Abstract
Tumor-infiltrating lymphocytes are associated with the response to neoadjuvent chemotherapy and prognosis in breast cancer. However, the distribution, interaction and prognostic value of tumor-infiltrating T cells, the main component of the tumor microenvironment, have seldom been reported. In the present study, surgical specimens of 72 breast cancer patients were analyzed. Tumor-infiltrating T cell subsets [cluster of differentiation (CD)4+T, CD8+T and regulatory T cells] and expression of their cytokines [interferon-γ, interleukin (IL)-4, and IL-17] were evaluated by flow cytometry. These parameters together with The Cancer Genome Atlas database were used to demonstrate the distribution, interaction and prognostic value of tumor-infiltrating T cells in breast cancer. Tumor-infiltrating lymphocytes were closely associated with histological grade (P=0.03), estrogen receptor status (P=0.006), human epidermal growth factor receptor 2 status (P=0.047) and molecular subtype in breast cancer (P=0.012). The gene expression of CD4, CD8A and forkhead box protein P3 in the tumor was increased compared with healthy breast tissue, and was positively associated with the prognosis of breast cancer patients. HER2+ and triple-negative breast cancer exhibited a significantly increased percentage of CD4+T cells (P=0.01) and regulatory T cells (P=0.035), and a decreased percentage of CD8+T cells (P=0.006) compared with the luminal subtype. Furthermore, the regulatory T cell number was positively correlated with CD8+T cell number in tumors (R=0.7, P=1.5×10−162) and significantly inhibited the cytokine secretion of T cells. These results reveal the distribution and interaction of tumor-infiltrating T cell subsets, and indicate that CD8+T cells and regulatory T cells may be used as reliable predictors of prognosis in breast cancer.
Keywords: tumor-infiltrating T cells, gene expression, distribution, prognosis, breast cancer
Introduction
The role of the immune system in the origin, development and metastasis of cancer is gradually being elucidated (1–3) and is being taken into consideration in anticancer treatment (4–6). For example, the predictive and prognostic value of tumor-infiltrating lymphocytes (TILs) in various tumors, especially breast cancer (BC), was recently investigated (7–9). The main infiltrating cells of the immune system in BC are T cells, and accumulating evidence suggests that immune activity mediated by T cells is critical for a sustained and effective antitumor response (10). A previous study involving semi-quantitative hematoxylin-eosin staining demonstrated that triple-negative breast cancer (TNBC) exhibited a high infiltration of cluster of differentiation (CD)4+T cells, CD8+T cells and regulatory T cells (Tregs) (11). However, the exact numbers of these cells and discrepancies between tumor, and healthy tissue are seldom reported. Therefore, a more accurate and detailed analysis is needed to determine the distribution of tumor-infiltrating T cells in BC.
Tregs are a subset of CD4+T cells that regulate immune responses to pathogens and maintain self-tolerance. The forkhead box P3 protein (FOXP3) controls immune system development and function, and serves a crucial role in the generation of Tregs (12). The infiltration of Tregs was reported in a variety of malignancies, including BC and can effectively inhibit anti-tumor responses (13–15). However, tumors are highly heterogeneous and the way in which Tregs influence other immune cells in BC remains unknown.
Mahmoud et al (16) demonstrated that tumor-infiltrating CD8+ T lymphocytes exhibited antitumor activity, as evidenced by their favorable effect on patient survival. However, Matkowski et al (17) demonstrated that the presence of CD8+ and CD4+ cells correlated with lymph node involvement and unfavorable prognosis in early BC. Similarly, Merlo et al (18) demonstrated that FOXP3 expression in tumors was associated with worse overall survival in BC. However, Ladoire et al (19) demonstrated that FOXP3 expression in tumor cells predicted a better survival in human epidermal growth factor receptor (HER)2-overexpressing BC patients treated with neoadjuvant chemotherapy. These data identified the controversy surrounding the prognostic value of tumor-infiltrating T cell subsets and demonstrated that further research is required to fully understand it.
Therefore, the present study aimed to assess the distribution and interaction of tumor-infiltrating T cell subsets in BC. Additionally, the prognostic value of CD4, CD8A and FOXP3 expression in BC was evaluated using The Cancer Genome Atlas (TCGA) database.
Materials and methods
Patient samples
The present study comprised 72 female BC patients who underwent breast-conserving surgery or mastectomy without neoadjuvant chemotherapy in the First People's Hospital of Yunnan Province between October 2016 and July 2017 (Kunming, China). All patient samples were diagnosed as invasive breast carcinoma by core needle biopsy prior to surgery. Patient characteristics are presented in Table I. The present study was approved by the Ethics Committee of The First People's Hospital of Yunnan Province and written informed consent was provided by each patient. All methods were performed in accordance with relevant guidelines and regulations.
Table I.
Association of tumor infiltrating lymphocytes with clinicopathological characteristics of breast cancer patients.
| Characteristics | Total N | TILs low N (%) | TILs high N (%) | P-value |
|---|---|---|---|---|
| Age | 0.976 | |||
| <50 years | 26 | 18 (69.2) | 8 (30.8) | |
| ≥50 years | 46 | 32 (69.6) | 14 (30.4) | |
| Tumor size | 0.100 | |||
| <2 cm | 30 | 24 (80.0) | 6 (20.0) | |
| ≥2 cm | 42 | 26 (61.9) | 16 (38.1) | |
| Lymph node | 0.409 | |||
| Negative | 38 | 28 (73.7) | 10 (26.3) | |
| Positive | 34 | 22 (64.7) | 12 (35.3) | |
| Histological grade | 0.030 | |||
| Low (I and II) | 40 | 32 (80.0) | 8 (20.0) | |
| High (III) | 32 | 18 (56.3) | 14 (43.8) | |
| ER | 0.006 | |||
| Negative | 23 | 11 (47.8) | 12 (52.2) | |
| Positive | 49 | 39 (79.6) | 10 (20.4) | |
| HER2 status | 0.047 | |||
| Negative | 48 | 37 (77.1) | 11 (22.9) | |
| Positive | 24 | 13 (54.2) | 11 (45.8) | |
| Ki-67 | 0.113 | |||
| <20% | 33 | 26 (78.8) | 7 (21.2) | |
| ≥20% | 39 | 24 (61.5) | 15 (38.5) | |
| Molecular subtype | 0.012 | |||
| Luminal | 37 | 31 (83.8) | 6 (16.2) | |
| Her2+ | 24 | 13 (54.2) | 11 (45.8) | |
| Triple-negative | 11 | 5 (45.5) | 6 (54.5) |
TILs, tumor infiltrating lymphocytes; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2.
