Abstract
Environmental change has been proposed as a factor that contributed to the extinction of the Neanderthals in Europe during MIS3. Currently, the different local environmental conditions experienced at the time when Anatomically Modern Humans (AMH) met Neanderthals are not well known. In the Western Pyrenees, particularly, in the eastern end of the Cantabrian coast of the Iberian Peninsula, extensive evidence of Neanderthal and subsequent AMH activity exists, making it an ideal area in which to explore the palaeoenvironments experienced and resources exploited by both human species during the Middle to Upper Palaeolithic transition. Red deer and horse were analysed using bone collagen stable isotope analysis to reconstruct environmental conditions across the transition. A shift in the ecological niche of horses after the Mousterian demonstrates a change in environment, towards more open vegetation, linked to wider climatic change. In the Mousterian, Aurignacian and Gravettian, high inter-individual nitrogen ranges were observed in both herbivores. This could indicate that these individuals were procured from areas isotopically different in nitrogen. Differences in sulphur values between sites suggest some variability in the hunting locations exploited, reflecting the human use of different parts of the landscape. An alternative and complementary explanation proposed is that there were climatic fluctuations within the time of formation of these archaeological levels, as observed in pollen, marine and ice cores.
Introduction
Marine Isotope stage 3 (MIS3) (60-25ka BP) was a period of instability with rapid and acute climatic changes1,2. Mid-late MIS3 was the time of the Middle-Upper Palaeolithic transition (c.45-25ka BP) (MP-UP), when late Neanderthal populations became extinct and were replaced by Anatomically Modern Humans (AMH). Neanderthal extinction is now known to have been relatively rapid, following a regional pattern, rather than a uniform pan-European one3. Climate cannot be claimed as a homogeneous, monolithic driver for their extinction in a single event across the continent as previously proposed by some4–7. How climate was expressed locally within the continental scale of Europe is not well understood, with climatic proxies identified from caves such as pollen, charcoal and plant remains, as well as microstratigraphies for this period all being relatively scarce, and although useful as environmental indicators8, they can be subject to taphonomic and diagenetic alterations9,10. There is a lack of radiometric chronologies for environmental and archaeological records independent of ice11 and marine cores12,13, which are not directly related to the localised conditions experienced at the archaeological sites. New methods have been recently proposed with great promise to overpass these limitations for a continental scale such as tephrochronology14 or atmospheric circulation modelling15, but they have some limitations and higher levels of precision are required.
Since the early 2000s, δ13C and δ15N analyses have been used to reconstruct animal palaeoecology and past environments16. Bone collagen δ13C and δ15N analysis within animal bones derive directly from diet consumed17, representing long-term feeding behaviour, informing on average environmental conditions throughout the period of bone growth18,19. Collagen δ34S analysis can be used as a locational tool, with values directly linked to local geology and soil type, proximity to the sea and rainfall20. These techniques have successfully been applied to European Palaeolithic environmental reconstructions directly related with human occupations to unravel the ecological conditions those populations confronted16,21–30.
An ideal location to apply this methodology to reconstruct the conditions faced by late Neanderthals and early AMH during MIS3 is the Cantabrian region in the Atlantic zone of northern Spain, which contains a high density of Middle and early Upper Palaeolithic sites31. The Cantabrian geographical region is formed by the Autonomous Communities of Asturias in the west, Cantabria in the centre and the Basque Country in the east. In this paper, we focus on the latter sector which is encompassed by the modern-day provinces of Gizpukoa, Bizkaia and Alava (Fig. 1). Gipuzkoa and Bizkaia are ecologically distinct from the western sectors of the Cantabrian region and are characterised by deep, steep-sided closed in mountains that often drop directly to the ocean. The Cantabrian Cordillera mountain range, relatively low in the Basque sector, separates the Atlantic coastal region from the Mediterranean-draining Ebro basin32. A recent chronological review of dates for MP-UP transitional sites undertaken using ultrafiltration radiocarbon method has provided a high-precision sequence of events for the timing of both human species’ activities in the region3,33–36. Therefore, it is now possible to obtain an accurate, independently dated environmental record in those archaeological sites by undertaking stable isotope analysis of ungulate bones exhibiting evidence of human manipulation (i.e. cut marks and fresh fractures) during the Mousterian, Châtelperronian, Aurignacian and Gravettian periods. The results can be integrated with the available sedimentology, palynological and micro and macromammal data. Results of bone collagen δ13C, δ15N and δ34S analyses on macromammals have identified temporal, intra- and inter-site trends in the climatic and environmental conditions directly experienced by late Neanderthals and early AMH in this archaeologically important region.
Figure 1.
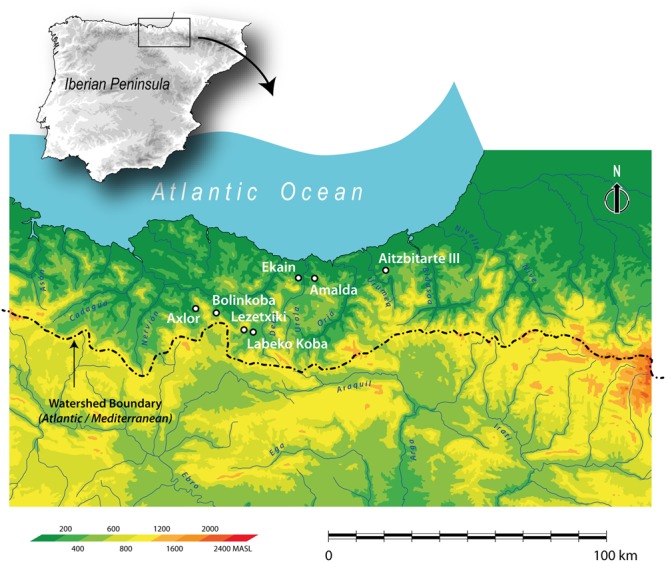
Map of the eastern Cantabrian region, northern Spain, showing the locations of the Bizkaia and Gipuzkoa sites studied. In Bizkaia: Axlor and Bolinkoba. In Gipuzkoa: Lezetxiki, Labeko Koba, Ekain, Amalda and Aitzbitarte III.
Sites and Materials
Geographical setting of the region
The sites analysed in this article are located in the two small coastal provinces of the Spanish Basque Country (the autonomous region of Euskadi): Gipuzkoa and Bizkaia. This is an eminently mountainous area straddling 43°10′ north latitude, bounded to the north by the Bay of Biscay, to the south by the Basque Mountain ranges of the Cantabrian Cordillera, to the east by the western end of the Pyrenees and to the west by the orographically less “chaotic”/structurally “banded” regions of Cantabria and Asturias. The coastal Basque provinces are connected to the SW corner of France via a very low pass between Monte Jaizkibel and Mont Rhune at the Bidasoa River and to the interior, upland Basque province of Alava and the autonomous region of Navarra via the lowest (600–900 m a.s.l.) passes across the entire Cantabrian Cordillera37. During the Last Glacial, there were mountain glaciers on some of the highest peaks of the Basque Mountains (e.g., Gorbea 1475 m a.s.l.; Aralar 1300 m a.s.l.), but with no continuous ice sheet, access to the semi-distant flint sources to the south of the Cordillera in Alava, Navarra and Treviño was always possible. The region is generally characterized by deep, narrow, short valleys, separated by high mountain ridges that often extend all the way to the shore. Under full glacial conditions, only a very narrow band of the inner continental shelf was exposed, but enough to provide what was probably the easiest east-west avenue of communication across the region and between it and the French Basque Country and Cantabria, respectively. Today characterized by a temperate, oceanic, pluvial climate (heavily dominated by the Gulf Stream), this region was probably always relatively humid even under the rigorously cold conditions of the Last Glacial (when the Gulf Stream was absent). In sharp contrast to the Mediterranean environments of the upper Ebro Basin to the south of the Cordillera, the Basque Country is in the Eurosiberian ecological zone, and differences (albeit attenuated) also existed during the Last Glacial.
Sites sampled
The eastern sector of the Cantabrian region contains several key sites pertaining to the late Mousterian, Châtelperronian, Aurignacian and Gravettian technocomplexes including Bolinkoba and Axlor in Bizkaia and Labeko Koba, Amalda, Aitzbitarte III, Lezetxiki and Ekain in Gipuzkoa (Table 1). Although there are other contemporaneous sites in those provinces, the sites analysed were selected because they are considered key regional sites with well-established stratigraphies that have been reviewed and recently dated by ultrafiltration33–36 (Table 2). Additionally, these sites have been the subject of technological studies and some taphonomic analyses that proved human presence38–43 and, also have environmental datasets44–49. Sites and levels sampled are presented in Table 1, with AMS radiocarbon dates shown in Table 2. Briefly, we describe the sites and levels included in this study and their chronology, from west (Bizkaia) to east (Gipuzkoa).
Table 1.
Summary of the specimens sampled per sites and archaeological levels and their chrono-cultural adscription.
| Broad cultural attribution | Temporal span (Ka BP uncal). | Site | Level | No. Samples for δ13C and δ15N analysis | No. aliquots analysed for δ34S | ||
|---|---|---|---|---|---|---|---|
| Red deer | Horse | Red deer | Horse | ||||
| Gravettian | 28 | Bolinkoba | VI/F | 4 | 7 | — | — |
| Amalda | VI | 5 | 6 | 1 | 3 | ||
| 31–29 | Aitzbitarte III | IV | 5 | — | 2 | — | |
| Va | 5 | — | 4 | — | |||
| Vb Upper | 1 | 2 | 1 | 1 | |||
| Aurignacian | 31 | Ekain | IXb | 4 | — | — | — |
| Aitzbitarte III | Vb Central | 5 | 2 | 2 | 1 | ||
| 35–32 | Labeko Koba | IV | — | 1 | — | — | |
| V | 5 | 5 | 2 | 2 | |||
| VI | 5 | 4 | 3 | — | |||
| Châtelperronian | 38–37 | IX Lower | 5 | 6 | 2 | 2 | |
| Mousterian | n/a | Lezetxiki | V | 4 | — | — | — |
| IV | 5 | — | — | — | |||
| 44–42 | Amalda | VII | 5 | 5 | 1 | 3 | |
| >49 | Axlor | IV | 12 | 2 | — | — | |
| 60–50 (based on typology) | VI | 12 | 6 | — | — | ||
| VIII | 10 | — | — | — | |||
| Totals | 92 | 46 | 18 | 12 | |||
Table 2.
Radiocarbon dates from sites and levels sampled, with laboratory codes and sample preparation methods used. AF: collagen extracted using the ultrafiltration method prior to analysis. Bone sample code is included for bones, that were both dated and analysed for stable isotopes.
| Culturalperiod | Site | Level | Radiocarbon date | Lab code | Dating method | Bone Sample code | Ref. |
|---|---|---|---|---|---|---|---|
| Gravettian | Bolinkoba | IV/F | 25280 ± 210 | OxA-32519 | AF | BOL03 | 33 |
| 29,950 ± 120 | Beta-426854 | AMS | — | 59 | |||
| 21,020 ± 90 | Beta-302981 | AMS | — | 59 | |||
| Amalda | VI | 27400 ± 1000 | I-11–665 | C14 | — | 74 | |
| 27400 ± 1100 | I-11-664 | C14 | — | 74 | |||
| 28540 ± 310 | OxA-32426 | AMS AF | AMA26 | 33 | |||
| 28710 ± 300 | OxA-34934 | AMS AF | AMA25 | 33 | |||
| Aitzbitarte III | IV | 28,320 ± 605 | Ua-18465 | AMS | — | 136 | |
| 22,420 ± 290 | Ua-24965 | AMS | — | 136 | |||
| 27,580 ± 550 | Ua-18464 | AMS | — | 136 | |||
| 26,260 ± 480 | Ua-37961 | AMS | — | 136 | |||
| 24,240 ± 365 | Ua-11146 | AMS | — | 136 | |||
| 25,815 ± 475 | Ua-11148 | AMS | — | 136 | |||
| 29,130 ± 310 | OxA-32422 | AMS AF | AIT26 | 33 | |||
| 29,020 ± 320 | OxA-32499 | AMS AF | AIT13 | 33 | |||
| Va | 31,210 ± 860 | Ua-18467 | AMS | — | 136 | ||
| 28,950 ± 655 | Ua-18466 | AMS | — | 136 | |||
| 28,530 ± 645 | Ua-37962 | AMS | — | 136 | |||
| 27,165 ± 520 | Ua-24966 | AMS | — | 136 | |||
| 26,350 ± 475 | Ua-24967 | AMS | — | 136 | |||
| 31,090 ± 400 | OxA-32420 | AMS AF | AIT7 | 33 | |||
| 31,300 ± 400 | OxA-32421 | AMS AF | AIT10 | 33 | |||
| Vb Upper | 31,950 ± 450 | OxA-32419 | AMS AF | AIT21 | 33 | ||
| 30,990 ± 390 | OxA-32416 | AMS AF | AIT2 | 33 | |||
| Aurignacian | Vb Central | 31,130 ± 390 | OxA-34932 | AMS AF | Only dated | 33 | |
| 31,600 ± 400 | OxA-32418 | AMS AF | AIT19 | 33 | |||
| 34,900 ± 400 | OxA-32417 | AMS AF | AIT05 | 33 | |||
| Ekain | IXb | >30,600 | I-11506 | C14 | — | 70 | |
| 31,140 ± 400 | OxA-32423 | AMS AF | EK02 | 33 | |||
| 31,110 ± 400 | OxA-32424 | AMS AF | EKA05 | 33 | |||
| Labeko Koba | Level IV | 33,550 ± 550 | OxA-21780 | AMS AF | — | 35 | |
| 33,600 ± 500 | OxA-21768 | AMS AF | — | 35 | |||
| Level V | 34,750 ± 600 | OxA-21767 | AMS AF | — | 35 | ||
| 34,650 ± 600 | OxA-21779 | AMS AF | — | 35 | |||
| Level VI | 32,200 ± 450 | OxA-21794 | AMS AF | — | 35 | ||
| 32,150 ± 450 | OxA-21841 | AMS AF | — | 35 | |||
| 35,100 ± 600 | OxA-21778 | AMS AF | — | 35 | |||
| Châtelperronian | Labeko Koba | IX Lower | 37,900 ± 900 | OxA-22564 | AMS AF | — | 35 |
| 37,400 ± 800 | OxA-22506 | AMS AF | — | 35 | |||
| 38,000 ± 900 | OxA-22561 | AMS AF | — | 35 | |||
| 38,100 ± 900 | OxA-22562 | AMS AF | — | 35 | |||
| 37,800 ± 900 | Oxa-22563 | AMS AF | — | 35 | |||
| Mousterian | Amalda | VII | 44,500 ± 2100 | OxA-32500 | AMS AF | AMA2 | 35 |
| 42,600 ± 1600 | OxA-34933 | AMS AF | AMA5 | 35 | |||
| Axlor | IV | 42,010 ± 1280 | Beta-144262 | AMS | — | 54 | |
| >43,000 | Beta-22586 | AMS | — | 54 | |||
| >49,300 | OxA-32428 | AMS AF | AXL39 | 33 | |||
| >49,900 | OxA-32429 | AMS AF | AXL42 | 33 |
Axlor in Dima (Bizkaia) is a rock-shelter that was excavated by J. M. Barandiarán between 1967 and 1974, revealing a long sequence of Mousterian Levels I-VIII50,51. Subsequent interventions between 1999 and 2004 linked a detailed stratigraphy (Levels B-N) to the levels recorded by Barandiarán52,53. In this study, Levels VIII, VI and IV from the Barandiarán’s excavations were analysed, and correlated to new Levels N, M and D, respectively38. New ultrafiltration dates were available for the uppermost of these levels, Level D, but were beyond the radiocarbon limit (>49,300 OxA-32428 and >49,900 OxA-32429). These dates are considerably older than previous ones achieved for this level using AMS but without ultrafiltration: 42,010 ± 1,280 BP (Beta-144,262) and >43,000 (Beta-22586)37,54,55. These new results suggest that the lower levels at Axlor are significantly older than previously thought. Based on artefact typology and technology, they are now estimated to date to around 60–50ka BP38. Macromammals represented are red deer, followed by Spanish ibex and large mammals (horse and bovines), with evidence of butchering marks and fresh breakage patterns50,51,56,57. Although a complete taphonomic analyses of the complete assemblage has not been undertaken, a recent the review of carnivore and bird bones have revealed evidence of manipulation by Neanderthals38 (Table 3)
Table 3.
