Abstract
Aconitum plants, which have analgesic, diuretic and anti-inflammatory effects, have been widely used to treat various types of disease. However, the apparent toxicity of Aconitum-derived agents, particularly in the cardiovascular system, has largely limited their clinical use. Thus, the present study investigated whether berberine (Ber), an isoquinoline alkaloid, may reduce myocardial injury induced by aconitine (AC) in rats and the underlying mechanisms. Rats (n=40) were randomly divided into four groups: Control, Chuan-wu and Chuan-wu + Ber (8 and 16 mg/kg doses). Electrocardiograms (ECG) of the rats were recorded and serum biomarkers of cardiac function [lactate dehydrogenase (LDH), creatine kinase (CK) and CK-MB] were assayed. Histopathological changes were assessed using myocardial tissue sectioning and hematoxylin and eosin staining. Additionally, the effects of Ber on AC-induced arrhythmias in rats were observed. The changes in ECG following AC perfusion were observed, and the types and onset time of arrhythmias were analyzed. Furthermore, the effects of Ber and AC on papillary muscle action potentials were observed. The results suggested that Ber ameliorated myocardial injury induced by Chuan-wu, which was indicated by reduced arrhythmias and decreased LDH, CK and CK-MB levels in serum. Furthermore, histological damage, including dilation of small veins and congestion, was also markedly attenuated by Ber. In addition, the occurrence of arrhythmias was significantly delayed, and the dosage of AC required to induce arrhythmias was also increased by Ber pretreatment. Additionally, AC-induced changes in action potential amplitude, duration of 30% repolarization and duration of 90% repolarization in the papillary muscle were attenuated by Ber. All of these results indicate that Ber had a preventive effect on acute myocardial injury induced by Chuan-wu and arrhythmias caused by AC, which may be associated with the inhibition of delayed depolarization and triggered activity caused by AC. Thus, combination treatment of Ber with Aconitum plants may be a novel strategy to prevent AC-induced myocardial injury in clinical practice.
Keywords: berberine, aconite, arrhythmias, aconitine, action potential
Introduction
Aconitum plants including Chuan-wu (Crude Radix Aconiti) which have analgesic, diuretic and anti-inflammatory effects, have been widely used to treat various diseases, including chronic neuralgia, rheumatoid arthritis, acute myocardial infarction, coronary heart disease and angina pectoris in China for thousands of years (1,2). Thus, numerous herbal medicines have been formulated containing aconitum plant extracts as the main ingredient. However, the apparent toxicity of these compounds has severely limited their clinical use (3,4). The toxic effects predominantly involve the central nervous and cardiovascular systems, particularly the cardiovascular system where aconitum plant extracts can cause polymorphous arrhythmias. Patients often succumb from arrhythmia and respiratory center paralysis. However, little information is available regarding the mechanisms of aconite poisoning.
The exact mechanisms involved in aconite-induced cellular damage are extremely complex and have not been fully elucidated. Aconitine (AC), a major bioactive diterpenoid alkaloid derived from aconitum plants, stimulates the majority of the cardiotoxic effects of aconitum plants (5). A previous study has shown that AC can increase the excitability of ectopic rhythms and lead to tachyarrhythmias (6). At the cellular level, AC has been shown to bind to voltage-gated Na+ channels and prolong their open state, favoring the entry of a large quantity of Na+ into the cytosol, which is accompanied by Ca2+ overload via sequential activation of electrogenic Na+-Ca2+ exchangers or L-type Ca2+ channels, eventually inducing delayed afterdepolarization (DAD) and triggered activity (TA) (7,8). Additionally, L-type Ca2+ channel inhibition contributes to the arrhythmic effects of AC in human cardiomyocytes (9). Therefore, it is important to identify more effective strategies against AC-induced cardiovascular complications, and search for therapeutic targets that could counteract the myocardial toxicity induced by AC.
Berberine (Ber), one of the main alkaloids extracted from Rhizoma coptidis, has been extensively used to treat various parasitic and fungal infections, and has a long history of use for treating diarrhea in traditional Chinese medicine (10). Additionally, it also exhibits positive inotropic, negative chronotropic and anti-arrhythmic properties (11,12). It has been reported that Ber improves cardiac function in patients with severe congestive heart failure (13). Ber is extracted from Rhizoma coptidis, and the latter has also been used to treat diabetes mellitus in China for >1,400 years (14). Additionally, it has also been demonstrated that Ber alleviates the acute cardiotoxicity and hepatorenal toxicity resulting from doxorubicin treatment in rats (15,16). However, to the best of our knowledge, no study to date has been performed concerning the protective effects of Ber on aconite-induced myocardial toxicity. Therefore, the present study was designed to investigate whether Ber has an antagonistic effect on myocardial damage and the associated arrhythmias caused by AC, and to elucidate the potential mechanisms of action in isolated guinea pig papillary muscle.
Materials and methods
Drugs and reagents
Chuan-wu was obtained from the Anguo medicine market (Shijiazhuang, China). AC was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Ber was obtained from Acros Organics (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Pentobarbital was obtained from Sigma-Aldrich (Merck KGaA). Creatine kinase (CK) and CK-MB isoenzyme diagnostic kits were all from Sysmex Corporation (Kobe, Japan). Lactate dehydrogenase (LDH) assay kit was obtained from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Animals
Sprague Dawley (SD) rats and guinea pigs were obtained from the Experimental Animal Center of Hebei Medical University [license no. SCXK (Hebei) 2013-003]. All experiments were approved by the Ethics Committee for the Use of Experimental Animals at Hebei Medical University (Shijiazhuang, Hebei, China).
