Abstract
Pristimerin, a quinonemethide triterpenoid, has demonstrated anticancer activity against a number of types of cancer, including breast cancer. However, its mechanism of action remains unclear. The present study investigated the autophagy-induced anticancer efficacy of pristimerin on MDA-MB-231 human breast cancer cells. Pristimerin inhibited the growth of these cells in a concentration-dependent manner. Treatment with pristimerin dose-dependently induced an increase of light chain 3B (LC3-II), whereas autophagy inhibitor 3-methyladenine (3-MA) inhibited pristimerin-induced LC3-II accumulation and cytotoxic effects. Autophagy was also activated by paclitaxel as observed by an elevated LC3-II level. Although 24 µM paclitaxel induced autophagy without cytotoxicity, combined with pristimerin it additively induced cell growth inhibition and autophagy induction. Autophagy induction was measured with an autophagy detection kit and LC3-II levels were monitored by western blot analysis. Treatment with 3-MA inhibited LC3-II accumulation and cell death induced by a combination of paclitaxel and pristimerin. Pristimerin and paclitaxel inhibited extracellular signal-regulated kinase (ERK)1/2/p90RSK signaling, consistent with autophagy indicators, namely p62 degradation and beclin 1 expression. In addition, ERK activator ceramide C6 treatment suppressed the LC3-II levels induced by a combination of paclitaxel and pristimerin. These results suggested that exposure to pristimerin induced autophagic cell death, whereas a combination treatment of pristimerin and paclitaxel resulted in an additive effect on ERK-dependent autophagic cell death.
Keywords: breast cancer, pristimerin, paclitaxel, autophagy, extracellular signal-regulated kinase
Introduction
Breast cancer, one of the most common types of cancer in women, is associated with high mortality (1). Treatment for breast cancer includes hormonal therapy, chemotherapy, radiotherapy, targeted therapy, surgery and various combinations of these strategies. However, the prognosis of certain subtypes remains poor (2).
Autophagy is a homeostatic cellular self-digestive process responsible for degrading unnecessary or dysfunctional cellular organelles and proteins in all living cells (3). Although autophagy promotes a cell survival response, it also serves a role in cell death (4). Previous studies have demonstrated that autophagic cell death is triggered by numerous signaling pathways including adenosine monophosphate-activated protein kinase pathway (5), mammalian target of rapamycin (mTOR) pathway (6), and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK)1/2 pathway (7).
Medicinal plants and their extracts are commonly used to prevent and treat numerous diseases, including cancer. The World Health Organization has reported that ~4 million people (80% of the population in developing countries), depend on medicinal plants for primary healthcare (8). Pristimerin, a quinonemethide triterpenoid compound, has long been used as an anti-inflammatory, antioxidant, antimalarial and insecticidal agent (9). Pristimerin also possesses promising clinical potential as a therapeutic and chemopreventive agent for numerous types of cancer, including colon cancer (10), prostate cancer (11), ovarian cancer (12) and breast cancer (13). Pristimerin induces cell death via several mechanisms, including proteasome inhibition (14), caspase activation (15), inhibition of the human epidermal growth factor receptor 2 (HER2) (13), inhibition of protein kinase B/nuclear factor-κB/mTOR signaling (12), cell cycle arrest (12) and inhibition of migration and invasion (10). However, the effect of pristimerin on autophagy in human breast cancer has not been reported yet to the best of our knowledge.
The present study aimed to evaluate whether pristimerin induces autophagy in human breast cancer cells. The results of the present study demonstrated that pristimerin inhibited cell proliferation by autophagy induction. The effects of pristimerin were enhanced when combined with paclitaxel treatment through suppression of ERK1/2/p90 ribosomal S6 kinase (p90RSK) signaling, which in turn activated autophagy. These results indicated that pristimerin has potential to treat breast cancer through autophagy and combination therapy can enhance paclitaxel-induced anticancer activities.