Breast cancer subtypes
The St. Gallen Expert Consensus (20) was used to classify BC subtypes and was optimised as: Luminal type [estrogen receptor (ER)+ and/or progesterone receptor (PR)+ and HER2–], HER2+ (any ER status, any PR status and HER2+), and triple negative (ER–, PR– and HER2–). For ER and PR, cases with ≥1% positive staining as performed according to a previously published protocol (11) were considered positive, and patients with HER2 3+ or the presence of HER2 amplification were considered HER2-positive. The Ki67 index was also determined in all patients (11).
Processing of genomic data from TCGA
Publicly available TCGA data including 1,085 BC patients was downloaded from http://www.cbioportal.org and used in this study (21). Gene Expression Profiling Interactive Analysis (21), an interactive web server, was used for cancer and normal gene expression profiling and interactive analysis. A total of 69 BC patients who are male, presented with distant metastasis or received neoadjuvant chemotherapy were excluded in the further survival analysis. The best cut-off for CD4, CD8A and FOXP3 mRNA expression was 10.32, 7.47 and 6.55 separately which was defined by the receiver operating characteristic curve. Information regarding the 291 normal patients referred to as healthy women without infectious disease in the last 6 months was also obtained from this site.
Hematoxylin and eosin (H&E) staining
Sections (4 µm) were deparaffinized with 2 changes of xylene for 10 min each. The BC sections were hydrated by passing through decreasing alcohol series (100, 95 and 70%). Slides were stained in hematoxylin for 8 min at room temperature and then washed in running tap water for 5 min. 1% acidified alcohol was used for differentiation (1% HCl in 70% alcohol) for 2 min. Sections were washed in running tap water until the sections were blue again by dipping in an alkaline solution followed by another tap water wash. Then the sections were stained in 1% eosin Y for 5 min at room temperature. Sections were washed in tap water for 3 min and dehydrated in increasing concentration of alcohols and cleared in xylene.
Quantification of TILs
A semi-quantitative H&E method was used to evaluate the TILs (22). Histopathological analysis of the lymphocyte infiltrate was performed on H&E-stained sections (22). All tumor H&E-stained slides were observed at ×100 or ×400 magnification (OLYMPUS CX23; Olympus Corporation, Tokyo, Japan). Stromal TILs were defined as the percentage of tumor stroma area containing a lymphocytic infiltrate without direct contact to tumor cells. In heterogenous tumors, different regions were evaluated and the average percentages of TILs were reported. During H&E evaluation, the cutoff percentages for low TILs and high TILs were <60% and >60% in BC tissue, respectively.
Flow cytometry
Flow cytometry was used to evaluate the density of TILs. This was evaluated as the average number of CD45+ cells per gram. In the present study the H&E slides with 60% TILs from the 72 patients were chosen for subsequent analysis, and the TIL density of the same patient was calculated by flow cytometry. Low and high groups were defined as CD45+ events/g tissue <300,000 and ≥300,000, respectively.
Freshly resected tissue was manually minced and then incubated for 60 min at 37°C in a rocking table bed (120 rpm/min) with 1.0 mg/ml collagenase type IV (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 100 µg/ml DNase I (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) diluted in RPMI Medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) without fetal bovine serum (FBS). Single cell suspensions were prepared by filtering through 70-µm nylon strainers (BD Biosciences, Franklin Lakes, NJ, USA). Zombie Yellow (BioLegend, Inc., San Diego, CA, USA) was used to discriminate live and dead cells according to the manufacturer's suggested dilutions (1:1,000) prior to surface marker staining for 30 min at 4°C.
For surface staining, filtered single cells were incubated for 30 min on ice with Fc Receptor Binding Inhibitor (eBioscience; Thermo Fisher Scientific, Inc.) diluted 1:10 in PBS. Cells were then incubated for 15 min in PBS containing 1.0 mM EDTA and 5% FBS (Gibco; Thermo Fisher Scientific, Inc.) together with the manufacturer's suggested dilutions of the following antibodies at room temperature: Alexa Fluor 700 anti-human CD3 (1:500; cat. no. 300324; Biolegend, Inc.), fluorescein isothiocyanate anti-human CD4 (1:500; cat. no. 317408; Biolegend, Inc.), PerCP/Cy5.5 anti-human CD8 (1:500; cat. no. 300924; Biolegend, Inc.), phycoerythrin anti-human CD25 (1:500; cat. no. 302606; Biolegend, Inc.), PE/Cy5 anti-human CD45 (1:500; cat. no. 304010; Biolegend, Inc.) and Brilliant Violet 650 anti-human CD127 (IL-7Rα; 1:500; cat. no. 351326; Biolegend, Inc.).
