Abstract
Astragalus membranaceus (AM) is a traditional Chinese medicinal herb, whose cytoprotective effects remain largely unknown. Here, the bacterial endotoxin lipopolysaccharide (LPS) was applied to a human pulmonary type II-like epithelial lung adenocarcinoma cell line, a human umbilical vein endothelial cell line, and a human bladder carcinoma cell line to construct in vitro models of intracellular oxidative stress. The authors assayed the cellular and mitochondrial cytoprotective effects of varying doses of AM root extract upon these cell lines. The cell lines were cultured as follows: LPS-only group, four LPS+AM groups treated with various AM concentrations plus LPS, and an untreated control group. Flow cytometry was used to assess apoptosis and cell cycle progression. A 2′,7′-dichlorofluorescein-diacetate assay was used to quantitate reactive oxygen species (ROS) generation. Mitochondrial membrane potential (Δψmit) was analyzed by Rhodamine 123 assay. Western blotting was performed to detect cleaved caspase-3, p53, and B cell lymphoma (Bcl)-2 levels. Across all cell lines, LPS significantly elevated apoptosis rates, shifted cells to S/G2 phase, increased ROS production, reduced Δψmit, upregulated cleaved caspase-3, upregulated p53, and downregulated Bcl-2 relative to controls (all P<0.05). As a general trend, increasing AM concentrations produced progressively greater reductions in the apoptosis rate, greater reductions in S/G2 phase %, greater reductions in ROS production, greater increases in Δψmit, greater reductions in cleaved caspase-3 and p53 expression, and greater increases in Bcl-2 expression. AM treatment protects human pulmonary and bladder epithelial cells, in addition to human endothelial cells, from LPS-induced apoptosis, in a dose-dependent manner.
Keywords: Astragalus membranaceus, Astragali Radix, Huangqi, traditional Chinese medicine, herb, herbal, lipopolysaccharide
Introduction
The dried root of Astragalus membranaceus (AM), alternatively termed Astragali Radix or Huangqi, is a globally popular medicinal herb primarily grown in Northern China, Mongolia and Korea (1). AM has been used in traditional Chinese medicine (TCM) for over two millennia, where it is traditionally believed to ease urination, reduce purulent discharge, and promote soft tissue repair (1,2). To this day, the water-soluble extract of AM is still applied for the alternative treatment of various conditions, including fatigue, anorexia, anemia, fever, allergies, gastric ulcers and diarrhea (1). There are >100 biologically-active compounds that have been identified in AM, including flavonoids, saponins, polysaccharides, amino acids and trace elements (1,3). It is hypothesized that this combination of bioactive compounds are responsible for AM's observed immunomodulatory, anti-hyperglycemic, anti-inflammatory, antioxidant and antiviral properties (1).
In particular, AM has been indicated to demonstrate antioxidant effects in diabetic nephropathy, heart disease and liver disease (1). Several previous studies have reported that AM root extract demonstrates antioxidant activity in vitro (4–6). Although these previous findings reveal AM's beneficial antioxidant properties, its cytoprotective effects at the cellular and mitochondrial levels remain largely unknown.
To address this question, in vitro administration of the bacterial endotoxin lipopolysaccharide (LPS) is a well-established experimental model for generating intracellular oxidative stress (7,8). In the present study, the authors applied LPS to a human pulmonary type II-like epithelial lung adenocarcinoma cell line, a human umbilical vein endothelial cell line and a human bladder carcinoma cell line in order to construct in vitro models of intracellular oxidative stress. They then assayed the cellular and mitochondrial cytoprotective effects of varying doses of AM root extract upon these three LPS-treated cell lines. These findings should better the understanding of AM's cytoprotective effects upon human cells.
Materials and methods
Cell culture and experimental group construction
The root extract of Astragalus membranaceus (AM) and lipopolysaccharide (LPS; 1 µg/ml) were purchased from Sigma-Aldrich (Merck KGaA; Darmstadt, Germany). The human pulmonary type II-like epithelial lung adenocarcinoma cell line A549, the human umbilical vein endothelial cell (HUVEC) line CRL-1730, and the ECV304 cell line were purchased from American Type Culture Collection (Manassas, VA, USA). Notably, the ECV304 cell line was originally thought to be derived from HUVECs from a healthy donor, however is now known to be cross-contaminated with the T24 bladder carcinoma cell line (9). Trypsin, propidium iodide (PI), and RNase A were purchased from Sigma-Aldrich. Fetal calf serum (FCS) was purchased from Hyclone; GE Healthcare Life Sciences (Logan, UT, USA), and Dulbecco's modified Eagles medium (DMEM) was purchased from Gibco; Thermo Fisher Scientific, (Waltham, MA, USA).