Materials and methods
Chemicals
Pristimerin was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) and dissolved in dimethyl sulfoxide (DMSO) to give a stock solution of 100 mM and stored at −20°C in aliquots. Paclitaxel was gifted by Boryung Co., Ltd. (Seoul, Korea). Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS) and penicillin/streptomycin were obtained from GE Healthcare Life Sciences HyClone (Logan, UT, USA). Trypsin/EDTA was purchased from Gibco™ (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The following primary antibodies were used: Rabbit polyclonal anti-human light chain (LC) 3-I/II (1:1,000; cat. no. 4108), rabbit polyclonal anti-human phosphorylated (phospho)-p44/p42 MAPK (ERK1/2; Thr202/Tyr204; 1:1,000; cat. no. 9101), rabbit monoclonal anti-human P-90RSK (1:1,000; cat. no. 9355), rabbit polyclonal anti-human phospho-p90RSK (Ser380; 1:1000; cat. no. 9314) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA) and rabbit polyclonal anti-human ERK (1:1,000; cat. no. sc-94), mouse monoclonal anti-human p62 (1:1,000; sc-48389), rabbit polyclonal anti-human beclin1 (1:1,000; cat. no. sc-11427) and rabbit polyclonal anti-human GAPDH (1:1,000; cat. no. sc-25778) were obtained from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). Horseradish peroxidase-conjugated anti-mouse and anti-rabbit antibodies were purchased from BD Biosciences, Pharmingen (San Diego, CA, USA). SuperSignal® West Pico Chemiluminescent Substrate was purchased from Pierce (Thermo Fisher Scientific, Inc.). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc., (Kumamoto, Japan) and the Autophagy Detection kit (cat. no. ab139484) was purchased from Abcam® (Cambridge, MA, USA). 3-MA, ceramide C6 and all other reagents were obtained from Sigma-Aldrich (Merck KGaA).
Cell line and cell culture
The MDA-MB-231 human breast cancer cell line was purchased from American Type Culture Collection (Manassas, MD, USA). Cells were grown in DMEM supplemented with 10% (v/v) FBS, penicillin (100 U/ml)/streptomycin (100 µg/ml) at 37°C in a humidified CO2 (5%)-controlled incubator.
Cell viability assay
Cells were seeded into 96-well microplates at a density of 5×103 cells/ml and allowed to attach for 24 h. Pristimerin (1, 2.5, 5 and 10 µM) and paclitaxel (6, 12, 24 and 30 µM) were added to the medium at various concentrations. Following treatment, the cell cytotoxicity and/or proliferation was assessed using the CCK-8 assay. CCK-8 (10 µl) was added to each well and incubated for 3 h at 37°C; cell proliferation and cytotoxicity were assessed by measuring the absorbance at a wavelength of 450 nm using a microplate reader (Corning, Inc., Corning, NY, USA). A total of three replicated wells were used per experimental condition.
Western blotting analysis
Cells were harvested using Trypsin-EDTA, washed twice with cold phosphate buffered saline (PBS), lysed with lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% TritonX-100, 0.5% NP-40, 1 mM PI, 1 mM DTT and 1 mM PMSF), placed on ice for 1 h with occasional vortexing and centrifuged at 13,000 × g for 10 min at 4°C to collect the supernatant. A Pierce BCA Protein Assay kit (Pierce; Thermo Fisher Scientific Inc.) was used to determine protein concentration. Cell lysates (50 µg) were subjected to 8, 10, and 15% SDS-PAGE and transferred to a polyvinylidene difluoride membrane. Blots were blocked with 5% skim milk in PBS containing 0.05% Tween-20 (PBST) for 1 h at 25°C and then incubated with primary antibodies (1:1,000) overnight at 4°C. After washing with PBST, membranes were incubated with anti-rabbit horseradish peroxidase-conjugated IgG (1:3,000) at room temperature for 2 h and visualized with enhanced chemiluminescence. Band intensity was quantified by densitometry using ImageJ (version 1.52) software (National Institutes of Health, Bethesda, MD, USA) and was normalized to loading controls. Quantification value was expressed as the fold change vs. band numbered 1.0.
Autophagy detection assay
Autophagy determination was performed using an Autophagy Detection kit according to the manufacturer's protocol. According to product overview by the company, the Autophagy Detection kit can measure autophagic vacuoles and monitor autophagic flux in live cells using a novel dye that selectively labels autophagic vacuoles. The dye has been optimized through identification of titratable functional moieties that allow for minimal staining of lysosomes while exhibiting bright fluorescence upon incorporation into pre-autophagosomes, autophagosomes and autolysosomes (autophagolysosomes). Cells were seeded into 8-well chamber slides at a density of 1×10 cells/ml and treated with indicated drugs. Following drug treatment, cells were washed with 1X assay buffer, following incubation with 100 µl microscopy dual detection reagent for 30 min at 37°C in the dark. Following the incubation, cells were washed with 1X assay buffer to remove unbound detection reagent and examined using a confocal microscope (LSM5; Carl Zeiss AG, Oberkochen, Germany).