Macromammal faunal assemblage NISP values represented in the levels of the archaeological sites sampled in this study, indicating when available references of taphonomic studies.
| Site and Level | Axlor | Amalda | Labeko Koba | Ekain | Aitzbitarte III | Bolinkoba | Amalda | NISP total per cultural periods | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIII | VI+V | IV | VII | IX lower | VI | V | IV | IXb | Vb central | Vb upper | Va | IV | VI/F | VI | |||||
| Cultural attribution | Mousterian | Châtelperronian | Aurignacian | Gravettian | Moust. | Châtel. | Aurig. | Gravet. | |||||||||||
| Mammuth primigenius | — | — | — | — | — | — | 6 | 5 | — | — | — | — | — | — | 0 | — | — | 11 | — |
| Equus ferus | 3 | 23 | 64 | 48 | 210 | 36 | 61 | 53 | — | 12 | — | — | 2 | — | 101 | 138 | 210 | 162 | 103 |
| Bovini | 2 | 61 | 160 | 58 | 143 | 175 | 290 | 161 | 21 | 51 | 53 | 105 | 180 | 1 | 99 | 281 | 143 | 698 | 438 |
| Capra pyrenaica | 28 | 117 | 179 | 61 | — | — | — | — | — | — | — | — | 9 | 236 | 385 | — | — | 245 | |
| Rupicapra rupicapra | 13 | 75 | 10 | 536 | — | 4 | 7 | 8 | 133 | 22 | 9 | 44 | 88 | 1 | 2769 | 634 | — | 174 | 2911 |
| Megaloceros giganteus | — | — | — | — | — | — | — | 1 | — | — | — | — | 0 | 0 | 0 | 0 | — | 1 | — |
| Cervus elaphus | 120 | 292 | 127 | 150 | 792 | 59 | 29 | 13 | 26 | 71 | 65 | 53 | 72 | 2 | 274 | 689 | 792 | 198 | 466 |
| Rangifer tarandus | 0 | 0 | 7 | 0 | 14 | 1 | — | 1 | — | — | — | 4 | 2 | 0 | 2 | 7 | 14 | 2 | 8 |
| Capreolus capreolus | 1 | 1 | 0 | 3 | 0 | — | — | 1 | 6 | 3 | — | 8 | 5 | 0 | 17 | 5 | — | 10 | 30 |
| Sus scrofa | 1 | 1 | 0 | 0 | 0 | — | — | — | — | — | — | 0 | 0 | 0 | 3 | 2 | — | — | 3 |
| Sub-Total | 168 | 570 | 547 | 856 | 1159 | 275 | 393 | 243 | 186 | 159 | 127 | 214 | 349 | 13 | 3501 | 2141 | 1159 | 1256 | 4204 |
| Canis lupus | — | 3 | 1 | 17 | — | 2 | 1 | — | 2 | — | — | 2 | 2 | — | 37 | 21 | — | 5 | 41 |
| Vulpes vulpes | — | — | 3 | 29 | 2 | 67 | 6 | 16 | 10 | — | 4 | 4 | — | — | 133 | 32 | 2 | 99 | 141 |
| Alopex lagopus | — | — | — | — | — | — | — | — | — | 5 | — | — | 23 | — | 1 | — | — | 5 | 24 |
| Cuon alpinus | — | — | — | 1 | — | — | — | — | — | — | — | — | — | — | 1 | 1 | — | — | 1 |
| Ursus spelaeus | 1 | 1 | 3 | 58 | 3 | 20 | — | 4 | 29 | — | — | 10 | 7 | — | 103 | 63 | 3 | 53 | 120 |
| Meles meles | — | — | 1 | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — |
| Mustela erminea | — | — | — | — | — | — | — | — | — | — | — | 1 | 5 | — | — | — | — | — | 6 |
| Mustela nivalis | — | — | — | — | — | — | — | — | — | 8 | 8 | 5 | 21 | — | — | — | — | 8 | 34 |
| Mustela putorius | — | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | 1 |
| Crocuta crocuta | — | — | — | 3 | 55 | 43 | 2 | 9 | 0 | 2 | 1 | — | 1 | — | 11 | 3 | 55 | 56 | 13 |
| Panthera leo | — | — | — | — | — | — | — | — | — | — | — | 1 | — | — | — | — | — | — | 1 |
| Panthera pardus | — | — | — | 3 | — | — | — | — | — | — | — | — | — | 1 | 3 | 3 | — | — | 4 |
| Lynx | — | 1 | — | — | — | — | — | — | — | — | — | — | 1 | — | — | 1 | — | — | 1 |
| Felis silvestris | — | — | — | — | — | — | 1 | — | — | — | — | — | 1 | — | — | — | — | 1 | 1 |
| Marmota marmota | 1 | 1 | — | — | — | — | — | — | — | — | — | — | — | — | 2 | — | — | — | |
| Sub-Total | 2 | 6 | 8 | 111 | 60 | 132 | 10 | 29 | 41 | — | 13 | 23 | 62 | 1 | 289 | 127 | 60 | 227 | 388 |
| TOTAL | 170 | 576 | 555 | 967 | 1219 | 407 | 403 | 272 | 227 | 174 | 140 | 237 | 411 | 14 | 3790 | 2268 | 1219 | 1483 | 4592 |
| Taphonomic analysis | NO | YES | YES | YES | YES | NO | YES | ||||||||||||
| References | 49, 54, 55 | 40, 41, 138 | 65, 81 | 53, 69 | 42 | 58 | 40, 41, 71 | ||||||||||||
Bolinkoba, in Abadiño (Bizkaia), was originally explored by J.M. Barandairán and T. Aranzadi in the early 1930s58, followed by excavations by J.M. de Urquijo in the 1940s and again recently, between 2008 and 2014 by M.J. Iriarte59. The site stratigraphy reveals a series of levels from the Gravettian to the Azilian. Pertinent to this project, Gravettian Level VI (F) from the Barandiarán excavations was selected and a new date indicates a Gravettian occupation (25,280 ± 210 OxA-32519)33, although AMS dates from new excavation have provided dates of 29,950 ± 120 (Beta-426854) and 21,020 ± 90 (Beta-302981) for the same level59 (Iriarte and Arrizabalaga 2015). Recent review of macromammal remains from the Barandiaran and Aranzadi collection and, from the recent excavations, indicate similar proportions and presence of ungulates between both assemblages, with Spanish ibex being the most common hunted species followed by red deer60 (Table 3). Despite the small representation of micromammals, these show cold palaeoenvironmental conditions during the Gravettian that included a modest decline in forest61. Palynological results did not provide palaeoenvironmental information due to the poor spore and pollen preservation62 (Table 4).
Table 4.
Summary of available environmental proxies for the studied sites, including references for the information provided. Dashes denote levels where datasets are missing.
| Broad cultural attribution | Temporal span (Ka uncal BP) | Site | Level | Pollen | Sediment interpretations | Macrofauna | Microfauna |
|---|---|---|---|---|---|---|---|
| Gravettian | 28 | Bolinkoba | VI/F | — | — | — | — |
| Amalda | VI | Pinus accounts for around 10% of pollen, with Betula present. Poaceae/Graminae grasses are common (although varying between 5-40% in samples), and Anthemidae and Cichorieae are represented. More temperate Corylus accounts for 15–20% of pollen76. | Cool/wet environment137 | Total ungulate NISP = 3501, chamois 79%, red deer 8%, spanish ibex 7%, bos/bison 3%, and horse 3%. Presence of cool indicator species reindeer and arctic fox138 |
Total NISP = 490, dominance of Microtus
arvalis-agrestis (74%). Cold adapted Microtus oeconomus present in smaller numbers than previously seen in the sequence (7%), and presence of P. lenki (>1%). Pitymys sp. (5%). Increased frequency of species that thrive in moist/temperate conditions including Sorex araneus (7%), A. terrestris (5%), Talpa europea and N. fodiens also present139 |
||
| 31–29 | Aitzbitarte III | IV | Low percentage of tree pollen 2.5% (Pinus declines slightly and Cupressaceae increases). Poaceae declines (9.5%) and an increase in the composites is seen (except Cedrela tubiflora). Spores decrease (3.5%)44 |
Slightly warmer climate compared to previous parts of the sequence140. | Total ungulate NISP = 354, bos/bison 52%, chamois 25%, red deer 12%, horse, roe deer and reindeer all present43 | Total NISP = 1471, Microtus arvalis-agrestis common (47%), and Microtus oeconomus well represented (17%). Sorex araneus (12%), Pitymys sp. (5%) and Talpa sp. (12%), also identified, alongside A. terrestris (4%). Presence of Neomys sp. and Sorex minutus79. | |
| Va | Tree cover remains stable. Steppic vegetation including grasses Poaceae/Gramineae (25%), umbelliferous plants (6%) and rosaceae (2%) present. Cedrela tubiflora and Compositae liguliflor (31%) common as seen previously. Spores up to 8%44 |
Conditions expressed were more severe79 | Total ungulate NISP = 211, bos/bison 49%, red deer 25%, chamois 20%, roe deer and reindeer also present43 | Total NISP = 1155, Microtus arvalis-agrestis common (51%) and Microtus oeconomus well represented (17%). Sorex araneus (15%), Pitymys sp. (7%) and Talpa sp. (6%) also identified, alongside A. terrestris (3%). Sorex minutus and Apodemus sp. present79. | |||
| Vb upper | Slight recovery in tree cover (up to 2.5%- Pinus and Cupressaceae) Steppic vegetation dominates, Poaceae starts to increase. Centaurea declines to 8%, Compositae liguliflor accounts for 41% pollen. Level generally similar in composition to levels Vb Central and Va44 | Slightly cooler conditions were experienced at this time140 | Total ungulate NISP = 127, red deer 51%, bos/bison 42%, chamois 7%43 |
— | |||
| Aurignacian | 31 | Ekain | IXb | — | — | Total ungulate NISP = 398, more temperate suite of fauna: chamois 65%, bos/bison 19%, red deer 15%72 |
Total NISP = 31, Arvicola most common species (NISP = 27), Pliomys lenki and Pitymys sp. also present73 |
| Aitzbitarte III | Vb Central | 1.6% tree cover, pine and juniper. Steppic vegetation dominates with of Centaurea common (32–35%) and Compositae, in addition to Cedrela tubiflora and Compositae liguliflor found in variable proportions within the samples44 | Possibly slightly cooler conditions experienced | Total ungulate NISP = 158, red deer 44%, bos/bison 32%, chamois 14%, horse 8%, and roe deer also present43. | Total NISP = 574, Microtus arvalis-agrestis common (51%), and Microtus oeconomus present in substantial quantities (15%). Sorex araneus (14%), Pitymys sp. (5%) and Talpa sp. (8%) also represented, alongside A. terrestris (7%)79. | ||
| 35–32 | Labeko Koba | IV | — | Conditions still cool, but indicate the start of a tempering environment141 | Total ungulate NISP = 237, bos/bison 68%, horse 22%, red deer 5%, chamois 3%, and reindeer present85 | Total NISP = 40. Arvicola terrestris (NISP = 38) and Talpa sp. (NISP = 2) present77. | |
| V | Pollen evidence sparse. Pinus present (15%) alongside steppic vegetation, such as Cedrela tubiflora (8%), Compositae liguliflor (25%) and Poaceae/Gramineae (15%)45 | Cold conditions remaining in this level141 | Total ungulate NISP = 387, bos/bison 75%, horse 16%, red deer 7%, and chamois 2%)85 | Total NISP = 74. Arvicola terrestris most common species (NISP 67) Microtus agrestris-arvalis, Talpa sp. Glis glis and present77. | |||
| VI | Climatic conditions interpreted as declining, decrease in the mid-thermophilic taxa observed including Poaceae/Gramineae (40–50%), Plantago (c. 10%), Cedrela tubiflora and Compositae liguliflor (both representing c.10% of samples taken). Low quantities of arboreal pollen, mostly Pinus (10–15%) persist45 | — | Total ungulate NISP = 275 bos/bison 64%, red deer 21%, horse 13%, and chamois present85 |
Total NISP = 43. Arvicola terrestris (NISP = 37) most common, Talpa sp., Microtus agrestris-arvalis present77 | |||
| Châtelperronian | 38–37 | Labeko Koba | IX Lower | Conditions fairly benign. Presence of mid-thermophilic species such as Corylus (5–7%). Increased evidence of steppic vegetation (60% in one sampled) Pinus also present (c.12–15%)45 |
— | Total ungulate NISP = 1159, red deer 68%, horse 18%, bos/bison 12%, and presence of reindeer85 | Total NISP = 50. Arvicola terrestris (NISP = 40) most common. Talpa sp. Glis glis, Microtus agrestris-arvalis present77 |
| Mousterian | 44–42 | Amalda | VII | Poor pollen preservation in this level, represented predominantly by Pinus (c.60%), with Cichorioideae accounting for c. 20% of total pollen and low frequencies of Chenopodiaceae76. | — | Total ungulate NISP = 856, chamois 63%, red deer 18%, bos/bison 7%, spanish ibex 7% and horse 6%138 | Total NISP = 22. Microtus arvalis- agrestis (NISP = 14) most common. Cool indicator species Pliomys lenki present in addition to A. terrestris, Apodemus sp., and Sorex139 |
| >49 | Axlor | IV | — | — | Total ungulate NIS = 109 Red deer 44%, bos/bison 30%, Spanish ibex 15%, horse 7%, and chamois 4%56,142 |
— | |
| VI | — | — | Total ungulate NISP = 585, bos/bison 15%, spanish ibex 15%, red deer 14%, horse 6% and chamois present56,142 | — | |||
| 60–50 | VIII | — | — | Total ungulate NISP 163, red deer 74%, spanish ibex 14%, chamois 8%, horse 2%, and presence of bos/bison, roe deer and wild boar56,142 | — |
Labeko Koba (Arrasate, Gipuzkoa) was discovered during the construction of a road and was the object of a programmed salvage excavation during 1987–88. The now-destroyed cave held one of the few Châtelperronian levels (Level IX lower) together with Morin Level 10 and Aranbaltza, in the entire Cantabrian region35,63,64. There is a lack of human remains in sites attributed to the Cantabrian Châtelperronian, although recent research has suggested that this techno-complex was the work of Neanderthals33. The Labeko Châtelperronian is followed by Early Upper Palaeolithic levels, with a sequence of Protoaurignacian (Level VII) and Early Aurignacian technocomplexes (Levels IV-VI)65–67. These have been recently dated, producing an accurate chronology of the modern human occupation at the site35. The archaeozoological and taphonomic analyses revealed that the site was alternatively used as an occasional hunting camp and a carnivore den68,69 (Table 3). Levels sampled for stable isotope analysis were IX lower (Châtelperronian) and Early Aurignacian Levels VI, V and IV. No well-preserved faunal remains were available from Level VII and Level IX upper was not sampled as it was archaeologically sterile35,66. Palynological remains from Level IX lower reveal the presence of mesothermophilic species, including Castanea, while the other levels display characteristically stadial botanical associations, with a low representation of arboreal taxa45 (Table 4).
