Abstract
Background
Organ failure determines outcome in acute pancreatitis (AP). It is controversial if infected pancreatic necrosis (IPN) is also an independent determinant of mortality. We hypothesized that the predictors of mortality in AP might have changed with advances in management and consequent decline in mortality over the past decades. Our objective was to study the predictors of mortality in patients with AP.
Methods
Consecutive patients with a first episode of AP hospitalized from January 2015 to December 2016 were included in an observational study. Patients with IPN were treated with a conservative first approach followed by intervention. Necrosectomy, if required, was delayed beyond 4 weeks and done primarily employing minimally invasive techniques. The primary outcome measure was independent predictors of in-hospital mortality.
Results
Of 209 patients with AP, 81 (39%) had persistent organ failure (OF) and 108 (52%) developed IPN. Overall, 46/209 (22%) patients died. Independent predictors of mortality were OF (odds ratio [OR]19; 95% CI: 6.1–58.8), and IPN due to infection with multidrug resistant (MDR) organisms (OR: 8.4; 95% CI:3.1–22.5). Infected pancreatic necrosis by itself was not found to be a significant predictor of mortality (OR 2; 95% CI: 0.4–9.5).
Conclusion
Persistent OF and complicated IPN due to MDR infection were independent predictors of mortality in patients with AP. Renewed efforts to prevent MDR infection with antibiotic stewardship and strategies for early control of sepsis are urgently required.
Introduction
Acute pancreatitis (AP) is a potentially fatal disease with a reported incidence varying from 12 to 38 per 100,000 population1,2. AP could either be interstitial or complicated by pancreatic and/or peri-pancreatic necrosis. Necrotic tissue and associated fluid collection(s), though initially sterile, may become infected in up to 40% of cases3. AP has a variable course of illness with most patients having a mild self-limiting disease. The mortality in AP ranges from 4 to 25%4–6. There are 2 peaks of severity of AP; an early peak associated with up to 50% of all deaths during first 2 weeks of illness and a late peak due to infected necrosis-induced sepsis7. The factors underlying the differences in clinical course and outcome in patients with AP need to be identified for reducing the mortality. In this regard, IPN and organ failure have been considered as important determinants of outcome8. Previous studies, including from our center, have shown IPN as an independent predictor of outcome in AP4,8. However, the management of AP has evolved over the past decades with primary conservative and minimally invasive approaches preferred over open surgical necrosectomy9–11. We have shown that severity adjusted mortality has decreased over time with a change in management approach12. Whether or not the predictors of outcome have changed in view of the evolving treatment strategies is, however, not clear. In the present study, our objective was to examine the predictors of mortality in patients with AP who were managed with the current standard of care.
Methods
Patients
In this prospective, single-center observational cohort study, all consecutive patients hospitalized to our center with a first episode of AP from January 2015 to December 2016 were included. We excluded patients with chronic pancreatitis, recurrent acute pancreatitis, those with onset of AP 3 months before hospitalization and those refusing consent. Institutional ethical approval was obtained (AIIMS: IESC/T-253).
Definitions
Acute pancreatitis
AP was diagnosed if two of the following three criteria were present: acute abdominal pain, serum amylase and/or lipase activity > 3 times the upper limit of normal, and characteristic findings of AP on trans-abdominal ultrasonography (USG)/computed tomography (CT) scan/magnetic resonance imaging (MRI) of the abdomen.
Severity of acute pancreatitis
The severity of AP was defined as per the revised Atlanta classification13 as follows:
Mild acute pancreatitis: if there was no organ failure, and no local or systemic complications.
Moderately severe acute pancreatitis: if there was transient organ failure and/or local complications.
Severe acute pancreatitis: if there was persistent organ failure (>48 h) with a score of 2 or more using the modified Marshall scoring system14.
Early severe acute pancreatitis (ESAP) was defined as development of organ failure within 7 days of onset of acute pancreatitis5.
Charlson co-morbidity index15 was used to record and stratify co-morbidities.
Pancreatic necrosis
Pancreatic necrosis was diagnosed as non-enhancing areas of the pancreas on a contrast enhanced CT (CECT) scan. The amount of pancreatic necrosis was graded as <30%, 30-50% and >50%. A CT severity score was calculated according to Balthazar et al16.