Experimental protocol of Chuan-wu induced cardiac injury
Male SD rats (8-week-old; certificate no. 1109105), weighing 250–300 g, were housed under controlled atmosphere (12-h light/dark cycle, relative humidity 50–60%, and 24±3°C) and food and water were provided ad libitum. Rats (n=40) were randomly divided into Control group (A), AC group (B), AC + Ber (8 mg/kg/d) group (C), and AC + Ber (16 mg/kg/d) group (D). Ber was administered intragastrically once a day for 7 consecutive days in groups C and D. Group A and B rats were administrated with an equal volume of distilled water. Chuan-wu (0.08 mg/kg) was administrated from day 4 during the Ber administration period by dissolving the Chuan-wu powder into distilled water; this was performed once a day for 3 days, while an equal volume of distilled water was given to the control group.
Electrocardiogram (ECG) analysis
On days 1 and 3 following Chuan-wu administration, 30 min following Ber administration, the rats were anesthetized with 45 mg/kg pentobarbital sodium via an intraperitoneal injection (i.p.). The electrodes were inserted into the subcutaneous tissue of the rat limb, and the ECG parameters (day 1) and the different types of arrhythmia (day 3) were recorded in each group using an RM 6240CD multi-channel physiological signal acquisition and processing system (Chengdu Instrument Factory, Chengdu, China) via standard limb lead II.
Determination of serum biochemical indicators
On day 3 following Chuan-wu administration, blood samples were collected from the abdominal aorta and stored on ice prior to centrifugation at 2,100 × g for 10 min within 1 h of collection. Subsequently, the supernatant was used for the determination of biochemical indicators. The levels of LDH, CK and CK-MB in the serum were assayed as sensitive indicators of myocardial damage, according to the manufacturer's protocol of the diagnostic kits (cat. nos., LDH, R1 1000331, R2 1000312; CK, R1 1000531, R2 1000512; CK-MB R1 1001611 and R2 1001612; Sysmex Corporation) using a CHEMIX-180 automatic biochemistry analyzer (Sysmex Corporation).
Histopathological analysis
At day 3 after Chuan-wu administration, the heart was rapidly removed and rinsed with ice-cold saline following the collection of blood samples. Heart samples were fixed in 4% formaldehyde immediately following excision for 7 days at room temperature, then dehydrated in an ascending series of ethanol (70, 80, 96 and 100%). Following paraffin embedding, transverse slides (5 µm thickness) were stained with hematoxylin and eosin at room temperature for 2 min respectively, and histological examination was conducted using a light microscope (Olympus BX-50; Olympus Corporation, Tokyo, Japan).
Effects of Ber on AC-induced arrhythmias in rats
Male SD rats (n=40, certificate no.1107071), weighing 250–300 g were randomly divided into 4 groups: Control group (A), AC group (B), AC + Ber (8 mg/kg/d) group (C), and AC + Ber (16 mg/kg/d) group (D). Ber was administered intragastrically once a day for 7 consecutive days in groups C and D. Rats in groups A and B received an equal volume of distilled water. On day 7, 30 min following Ber administration, the rats were anesthetized with 45 mg/kg pentobarbital sodium (i.p.). The surface lead II ECG was recorded continuously with three subcutaneous limb electrodes using the RM 6240CD multi-channel physiological signal acquisition and processing system. The femoral vein was cannulated for administration of AC (10 µg/ml) at a rate of 0.08 ml/min in groups B, C and D, while an equal volume of normal saline was given to group A. The time onset of the first ventricular premature contraction discrete (VPCD), ventricular premature contraction bigeminy (VPB), successive ventricular tachycardia (VT), ventricular fibrillation (VF) and mortality (DD) was recorded, and the amount of AC required to induce arrhythmia was calculated.
Effects of Ber and AC on papillary muscle action potential
Male guinea pigs (4–5 weeks of age, n=6), weighing 200–250 g, were housed under controlled atmosphere (12-h light/dark cycle, relative humidity 50~60% and 22±2°C), and food and water were provided ad libitum. The animals were anesthetized with 40 mg/kg pentobarbital sodium (i.p), then the heart was rapidly removed and put into a preparation dish containing ice-cold K-H solution (140 mmol/l NaCl, 5.4 mmol/l KCl, 1 mmol/l MgCl2, 2 mmol/l CaCl2, 10 mmol/l HEPES and 10 mmol/l glucose). The right ventricle was opened and columnar papillary muscles were removed. The papillary muscles were then transferred into the tissue chamber and perfused with K-H solution, which was continually gassed with 100% O2. The temperature was maintained at 36°C. A conventional microelectrode recording technique was used to record the action potential of the papillary muscle of guinea pigs under the stimulation frequency of 1 Hz. The following action potential parameters were calculated: Action potential amplitude (APA), duration of 30% repolarization (APD30) and duration of 90% repolarization (APD90). The hearts were divided into two groups (n=3 in each group): Control and Ber treatment groups. Once a normal action potential was continuously recorded in the control group, Tyrode's solution containing AC (3×10−7 mol/l) was perfused, and DAD and TA by AC were observed. While in the Ber treatment group, once a normal action potential was continuously recorded, solution containing Ber (1×10−5 mol/l) was perfused. Following 5 min, the action potential was recorded. Then, AC (3×10−7 mol/l) was perfused, and the changes in action potential following AC perfusion were recorded. If no change appeared, 15 min later, 1×10−6 mol/l AC was added to observe the change in the action potential.