The three logarithmic-phase cell lines were seeded onto six-well plates at a density of 1×106 cells/ml and cultured in DMEM supplemented with 5% FCS at 37°C to the point of adherence. Then, adherent cells were divided into six experimental groups: LPS group treated with LPS (1 µg/ml) alone, four LPS+AM groups treated with various concentrations of AM (25, 50, 100, and 200 µg/ml) in addition to LPS (1 µg/ml), and a control group receiving neither LPS nor AM. The three cell lines were cultured under these conditions at 37°C for 24 h prior to performance of the following assays.
Cell apoptosis assay
As previously described with minor modifications (10), flow cytometric analysis was used to differentiate early and late apoptotic cells using an Annexin V-FITC/PI apoptosis detection kit (Nanjing KGI Biological Technology Development Co., Ltd., Nanjing, China) according to the manufacturer's instructions. Briefly, cells (1×106 cells/ml) were trypsinized, and the cell suspension was transferred into a centrifuge tube for centrifugation (200 × g, 30 min, 4°C). The supernatant was aspirated out, and the cells were washed three times with phosphate-buffered saline (PBS). A total of 100,000 cells were resuspended in 100 µl binding buffer containing Annexin V-FITC and PI. Samples were incubated for 5 min at room temperature in the dark. Quantification of Annexin V-FITC and PI binding was performed using a BD-FACS Canto™ II flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). BD CellQuest™ Pro version 5.2.1 (BD Biosciences) was then used to perform the quadrant analysis. Experiments were repeated three times.
Cell cycle progression assay
Cell cycle progression was determined by flow cytometry following PI staining, as previously described with minor modifications (11). Cells were trypsinized, and the cell suspension was transferred into a centrifuge tube for centrifugation (1,000 × g, 5 min, 37°C). The supernatant was aspirated out, the cells were washed three times with PBS. Cells were fixed in 70% ethanol at 4°C for 24 h. The cells were then stained with a PI/RNase staining buffer for 1 h at 37°C. Stained cells were analyzed on a BD-FACS Canto™ II flow cytometer (BD Biosciences) to calculate the proportion of cells in the various phases of the cell cycle using Mod Fit LT version 3.0 (Verity Software House Inc., Topsham, ME, USA). Experiments were repeated three times.
Reactive oxygen species (ROS) detection assay
The 2′,7′-dichlorofluorescein-diacetate (DCFH-DA) kit was used to quantitate ROS generation as an index for cytotoxicity, and was purchased from Cell Biolabs, Inc. (San Diego, CA, USA). ROS generation was analyzed with a DCFH-DA assay as previously described, with minor modifications (12). The culture medium was aspirated out, cells were washed three times in PBS, and then incubated in ROS staining solution (DCFH-DA) at 37°C for 20 min. Following washing three times with PBS, DCFH fluorescence intensity was detected with a BD-FACS Canto™ II flow cytometer (BD Biosciences) at a 488 nm excitation wavelength and a 525 nm emission wavelength (channel FL1). Results were calculated relative to the mean fluorescence intensity of channel FL1 in the untreated control group. Experiments were repeated three times.
Mitochondrial membrane potential (Δψmit) assay
Rhodamine 123 is a mitochondrial-specific fluorescent probe that stains in a membrane potential-dependent fashion. This was used in the present study and was. The mitochondrial membrane potential (Δψmit) was analyzed with a rhodamine 123 assay purchased from Thermo Fisher Scientific, Inc, as previously described with minor modifications (13). Cells were trypsinized, and the cell suspension was transferred into a centrifuge tube for centrifugation (400 × g, 15 min, 37°C). The supernatant was aspirated, washed three times with PBS, and incubated with 5 µM rhodamine 123 at 37°C for 30 min in the dark. Rhodamine 123 fluorescence intensity was then detected with a BD-FACS Canto™ II flow cytometer (Becton-Dickinson) and CellQuest™ Pro version 5.2.1 (BD Biosciences). Experiments were repeated three times.