For cytokine staining, filtered single cells were incubated for 5 h with phorbol-12-myristate-13-acetate (50 ng/ml), ionomycin (1 µg/ml) and Brefeldin A (1:1,000) diluted in RPMI Medium (HyClone; GE Healthcare Life Sciences) containing 10% FBS and penicillin-streptomycin (1:100). Surface markers were stained as described above. Cells were fixed with fixation/permeabilization buffer (eBioscience; Thermo Fisher Scientific, Inc.) for 20 min at 4°C according to the manufacturer's protocol. Then the intracellular staining plate containing Pacific Blue-conjugated anti-human IFN-γ (1:500; cat. no. 502517; Biolegend, Inc.), PECy7-conjugated anti-human IL-4 (1:500; cat. no. 500817; Biolegend, Inc.) and Brilliant Violet 510-conjugated anti-human IL-17 (1:500; cat. no. 512307; Biolegend, Inc.) was placed at 4°C for 30 min, washed twice with fixation/permeabilization buffer (eBioscience; Thermo Fisher Scientific, Inc.) diluted in PBS (1:1,000) and stored at 4°C until required for analysis with the CytoFLEX flow cytometer (Beckman Coulter, Inc., Brea, CA, USA). Results were analyzed using FlowJo software v9.3.2 (Tree Star, Inc., Ashland, OR, USA).
Statistical analysis
All statistical analysis was conducted using IBM SPSS Statistics software (version 22.0; IBM Corp, Armonk, NY, USA). Continuous data are presented as the mean ± standard deviation. Non-continuous data were compared by the Chi-square test or Fisher's exact (two-sided) test and continuous data were analyzed by the Mann-Whitney U test (two groups) or Kruskal-Wallis test followed by Tukey's post hoc test (>two groups). Kaplan-Meier curves and the log-rank test were used for survival analysis. Pearson's correlation analysis was used to evaluate the correlation between two variables. Univariate and multivariate Cox regression analyses were used to evaluate the significance of various parameters for survival. P<0.05 was considered to indicate a statistically significant difference. All experiments were repeated three times.
Results
TILs are closely associated with the clinicopathological characteristics of BC patients
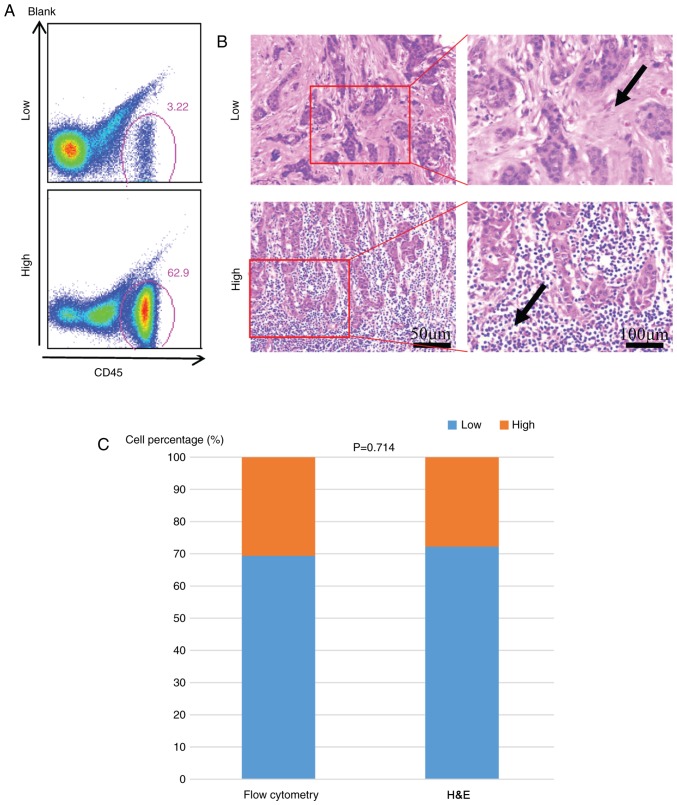
A total of 72 patients were divided into two groups (low and high) according to their TIL density. There were 22 patients with high TILs using the quantitative method and this number was 20 when using the semiquantitative H&E staining. The result was comparable in evaluating the TILs by flow cytometry (Fig. 1A) and hematoxylin-eosin staining evaluation (Fig. 1B and C). The association between TIL infiltration (low vs. high) and various clinicopathological parameters of BC patients are presented in Table I. A high infiltration of TILs was significantly associated with histological grade (P=0.03), ER negativity (P=0.006), HER2 positivity (P=0.047) and BC molecular subtypes (P=0.012). The infiltration of lymphocytes demonstrated no correlation with age, tumor size, lymph node status, or Ki-67 proliferation index (Table I).
Figure 1.
Evaluation of TILs in breast cancer by different methods. Flow cytometry (A) and (B) H&E staining was used to evaluate the TIL infiltration in breast cancer separately. (C) Comparison of the methods of flow cytometry and H&E staining. The arrow indicated the TILs. The magnification used to analyze the low and high groups was the same. All experiments were repeated three times. TILs, tumor-infiltrating lymphocytes; CD, cluster of differentiation; H&E, hematoxylin and eosin.