Statistical analysis
All values are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance and Dunnett's test and SPSS v11.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons of the same parameters prior to and following administration were performed using a paired t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of Ber on ECG in rats with Chuan-wu-induced cardiac injury
During the experiments, no animals succumbed in the control or Ber 16 mg/kg treatment groups; whereas, 3 animals in the Chuan-wu group and 1 animal in the Ber 8 mg/kg group perished.
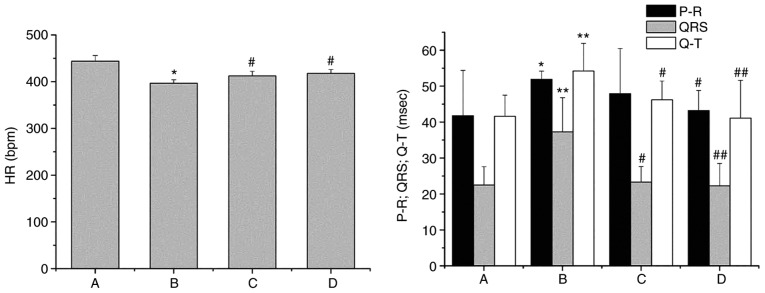
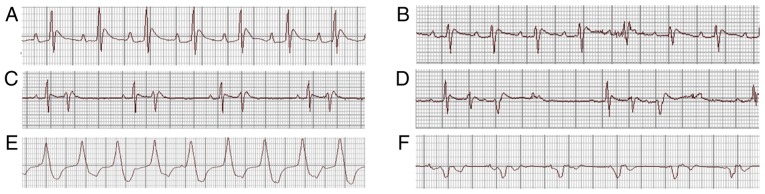
On day 1 following Chuan-wu administration, the analysis of ECG parameters revealed that the heart rate was decreased (P<0.05) and the P-R, QRS and QT intervals were all prolonged in the Chuan-wu group (P<0.05 or P<0.01), compared with that observed in control group. The effects of Chuan-wu on heart rate, P-R, QRS and QT intervals in the 8 and 16 mg/kg Ber treatment groups were all reversed by different degrees when compared with the Chuan-wu group (P<0.05 or P<0.01; Fig. 1). On day 3 following Chuan-wu administration, there was no ECG abnormality in the control group. However, in the Chuan-wu group, ventricular premature beat, bigeminies or trigeminies were observed in 3 rats, typical ventricular tachycardia was observed in 4 rats, and 3 rats exhibited ventricular tachycardia and then succumbed following ventricular fibrillation. Additionally, in the Ber 8 mg/kg group, 4 rats exhibited ventricular premature beat-bigeminies, 3 rats presented typical ventricular tachycardia, and 1 rat perished from ventricular fibrillation. In the Ber 16 mg/kg group, 2 rats exhibited ventricular premature beat, bigeminies or trigeminies, and 1 rat presented with typical ventricular tachycardia, with no evident abnormalities. Typical arrhythmias are presented in Fig. 2. The summaries of the arrhythmias and mortalities in each group are presented in Table I.
Figure 1.
Effects of Ber on the electrocardiogram parameters 1 day following Chuan-wu administration in rats. Group A: control; group B: Chuan-wu; group C: Ber 8 mg/kg; and group D: Ber 16 mg/kg. *P<0.05 and **P<0.01 vs. control group (group A); #P<0.05 and ##P<0.01 vs. Chuan-wu group (group B). Ber, Berberine.
Figure 2.
Representative ECGs showing the observed arrhythmias recorded using a RM 6240B/C biological signal recorder system; II lead, 50 mm/sec, 20 mm/mV. (A) Normal ECG; (B) ventricular premature beat; (C) ventricular premature beats-bigeminies; (D) ventricular premature beats-trigeminies; (E) ventricular tachycardia; and (F) ventricular fibrillation. ECG, electrocardiogram.
Table I.
Influence of Berberine on the occurrence rate of arrhythmias and mortality rate on day 3 following Chuan-wu administration in rats.
| Group | Normal (no. of cases) | VPC (no. of cases) | VT (no. of cases) | Mortalities (no. of cases) |
|---|---|---|---|---|
| Control | 10 | 00 | 00 | 00 |
| Chuan-wu | 0 | 33 | 4 | 3 |
| Ber 8 mg/kg | 2 | 4 | 3 | 1 |
| Ber 16 mg/kg | 7 | 2 | 1 | 0 |
VPC, ventricular premature contractions; VT, ventricular tachycardia; Ber, Berberine.
