Abstract
The genomic lesions found in malignant tumours exhibit a striking degree of heterogeneity. Many tumours lack a known driver mutation, and their genetic basis is unclear. By mapping the somatic mutations identified in primary lung adenocarcinomas onto an independent coexpression network derived from normal tissue, we identify a critical gene network enriched for metastasis-associated genes. While individual genes within this module were rarely mutated, a significant accumulation of mutations within this geneset was predictive of relapse in lung cancer patients that have undergone surgery. Since it is the density of mutations within this module that is informative, rather than the status of any individual gene, these data are in keeping with a ‘mini-driver’ model of tumorigenesis in which multiple mutations, each with a weak effect, combine to form a polygenic driver with sufficient power to significantly alter cell behaviour and ultimately patient outcome. These polygenic mini-drivers therefore provide a means by which heterogeneous mutation patterns can generate the consistent hallmark changes in phenotype observed across tumours.
Introduction
Large-scale cancer sequencing projects have led to a rapid expansion in the catalogue of putative driver mutations and revealed striking genetic heterogeneity across the sets of mutations identified in different patients1. While a small number of genes are frequently mutated at rates >20%, the majority occur at low frequencies2. In lung adenocarcinoma (LUAD), for example, the most frequently mutated gene, TP53, is altered in only ~50% of patients3. Despite this extensive heterogeneity, cancer is characterized by a set of ‘hallmark’ changes in phenotype4,5, even in the absence of a known driver mutation that can be linked directly to one of these canonical processes2. A critical question, therefore, is how these consistent phenotypes emerge from such underlying genetic diversity, and the task of identifying the mutations that contribute to these changes remains challenging6.
One possibility, as hypothesized by Castro-Giner et al.7, is that in tumours lacking a clear major driver, oncogenic transformation may instead occur through the collaborative action of a set of ‘mini-drivers’, each with a weak individual effect. In this model, these mini-driver mutations accumulate to form a polygenic driver with sufficient power to lead to tumour promoting effects and phenotypic changes7,8. Polygenic mini-drivers may also function in combination with conventional driver mutations. This model of the disease is in keeping with findings from large scale Genome Wide Association Studies (GWAS) that have shown, for typical traits, that even the most significant loci explain only a fraction of the predicted genetic variation9; the remaining “missing heritability” is provided by the contribution of multiple Single Nucleotide Polymorphisms (SNPs) that each fall below the threshold for genome-wide significance9. While it is tempting to speculate that cancer genes may behave similarly, there has been little experimental evidence so far to support this polygenic ‘mini-driver’ model of oncogenesis.
One of the challenges with validating such a model arises from the need to consider mutations with weak effects. Current approaches that attempt to predict driver mutations from mutation frequency do so by seeking mutations significantly more abundant than the estimated background mutation rate2. Since the background rate is dependent on multiple factors including genomic position, the mutation load in an individual patient, and gene size, these factors are represented as additional covariates in the numerical model1,2. This tends to raise the bar for long genes and/or those located in regions with higher mutation frequencies. Overall these techniques successfully remove multiple artefacts from candidate driver lists, including false positives such as TTN, which is extremely large, or olfactory receptors that are not expressed in the tissue of interest1.
While these models have significantly advanced the state of the art, they are focused on finding conventional drivers with significant individual effect. In the context of a mini-driver model, where individual components of a polygenic driver are each expected to have a minimal effect, these genes would not be expected to pass the threshold for overall significance. Similarly, approaches that aim to predict high impact changes to the encoded protein sequence (e.g.10,11) will tend to ignore the weak-effect mutations of most interest when searching for polygenic drivers. Thus, taken together, these data suggest that alternative mathematical models might prove useful when seeking to expand the catalogue of driver mutations to include polygenic drivers.
Here, we use Complex Network analysis to test the ‘mini-driver’ model proposed by Castro-Giner and colleagues7. We do this by building models that integrate somatic mutations with gene-expression data from LUAD patients. Complex networks are frequently used to represent natural systems. They encompass fields as diverse as social network analysis12, epidemiology13, biology14 and computing15. Complex networks exhibit community structure: a modular organization comprising discrete, highly connected sets of nodes with sparse interconnections16,17. Irrespective of the domain of interest, nodes within the same module, or ‘community’, typically share functionality14,18. They thus offer a powerful framework with which to explore interactions within systems and to identify common patterns of activity within heterogeneous populations19. Gene regulatory interactions also lend themselves well to complex network models, since co-expression is often indicative of common patterns of regulatory control20,21. This results in a modular structure in which functionally related genes congregate as sets of densely interconnected nodes within the network9,16.
Since cancers exhibit a set of consistent hallmark phenotypes, irrespective of their underlying mutation profiles4, we speculated that these might sometimes arise through functional changes resulting from the accumulation of multiple weak insults to the same cellular subsystems. We therefore hypothesized that if present, polygenic mini-driver mutations would tend to occur within sets of functionally related genes, and thus congregate within specific modules in a co-expression network.