Ekain, located in Deba (Gipuzkoa), has a stratigraphic sequence ranging from the Early Upper Palaeolithic through the Upper Magdalenian and Azilian, and is well-known for its late Upper Palaeolithic cave art70. The lower part of the sequence contains evidence of human presence during the Initial Upper Palaeolithic. Relevant to this study, Level IXb was interpreted as an Early Aurignacian sporadic camp with a small lithic assemblage, probably accumulated in no more than a single occupation episode71. Level IXb was dated by conventional 14C to >30,600 (I-11506) but recent AMS ultrafiltered dates of 31,140 ± 400 (OxA-32423) and 31,110 ± 400 (OxA-32424) show that this level is contemporaneous with the Evolved Aurignacian from Aitzbitarte III (entrance) Vb central (Gipuzkoa) and La Viña Levels XIII and XII (Asturias)33. Ursus spelaeus is abundant in this level (48%), but this is a lower relative frequency than in Level Xa (91%). Other carnivores are also present, including: fox, wolf, hyena and panther. Ungulates such as bovines, chamois and red deer are represented in the assemblage and have been interpreted as occasional elements of human diet72 (Table 3). Within the micromammal assemblage Arvicola sp. has been identified73 (Table 4).
Amalda in Zestoa (Gipuzkoa) is known for its succession of Mousterian and Gravettian levels, as well as Late Upper Palaeolithic occupations74. Mousterian Level VII was once interpreted to have been an anthropogenic occupation level. Subsequent, taphonomic analysis proposed that this level was the deposit of a carnivore den visited sporadically by Neanderthals40,41. However, recent reanalysis reaffirmed that the level’s contents were mainly human-derived, but with occasional carnivore activity42,74,75 (Table 3). Recent AMS ultrafiltered dates of 44,500 ± 2100 (OxA-32500) and 42,600 ± 1600 (OxA-34933) confirm its Mousterian chronology33. The Gravettian technocomplex is present in Level VI, which is dated to between 28,540 ± 310 (OxA-32426) and 28,710 ± 300 (OxA-34934). All animal samples analysed from this site were selected from areas where stratigraphic integrity remained intact. Despite poor pollen preservation, the spectrum from Level VII is represented predominantly by pine trees (c.60%), with Cichorioideae accounting for c. 20% of total pollen and low frequencies of Chenopodiaceae. There are more temperate pollen species in Level VI including Corylus and Betula76 (Dupré 1990). The scarce micromammal remains recovered in Level VII are from Microtus arvalis-agrestis and Pliomys lenki, Arvicola terrestris, Apodemus sp. and Sorex and include cool climate indicator species. In Level VI the representation of cold adapted species is lower and there is an increased frequency of species that thrive in moist/temperate conditions including Sorex araneus (7%), A. terrestris (5%), with Talpa europea and N. fodiens also present77 (Table 4).
Aitzbitarte III is part of a karstic system with five different caves in Errenteria, Gipuzkoa, near San Sebastián. The most recent excavations and analyses of material from the site were undertaken in the entrance area of the cave between 1994 and 2002, revealing a cultural sequence of Evolved Aurignacian (Level Vb central) and Gravettian (Levels Vb upper, Va and IV)78. Ultrafiltration dates reveal a rapid transition from the Aurignacian to the Gravettian. The dates at the cave entrance show that these levels represent a short span of time and were formed of discrete periods of occupation. The dates for Level Vb Central are 31,130 ± 390 (OxA-34932) and 31,600 ± 400 (OxA-32418)33 which are coherent with previous AMS dates78 and the cultural attribution to an Evolved Aurignacian phase. Dates obtained from Level Vb upper of 31,950 ± 450 (OxA-32419) and 30,990 ± 390 (OxA-32416) are similar to those obtained in Level Vb Central, indicating either an Evolved Aurignacian occupation that already shows some features transitional to the Gravettian or the first manifestation of an Early Gravettian at the site (Rios-Garaizar et al. 2013). New dates in Level Va, previously identified as Early Gravettian with Noailles burins33,71, appear to support a quick transition towards the Gravettian, with both dates being very similar: 31,300 ± 400 (OxA-32421) and 31,090 ± 400 (OxA-32420), and are consistent with other dates obtained in Levels Vb Central and Vb Upper. Finally, for Level IV, also classified as Early Gravettian with Noailles burins, the new dates of 29,130 ± 310 (OxA-32422) and 29,020 ± 320 (OxA-32499) are consistent with other Early Gravettian dates in the region. Archaeozoological and taphonomic analyses indicate an intense exploitation of red deer and bovines, followed by chamois and horse during the Aurignacian, while during the Gravettian bovines are the most common taxa, followed by red deer, chamois and horse43 (Table 3). Micromammals have a similar faunal composition during both periods; Microtus agrestis-arvalis is the dominant group and Pliomys lenki is sparsely found. These species reflect harsh climatic conditions, cold and humid, with open landscapes and scarce woodlands79. Pollen also reflect cold conditions with variations in the moisture, that indicate a landscape dominated by herbaceous-shrub taxa, with very little representation of trees44. Sedimentological studies correlate with the environmental proxies suggesting severe conditions80 (Table 4).
Lezetxiki in Arrasate, Gipuzkoa, was excavated by J. M Barandiarán between 1956 and 1968 and since 1996 has been excavated by A. Arrizabalaga. The site contains several levels corresponding to the Middle Palaeolithic from some of which a Homo heidelbergensis humerus and two Neanderthal teeth were recovered81–83. Mousterian Levels IV and V were sampled to provide an indication of the environments during that period. Collagen preservation was poor with these levels and samples did not yield sufficient collagen for radiocarbon dating and isotopic analysis, and will not be discussed further, but this serves to provide caution to other researchers contemplating analysis of remains from this site. Besides, Aurignacian Level IIIa, from the original excavation, was not sampled due to its possible mixing with Mousterian Level IIIb34, in addition to it having a possible Solutrean component.
Materials
Bones of red deer (Cervus elaphus) and horse (Equus sp.), two of the most common mammal species represented during the regional Middle and Early Upper Palaeolithic43,84–86, were sampled. These specimens were selected strategically to measure the impact of broader climate on the isotopic values of two ecologically different species; grazers (horse) and intermediate feeders (red deer) to be observed. No contemporary browsers were available for comparison as they are scarcely represented during the period of study in the region. Specimens with evidence of anthropogenic modification (i.e., cut marks and/or anthropogenic breakage), associated with stone tools were targeted. Fused long bones of mature individuals were selected to prevent analysis of juveniles that might have prevailing weaning signatures87,88. In total, 138 animal individuals belonging to Mousterian (n = 61), Châtelperronian (n = 11), Aurignacian (n = 31) and Gravettian (n = 35) levels at six archaeological sites were analysed for δ13C and δ15N and 30 of these specimens were also analysed for δ34S (Table 1). The 12 horse and 18 red deer specimens selected for δ34S all contained >5 mg of collagen required for analysis and had δ15N values from the lower, middle and upper ranges of the dataset to explore the relationship between δ15N values and feeding locations. At the beginning of this project, radiocarbon dating using the AMS ultrafiltration method was undertaken to confirm the chronological attribution of the levels sampled33. Although some of the archaeological sites already had radiocarbon dates (mostly completed in the 1990s and early 2000s), new dates were run with ultrafiltration method (Table 2), which has greatly improved the reliability of the ages obtained due to the more effective removal of low molecular weight contaminants89,90, especially during this period of study.
Results
Collagen preservation and sample integrity
Collagen preservation within the >30ka fraction was excellent. Of the 138 archaeological specimens analysed, 124 provided sufficient collagen for analysis (82 red deer and 42 horse). Values discussed within this paper had C: N values between 2.9 and 3.6, suggesting in vivo collagen91, with 121 of these complying with the more rigid criteria of 2.9–3.492. For δ34S analysis, all specimens had C:S collagen values between 600 ± 300 and atomic N:S collagen values between 200 ± 1093. Raw data, quality indicators and information for the samples that failed are provided within Supplementary Information Table 1.
Results of the δ13C and δ15N analyses
Broad temporal trends
The δ15N values of both species range between 1.3‰ and 9.2‰, which is higher than typically observed for animals feeding within the same trophic level in the same geographical location94. The δ13C values range between −21.6‰ and −19.2‰. To explore wider broader environmental trends, specimens from each cultural period, defined by archaeological assemblage characteristics were grouped and populations were compared statistically, as outlined in the methods section at the end of this article.
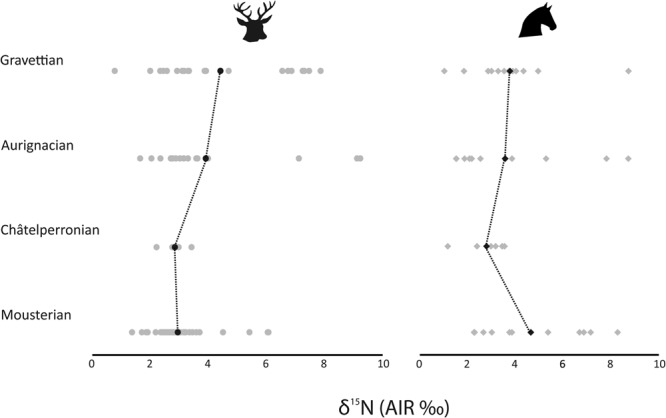
There is an increase in mean red deer δ15N values from the Mousterian (3.4‰) and Châtelperronian (3.3‰) to the Aurignacian (4.3‰) and Gravettian (4.7‰), although these means are likely influenced by the presence of individuals with elevated δ15N values (Table 5; Figs 2–5). The Mousterian red deer populations were statistically significantly different from those in the Aurignacian (p = 0.02) and Gravettian (p = 0.01) (Table 6). For the horse, the highest mean δ15N values are in the Mousterian (4.5‰), decrease in the Châtelperronian (2.9‰) and stay at a lower level throughout the Aurignacian (3.6‰) and Gravettian (3.8‰), but no statistically significant differences between δ15N values of horse populations is observed between any of these cultural levels (Table 5).
Table 5.
Summary statistics for red deer and horse specimens for each cultural period analysed.
| Cultural attribution | N | Red deer δ13C | Red deer δ15N | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | 1σ | Mean | Min | Max | 1σ | ||
| Mousterian | 39 | −20.3 | −21.6 | −19.5 | 0.4 | 3.4 | 1.9 | 6.3 | 1.0 |
| Châtelperronian | 4 | −19.9 | −20.1 | −19.8 | 0.1 | 3.3 | 2.7 | 3.8 | 0.5 |
| Aurignacian | 19 | −20.2 | −20.9 | −19.7 | 0.3 | 4.3 | 2.2 | 9.2 | 2.0 |
| Gravettian | 20 | −20.1 | −21.0 | −19.5 | 0.3 | 4.7 | 1.3 | 8.0 | 2.1 |
| Horse δ 13 C | Horse δ 15 N | ||||||||
| Mousterian | 12 | −20.2 | −21.0 | −19.2 | 0.6 | 4.5 | 2.5 | 7.6 | 1.8 |
| Châtelperronian | 6 | −20.7 | −21.1 | −20.2 | 0.3 | 2.9 | 1.5 | 3.6 | 0.8 |
| Aurignacian | 11 | −20.6 | −21.2 | −19.7 | 0.5 | 3.6 | 1.8 | 8.0 | 2.1 |
| Gravettian | 13 | −20.9 | −21.3 | −20.0 | 0.4 | 3.8 | 1.4 | 8.0 | 1.6 |
Figure 2.
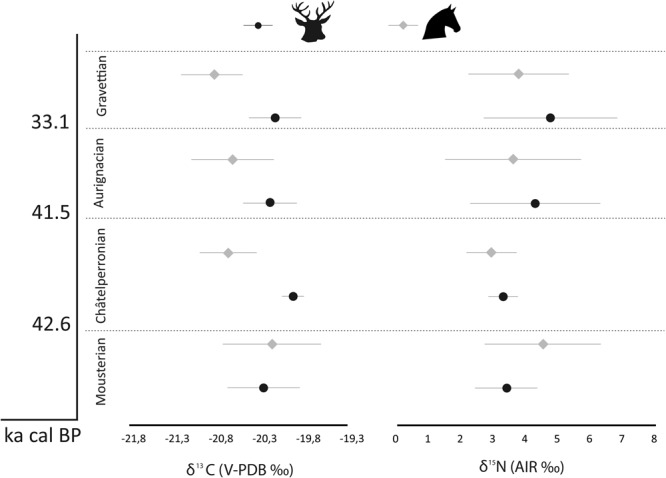
Mean red deer (denoted by black circles) and horse (denoted by grey diamonds) values with error bars showing 1σ for each cultural period. The chronological data are from Marín-Arroyo et al.33 which states that the Mousterian disappeared in the region by 47.9–45.1ka cal BP, while the Châtelperronian lasted between 42.6k and 41.5ka cal BP and the Mousterian and Châtelperronian did not overlap. The Aurignacian appears between 43.3–40.5ka cal BP overlapping with the Châtelperronian and ended around 34.6–33.1ka cal BP, after the Gravettian had already been established in the region.