IPN was suspected when a patient with necrotizing pancreatitis developed persistent fever of >38 °C beyond the first week of illness without any other focus of sepsis along with leukocytosis and deterioration in clinical condition. The diagnosis of IPN was confirmed when pancreatic necrotic tissue/fluid showed presence of bacteria on culture or if there was presence of extra-intestinal air in the pancreatic bed on abdominal CT scan. Culture sensitivity of the organism from the sample collected during the first intervention procedure is being reported.
Multidrug-resistant (MDR) infection was defined if the organism was resistant to at least one antimicrobial agent in three or more antimicrobial categories. Methicillin resistant staphylococcus aureus was considered MDR irrespective of resistance to other antimicrobials.
Extensively drug-resistant organisms (XDR) was defined when the organism was resistant to at least one antimicrobial agent in all except 2 antimicrobial categories which are considered effective against it17. The details of culture techniques are described in supplementary appendix.
Extra-pancreatic infection
Extra-pancreatic infection was defined as documented infection at any extra-pancreatic site such as pneumonia, urinary tract infection, and cholangitis during hospitalization.
Laboratory investigations
Standard lab investigations were done in all subjects that included complete blood count, liver and renal function tests, electrolytes, blood gases and pH, USG of the abdomen and a chest X-ray. A CECT scan of the abdomen was done primarily to assess the severity and complications of pancreatitis as and when clinically indicated.
Management of patients
All patients were managed according to a predefined management protocol10. They were treated conservatively with analgesics, intravenous fluids, and supportive treatment. Antibiotics were prescribed if: (a) patients had infected necrosis, (b) there was documented infection at extra-pancreatic sites such as cholangitis, and (c) patients had signs of sepsis in the form of fever and leukocytosis even in the absence of documented infection, provided the fever persisted for more than 2 days. The antibiotics chosen were according to the culture and sensitivity report whenever available. In the absence of a sensitivity report, a broad-spectrum antibiotic was used. Patients with clinically severe acute pancreatitis were treated in an intensive care unit with all possible organ support systems, including ventilatory support, vasopressors, and dialysis as and when required. Those with sterile necrosis were continued on conservative management. Patients with IPN were treated with a conservative first approach followed by percutaneous drainage (PCD) of the collections9. If there was no improvement in sepsis, necrosectomy was done. It was delayed at least beyond 4 weeks and achieved preferably by a minimally invasive technique either by video-assisted retroperitoneal debridement or endoscopic necrosectomy18. The decision about the choice of therapy was taken in consultation with a multi-disciplinary team comprising of a gastroenterologist, radiologist and gastrointestinal surgeon.
Statistical analysis
The baseline data were recorded as mean + /- SD or median (range) as appropriate. Chi square test was used to compare the 2 groups for categorical variables and Student’s t test was used for continuous variables with normal distribution while Wilcoxon-Mann–Whitney U test was used for continuous variables without normal distribution. A p value of <0.05 was considered statistically significant. Univariable and multivariable regression analyses were done with mortality as the dependent variable and predefined prognostic factors as independent variables, which included age, sex, etiology, Charlson co-morbidity index, APACHE II score at admission, referral status, prophylactic antibiotic use, delay in admission, intervention prior to transfer, persistent OF, modified CT severity index (CTSI), infected pancreatic necrosis, infection due to MDR organism, fungal infection, extra-pancreatic infection, air in collection prior to intervention and type of collection (acute necrotic collection vs. walled off necrosis).Variables with a p value <0.1 on univariable analysis were considered for multivariable analysis. Odds ratio (OR) with 95% confidence interval (95% CI) was calculated. A CECT scan was not done in 70 patients. For the purpose of analysis, 43 of these 70 patients with mild AP were assumed to have interstitial pancreatitis and 27 patients with severe AP were excluded from the analysis. Receiver operating characteristic (ROC) curve was plotted between sensitivity and 1- specificity for prediction of outcome by variables.