Statistical analysis
All results presented were confirmed in at least three independent experiments. Data were presented as the mean ± standard deviation. Statistical differences were analyzed by one-way analysis of variance followed by a Tukey test using IBM® SPSS® Statistics Version 24.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Pristimerin enhances cell death and activates autophagy in MDA-MB-231 cells
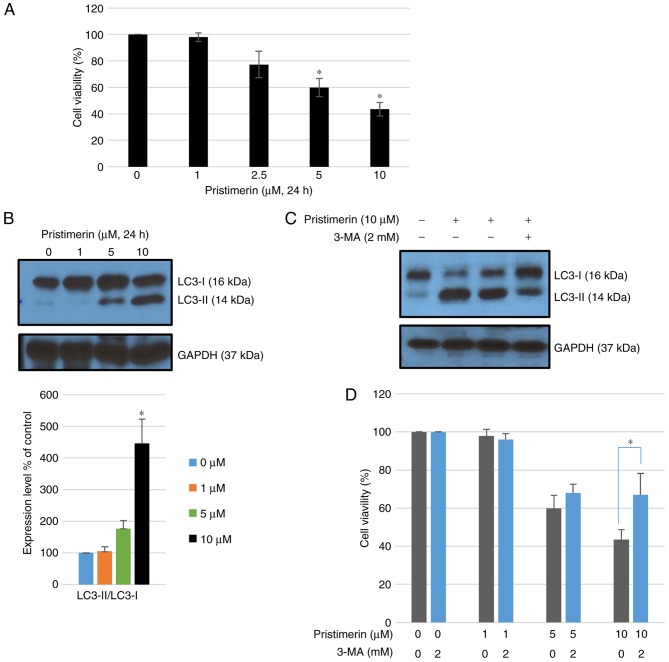
Exposure to pristimerin for 24 h significantly inhibited growth of MDA-MB-231 cells in a concentration-dependent manner compared with control treatment with a vehicle (P<0.05; Fig. 1A). Whether pristimerin-mediated inhibition of MDA-MB-231 cells originated from autophagy was further examined. Autophagy can be accurately measured by assessing the expression of microtubule-associated protein light chain 3 (LC3), namely the conversion of LC3-I to LC3-II using western blot analysis. Following treatment with pristimerin at 10 µM for 24 h, the ratio of LC3-II/LC3-I as well as LC3-II levels were increased (Fig. 1B). Co-treatment with 2 mM 3-MA (an autophagy inhibitor) inhibited LC3-II accumulation induced by 10 µM pristimerin (Fig. 1C) and promoted cell viability (Fig. 1D). These results suggested that pristimerin exposure induced autophagic cytotoxicity in MDA-MB-231 cells whereas inhibition of pristimerin-induced autophagy by 3-MA increased cell viability.
Figure 1.
Pristimerin-induced cell death is associated with activation of autophagy. (A) Cell viability following treatment with pristimerin (0, 1, 2.5, 5 and 10 µM) in MDA-MB-231 human breast cancer cells. *P<0.05 vs. pristimerin (0 µM). (B) MDA-MB-231 breast cancer cells were treated with 0, 1, 5 and 10 µM pristimerin for 24 h and expression levels of LC3-I and LC3-II were detected by western blot analysis. GAPDH was used as a loading control. Values are expressed as the mean ± standard deviation. *P<0.05 vs. control. (C) Cells were pre-incubated with or without 3-MA (2 mM) for 3 h and then incubated with pristimerin (10 µM) for 24 h, following which LC3-I and LC3-II levels were detected by western blotting analysis. (D) Cells were pre-incubated with or without 3-MA (2 mM) for 3 h and then incubated with 0, 1, 5 and 10 µM pristimerin, and cell viability was detected using a Cell Counting Kit-8 assay. Data are expressed as the mean ± standard deviation. *P<0.05 vs. pristimerin single treatment. 3-MA, 3-methyladenine; LC3-I, light chain 3.
Paclitaxel induces autophagy in MDA-MB-231 cells
To examine the inhibitory effect of paclitaxel on the proliferation of MDA-MB-231 cells breast cancer cells, various concentrations (0, 6, 12, 24 and 30 µM) of paclitaxel were evaluated by CCK-8 viability assay. Paclitaxel exhibited no significant toxicity to MDA-MB-231 cells at any concentration evaluated up to 24 µM (Fig. 2A). However, high concentrations of paclitaxel (over 48 µM) demonstrated strong toxicity (data not shown). Paclitaxel can modulate autophagy (16,17), although its mode of action remains controversial. In the present study, the effect of various concentrations of paclitaxel on autophagy in MDA-MB-231 cells was examined. Following treatment with paclitaxel at 12, 24, or 30 µM for 24 h, the ratio of LC3-II/LC3-I was significantly increased (P<0.05; Fig. 2B). These results demonstrated that paclitaxel induced autophagy in MDA-MB-231 cells without demonstrating significant cytotoxicity.
Figure 2.