Expression of tumor infiltrating T cell genes in BC
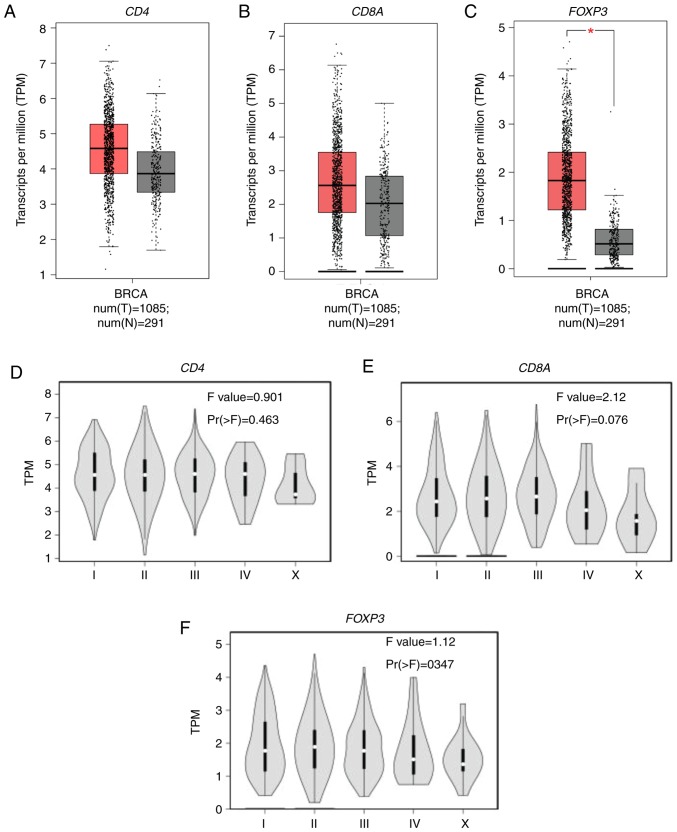
The expression of CD4, CD8A and FOXP3 mRNA as measured by transcripts per million, reflects the infiltration of CD4+T cells, CD8+T cells, and Tregs, respectively. A total of 1,085 BC samples and 291 healthy breast tissue samples were analyzed, and increased CD4, CD8A, and significantly increased FOXP3 mRNA expression (P<0.01) was detected in BC samples compared with the healthy breast tissue (Fig. 2A-C). The association between CD4, CD8A and FOXP3 mRNA expression and clinical stage of BC patients was also evaluated as determined by the American Joint Committee on Cancer staging system. There was no significant difference in the mRNA expression of CD4 (P=0.463; Fig. 2D) or FOXP3 (P=0.347; Fig. 2F) in BC patients with different clinical stages. However, stage IV BC patients tended to exhibit decreased CD8A mRNA expression compared with early BC patients (P=0.076; Fig. 2E).
Figure 2.
CD4, CD8A and FOXP3 mRNA expression in breast cancer. TPM of (A) CD4, (B) CD8A and (C) FOXP3 obtained from The Cancer Genome Atlas database were compared between BC and healthy breast tissue samples. TPM of (D) CD4, (E) CD8A and (F) FOXP3 were compared among BC patients at different clinical stages. *P<0.01. All experiments were repeated three times. CD, cluster of differentiation; BRCA, Breast invasive carcinoma; BC, breast cancer; FOXP3, forkhead box protein P3; TPM, transcripts per million; num(T), number of tumor samples; num(N), number of normal tissue samples.
Distribution of tumor-infiltrating T cells in different molecular subtypes of BC patients
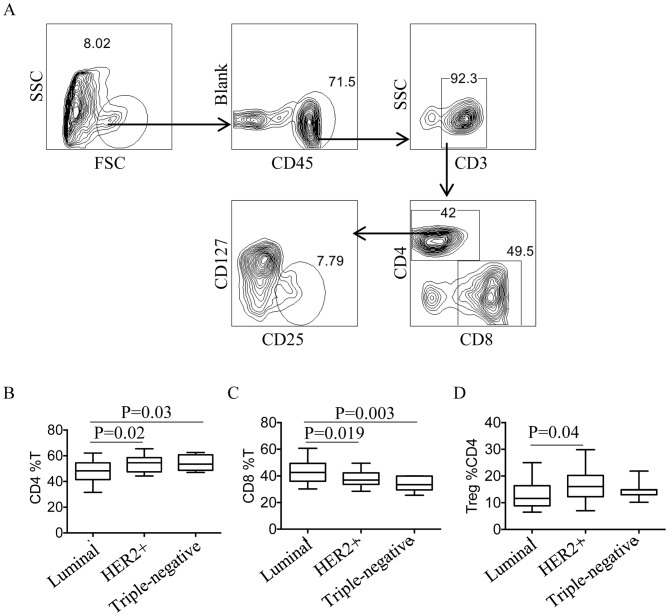
Flow cytometry was used to quantify CD4+T cells, CD8+T cells and Tregs, and the gating strategy is presented in Fig. 3A. First, total lymphocytes were gated, then CD3+T cells were distinguished from CD45+T cells. Tregs were defined as CD4+CD25+CD127low T cells. The quantity of CD4+T cells, CD8+T cells and Tregs was compared between different subtypes of BC, and the percentage of CD4+T cells was demonstrated to be significantly increased in HER2+ and TNBC compared with the luminal subtype (P=0.02 and P=0.03 respectively; Fig. 3B. However, the percentage of CD8+T cells was significantly reduced in HER2+ and TNBC patients compared with patients with the luminal subtype (P=0.019 and P=0.003, respectively; Fig. 3C). The percentage of Tregs was also significantly increased in HER2+ patients compared with patients with the luminal subtype (P=0.04, Fig. 3D).
Figure 3.
Distribution of tumor-infiltrating T cell subsets in BC patients with different molecular subtypes. (A) The gating strategy for tumor-infiltrating T cell subsets by flow cytometry. Quantity comparison of (B) CD4+T cells, (C) CD8+T cells and (D) Tregs in BC patients with different molecular subtypes. All experiments were repeated three times. CD, cluster of differentiation; FOXP3, forkhead box protein P3; BC, breast cancer; Treg, regulatory T cell; SSC, side scatter; FSC, forward scatter; HER2+, human epidermal growth factor receptor.