Results
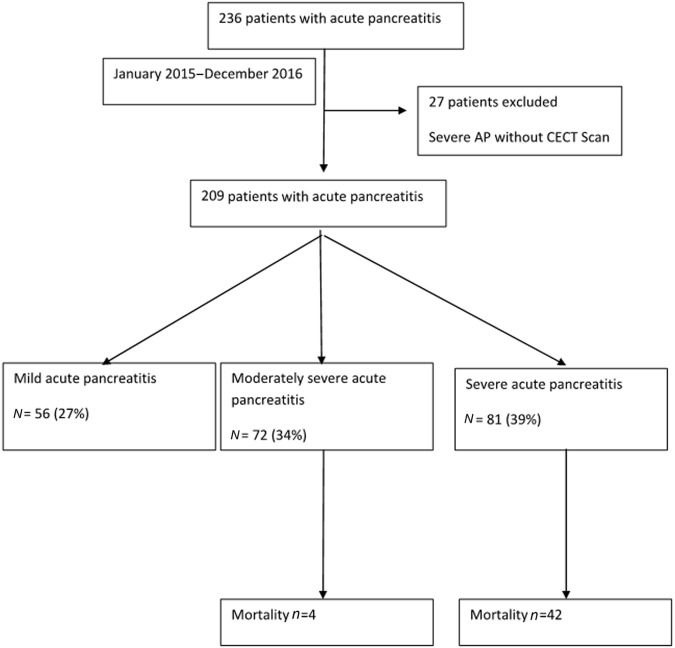
A total of 236 patients with AP were hospitalized to our institution from January 2015 to December 2016; 27 patients with severe AP who did not undergo a CECT scan were excluded from the analysis, and thus 209 patients formed the study cohort (Fig. 1).
Fig. 1.
Flow chart of study population
Baseline demographic characteristics
The mean age of our patients was 40.2 ± 14.1 years; 61% were male (Table 1). There was no difference in age and sex between survivors and non-survivors. 145/209 (69%) of our patients were referred from other hospitals with a median delay of 8 (1–90) days.
Table 1.
Comparison of baseline demographic, clinical and laboratory characteristics between survivors and non-survivors
| Variable | Total (n = 209) | Survivors (n = 163) | Non-survivors (n = 46) | p value |
|---|---|---|---|---|
| Age (year) | 40.2 ± 14.1 | 39.5 ± 13.6 | 42.8 ± 15.8 | 0.20 |
| Male | 124 (59) | 99 (61) | 25 (54) | 0.4 |
| Etiology | 0.5 | |||
| Biliary | 107 (51) | 79 (49) | 28 (61) | |
| Alcohol | 50 (24) | 39 (24) | 11 (24) | |
| Idiopathic | 33 (16) | 26 (16) | 7 (15) | |
| Others | 19 (9) | 19 (11) | 0 | |
| Referred patient | 144 (69) | 101 (62) | 43 (94) | 0.001 |
| Delay in presentation (days) | 8 (0–90) | 6 (0–90) | 17 (2–90) | 0.001 |
| Intervention prior to referral (number of patients) | 10 (7) | 6 (6) | 4 (9) | 0.4 |
| CCI > 2 | 24 (11) | 11 (7) | 13 (28) | 0.001 |
| Prophylactic antibiotic use | 65 (31) | 43 (26) | 22 (48) | 0.001 |
| APACHE II at admission | 6 (0–28) | 6 (0–19) | 11 (3–28) | 0.001 |
| Marshall score at admission | 0 (0–7) | 0 (0–5) | 3 (0–7) | 0.001 |
| Modified CTSI | 8 (0–10) | 8 (0–10) | 10 (4–10) | 0.001 |
| Necrosis | 112 (54) | 75 (46) | 37 (80.5) | 0.001 |
| Persistent OF | 81 (39) | 39 (24) | 42 (91) | 0.001 |
| Early OF | 58 (28) | 33 (20) | 25 (54.3) | 0.001 |
| IPN | 108 (52) | 69 (42) | 39 (85) | 0.001 |
| MDR organism | 80 (38) | 41 (25) | 39 (85) | 0.001 |
| XDR organism | 52 (25) | 25 (15) | 27 (59) | 0.001 |
| Fungal infection | 27 (13) | 14 (9) | 13 (28) | 0.001 |
| Extra-pancreatic infection | 29 (14) | 17 (11) | 12 (26) | 0.01 |
| Air in collection prior to interventiona | 32 (24) | 27 (30) | 5 (12) | 0.03 |
| Need for any intervention | 93 (45) | 54 (33) | 39 (85) | 0.001 |
| Surgical intervention | 28 (13) | 12 (7) | 16 (35) | 0.001 |
| Walled off necrosisa | 54 (41) | 40 (44) | 14 (34) | 0.3 |
| Duration of hospital stay (day) | 14 (3–120) | 12 (3–120) | 27 (5–100) | 0.001 |
Values as mean ± SD, median (range), and n (%) as appropriate
CCI Charlson co morbidity index, MDR multidrug resistant, OF organ failure, IPN infected pancreatic necrosis, CTSI CT severity index
aCollection present in 133 patients
The most common etiology of AP was gallstones in 51% followed by alcohol in 24%, idiopathic in 16% and miscellaneous in 9% of patients. There was no difference in the etiology of AP between survivors and non-survivors.