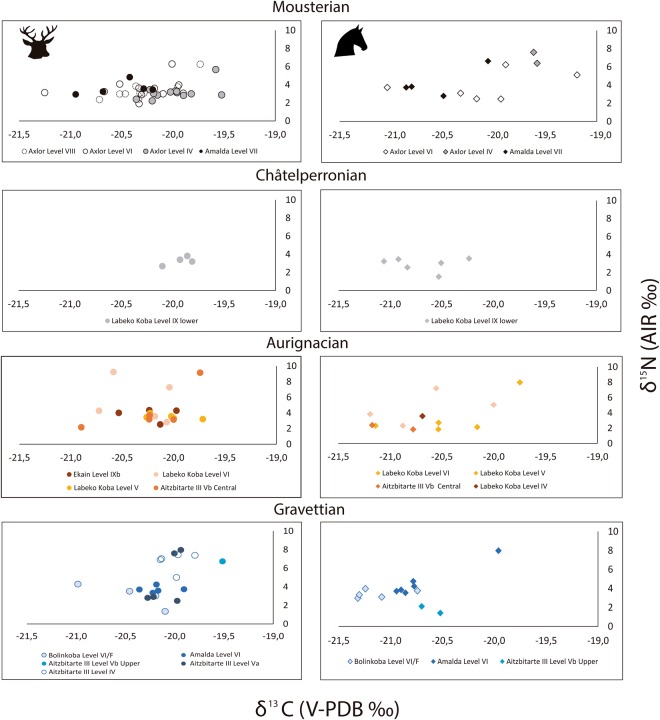
Figure 5.
Bone collagen δ13C and δ15N values of red deer and horse specimens from the sites analysed in this study, for each cultural period and archaeological level. Red deer values are plotted on the graphs to the left (denoted as circles) and horse ones to the right (denoted as diamonds).
Table 6.
Statistical test results comparing red deer δ15N and δ13C values within each cultural period. Statistically significant p values are highlighted in bold.
| Statistical comparisons of red deer δ15N values (p values) | |||||
|---|---|---|---|---|---|
| Mousterian | Châtelperronian | Aurignacian | Gravettian | ||
| Statistical comparisons of red deer δ13C values (p values) | Mousterian | — | 0.71 | 0.02 | 0.01 |
| Châtelperronian | 0.04 | — | 0.33 | 0.20 | |
| Aurignacian | 0.50 | 0.05 | — | 0.51 | |
| Gravettian | 0.17 | 0.08 | 0.57 | — | |
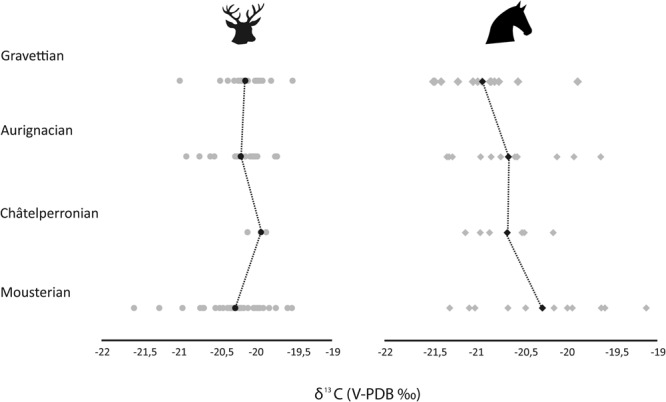
There is little temporal change in δ13C values. In the Châtelperronian, red deer have lower δ13C values than in the Mousterian (Table 5) and the populations were statistically different (p = 0.04) (Table 6). Horses had slightly lower δ13C values in the Châtelperronian compared to the Mousterian, but no statistically significant differences between populations within consecutive temporal periods were observed (Table 7).
Table 7.
Statistical test results comparing horse δ15N and δ13C values within each cultural period. Statistically significant p values are highlighted in bold.
| Statistical comparisons of horse δ15N values (p values) | |||||
|---|---|---|---|---|---|
| Mousterian | Châtelperronian | Aurignacian | Gravettian | ||
| Statistical comparisons of horse δ13C values (p values) | Mousterian | — | 0.09 | 0.09 | 0.53 |
| Châtelperronian | 0.07 | — | 0.93 | 0.15 | |
| Aurignacian | 0.06 | 0.96 | — | 0.38 | |
| Gravettian | 0.00 | 0.27 | 0.17 | — | |
When comparing the red deer and horse values, both species have similar mean δ13C values in the Mousterian and horses have a mean δ15N value 1‰ higher than the red deer (Fig. 2). In the Châtelperronian, Aurignacian and Gravettian, there is a shift in the diet of horses relative to the red deer and the mean horse values are lower in δ13C and δ15N values than red deer ones (Table 5) (Fig. 2).
Period-specific trends
Mousterian
Within the Mousterian, both the red deer and the horses display wide ranges in the δ15N and δ13C values observed. A group of horses and red deer have δ15N values between 1.8‰ and 5.1‰. There is another cluster of horse and red deer specimens with δ15N values within ± 1σ of the mean, ranging between 5.7‰ and 9.5‰ (Figs 2, 4), which have been identified as belonging to different groups using cluster analysis (horse cophenetic correlation coefficient = 0.91; red deer = 0.87) The latter is outside of the range typically expected for individuals feeding within the same geographical location94. This pattern is particularly observed in Mousterian Levels IV, VI, VIII of Axlor and in Amalda Level VII. There is little difference in the δ13C values of either species and a clear overlap is seen (Figs 3, 5).
Figure 4.
Mean and individual red deer (left) and horse (right) δ15N values plotted for each cultural period. Circles denote red deer, diamonds denote horses. Mean values are displayed in black and individuals sampled are displayed in grey.
Figure 3.
Mean and individual red deer (left) and horse (right) δ13C values plotted for each cultural period. Circles denote red deer, diamonds denote horses. Mean values are displayed in black and individuals sampled are displayed in grey.
Châtelperronian
Level IX lower at Labeko Koba is the unique Châtelperronian level in the eastern Cantabrian region dated by ultrafiltration35. All horses and red deer plot within the same δ15N trophic range (horse: 1.5–3.6‰, red deer: 2.7–3.8‰) (Figs 4, 5.). There is no overlap between the two species in the δ13C values (red deer min −20.1‰, max −19.8‰, horse min −21.0‰, max −20.2‰). Red deer δ13C values are consistently higher relative to the horse analysed in this level and difference in δ13C values between the two populations is statistically significant (p = 0.01) (Table 8).
Table 8.
Statistical test results comparing red deer and horse δ15N and δ13C values within each cultural period. Statistically significant p values are highlighted in bold.
| Statistical comparisons of red deer and horse δ15N values (p values) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mousterian | Châtelperronian | Aurignacian | Gravettian | |||||||
| Red deer | Horse | Red deer | Horse | Red deer | Horse | Red deer | Horse | |||
| Statistical comparisons of red deer and horse δ13C values (p values) | Mousterian | Red deer | 0.06 | 0.71 | 0.66 | 0.02 | 0.33 | 0.01 | 0.13 | |
| Horse | 0.60 | 0.39 | 0.09 | 0.76 | 0.09 | 0.68 | 0.53 | |||
| Châtelperronian | Red deer | 0.04 | 0.39 | 0.52 | 0.33 | 0.58 | 0.20 | 0.43 | ||
| Horse | 0.01 | 0.07 | 0.01 | 0.06 | 0.93 | 0.05 | 0.15 | |||
| Aurignacian | Red deer | 0.50 | 0.85 | 0.05 | 0.01 | 0.11 | 0.51 | 0.66 | ||
| Horse | 0.01 | 0.06 | 0.02 | 0.96 | 0.01 | 0.07 | 0.38 | |||
| Gravettian | Red deer | 0.18 | 0.91 | 0.08 | 0.00 | 0.57 | 0.00 | 0.36 | ||
| Horse | 0.00 | 0.00 | 0.01 | 0.27 | 0.00 | 0.17 | 0.00 | |||
Aurignacian
Aurignacian Levels VI and V in Labeko Koba and Level Vb Central of Aitzbitarte III, exhibit a similar pattern to the Mousterian, with a group of individuals with lower δ15N values (range; 2.3‰ to 5.0‰) and another with elevated δ15N values (range; 6.7‰ to 9.2‰) (Fig. 4), and cluster analysis showed these individuals as belonging to different groups (horse cophenetic correlation coefficient = 0.93; red deer = 0.95). Within Level V at Labeko Koba all individuals analysed plot within the lower nitrogen range, except for one horse and Level VI contains two red deer with elevated δ15N values (Fig. 5). The δ13C ranges of the individuals with high and lower δ15N values fall within the same range (−21.2‰ to −20.5‰, Fig. 3) and there is a clear differentiation in the δ13C values of horse and red deer, with horse having consistently lower values (Fig. 2).
Gravettian
In the Gravettian horse and red deer are found to have lower δ15N values (range 1.3‰−5.1‰), apart from a group of red deer with higher values (6.9‰−9.5‰) observed within Aitzbitarte III Level IV, Amalda Level VI and at Bolinkoba Level VI(F) (Figs 4, 5). The red deer and horses with higher δ15N values from this period were identified using cluster analysis as belonging to a different group (horse cophenetic correlation coefficient = 0.94; red deer = 0.91). This is consistent in the preceding periods, although, unlike the earlier periods, no horses show higher values. As seen in the Châtelperronian and Aurignacian, horses have lower δ13C values than red deer (Fig. 2).
Results of the δ34S analysis
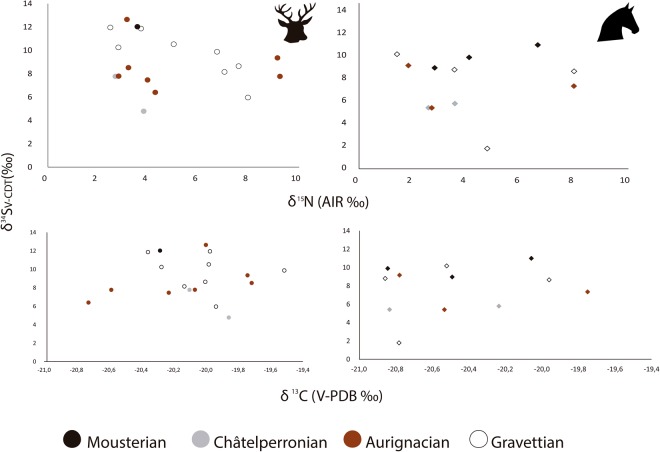
The δ34S values of the red deer and horse analysed ranged between 1.8‰ and 12.6‰, with most specimens falling between 5‰ and 11‰ and are consistent with animals inhabiting terrestrial ecosystems20. There is no linear correlation between δ34S and δ15N values (r = 0.049) or δ34S and δ13C values (r = 0.076), which would be expected if there was a link between the sulphur isozones and the carbon/nitrogen zones being exploited (Fig. 6) (Supplementary Information Table 2), and no clear groupings of values were identified using cluster analysis. Sulphur values within local food webs are controlled by the bedrock composition, atmospheric deposition and microbial processes95, rather than by dietary behaviour, enabling both herbivore species to be directly compared.
Figure 6.
Bone collagen δ34S values plotted with the δ15N values (above) and δ34S values and δ13C values (below) for each of the cultural periods sampled within the eastern Cantabrian region. Red deer values are plotted in the graphs on the left (denoted as circles) and horse values are plotted on the right (denoted as diamonds).
Within Châtelperronian Level IX Lower at Labeko Koba, the δ34S values range between 4.8‰ and 7.8‰. At Labeko Koba, within Level V, values ranged between 5.4 and 8.5‰ and for Level VI individuals have values between 6.4‰ and 7.8‰.
In Amalda the values were generally higher than at Labeko Koba. In Amalda Level VII, δ34S values ranged between 9‰ and 12‰ and in Amalda Level VI, the δ34S values from the bulk of the individuals ranged between 8.7‰ and 11.9‰ (Fig. 7), with only one specimen showing an uncharacteristically low δ34S value of 1.8‰ with a δ15N value of 4.8‰ (Fig. 7). This was inconsistent with any of the other specimens analysed in this study.
Figure 7.
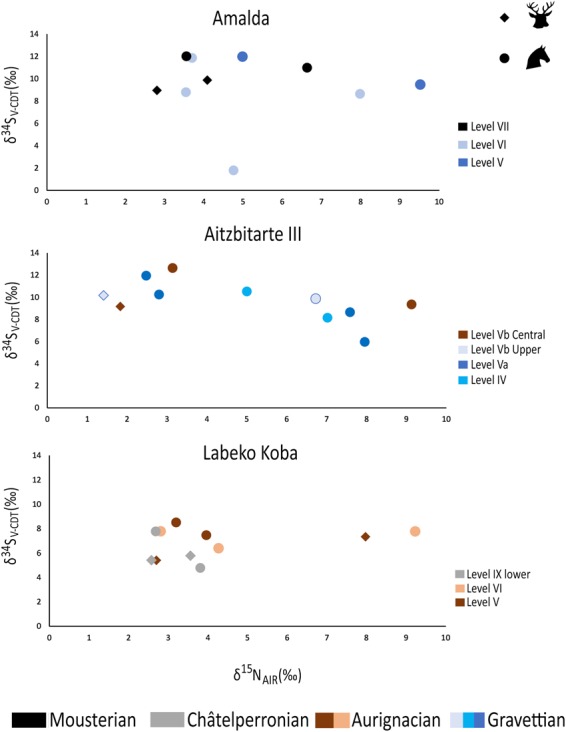
Red deer and horse δ34S values plotted with the δ15N ones for Aitzbitarte III, Labeko Koba and Amalda showing the distribution of values for each archaeological level and cultural period. Red deer are plotted as circles and horses are plotted as diamonds.
At Aitzbitarte III, δ34S values ranged between 6‰ and 12.6‰ (Fig. 7). The Evolved Aurignacian samples in Level Vb Central ranged between δ34S values of 9.4‰ and 12.6‰. For the Gravettian at the site, Level Va one individual had a lower δ34S value of 6‰ with a δ15N value of 8‰. All other specimens from Levels Va, IV and Vb Upper fall within a δ34S range of 8.2‰−11.9‰.
Discussion
Broad trends in bone collagen values and its impact for environmental reconstruction
Within the red deer and horses sampled no δ13C value is higher than −22.5‰, meaning that they are consistent with the consumption of C3 plants in an open landscape, according to Drucker et al.24. They are also stable through time, indicating that there was little change in tree cover throughout the period of study (i.e., late MIS3). The higher δ13C values of horses, in comparison to red deer, observed in the Mousterian, and an opposite trend afterwards suggest a change in environment in the temporal sequence related to the horse niche. Typically horses persistently select poor quality, low-protein, high-fibre browse a fact which results in a relative depletion in the δ15N and δ13C values96, as seen in this study in the Châtelperronian onwards. A similar pattern has been observed in other Palaeolithic faunas in Central Europe, where horses are consistently depleted in 13C and 15N relative to red deer due to this dietary preference21,22,97–99. This change in the horse relative δ13C values demonstrate a shift in the suite of vegetation, resulting in niche separation of red deer and horses, likely expressing environmental shifts, resulting from wider climatic changes between the Mousterian and the later periods studied. Other palaeoenvironmental records in the region during this period of study are sparse (Table 4). The only Mousterian level with pollen information is Amalda VII, and it is mainly represented by Pinus and the Cichorioideae, although pollen preservation at the site was poor and pollen samples were selected from an area of the site with possible disturbance. During the Châtelperronian (represented by Level IX Lower at Labeko Koba) pollen evidence indicates that steppic vegetation dominated (Table 4). Similarly, trends based on the data from the sites with Aurignacian and Gravettian levels indicate that low tree cover, and steppic vegetation, such as the Poaceae/Gramineae grasses Cedrela tubiflora and Compositae liguliflor, dominated the landscape (Table 4). The shift towards more open, steppic landscapes, after the end of the Mousterian, environments more favourable to the niche of horse, may explain this change in the diet of horses, although its representation in the macromammal assemblages remained stable through the period of study in the eastern Cantabrian region (Table 3) and could indicate a climatic change at this time.
