Abstract
Angiotensin II (Ang II) is a principal molecule of the renin-angiotensin system, which promotes hypertrophy and fibrosis. It has been demonstrated that Ang II upregulates the expression of cyclophilin A (CypA), which is a potential myocardial hypertrophy factor. However, the mechanisms by which Ang II induces the expression of CypA in cardiomyocytes remain unclear. In the present study, reactive oxygen species (ROS) were detected by fluorescence microscopy, and western blot analysis and ELISA were used to measure CypA expression. It was identified that Ang II enhanced the production of ROS in rat cardiomyocytes. ROS, in turn, promoted CypA expression and secretion. Notably, the action of Ang II was primarily dependent on the angiotensin type 2 receptor (AT2R), not the type 1 receptor. These results provided an insight into the role of the AT2R signaling pathway in Ang II-induced myocardial hypertrophy.
Keywords: Ang II, CypA, ROS, AT2R
Introduction
Cardiac remodeling is associated with pathological alterations to the heart that are caused by myocardial infarction and hypertension, primarily via cardiomyocyte and myocardial interstitial remodeling (1). Cardiomyocyte remodeling is characterized by compensatory hypertrophy, cell apoptosis and necrosis. Myocardial interstitial remodeling primarily refers to alterations in the extracellular matrix of cardiomyocytes, including fibroblast proliferation and fibrosis (2,3). The renin-angiotensin system (RAS) has been identified to serve an important role in cardiac remodeling. Angiotensin II (Ang II) is a principal functional molecule in RAS, which is involved in myocardial fibrosis and accelerated myocardial remodeling (4–6).
Cyclophilin A (CypA), which was initially discovered in 1984, is the primary target molecule of the immunosuppressant cyclosporine A (CsA) (7). CypA is involved in numerous biological activities, including protein folding (8,9), inflammation (10–13), immunosuppression (14,15), apoptosis (16–19), and viral infection and replication (20,21). A previous study conducted by Venkatesan et al (22) demonstrated that CypA is able to promote myocardial hypertrophy and exacerbate the severity of Ang II-induced myocardial hypertrophy. In rats, CsA effectively blocks or alleviates Ang II-induced myocardial hypertrophy by binding with CypA to form a dimer complex and inhibiting Ang II activity by binding to calcineurin (1,22). Collectively, these results indicate that CypA is involved in Ang II-induced myocardial hypertrophy.
Reactive oxygen species (ROS) are active oxygen-containing compounds that are generated during biological aerobic metabolism. A series of responses, including cell proliferation, differentiation, migration, injury, matrix remodeling, apoptosis and necrosis, result from ROS production. ROS participate in signal transduction processes that control gene expression, cell growth and apoptosis (23–25). Furthermore, increased ROS production is considered to be a mechanism underlying Ang II-induced myocardial hypertrophy (26); however, the signaling pathways leading to Ang II-induced ROS production are not well understood (27).
Ang II is primarily recognized by two principal receptors in the cell membrane: Angiotensin type 1 receptor (AT1R) and angiotensin type 2 receptor (AT2R). Previously, Satoh et al demonstrated that, during the Ang II-induced formation of abdominal aortic aneurysm (AAA), CypA synergistically elevates ROS production (28). In the present study, the aim was to explore the mechanism of Ang II-induced myocardial hypertrophy. The results demonstrated that Ang II increased ROS production via the AT2R pathway in rat cardiomyocytes. ROS production, in turn, promoted CypA expression and secretion. These results suggested that ROS may serve an important role in the upregulation of CypA by Ang II.
Materials and methods
Rat cardiomyocyte culturing
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (cat. no. FSP500; Shanghai ExCell Biology, Inc., Shanghai, China) and F12 factor (HyClone; GE Healthcare, Chicago, IL, USA) was used to culture H9C2 rat cardiomyocytes, which were provided by the State Key Laboratory of Natural Resource Conservation and Utilization (Kunming, China) in a 25-ml filtered cell culture flask in an incubator containing 5% CO2 at 37°C. The cultures were observed daily and culture medium was changed every 2–3 days. When the cells had reached ~80% confluence, the cells were washed three times in PBS and treated with 0.25% trypsin (HyClone; GE Healthcare) for digestion and passaging. The present study used the second and third passages of H9C2 cells for experimentation.