Severity of AP
Of the 209 patients, 56 (27%) had mild pancreatitis, 72 (34%) had moderately severe acute pancreatitis and 81 (39%) had severe AP. Among patients with severe AP, 58 (72%) had early severe AP (ESAP) and 23 (28%) had late OF associated with IPN. The median APACHE II score was 6 (0–28) and median Marshall score was 0 (0–7) at admission. Twenty-four (11%) patients had Charlson co-morbidity index (corrected for age) of >2.
Pancreatic necrosis
A CECT of the abdomen was done in 166 (80%) patients. Of these, 143 (86%) had necrotizing pancreatitis. In necrotizing pancreatitis subgroup, < 30%, 30–50%, and >50% pancreatic necrosis was present in 31 (22%), 36 (25%), 76 (53%) patients, respectively and the median modified CTSI was 8 (0–10).
Infected pancreatic necrosis
IPN developed in 108/209 (52%) patients in our cohort. Extra-pancreatic infections were seen in 14% (29/209) of patients with chest infection being the most common (25/29, 86%) followed by urinary tract infection (2/29), cholangitis (1/29) and infected ascites (1/29).
Microbiological profile of patients with IPN
Eighty-seven out of 108 (81%) patients had culture positive IPN and 86% of cultures grew MDR organism(s). Fungal infection was present in 13% (27/209) of patients. Seventy-five out of 87 (86%) patients had Gram-negative bacteria. Poly-microbial infection was seen in 44/87 (51%) patients. The most common Gram-negative bacterium was Escherichia coli in 48/149 (32%) isolates and most common Gram-positive bacterium was Enterococcus faecium in 10/149 (7%) isolates (Table 2). 64 out of 149 (42%) isolates were XDR isolates in 58/209 (25%) patients. Patients who died had a higher rate of XDR organisms compared to those who survived (59% vs. 15%, p = 0.001). Of the 144 patients referred to our center, 65 (44%) had received prophylactic antibiotics in the 1st week of illness; 97 of the 144 patients developed IPN. Among those who received prophylactic antibiotics, the rate of isolation of MDR organisms was not significantly different from those who did not receive prophylactic antibiotics (70% vs. 66%) (p = 0.5). Only 10 patients had an intervention in the form of percutaneous drainage prior to referral which did not influence the development of MDR infection. The proportion of MDR organisms in patients who underwent PCD ≤ 4 weeks and those > 4 weeks after the onset of AP was similar i.e. 43/50 (61%) vs. 28/38 (73%), respectively (p = 0.2).
Table 2.
Microbiology profile of organisms in infected pancreatic necrosis
| Organisms | Number of isolates (n = 149) | Multidrug-resistant isolates (n = 127) |
|---|---|---|
| Gram negative | ||
| Escherichia coli | 49 | 38 |
| Acinetobacter baumannii | 28 | 25 |
| Klebsiella pneumonia | 27 | 24 |
| Pseudomonas aeruginosa | 17 | 14 |
| Enterobacterspp. | 10 | 10 |
| Proteus spp. | 3 | 3 |
| Citrobacterspp. | 1 | 1 |
| Klebsiella oxytoca | 1 | 1 |
| Gram positive | ||
| Enterococcus faecium | 10 | 8 |
| Enterococcus faecalis | 1 | 1 |
| Staphylococcus aureus | 2 | 2 |
Management of patients with IPN
Of the 108 patients who developed IPN, 15 (14%) were managed conservatively with antibiotics alone, 37 (34%) were treated with antibiotics plus PCD, 34 (32%) underwent percutaneous endoscopic necrosectomy (PEN) after PCD, one underwent per-oral endoscopic necrosectomy, and 27 (25%) underwent surgical necrosectomy – 16 after PCD alone, 6 after PEN and 5 direct surgery. Two patients underwent surgery for complications: one for colonic perforation and another for endoscopic cystogastrostomy induced bleeding. The mean time to first intervention i.e. PCD was 32 ± 23 days and the mean time to next intervention (necrosectomy) was 55 ± 27 days from the onset of AP.
