Abstract
We conducted a comprehensive analysis to evaluate clinical utility of decarboxylation prothrombin combined with α-fetoprotein (AFP) for diagnosing primary hepatocellular carcinoma (HCC). Systematical searches were performed in PubMed, Web of Science, China National Knowledge Internet, and Wangfang databases. The bivariate random-effect model was used to calculate the pooled sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood, diagnostic odds ratio (DOR), and summary area under the curve (AUC). Fourteen studies were included in the meta-analysis. For decarboxylation prothrombin, the overall pooled parameters are as follows: sensitivity: 79% (95% confidence interval (CI): 74–84%), specificity: 91% (95%CI: 87–93%), PLR: 8.42 (95%CI: 5.79–12.23), negative likelihood ratio (NLR): 0.23 (95%CI: 0.17–0.30), DOR: 37.09 (95%CI: 21.37–64.36), summary AUC: 0.92 (95%CI: 0.89–0.94); for combined diagnostic, the overall pooled parameters were as follows: sensitivity: 91% (95%CI: 85–95%), specificity: 83% (95%CI: 74–89%), PLR: 5.26 (95%CI: 3.53–7.83), NLR: 0.11 (95%CI: 0.07–0.18), DOR: 47.14 (95%CI: 30.09–73.85), summary AUC: 0.94 (95%CI: 0.91–0.95). The serum decarboxylation prothrombin showed a relatively higher diagnostic specificity for primary HCC and decarboxylation prothrombin combined with AFP exhibited can improve sensitivity for HCC than any of the biomarkers alone.
Keywords: Alpha-fetoprotein, Decarboxylation prothrombin, Hepatocellular cancer, Meta-analysis
Introduction
The hepatocellular carcinoma (HCC) is the fifth most common cancer in men and seventh in women (17.4 and 6.7 per 100000 persons per year, respectively), and ~750 thousand new cases were diagnosed each year around the world [1]. Seventy percent of all new HCCs worldwide occur in Asia, with patients in the People’s Republic of China accounting for 55% of liver cancer deaths each year [2]. HCC mostly occurs in people with cirrhosis of the liver, and so risk factors generally include factors which cause chronic liver disease that may lead to cirrhosis: chronic viral hepatitis, alcohol abuse, aflatoxin, non-alcoholic steatohepatitis, α1-antitrypsin deficiency, and so on [3]. HCC is the leading cause of cancer-related deaths in some developing countries, with higher degree of malignancy and poor prognosis (almost without exception, those who develop HCC each year die within 12 months), and severely threatens the public health. The data from epidemiological investigations indicate that HCC is a complex and multistage process disease with high incidence, high mortality, highly malignant and invasive, and metastatic [4]. The mortality of HCC was the second to gastric cancer [5]. However, effective treatment with less side effects is scarce [6]. More and more researchers pay more attention on early diagnostic with the expectation of early intervention. Serum biomarkers were still commonly used for tumor diagnostic. Some traditional tumor biomarkers such as α-fetoprotein (AFP) had been widely used in clinical practice. Many studies also reported the diagnostic accuracy of AFP for HCC. The practitioner found that solely AFP still had some false positive (FP) or false negative (FN) rate. It was reported that the AFP level would increase when patients experienced some special disease activities such as active liver injury, some gastrointestinal tumors, and even during pregnancy. Therefore, we further considered the combined diagnostic of serum biomarkers [7]. Des-γ-carboxy-prothrombin (DCP) was a kind of des-γ protein induced by vitamin K absence or antagonist-II (PIVKA-II) and was first suggested to have high sensitivity for HCC in 1984. Up to now, lots of studies have reported that the diagnostic ability of DCP was significantly higher than AFP for HCC, and combined application can further improve the accuracy [8–10]. However, the accurate estimations of DCP and combined diagnostic have not been reported because single study still has some limitations such as population, sample size, and application of different gold standards. We conducted a comprehensive analysis to evaluate clinical utility of decarboxylation prothrombin combined with AFP for diagnosing HCC.
Materials and methods
Search strategy
We performed literatures search in PubMed, Web of Science, China National Knowledge Internet, and Wangfang databases from 31 May 1984 to 20 March 2018. We used the following search terms: DCP, protein induced by vitamin K absence or antagonist-II, primary HCC, diagnostic test, DCP, and AFP. Two investigators independently searched the literatures. The search was limited to English and Chinese publications. We also retrieved the references lists of some reviews and articles for potentially included studies. The search strategy was presented in Supplementary material S1.