ROS detection
H9C2 cells in the logarithmic growth phase were seeded at a density of 1×105 cells/well in 12-well plates and cultured at 37°C in 5% CO2. Following cell adhesion, cells were subjected to different treatments: i) untreated; ii) 24-h incubation with 0.1 µM Ang II (cat. no. A9290; Beijing Solarbio Science & Technology Co., Ltd., Beijing, China); iii) 24-h incubation with 3 mM glutathione ethyl ester (GEE; cat. no. 14953; Cayman Chemical Company, Ann Arbor, MI, USA) and 0.1 µM Ang II; iv) 24-h incubation with 0.4 mM butyrate (cat. no. B110438; Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China); v) pre-incubation with 10 µM Valsartan (cat. no. MB1341-S; Dalian Meilun Biotech Co., Ltd., Dalian, China) for 1 h, followed by 24-h incubation with 0.1 µM Ang II; vi) 1-h pre-incubation with 1 mM PD123319 (cat. no. MB5078; Dalian Meilun Biotech Co., Ltd.), followed by 24-h incubation with 0.1 µM Ang II. Subsequently, the H9C2 cells were treated with 50 µM BES-H2O2-Ac (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 1 h prior to measuring the fluorescence intensity by fluorescence microscopy (29,30).
Western blot analysis
H9C2 cells in the logarithmic growth phase were used to seed a density of 2×105 cells into each well of a 6-well plate. Following cell adhesion, cells were treated as described above. After the 24-h treatment, the H9C2 cells were washed with PBS and harvested to extract total protein using radioimmunoprecipitation lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.). A bicinchoninic acid assay was used to quantify total protein concentration. Total protein (50 µg) was loaded into each lane of an SDS-PAGE gel (10% separation gel, 4% stacking gel), and the proteins were separated by electrophoresis at 90 V for 100 min. Subsequently, proteins were transferred to nitrocellulose membranes at 300 mA for 100 min, and the membranes were blocked with 4% skim milk at room temperature for 1 h. After washing two times with PBS plus 0.05% Tween-20 (PBST; 5 min/wash), the membranes were incubated with CypA-specific antibody, which was a rabbit polyclonal antibody generated for CypA, cloned from Microplitis bicoloratus hemocytes and expressed in Escherichia coli using the pET-32a-CypA plasmid (1:2,000; Bioworld Technology, Inc., Nanjing, China) and β-actin-specific antibody (1:2,000; cat. no. BS6007M; Bioworld Technology, Inc.) at 4°C overnight with agitation. After washing with PBST twice, the membranes were incubated with Alexa Fluor® 568 Goat Anti-Rabbit Immunoglobulin G (Heavy+Light) secondary antibody (1:2,000; cat. no. A0208; Beyotime Institute of Biotechnology, Haimen, China) at room temperature for 1 h, followed by two washes with PBST for 10 min. The protein bands were visualized with enhanced chemiluminescent reagent (Tanon Science and Technology Co., Ltd., Shanghai, China) using a Tanon 5200 imager (Tanon Science and Technology Co., Ltd.). Semi-quantification of the bands was performed using ImageJ software version 1.46r (National Institutes of Health, Bethesda, MD, USA), with the intensity values normalized to the corresponding β-actin band.
ELISA measurement of CypA expression in the culture medium
H9C2 cells in the logarithmic growth phase were used to seed a density of 2×105 cells into each well of a 6-well plate. Following cell adhesion, cells were treated as described above. The cell culture supernatant was collected in a 1.5-ml sterile centrifuge tube and centrifuged at 800 × g for 20 min at room temperature. The supernatant was transferred to a new 1.5-ml centrifuge tube and CypA protein content was measured using ELISA (cat. no. HZ-EL-R0298c; eBioscience; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Statistical analysis
All experiments were repeated three times and the data are presented as the means ± standard deviation. The green fluorescence signals of ROS were randomly collected and analyzed from three fields of vision using ImageJ software version 1.46r (National Institutes of Health). GraphPad Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA) software was used for statistical analysis of all data in the present study. Statistical differences between the groups were analyzed using one-way analysis of variance followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Ang II upregulates CypA in rat cardiomyocytes
Untreated mock H9C2 cells exhibited relatively low CypA expression compared with the cells treated with Ang II. After a 24-h incubation with 0.1 µM Ang II, H9C2 cells exhibited significantly upregulated CypA expression and increased CypA content in the supernatant (P<0.05; Fig. 1). These data suggested that Ang II may promote the expression and secretion of CypA in rat cardiomyocytes.
Figure 1.