Cell viability following treatment with paclitaxel in MDA-MB-231 human breast cancer cells. (A) Cell viability following treatment with various concentrations (0, 6, 12, 24 and 30 µM) of paclitaxel. (B) Cells were treated with paclitaxel for 24 h and the expression levels of LC3-II/LC3-I were detected by western blot analysis. GAPDH was used as a loading control. Data are expressed as the mean ± standard deviation. *P<0.05 vs. 0 µM treatment. LC3-I, light chain 3.
Involvement of autophagy in the additive action of pristimerin in combination with paclitaxel
As illustrated in Fig. 3A, combination treatment with 10 µM pristimerin and 24 µM paclitaxel additively inhibited cell viability. Further experiments were performed to observe whether paclitaxel influenced autophagic-cell death following treatment combined with pristimerin. Cells were pretreated with paclitaxel for 2 h and then treated with pristimerin for another 24 h. It was observed that the ratio of LC3-II/LC3-I as well as LC3-II levels were significantly additively increased (P<0.05; Fig. 3B). Chemical inhibition of autophagy using 3-MA significantly inhibited LC3-II accumulation induced by the combination of pristimerin and paclitaxel (P<0.05; Fig. 3B). Autophagy was further assessed by a detection assay where a population of green detection reagent-labeled vesicles co-localized with LC3, a specific autophagosome marker. It was revealed that paclitaxel or pristimerin treatment for 24 h resulted in the appearance of green detection reagent while their combination exhibited stronger green fluorescence (Fig. 3C). Autophagy inhibitor (3-MA) inhibited the autophagy reaction (Fig. 3C). Co-treatment with 3-MA significantly increased cell viability, even in the presence of a combination of pristimerin and paclitaxel (P<0.05; Fig. 3D). These results suggested that pristimerin enhanced the paclitaxel-induced growth inhibition of MDA-MB-231 cells by enhancing cytotoxic autophagic cell death.
Figure 3.
Involvement of autophagy in the additive action of pristimerin and paclitaxel. (A) Cell viability following treatment with pristimerin (0, 1, 2.5, 5 and 10 µM) and paclitaxel (24 µM) in MDA-MB-231 human breast cancer cells. *P<0.05 vs. pristimerin (10 µM) single treatment. (B) Cells were treated with paclitaxel and pristimerin for 24 h and expression levels of LC3-II/LC3-I were detected by western blot analysis. GAPDH was used as a loading control. Data are expressed as the mean ± standard deviation. *P<0.05 vs. paclitaxel and &P<0.05 vs. pristimerin. #P<0.05 vs. paclitaxel and pristimerin combined treatment. (C) An autophagy detection kit was used for the detection of autophagy in cells following treatment with indicated drugs. (D) Cells were pre-incubated with or without 3-MA (2 mM) for 3 h and then incubated with pristimerin (10 µM), paclitaxel, and a combination of the two for 24 h. Cell viability was then measured. Data are expressed as the mean ± standard deviation. *P<0.05 vs. combined treatment with pristimerin and paclitaxel. #P<0.05 vs. pristimerin (10 µM) single treatment. LC3-I, light chain 3; 3-MA, 3-methyladenine.
Regulation of ERK1/2 signaling contributes to pristimerin-induced autophagy in MDA-MB-231 cells
It is well known that ERK1/2 and autophagy are closely linked (18). Whether pristimerin treatment of MDA MB-231 cells could affect the ERK 1/2 signaling pathway was investigated. Cells were treated with various concentrations of pristimerin for 24 h, following which, phospho-ERK 1/2 and p90RSK, one of the potentially important substrates of ERK, were assessed by western blot analysis. Pristimerin dose-dependently inhibited the phosphorylation of ERK1/2 and p90RSK (Fig. 4A). It was also observed that beclin 1 expression and p62 degradation, both autophagic proteins, were increased by pristimerin treatment (Fig. 4A). Paclitaxel treatment (24 µM) alone also inhibited ERK1/2/p90RSK phosphorylation levels and increased the beclin 1 expression and p62 degradation (Fig. 4A). Combined treatment of pristimerin and paclitaxel additively inhibited ERK1/2 phosphorylation and significantly increased the ratio of LC3-II/LC3-I (P<0.05; Fig. 4B). Co-treatment with an ERK activator (ceramide C6) increased phosphorylation of ERK and inhibited LC3-II accumulation in combination treatment of pristimerin and paclitaxel (Fig. 4B). These results suggested that pristimerin-induced autophagy was associated with ERK1/2 signaling and paclitaxel additively enhanced pristimerin-induced autophagic cell death in MDA-MB-231 cells.
Figure 4.