Effects of Ber on cardiac function indexes in rats
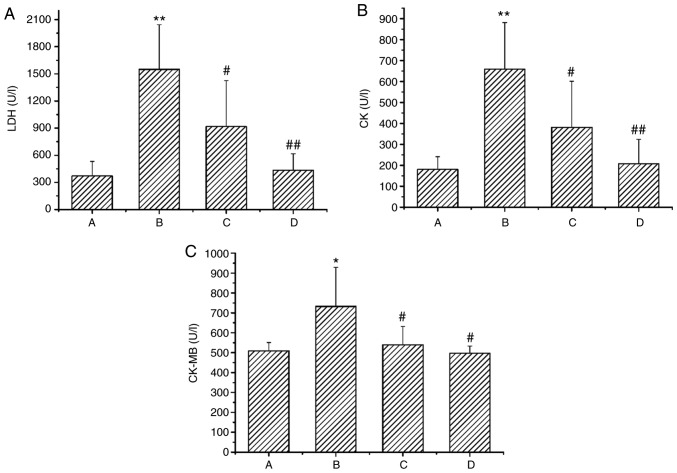
Compared with the control group, the levels of serum CK, CK-MB and LDH were significantly increased in the Chuan-wu group (B; P<0.05 or P<0.01). Whereas, in the 8 and 16 mg/kg Ber treatment groups (C and D), the levels of serum CK, CK-MB and LDH were all markedly reduced when compared with the Chuan-wu group, particularly in the Ber 16 mg/kg group (P<0.05 or P<0.01; Fig. 3).
Figure 3.
Effects of Ber on the activity of (A) LDH, (B) CK and (C) CK-MB in the serum of rats with acute cardiac injury induced by Chuan-wu. Group A: control; group B: Chuan-wu; group C: Ber 8 mg/kg; and group D: Ber 16 mg/kg. *P<0.05 and **P<0.01 vs. control group (group A); #P<0.05 and ##P<0.01 vs. Chuan-wu group (group B). Ber, Berberine; LDH, lactate dehydrogenase; CK, creatine kinase.
Effects of Ber on the heart histopathology of rats with Chuan-wu induced cardiac injury
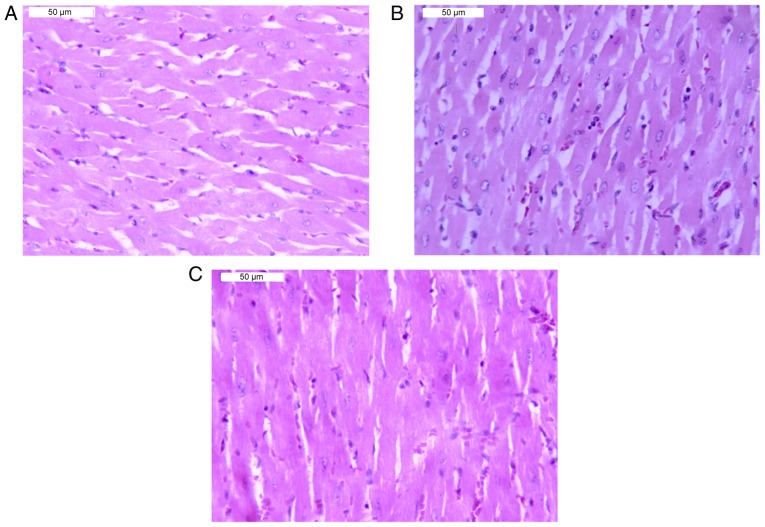
Histopathological examination of the heart tissue revealed that there were no evident abnormalities in the myocardium of the control group (Fig. 4A). Whereas, following Chuan-wu administration for 3 days, small vein dilation, congestion, ventricular dilatation and congestion were observed in myocardial tissue. Additionally, some muscle fibers exhibited mild atrophy and inflammatory cell infiltration was observed in the muscle. The representative images of congestion in veins and slight inflammatory cell infiltration are shown in Fig. 4B. Compared with the Chuan-wu group, the congestion was reduced in the Ber 8 and 16 mg/kg groups. Notably, in the Ber 16 mg/kg group, no marked congestion was observed and only minor infiltration of inflammatory cells in myocardial tissues was exhibited (Fig. 4C).
Figure 4.
Morphology of cardiac tissue in rats under an optical microscope. Hematoxylin and eosin staining (magnification, ×200). (A) Control group. (B) Chuan-wu treated group: Veins were dilated and congested, and slight inflammatory cell infiltrations were observed. (C) Ber 16 mg/kg group: A normal myocardium was exhibited.
Effects of Ber on AC-induced arrhythmias in rats
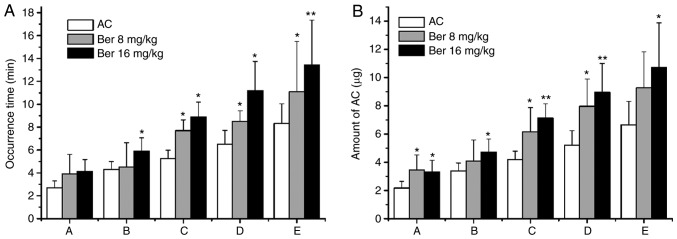
In the control group, an ECG was recorded continuously for 120 min with no evident abnormalities observed. In all of the other groups, no abnormal ECG features were observed prior to the administration of AC. However, in the AC group, VPCD, VPB, VT, VF and DD occurred successively following the administration of AC. Compared with the AC group, the occurrence time of VT, VF and DD was significantly prolonged in the Ber 8 mg/kg pretreatment group (P<0.05). Ber 16 mg/kg pretreatment also significantly prolonged the occurrence of all types of arrhythmias mentioned above (P<0.05 or P<0.01; Fig. 5A). Therefore, pretreatment with Ber 8 and 16 mg/kg increased the dosage of AC required to induce arrhythmias (P<0.05 or P<0.01; Fig. 5B).