Mortality
The overall mortality was 22%. All the 56 patients with mild AP survived, 4 of 72 (5%) patients with MSAP died and 42 of the 81(52%) patients with severe AP died (p = 0.001). Among patients with severe AP, patients with ESAP had a mortality of 43%. Of these patients with ESAP, 46 (79%) had single OF, 12 (21%) had 2 OF with higher mortality in those with multiple OF. There was no difference among patients outcome according to the type of OF. Among 15 patients with IPN managed conservatively, all survived; 19 of 37 patients treated with antibiotics and PCD died; they did not undergo necrosectomy because either they were too sick with persistent organ failure and/or had no major drainable collections. Five patients died after PEN and 15 patients died after surgery.
Of the 209 patients, 45 (22%) with ESAP and IPN (the so-called critical AP) had a mortality of 47% (21/45 patients), which was similar to the mortality in patients with ESAP alone (4/13, 30.7%). There was a trend towards a higher mortality among patients with infected acute necrotic collections (ANC) (42%, 26/62) compared with infected walled off necrosis (33%, 13/46) but the difference was not statistically significant [p = 0.18].
Primary outcome measure: Predictors of mortality
Univariable analysis
On univariable analysis, patient referred from other institutes, delay in presentation from onset of AP, prophylactic antibiotic use,Charlson co-morbidity index >2, APACHE II at admission of >9, persistent OF, modified CTSI > 8, pancreatic necrosis > 30%, air in the collection prior to intervention, infected pancreatic necrosis, infection due to MDR organism, infection due to XDR organisms, fungal infection and extra-pancreatic infection were predictors of mortality. CCI > 2 was present in only 13% of our patients. Since the co-morbidities were already included in the APACHE II score, CCI was not considered in the multivariable analysis.Variables found significant on univariable analysis (p < 0.1) i.e., referral status of patient, prophylactic antibiotic use, OF, IPN, air in collection prior to intervention, extra-pancreatic infection, MDR organism, fungal infection, were included in multivariable analysis. We did not include delay in presentation, APACHE II, early OF, CTSI, infection with XDR organisms, presence of pancreatic necrosis, surgical intervention and need for any intervention due to multi-collinearity.
Multivariable analysis
On step wise regression analysis, only persistent OF (OR 19; 95% CI: 6.1–59.8) and IPN complicated by MDR organisms (OR 8.4; 95% CI: 3.1–22.5) were independent predictors of mortality (Table 3). IPN per se did not turn out to be an independent predictor of mortality (OR 2; 95% CI: 0.4–9.5).
Table 3.
Multivariable analysis for independent predictors of mortality
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Persistent OF | 33.3 (10–100) | 0.001 | 19 (6.1– 59.8) | 0.001 |
| MDR organism(s) | 16.7 (6.9–40) | 0.001 | 8.4 (3.1–22.5) | 0.001 |
| Prophylactic antibiotic use | 2.6 (1.3–5) | 0.001 | 1.7 (0.6–4.7) | 0.3 |
| Fungal infection | 4.2 (1.8–9.7) | 0.001 | 1.2 (0.4–3.9) | 0.7 |
| Extra-pancreatic infection | 3 (1.3–6.9) | 0.01 | 1.1 (0.4–3.5) | 0.8 |
| IPN | 7.5 (3.2–17.8) | 0.001 | 2 (0.4–9.5) | 0.4 |
| Referred patients | 8.5 (2.6–28.6) | 0.001 | 2.2 (0.4–10.8) | 0.4 |
| Air in collection prior to intervention | 0.3 (0.1–0.8) | 0.01 | 0.3 (0.1–1.1) | 0.1 |
OF organ failure, MDR multidrug resistant, IPN infected pancreatic necrosis, OR odds ratio, CI confidence interval
Discussion
Acute pancreatitis is associated with significant morbidity and mortality particularly in patients with severe acute pancreatitis5. According to the revised Atlanta classification13, presence of organ failure defines severe AP. Organ failure is thus considered a sine quo non of severity. Two major determinants of mortality in acute pancreatitis are organ failure and infected pancreatic necrosis (IPN). The revised Atlanta classification does not consider infected necrosis alone as a criterion for severe AP. The management of AP has undergone a significant change over the past several years. In particular, the management of infected pancreatic necrosis has evolved from surgical necrosectomy in all patients to conservative first approach that entails a step-up treatment involving antibiotics, percutaneous drainage and only if required, necrosectomy9,10. With the changing management strategy, the outcome of AP is improving. We have previously shown a reduction in severity adjusted mortality over a time period of 16 years12. The major changes in the management of AP have been in the treatment approach to IPN. The practice of early surgery, open surgical necrosectomy and multiple operations has largely been replaced by a delayed and minimally invasive approach. It is therefore important to understand if IPN still remains a major determinant of outcome.