Pristimerin-induced autophagy is regulated by ERK1/2 signaling in MDA-MB-231 cells. (A) Cells were treated with pristimerin and paclitaxel for 24 h and expression levels of p-ERK1/2, ERK1/2, p-p90RSK, p90RSK, beclin 1, and p62 were detected by western blot analysis. GAPDH was used as a loading control. *P<0.05 vs. pristimerin (0 µM) (Lane no. 1) and #P<0.05 vs. paclitaxel (0 µM) (Lane no. 5). (B) Cells were treated with pristimerin and paclitaxel and expression levels of p-ERK1/2, ERK1/2, LC3-II/LC3-I were detected by western blot analysis. Cells were pretreated with ERK activator ceramide C6 for 3 h prior to combined treatment with pristimerin and paclitaxel. GAPDH was used as a loading control. Data are expressed as the mean ± standard deviation. *P<0.05 vs. paclitaxel (lane no. 3), &P<0.05 vs. pristimerin (lane no. 2) and ##P<0.01 vs. pristimerin and paclitaxel combined treatment without ceramide C6 (lane no. 4). p-ERK, phosphorylated extracellular signal-regulated kinase; LC3, light chain 3; p90RSK, p90 ribosomal S6 kinase.
Discussion
Breast cancer is a complicated and heterogeneous disease with a number of biomarkers, including estrogen receptor, progesterone receptor, HER2 and triple-negative breast cancer (19). Each of them has different treatment strategies and prognosis (19). A high mortality rate is associated with breast cancer patients (19). Increasing effort has been focused on the identification of novel anti-breast cancer agents. Medicinal plants and their extracts are commonly used to prevent and treat a number of diseases, including cancer. Developing novel therapeutic agents from plants with fewer side-effects and high efficacy is a promising strategy to reduce the mortality rate of breast cancer.
Pristimerin, a quinonemethide triterpenoid, has been isolated from several plants including Maytenus chuchuhuasca and M. ilicifolia in South Africa (20). Promising anticancer activities of pristimerin have been emphasized in terms of its therapeutic potential for breast cancer (21). Previous studies have demonstrated that pristimerin is involved in apoptotic cell death of MDA-MB-231 (15) and SKBR3 human breast cancer cells (13). It was demonstrated that the apoptotic activity of pristimerin and pristimerin induced apoptosis in MDA-MB-231 cells, as expected. However, the effect of pristimerin on autophagy in human breast cancer has not been fully understood. Certain studies have reported that triterpenoids can cause cell death by autophagy, including cimigenol (KY17) (22), 2α, 3α, 24-thrihydroxyurs-12-en-28-oicacid (23), ursolic acid (24) and cucurbitane (25). In the present study, the autophagic effect of pristimerin on MDA-MB-231 human breast cancer cells was examined.
Autophagy has been established as a type of programmed cell death involving self-destruction characterized by distinct morphological and biochemical features. Autophagy is generally considered to be pro-survival associated, or cytoprotective under stressful conditions such as g-radiation and chemotherapy (26). However, it is frequently activated in response to a number of environmental stresses, thereby leading to cell death (27). LC3 is considered to be a strong marker of autophagy. The conversion of LC3-I to LC3-II and LC3 puncta usually demonstrate an activation of autophagy (28). In the present study, pristimerin-induced autophagy in MDA-MB-231 human breast cancer cells was examined using western blot analysis. As demonstrated in the results, LC3-II/LC3-I levels were increased, which indicated that induction of autophagy was concentration-dependent. This autophagy induction has the same pattern as pristimerin-induced cell death. Furthermore, it was observed that autophagy inhibition by 3-MA partially decreased pristimerin-induced cytotoxicity and undermined LC3-II levels. These data suggested that pristimerin-induced autophagy can serve as a cell death pathway.