For the δ15N values, the presence of individuals with elevated values during the Mousterian, Aurignacian and Gravettian prevents broad patterns from being clearly observed. In the neighbouring region of SW France, a population level increase in δ15N values of between 2‰ and 4‰ during the Aurignacian was identified at around 31–35 ka uncal BP, being interpreted as a climatic change in the Early Aurignacian, potentially relating to episodes of aridity21.
Larger ranges in δ15N values in the Cantabrian Region
The wide inter-individual nitrogen ranges observed within archaeological levels during the Mousterian, Aurignacian and Gravettian levels established in both the red deer and horse requires further exploration. It is not seen during Châtelperronian, but this may be a product of the small sample, consisting of only one archaeological level from one site.
Typically differences between herbivorous individuals of up to 2‰ in δ15N are expected on an intra-site level94. In this study, however, the dataset shows a δ15N value range of 5‰ and above for both species. Furthermore, the site of Antoliñako Koba, also in Gipuzkoa, exhibited a similar phenomenon to the current study with six red deer dating to the Gravettian period having δ15N values greater than 7‰ (maximum value 8.1‰, range 4.8‰, σ = 1.8, Table 6 in100), alongside a group of five individuals with lower values (3–5‰)97. Comparative red deer datasets of archaeological specimens, predominantly from the Late Upper Palaeolithic, all fall within lower δ15N ranges (typically between 3 and 5‰) with smaller standard deviations (Table 6)100.
The causes of elevated δ15N values in bone collagen can be related to either physiological or environmental factors. Regarding physiology, juvenile animals being nursed produce a δ15N value around one trophic level above that of their mothers87,88,101. However, all individuals analysed in this study were archaeozoologically determined to be adults, based on bone fusion, so this factor can be disregarded. Short-term stress episodes such as starvation or pregnancy can impact on δ15N values within an individual102,103, although to register in the long-term bone collagen signature, this had to have been experienced over long durations. Physiological factors are not sufficient to explain the pattern.
In environmental terms, altitude has been observed to have both positive104,105 and negative effects106 on δ15N values of plants and animal tissues. Positive correlations between plant δ15N values and altitude, related to soil activity and temperature differences, could exist, although a change in δ13C might also be expected. Altitudinal differences of up to 1,000–4,000 m are needed to have a substantial impact on isotopic values in plants104,107. Altitudes of up to 2,000 m in the Cantabrian Cordillera exist, but only in the west31, although none of the sites in our sample are located over 350 m.a.s.l. Additionally, feeding habits at high altitudes are not consistent with the ecology of horse and red deer, which typically do not inhabit higher elevations for prolonged periods of time. Modern ecological studies of red deer populations inhabiting mountainous regions demonstrate that red deer typically do not reach altitudes above 500 m108, although caution must be considered when using modern ecological analogues. Nonetheless altitude differences are not sufficient to explain the large differences in δ15N values seen within this dataset. Another environmental factor is the difference in the parts of plants consumed by the animals. An increase in leaf consumption or a shift from eating shrubs or trees to grasses could both explain the increased δ15N values109,110. Vegetation change was cited as an explanation for increased ranges in δ15N values of red deer in the Jura uplands of eastern France during the Late Glacial period23. If regular consumption of different types of vegetation by individuals could cause differences in the δ15N values, we would expect this to typically affect the δ13C values as well111 which is not observed in this study, meaning that other environmental factors must be at play.
Differing mycorrhiza in ecosystems depending on localised conditions can impact on 15N concentrations absorbed by plants112,113. Soil activity of nitrogen fixing mycorrhizal is reduced within colder environments and consequently, a temperature decrease is observed114,115, and decreased δ15N is often associated with lower mean annual temperatures116. Soil nitrogen content within different floodplains can also vary depending on water table height117, impacting on plants growing in different valley systems. Additionally, several worldwide studies demonstrate a negative correlation between plant δ15N values and precipitation116,118–120. Increased aridity due to reduced precipitation can produce 15N-enriched values105,121–124. Consequently, animals habitually feeding in drier environments would be expected to have higher δ15N values than those consistently feeding in wetter environments.
Given the above, to explain differing δ15N values in bone collagen within individuals in the same archaeological level, there are two plausible scenarios. Firstly, it could be that animals brought to the caves were being hunted in two isotopically distinct environments (‘isozones’), producing a mixed assemblage of animals with different isotopic signatures within the same archaeological levels, as has been recently proposed for contemporary regional sites in the central area of the Cantabrian region125. At early times in site occupation, humans might have consumed “trail food”—joints of transported meat acquired in their area of hunting—before they acquired local game. It is conceivable that human groups moved across the low Basque Mountains between the upper Ebro drainage and the Cantabrian coastal region as part of seasonal rounds (as suggested by the presence of Trans-Cordilleran flints (Urbasa, Treviño, etc.) in the sites of Bizkaia and Gipuzkoa. Secondly, it is conceivable that environmental fluctuations occurred during the formation of the archaeological levels, with changing environmental conditions of temperature and aridity, causing the accumulation of individual animals with distinctly different stable isotope signatures, as proposed by Bocherens et al.21 for the ungulate assemblage studied in the SW France during the Middle to Upper Palaeolithic transition. The long-time formation of the archaeological levels analysed converted them into thick palimpsests which could represent multiple human occupations perhaps over centuries, and this fact might also explain the changes in the observed climatic conditions.
Regarding the first hypothesis, the δ15N results could be a result of animals feeding in two isotopically distinct territories, with human activity being responsible for bringing these two groups of animals habitually feeding within different geographical regions: one, with lower δ15N values and another, with higher δ15N values. Animals may have been procured from different valley systems, which may have differing soil δ15N values due to mycorrhiza112,113 or with varying baseline nitrogen values related to water table height117. The lithology in the region is highly varied, with Jurassic and Cretaceous rocks predominating in the coastal regions of the Basque country, interspersed with pockets of Triassic clays, gypsum and seams of tertiary rocks within the Ebro basin and further south, comprising predominantly of Tertiary rocks126,127. This high diversity in rock types, coupled with distinctive topographical conditions, could support the existence of micro-environments within the region, as seen in the province of Cantabria to the West125. The specimens from Antoliñako Koba in Bizkaia, showed a similar scenario of higher and lower ungulates δ15N values during the Gravettian100 (Table 9). The explanation provided was that those animals might have been obtained from different hunting locations. Lower δ15N values are seen during the Châtelperronian of Labeko Koba (Level IX lower), where ungulates brought to the site by carnivores and alter scavenged by humans, are indicative of a local δ15N signature at that time. Another possibility is that the outlying individuals came from a drier region, such as the area to the south of the Cantabrian cordillera, potentially the Llanada Alavesa in Alava, Province of the Basque Autonomous Community (Fig. 1), in the rain shadow of the mountains and outside the reach of the Foehn effect. Proposed hunting ranges during the Middle and Upper Palaeolithic, based on the Optimal Foraging Theory, suggest that hunting territories were modified to target higher ranked prey, with movements between the coast and the mountains86, although the mobility in this particular region based on predictive models to calculate the potential distribution of ungulates, according to topography and related vegetation, indicate an area of exploitation within the two-hour walking territories of the sites128. At Axlor and Amalda studies suggest that, due to the steep topography surrounding these sites, occupants would have had extended territories to target specially selected prey128. Based on this, hunting in various locations within the wider sites was an option as reveal by the catchment territory of lithic raw materials in the sites analysed38. Thus, macromammal assemblages from the studied sites represent taxa related to the topographic location of each site, with high representation of montane (or at least, steep, rock environment) ungulates, fluvial valleys and plains or all of the above. In the sites studied, red deer is the most common taxon (24%), followed by bovines (18%), horses (7%), Spanish ibex (7%) (Table 3) and exceptionally, chamois (at most 42%) is highly represented in Amalda Levels VI-VII attributed by one researcher to a carnivore accumulation with sporadically human occupations40,42. Taphonomic analyses in Aitzbitarte III show that prey transport was mostly dependent on body size. Age profiles indicate exploitation of prime-age individuals, although infantile and juveniles are also represented43. Bone marrow extraction has been documented in all the sites, in combination with butchering marks on the ungulates and even on some carnivores41,42. For instance, in Aitzbitarte III Level Vb, cut marks were identified in an ulna of cave bear and, in Axlor exploitation of carnivores (wolf and dhole) and birds (raven and golden eagle) by Neanderthals were also documented39.
Table 9.
Red deer comparisons of mean, minimum, maximum and range δ15N values from existing isotopic studies in the Cantabrian Region. No horse specimens were available as a comparison.
| Province | Eastern Cantabria Region Middle Pal and Early Upper Pal. (This Study) | El Castillo Cave Middle Pal and Early Upper Pal121 | Covalejos Cave Middle Pal and Early Upper Pal121 | Kiputz IV Paleontological site (~25-13 ka cal BP)143 | Antoliña Koba Upper Pal (~36–12 ka cal BP)97 | El Mirón Late Upper Palaeolithic30 | La Paloma (Late Upper Pal.)144 |
|---|---|---|---|---|---|---|---|
| Gipuzkoa and Bizkaia | Cantabria | Cantabria | Gipuzkoa | Bizkaia | Cantabria | Asturias | |
| n= | 88 | 45 | 33 | 29 | 28 | 127 | 18 |
| Mean | 4.0 | 3.8 | 4.4 | 3.7 | 4.7 | 2.9 | 4.9 |
| Minimum | 1.3 | 1.0 | 1.4 | 2.4 | 3.4 | 0.6 | 3.9 |
| Max | 9.2 | 5.2 | 7.9 | 5.5 | 8.1 | 4.5 | 5.9 |
| Range | 7.9 | 4.2 | 6.5 | 3.1 | 4.8 | 3.9 | 2.1 |
| 1σ | 1.6 | 0.9 | 1.4 | 0.9 | 1.8 | 0.6 | 0.6 |
To evaluate animal provenance further, δ34S analyses was undertaken. Sulphur studies of modern ecosystems note a 1.9‰ difference within ungulate populations, with higher variability in archaeological studies of 2.4‰ variation observed20. At Labeko Koba (Level IX lower) a 3‰ difference was observed between specimens, all of which resulted from a hyena-formed deposit, later scavenged by humans68 and can be assumed to represent animals accumulated from the local area. For the other sites studied, excluding outliers, a range of 4‰ was observed. This suggests that the level of variation for animals feeding within the same region could be up to 3-4‰, particularly in landscapes within deep, narrow, sinuous valleys. The δ34S values from all levels at Amalda and Aitzbitarte III were typically higher than those seen within the levels analysed at Labeko Koba and may not have derived from the same locality. All three sites are in different, but adjacent valleys, which may explain the differences in baseline δ34S values observed between them (Fig. 7). One red deer from Amalda Level VI and one from Aitzbitarte III Level Va had very low δ34S values in comparisons to other analysed from the same levels, suggesting procurement of animals from at least two locations by the inhabitants of these sites (Fig. 7).
There was no linear correlation between δ15N and δ34S values, suggesting that if the animals with higher δ15N were being hunted from elsewhere, they were not necessarily coming from a different sulphur isoscape. Considering that 34S systems are dictated by geographically determined factors, such as rock type and precipitation20,95 and δ15N systems are controlled by more biological factors, such as nitrogen fixation by plants, nitrogen and sulphur isoscapes may not necessarily be the same.
The hypothesis of multiple catchment territories with different geographical and environmental conditions producing distinctive isotopic values (i.e. from the Llanada Alavesa) could be considered less feasible, when contemplating hunting efficiency for these hunter-gatherer groups in relation to the distance to the camp site and prey size hunted, among other factors129.
Regarding the second hypothesis, the high variability of δ15N values of the red deer and horses within the same archaeological levels could be a product of cycling environmental conditions within these archaeological levels (accumulated usually during multiple human occupations along hundreds of years and sometimes, thousands), potentially relating to fluctuating temperatures and cycling periods of aridity. The second part of MIS3 was a time of remarkable instability with abrupt and rapid climatic changes. The Heinrich events produced a sequence of cooling and warming cycles2 and the Dansgaard-Oeschger cycles presented periods of climatic fluctuation11, occurring sporadically within millennial scales1,4,130. Offshore records from the NW of the Iberian Peninsula exhibit cycles of wetter-drier periods13. Marine core MD95-2042, from the SW Margin of the Iberian Peninsula, demonstrated millennial scale climatic changes, ranging from temperate and humid to cold and dry continental conditions130. Available environmental proxies for these sites do not exhibit evidence of intra-level fluctuations (Table 4), although these indicators may not be sensitive enough to reflect these smaller changes on this temporal scale. Isotopic values obtained in this study provide a direct indication of past environment, directly related to evidence of human occupation.
In terms of chronology of the timescales of the levels involved with the fluctuating cycling climatic conditions, the Mousterian levels within Axlor date beyond the radiocarbon limit, and it is possible that these represent thick deposits spanning several millennia. For the other archaeological levels analysed, the chronologies represented are much tighter. At Aitzbitarte III Level Vb Upper to c. 32-31 ka BP and Level Vb Central dated to c. 31 ka BP, Level Va it is 31–26 ka BP and Level IV it is 29 ka BP. For Labeko Koba, the chronologies are equally well defined, with Level IV dating to 33.5 ka BP, Level V to 34.6 ka BP, Level VI to c. 35 ka BP and Level IX Lower to 38-37 ka BP35 (Table 2). The tight chronological resolution of these levels, means that environmental oscillations would had to have occurred in a timeframe of hundreds of years, and the abrupt nature of these changes could have implications for human subsistence and behaviour. Other contemporaneous European sites with analyses of both red deer and horses21,23,131 do not show evidence of rapid frequency of environmental shifts, a fact which may be related to site formation chronologies or local environmental conditions in the particular studied regions. Further exploration of this hypothesis is required, with more precise chronological timescale, in additional regions occupied by Neanderthals and AMH that represent similar timeframes, as presented here, would be beneficial in the future.