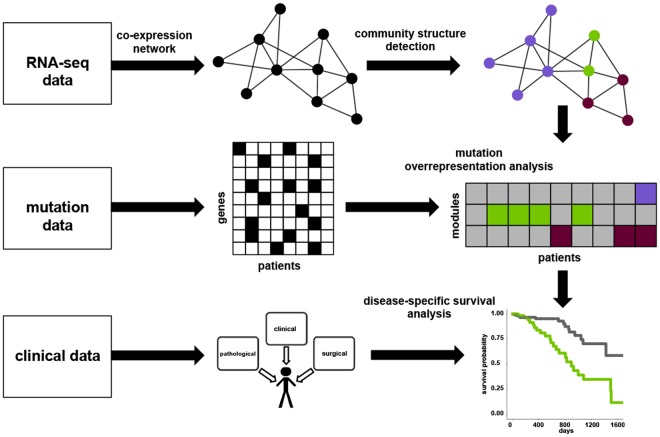
We sought to test this hypothesis as follows (summarized in Fig. 1): We first built independent co-expression networks representing LUAD tumour and matched normal samples. We segmented these networks using the Louvain greedy optimization algorithm22. We then mapped mutations from an independent cohort of LUAD patients onto these modular networks. Rather than assessing the significance of each individual mutation, as would be appropriate when seeking high impact single gene drivers, we instead use complex networks to model potential relationships between genes. We then seek regions of the networks that are significantly enriched for mutations. In this way we consider statistical significance in terms of the aggregate behaviour of multiple mutations, rather than on an individual gene basis. This makes it possible to consider genes that would otherwise be dismissed using conventional ranking algorithms because their individual effect is too small to raise them above the score threshold for individual significance.
Figure 1.
Overall strategy. Co-expression networks were generated from RNA-seq data. Community structure detection was applied to partition the networks into modules (purple, green and maroon nodes). For each patient, genes with non-synonymous mutations were enumerated, then mapped to the network modules, and overrepresentation analysis was performed. Modules with (purple, green and maroon cells), or without (grey cells) significant enrichment for mutations were identified. Disease-specific survival analysis was then conducted.
Finally, we validated the approach by assessing the biological relevance of these aggregate gene sets by asking whether they were associated with patient outcome. This allowed us to derive a polygenic mutation pattern predictive of disease-specific survival, thus confirming the biological relevance of the signature.
Results
We enumerated all non-synonymous mutations across 660 LUAD samples23. Mutation frequency distributions for both genes and residues exhibited little overlap between tumours (Supplementary Fig. 1). Only 11 protein-coding genes were mutated in more than 25% of tumours, and only one, TP53, harboured a mutation in more than 50% of samples (347/660; Supplementary Fig. 1a). At the residue level, while canonical mutations such as KRASG12, EGFRL858R, TP53R158P and BRAFV600E were detected, no individual non-synonymous Single Nucleotide Variants (SNVs) occurred in more than 16% of tumours (101/660; Supplementary Fig. 1b). Thus, in keeping with previous reports1, these patients exhibited a high degree of heterogeneity between their individual mutation spectra.
Lung cancer co-expression network exhibits a higher degree of modular structure
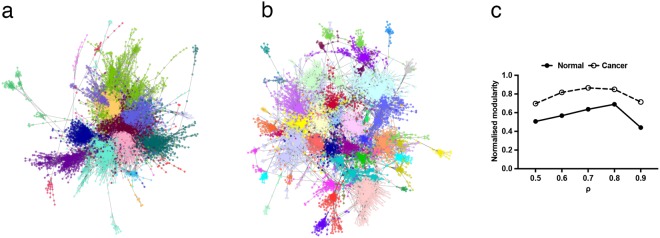
Co-expression networks were then derived from a dataset comprising 58 LUAD matched tumour normal pairs24. Tumour and normal samples were treated independently, using Pearson correlation, ρ, as the underlying distance metric, since in this context it outperforms other more complex mathematical relationships25. Co-expression networks were constructed with threshold values, ρ = 0.5, 0.6, 0.7, 0.8 and 0.9 (network properties summarized in Supplementary Table 1). Modularity quantifies the strength of community structure exhibited by a network16. The Louvain greedy modularity optimization algorithm22 was used to partition the network into modules, thus providing an unbiased definition of individual communities within the tumour and normal networks (Fig. 2a and b; gene-module memberships for ρ = 0.8 given in Supplementary Table 2). These data revealed a striking increase in normalized modularity with respect to matched normal samples, irrespective of the Pearson cut off (Fig. 2c). Since these organizational changes were consistent at multiple scales, all subsequent data are presented at ρ = 0.8.
Figure 2.
Network visualization and community structure detection. (a) Normal tissue derived co-expression network. Edges included at ρ ≥ 0.8. Networks were visualised using the Cytoscape(49) perfuse force-directed layout algorithm, without knowledge of the identified community structure. Each community was assigned a colour according to the partitions identified by the Louvain algorithm. (b) Cancer tissue derived co-expression networks. All parameters as a. (c) Normalised modularity values for normal and tumour derived networks at different values of ρ.