Western blotting
Western blotting was performed as previously described, with minor modifications (14). Cells were lysed for 30 min in MBST/OG buffer (25 mM MES; 150 mM NaCl; 60 mM octylglucopyranoside; 1% Triton X-100; pH 6.4) on ice. The lysate was boiled for 10 min to denature the proteins and then cooled. The protein concentration was determined using the Bradford assay, and equal amounts of lysate protein (50 µg/lane) were then resolved by 10% SDS-PAGE. Following electrophoresis, the proteins were transferred to a polyvinylidene difluoride membrane, which was blocked for 1 h in 5% skim milk solution in Tris-buffered saline containing 0.1% Tween-20 at room temperature. The membrane was incubated overnight at 4°C with primary antibodies (Abcam, Cambridge, UK) against human cleaved caspase-3 (1:100; cat. no. ab2302), human p53 (1:500; cat. no. ab26), human B cell lymphoma (Bcl)-2 (1:1,000; cat. no. ab32124), and human β-actin (1:500; cat. no. ab8226). The membrane was subsequently incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibodies (1:5,000; anti-rabbit, cat. no. ab7090; anti-mouse, cat. no. ab97040; Abcam). The immunoreactive bands were visualized using an enhanced chemiluminescence detection kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Band intensity was quantified using ChemiDoc XRS (Bio-Rad, Laboratories, Inc.) and normalized to β-actin. Experiments were repeated three times.
Statistical analysis
Statistical analysis was performed using SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation from at least three independent experiments. Statistically significant differences between experimental groups were assessed by one-way analysis of variance followed by Bonferroni's multiple comparison test. P<0.05 was considered to indicate a statistically significant difference.
Results
AM therapy reduces apoptosis rate
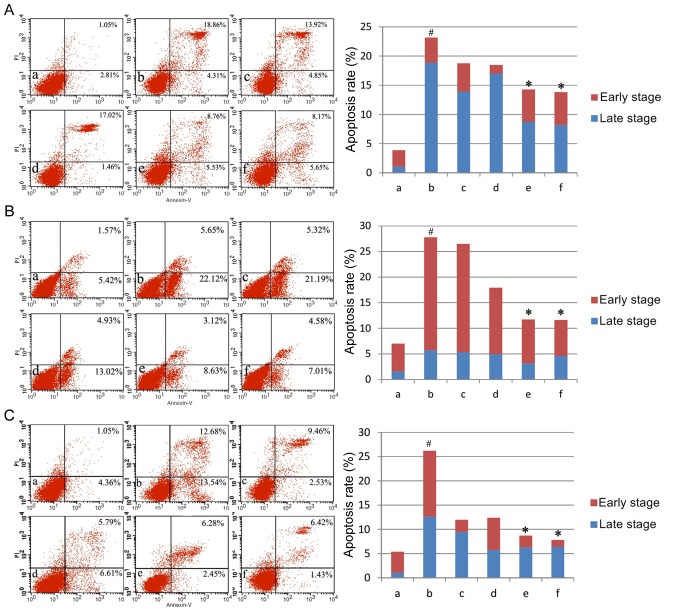
The present study first assayed apoptosis rates under varying doses of AM root extract in the three LPS-treated human cell lines. Across all three cell lines, LPS administration significantly increased apoptosis rates compared with controls (P<0.05; Fig. 1). The addition of either 100 µg/ml or 200 µg/ml AM therapy significantly reduced apoptosis rates in LPS-treated cells (P<0.05; Fig. 1). As a general trend, increasing concentrations of AM therapy produced progressively greater reductions in the apoptosis rate.
Figure 1.
Apoptosis rates in the three cell lines. Apoptosis rates of (A) the human pulmonary type II-like epithelial lung adenocarcinoma cell line A549, (B) the human umbilical vein endothelial cell line CRL-1730, and (C) the human bladder carcinoma cell line ECV304. A, Untreated control group (no LPS, no AM); b, LPS group (1 µg/ml), and the four LPS+AM groups treated with LPS (1 µg/ml); plus c, 25; d, 50; e, 100; and f, 200 µg/ml of AM. Data are presented as the mean ± standard deviations from three independent experiments. #P<0.05 vs. control group a, and *P<0.05 vs. LPS group b, with one-way analysis of variance followed by Bonferroni post-hoc test. AM, Astragalus membranaceus; LPS, lipopolysaccharide; PI, propidium iodide.