Conclusions
The results of this study of bone collagen δ13C, δ15N and δ34S values conducted on animal remains, with evidence of human manipulation, pertaining to the Middle-Upper Palaeolithic transition in the Cantabrian Region of Northern Spain provide a human-related reconstruction of the past environmental conditions at the time the replacement of late Neanderthals by the anatomically modern human populations took part in the Atlantic zone of Iberia. During the Palaeolithic, the Cantabrian region was special in providing a variety of habitats to exploit for both human species, and whilst MIS3 was a period of climatic instability in Europe, this did not prevent either human populations from successfully occupying this region. The δ13C values results show that after the conclusion of the Mousterian, a shift in climate, was expressed in the eastern part of the Cantabrian region by a change in the dominant vegetation, generating more open landscapes, with the horse niche changing to favour the lower-quality browse that they preferentially consume, an hypothesis which is supported by the other environmental proxies including sedimentology, pollen and micromammals. The δ15N results show high inter-individual variability within the same archaeological levels in the Mousterian, Aurignacian and Gravettian. This could be linked to animals hunted in different territories, although whether this represents micro-environments within the eastern Cantabrian region, and the same valley system, as proposed for the central region of Cantabria or animal carcasses (or joints of meat) being brought from further afield as trail food, is difficult to determine. However, we do not discard the possibility that these large differences in δ15N values are more a reflection of changing environments during the formation of these archaeological levels, representing several generations of human occupation activity. If so, changing environmental conditions across the transition would have had implications for human evolution and adaptive skills. Further research is required to explore, involving high resolution sampling and dating to observe the difference variation in isotopic values among the animals consumed by human groups during the period of study in this region.
Methods summary
Collagen extraction was undertaken following procedures outlined in Richards and Hedges132 with an ultra-filtration step133. Bone fragments between 0.5 and 0.8 g were cleaned using aluminium oxide air abrasion, before demineralisation in 0.5 M HCL at 6–8 °C for between 3–10 days,and were washed three times using de-ionised water. Samples were gelatinised in a weak acidic solution (pH3 HCL) at 70 °C for 48 hours, then filtered with 5–8 μm Ezee® filters, prior to ultrafiltration to separate out the larger >30ka collagen chains. The >30ka fraction was frozen and lyophilized for 48 hours. Collagen was analysed in duplicate, using a Delta XP mass spectrometer coupled to a Flash EA 2112 elemental analyser at the Department of Human Evolution, Max Planck Institute for Evolutionary Anthropology (Leipzig, Germany). The δ13C values and δ15N values are reported relative to the V-PDB and AIR standards. International and internal standards were used to calculate analytical error which was ± 0.1‰ (1σ) or better. The mean difference observed between duplicate measurements was 0.03 for δ13C, and 0.01 for δ15N. Sufficient collagen was not available for duplicate analysis for specimens: AXL01, LAB06, and EK02, EK04, EK05 and EK06. Analysis for δ34S values was undertaken at the University of British Columbia Stable isotope laboratory in Vancouver using a MicroCube IsoPrime 100 DI mass spectrometer.
Isotopic values were analysed statistically using a Mann-Whitney U test, with a post-hoc Holm-Bonferroni correction134. A p value of <0.05 or less was deemed to be indicative of a statistically significant result. To compare nitrogen value groupings within the dataset classical cluster analysis was used. All tests were undertaken using the statistical package PAST135.
A summary of environmental proxies at the sites investigated in this study, including pollen, microfauna, macrofauna and sedimentology, when available, have been included in the discussion to enhance interpretation of the results and provide general environmental context (Table 4).
Electronic supplementary material
Acknowledgements
This research was funded by the European Commission through a Marie Curie Career Integration Grant (FP7-PEOPLE-2012-CIG-322112), by the Spanish Ministry of Economy and Competitiveness (HAR2012-33956 and Ramon y Cajal-2011-00695), the University of Cantabria and Campus International to ABMA. Radiocarbon dating at ORAU was funded by MINECO-HAR2012-33956 project. J.J was supported initially by the FP7-PEOPLE-2012-CIG-322112 and later by a Marie Curie Individual Fellowship (H2020-MSCA-IF-2014-656122). Laboratory work, associated research expenses and isotopic analysis were kindly funded by the Max Planck Society to M.R. The authors would like to thank Museo de Arqueología de Bizkaia and Centro de Custodia de los Materiales Arqueológicos de Gipuzkoa (Basque Government) for access to the collections. Annabell Reiner provided essential lab support in the MPI-EVA laboratories, and Sven Steinbrenner supported the mass spectrometry aspects of the project. Many thanks also to Reba McDonald and Megan Wong (UBC) for assisting with the sulphur analysis undertaken and Luis Teira and Lucia Agudo for their help with pictures and figures. Supporting data can be accessed in the Supporting Material file.
Author Contributions
Conceptualization: A.B.M.A., Data curation: J.J., H.R., A.B.M.A., Formal analysis: A.B.M.A., J.J., Funding acquisition: A.B.M.A., M.R., Investigation: A.B.M.A., J.J., H.R., M.R., L.G.S., J.A., K.M., Methodology: M.R., J.J., H.R., Project administration: A.B.M.A., Resources: A.B.M.A., M.R., Writing – original draft: J.J., A.B.M.A., Writing – review & editing: J.J., M.R., L.G.S., H.R., J.A., K.M., A.B.M.A.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-32493-0.
References
- 1.Ditlevsen PD, Ditlevsen S, Andersen KK. The fast climate fluctuations during the stadial and interstadial climate states. Ann. of Glaciol. 2002;35:457–462. doi: 10.3189/172756402781816870. [DOI] [Google Scholar]
- 2.Kindler P, et al. Temperature reconstruction from 10 to 120 kyr b2k from the NGRIP ice core. Clim. of the Past. 2014;10:887–902. doi: 10.5194/cp-10-887-2014. [DOI] [Google Scholar]
- 3.Higham TFG, et al. The timing and spatio-temporal patterning of Neanderthal disappearance. Nature. 2014;512:306–309. doi: 10.1038/nature13621. [DOI] [PubMed] [Google Scholar]
- 4.d’Errico F, Goñi MFS. Neandertal extinction and the millennial scale climatic variability of OIS 3. Quat. Sci. Rev. 2003;22:769–788. doi: 10.1016/S0277-3791(03)00009-X. [DOI] [Google Scholar]
- 5.Finlayson C. Neanderthals and Modern Humans: An Ecological and Evolutionary Perspective (Cambridge University Press, 2004).
- 6.Wolf D, et al. Climate deteriorations and Neanderthal demise in interior Iberia. Scientific Reports. 2018;8(1):7048. doi: 10.1038/s41598-018-25343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banks WE, et al. Neanderthal Extinction by Competitive Exclusion. PLoS One. 2008;3:3972. doi: 10.1371/journal.pone.0003972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrión JS, et al. The palaeoecoloical potential of pollen records in caves: the case of Mediterranean Spain. Quat. Sci. Revs. 1999;18:1061–1073. doi: 10.1016/S0277-3791(98)00002-X. [DOI] [Google Scholar]
- 9.Bryant VM, Jr., Holloway RG. The role of palynology in archaeology. Adv. In Arch. Method and Theory. 1983;6:191–224. [Google Scholar]
- 10.Coles GM, Gilbertson DD, Hunt CO, Jenkinson RDS. Taphonomy and the palynology of cave sediments. Cave Sci. 1989;16:83–8. [Google Scholar]
- 11.Dansgaard W, et al. Evidence for general instability of past climate from a 250-kyr ice-core record. Nature. 1993;364:218–220. doi: 10.1038/364218a0. [DOI] [Google Scholar]
- 12.Sanchez Goñi MFS, Turon JL, Eynaud F, Gendreau S. European climatic response to millennial-scale changes in the atmosphere–ocean system during the Last Glacial period. Quat. Res. 2000;54:394–403. doi: 10.1006/qres.2000.2176. [DOI] [Google Scholar]
- 13.Naughton F, et al. Wet to dry climatic trend in north-western Iberia within Heinrich events. Adv. in Arch. Method and Theory. 2009;284:329–342. [Google Scholar]
- 14.Blockley SP, et al. Tephrochronology and the extended intimate (integration of ice-core, marine and terrestrial records) event stratigraphy 8–128 ka b2k. Quat. Sci. Revs. 2014;106:88–100. doi: 10.1016/j.quascirev.2014.11.002. [DOI] [Google Scholar]
- 15.Sepulchre P, et al. H4 abrupt event and late Neanderthal presence in Iberia. Earth and Planet. Sci. Letters. 2007;258:283–292. doi: 10.1016/j.epsl.2007.03.041. [DOI] [Google Scholar]
- 16.Hedges REM, Stevens RE, Richards MP. Bone as a stable isotope archive for local climatic information. Quat. Sci. Rev. 2004;23:959–965. doi: 10.1016/j.quascirev.2003.06.022. [DOI] [Google Scholar]
- 17.Ambrose, S. H., Norr, L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate in Prehistoric Human Bone Archaeology at the Molecular Level (eds Grupe, G. & Lambert, J. L.) 1–13 (Springer-Verlag, 1993).
- 18.Hedges REM, Clement JG, Thomas DL, O’Connell TC. Collagen turnover in the adult femoral mid-shaft: modelled from anthropogenic radiocarbon tracer measurements. Am. J. Phys. Anthropol. 2007;133:808–816. doi: 10.1002/ajpa.20598. [DOI] [PubMed] [Google Scholar]
- 19.Stenhouse, M.J., Baxter, M.S. The uptake of bomb 14C in humans in Radiocarbon Dating (eds Suess, H. E., Berger, R.) 324–341 (Berkeley, 1979).
- 20.Nehlich O. The application of sulphur isotope analyses in archaeological research: a review. Earth-Sci. Rev. 2015;142:1–17. doi: 10.1016/j.earscirev.2014.12.002. [DOI] [Google Scholar]
- 21.Bocherens H, Drucker DG, Madelaine S. Evidence for a 15N positive excursion in terrestrial foodwebs at the Middle to Upper Palaeolithic transition in south-western France: Implications for early modern human palaeodiet and palaeoenvironment. J. Hum. Evol. 2014;69:31–43. doi: 10.1016/j.jhevol.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Britton KH, Gaudzinski-Windheuser S, Roebroeks W, Kindler L, Richards MP. Stable isotope analysis of well-preserved 120,000-year-old herbivore bone collagen from the Middle Palaeolithic site of Neumark-Nord 2, Germany reveals niche separation between bovids and equids. Palaeogeogr. Palaeoclimotol. Palaeoecol. 2012;333–334:168–177. doi: 10.1016/j.palaeo.2012.03.028. [DOI] [Google Scholar]
- 23.Drucker DG, Bocherens H, Billiou D. Evidence for shifting environmental conditions in Southwestern France from 33 000 to 15 000 years ago derived from carbon-13 and nitrogen-15 natural abundances in collagen of large herbivores. Earth and Planet. Sci. Letters. 2003;216:163–173. doi: 10.1016/S0012-821X(03)00514-4. [DOI] [Google Scholar]
- 24.Drucker D, Bridault A, Hobson KA, Szuma E, Bocherens H. Can carbon-13 abundances in large herbivores track canopy effect in temperate and boreal ecosystems? Evidence from modern and ancient ungulates. Palaeogeog. Palaeoclim. Palaeoecol. 2008;266:69–82. doi: 10.1016/j.palaeo.2008.03.020. [DOI] [Google Scholar]
- 25.Drucker DG, Bridault A, Cupillard C, Hujic A, Bocherens H. Evolution of habitat and environment of red deer (Cervus elaphus) during the Late-glacial and early Holocene in eastern France (French Jura and the western Alps) using multi-isotope analysis (δ13C, δ15N, δ18O, δ34S) of archaeological remains. Quat. Int. 2011;245:268–278. doi: 10.1016/j.quaint.2011.07.019. [DOI] [Google Scholar]
- 26.Drucker DG, et al. Tracking possible decline of woolly mammoth during the Gravettian in Dordogne (France) and the Ach Valley (Germany) using multi-isotope tracking (13 C, 14 C, 15 N, 34 S, 18 O) Quat. Int. 2015;359:304–317. doi: 10.1016/j.quaint.2014.11.028. [DOI] [Google Scholar]
- 27.Richards MP, Hedges REM. Variations in bone collagen N13C and N15N values of fauna from Northwest Europe over the last 40 000 years. Palaeogeo. Palaeoclim. Palaeoecol. 2003;193:261–26. doi: 10.1016/S0031-0182(03)00229-3. [DOI] [Google Scholar]
- 28.Stevens RE, Hedges REM. Carbon and Nitrogen Stable Isotope Analysis of Northwest European Horse Bone and Tooth Collagen, 40,000 BP-Present: Palaeoclimatic Interpretations. Quat. Sci. Revs. 2004;23:977–991. doi: 10.1016/j.quascirev.2003.06.024. [DOI] [Google Scholar]
- 29.Szpak P, et al. Regional differences in bone collagen δ13C and δ15N of Pleistocene mammoths: implications for paleoecology of the mammoth steppe. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010;286:88–96. doi: 10.1016/j.palaeo.2009.12.009. [DOI] [Google Scholar]
- 30.Stevens RE, Hermoso-Buxán XL, Marín-Arroyo AB, González-Morales MR, Straus LG. Investigation of Late Pleistocene and Early Holocene palaeoenvironmental change at El Mirón cave (Cantabria, Spain): Insights from carbon and nitrogen isotope analyses of red deer. Palaeogeogr. Palaeoclimotol, Palaeoecol. 2014;414:46–60. doi: 10.1016/j.palaeo.2014.05.049. [DOI] [Google Scholar]
- 31.Straus LG. Recent developments in the study of the Upper Paleolithic of Vasco-Cantabrian Spain. Quat. Int. 2015;364:255–271. doi: 10.1016/j.quaint.2014.05.008. [DOI] [Google Scholar]
- 32.Straus, L.G. Iberia before the Iberians. Stone Age Prehistory of Cantabrian Spain (University New Mexico Press, 1992).
- 33.Marín-Arroyo AB, et al. Chronological reassessment of the Middle to Upper Paleolithic transition and Early Upper Paleolithic cultures in Cantabrian Spain. PLoS ONE. 2018;13:194708. doi: 10.1371/journal.pone.0194708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maroto J, et al. Current issues in late Middle Palaeolithic chronology: New assessments from Northern Iberia. Quat. Int. 2012;247:15–25. doi: 10.1016/j.quaint.2011.07.007. [DOI] [Google Scholar]
- 35.Wood RE, et al. The chronology of the earliest Upper Palaeolithic in northern Iberia: New insights from L’Arbreda, Labeko Koba and La Viña. J. Hum. Evol. 2014;69:91–109. doi: 10.1016/j.jhevol.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Wood R, et al. El Castillo (Cantabria, northern Iberia) and the Transitional Aurignacian: Using radiocarbon dating to assess site taphonomy. Quat. Int. 2018;474:56–70. doi: 10.1016/j.quaint.2016.03.005. [DOI] [Google Scholar]
- 37.Ábalos B. Geologic map of the Basque-Cantabrian Basin and a new tectonic interpretation of the Basque Arc. Int J Earth Sci (Geol Rundsch) 2016;105:2327. doi: 10.1007/s00531-016-1291-6. [DOI] [Google Scholar]
- 38.Rios-Garaizar J. A new chronological and technological synthesis for Late Middle Paleolithic of the Eastern Cantabrian Region. Quat. Int. 2017;433:50–63. doi: 10.1016/j.quaint.2016.02.020. [DOI] [Google Scholar]
- 39.Gomez-Olivencia A, et al. First data of Neandertal bird and carnivore exploitation in the Cantabrian Region (Axlor; Barandiaran excavations; Dima, Biscay, Northern Iberian Peninsula) Scientific Rep. 2018;8:10551. doi: 10.1038/s41598-018-28377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yravedra J. Acumulaciones biológicas en yacimientos arqueológicos: Amalda VII y Esquilleu III-IV. Trabajos de prehistoria. 2006;63:55–78. [Google Scholar]
- 41.Yravedra J. Nuevas contribuciones en el comportamiento cinegético de la Cueva de Amalda. Munibe (Antropologia-arkeologia) 2007;58:43–88. [Google Scholar]
- 42.Altuna J, Mariezkurrena K. Tafocenosis en yacimientos del País Vasco con predominio de grandes carnívoros. Consideraciones sobre el yacimiento de Amalda. Zona arqueológica. 2010;13:214–228. [Google Scholar]
- 43.Altuna, J. & Mariezkurrena, K. Estudio de los Macromamíferos del Yacimiento de Aitzbitarte III (Excavación De La Entrada) in Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva. (eds Altuna, J., Mariezkurrena, K. & Rios, J.) 395–478 (Colección de Patrimonio Cultural vasco 5, 2013).