The present prospective study has shown that the most important independent predictors of mortality are –persistent OF and IPN complicated by MDR organisms. Early-onset OF develops due to an exaggerated proinflammatory host response to injury, leading to a high mortality. Early severe acute pancreatitis (ESAP) develops at a time when the local injury is evolving and pancreatic necrosis is setting in. We and others have shown that patients with ESAP, particularly those with fulminant pancreatitis, have a high mortality early in the course of AP5,19. Although improvements in critical care might have resulted in some reduction in mortality, the outcome of patients with multi-organ failure still remains poor. Until targeted therapy aimed at controlling severe systemic inflammation is developed, significant reduction in mortality is unlikely with supportive care alone.
Necrotizing pancreatitis is known to be associated with a worse outcome compared with interstitial pancreatitis20. The extent of necrosis has been correlated with organ failure and mortality. In a previous study, all of the 37 patients with organ failure had necrosis on CECT scan4. In the present study too, of the 58 patients with organ failure who had a CECT done, all had necrosis. Pancreatic and extra-pancreatic necrosis is not only a marker of the severity of the local injury, it also predisposes to further complications such as infection, pseudoaneurysm, and bowel fistula. Furthermore, it has now been shown to worsen systemic inflammation and organ failure due to release of unsaturated fatty acids21. The present study has shown that complicated IPN was a major determinant of outcome. Surprisingly, IPN itself did not come out as an independent predictor of mortality in the present study unlike previous data from our own and other centers4,8. However, a few other recent studies have also not found IPN as an independent predictor of mortality in AP20,22. Infection with MDR organisms in IPN turned out to be an important factor for mortality. This may look contradictory at first but on closer look it reflects the reality of the present day management of patients with IPN. It has been shown that a conservative first strategy with supportive treatment, antibiotics, and percutaneous drainage resulted in improved outcome of patients with IPN9–11. In the present study also, 15 patients with either confirmed or suspected IPN could be treated successfully with antibiotics alone and 50% of patients who underwent PCD improved without additional treatment. In addition, minimally invasive treatment in the form of percutaneous endoscopic necrosectomy led to satisfactory results in patients requiring necrosectomy. These measures have improved the prognosis of patients with IPN. We believe that IPN per se did not come out as a predictor of mortality due to conservative first and minimally invasive approach. On the other hand, infection with MDR organism emerged as an important factor, leading to what we have termed as ‘complicated IPN’. Infection with MDR organisms is a growing threat and there is an urgent need to curb its menace. Increasing use of prophylactic antibiotics such as carbipenem and third generation cephalosporins has resulted in more resistant infections. In fact, 65 out of 144 (44%) patients had received prophylactic antibiotics before referral to our hospital. The proportion of patients with resistant infections is increasing over the years and there is an alarming increase in community acquired MDR strain in both developing23–25 and developed countries26. Late referral to a tertiary care center is another factor that compounds the problem.
Lee et al.27 found MDR isolates in 29/46 (63%) patients with IPN without any significant difference in mortality in those with MDR infection than those without it. Similar to our findings, a recent study from our institution which looked at the microbiological profile in pancreatic and extra-pancreatic infections among patients with AP found MDR infection in 164/189 (87%) patients with IPN and XDR infection in 94/189 (49%) of isolates28. The objective of the present study was to examine the predictors of mortality in patients with AP who were managed with the current standard of care and MDR infection was found to be an independent predictor of mortality.
The difference between the present study and the prior studies is that we demonstrate for the first time that it is the infection with MDR organisms and not IPN per se that is an independent predictor of mortality in our patients. This finding has important implication for the management of patients with IPN.