Figure 5.
Effects of Ber on the occurrence time of arrhythmias and the dose of AC for inducing arrhythmias in rats. (A) Occurrence times and (B) amount of AC. Group A: Ventricular premature contraction discrete; Group B: Ventricular premature contraction bigeminy; Group C: Successive ventricular tachycardia; Group D: Ventricular fibrillation; Group E: Mortality. *P<0.05 and **P<0.01 vs. AC group. Ber, Berberine; AC, aconitine.
Effects of Ber and AC on papillary muscle action potential
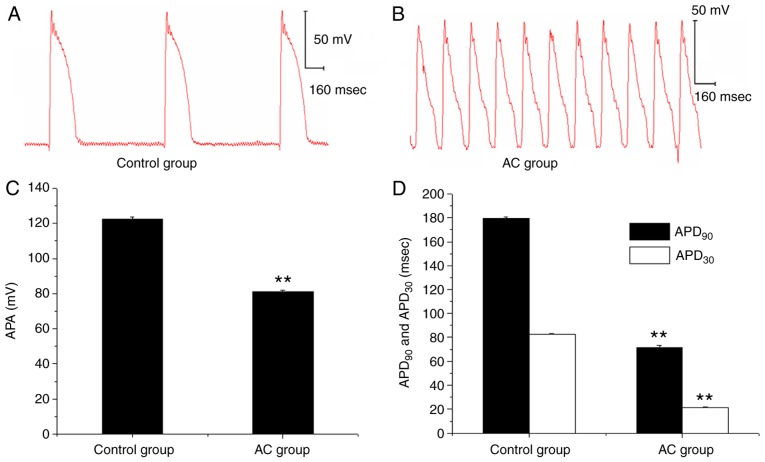
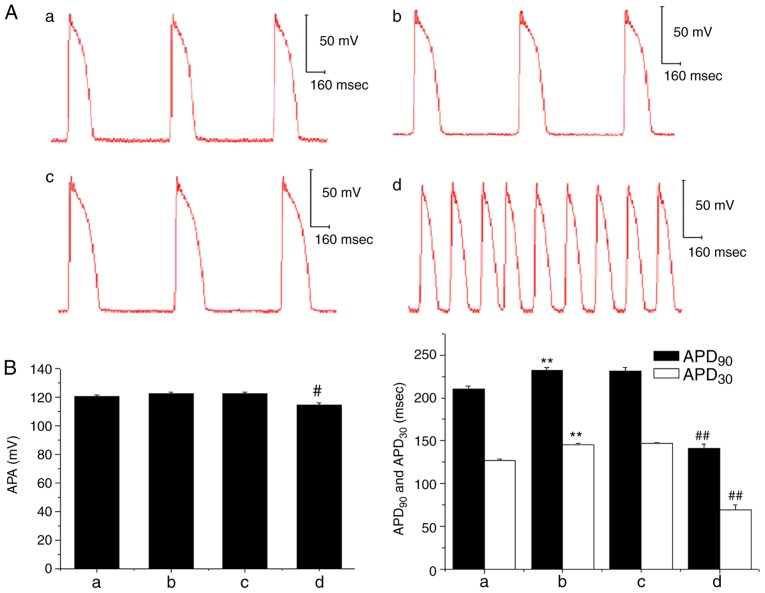
In the ex vivo experiment, the APA of the papillary muscle action potential was decreased following the administration of 3×10−7 mol/l AC, and the APD30 and APD90 were also significantly shortened when compared with prior to AC treatment; DAD and TA was also subsequently observed (Fig. 6). However, when Ber 1×10−5 mol/l pre-administration was performed, there were no marked changes in APA, APD30 and APD90, and no DAD occurred following AC perfusion. Subsequently, when the concentration of AC was increased to 1×10−6 mol/l, a decrease in APA and a shortening in APD90 and APD30 were induced (P<0.01); and DAD and TA were also observed (Fig. 7).
Figure 6.
Effects of AC on the action potential parameters of the papillary muscle in guinea pigs. (A and B) Representative recordings of action potentials in guinea pig papillary muscles with or without AC (3×10−7mol/l) at stimulation frequency of 1 Hz. (C and D) Effects of AC on the action potential parameters (C) APA, (D) APD30 and APD90. **P<0.01 vs. Control group. AC, aconitine.
Figure 7.
Effects of Ber on AC-induced abnormal action potential of guinea pig papillary muscles. (A) Representative figures of action potentials in guinea pig papillary muscles recorded prior to (image a: control) and following the addition of Ber 1×10−5 mol/l (image b) or adding AC 3×10−7 mol/l (image c) and AC 1×10−6 mol/l (image d) in the presence of Ber 1×10−5 mol/l at stimulation frequency of 1 Hz. (B) Effects of Ber on the changes in APA, APD30 and APD90 induced by AC. Group a: control; group b: Ber 1×10−5 mol/l; group c: Ber 1×10−5 mol/l+AC 3×10−7 mol/l; group d: Ber 1×10−5 mol/l+AC 1×10−6 mol/l. **P<0.01 vs. control group (group a); #P<0.05 and ##P<0.01 vs. Ber group (group b). Ber, Berberine; AC, aconitine; APA, action potential amplitude; APD30/90, duration of 30/90% repolarization.