Tregs inhibit T cell cytokine secretion in BC
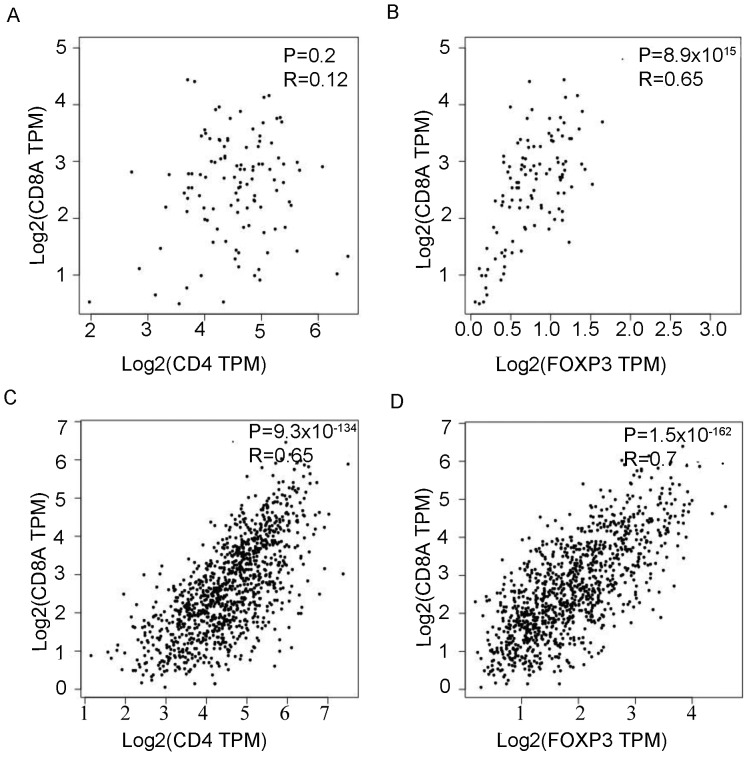
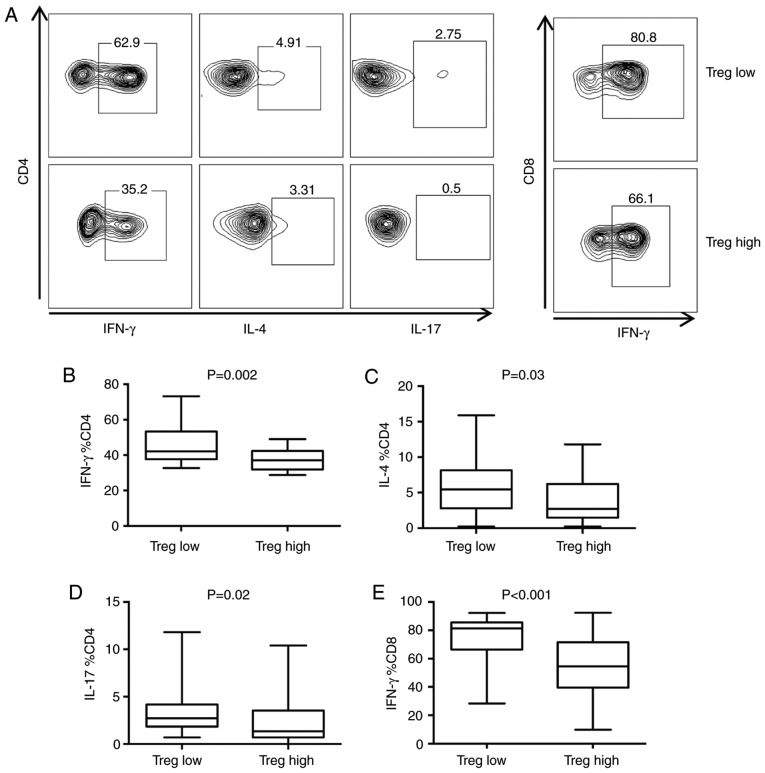
The correlation of mRNA expression in CD4+T cells, CD8+T cells and Tregs in BC and healthy breast tissue was next evaluated. In healthy breast tissue, there was no association between CD4 and CD8A mRNA expression (P=0.2; Fig. 4A). However, CD8A mRNA expression was significantly, positively correlated with FOXP3 expression (P=8.9×10−15; Fig. 4B). In BC, CD8A mRNA expression was significantly, positively correlated with CD4 (P=9.3×10−134; R=0.65; Fig. 4C) and FOXP3 (P=1.5×10−162; R=0.7; Fig. 4D) mRNA expression. Furthermore, the association between the number of Tregs and cytokine secretion of T cells was studied (Fig. 5A). The median number of Tregs was used as the cutoff to divide cells into Tregs high and Tregs low groups. CD4+T cells secreted significantly more interferon (IFN)-γ (P=0.002; Fig. 5B), IL-4 (P=0.03; Fig. 5C) and IL-17 (P=0.02; Fig. 5D), while CD8+T cells secreted significantly more IFN-γ (P<0.001; Fig. 5E) in the Tregs low group compared with the Tregs high group.
Figure 4.
The reciprocal quantity association of CD4, CD8A and FOXP3 mRNA expression. The reciprocal association of (A) CD4 and (B) FOXP3 with CD8A mRNA expression in healthy breast tissue. The reciprocal association of (C) CD4 and (D) FOXP3 with CD8A mRNA expression in BC tissue. All experiments were repeated three times. TPM, transcript per million; CD, cluster of differentiation; FOXP3, forkhead box protein P3; BC, breast cancer.
Figure 5.
Influence of Tregs on the cytokine secretion of T cells. (A) Representative flow cytometry demonstrating the association between Treg quantity and cytokine secretion of T cells in tumors. Comparison of CD4+T cell secretion of (B) IFN-γ, (C) IL-4 and (D) IL-17 and (E) CD8+T cell cytokine secretion between Treg low and Treg high BC patients. All experiments were repeated three times. Treg, regulatory T cell; BC, breast cancer; CD, cluster of differentiation; IFN, interferon; IL, interleukin.