Paclitaxel is isolated from the bark of the yew tree. It inhibits the growth of tumor cells. It is an important therapeutic drug in the treatment of a number of types of cancer, including breast cancer (29). It is known to stabilize microtubules during DNA synthesis, thereby suppressing mitosis of cancer cells. Paclitaxel is capable of inducing mitochondria-mediated apoptosis involving caspase-dependent (via caspase-3) and caspase-independent pathways (via apoptosis inhibitory factor) (30). Apoptosis is frequently closely associated with autophagy in cancer (31). Since autophagy has a housekeeping role in clearing damaged organelles and eliminating intracellular pathogens, autophagy is generally regarded as a survival mechanism. On the other hand, autophagy has a key role in tumorigenesis, progression and oncotherapy (30). Paclitaxel can induce autophagy in human osteosarcoma cells (MG-63) (30), non-small cell lung cancer cells (A549) (16) and cervical cancer (HeLa) (32). In the present study, paclitaxel treatment promoted autophagy in MDA-MB-231 cells at concentrations over 12 µM, and did not demonstrate cytotoxicity at 12 µM. However, higher concentrations of paclitaxel (48 and 60 µM) demonstrated strong cytotoxicity along with autophagy induction (data not shown). A relatively high concentration of paclitaxel was used in the present experiment compared with other studies. There may be certain differences in drug use. Paclitaxel was obtained for intravenous use from Boryung Co., Ltd. (Seoul, Korea). Other investigations purchased the drug from Sigma-Aldrich; Merck KGaA. For unknown reasons, in the present experiment, MDA-MB-231 cancer cell lines did not respond to low concentrations at all, in contrary to results of other papers. It was demonstrated that another previous study also used a high concentration of paclitaxel (33). The clinical use of paclitaxel is frequently limited due to acquisition of anticancer drug resistance (34). Therefore, combined treatment is often used to enhance the effectiveness of chemotherapy and avoid chemo-resistance to a single agent. All single anticancer agents could similarly be used at reduced concentrations when they are combined with others to synergistically induce cancer cell death (35). Pristimerin in combination with taxol can synergistically induce the death of cervical cancer cells (35). In the present study, pristimerin additively enhanced paclitaxel-induced cell death by autophagic induction in MDA-MB-231 cells. To the best of our knowledge, the present study is the first to propose that autophagy in breast cancer cells may be stimulated by pristimerin alone, as well as in combination with paclitaxel.
In the present experimental data (not shown), pristimerin-induced apoptotic activity was increased by addition of paclitaxel. The mechanism involved in these effects is still being investigated. There is a complex crosstalk between autophagy and apoptosis (31). It is frequently unclear which specific interactions may contribute to cancer cell death. Cancer cell death in this experiment is not the effect of only one mechanism, namely autophagy. However, autophagy may be one of the mechanisms that contribute to cancer cell death, which can be detected by a number of methods.
GTPase HRas/Raf proto-oncogene serine/threonine protein kinase/Dual specificity mitogen-activated protein kinase kinase mek/ERK pathway serves an important role in autophagy. ERK phosphorylates and inhibits TSC1/TSC2 which then activates C1 and induces autophagy (36). Recently, it has been demonstrated that a synthetic antihepatitis drug (Bicyclol) can induce autophagy via the ERK signaling pathway in HepG2 hepatocellular carcinoma cells (37). To understand the signaling cascade that mediates the autophagic effect of pristimerin on MDA-MB-231 cells, modulation of the activation of ERK1/2 by pristimerin was examined. Pristimerin treatment suppressed phospho-ERK1/2 and phospho-p90RSK levels in a dose-dependent manner, although without affecting total ERK1/2 and total p90RSK expression. The function of pristimerin on ERK regulation remains controversial. A previous study suggested that pristimerin can decrease the level of p-ERK1/2 and mTOR to induce cell death in SKBR3 breast cancer cells (13). However, another study demonstrated that ERK phosphorylation is not altered by pristimerin treatment in HeLa cervical cancer cells (35). In the present study, ERK1/2 inhibition by pristimerin, paclitaxel, or the combination was confirmed to induce autophagy (increased p62 degradation and increased beclin1 expression). These effects were reversed by treatment with an ERK activator. These results suggested that pristimerin-induced autophagy served as a cell death pathway via ERK1/2 inhibition, and that non-toxic doses of paclitaxel can additively enhance these activities.