There are some limitations of our study. The duration of antibiotic exposure could have influenced the emergence of MDR organisms. Most patients with acute necrotizing pancreatitis receive prophylactic or therapeutic antibiotics. We had shown previously that 67% of patients had received prophylactic antibiotics in a multi-center study which was similar to reports from other centers29,30. In addition, all patients with suspected or proven infected collections receive antibiotics. Since the majority of patients were referred to our tertiary care center after a delay, we do not have precise duration of antibiotic exposure from onset of pancreatitis to drainage. Another reason for emergence of MDR organisms could be inadequate source control in our patients resulting in prolonged exposure to antibiotics. Although PCD was done as early as required and necrosectomy was undertaken as dictated clinically, it is possible that our approach was not as effective as would have been ideal. An important reason for the sub-optimal outcome however could be late referral from other hospitals.
Thus, we conclude that persistent OF and ‘complicated IPN’ due to infection with MDR organism were independent predictors of mortality in patients with AP. Renewed efforts to prevent MDR infection and use of antibiotic stewardship programs are of paramount importance if mortality from this condition is to be reduced further.
Study Highlights
What is current knowledge?
✓Organ failure is a determinant of outcome in acute pancreatitis.
✓Infected pancreatic necrosis alone as a determinant of mortality in acute pancreatitis is controversial.
✓It is unclear whether predictors of mortality in acute pancreatitis have changed with evolving management strategies.
What are the new findings
✓Persistent organ failure is an independent predictor of mortality in AP
✓Infected pancreatic necrosis complicated by MDR organisms was found to be an independent predictor of mortality.
✓Infected pancreatic necrosis per se was not found to be an independent predictor of mortality in acute pancreatitis.
Electronic supplementary material
Acknowledgements
We are grateful to our patients, colleagues, nursing, pharmacy, and clerical staff, and allied professionals who collectively supported our gastroenterology ward and ICU, and enabled the study to be performed.
Conflict of Interest
Guarantor of article: Pramod Kumar Garg, MD, DM.
Specific author contributions: Saransh Jain: Acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision and final approval of the manuscript. Soumya Jagannath Mahapatra: acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision and final approval of the manuscript. Swatantra Gupta: acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision and final approval of the manuscript. Shalimar: Critical revision and final approval of the manuscript. Pramod Kumar Garg: Study concept and design, analysis and interpretation of data; study supervision, drafting of the manuscript, critical revision and final approval of the manuscript.
Financial support: None.
Potential competing interests: None.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
The online version of this article (10.1038/s41424-018-0056-x) contains supplementary material, which is available to authorized users.
References
- 1.Frey CF, et al. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994-2001. Pancreas. 2006;33:336–344. doi: 10.1097/01.mpa.0000236727.16370.99. [DOI] [PubMed] [Google Scholar]
- 2.Eland IA, et al. Incidence and mortality of acute pancreatitis between 1985 and 1995. Scand. J. Gastroenterol. 2000;35:1110–1116. doi: 10.1080/003655200451261. [DOI] [PubMed] [Google Scholar]
- 3.Uhl W, et al. IAP Guidelines for the surgical management of acute pancreatitis. Pancreatology. 2002;2:565–573. doi: 10.1159/000067684. [DOI] [PubMed] [Google Scholar]
- 4.Garg PK, et al. Association of extent and infection of pancreatic necrosis with organ failure and death in acute necrotizing pancreatitis. Off. Clin. Gastroenterol. Hepatol. 2005;3:159–166. doi: 10.1016/S1542-3565(04)00665-2. [DOI] [PubMed] [Google Scholar]
- 5.Sharma M, Banerjee D, Garg PK. Characterization of newer subgroups of fulminant and subfulminant pancreatitis associated with a high early mortality. Am. J. Gastroenterol. 2007;102:2688–2695. doi: 10.1111/j.1572-0241.2007.01446.x. [DOI] [PubMed] [Google Scholar]
- 6.Bota S, et al. Predictive factors for severe evolution in acute pancreatitis and a new score for predicting a severe outcome. Ann. Gastroenterol. 2013;26:156–162. [PMC free article] [PubMed] [Google Scholar]