Two additional global metrics were also considered: Network Density, and Average Path Length. Network density refers to the number of edges in a network scaled by the maximum number of possible edges, while average path length is computed as the mean of the shortest paths between all pairs of nodes in the network. Both measures indicate the degree of connectivity of a network. In keeping with their increased modularity, the cancer networks also exhibited reduced density (normal = 0.0092, cancer = 0.0049) and increased average path length (normal = 5.24, cancer = 8.09). Together, these data demonstrate a system-wide loss of connective elements from the normal networks. We therefore reasoned that the network generated from the matched normal material, which includes elements perturbed or lost following oncogenic transformation, would provide a better baseline for understanding how cancer disturbs the normal functioning of the cell26. This is particularly important in the context of tumours, because many mutations result in transcripts being lost from the cancer-derived coexpression network, either through altered patterns of expression and loss of overall correlation, or through the creation of truncated or rapidly degraded transcripts27 that are no longer detected by sequencing. Many mutated genes, including tumour suppressors27, are consequently not represented in the cancer networks.
We then used the modular architecture of the normal network as a map onto which to project the mutational profiles from individual LUAD patients.
The normal network acts as a unifying framework for heterogeneous LUAD patients
We generated the mutational spectra for 602 LUAD patients23 by enumerating, for each sample, all genes with a non-synonymous somatic mutation. These samples were independent from those used to derive the expression networks. We then mapped the mutation profile for each patient onto the baseline normal network and performed a mutation overrepresentation analysis, seeking modules with a disproportionate number of mutations. Importantly, we did not filter mutations using existing models of significance focused on single-gene drivers, since the goal was to consider genetic lesions with weak effects that would be expected to fall at or below their significance threshold. Instead, we estimated statistical significance by using the normal networks to provide an unbiased grouping of genes into discrete subsystems, and then testing whether regions of the network were consistently enriched for mutations across the patient cohort.
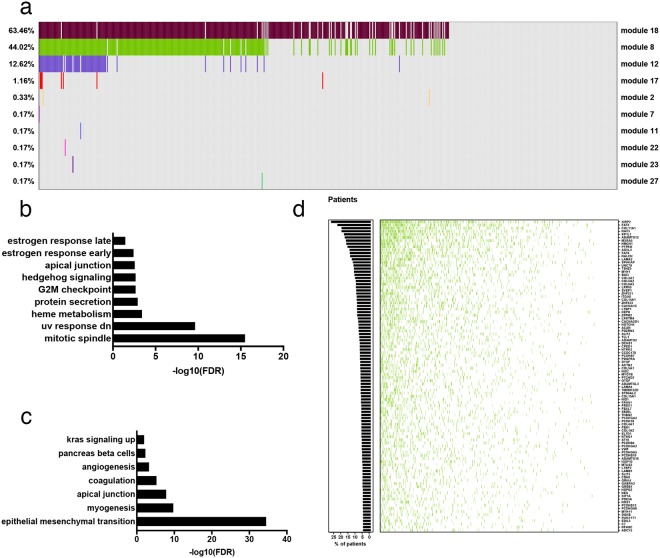
Since the majority of mutated genes would be expected to harbour only passenger mutations28 that confer no proliferative or survival advantage, a reasonable a priori expectation would be for them to be randomly distributed across the network. Instead, we observed a pattern in which specific network modules were frequently enriched for mutations relative to the overall mutational load for that patient (FDR < 0.05). In particular, two modules in the normal networks repeatedly exhibited a disproportionate accumulation of mutations in 63% and 44% of patients respectively (modules 18, 8; Fig. 3a). Importantly, only a weak pattern of enrichment was observed in the tumour-derived network (Supplementary Fig. 2a), demonstrating the additional analytical power lent by projecting the mutation data onto the normal network.
Figure 3.
Mutation overrepresentation analysis. (a) Modules with a statistically significant number of mutated genes in the normal network (non-grey cells). Module number is indicated on the right hand side of the figure, overall representation within the cohort on the left. Colours correspond to the modules in Fig. 2a. Only modules enriched for mutations in at least one patient are shown. (b) Broad Institute Hallmark genesets enriched in module 18. (c) Broad Institute Hallmark genesets enriched in module 8. (d) The top 100 most frequently mutated genes in module 8. Each row corresponds to a gene, columns, patients. Green cells indicate that a gene is mutated in the corresponding patient. The histogram shows the percentage of patients with at least one mutation in each gene.
The majority of tumours have a significant overrepresentation of mutations in modules enriched for mitotic spindle or metastasis associated genes
In total 71% of samples were enriched for mutations in either module 18 or 8. The most frequently enriched module (module 18) comprised a significant number of genes important in mitotic spindle assembly and the G2 DNA damage checkpoint (Broad Institute Hallmark genesets shown in Fig. 3b; top 20 gProfileR results in Supplementary Fig. 2b and full results in Supplementary Table 3). This module included several of the canonical oncogenes and tumour suppressors (as defined in24; EGFR, ERBB2, PIK3CA, NF1, and RB1).