- 44.Iriarte Chiapusso, J. El medio vegetal del yacimiento de Aitzbitarte III (Rentería, País Vasco). A partir de su análisis palinológico in Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva. (eds Altuna, J., Mariezkurrena, K., & Rios, J.) 57–75 (Colección de Patrimonio Cultural vasco 5, 2013).
- 45.Iriarte MJ. El entorno vegetal del yacimiento paleolítico de Labeko Koba (Arrasate, País Vasco): análisis polínico. Munibe (Antropologia-Arkeologia) 2000;52:89–106. [Google Scholar]
- 46.Uzquiano, P. El registro antracológico durante la transición Musteriense-Paleolítico Superior Inicial en la Región Cantábrica: vegetación, paleoambiente y modos de vida alrededor del fuego in El Paleolítico Medio cantábrico: Hacia una revisión actualizada de su problemática (eds Montes Barquín, R. & Lasheras Corruchaga, J. A.) 255–274 (Museo Nacional y Centro de Investigación de Altamira, 2005).
- 47.Uzquiano, P. Domestic fires and vegetation among Neanderthals and Anatomically Modern Humans (>53-30 kyrs BP) in the Cantabrian Region, Cantabria, N Spain. 273–285 (BAR International Series, 2008).
- 48.López-García, et al. Palaeoenvironment and palaeoclimate of the Mousterian–Aurignacian transition in northern Iberia: The small-vertebrate assemblage from Cueva del Conde (Santo Adriano, Asturias) J. Hum. Evol. 2011;61:108–116. doi: 10.1016/j.jhevol.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Cuenca-Bescós G, Straus L, González Morales MR, Garcia- Pimienta J. The reconstruction of past environments through small mammals: from the Mousterian to the Bronze Age in El Mirón Cave (Cantabria, Spain) J. Arch. Sci. 2009;36:947–955. doi: 10.1016/j.jas.2008.09.025. [DOI] [Google Scholar]
- 50.Barandiarán Ayerbe, J. M. Excavaciones en Axlor. 1967–1974. In Obras Completas de José Miguel de Barandiarán Tomo XVII (edBarandiarán, J. M.) 127–384 (La Gran Enciclopedia Vasca (1980).
- 51.Baldeón A. El Abrigo de Axlor (Bizkaia, País Vasco). Las industrias líticas de sus niveles musterienses. Munibe (Antropologia-Arkeologia) 1999;51:9–121. [Google Scholar]
- 52.González Urquijo, E., Ibáñez Estévez, J. J., Rios Garaziar, J., & Bourguignon, L. Aportes de las nuevas excavaciones en Axlor sobre el final del Paleolítico In En el centenario de la cueva de El Castillo: el ocaso de los neandertales (eds Cabera Valdés, V., Quirós Guidotti, F. B., Bernaldo de Quirós, F. and Maíllo Fernández, F.) 269–290 (Centro Asociado a la Universidad Nacional de Educación a Distancia en Cantabria, 2005).
- 53.Rios-Garaizar J, González-Urquijo J, Ibáñez-Estévez JJ. Abrigo de Axlor (Dima) Arkeoikuska. 2004;2004:75–79. [Google Scholar]
- 54.González-Urquijo JE, Ibáñez-Estévez JJ. 2002. Abrigo de Axlor (Dima) Arkeoikuska: Investigación arqueológica. 2001;2001:90–93. [Google Scholar]
- 55.Rios-Garaizar, J. Industria lítica y sociedad del paleolítico medio al superior en torno al golfo de Bizkaia (Vol. 3). (Universidad de Cantabria, 2012).
- 56.Altuna, J. Subsistance d’origine animale pendant le Mousterien dans la Region Cantabrique (Espagne) In L’Homme de Neandertal. La Subsistance. Actes du Colloque International de Liège, vol. 6 (eds Pathou, M. & Freeman, L. G.) 41-43 (ERAUL, 1989).
- 57.Castaños, P. Revisión actualizada de las faunas de macromamíferos del Würm antiguo en la Región Cantábrica in Actas de La Reunión Científica: Neandertales Cantábricos, Estado de La Cuestión (eds Montes, R. & Lasheras, J. A) 201–207 (Ministerio de Cultura, 2005).
- 58.Barandiarán, J. M. Bolinkoba y otros yacimientos paleolíticos en la sierra de Amboto (Vizcaya) (Seminario de Historia Primitiva del Hombre, 1950).
- 59.Iriarte-Chiapusso, M. J. & Arrizabalaga, A. El yacimiento arqueológico de Bolinkoba (Abadiño, Bizkaia). Crónica de las investigaciones en la cavidad. Secuencia estratigráfica y cronología numérica in Bolinkoba (Abadiño) Y su Yacimiento Arqueológico: Arqueología de La Arqueología Para La Puesta En Valor de Su Depósito, a La Luz de Las Excavaciones Antiguas Y Recientes (eds Iriarte-Chiapusso, M. J. & Arrizabalaga, A.) 5–88 (Kobie Serie BAI 6, 2015).
- 60.Castaños; P. & Castaños, J. Estudio de los macromamíferos del yacimiento de Bolinkoba (Abadiño, Bizkaia) In Bolinkoba (Abadiño) Y su Yacimiento Arqueológico: Arqueología de La Arqueología Para La Puesta En Valor de Su Depósito, a La Luz de Las Excavaciones Antiguas Y Recientes (eds Iriarte-Chiapusso, M. J., Arrizabalaga, A.) 103–113 (Kobie Serie BAI 6, 2015).
- 61.Garcia-Ibaibarriaga, N., Suarez-Bilbao, A., Ordiales, A., & Murelaga, X. Estudio de los microvertebrados del Pleistoceno superior de la cueva de Bolinkoba (Abadiño, Bizkaia) In Bolinkoba (Abadiño) Y su Yacimiento Arqueológico: Arqueología de La Arqueología Para La Puesta En Valor de Su Depósito, a La Luz de Las Excavaciones Antiguas Y Recientes (eds Iriarte-Chiapusso, M. J., Arrizabalaga, A.) 113–120 (Kobie Serie BAI 6, 2015).
- 62.Iriarte-Chiappusso, M. J. & Ayerdi, M. El registro arqueobotánico del yacimiento de Bolinkoba (Abadiño, Bizkaia), a la luz de las últimas excavaciones in Bolinkoba (Abadiño) Y su Yacimiento Arqueológico: Arqueología de La Arqueología Para La Puesta En Valor de Su Depósito, a La Luz de Las Excavaciones Antiguas Y Recientes (eds Iriarte-Chiapusso, M. J., Arrizabalaga, A.) 121–126 (Kobie Serie BAI 6. Diputación Foral de Bizkaia, 2015).
- 63.Arrizabalaga, A. & Altuna, J. Labeko Koba (País Vasco). Hienas y Humanos en los Albores del Paleolítico Superior. (Munibe Antropologia-Arkeologia 52 Sociedad de Ciencias Aranzadi, 2000).
- 64.Ríos Garaizar J, Libano Silvente I, Garate Maidagan D. El yacimiento chatelperroniense al aire libre de Aranbaltza (Barrika, Euskadi) Munibe (Antropologia-Arkeologia) 2012;63:81–92. [Google Scholar]
- 65.Arrizabalaga, A. Los tecnocomplejos líticos del yacimiento arqueológico de Labeko Koba (Arrasate, País Vasco). In Labeko Koba (País Vasco). Hienas y Humanos en los Albores del Paleolítico Superior (eds Arrizabalaga, A. & Altuna, J.) 193–343 (Munibe Antropologia-Arkeologia 52, Sociedad de Ciencias Aranzadi, 2000).
- 66.Arrizabalaga, A. El yacimiento arqueológico de Labeko Koba (Arrasate, País Vasco). Entorno. Crónica de las investigaciones. Estratigrafía y estructuras. Cronología absoluta. In Labeko Koba (País Vasco). Hienas y Humanos en los Albores del Paleolítico Superior (eds Arrizabalaga, A. & Altuna, J.) 15–72 (Munibe Antropologia-Arkeologia 52, Sociedad de Ciencias Aranzadi, 2000).
- 67.Zilhão J. Chronostratigraphy of the Middle-to-Upper Paleolithic transition in the Iberian Peninsula. Pyrenae. 2006;37:7–84. [Google Scholar]
- 68.Villaluenga A, Arrizabalaga A, Rios-Garaizar J. Multidisciplinary approach to two Châtelperronian series: lower IX layer of Labeko Koba and X Level of Ekain (Basque country, Spain) J. Taphon. 2012;10:525–548. [Google Scholar]
- 69.Rios-Garaizar J, Arrizabalaga A, Villaluenga A. Haltes de chasse du Châtelperronien de la Péninsule Ibérique: Labeko Koba et Ekain (Pays Basque Péninsulaire) L’Anthropologie. 2012;116:532–549. doi: 10.1016/j.anthro.2012.10.001. [DOI] [Google Scholar]
- 70.Altuna, J. & Merino, J. M. (eds) El yacimiento prehistórico de la cueva de Ekain (Deba, Guipúzcoa). (Sociedad de Estudios Vascos, Serie B1, 1984).
- 71.Rios-Garaizar J. El nivel IXb de Ekain (Deba, Gipuzkoa): Una ocupación efímera del Auriñaciense Antiguo. Munibe (Antropologia-Arkeologia) 2011;62:87–100. [Google Scholar]
- 72.Altuna, J., & Mariezkurrena, K. Bases de subsistencia de origen animal en el yacimiento de Ekain in El yacimiento prehistórico de la cueva de Ekain (Deba, Guipúzcoa) (eds Altuna J. and Merino, J. M.) 211–280 (Sociedad de Estudios Vascos, 1984).
- 73.Zabala, J. Los micromamíferos del yacimiento de Ekain in El yacimiento prehistórico de la cueva de Ekain (Deba, Guipúzcoa) (eds Altuna J. and Merino, J. M.) 317–330 (Sociedad de Estudios Vascos, 1984).
- 74.Altuna, J, Baldeón, A., & Mariezkurrena, K. La Cueva de Amalda (Zestoa, País Vasco): Ocupaciones Paleolíticas y Postpaleolíticas. Eusko Ikaskuntza, 1990).
- 75.Rios-Garaizar J. Organización económica de las sociedades Neandertales: el caso del nivel VII de Amalda (Zestoa, Gipuzkoa) Zephyrus. 2010;LXV:15–37. [Google Scholar]
- 76.Dupre, M. Análisis polínico de la cueva de Amalda in Cueva de Amalda (Zestoa, País Vasco). Ocupaciones Paleolíticas y Postpaleolíticas (eds Altuna, J., Baldeon, A. & Mariezkurrena, K.)149–192 (Sociedad de Estudios Vascos Serie B 4, 1990).
- 77.Pemán E. Los micromamíferos de Labeko Koba (Arrasate, País Vasco) Munibe Ciencias Naturales. 2000;52:183–185. [Google Scholar]
- 78.Altuna, J., Mariezkurrena, K. & Rios, J. (eds) Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva (Colección de Patrimonio Cultural vasco 5, 2013).
- 79.Pemán, E. Los Micromamíferos del Yacimiento de Aitzbitarte III (Rentería, Gipuzkoa) (zona de entrada) In Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva. (eds Altuna, J., Mariezkurrena, K., & Rios, J.) 481–491 (Colección de Patrimonio Cultural vasco 5, 2013).
- 80.Areso, P. & Uriz, A. Estudio del Sedimento del Yacimiento De Aitzbitarte III (Zona De Entrada) In Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva. (eds Altuna, J., Mariezkurrena, K., & Rios, J.) 39–54 (Colección de Patrimonio Cultural vasco 5, 2013).
- 81.Barandiarán, J. Exploración de la cueva de Lezetxiki en Mondragón (Memorias de los trabajos de 1956, 1957, 1959 y 1960 y 1961 a 1968). Obras Completas 423–480 (1978).