In conclusion, the results of the present study elucidated the anti-cancer mechanism of pristimerin, and demonstrated that non-toxic paclitaxel doses induced autophagy in breast cancer cells. The current study provides sufficient evidence that an autophagy inducer may be used as an adjuvant modality during anti-cancer pharmacological treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by research fund of Chungnam National University (grant no. 2015088201).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YL and JN designed the study and prepared the manuscript. ML, EC and JP performed the experiments and analyzed the data. JS and JL were involved in the study conception and design and revised the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zagouri F, Sergentanis TN, Tsigginou A, Dimitrakakis C, Zografos GC, Dimopoulos MA, Psaltopoulou T. Female breast cancer in Europe: Statistics, diagnosis and treatment modalities. J Thorac Dis. 2014;6:589–590. doi: 10.3978/j.issn.2072-1439.2014.06.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Majeed W, Aslam B, Javed I, Khaliq T, Muhammad F, Ali A, Raza A. Breast cancer: Major risk factors and recent developments in treatment. Asian Pac J Cancer Prev. 2014;15:3353–3358. doi: 10.7314/APJCP.2014.15.8.3353. [DOI] [PubMed] [Google Scholar]
- 3.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, Thompson CB. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–248. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 4.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi WY, Xiao D, Wang L, Dong LH, Yan ZX, Shen ZX, Chen SJ, Chen Y, Zhao WL. Therapeutic metformin/AMPK activation blocked lymphoma cell growth via inhibition of mTOR pathway and induction of autophagy. Cell Death Dis. 2012;3:e275. doi: 10.1038/cddis.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fénichel P, Hofman P, Poujeol P, Mograbi B. Control of the autophagy maturation step by the MAPK ERK and p38: Lessons from environmental carcinogens. Autophagy. 2007;3:57–59. doi: 10.4161/auto.3424. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, Zhu R, Tian S, Wang Y, Lou S, Zhao H. Jatamanvaltrate P induces cell cycle arrest, apoptosis and autophagy in human breast cancer cells in vitro and in vivo. Biomed Pharmacother. 2017;89:1027–1036. doi: 10.1016/j.biopha.2017.02.065. [DOI] [PubMed] [Google Scholar]
- 9.Dirsch VM, Kiemer AK, Wagner H, Vollmar AM. The triterpenoid quinonemethide pristimerin inhibits induction of inducible nitric oxide synthase in murine macrophages. Eur J Pharmacol. 1997;336:211–217. doi: 10.1016/S0014-2999(97)01245-4. [DOI] [PubMed] [Google Scholar]
- 10.Yousef BA, Hassan HM, Guerram M, Hamdi AM, Wang B, Zhang LY, Jiang ZZ. Pristimerin inhibits proliferation, migration and invasion and induces apoptosis in HCT-116 colorectal cancer cells. Biomed Pharmacother. 2016;79:112–119. doi: 10.1016/j.biopha.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Liu YB, Gao X, Deeb D, Pindolia K, Gautam SC. Role of telomerase in anticancer activity of pristimerin in prostate cancer cells. J Exp Ther Oncol. 2015;11:41–49. [PubMed] [Google Scholar]
- 12.Yousef BA, Guerram M, Hassan HM, Hamdi AM, Zhang LY, Jiang ZZ. Pristimerin demonstrates anticancer potential in colorectal cancer cells by inducing G1 phase arrest and apoptosis and suppressing various pro-survival signaling proteins. Oncol Rep. 2016;35:1091–1100. doi: 10.3892/or.2015.4457. [DOI] [PubMed] [Google Scholar]
- 13.Lee JS, Yoon IS, Lee MS, Cha EY, Thuong PT, Diep TT, Kim JR. Anticancer activity of pristimerin in epidermal growth factor receptor 2-positive SKBR3 human breast cancer cells. Biol Pharm Bull. 2013;36:316–325. doi: 10.1248/bpb.b12-00685. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Landis-Piwowar KR, Lu D, Yuan P, Li L, Reddy GP, Yuan X, Dou QP. Pristimerin induces apoptosis by targeting the proteasome in prostate cancer cells. J Cell Biochem. 2008;103:234–244. doi: 10.1002/jcb.21399. [DOI] [PubMed] [Google Scholar]
- 15.Wu CC, Chan ML, Chen WY, Tsai CY, Chang FR, Wu YC. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther. 2005;4:1277–1285. doi: 10.1158/1535-7163.MCT-05-0027. [DOI] [PubMed] [Google Scholar]
- 16.Klimaszewska-Wisniewska A, Halas-Wisniewska M, Tadrowski T, Gagat M, Grzanka D, Grzanka A. Paclitaxel and the dietary flavonoid fisetin: A synergistic combination that induces mitotic catastrophe and autophagic cell death in A549 non-small cell lung cancer cells. Cancer Cell Int. 2016;16:10. doi: 10.1186/s12935-016-0288-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veldhoen RA, Banman SL, Hemmerling DR, Odsen R, Simmen T, Simmonds AJ, Underhill DA, Goping IS. The chemotherapeutic agent paclitaxel inhibits autophagy through two distinct mechanisms that regulate apoptosis. Oncogene. 2013;32:736–746. doi: 10.1038/onc.2012.92. [DOI] [PubMed] [Google Scholar]