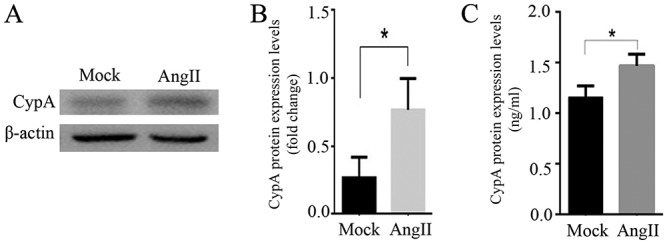
Ang II upregulates CypA in rat cardiomyocytes. (A) Western blotting and (B) semi-quantification analysis demonstrated that CypA expression was stimulated by Ang II in cultured H9C2 cells. (C) Ang II increased CypA content in the supernatant of cultured H9C2 cells. n=3/group. *P<0.05. Ang II, angiotensin II; CypA, cyclophilin A.
Ang II upregulates CypA via ROS
After a 24-h treatment with 0.1 µM Ang II, ROS production was significantly increased in H9C2 cells (P<0.05; Fig. 2A and B). When ROS production was inhibited by treatment of H9C2 cells with 3 mM of the antioxidant glutathione ethyl ester, the expression of CypA were significantly decreased (P<0.05; Fig. 2C and D). These results suggested that Ang II may upregulate CypA by enhancing ROS production in rat cardiomyocytes.
Figure 2.
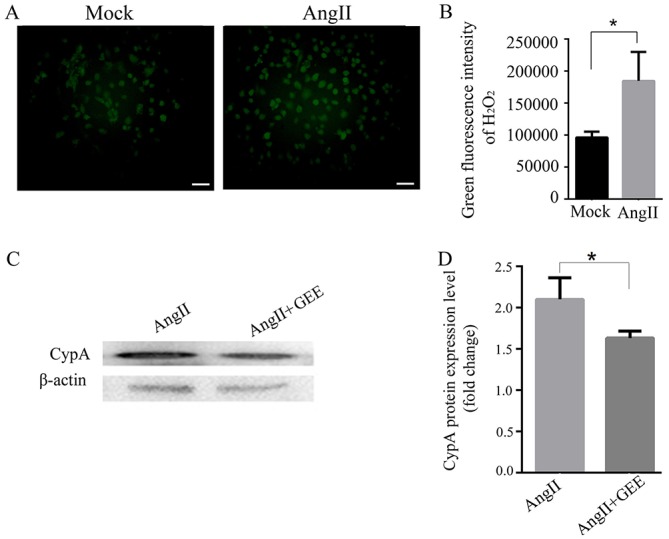
Ang II upregulates CypA via ROS. (A) Representative images and (B) analysis demonstrating that Ang II increased ROS production in cultured H9C2 cells. n=3/group. Green fluorescence represents ROS; scale bar, 50 µm. (C) Western blotting and (D) semi-quantification analysis demonstrated that Ang II-induced CypA expression was inhibited by GEE, a ROS inhibitor. *P<0.05. Ang II, angiotensin II; CypA, cyclophilin A; GEE, glutathione ethyl ester; ROS, reactive oxygen species.
Increased ROS induce the expression of CypA in rat cardiomyocytes
Butyrate, an oxidant, promotes ROS production in numerous types of cells (31,32). In order to validate the role of ROS in upregulating CypA expression, 0.4 mM butyrate was added to cells to stimulate ROS production. A ROS fluorescence probe (BES-H2O2-Ac) was used to evaluate ROS production over the course of 1 h under a fluorescence microscope. As hypothesized, ROS production was increased in H9C2 cells following treatment with 0.4 mM butyrate compared with in the control group (P<0.05; Fig. 3A and B). Under butyrate stimulation, CypA in H9C2 cells and its content in the supernatant were significantly upregulated (P<0.05; Fig. 3C-E). These results indicated that increased ROS levels may promote the expression and secretion of CypA in rat cardiomyocytes.
Figure 3.
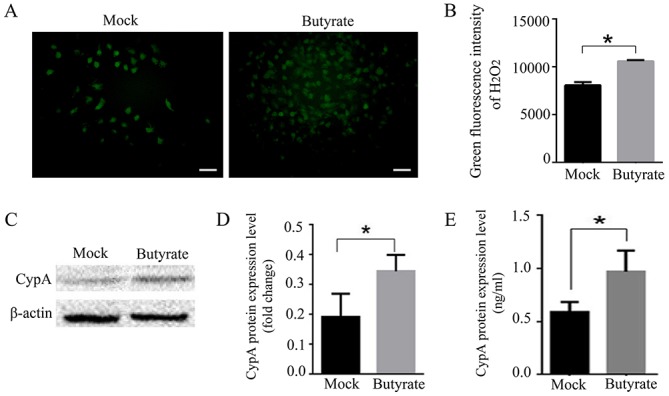
Elevated ROS induce the expression of CypA. (A) Representative images and (B) analysis demonstrating that butyrate promoted ROS production in cultured H9C2 cells. n=3/group; scale bar, 50 µm. (C) Western blotting and (D) semi-quantification analysis of CypA expression in H9C2 cells following treatment with butyrate. (E) CypA content analysis in the supernatant of H9C2 cells following treatment with butyrate. *P<0.05. CypA, cyclophilin A; ROS, reactive oxygen species.