AM therapy affects cell cycle progression
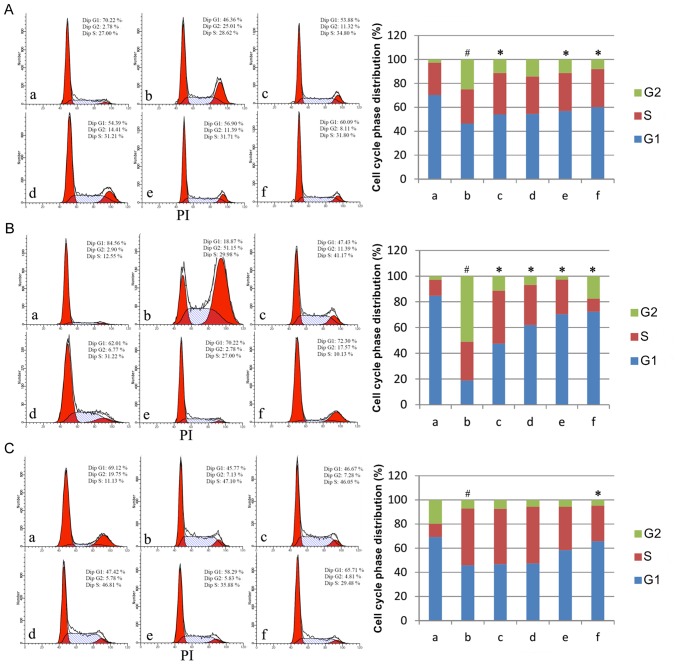
Having established that AM therapy significantly reduces apoptosis rate in LPS-treated cells, the present study assayed cell cycle phase distributions under varying doses of AM root extract in the three LPS-treated human cell lines. Across all three cell lines, LPS administration produced a significant shift to S/G2 phase relative to controls (P<0.05; Fig. 2). In A549 cells, the addition of either 25, 100 or 200 µg/ml AM therapy significantly reduced the S/G2 phase % in LPS-treated cells (P<0.05; Fig. 2A). In CRL-1730 cells, the addition of all AM therapy doses significantly reduced the S/G2 phase % in LPS-treated cells (P<0.05; Fig. 2B). In ECV304 cells, the addition of 200 µg/ml AM therapy significantly reduced the S/G2 phase % in LPS-treated cells (P<0.05; Fig. 2C). As a general trend, increasing concentrations of AM therapy produced progressively greater reductions in S/G2 phase %.
Figure 2.
Cell cycle phase distributions in the three cell lines. Cell cycle phase distributions of (A) the human pulmonary type II-like epithelial lung adenocarcinoma cell line A549, (B) the human umbilical vein endothelial cell line CRL-1730, and (C) the human bladder carcinoma cell line ECV304. A, Untreated control group (no LPS, no AM); b, LPS group (1 µg/ml), and the four LPS+AM groups treated with LPS (1 µg/ml); plus c, 25; d, 50; e, 100; and f, 200 µg/ml of AM. Data are presented as the mean ± standard deviations from three independent experiments. #P<0.05 vs. control group a, and *P<0.05 vs. LPS group b, with one-way analysis of variance followed by Bonferroni post-hoc test. AM, Astragalus membranaceus; LPS, lipopolysaccharide.
AM therapy reduces ROS production
The present study next assayed ROS production under varying doses of AM root extract in the three LPS-treated human cell lines. Across all three cell lines, LPS administration significantly increased ROS production relative to controls (P<0.05; Fig. 3A). Across all three cell lines, the addition of either 100 µg/ml or 200 µg/ml AM therapy significantly reduced ROS production in LPS-treated cells (P<0.05; Fig. 3A). As a general trend, increasing concentrations of AM therapy produced progressively greater reductions in ROS production.
Figure 3.