By contrast, module 8 comprised genes associated with extracellular matrix organization, epithelial-mesenchymal transition, TGFβ receptor signalling pathway, collagen metabolism, blood vessel development, blood circulation, and angiogenesis (Broad Institute Hallmark genesets shown in Fig. 3c; top 20 gProfileR results in Supplementary Fig. 2c; full results in Supplementary Table 3). These are all processes involved in tumour dissemination, the establishment of metastases and, ultimately, poorer prognosis29.
Importantly, while the most frequently mutated gene in module 8, XIRP2, is mutated in 27% of patients, and only 24 genes mutated in at least 10% of tumours, the majority of genes in the module are altered only in a small proportion of cases (Fig. 3d; similarly for module 18, Supplementary Fig. 2d). Thus, viewing these heterogeneous samples in the context of the modular landscape of the normal network reveals unifying patterns not evident at the level of individual genes.
While expression-based signatures associated with metastasis have been proposed, a mutation-based signature of metastasis remains elusive28. This mutation pattern, which occurs within a network module associated with tumour progression and metastasis, was therefore of substantial interest. We next asked whether the frequent enrichment of mutations within this module might provide evidence in support of a mini-driver model of cancer, reasoning that if the accumulation of mutations within module 8 was indeed biologically relevant, it would correlate with patient characteristics. Since metastasis is the most common cause of cancer-death28, we focused on disease specific survival.
Accumulation of mutations within the metastasis-associated module predicts worse 5-year disease-specific survival
Clinical characteristics23,30, including known independent prognostic factors, were available for a subset of the LUAD cohort (296 patients; Table 1). A univariate Cox proportional hazards regression analysis was used to determine potential factors contributing towards di sease-specific survival, including overrepresentation of mutations in modules 8 and 18 (Fig. 4a and Supplementary Table 4). TNM stage31, T stage (T; tumour size), N stage (N; degree of spread of cancer to the lymph nodes) and Enrichment of mutations in module 8 (‘E status’) had a significant negative correlation with survival (P < 0.05; Kaplan-Meier curves in Fig. 4b–e).
Table 1.
Summary of clinical features of 296 LUAD patients.
| Age, yr | |
| Median (IQR) | 66 (59–72) |
| Gender (%) | |
| Male | 139 (47) |
| Female | 157 (53) |
| TNM stage (%) | |
| Stage I | 162 (54.7) |
| Stage II | 68 (23) |
| Stage III | 47 (15.9) |
| Stage IV | 17 (5.7) |
| Unknown | 2 (0.7) |
| T stage (%) | |
| T1 | 96 (32.4) |
| T2 | 154 (52) |
| T3 | 31 (10.5) |
| T4 | 14 (4.7) |
| TX | 1 (0.3) |
| M stage (%) | |
| M0 | 200 (67.6) |
| M1 | 16 (5.4) |
| MX | 77 (26) |
| Unknown | 3 (1) |
| N stage (%) | |
| N0 | 196 (66.2) |
| N1 | 53 (17.9) |
| N2 | 40 (13.5) |
| N3 | 1 (0.3) |
| NX | 5 (1.7) |
| Unknown | 1 (0.3) |
| Surgical margin status (%) | |
| R0 | 198 (67) |
| R1 | 9 (3) |
| R2 | 1 (0.3) |
| RX | 13 (4.4) |
| Unknown | 75 (25.3) |
| Smoking history | |
| Smoker | 240 (81) |
| Never | 49 (17) |
| Unknown | 7 (2) |
| Overall mutational load | |
| Median (IQR) | 192.5 (90.75, 378) |
| Number of mutations in module 8 | |
| Median (IQR) | 16 (6, 32) |
| Total no. of deaths | 45 |
| No. of deaths with tumour | 36 |
Figure 4.
Five-year disease-specific survival. (a) Forest plot with hazard ratios from the univariate Cox proportional hazards regression analysis. Significance values are the global model log-rank p-values. E status in red. Kaplan-Meier curves with log-rank p-value for (b) TNM stage, (c) T stage, (d) N stage, (e), E status (E = “Yes” indicates a significant number of mutations in module 8).
While M stage (M; spread of cancer to another part of the body) is a known prognostic factor31, it was not associated with survival in this analysis. This is a consequence of the high proportion of patients without metastatic disease (M0 = 68%, M unknown = 27%). Further, 27% of M-unknown patients are highly likely to represent M0 disease, as the TCGA dataset is comprised almost exclusively of samples collected at the time of curative intent surgery, which is not routinely used as a treatment for metastatic (M1) disease.