Ang II increases ROS production via AT2R
Ang II activates at least two pharmacologically distinct receptors, AT1R and AT2R. To investigate which receptor is involved in ROS production, Valsartan and PD123319, which are antagonists of AT1R and AT2R, respectively, were used in the present study. H9C2 cells were pre-incubated with 10 µM Valsartan or 1 mM PD123319 for 1 h prior to stimulation with 0.1 µM Ang II. The results indicated that PD123319 significantly inhibited Ang II-induced ROS production (P<0.001; Fig. 4A and B). Valsartan additionally inhibited Ang II-induced ROS production; however, this effect was not statistically significant (P=0.0637; Fig. 4A and B). Notably, PD123319 significantly suppressed the CypA expression in H9C2 cells (P<0.05; Fig. 4C and D). Conversely, Valsartan did not significantly decrease CypA expression (P=0.823). These results suggested that Ang II upregulated CypA by enhancing ROS production primarily via the AT2R pathway in rat cardiomyocytes.
Figure 4.
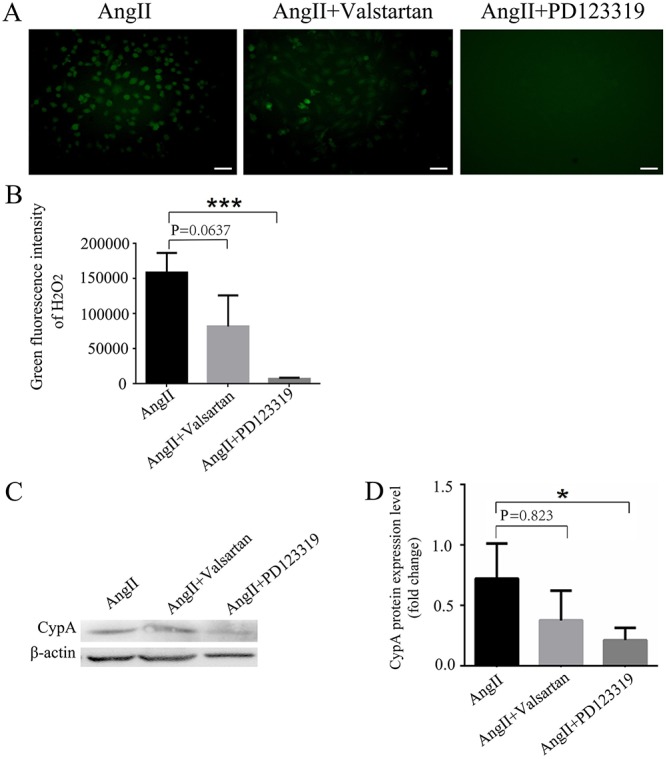
Ang II enhances ROS production via AT2R. (A) Representative images and (B) analysis of ROS production in Ang II-induced H9C2 cells pretreated with Valsartan (angiotensin receptor 1 antagonist) and PD123319 (AT2R antagonist). Scale bar, 50 µm. PD123319 significantly inhibited Ang II-induced ROS production. n=3/group. (C) Western blotting and (D) semi-quantification analysis of CypA expression in Ang II-induced H9C2 cells pretreated with Valsartan and PD123319. PD123319 significantly suppressed Ang II-induced CypA expression in H9C2 cells. n=3/group. *P<0.05, ***P<0.01. Ang II, angiotensin II; AT2R, angiotensin receptor 2; CypA, cyclophilin A; ROS, reactive oxygen species.