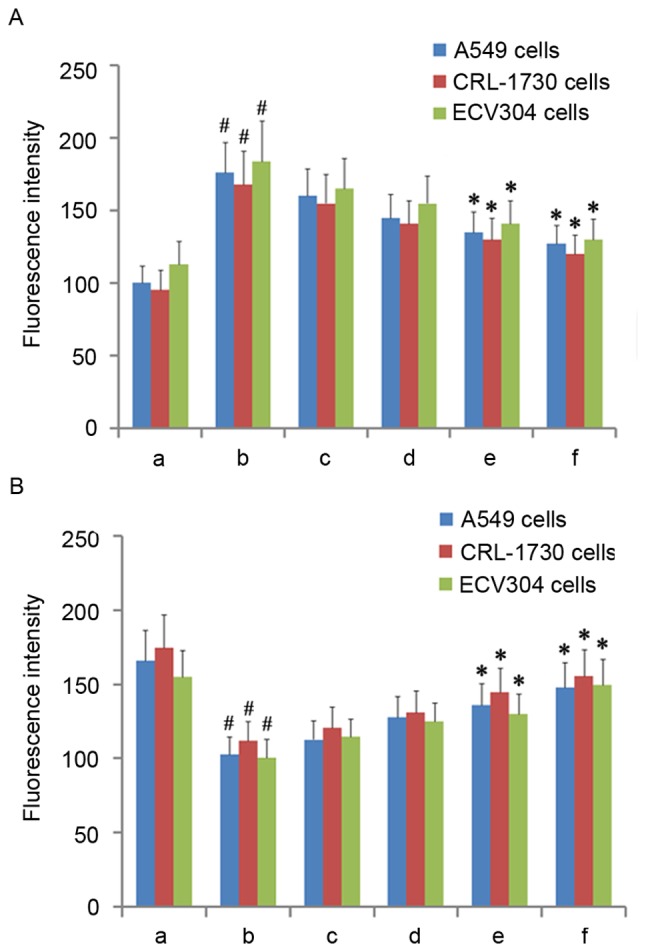
ROS and mitochondrial membrane potential in the three cell lines. (A) Fluorescence intensity of ROS in the three cell lines. (B) Fluorescence intensity of rhodamine 123 in the three cell lines. A, Untreated control group (no LPS, no AM); b, LPS group (1 µg/ml), and the four LPS+AM groups treated with LPS (1 µg/ml); plus c, 25; d, 50; e, 100; and f, 200 µg/ml of AM. Data are presented as the mean ± standard deviations from three independent experiments. #P<0.05 vs. control group a, and *P<0.05 vs. LPS group b, with one-way analysis of variance followed by Bonferroni post-hoc test. AM, Astragalus membranaceus; LPS, lipopolysaccharide; ROS, reactive oxygen species.
AM therapy increases Δψmit levels
Having established that AM therapy significantly reduces ROS production in LPS-treated cells, Δψmit levels were then assayed under varying doses of AM root extract in the three LPS-treated human cell lines. As diffusion of the cationic dye rhodamine 123 is directly proportional to the degree of membrane polarization, Δψmit levels were examined by measuring the relative differences in rhodamine 123 fluorescence intensity between the experimental groups (15). Across all three cell lines, LPS administration significantly reduced Δψmit relative to controls (P<0.05; Fig. 3B). Across all three cells lines, the addition of either 100 µg/ml or 200 µg/ml AM therapy significantly increased Δψmit in LPS-treated cells (P<0.05; Fig. 3B). As a general trend, increasing concentrations of AM therapy produced progressively greater increases in Δψmit.
AM therapy affects expression of apoptosis-associated proteins
Having established that AM therapy significantly increases Δψmit levels in LPS-treated cells, the expression of several apoptosis-associated proteins (cleaved caspase-3, p53, and Bcl-2) were assayed under varying doses of AM root extract in the three LPS-treated human cell lines. In all three cell lines, LPS administration significantly increased cleaved caspase-3 and p53 expression and significantly decreased Bcl-2 expression, relative to controls (P<0.05; Fig. 4). In A549 cells, the addition of all AM therapy doses significantly reduced cleaved caspase-3 and p53 expression (P<0.05; Fig. 4A); however, only 200 µg/ml AM therapy significantly increased Bcl-2 expression in LPS-treated A549 cells (P<0.05; Fig. 4A). In CRL-1730 cells, the addition of all AM therapy doses significantly reduced cleaved caspase-3 and p53 expression (P<0.05; Fig. 4B); however, only the addition of either 100 µg/ml or 200 µg/ml AM therapy significantly increased Bcl-2 expression in LPS-treated CRL-1730 cells (P<0.05; Fig. 4B). In ECV304 cells, the addition of either 100 µg/ml or 200 µg/ml AM therapy significantly reduced cleaved caspase-3 and p53 expression (P<0.05; Fig. 4C); however, only the addition of 200 µg/ml AM therapy significantly increased Bcl-2 expression in LPS-treated ECV304 cells (P<0.05; Fig. 4C). Interestingly, the addition of 25 µg/ml AM therapy increased p53 expression, and 50 µg/ml AM therapy increased cleaved caspase-3 and p53 expression; however, these alterations were not significant (P>0.05; Fig. 4C). As a general trend, increasing concentrations of AM therapy produced progressively reduced cleaved caspase-3 and p53 expression, and increased Bcl-2 expression levels.
