U-test was used to compare medians in skewed distributed parameters. A general linear model was applied to account for a possible confounding bias caused by the strong correlation between GFAP levels and age. The Spearman’s rho test was used to measure correlation. A p-value ≤ 0.05 was considered as statistically significant. Figures were made using GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA).
Ethical approval
The study was reviewed by the ethics committee of the University of Ulm and all experimental protocols were approved (approval number 20/10). Our study was performed in accordance with the ethical standards the 1964 Declaration of Helsinki. Written informed consent for the study participation was obtained from all patients participating in this study.
Acknowledgements
We would like to thank Benjamin Mayer for his assistance with the statistical methods.
Author Contributions
M.O., H.T. study concept. A.A., A.H. data acquisition. A.A., A.H. data analysis and interpretation. A.A., A.H. drafting of the manuscript. M.O., H.T., J.K. Study supervision and critical revision. All authors critically reviewed and approved the manuscript.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A. Abdelhak and A. Huss contributed equally.
H. Tumani and M. Otto jointly supervised this work.
Change history
6/5/2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
- 1.Correale J, Farez MF. The Role of Astrocytes in Multiple Sclerosis Progression. Front. Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassmann H. Multiple sclerosis: lessons from molecular neuropathology. Exp. Neurol. 2014;262(Pt A):2–7. doi: 10.1016/j.expneurol.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petzold A, et al. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain: a journal of neurology. 2002;125:1462–1473. doi: 10.1093/brain/awf165. [DOI] [PubMed] [Google Scholar]
- 5.Axelsson M, et al. Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. Journal of neurology. 2011;258:882–888. doi: 10.1007/s00415-010-5863-2. [DOI] [PubMed] [Google Scholar]
- 6.Martinez MA, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Multiple sclerosis. 2015;21:550–561. doi: 10.1177/1352458514549397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mane-Martinez MA, et al. Glial and neuronal markers in cerebrospinal fluid in different types of multiple sclerosis. Journal of neuroimmunology. 2016;299:112–117. doi: 10.1016/j.jneuroim.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Kassubek R, et al. GFAP in early multiple sclerosis: A biomarker for inflammation. Neurosci Lett. 2017;657:166–170. doi: 10.1016/j.neulet.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Linker RA, et al. Proteome profiling in murine models of multiple sclerosis: identification of stage specific markers and culprits for tissue damage. PloS one. 2009;4:e7624. doi: 10.1371/journal.pone.0007624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avsar T, et al. Protein biomarkers for multiple sclerosis: semi-quantitative analysis of cerebrospinal fluid candidate protein biomarkers in different forms of multiple sclerosis. Multiple sclerosis. 2012;18:1081–1091. doi: 10.1177/1352458511433303. [DOI] [PubMed] [Google Scholar]
- 11.Bogoslovsky T, et al. Increases of Plasma Levels of Glial Fibrillary Acidic Protein, Tau, and Amyloid beta up to 90 Days after Traumatic Brain Injury. J. Neurotrauma. 2017;34:66–73. doi: 10.1089/neu.2015.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelhak A, et al. Brain-Specific Cytoskeletal Damage Markers in Cerebrospinal Fluid: Is There a Common Pattern between Amyotrophic Lateral Sclerosis and Primary Progressive Multiple Sclerosis? In.t J. Mol. Sci. 2015;16:17565–17588. doi: 10.3390/ijms160817565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norgren N, et al. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63:1586–1590. doi: 10.1212/01.WNL.0000142988.49341.D1. [DOI] [PubMed] [Google Scholar]
- 14.Rosengren LE, Lycke J, Andersen O. Glial fibrillary acidic protein in CSF of multiple sclerosis patients: relation to neurological deficit. Journal of the neurological sciences. 1995;133:61–65. doi: 10.1016/0022-510X(95)00152-R. [DOI] [PubMed] [Google Scholar]
- 15.Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J. Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology. 2003;61:1720–1725. doi: 10.1212/01.WNL.0000098880.19793.B6. [DOI] [PubMed] [Google Scholar]
- 16.Madeddu R, et al. Cytoskeletal proteins in the cerebrospinal fluid as biomarker of multiple sclerosis. Neurol. Sci. 2013;34:181–186. doi: 10.1007/s10072-012-0974-4. [DOI] [PubMed] [Google Scholar]
- 17.Burman J, et al. Assessing tissue damage in multiple sclerosis: a biomarker approach. Acta Neurol Scand. 2014;130:81–89. doi: 10.1111/ane.12239. [DOI] [PubMed] [Google Scholar]
- 18.Disanto G, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Annals of neurology. 2017 doi: 10.1002/ana.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhle J, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology. 2017;88:826–831. doi: 10.1212/WNL.0000000000003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhle J, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016;54:1655–1661. doi: 10.1515/cclm-2015-1195. [DOI] [PubMed] [Google Scholar]
- 21.Porchet R, et al. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer’s disease. Proteomics. 2003;3:1476–1485. doi: 10.1002/pmic.200300456. [DOI] [PubMed] [Google Scholar]
- 22.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. The Lancet. Neurology. 2015;14:183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 23.Abdelhak A, Weber MS, Tumani H. Primary Progressive Multiple Sclerosis: Putting Together the Puzzle. Front Neurol. 2017;8:234. doi: 10.3389/fneur.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: pathology and pathogenesis. Nature reviews. Neurology. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 25.Holley JE, Gveric D, Newcombe J, Cuzner ML, Gutowski NJ. Astrocyte characterization in the multiple sclerosis glial scar. Neuropathology and applied neurobiology. 2003;29:434–444. doi: 10.1046/j.1365-2990.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 26.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002;200:629–638. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fog T. On the Vessel-Plaque Relationships in the Brain in Multiple Sclerosis. Acta. Neurol. Scand. Suppl. 1964;40(SUPPL 10):19–15. [PubMed] [Google Scholar]
- 29.Tallantyre EC, et al. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology. 2008;70:2076–2078. doi: 10.1212/01.wnl.0000313377.49555.2e. [DOI] [PubMed] [Google Scholar]
- 30.Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957–967. doi: 10.1016/j.immuni.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Novakova L, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology. 2017;89:2230–2237. doi: 10.1212/WNL.0000000000004683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson AJ, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. The Lancet. Neurology. 2017 doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 33.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manouchehrinia A, et al. Age Related Multiple Sclerosis Severity Score: Disability ranked by age. Multiple sclerosis. 2017;23:1938–1946. doi: 10.1177/1352458517690618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teunissen CE, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73:1914–1922. doi: 10.1212/WNL.0b013e3181c47cc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reiber H, Otto M, Trendelenburg C, Wormek A. Reporting cerebrospinal fluid data: knowledge base and interpretation software. Clin. Chem. Lab. Med. 2001;39:324–332. doi: 10.1515/CCLM.2001.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.