Discussion
Accumulating evidence indicates that Ang II serves an important role in myocardial fibrosis and hypertrophy, which are important features in cardiac remodeling (6,27,33). Satoh et al (28) demonstrated that CypA serves a key role in Ang II-induced AAA formation using gene knockout animal models. AAA formation is a typical manifestation of vascular remodeling that is caused by atherosclerosis. These results suggested that CypA is likely to serve the same role in Ang II-induced cardiac remodeling; however, the mechanism underlying Ang II-induced CypA expression remains unclear. Previously, Seko et al (34) demonstrated that hypoxia followed by reoxygenation may promote CypA secretion in cultured rat cardiomyocytes. In the present study, it was identified that Ang II promoted CypA expression and secretion, in addition to ROS production in H9C2 cells. These results suggested that CypA expression may be associated with ROS formation. Notably, when the formation of ROS was inhibited by the antioxidants GEE and PD123319, CypA expression was downregulated in Ang II-treated rat cardiomyocytes. Furthermore, it was identified that an increase in ROS formation induced by butyrate upregulated the expression of CypA. These results indicated that Ang II upregulated CypA in a ROS-dependent manner.
The biological activities of Ang II, a primary functional molecule of the RAS, are primarily mediated by its two G-protein-coupled receptors, AT1R and AT2R (35). AT1R is documented to mediate the majority of the biological functions of Ang II, whereas the roles of AT2R are considered to counterbalance the effects mediated by activation of AT1R. Previous studies have demonstrated that long-term Ang II injection causes elevated blood pressure in mice, leading to myocardial hypertrophy, fibrosis and activation of AT1R (6,36). Ang II promotes cardiomyocyte proliferation and growth, collagen formation, extracellular matrix deposition and myocardial fibrosis via AT1R (37). Therefore, Ang II is involved in cardiac remodeling through the AT1R pathway. However, AT2R expression is upregulated in pathological circumstances, including in heart failure, experimental cardiac hypertrophy, myocardial infarction and vascular injury (38,39). In the present study, it was observed that Ang II induced ROS production primarily via AT2R, which is consistent with results of Thakur et al (40); in their research, Ang II induced ROS production by NADPH oxidase 2 in endothelial cells. ROS, in turn, may promote CypA expression and secretion. Therefore, it may be suggested that AT2R exhibits its function in Ang II-mediated myocardial hypertrophy, at least in part, by upregulating CypA expression (Fig. 5).
Figure 5.
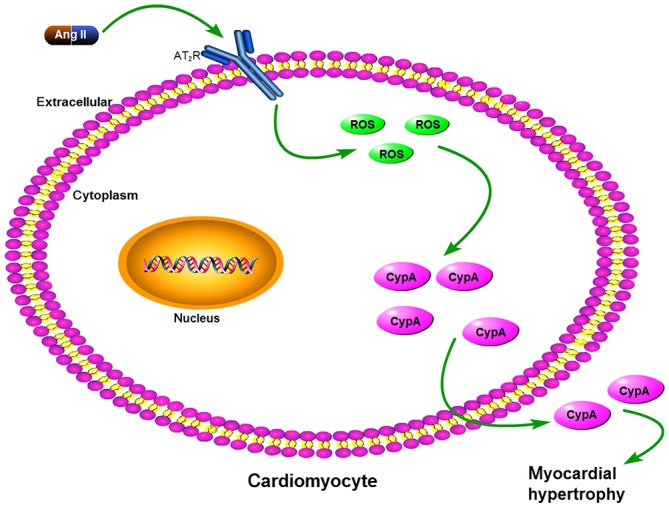
Proposed mechanism of how Ang II upregulates CypA. Ang II increased ROS levels via AT2R in cardiomyocytes; increased ROS, in turn, promoted CypA expression and secretion. Ang II, angiotensin II; AT2R, angiotensin type 2 receptor; CypA, cyclophilin A; ROS, reactive oxygen species.
Cardiac remodeling is associated with the apoptosis and necrosis of a large number of cardiomyocytes. In the present study, it was revealed that Ang II may promote CypA expression by stimulating ROS production. Satoh et al (28) suggested that CypA may increase ROS production in vascular tissue, and Seko et al (34), demonstrated that cultured rat cardiomyocytes are able to promote CypA secretion during reoxidation, thus indicating that during Ang II stimulation in rat cardiomyocytes the following cycle may be exhibited: Ang II binds to AT2R and upregulates CypA expression and secretion by promoting ROS production, in turn, CypA may increase ROS production. The positive association between CypA expression and ROS production is consistent with the results of Perrucci et al (41); during the course of CypA-promoted angiogenesis, low CypA concentrations promote the proliferation of bone marrow-derived cluster of differentiation (CD)117+ cells, whereas high CypA concentrations stimulate CD117+ cell death. Therefore, it is reasonable to speculate that, during Ang II-induced cardiac remodeling, the positive association between CypA and ROS may result in CypA and ROS accumulation. A continuous increase of these two molecules promotes cell death, thereby accelerating the processes of cardiac reconstruction. An in vitro study suggested that CypA is an oxidative stress-inducing factor, whereas in vascular smooth muscle cells, ROS activates a vesicle-containing pathway that leads to CypA secretion; this process requires the involvement of Rho GTPases (including transforming protein RhoA, cell division control protein 42 homolog and Ras-related C3 botulinum toxin substrate 1) (42).