CD8A and FOXP3 mRNA expression as prognostic markers in BC
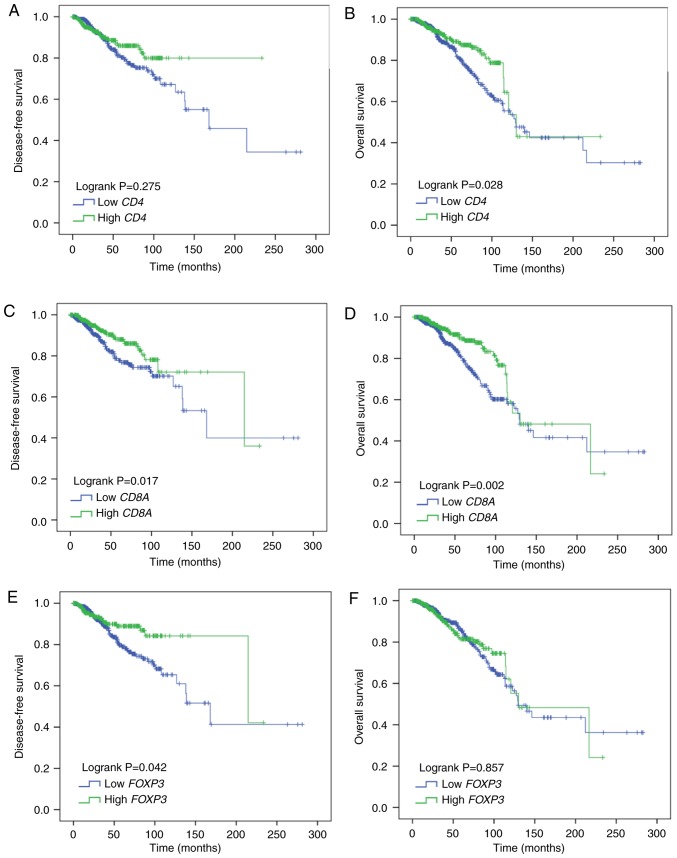
A total of 1,029 BC patients were evaluated for disease-free survival (DFS) and overall survival (OS). CD4 mRNA expression was not correlated with DFS (Log rank P=0.275; Fig. 6A); however, it was significantly, positively correlated with OS (Log rank P=0.028; Fig. 6B). CD8A mRNA expression was both significantly, positively correlated with DFS (Log rank P=0.017; Fig. 6C) and OS (Log rank P=0.002; Fig. 6D). As for FOXP3 mRNA, its expression was significantly, positively correlated with DFS (Log rank P=0.042; Fig. 6E) and had no correlation with OS (Log rank P=0.857; Fig. 6F). In univariate analysis, correlations between DFS, OS and each clinicopathological parameter were examined for BC patients. CD8A, FOXP3 demonstrated a significant association with DFS (P=0.018 and 0.044) and CD4. CD8A demonstrated a significant association with OS (P=0.029 and 0.003; Table II). In multivariate analysis, CD8A, FOXP3 remained statistically significant (P=0.013 and 0.028) in the analysis for DFS and CD8A remained statistically significant (P=0.028) in the analysis for OS (Table II). These data indicate that CD8A and FOXP mRNA expression could serve as independent prognosis markers in BC patients.
Figure 6.
Prognostic ability of CD4, CD8A and FOXP3 mRNA expression in BC patients. Kaplan-Meier survival curves of (A) disease-free survival and (B) overall survival with CD4 expression level. (C) Disease free survival and (D) overall survival with CD8A and (E) disease free survival and (F) overall survival with FOXP3 in BC patients. P-values were calculated using the log-rank method. All experiments were repeated three times. CD, cluster of differentiation; FOXP3, forkhead box protein P3; BC, breast cancer.
Table II.
Univariate and multivariate Cox regression analyses of DFS and OS in patients with breast cancer.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Covariates | HR (95% CI) | P-value | HR (95% CI) | P-value |
| A, DFS | ||||
| Age | ||||
| 41–65 vs. ≤40 years old | 0.434 (0.257–0.734) | 0.002 | 0.518 (0.299–0.895) | 0.018 |
| >65 vs. ≤40 years old | 0.625 (0.339–1.151) | 0.131 | 0.717 (0.377–1.366) | 0.312 |
| Menopause status (post vs. pre) | 1.002 (0.635–1.582) | 0.992 | ||
| Histology | ||||
| ILC vs. IDC | 1.297 (0.8–2.105) | 0.292 | ||
| Others vs. IDC | 1.667 (0.83–3.349) | 0.151 | ||
| pT stage | ||||
| T2 vs. T1 | 1.794 (1.041–3.093) | 0.035 | 1.678 (0.957–2.942) | 0.071 |
| T3 vs. T1 | 2.579 (1.338–4.97) | 0.005 | 2.408 (1.194–4.855) | 0.014 |
| T4 vs. T1 | 7.541 (3.068–18.531) | 0.000 | 3.784 (1.447–9.899) | 0.007 |
| pN stage (positive vs. negative) | 1.969 (1.295–2.993) | 0.002 | 1.808 (1.159–2.822) | 0.009 |
| ER (positive vs. negative) | 0.535 (0.351–0.815) | 0.004 | 0.782 (0.418–1.464) | 0.443 |
| PR (positive vs. negative) | 0.514 (0.342–0.771) | 0.001 | 0.574 (0.316–1.041) | 0.068 |
| HER2 (positive vs. negative) | 0.781 (0.412–1.484) | 0.451 | ||
| CD4 expression level (high vs. low) | 0.794 (0.525–1.202) | 0.276 | ||
| CD8A expression level (high vs. low) | 0.608 (0.403–0.918) | 0.018 | 0.575 (0.371–0.891) | 0.013 |
| FOXP3 expression level (high vs. low) | 0.634 (0.406–0.988) | 0.044 | 0.595 (0.374–0.947) | 0.028 |
| B, OS | ||||
| Univariate analysis | Multivariate analysis | |||
| Covariates | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | ||||
| 41–65 vs. ≤40 years old | 0.58 (0.331–1.016) | 0.057 | 0.553 (0.284–1.076) | 0.081 |
| >65 vs. ≤40 years old | 1.785 (1.021–3.123) | 0.042 | 1.73 (0.806–3.71) | 0.159 |
| Menopause status (post versus pre) | 2.135 (1.246–3.661) | 0.006 | 1.638 (0.831–3.23) | 0.154 |
| Histology | ||||
| ILC vs. IDC | 1.082 (0.688–1.699) | 0.734 | ||
| Others vs. IDC | 1.521 (0.812–2.849) | 0.191 | ||
| pT stage | ||||
| T2 vs. T1 | 1.361 (0.875–2.116) | 0.172 | 1.35 (0.851–2.141) | 0.203 |
| T3 vs. T1 | 1.559 (0.884–2.749) | 0.125 | 1.347 (0.736–2.467) | 0.334 |
| T4 vs. T1 | 3.277 (1.524–7.046) | 0.002 | 1.945 (0.861–4.39) | 0.109 |
| pN stage (positive vs. negative) | 1.884 (1.301–2.727) | 0.001 | 1.852 (1.244–2.757) | 0.002 |
| ER (positive vs. negative) | 0.631 (0.427–0.933) | 0.021 | 0.574 (0.381–0.865) | 0.008 |
| PR (positive vs. negative) | 0.733 (0.506–1.062) | 0.101 | ||
| HER2 (positive vs. negative) | 1.391 (0.852–2.271) | 0.187 | ||
| CD4 expression level (high vs. low) | 0.649 (0.440–0.957) | 0.029 | 0.781 (0.522–1.169) | 0.229 |
| CD8A expression level (high vs. low) | 0.562 (0.385–0.82) | 0.003 | 0.637 (0.426–0.953) | 0.028 |
| FOXP3 expression level (high vs. low) | 0.967 (0.668–1.398) | 0.857 | ||