- 7.Carnovale A, et al. Mortality in acute pancreatitis: is it an early or a late event? JOP. 2005;6:438–444. [PubMed] [Google Scholar]
- 8.Petrov MS, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. 2010;139:813–820. doi: 10.1053/j.gastro.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Mouli VP, Sreenivas V, Garg PK. Efficacy of conservative treatment, without necrosectomy, for infected pancreatic necrosis: a systematic review and meta-analysis. Gastroenterology. 2013;144:333–340.e2. doi: 10.1053/j.gastro.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Garg PK, et al. Primary conservative treatment results in mortality comparable to surgery in patients with infected pancreatic necrosis. Clin. Gastroenterol. Hepatol. 2010;8:1089–1094.e2. doi: 10.1016/j.cgh.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Santvoort HC, et al. A step-up approach or open necrosectomy for necrotizing pancreatitis. N. Engl. J. Med. 2010;362:1491–1502. doi: 10.1056/NEJMoa0908821. [DOI] [PubMed] [Google Scholar]
- 12.Agarwal S, et al. Reduction in mortality in severe acute pancreatitis: A time trend analysis over 16 years. Pancreatology. 2016;16:194–199. doi: 10.1016/j.pan.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Banks PA, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 14.Marshall JC, et al. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit. Care Med. 1995;23:1638–1652. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 16.Balthazar EJ, et al. Acute pancreatitis: prognostic value of CT. Radiology. 1985;156:767–772. doi: 10.1148/radiology.156.3.4023241. [DOI] [PubMed] [Google Scholar]
- 17.Magiorakos AP, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 18.Dhingra R, et al. Single or multiport percutaneous endoscopic necrosectomy performed with the patient under conscious sedation is a safe and effective treatment for infected pancreatic necrosis (with video) Gastrointest. Endosc. 2015;81:351–359. doi: 10.1016/j.gie.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 19.Isenmann R, Rau B, Beger HG. Bacterial infection and extent of necrosis are determinants of organ failure in patients with acute necrotizing pancreatitis. Br. J. Surg. 1999;86:1020–1024. doi: 10.1046/j.1365-2168.1999.01176.x. [DOI] [PubMed] [Google Scholar]
- 20.Guo Q, et al. The role of organ failure and infection in necrotizing pancreatitis: a prospective study. Ann. Surg. 2014;259:1201–1207. doi: 10.1097/SLA.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 21.Noel P, et al. Peripancreatic fat necrosis worsens acute pancreatitis independent of pancreatic necrosis via unsaturated fatty acids increased in human pancreatic necrosis collections. Gut. 2016;65:100–111. doi: 10.1136/gutjnl-2014-308043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bumbasirevic V, et al. Severe acute pancreatitis: overall and early versus late mortality in intensive care units. Pancreas. 2009;38:122–125. doi: 10.1097/MPA.0b013e31818a392f. [DOI] [PubMed] [Google Scholar]
- 23.Lim C., et al. Epidemiology and burden of multidrug-resistant bacterial infection in a developing country. eLife 5, e18082 (2016). [DOI] [PMC free article] [PubMed]
- 24.Joshi S, et al. Methicillin resistant Staphylococcus aureus (MRSA) in india: prevalence & susceptibility pattern. Indian J. Med. Res. 2013;137:363–369. [PMC free article] [PubMed] [Google Scholar]
- 25.Sabharwal ER. Antibiotic susceptibility patterns of uropathogens in obstetric patients. N. Am. J. Med. Sci. 2012;4:316–319. doi: 10.4103/1947-2714.98591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duin D, van, Paterson DL. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin. North Am. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HS, et al. Emergence of multidrug resistant infection in patients with severe acute pancreatitis. Pancreatology. 2014;14:450–453. doi: 10.1016/j.pan.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Moka P, et al. Impact of antibiotic-resistant bacterial and fungal infections in outcome of acute pancreatitis. Pancreas. 2018;47:489–494. doi: 10.1097/MPA.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 29.Talukdar R, et al. Antibiotic use in acute pancreatitis: an Indian multicenter observational study. Indian J. Gastroenterol. 2014;33:458–465. doi: 10.1007/s12664-014-0494-7. [DOI] [PubMed] [Google Scholar]
- 30.Vlada AC, et al. Failure to follow evidence-based best practice guidelines in the treatment of severe acute pancreatitis. HPB. 2013;15:822–827. doi: 10.1111/hpb.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.