In conclusion, to the best of our knowledge, the present study is the first to demonstrate that Ang II may induce CypA expression and secretion by enhancing ROS production. In addition, Ang II promoted ROS formation primarily via the AT2R signaling pathway in rat cardiomyocytes. These results provided further insight into the role of AT2R signaling in Ang II-induced myocardial hypertrophy and may aid in the development of novel therapeutic strategies for treatment of cardiovascular disease.
Acknowledgements
The authors would like to thank Professor Chenggang Zou from the State Key Laboratory of Natural Resource Conservation and Utilization (Kunming, China) for providing the H9C2 cells. The authors are grateful to Dr Zhiwen Zhu from Tsinghua-Peking Center for Life Sciences, School of Life Sciences, Tsinghua University (Beijing, China) for discussions regarding the present study.
Glossary
Abbreviations
- ROS
reactive oxygen species
- Ang II
angiotensin II
- CypA
cyclophilin A
- AT1R
angiotensin type 1 receptor
- AT2R
angiotensin type 2 receptor
- GEE
glutathione ethyl ester
- AAA
abdominal aortic aneurysm
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant no. 31360454 and 31560528) and a grant (grant no. 2012FB120) from the Yunnan Department of Science and Technology.
Availability of data and materials
The analyzed data sets generated during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
ML and QH conceived the present study. HT and DY performed the experiments and collected and analyzed the data. HT and ML analyzed the data, wrote the manuscript and discussed the paper. YH and PZ performed cell culture experiments. YY provided guidance on fluorescence microscopy and analyzed the fluorescence data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Satoh K, Shimokawa H, Berk BC. Cyclophilin A-promising new target in cardiovascular therapy. Circ J. 2010;74:2249–2256. doi: 10.1253/circj.CJ-10-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: Experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Diwan A, Dorn GW., II Decompensation of cardiac hypertrophy: Cellular mechanisms and novel therapeutic targets. Physiology (Bethesda) 2007;22:56–64. doi: 10.1152/physiol.00033.2006. [DOI] [PubMed] [Google Scholar]
- 4.Olson ER, Shamhart PE, Naugle JE, Meszaros JG. Angiotensin II-induced extracellular signal-regulated kinase 1/2 activation is mediated by protein kinase Cdelta and intracellular calcium in adult rat cardiac fibroblasts. Hypertension. 2008;51:704–711. doi: 10.1161/HYPERTENSIONAHA.107.098459. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Matsumoto E, Sasaki S, Kinoshita H, Kito T, Ohta H, Konishi M, Kuwahara K, Nakao K, Itoh N. Angiotensin II-induced cardiac hypertrophy and fibrosis are promoted in mice lacking Fgf16. Genes Cells. 2013;18:544–553. doi: 10.1111/gtc.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Handschumacher R, Harding M, Rice J, Drugge R, Speicher D. Cyclophilin: A specific cytosolic binding protein for cyclosporin a. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 8.Göthel S, Marahiel M. Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts. Cell Mol Life Sci. 1999;55:423–436. doi: 10.1007/s000180050299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ivery MT. Immunophilins: Switched on protein binding domains? Med Res Rev. 2000;20:452–484. doi: 10.1002/1098-1128(200011)20:6<452::AID-MED2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Li C, Cai C, Xiang J, Cao Z. Cyclophilin a (CypA) is associated with the inflammatory infiltration and alveolar bone destruction in an experimental periodontitis. Biochem Biophys Res Commun. 2010;391:1000–1006. doi: 10.1016/j.bbrc.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Seizer P, Geisler T, Bigalke B, Schneider M, Klingel K, Kandolf R, Stellos K, Schreieck J, Gawaz M, May AE. EMMPRIN and its ligand cyclophilin a as novel diagnostic markers in inflammatory cardiomyopathy. Int J Cardiol. 2013;163:299–304. doi: 10.1016/j.ijcard.