CI, confidence interval; HR, hazard ratio; DFS, disease-free survival; OS, overall survival; BC, breast cancer; ILC, invasive lobular carcinoma; IDC, invasive ductal carcinoma; pT stage, pathological stage according to the tumor size; T1, tumor size ≤2 cm; T2, tumor size 2–5 cm; T3, tumor size >5 cm; T4, tumor invades the chest wall or skin; pN stage, pathological stage according to the lymph node metastasis; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor-2; FOXP3, forkhead box P3; CD, cluster of differentiation.
Discussion
Immune cells that infiltrate in BC maintain tissue homeostasis by continuous immunosurveillance and the initiation of inflammatory reactions (10,23). The assessment of TILs and their subsets in BC patients by histological methods (hematoxylin-eosin or immunohistochemistry staining) has been widely used in previous studies (7–9,11,16), and suggestions have been made to improve their evaluation (22). Although flow cytometry was seldom used to determine the distribution of TILs and their subsets, it has the advantage of being a quantitative technique that emphasizes the relative percentages of immune cell subsets. This value may be as important as the absolute number of immune cell subsets given that immune cells interact with each other in the tissue.
A number of studies have focused on the distribution of TILs and demonstrated that they are particularly prevalent in ductal carcinoma and in ER negative, high Ki67 BC patients with a high histological grade (24,25). Similar results were observed in the present study. TIL recruitment is influenced by a number of factors including C-X-C motif chemokine 9 expression (26,27), HLA class I histocompatibility antigen (28), the presence of high endothelial venules in the tissue (29), indoleamine 2,3-dioxygenase levels (30) and epithelial-mesenchymal transition (11).
TIL subsets also have a particular distribution in BC. Seo et al (11) analyzed the correlation between absolute CD4+T cells, CD8+T cells and FOXP3+ TIL numbers, and the clinicopathological characteristics of tumors. In the present study, it was demonstrated that the percentage of tumor-infiltrating T cell subsets was associated with the BC molecular subtype. However, further study is required to determine the specific mechanism influencing the distribution of T cell subsets. CD4, CD8A and FOXP3 mRNA expression was also demonstrated to be increased in BC compared with healthy breast tissue, indicating the important role of tumor-infiltrating T cells in BC.
The prognostic value of tumor-infiltrating T cells, especially Tregs, in tumors is controversial. Tregs are usually considered an unfavorable factor because of their inhibitory function on other effector T cells. However, a high infiltration of Tregs was associated with a pathological complete response in BC (31,32). Seo et al (11) postulated that the suppression of Treg inhibitory function by chemotherapy facilitated the CD8+ cytotoxic T cell attack on the tumor, aiding the achievement of pathologic complete response. In the present study, the prognostic value of FOXP3 mRNA expression was evaluated in 1,029 BC patients and it was demonstrated to be positively correlated with DFS but not OS. Therefore, the prognostic value of Tregs remains to be validated.
There are certain limitations in the present study. This was only a preliminary study and more patients are required to validate the conclusion. In addition, more studies are needed to investigate how the tumor-infiltrating T cells serve a positive role in BC.
In conclusion, the present study elucidated the distribution and interaction of TILs and their subsets in BC patients. The results of the present study also suggest that CD8+T cells and Tregs may be used as reliable predictors of prognosis in BC, although further study is needed to determine the underlying mechanism. The present study identified potential targets for BC treatments that may provide more clinical choice in the future.
Acknowledgements
The authors would like to thank Sarah Williams for language editing this manuscript.
Funding
This study was supported by the Science and Technology Planning Project of Yunnan Province (grant no. 2015FB093).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
KY designed the study. SM and LL performed the experiments, SM, LL, MZ, WJ and HN analyzed the data. SM, LL and KY wrote the manuscript. All authors reviewed the manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of The First People's Hospital of Yunnan Province and written informed consent was provided by each patient. All methods were performed in accordance with relevant guidelines and regulations.
Patient consent for publication
Written informed consent was provided by each patient.
Competing interests
The authors declare they have no competing interests.