Figure 4.
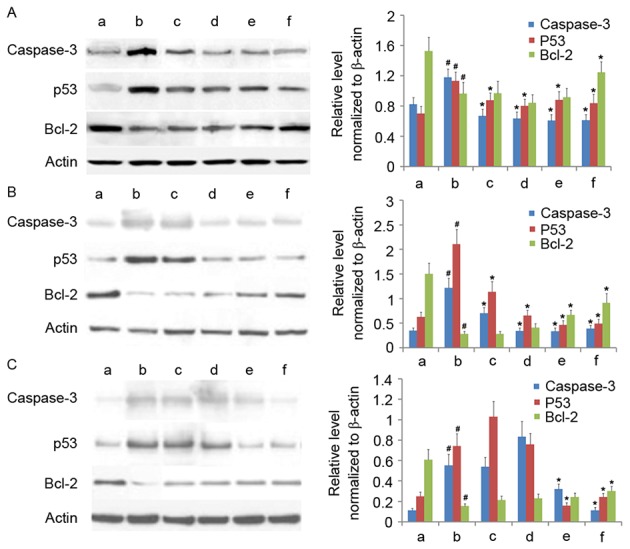
Western blotting of cleaved caspase-3, p53 and Bcl-2 in the three cell lines. Western blotting of (A) the human pulmonary type II-like epithelial lung adenocarcinoma cell line A549, (B) the human umbilical vein endothelial cell line CRL-1730, and (C) the human bladder carcinoma cell line ECV304. A, Untreated control group (no LPS, no AM); b, LPS group (1 µg/ml), and the four LPS+AM groups treated with LPS (1 µg/ml); plus c, 25; d, 50; e, 100 and f, 200 µg/ml of AM. β-actin served as a loading control. Data are presented as the mean ± standard deviations from three independent experiments. #P<0.05 vs. control group a and *P<0.05 vs. LPS group b with one-way analysis of variance followed by Bonferroni post-hoc test. AM, Astragalus membranaceus; LPS, lipopolysaccharide; Bcl-2, B cell lymphoma-2.
Discussion
Oxidative stress is defined as the excess production of ROS (and/or other oxidant species) that overwhelms the cell's antioxidant capacity, with mitochondrial electron transport recognized as an important source of ROS in the majority of eukaryotic cell types (16,17). Oxidative stress induces macromolecular damage (oxidative damage of DNA bases, polyunsaturated fatty acids, and amino acid residues) and is involved in the pathogenesis of several disorders, including inflammatory diseases, atherosclerosis, diabetes, and cancer (16–18). Although several synthetic compounds have been developed and marketed to address oxidative stress (butylated hydroxytoluene, butylated hydroxyanisole, and nordihydroguaiaretic acid), these synthetic compounds may produce adverse side effects (18,19). As a result, there is increasing interest in the discovery and investigation of non-synthetic, naturally-occurring therapies to address oxidative stress.
The TCM herb Astragalus membranaceus (AM, Astragali Radix, or Huangqi) has been demonstrated to exhibit antioxidant effects in vitro (4–6). Previous research on eight TCM herb pairs demonstrated that antioxidant activity is largely driven by alterations in total flavonoid content (20). In accordance with this, antioxidant assays on five AM extracts (ABTS, DPPH, reducing power, and •OH assays) demonstrated that AM's phenolic compounds (including flavonoids) are primarily responsible for its antioxidant effects (4). However, there is little available information on the cellular and mitochondrial cytoprotective effects of AM root extract in human cells.
Therefore, the present study examined the cellular and mitochondrial cytoprotective effects of varying doses of AM root extract applied to three LPS-treated human cell lines. Across all three cell lines, it was demonstrated that AM treatment reduced the negative cellular and mitochondrial effects induced by LPS in a dose-dependent manner, through reduced apoptosis rates, reduced S/G2 phase arrest, reduced ROS production, increased Δψmit, reduced cleaved caspase-3, reduced p53 expression and increased Bcl-2 expression. As increased ROS production and loss of Δψmit have been associated with the activation of caspase-induced apoptotic cascades (15,21), these results suggested that AM therapy reduced apoptosis through dampening ROS production and dampening Δψmit loss, which downregulated the p53-induced caspase-mediated pro-apoptotic pathway and upregulated the nuclear factor-κB-induced Bcl-2-mediated anti-apoptotic pathway. Accordingly, this precise pattern of anti-apoptotic findings has been previously observed with other naturally-occurring therapies in vitro, including isofraxidin, quercetin, cyanidin, arjunolic acid, and taurine (21–25).