Importantly, a significant number of mutations in module 8 (i.e. E = “yes”) was negatively associated with disease-specific survival (P = 0.0287, HR = 2.1, CI: 1.1–4.2; Fig. 4e, Supplementary Table 4), confirming the prognostic significance, and therefore biological relevance, of the mini-driver pattern. No such correlation was found for module 18. Given the core cell cycle role of genes within this module, it may be that mutations here are indicative solely of the presence of a tumour, rather than of its aggressiveness.
Overall mutational load was not associated with survival (Supplementary Table 4), establishing that the signature was not simply a proxy for genome-wide mutation rates. Furthermore, while enrichment for mutations in module 8 was associated with survival, absolute mutation count within the module was not (Supplementary Table 4). This may be because when mutation rates are high throughout the cell, rather than simply in module 8, there is a general loss of fitness, reducing cell survival, and thus making relapse less likely. Alternatively, these cells may present better targets to the immune system32.
We next performed a multivariate analysis considering all covariates from the univariate analysis with P < 0.2. At this threshold, surgical margin status (R; degree of residual tumour after surgery) was also included in the initial model, in addition to TNM stage, T stage, N stage, and E status, as before. Backward stepwise selection was then used to identify the best model, with goodness of fit determined using the AIC score. Data with missing values were removed, leaving 204 patients in the analysis. The final model included N stage and enrichment of mutations in module 8 (E status), with AIC = 219.5 (Fig. 5). Taken together, these data thus demonstrate the prognostic value of E status in patients with nodal disease.
Figure 5.
Multivariate model combining nodal status and module 8 enrichment. Five-year disease-specific survival. Kaplan-Meier curve with log-rank p-value for multivariate model incorporating N and E status (E = ‘yes’ indicates significant enrichment of mutations in module 8).
Enrichment of mutations in the metastasis module predicts worse 5-year disease-specific survival in patients with N2 lymph node disease
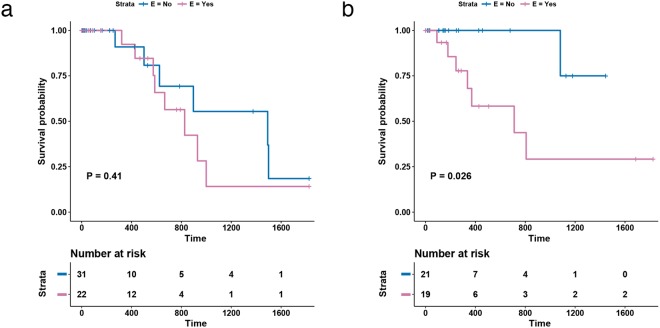
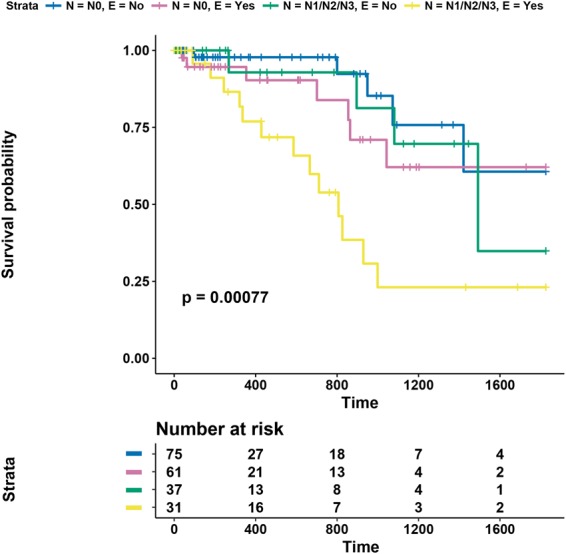
We further investigated the subset of patients with lymph node metastases. Lymph node involvement is an important prognostic factor; with increasing N stage, cure rate following surgery decreases33. We inspected N1 and N2 patients individually and stratified samples based on E status (there was only a single N3 patient; Fig. 6a,b). In patients with N1 disease, E status was not associated with survival (P = 0.4129, HR = 1.6, CI: 0.5–4.6; Fig. 6a). In patients with N2 involvement, E status significantly partitioned samples based on 5-year disease-specific survival (P = 0.0264; Fig. 6b), with only one cancer-specific death in patients with negative E status. However, due to the small sample size of N2 patients, there is a large 95% confidence interval for the hazard ratio (HR = 7.7, CI: 0.9–62.9). Therefore, while this result requires further validation, it indicates that N2 patients with an accumulation of mutations in the metastasis module are more likely to relapse and die with tumour following surgery.
Figure 6.
5-year disease-specific survival results for N-stage patients. Kaplan-Meier curves with log-rank p-value for patients with N stage equal to (a) N1 and (b) N2, stratified by E status.
These data are of clinical interest because the optimum treatment strategy for Stage IIIA N2 disease is unclear34. Dependent on clinical characteristics, primary treatment may involve surgery or chemoradiotherapy. In those patients who undergo surgery, the benefit of postoperative radiotherapy (PORT) is uncertain and current practice is to consider this adjuvant approach based on an individual assessment of relapse risk. Our data reveal a set of genes associated with poor outcome, with the potential to stratify postoperative Stage IIIA N2 patients for a prospective clinical trial of PORT efficacy in this setting.