Discussion
In recent years, increasing attention has been paid to the study of toxicity-reducing effects that can be achieved by co-administration of Chinese herbal medicines. Aconitum drugs have served an important role in the treatment of acute myocardial infarction, coronary heart disease and angina pectoris (1,2). However, the therapeutic and toxic doses of this type of medicine are very close, which can easily lead to damaging effects in patients (17). Arrhythmias are a major side-effect of Aconitum drugs (18).
Berberine hydrochloride, also known as Ber, is the main active ingredient of Rhizoma coptidis. Previous studies have revealed that Ber has an antagonistic effect on isoproterenol-induced myocardial damage and the ischemic arrhythmia caused by ligation of the anterior descending branch of the left coronary artery in rat models (19,20). In the present study, the antagonistic effects of Ber on Chuan-wu-induced acute myocardial injury were observed in rats. The results revealed that ventricular arrhythmias, including premature ventricular bigeminy, trigeminy or ventricular tachycardia, were all observed in rats following treatment with Chuan-wu for 3 days. In addition, the serum levels of CK, CK-MB and LDH, which are indicators of cardiac function, were all increased following Chuan-wu administration. The serum levels of CK, CK-MB and LDH are quantitative indicators of loss of membrane integrity during myocardial damage (21). The increase in CK, CK-MB and LDH suggested that Chuan-wu can affect the integrity of myocardial cell membranes. The histological findings of the myocardium also revealed dilation of small veins and the ventricular lumen, congestion of the heart cavity and some atrophy of muscle fibers appeared following the administration of Chuan-wu for 3 days, which is consistent with the literature (22,23). The results suggested that Chuan-wu disrupts the integrity of myocardial cell membranes and causes cell damage. Ber significantly reduced the degree of arrhythmia induced by Chuan-wu, and improved the survival rate of rats. Analysis of myocardial enzymes and histopathological examination demonstrated marked improvements in Ber pretreatment groups. This result indicated that Ber may have clear beneficial effects against Chuan-wu-induced myocardial injury; however, this requires further confirmation.
The toxic components of Chuan-wu include AC and mesaconitine. AC can stimulate the vagus nerve, and inhibit the sinoatrial node and conduction system, resulting in a slowed heart rate and conduction block (24,25). In addition, AC can act directly on myocardial cells, promoting the opening of Na+ channels, accelerating the ion influx, inducing cell membrane depolarization and improving the automaticity of fast response cells, including atrioventricular bundles and Purkinje fibers, thereby causing one-source or multifocal ectopic rhythms (26,27). It was also demonstrated that AC significantly increased Ca2+ influx, inhibited outward K+ currents, prolonged repolarization, and produced DAD and re-entry, resulting in arrhythmias (28). Therefore, the arrhythmia caused by Chuan-wu in rats may be associated with the presence of AC-type alkaloids.
As AC is a common arrhythmogenic toxin within Aconitum drugs, the present study investigated whether Ber has antagonistic effects on AC-induced arrhythmia. The in vivo ECG recording experiments demonstrated that AC administration decreased the heart rate, and prolonged the S-T and Q-T intervals, which lead to ventricular premature beat, coupled rhythm, trigeminy and tachycardia; these ultimately induced ventricular fibrillation and rat mortality. These observations are consistent with the literature (6). Pre-administration of Ber effectively delayed the onset of ventricular premature beat and coupled rhythm, and in turn prolonged the survival time of rats.
Considering guinea pig papillary muscle is a suitable preparation for the assessment of action potential in drug-induced Torsades De Pointes liability in humans (29), the present study observed the effects of Ber and AC on action potentials to investigate the mechanism of the anti-arrhythmic effects of Ber with papillary muscle in guinea pigs. The results revealed that AC decreased the APA of papillary muscle action potentials, shortened the APD90 and APD30, and DAD and TA were observed. However, Ber pretreatment completely inhibited the DAD induced by the same concentration of AC. However, when the dose of AC was increased, DAD and TA still occurred, even when Ber was pre-administered. From these results it was concluded that Ber may competitively counteract DAD and TA induced by AC. At the molecular level, it is generally believed that AC can accelerate the influx of Na+ ions into cardiomyocytes, promote the depolarization of the cell membrane and accelerate the automaticity of the pacemaker (30). It has also been reported that the cardiac toxicity of AC is associated with an increase in free radicals caused by oxidative stress (8). Additionally, Ca2+ overload and apoptosis via p38 signaling were also involved in the cardiotoxicity of AC (31). Therefore, AC-induced arrhythmias may be the result of a combination of multiple mechanisms. Modulation of apoptosis or cell signaling may also be involved in the underlying mechanisms of Ber's protective effects; the complete molecular mechanisms still require validation in further experiments. Therefore, a lack of a more thorough mechanistic analysis of the underlying pathway was a limitation of the present study.