There are several limitations to the present study. Firstly, the present study only analyzed three human cell lines, therefore results are restricted to the three cell lines and AM therapy may have different effects in other cell types. Secondly, the in vitro LPS model used here may not precisely match the oxidative stress conditions for the three cell types in vivo. Finally, an AM root extract solution that contains numerous bioactive compounds was applied; therefore, the present study did not establish which compound(s) in the AM root extract were responsible for the observed effects. Future studies on AM root extract should apply high performance liquid chromatography and mass spectrometry analytical techniques in order to identify the constituent bioactive compounds responsible for AM's beneficial effects (26). In addition, although the results suggest it, the present study did not conclusively establish that AM therapy reduces apoptosis through downregulating the p53-induced caspase-mediated pro-apoptotic pathway and/or upregulating the NF-κB-induced Bcl-2-mediated anti-apoptotic pathway. Therefore, further experiments involving gene silencing and overexpression are required to establish the precise molecular mechanism(s) underlying AM's beneficial effects.
In conclusion, AM treatment protects human pulmonary and bladder epithelial cells as well as human endothelial cells from LPS-induced apoptosis in a dose-dependent manner. The present evidence suggests that AM therapy reduces ROS production and mitochondrial membrane depolarization, thereby preventing the activation of caspase-induced apoptotic cascades. Further research on the molecular effects of AM's constituent compounds are needed to better understand the molecular mechanism(s) underlying AM's beneficial effects.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Heilongjiang Province Research Program (grant no. D201179). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during the present study are included in this published article.
Author's contributions
XW and YL conceived of and designed the study. XW, WZ, QW and PC performed the experiments. XW, WZ, QW and PC analyzed and interpreted the data. XW wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have competing interests.
References
- 1.Fu J, Wang Z, Huang L, Zheng S, Wang D, Chen S, Zhang H, Yang S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi) Phytother Res. 2014;28:1275–1283. doi: 10.1002/ptr.5188. [DOI] [PubMed] [Google Scholar]
- 2.Zhang HW, Lin ZX, Xu C, Leung C, Chan LS. Astragalus (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst Rev. 2014;22:CD008369. doi: 10.1002/14651858.CD008369.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma XQ, Shi Q, Duan JA, Dong TT, Tsim KW. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J Agric Food Chem. 2002;50:4861–4866. doi: 10.1021/jf0202279. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Chen D, Mai Y, Wen B, Wang X. Concordance between antioxidant activities in vitro and chemical components of Radix Astragali (Huangqi) Nat Prod Res. 2012;26:1050–1053. doi: 10.1080/14786419.2010.551771. [DOI] [PubMed] [Google Scholar]
- 5.Wu HW, Fang J, Tang LY, Lu P, Xu HY, Zhao Y, Li DF, Fu MH, Yang HJ. Quality evaluation of astragali radix based on DPPH radical scavenging activity and chemical analysis. Chin Herbal Med. 2014;6:282–289. doi: 10.1016/S1674-6384(14)60043-5. [DOI] [Google Scholar]
- 6.Jiao J, Gai QY, Fu YJ, Ma W, Peng X, Tan SN, Efferth T. Efficient production of isoflavonoids by Astragalus membranaceus hairy root cultures and evaluation of antioxidant activities of extracts. J Agric Food Chem. 2014;62:12649–12658. doi: 10.1021/jf503839m. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Wang GZ, Rabinovitch PS, Tabas I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-κB-mediated inflammation in macrophages. Circ Res. 2014;114:421–433. doi: 10.1161/CIRCRESAHA.114.302153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller JM, Ziegler-Heitbrock HL, Baeuerle PA. Nuclear factor kappa B, a mediator of lipopolysaccharide effects. Immunobiology. 1993;187:233–256. doi: 10.1016/S0171-2985(11)80342-6. [DOI] [PubMed] [Google Scholar]