Discussion
By building network models representing the expression profiles of normal samples, and then mapping the mutation spectra of individual lung cancer patients onto these networks, we were able to place somatic mutations into the context of lung tissue before the system-wide reorganization of gene expression that occurs during oncogenesis. This allowed us to identify a set of genes with a mutation pattern predictive of disease specific survival that, in combination with nodal status (N-stage), led to an improved classifier. A significant number of genes within this set were associated with the ECM, EMT, tumour dissemination, and metastasis (Fig. 3c and Supplementary Fig. 2c), in keeping with its significance in patients who relapse following surgery. Further, the enrichment for mutations within this gene set has the potential to better stratify patients with N2 nodal disease.
While 44% of tumours harboured a statistically significant number of mutations within the ECM module (module 8), individual genes were mutated at much lower rates (Fig. 3d), in keeping with a ‘mini-driver’ model in which it is the polygenic combination of multiple genes within the module that leads to the observed effect. We also detected substantial enrichment in the network module associated with the mitotic spindle and DNA damage repair (module 18; Fig. 3a and b and Supplementary Fig. 2b). This was observed for 84% of patients for which the metastatic module was enriched, and in over half (63%) of all patients studied.
These data also demonstrate the utility of alternative models for assessing the significance of mutations. Recently Waks and colleagues were able to identify a set of candidate tumour suppressors encoding extremely long chromatin regulators35. They achieved this in part by relaxing gene-length constraints in favour of additional functional annotation data. Here we make use of the extra structure provided by co-expression networks to find significant sets of mutations.
The complex network approach of integrating expression and somatic mutation data also demonstrates the importance of looking beyond a binary driver versus passenger view of cancer and to integrate the concept of mini-drivers into models of tumorigenesis. Importantly, mapping mutations onto expression data derived from tumour samples did not yield similar patterns (including prognostic significance; data not shown), thus highlighting the utility of using normal samples to provide a reference framework. Our approach therefore differs from previous studies, which have tended to focus on the gene expression profiles derived from tumour cells, either to develop expression-based signatures, e.g.36–40, or to consider them in combination with somatic mutation profiles41.
Taken together, these data therefore provide significant evidence in support of the mini-driver models of cancer proposed by Castro-Giner and colleagues7, and a potential explanation of how the same hallmark phenotypes can emerge in different tumours with a high degree of heterogeneity. This has significant implications for therapeutic strategies, since monogenic approaches that target an individual driver gene are unlikely to be beneficial in the context of a tumour driven by a set of collaborating mini-drivers. Instead, for these tumours, multi-target and pathway focused strategies that aim to treat cellular subsystems, rather than a gene, are more likely to be effective.
Methods
RNA-seq data
The Cancer Genome Atlas (TCGA) LUAD expression dataset comprised 116 matched-normal lung adenocarcinoma samples (58 normal and 58 cancer)24. Raw data for were aligned using MapSplice42 against human reference genome hg19 (minimal filtering) and read counts were generated at the exon level (Ensembl version 70) and transformed into RPKM values.
Co-expression network construction and analysis
The expression datasets of normal and cancer samples were analysed separately. Genes in the 40th percentile of summed RPKM values were discarded due to low levels of expression. Pearson correlations were calculated between the remaining gene expression profiles, and normal and cancer co-expression networks were constructed with the following threshold values, ρ = 0.5, 0.6, 0.7, 0.8 and 0.9. Network analysis was carried out using the igraph package in R43. The greedy modularity optimization method, Louvain22, implemented in the ‘multilevel.community’ function, was used for community structure detection. For both co-expression networks, randomly rewired networks that preserved the original network’s degree distribution were generated repeatedly using a computationally optimized implementation of the ‘rewire.edges’ function. In order to compare modularity across networks with different size and connectivity, modularity was normalized using the randomly generated networks, as described in44,45.
Mutation data
Mutation data for 1144 non-small cell lung cancer patients were downloaded from cBioPortal23,30. This dataset included both lung squamous and lung adenocarcinoma samples. 660 LUAD patients were selected for further analysis: 501 from the TCGA cohort24, and 159 from Imielinski et al.3. For each patient, non-synonymous somatic mutations were enumerated. Silent mutations were not included (Variant_Classification = ‘Silent’), while non-silent mutations (Variant_Classification equal to any of the following: Missense_Mutation, Nonsense_Mutation, Frame_Shift_Del, Frame_Shift_Ins, In_Frame_Ins, In_Frame_Del, Missense, Splice_Site, Translation_Start_Site, Nonstop_Mutation) were considered non-synonymous. Only Single Nucleotide Variants (Variant_Type = ‘SNP’) were used to generate calculate mutation frequency distributions for residues.