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 12.Seizer P, Klingel K, Sauter M, Westermann D, Ochmann C, Schönberger T, Schleicher R, Stellos K, Schmidt EM, Borst O, et al. Cyclophilin a affects inflammation, virus elimination and myocardial fibrosis in coxsackievirus B3-induced myocarditis. J Mol Cell Cardiol. 2012;53:6–14. doi: 10.1016/j.yjmcc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Takapoo M, Chamseddine AH, Bhalla RC, Miller FJ., Jr Glutathione peroxidase-deficient smooth muscle cells cause paracrine activation of normal smooth muscle cells via cyclophilin a. Vascul Pharmacol. 2011;55:143–148. doi: 10.1016/j.vph.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jabri B, Barreiro LB. Don't move: LRRK2 arrests NFAT in the cytoplasm. Nat Immunol. 2011;12:1029–1030. doi: 10.1038/ni.2139. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Pink MD, Murphy JG, Stein A, Dell'Acqua ML, Hogan PG. Balanced interactions of calcineurin with AKAP79 regulate Ca2+-calcineurin-NFAT signaling. Nat Struct Mol Biol. 2012;19:337–345. doi: 10.1038/nsmb.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Candé C, Vahsen N, Kouranti I, Schmitt E, Daugas E, Spahr C, Luban J, Kroemer RT, Giordanetto F, Garrido C, et al. AIF and cyclophilin a cooperate in apoptosis-associated chromatinolysis. Oncogene. 2004;23:1514–1521. doi: 10.1038/sj.onc.1207279. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Shimazaki H, Kimura M, Izuta H, Tsuruma K, Shimazawa M, Hara H. Apoptosis-inducing factor and cyclophilin a cotranslocate to the motor neuronal nuclei in amyotrophic lateral sclerosis model mice. CNS Neurosci Ther. 2011;17:294–304. doi: 10.1111/j.1755-5949.2010.00180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, Neagu MR, Nilsson M, Eriksson PS, Hagberg H, et al. Cyclophilin a participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007;204:1741–1748. doi: 10.1084/jem.20070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piao CS, Loane DJ, Stoica BA, Li S, Hanscom M, Cabatbat R, Blomgren K, Faden AI. Combined inhibition of cell death induced by apoptosis inducing factor and caspases provides additive neuroprotection in experimental traumatic brain injury. Neurobiol Dis. 2012;46:745–758. doi: 10.1016/j.nbd.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He H, Zhou D, Fan W, Fu X, Zhang J, Shen Z, Li J, Li J, Wu Y. Cyclophilin a inhibits rotavirus replication by facilitating host IFN-I production. Biochem Biophys Res Commun. 2012;422:664–669. doi: 10.1016/j.bbrc.2012.05.050. [DOI] [PubMed] [Google Scholar]
- 21.Zhou D, Mei Q, Li J, He H. Cyclophilin a and viral infections. Biochem Biophys Res Commun. 2012;424:647–650. doi: 10.1016/j.bbrc.2012.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venkatesan B, Valente AJ, Prabhu SD, Shanmugam P, Delafontaine P, Chandrasekar B. EMMPRIN activates multiple transcription factors in cardiomyocytes, and induces interleukin-18 expression via Rac1-dependent PI3K/Akt/IKK/NF-kappaB andMKK7/JNK/AP-1 signaling. J Mol Cell Cardiol. 2010;49:655–663. doi: 10.1016/j.yjmcc.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Gasparo M, Siragy HM. The AT2 receptor: Fact, fancy and fantasy. Regul Pept. 1999;81:11–24. doi: 10.1016/S0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 24.Ma Y, Kong L, Qi S, Wang D. Atorvastatin blocks increased l-type Ca2+current and cell injury elicited by angiotensin II via inhibiting oxide stress. Acta Biochim Biophys Sin (Shanghai) 2016;48:378–384. doi: 10.1093/abbs/gmw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martens GA, Cai Y, Hinke S, Stangé G, Van de Casteele M, Pipeleers D. Glucose suppresses superoxide generation in metabolically responsive pancreatic beta cells. J Biol Chem. 2005;280:20389–20396. doi: 10.1074/jbc.M411869200. [DOI] [PubMed] [Google Scholar]