- 18.Corcelle E, Djerbi N, Mari M, Nebout M, Fiorini C, Fénichel P, Hofman P, Poujeol P, Mograbi B. Control of the autophagy maturation step by the MAPK ERK and p38: Lessons from environmental carcinogens. Autophagy. 2007;3:57–59. doi: 10.4161/auto.3424. [DOI] [PubMed] [Google Scholar]
- 19.Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirota O, Morita H, Takeya K, Itokawa H. Cytotoxic aromatic triterpenes from Maytenus ilicifolia and Maytenus chuchuhuasca. J Nat Prod. 1994;57:1675–1681. doi: 10.1021/np50114a009. [DOI] [PubMed] [Google Scholar]
- 21.Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: Natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65:2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dai X, Liu J, Nian Y, Qiu MH, Luo Y, Zhang J. A novel cycloartane triterpenoid from Cimicifuga induces apoptotic and autophagic cell death in human colon cancer HT-29 cells. Oncol Rep. 2017;37:2079–2086. doi: 10.3892/or.2017.5444. [DOI] [PubMed] [Google Scholar]
- 23.Zhang D, Gao C, Li R, Zhang L, Tian J. TEOA, a triterpenoid from Actinidia eriantha, induces autophagy in SW620 cells via endoplasmic reticulum stress and ROS-dependent mitophagy. Arch Pharm Res. 2017;40:579–591. doi: 10.1007/s12272-017-0899-9. [DOI] [PubMed] [Google Scholar]
- 24.Hen S, Zhang Y, Zhang R, Tu X, Gong X. Ursolic acid induces autophagy in U87MG cells via ROS-dependent endoplasmic reticulum stress. Chem Biol Interact. 2014;218:28–41. doi: 10.1016/j.cbi.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Weng JR, Bai LY, Chiu CF, Hu JL, Chiu SJ, Wu CY. Cucurbitane Triterpenoid from Momordica charantia Induces Apoptosis and Autophagy in Breast Cancer Cells, in Part, through Peroxisome Proliferator-Activated Receptor γ Activation. Evid Based Complement Alternat Med. 2013;2013:935675. doi: 10.1155/2013/935675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen HY, White E. Role of autophagy in cancer prevention. Cancer Prev Res (Phila) 2011;4:973–983. doi: 10.1158/1940-6207.CAPR-10-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denton D, Nicolson S, Kumar S. Cell death by autophagy: Facts and apparent artefacts. Cell Death Differ. 2012;19:87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dank M, Budi L, Piko B, Mangel L, Erfan J, Cseh J, Ruzsa A, Landherr L. First-line bevacizumab-paclitaxel in 220 patients with metastatic breast cancer: Results from the AVAREG study. Anticancer Res. 2014;34:1275–1280. [PubMed] [Google Scholar]
- 30.Guo Y, Huang C, Li G, Chen T, Li J, Huang Z. Paxilitaxel induces apoptosis accompanied by protective autophagy in osteosarcoma cells through hypoxia-inducible factor-1α pathway. Mol Med Rep. 2015;12:3681–3687. doi: 10.3892/mmr.2015.3860. [DOI] [PubMed] [Google Scholar]
- 31.Zambrano J, Yeh ES. Autophagy and apoptotic crosstalk: Mechanism of therapeutic resistance in HER2-positive breast cancer. Breast Cancer (Auckl) 2016;10:13–23. doi: 10.4137/BCBCR.S32791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi EY, Viriyapak B, Kwack HS, Lee YK, Kim SI, Lee KH, Park TC. Regulation of paclitaxel-induced programmed cell death by autophagic induction: A model for cervical cancer. Obstet Gynecol Sci. 2013;56:84–92. doi: 10.5468/OGS.2013.56.2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu C, Zhang W, Zheng G, Zhang Z, Yin J, He Z. Metformin reverses multidrug resistance and epithelial-mesenchymal transition (EMT) via activating AMP-activated protein kinase (AMPK) in human breast cancer cells. Mol Cell Biochem. 2014;386:63–71. doi: 10.1007/s11010-013-1845-x. [DOI] [PubMed] [Google Scholar]
- 34.Koziara JM, Whisman TR, Tseng MT, Mumper RJ. In-vivo efficacy of novel paclitaxel nanoparticles in paclitaxel-resistant human colorectal tumors. J Control Release. 2006;112:312–319. doi: 10.1016/j.jconrel.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Eum DY, Byun JY, Yoon CH, Seo WD, Park KH, Lee JH, Chung HY, An S, Suh Y, Kim MJ, Lee SJ. Triterpenoid pristimerin synergizes with taxol to induce cervical cancer cell death through reactive oxygen species-mediated mitochondrial dysfunction. Anticancer Drugs. 2011;22:763–773. doi: 10.1097/CAD.0b013e328347181a. [DOI] [PubMed] [Google Scholar]
- 36.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Nie H, Zhao X, Qin Y, Gong X. Bicyclol induces cell cycle arrest and autophagy in HepG2 human hepatocellular carcinoma cells through the PI3K/AKT and Ras/Raf/MEK/ERK pathways. BMC Cancer. 2016;16:742. doi: 10.1186/s12885-016-2767-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.