Mutation overrepresentation analysis
Only 602/660 LUAD samples were considered, since 58 of the TCGA patients from the cBioPortal30 data were also included in the cohort used to generate the co-expression networks. For each remaining patient, their set of mutated genes was mapped onto the partitioned networks. A one-tailed Fisher’s exact test identified modules comprising a significantly disproportionate number of mutated genes relative to the background population of the entire network and the patient’s overall mutational load. P-values were adjusted using the Benjamini-Hochberg (BH) method for multiple testing to control the false discovery rate (FDR). A significance threshold of FDR < 0.05 was used.
Functional enrichment
The gProfileR package in R46 was used to identify overrepresented Gene Ontology Biological Processes47 and additionally, Broad Institute Hallmark genesets48 using a hypergeometric distribution (background population: the whole network; FDR < 0.05).
Clinical data and 5-year disease-specific survival analysis
Clinical annotation for the 660 LUAD patients were downloaded from cBioPortal23,30. Data corresponding to patient tumours for which gene expression data were used to generate the correlation networks were removed, leaving 602 patient samples. Disease-specific survival analysis requires the following information: ‘time’ taken as ‘days to death’ if available and ‘days to last follow-up’ otherwise, vital status, and whether the patient was with tumour or tumour free. Patients ‘with tumour’ at time of death were classified as having disease-specific death. LUAD patients from Imielinski et al.3 were not included in the survival analysis, since these data were not available. Tumour status (with tumour or tumour free) for the TCGA patients was obtained from the RTCGA package in R49, as was residual tumour status. A total of 296 patients had the required data and were included in the analysis.
Survival analysis was carried out using the ‘survival’ package in R50. Univariate and multivariate Cox proportional hazards regression analyses identified factors with significant impact on disease-specific survival. Multivariate analysis was performed by first identifying significant variables (log-rank p-value < 0.2) in a univariate analysis. These significant covariates were then used to form an initial multivariate model. Backwards stepwise regression was then performed, using the ‘stepAIC’ function from the ‘MASS’ package in R, to refine the model51. Log-rank p-values (P), associated hazard ratios (HR) and 95% confidence intervals (CI) were reported. Kaplan-Meier curves were generated for significant variables from the multivariate analysis.
Electronic supplementary material
Acknowledgements
The results published here are part based upon data generated by TCGA managed by the NCI and NHGRI. Information about TCGA can be found at http://cancergenome.nih.gov. This work was funded by Cancer Research UK Manchester Institute (C5759/A20971) and (MH) Cancer Research UK Lung Centre (C5759/A20465).
Author Contributions
L.B. performed the majority of the data analysis. M.H. contributed clinical insight. D.M. re-aligned the TCGA data. C.S. contributed novel, efficient, graph-searching code. C.Z. contributed to the multivariate analysis. L.B., C.M. devised and planned the experiments. L.B., M.H., C.M. wrote the manuscript.
Data Availability
All data are available via TCGA.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33276-3.
References
- 1.Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imielinski M, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150:1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell (2000). [DOI] [PubMed]
- 5.Garraway LA, Lander ES. Lessons from the cancer genome. Cell. 2013;153:17–37. doi: 10.1016/j.cell.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Hudson AM, et al. Discrepancies in cancer genomic sequencing highlight opportunities for driver mutation discovery. Cancer Res. 2014;74:6390–6396. doi: 10.1158/0008-5472.CAN-14-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro-Giner F, Ratcliffe P, Tomlinson I. The mini-driver model of polygenic cancer evolution. Nature Reviews. Cancer. 2015;15:680–685. doi: 10.1038/nrc3999. [DOI] [PubMed] [Google Scholar]
- 8.Li, X. & Thirumalai, D. Interplay of Driver, Mini-Driver, and Deleterious Passenger Mutations on Cancer Progression. bioRxiv 084392 (2016).
- 9.Boyle EA, Li YI, Pritchard JK. An Expanded View of Complex Traits: From Polygenic to Omnigenic. Cell. 2017;169:1177–1186. doi: 10.1016/j.cell.2017.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic acids research. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ediger, D. et al. Massive Social Network Analysis: Mining Twitter for Social Good. In 583–593, 10.1109/ICPP.2010.66 (2010).
- 13.Stattner, E. & Vidot, N. Social network analysis in epidemiology: Current trends and perspectives. 2011 Fifth International Conference on Research Challenges in Information Science 1–11 (2011).