In conclusion, Ber had preventive effects on Chuan-wu-induced acute myocardial injury, alleviated the symptoms of arrhythmia and improved the animal survival rate. Additionally, Ber also exhibited marked antagonistic effects on AC-induced arrhythmias, which may be associated with the inhibition of DAD and TA induced by AC. Thus, combination treatment of Ber with Aconitum plants is a novel strategy that has the potential for protection against Aconite-induced myocardial injury in clinical practice.
Acknowledgements
We would like to thank Professor Yongli Wang, Professor Hailin Zhang and Professor Yanfang Xu (all from the Department of Pharmacology, Hebei Medical University) for providing general support.
Funding
The present study was supported by the Natural Science Foundation of China (grant nos. 81773828, 81273600 and 81473292) and the Natural Science Foundation of Hebei Province (grant nos. H2018206297, C2011206145 and H2013206147).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
Study design: SS and QM; study conduct and data collection: HG, QL, KX, XC, YZ, XZ and QM; data analysis: QL, XC, ZZ and HL; manuscript writing: XC, QL and SS; manuscript review and editing: SS, QM and XC. All authors read and approved the manuscript.
Ethics approval and consent to participate
All experiments were approved by the Ethics Committee for the Use of Experimental Animals at Hebei Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zhou G, Tang L, Zhou X, Wang T, Kou Z, Wang Z. A review on phytochemistry and pharmacological activities of the processed lateral root of Aconitum carmichaelii Debeaux. J Ethnopharmacol. 2015;160:173–193. doi: 10.1016/j.jep.2014.11.043. [DOI] [PubMed] [Google Scholar]
- 2.Lin CC, Phua DH, Deng JF, Yang CC. Aconitine intoxication mimicking acute myocardial infarction. Hum Exp Toxicol. 2011;30:782–785. doi: 10.1177/0960327110385960. [DOI] [PubMed] [Google Scholar]
- 3.Chan TY. Aconitum alkaloid content and the high toxicity of aconite tincture. Forensic Sci Int. 2012;222:1–3. doi: 10.1016/j.forsciint.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Chan TY. Incidence and causes of aconitum alkaloid poisoning in Hong Kong from 1989 to 2010. Phytother Res. 2015;29:1107–1111. doi: 10.1002/ptr.5370. [DOI] [PubMed] [Google Scholar]
- 5.Sheth S, Tan EC, Tan HH, Tay L. Herb-induced cardiotoxicity from accidental aconitine overdose. Singapore Med J. 2015;56:e116–e119. doi: 10.11622/smedj.2015114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu M, Dong YH, Han F, Qin JM, Zhang HN, Du JX, Hao XM, Yang YM. Influence of total flavonoids derived from Choerospondias axillaris folium on aconitine-induced antiarrhythmic action and hemodynamics in Wistar rats. J Toxicol Environ Health A. 2016;79:878–883. doi: 10.1080/15287394.2016.1193117. [DOI] [PubMed] [Google Scholar]
- 7.Zhou YH, Piao XM, Liu X, Liang HH, Wang LM, Xiong XH, Wang L, Lu YJ, Shan HL. Arrhythmogenesis toxicity of aconitine is related to intracellular ca(2+) signals. Int J Med Sci. 2013;10:1242–1249. doi: 10.7150/ijms.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YJ, Chen BS, Lin MW, Lin AA, Peng H, Sung RJ, Wu SN. Time-dependent block of ultrarapid-delayed rectifier K+ currents by aconitine, a potent cardiotoxin, in heart-derived H9c2 myoblasts and in neonatal rat ventricular myocytes. Toxicol Sci. 2008;106:454–463. doi: 10.1093/toxsci/kfn189. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Wang X, Chung YY, Koh CH, Liu Z, Guo H, Yuan Q, Wang C, Su S, Wei H. L-type calcium channel inhibition contributes to the proarrhythmic effects of aconitine in human cardiomyocytes. PLoS One. 2017;12:e0168435. doi: 10.1371/journal.pone.0168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: An analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther. 2012;12:1113–1124. doi: 10.1517/14712598.2012.704014. [DOI] [PubMed] [Google Scholar]
- 11.Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev. 2001;19:234–244. doi: 10.1111/j.1527-3466.2001.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoudvand H, Ayatollahi Mousavi SA, Sepahvand A, Sharififar F, Ezatpour B, Gorohi F, Saedi Dezaki E, Jahanbakhsh S. Antifungal, antileishmanial and cytotoxicity activities of various extracts of berberis vulgaris (Berberidaceae) and its active principle berberine. ISRN Pharmacol. 2014;2014:602436. doi: 10.1155/2014/602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan F, Zhang X, Chen L. Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life Sci. 2014;98:24–30. doi: 10.1016/j.lfs.2013.12.211. [DOI] [PubMed] [Google Scholar]