- 82.Arrizabalaga A. Las primeras ocupaciones humanas en el Pirineo Occidental y Montes Vascos. Un estado de la cuestión en 2005. Munibe (Antropologia-Arkeologia) 2005;57:53–70. [Google Scholar]
- 83.De la Rua C, Altuna J, Hervella M, Kinsley L, Grün R. Direct U-series analysis of the Lezetxiki humerus reveals a Middle Pleistocene age for human remains in the Basque Country (northern Iberia) J. Hum. Evol. 2016;93:109–119. doi: 10.1016/j.jhevol.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 84.Altuna J. Fauna de mamíferos de los yacimientos prehistóricos de Guipúzcoa. Munibe. 1972;24:3–41. [Google Scholar]
- 85.Altuna J, Mariezkurrena K. Macromamíferos del yacimiento de Labeko Koba (Arrasate, País Vasco) Munibe. 2000;52:107–181. [Google Scholar]
- 86.Marín-Arroyo AB. The use of Optimal Foraging Theory to estimate Late Glacial site catchment areas from a central place: The case of eastern Cantabria. Spain. J. Anth. Arch. 2009;28:27–36. doi: 10.1016/j.jaa.2008.11.001. [DOI] [Google Scholar]
- 87.Schurr MR. Using Stable Nitrogen-Isotopes to study weaning behaviour in past Populations. World Arch. 1988;30:327–342. doi: 10.1080/00438243.1998.9980413. [DOI] [PubMed] [Google Scholar]
- 88.Schurr MR. Stable Nitrogen Isotopes as Evidence for the Age of Weaning at the Angel Site: A Comparison of Isotopic and Demographic Measures of WeaningAge. J. Arch. Sci. 1997;24:919–927. doi: 10.1006/jasc.1996.0171. [DOI] [Google Scholar]
- 89.Higham T, et al. AMS radiocarbon dating of ancient bone using ultrafiltration. Radiocarbon. 2006;48:179–195. doi: 10.1017/S0033822200066388. [DOI] [Google Scholar]
- 90.Higham T. European Middle and Upper Palaeolithic radiocarbon dates are often older than they look: problems with previous dates and some remedies. Antiquity. 2011;85:235–249. doi: 10.1017/S0003598X00067570. [DOI] [Google Scholar]
- 91.DeNiro MJ. Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature. 1985;317:806–809. doi: 10.1038/317806a0. [DOI] [Google Scholar]
- 92.van Klinken GJ. Bone Collagen Quality Indicators for Palaeodietary and Radiocarbon Measurements. J. Archeol. Sci. 1999;26:687–695. doi: 10.1006/jasc.1998.0385. [DOI] [Google Scholar]
- 93.Nehlich O, Richards MP. Establishing collagen quality criteria for sulphur isotope analysis of archaeological bone collagen. Arch. and Anth. Sci. 2009;1:59–75. [Google Scholar]
- 94.Hedges RE, &Reynard LM. Nitrogen isotopes and the trophic level of humans in archaeology. J. Archeol. Sci. 2007;34:1240–1251. doi: 10.1016/j.jas.2006.10.015. [DOI] [Google Scholar]
- 95.Richards MP, Fuller BT, Sponheimer M, Robinson T, Ayliffe L. Sulphur isotopes in palaeodietary studies: a review and results from a controlled feeding experiment. Int J Osteoarchaeol. 2003;13:37–45. doi: 10.1002/oa.654. [DOI] [Google Scholar]
- 96.Sponheimer M, et al. Nitrogen isotopes in mammalian herbivores: hair δ15N values from a controlled feeding study. Int. J. of Osteoarchaeol. 2003;13:80–87. doi: 10.1002/oa.655. [DOI] [Google Scholar]
- 97.Bocherens H, Drucker DG, Billiou D, Patou-Mathis M, Vandermeersch B. Isotopic evidence for diet and subsistence pattern of the Saint-Césaire I Neanderthal: review and use of a multi-source mixing model. J. Hum. Evol. 2005;49:71–87. doi: 10.1016/j.jhevol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 98.Feranec R, García N, Díez JC, Arsuaga JL. Understanding the ecology of mammalian carnivorans and herbivores from Valdegoba cave (Burgos, northern Spain) through stable isotope analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010;297:263–272. doi: 10.1016/j.palaeo.2010.08.006. [DOI] [Google Scholar]
- 99.Fizet M, et al. Effect of diet, physiology and climate on carbon and nitrogen stable isotopes of collagen in late pleistocence anthropic palaeoecosystem: Marillac, Charente. France. J. Archaeol. Sci. 1995;22:67–79. doi: 10.1016/S0305-4403(95)80163-4. [DOI] [Google Scholar]
- 100.Rofes J, et al. Combining small-vertebrate, marine and stable-isotope data to reconstruct past environments. Scientific Rep. 2015;5:14219. doi: 10.1038/srep14219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dürrwächter C, Craig OE, Collins MJ, Burger J, Alt KW. Beyond the grave: variability in Neolithic diets in Southern Germany? J. Archaeol. Sci. 2005;33:39–48. doi: 10.1016/j.jas.2005.06.009. [DOI] [Google Scholar]
- 102.Fuller BT, et al. Nitrogen balance and d15N: why you’re not what you eat during nutritional stress. Rapid Commun. Mass Spectrom. 2005;19:2497–2506. doi: 10.1002/rcm.2090. [DOI] [PubMed] [Google Scholar]
- 103.Hauber D, Langel R, Scheu S, Ruess L. Effects of food quality, starvation and life stage on stable isotope fractionation in Collembola. Pedobiologia. 2005;49:229–237. doi: 10.1016/j.pedobi.2004.11.001. [DOI] [Google Scholar]
- 104.Mariotti A, Pierre D, Vedy JC, Bruckert S, Guillemot J. The abundance of natural nitrogen 15 in soils along an altitudinal gradient. Catena. 1980;7:293–300. doi: 10.1016/S0341-8162(80)80020-8. [DOI] [Google Scholar]
- 105.Hartman G, Danin A. Isotopic values of plants in relation to water availability in the Eastern Mediterranean region. Oecologia. 2010;162:837–852. doi: 10.1007/s00442-009-1514-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Männel TT, Auerswald K, Schnyder H. Altitudinal gradients of grassland carbon and nitrogen isotope composition are recorded in the hair of grazers. Global Ecol. and Biogeogr. 2007;16(5):583–592. doi: 10.1111/j.1466-8238.2007.00322.x. [DOI] [Google Scholar]
- 107.Zech M, et al. Human and climate impact on 15N natural abundance of plants and soils in high-mountain ecosystems: a short review and two examples from the Eastern Pamirs and Mt. Kilimanjaro. Isotopes Environ. Health Stud. 2011;47:286–296. doi: 10.1080/10256016.2011.596277. [DOI] [PubMed] [Google Scholar]
- 108.San José C, Braza F, Aragón S, Delibes JR. Habitat use by roe and red deer in Southern Spain. Miscellania Zoologica. 1997;20:27–38. [Google Scholar]
- 109.Michelsen A, Schmidt IK, Jonasson S, Quarmby C, Sleep D. Leaf 15N abundance of subarctic plants provides field evidence that ericoid, ectomycorrhizal and non- and arbuscular mycorrhizal species access different sources of soil nitrogen. Oecologia. 1996;105:53–63. doi: 10.1007/BF00328791. [DOI] [PubMed] [Google Scholar]
- 110.Hogberg P. 15N natural abundance in soil-plant systems. New Phytologist 137: 179-203 Keller M, Kaplan WA & Wofsy SC (1986) Emissions of N20, CH4, and CO2 from tropical soils. J. Geophys. Res. 1997;91:11791–11802. doi: 10.1046/j.1469-8137.1997.00808.x. [DOI] [PubMed] [Google Scholar]
- 111.Aerts R, Callaghan TV, Dorrepaal E, van Logtestijn RSP, Cornelissen JHC. Seasonal climate manipulations result in species-specific changes in leaf nutrient levels and isotopic composition in a sub-arctic bog. Function. Ecol. 2009;23:680–688. [Google Scholar]
- 112.Hobbie EA, Jumpponen A, Trappe J. Foliar and fungal 15N:14N ratios reflect development of mycorrhizae and nitrogen supply during primary succession: testing analytical models. Oecologia. 2005;146:258–268. doi: 10.1007/s00442-005-0208-z. [DOI] [PubMed] [Google Scholar]
- 113.Szpak P. Complexities of nitrogen isotope biogeochemistry in plant-soil systems: implications for the study of ancient agricultural and animal management practices. Frontiers in Plant Sci. 2014;5:288–298. doi: 10.3389/fpls.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Handley LL, et al. The 15-N natural abundance (δ15N) of ecosystem samples reflects measures of water availability. Austral. J. of Plant Physiol. 1999;26:185–199. doi: 10.1071/PP98146. [DOI] [Google Scholar]
- 115.Hobbie EA, Macko SA, Shugart HH. Patterns in N dynamics andN isotopes during primary succession in Glacier Bay. Alaska. Chem. Geol. 1998;152:3–11. doi: 10.1016/S0009-2541(98)00092-8. [DOI] [Google Scholar]
- 116.Amundson R, et al. Global patterns of the isotopic composition of soil and plant nitrogen. Glob. Biogeochem. Cycles. 2003;17:31–36. doi: 10.1029/2002GB001903. [DOI] [Google Scholar]
- 117.Hefting M, et al. Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry. 2004;67:113–134. doi: 10.1023/B:BIOG.0000015320.69868.33. [DOI] [Google Scholar]
- 118.Austin A, Vitousek PM. Nutrient dynamics on a precipitation gradient. Oecologia. 1998;113:519–529. doi: 10.1007/s004420050405. [DOI] [PubMed] [Google Scholar]
- 119.Aranibar JN, et al. Nitrogen cycling in the soil–plant system along a precipitation gradient in the Kalahari sands. Global Change Biology. 2004;10:359–373. doi: 10.1111/j.1365-2486.2003.00698.x. [DOI] [Google Scholar]
- 120.Pardo, L. H. & Nadelhoffer, K. J. Using nitrogen isotope ratios to assess terrestrial ecosystems at regional and global scales in Isoscapes: Understanding Movement, Pattern, and Process on Earth through Isotope Mapping (eds West, J., Bowen, G. J., Dawson, T. E. & Tu, K. P.) 221–249 (Springer, 2010).
- 121.Ambrose SH, DeNiro MJ. Reconstruction of African human diet using bone collagen carbon and nitrogen isotope ratios. Nature. 1986;319:321–324. doi: 10.1038/319321a0. [DOI] [Google Scholar]
- 122.Cormie AB, Schwarcz HP. Effects of climate on deer bone δ 15 N and δ 13 C: lack of precipitation effects on δ15N for animals consuming low amounts of C 4plants. Geochim. et Cosmochim. Acta. 1996;60:4161–4166. doi: 10.1016/S0016-7037(96)00251-7. [DOI] [Google Scholar]
- 123.Gröcke DR, Bocherens H, Mariotti A. Annual rainfall and nitrogen-isotope correlation in macropod collagen: application as a palaeoprecipitation indicator. Earth Planet. Sci. Lett. 1997;153:279–285. doi: 10.1016/S0012-821X(97)00189-1. [DOI] [Google Scholar]
- 124.Heaton THE, Vogel JC, von la Chevallerie G, Collett G. Climatic influence on the isotopic composition of bone collagen. Nature. 1986;322:822–823. doi: 10.1038/322822a0. [DOI] [Google Scholar]
- 125.Jones, J. R., Richards, M., Reade, H., Bernaldo de Quirós, F. & Marín-Arroyo, A. B. Multi-Isotope investigations of ungulate bones and teeth from El Castillo and Covalejos caves (Cantabria, Spain): Implications for paleoenvironment reconstructions across the Middle-Upper Palaeolithic transition. J. Archaeol. Sci. Rep., 10.1016/j.jasrep.2018.04.014 (2018).
- 126.Poprawski Y, et al. Sedimentary and structural record of the Albian growth of the Bakio salt diapir (the Basque Country, northern Spain) Basin Res. 2014;26:746–766. doi: 10.1111/bre.12062. [DOI] [Google Scholar]
- 127.Bodego, A. (ed.) Geología De La Cuenca Vasco-Cantábrica (Universidad del País Vasco, 2014).
- 128.Rios-Garaizar, J. & García-Moreno, A. Middle Paleolithic mobility patterns and settlement system variability in the eastern Cantabrian region (Iberian Peninsula): a GIS-based resource patching model In Settlement Dynamics of the Middle Paleolithic and Middle Stone Age (eds Conard, N. J. & Delagnes, A.) 329–360 (Kerns Verlag, 2015).
- 129.O’Connell JF, Hawkes K. Hadza hunting, butchering and bone transport and their archaeological implications. J. Archaeol. Res. 1988;44:113–161. [Google Scholar]
- 130.Sánchez-Goñi MF, Harrison SP. Millennial-scale climate variability and vegetation changes during the Last Glacial: concepts and terminology. Quatern. Sci. Rev. 2010;29:2823–2827. doi: 10.1016/j.quascirev.2009.11.014. [DOI] [Google Scholar]
- 131.Drucker D, Bocherens H. Carbon and nitrogen stable isotopes as tracers of change in diet breadth during Middle and Upper Palaeolithic in Europe. Int. J. of Osteoarch. 2004;14:162–177. doi: 10.1002/oa.753. [DOI] [Google Scholar]
- 132.Richards MP, Hedges REM. Stable isotope evidence for similarities in the types of marine foods used by Late Mesolithic humans at sites along the Atlantic coast of Europe. J. Archaeol. Sci. 1999;26:717–722. doi: 10.1006/jasc.1998.0387. [DOI] [Google Scholar]
- 133.Brown TA, Nelson DE, Vogel JS, Southon JR. Improved collagen extraction by modified Longin method. Radiocarbon. 1988;30:171–177. doi: 10.1017/S0033822200044118. [DOI] [Google Scholar]
- 134.Holm S. A simple sequentially rejective multiple test procedure. Scandin. J. of Stats. 1979;6:65–70. [Google Scholar]
- 135.Hammer Ø, Harper DAT, Ryan PD. PAST: paleontological statistics software package for education and data analysis. Palaeontologia electronica. 2001;4:9–40. [Google Scholar]
- 136.Altuna, J. Aitzbitarte III situación. Descripción del valle y de la cueva. Historia de las excavaciones recientes. Descripción del relleno (zona de entrada). Dataciones de radiocarbono in Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva (eds Altuna, J., Mariezkurrena, K., and Rios, J.) 23–38 (Colección de Patrimonio Cultural vasco 5, 2013).
- 137.Areso, P., Aranzasti, M., Olaskoaga, M. and Uriz, A. Sedimentología de la cueva de Amalda in La Cueva de Amalda (Zestoa, País Vasco) Ocupaciones Paleolíticas y postpaleolíticas (eds Altuna, J., Baldeón, A. and Mariezkurrena, K.) 33–48 (Sociedad de Estudios Vascos Serie B4, 1990).
- 138.Altuna, J. Caza y alimentación procedente de Macromamíferos durante el Paleolítico de Amalda in La Cueva de Amalda (Zestoa, País Vasco) Ocupaciones Paleolíticas y postpaleolíticas (eds Altuna, J., Baldeón, A., and Mariezkurrena, K.) 149–192 (Sociedad de Estudios Vascos Serie B4, 1990).
- 139.Pemán, E. La cueva de Amalda (Zestoa, País Vasco): ocupaciones paleolíticas y postpaleolíticas in La Cueva de Amalda (Zestoa, País Vasco) Ocupaciones Paleolíticas y postpaleolíticas (eds Altuna, J., Baldeón, A. and Mariezkurrena, K.) 225–238 (Sociedad de Estudios Vascos Serie B4, 1990).
- 140.Areso, P. & Uriz, A. Estudio del Sedimento del Yacimiento De Aitzbitarte III (Zona De Entrada). In Ocupaciones humanas en Aitzbitarte III (Pais vasco) 33.600-18.400BP. Zona de entrada a la cueva. (eds Altuna, J., Mariezkurrena, K. & Rios, J.) 481–491 (Colección de Patrimonio Cultural vasco 5, 2013).
- 141.Areso P, Uriz A. Estudio sedimentológico del yacimiento de Labeko Koba (Arrasate, País Vasco) Munibe (Antropologia-Arkeologia) 2000;52:79–88. [Google Scholar]
- 142.Altuna, J. Fauna de Axlor. Campaña de 1969 in excavaciones en Axlor. Campaña de 1969. Obras Completas XVII (ed. Barandiaran, J. M.) 219–225 (La Gran Enciclopedia Vasca, 1980).
- 143.Castaños P. Carbon and nitrogen stable isotopes of bone collagen of large herbivores from the Late Pleistocene Kiputz IX cave site (Gipuzkoa, north Iberian Peninsula) for palaeoenvironmental reconstruction. Quat. Int. 2014;339–340:131–138. doi: 10.1016/j.quaint.2013.10.006. [DOI] [Google Scholar]
- 144.Domingo L, et al. Late Quaternary climatic and environmental conditions of northern Spain: An isotopic approach based on the mammalian record from La Paloma cave. Palaeogeogr., Palaeoclimatol., Palaeoecol. 2015;440:417–430. doi: 10.1016/j.palaeo.2015.09.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.