- 9.MacLeod RA, Dirks WG, Matsuo Y, Kaufmann M, Milch H, Drexler HG. Widespread intraspecies cross-contamination of human tumor cell lines arising at source. Int J Cancer. 1999;83:555–563. doi: 10.1002/(SICI)1097-0215(19991112)83:4<555::AID-IJC19>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Scharstuhl A, Mutsaers HA, Pennings SW, Russel FG, Wagener FA. Involvement of VDAC, Bax and ceramides in the efflux of AIF from mitochondria during curcumin-induced apoptosis. PLoS One. 2009;4:e6688. doi: 10.1371/journal.pone.0006688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arora S, Bhardwaj A, Srivastava SK, Singh S, McClellan S, Wang B, Singh AP. Honokiol arrests cell cycle, induces apoptosis, and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS One. 2011;6:e21573. doi: 10.1371/journal.pone.0021573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang DY, Wang HJ, Tan YZ. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One. 2011;6:e21397. doi: 10.1371/journal.pone.0021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Zhang Y, Zhuang Y, Wang J, Ye J, Zhang S, Wu J, Yu K, Han Y. Matrine induces apoptosis in human acute myeloid leukemia cells via the mitochondrial pathway and Akt inactivation. PLoS One. 2012;7:e46853. doi: 10.1371/journal.pone.0046853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scotland RL, Allen L, Hennings LJ, Post GR, Post SR. The ral exchange factor rgl2 promotes cardiomyocyte survival and inhibits cardiac fibrosis. PLoS One. 2013;8:e73599. doi: 10.1371/journal.pone.0073599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao J, Liu Y, Jia L, Zhou HM, Kong Y, Yang G, Jiang LP, Li QJ, Zhong LF. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic Biol Med. 2007;43:968–975. doi: 10.1016/j.freeradbiomed.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hekimi S, Lapointe J, Wen Y. Taking a ‘good’ look at free radicals in the aging process. Trends Cell Biol. 2011;21:569–576. doi: 10.1016/j.tcb.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin M, Zhao K, Huang Q, Shang P. Structural features and biological activities of the polysaccharides from Astragalus membranaceus. Int J Biol Macromol. 2014;64:257–266. doi: 10.1016/j.ijbiomac.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Yang WJ, Li DP, Li JK, Li MH, Chen YL, Zhang PZ. Synergistic antioxidant activities of eight traditional Chinese herb pairs. Biol Pharm Bull. 2009;32:1021–1026. doi: 10.1248/bpb.32.1021. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Zhao QL, Wu LH, Jawaid P, Jiao YF, Kadowaki M, Kondo T. Isofraxidin, a potent reactive oxygen species (ROS) scavenger, protects human leukemia cells from radiation-induced apoptosis via ROS/mitochondria pathway in p53-independent manner. Apoptosis. 2014;19:1043–1053. doi: 10.1007/s10495-014-0984-1. [DOI] [PubMed] [Google Scholar]
- 22.Sharma DR, Sunkaria A, Verma D, Gill KD. Quercetin attenuates aluminum-induced apoptosis in rat hippocampus, by preventing cytochrome c translocation, Bcl-2 decrease, bax elevation, caspase-3 and p53 activation. Free Radical Biol Med. 2012;53:S44–S45. doi: 10.1016/j.freeradbiomed.2012.08.514. [DOI] [Google Scholar]
- 23.Gao S, Chen T, Choi MY, Liang Y, Xue J, Wong YS. Cyanidin reverses cisplatin-induced apoptosis in HK-2 proximal tubular cells through inhibition of ROS-mediated DNA damage and modulation of the ERK and AKT pathways. Cancer Lett. 2013;333:36–46. doi: 10.1016/j.canlet.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh J, Das J, Manna P, Sil PC. The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis. Biomaterials. 2011;32:4857–4866. doi: 10.1016/j.biomaterials.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 25.Chang CY, Shen CY, Kang CK, Sher YP, Sheu WH, Chang CC, Lee TH. Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol Appl Pharmacol. 2014;279:351–363. doi: 10.1016/j.taap.2014.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Xu X, Li F, Zhang X, Li P, Zhang X, Wu Z, Li D. In vitro synergistic antioxidant activity and identification of antioxidant components from Astragalus membranaceus and Paeonia lactiflora. PLoS One. 2014;9:e96780. doi: 10.1371/journal.pone.0096780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article.