- 14.Ren LM, Zhuo YJ, Hao ZS, He HM, Lu HG, Zhao D. Berberine improves neurogenic contractile response of bladder detrusor muscle in streptozotocin-induced diabetic rats. J Ethnopharmacol. 2013;150:1128–1136. doi: 10.1016/j.jep.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 15.Xiong C, Wu YZ, Zhang Y, Wu ZX, Chen XY, Jiang P, Guo HC, Xie KR, Wang KX, Su SW. Protective effect of berberine on acute cardiomyopathy associated with doxorubicin-treatment. Oncol Lett. 2018;15:5721–5729. doi: 10.3892/ol.2018.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Zhang Y, Zhu Z, Liu H, Guo H, Xiong C, Xie K, Zhang X, Su S. Protective effect of berberine on doxorubicin-induced acute hepatorenal toxicity in rats. Mol Med Rep. 2016 May;13:3953–3960. doi: 10.3892/mmr.2016.5017. [DOI] [PubMed] [Google Scholar]
- 17.Kasahara Y, Itou T, Numazawa T, Wada A. Aconitine analogues in wild Aconitum plants: Contents toxicity to mice and decrease by boiling. Shokuhin Eiseigaku Zasshi. 2013;54:364–369. doi: 10.3358/shokueishi.54.364. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 18.Zhu L, Wu J, Zhao M, Song W, Qi X, Wang Y, Lu L, Liu Z. Mdr1a plays a crucial role in regulating the analgesic effect and toxicity of aconitine by altering its pharmacokinetic characteristics. Toxicol Appl Pharmacol. 2017;320:32–39. doi: 10.1016/j.taap.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhang T, Yang S, Du J. Protective effects of berberine on isoproterenol-induced acute myocardial ischemia in rats through regulating HMGB1-TLR4 axis. Evid Based Complement Alternat Med. 2014;2014:849783. doi: 10.1155/2014/849783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LH, Li XL, Li Q, Fu Y, Yu HJ, Sun YQ, Zhang L, Shan HL. Berberine alleviates ischemic arrhythmias via recovering depressed I(to) and I(Ca) currents in diabetic rats. Phytomedicine. 2012;19:206–210. doi: 10.1016/j.phymed.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Gautam PL, Luthra N, Kaur M, Singh J, Wander GS, Tandon R, Gautam N. Evaluation of myocardial injury using standard diagnostic tools and tissue doppler imaging in blunt trauma chest. J Clin Diagn Res. 2017;11:OC33–OC36. doi: 10.7860/JCDR/2017/22746.10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge YB, Jiang Y, Zhou H, Zheng M, Li J, Huang XJ, Gao Y. Antitoxic effect of Veratrilla baillonii on the acute toxicity in mice induced by Aconitum brachypodum, one of the genus Aconitum. J Ethnopharmacol. 2016;179:27–37. doi: 10.1016/j.jep.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Gao Y, Tan G, Wu S, Dong X, Lou Z, Zhu Z, Chai Y. Myocardial lipidomics profiling delineate the toxicity of traditional Chinese medicine Aconiti Lateralis radix praeparata. J Ethnopharmacol. 2013;147:349–356. doi: 10.1016/j.jep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Ono T, Hayashida M, Tezuka A, Hayakawa H, Ohno Y. Antagonistic effects of tetrodotoxin on aconitine-induced cardiac toxicity. J Nippon Med Sch. 2013;80:350–361. doi: 10.1272/jnms.80.350. [DOI] [PubMed] [Google Scholar]
- 25.Jung BC, Lee SH, Cho YK, Park HS, Kim YN, Lee YS, Shin DG. Role of the alternans of action potential duration and aconitine-induced arrhythmias in isolated rabbit hearts. J Korean Med Sci. 2011;26:1576–1581. doi: 10.3346/jkms.2011.26.12.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright SN. Comparison of aconitine-modified human heart (hH1) and rat skeletal (mu1) muscle Na+ channels: An important role for external Na+ ions. J Physiol. 2002;538:759–771. doi: 10.1113/jphysiol.2001.012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki K, Matsumoto A, Nishida H, Reien Y, Maruyama H, Nakaya H. Termination of aconitine-induced atrial fibrillation by the KACh-channel blocker tertiapin: Underlying electrophysiological mechanism. J Pharmacol Sci. 2014;125:406–414. doi: 10.1254/jphs.14023FP. [DOI] [PubMed] [Google Scholar]
- 28.Mitamura M, Horie S, Sakaguchi M, Someya A, Tsuchiya S, Van de Voorde J, Murayama T, Watanabe K. Mesaconitine-induced relaxation in rat aorta: Involvement of Ca2+ influx and nitric-oxide synthase in the endothelium. Eur J Pharmacol. 2002;436:217–225. doi: 10.1016/S0014-2999(01)01623-5. [DOI] [PubMed] [Google Scholar]
- 29.Ducroq J. Sensitivity and specificity of the in vitro guinea pig papillary muscle action potential duration for the assessment of drug-induced torsades de pointes liability in humans. Handb Exp Pharmacol. 2015;229:205–219. doi: 10.1007/978-3-662-46943-9_8. [DOI] [PubMed] [Google Scholar]
- 30.Coulson JM, Caparrotta TM, Thompson JP. The management of ventricular dysrhythmia in aconite poisoning. Clin Toxicol (Phila) 2017;55:313–321. doi: 10.1080/15563650.2017.1291944. [DOI] [PubMed] [Google Scholar]
- 31.Sun GB, Sun H, Meng XB, Hu J, Zhang Q, Liu B, Wang M, Xu HB, Sun XB. Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats. Toxicol Appl Pharmacol. 2014;279:8–22. doi: 10.1016/j.taap.2014.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.