- 14.Mitra K, Carvunis A-R, Ramesh SK, Ideker T. Integrative approaches for finding modular structure in biological networks. Nat Rev Genet. 2013;14:719–732. doi: 10.1038/nrg3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortuna, M. A., Bonachela, J. A. & Levin, S. A. Evolution of a modular software network. 108, 19985–19989 (2011). [DOI] [PMC free article] [PubMed]
- 16.Girvan M, Newman MEJ. Community structure in social and biological networks. PNAS. 2002;99:7821–7826. doi: 10.1073/pnas.122653799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman AM, et al. An ultrasensitive method for quantitating circulating tumour DNA with broad patient coverage. Nat. Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alon U. Biological Networks: The Tinkerer as an Engineer. Science. 2003;301:1866–1867. doi: 10.1126/science.1089072. [DOI] [PubMed] [Google Scholar]
- 19.Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- 20.Allocco, D. J., Kohane, I. S. & Butte, A. J. Quantifying the relationship between co-expression, co-regulation and gene function. BMC Evolutionary Biology (2004). [DOI] [PMC free article] [PubMed]
- 21.Castro MAA, et al. Regulators of genetic risk of breast cancer identified by integrative network analysis. Nat Genet. 2016;48:12–21. doi: 10.1038/ng.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blondel VD, Guillaume J-L, Lambiotte R, Lefebvre E. Fast unfolding of communities in large networks. J. Stat. Mech. 2008;2008:P10008. doi: 10.1088/1742-5468/2008/10/P10008. [DOI] [Google Scholar]
- 23.Campbell JD, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. 2016;48:607–616. doi: 10.1038/ng.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collisson EA, et al. Comprehensive molecular profiling of lung adenocarcinoma: The cancer genome atlas research network. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballouz, S., Verleyen, W. & Gillis, J. Guidance for RNA-seq co-expression network construction and analysis: safety in numbers. Bioinformatics (Oxford, England) (2015). [DOI] [PubMed]
- 26.Krogan NJ, Lippman S, Agard DA, Ashworth A, Ideker T. The cancer cell map initiative: defining the hallmark networks of cancer. Mol. Cell. 2015;58:690–698. doi: 10.1016/j.molcel.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popp MW-L, Maquat LE. The dharma of nonsense-mediated mRNA decay in mammalian cells. Mol. Cells. 2014;37:1–8. doi: 10.14348/molcells.2014.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venning FA, Wullkopf L, Erler JT. Targeting ECM Disrupts Cancer Progression. Front Oncol. 2015;5:224. doi: 10.3389/fonc.2015.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerami E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discovery. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edge SB, Compton CC. The American Joint Committee on Cancer: the7th edition of the AJCC cancer staging manual and the future of TNM. Ann. Surg. Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 32.Goodman AM, et al. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi N, et al. The signature from messenger RNA expression profiling can predict lymph node metastasis with high accuracy for non-small cell lung cancer. J Thorac Oncol. 2006;1:622–628. [PubMed] [Google Scholar]
- 34.Postmus PE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 35.Waks Z, et al. Driver gene classification reveals a substantial overrepresentation of tumour suppressors among very large chromatin-regulating proteins. Sci Rep. 2016;6:38988. doi: 10.1038/srep38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Jia P, Zhao Z, Shen B. Key regulators in prostate cancer identified by co-expression module analysis. BMC Genomics. 2014;15:1015. doi: 10.1186/1471-2164-15-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y, et al. Gene co-expression network analysis reveals common system-level properties of prognostic genes across cancer types. Nat Commun. 2014;5:3231. doi: 10.1038/ncomms4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khirade MF, Lal G, Bapat SA. Derivation of a fifteen gene prognostic panel for six cancers. Sci Rep. 2015;5:13248. doi: 10.1038/srep13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, et al. A new strategy for exploring the hierarchical structure of cancers by adaptively partitioning functional modules from gene expression network. Sci Rep. 2016;6:28720. doi: 10.1038/srep28720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pepke S, Ver Steeg G. Comprehensive discovery of subsample gene expression components by information explanation: therapeutic implications in cancer. BMC Med Genomics. 2017;10:12. doi: 10.1186/s12920-017-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofree M, Shen JP, Carter H, Gross A, Ideker T. Network-based stratification of tumour mutations. Nat. Methods. 2013;10:1108–1115. doi: 10.1038/nmeth.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang, K. et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. 38, e178 (2010). [DOI] [PMC free article] [PubMed]
- 43.Csardi, G. & Nepusz, T. The igraph software package for complex network research. InterJournal Complex Systems (2006).
- 44.Parter M, Kashtan N, Alon U. Environmental variability and modularity of bacterial metabolic networks. BMC Evolutionary Biology. 2007;7:169. doi: 10.1186/1471-2148-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takemoto K, Kihara K. Modular organization of cancer signaling networks is associated with patient survivability. Biosystems. 2013;113:149–154. doi: 10.1016/j.biosystems.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Reimand, J. et al. g: Profiler—a web server for functional interpretation of gene lists (2016 update). 44, gkw199–W89 (2016). [DOI] [PMC free article] [PubMed]
- 47.Botstein, D., Cherry, J. M., Ashburner, M. & Ball, C. A. Gene Ontology: tool for the unification of biology. Nat Genet (2000). [DOI] [PMC free article] [PubMed]
- 48.Subramanian, A. et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed]
- 49.Kosinski, M. & Biecek, P. RTCGA: the Cancer Genome Atlas Data Integration R package.
- 50.Therneau, T. M. A package for survival analysis in S (2014).
- 51.Venables, W. N. & Ripley, B. D. Modern Applied Statistics with S. (Springer Science & Business Media, 2003).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available via TCGA.