References
- 1.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, Verstegen NJM, Ciampricotti M, Hawinkels LJAC, Jonkers J, de Visser KE. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jia Y, Xu L, Lin Q, Zhu M, Ding L, Wu K, Lu Y. Levels of lymphocyte subsets in peripheral blood prior treatment are associated with aggressive breast cancer phenotypes or subtypes. Med Oncol. 2014;31:981. doi: 10.1007/s12032-014-0981-9. [DOI] [PubMed] [Google Scholar]
- 3.Koh CH, Bhoo-Pathy N, Ng KL, Jabir RS, Tan GH, See MH, Jamaris S, Taib NA. Utility of pre-treatment neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as prognostic factors in breast cancer. Br J Cancer. 2015;113:150–158. doi: 10.1038/bjc.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Mayes PA, Eastman S, Shi H, Yadavilli S, Zhang T, Yang J, Seestaller-Wehr L, Zhang SY, Hopson C, et al. The BRAF and MEK inhibitors dabrafenib and trametinib: Effects on immune function and in combination with immunomodulatory antibodies targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res. 2015;21:1639–1651. doi: 10.1158/1078-0432.CCR-14-2339. [DOI] [PubMed] [Google Scholar]
- 5.von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 6.Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, Karin M. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–553. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 8.Denkert C, von Minckwitz G, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 9.Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, Rouas G, Francis P, Crown JP, Hitre E, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 10.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109:2796–2801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY. Tumour-infiltrating CD8+ lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–2713. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 13.Winerdal ME, Marits P, Winerdal M, Hasan M, Rosenblatt R, Tolf A, Selling K, Sherif A, Winqvist O. FOXP3 and survival in urinary bladder cancer. BJU Int. 2011;108:1672–1678. doi: 10.1111/j.1464-410X.2010.10020.x. [DOI] [PubMed] [Google Scholar]
- 14.Jensen HK, Donskov F, Nordsmark M, Marcussen N, von der Maase H. Increased intratumoral FOXP3-positive regulatory immune cells during interleukin-2 treatment in metastatic renal cell carcinoma. Clin Cancer Res. 2009;15:1052–1058. doi: 10.1158/1078-0432.CCR-08-1296. [DOI] [PubMed] [Google Scholar]
- 15.Kang MJ, Kim KM, Bae JS, Park HS, Lee H, Chung MJ, Moon WS, Lee DG, Jang KY. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol. 2013;6:282–289. doi: 10.1593/tlo.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 17.Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, Pudelko M, Szynglarewicz B, Szelachowska J, Zolnierek A, Kornafel J. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–2451. [PubMed] [Google Scholar]
- 18.Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Mènard S, Tagliabue E, Balsari A. FOXP3 expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–1752. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 19.Ladoire S, Arnould L, Mignot G, Coudert B, Rébé C, Chalmin F, Vincent J, Bruchard M, Chauffert B, Martin F, et al. Presence of Foxp3 expression in tumor cells predicts better survival in HER2-overexpressing breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;125:65–72. doi: 10.1007/s10549-010-0831-1. [DOI] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, Panel members Strategies for subtypes-dealing with the diversity of breast cancer: Highlights of the St. Gallen International expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs working group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demaria S, Pikarsky E, Karin M, Coussens LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo E, et al. Cancer and inflammation: promise for biologic therapy. J Immunother. 2010;33:335–351. doi: 10.1097/CJI.0b013e3181d32e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tung NM, Winer EP. Tumor-infiltrating lymphocytes and response to platinum in triple-negative breast cancer. J Clin Oncol. 2015;33:969–971. doi: 10.1200/JCO.2014.59.6031. [DOI] [PubMed] [Google Scholar]
- 25.Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107:864–873. doi: 10.1038/bjc.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walser TC, Ma X, Kundu N, Dorsey R, Goloubeva O, Fulton AM. Immune-mediated modulation of breast cancer growth and metastasis by the chemokine Mig (CXCL9) in a murine model. J Immunother. 2007;30:490–498. doi: 10.1097/CJI.0b013e318031b551. [DOI] [PubMed] [Google Scholar]
- 27.Ohtani H, Jin Z, Takegawa S, Nakayama T, Yoshie O. Abundant expression of CXCL9 (MIG) by stromal cells that include dendritic cells and accumulation of CXCR3+ T cells in lymphocyte-rich gastric carcinoma. J Pathol. 2009;217:21–31. doi: 10.1002/path.2448. [DOI] [PubMed] [Google Scholar]
- 28.Dong DD, Yie SM, Li K, Li F, Xu Y, Xu G, Song L, Yang H. Importance of HLA-G expression and tumor infiltrating lymphocytes in molecular subtypes of breast cancer. Hum Immunol. 2012;73:998–1004. doi: 10.1016/j.humimm.2012.07.321. [DOI] [PubMed] [Google Scholar]
- 29.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 30.Jacquemier J, Bertucci F, Finetti P, Esterni B, Charafe-Jauffret E, Thibult ML, Houvenaeghel G, van den Eynde B, Birnbaum D, Olive D, Xerri L. High expression of indoleamine 2,3-dioxygenase in the tumour is associated with medullary features and favourable outcome in basal-like breast carcinoma. Int J Cancer. 2012;130:96–104. doi: 10.1002/ijc.25979. [DOI] [PubMed] [Google Scholar]
- 31.Oda N, Shimazu K, Naoi Y, Morimoto K, Shimomura A, Shimoda M, Kagara N, Maruyama N, Kim SJ, Noguchi S. Intratumoral regulatory T cells as an independent predictive factor for pathological complete response to neoadjuvant paclitaxel followed by 5-FU/epirubicin/cyclophosphamide in breast cancer patients. Breast Cancer Res Treat. 2012;136:107–116. doi: 10.1007/s10549-012-2245-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16:32–39. doi: 10.4048/jbc.2013.16.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.