- 26.Perrotta I, Aquila S. The role of oxidative stress and autophagy in atherosclerosis. Oxid Med Cell Longev. 2015;2015:130315. doi: 10.1155/2015/130315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehta PK, Griendling KK. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 28.Satoh K, Nigro P, Matoba T, O'Dell MR, Cui Z, Shi X, Mohan A, Yan C, Abe J, Illig KA, Berk BC. Cyclophilin a enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009;15:649–656. doi: 10.1038/nm.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda H, Yamamoto K, Kohno I, Hafsi L, Itoh N, Nakagawa S, Kanagawa N, Suzuki K, Uno T. Design of a practical fluorescent probe for superoxide based on protection-deprotection chemistry of fluoresceins with benzenesulfonyl protecting groups. Chemistry. 2007;13:1946–1954. doi: 10.1002/chem.200600522. [DOI] [PubMed] [Google Scholar]
- 30.Maeda H, Yamamoto K, Nomura Y, Kohno I, Hafsi L, Ueda N, Yoshida S, Fukuda M, Fukuyasu Y, Yamauchi Y, Itoh N. A design of fluorescent probes for superoxide based on a nonredox mechanism. J Am Chem Soc. 2005;127:68–69. doi: 10.1021/ja047018k. [DOI] [PubMed] [Google Scholar]
- 31.Chang MC, Tsai YL, Chen YW, Chan CP, Huang CF, Lan WC, Lin CC, Lan WH, Jeng JH. Butyrate induces reactive oxygen species production and affects cell cycle progression in human gingival fibroblasts. J Periodontal Res. 2013;48:66–73. doi: 10.1111/j.1600-0765.2012.01504.x. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Shimoyama T, Suzuki K, Umeda T, Nakaji S, Sugawara K. Effect of sodium butyrate on reactive oxygen species generation by human neutrophils. Scand J Gastroenterol. 2001;36:744–750. doi: 10.1080/003655201300192012. [DOI] [PubMed] [Google Scholar]
- 33.Wang LP, Wang Y, Zhao LM, Li GR, Deng XL. Angiotensin II upregulates K(Ca)3.1 channels and stimulates cell proliferation in rat cardiac fibroblasts. Biochem Pharmacol. 2013;85:1486–1494. doi: 10.1016/j.bcp.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 34.Seko Y, Fujimura T, Taka H, Mineki R, Murayama K, Nagai R. Hypoxia followed by reoxygenation induces secretion of cyclophilin a from cultured rat cardiac myocytes. Biochem Biophys Res Commun. 2004;317:162–168. doi: 10.1016/j.bbrc.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 35.Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med. 2011;13:e11. doi: 10.1017/S1462399411001815. [DOI] [PubMed] [Google Scholar]
- 36.Mercure C, Yogi A, Callera GE, Aranha AB, Bader M, Ferreira AJ, Santos RA, Walther T, Touyz RM, Reudelhuber TL. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 37.Vinturache AE, Smith FG. Angiotensin type 1 and type 2 receptors during ontogeny: Cardiovascular and renal effects. Vascul Pharmacol. 2014;63:145–154. doi: 10.1016/j.vph.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Lopez JJ, Lorell BH, Ingelfinger JR, Weinberg EO, Schunkert H, Diamant D, Tang SS. Distribution and function of cardiac angiotensin AT1- and AT2-receptor subtypes in hypertrophied rat hearts. Am J Physiol. 1994;267:H844–H852. doi: 10.1152/ajpheart.1994.267.2.H844. [DOI] [PubMed] [Google Scholar]
- 39.Ohkubo N, Matsubara H, Nozawa Y, Mori Y, Murasawa S, Kijima K, Maruyama K, Masaki H, Tsutumi Y, Shibazaki Y, et al. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.CIR.96.11.3954. [DOI] [PubMed] [Google Scholar]
- 40.Thakur S, Du J, Hourani S, Ledent C, Li JM. Inactivation of adenosine A2A receptor attenuates basal and angiotensin II-induced ROS production by Nox2 in endothelial cells. J Biol Chem. 2010;285:40104–40113. doi: 10.1074/jbc.M110.184606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perrucci GL, Straino S, Corlianò M, Scopece A, Napolitano M, Berk BC, Lombardi F, Pompilio G, Capogrossi MC, Nigro P. Cyclophilin a modulates bone marrow-derived CD117(+) cells and enhances ischemia-induced angiogenesis via the SDF-1/CXCR4 axis. Int J Cardiol. 2016;212:324–335. doi: 10.1016/j.ijcard.2016.03.082. [DOI] [PubMed] [Google Scholar]
- 42.Nagai T, Anzai T, Kaneko H, Mano Y, Anzai A, Maekawa Y, Takahashi T, Meguro T, Yoshikawa T, Fukuda K. C-reactive protein overexpression exacerbates pressure overload-induced cardiac remodeling through enhanced inflammatory response. Hypertension. 2011;57:208–215. doi: 10.1161/HYPERTENSIONAHA.110.158915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the present study are available from the corresponding author upon reasonable request.