Criteria for inclusion and exclusion
The included studies must meet the following criteria: (i) all patients were confirmed by pathologic biopsy and did not receive relevant treatment; (ii) evaluating diagnostic ability of DCP or AFP, or both for HCC; (iii) providing the four-folds data for further analysis (TP, true positive; FP, false positive; TN, true negative; FN). Studies which could not supply enough data, replicates with secondary HCC or received treatment were excluded. Non-diagnostic studies, reviews, comments, case reports, meeting materials, and animal experiments were also excluded. For replicates’ studies, the latest data were used.
Data extraction and assessment of quality
A standard Excel datasheet was built for information extraction. Two investigators independently conducted the data extraction. For each included study, the following information was extracted: the first author of study, year of publication, source of sample, methods of examination, gold standard, sample size (case/control), total sample size, index, four-folds data (TP, FP, TN, FN), sensitivity, and specificity. We used the updated Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) to assess the qualities of selected studies. This scale evaluated the quality of study from four domains: patient selection index test, reference standard, and flow of patients. Each domain item was further broken down into several subitems designated as low risk, high risk, or unclear risk according to the study [11].
Statistical analysis
We used the Stata 14 version (StataCorp LP, College Station, TX, U.S.A.) to conduct all the analyses. Spearman correlation coefficient was used to evaluate the threshold effect [12]. The heterogeneity was assessed via Chi-square and I2 statistic. P<0.05 or I2 > 50% means the existence of heterogeneity [13]. A bivariate random-effect model was used to calculate all pooled parameters including sensitivity, specificity, positive likelihood ratios (PLRs), negative likelihood ratios (NLRs), diagnostic odds ratios (DORs), and their 95% confidence interval (CI) [14]. We also estimated the summary receiver operator characteristic curve to evaluate the diagnostic ability. The area under the curve (AUC) reflects the discrimination ability. The AUC ranged from 0 to 1. The diagnostic ability could be useful when AUC > 0.5 or represents a poor test [15,16]. We used linear regression test to evaluate the publication bias [17]. P<0.05 was considered as a significant level.
Results
Study selection
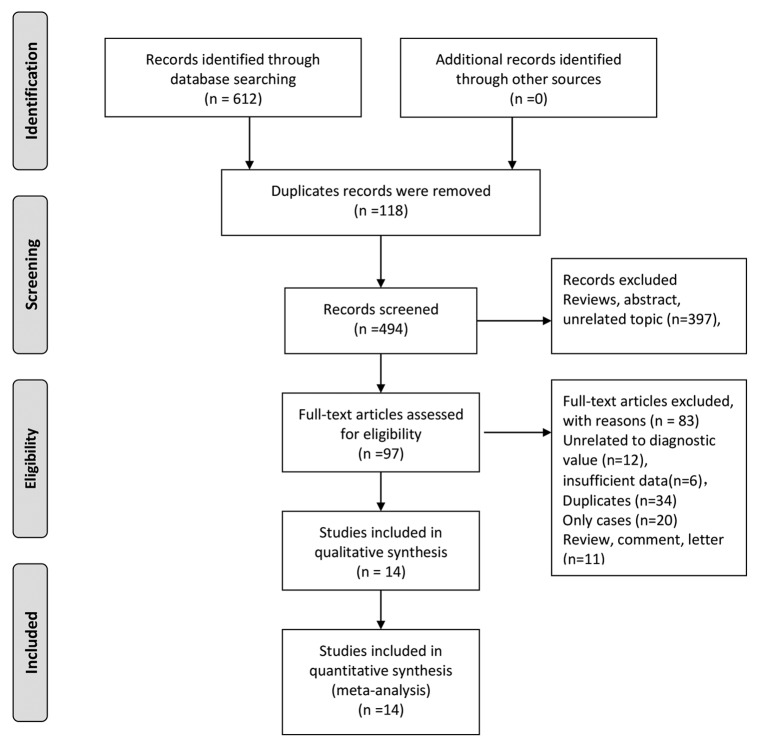
Two investigators performed literature searches in the online databases. Our initial search returned 612 records and identified zero record through additional methods. One hundred and eighteen records were excluded because of duplicates. Three hundred and ninety-seven records were excluded because of different kinds of reasons such as reviews and unrelated topics. Ninety-seven articles were assessed via full-text articles, and 83 articles were excluded, including 12 unrelated to diagnostic value, 6 records with insufficient data, 34 duplicates, 20 case reports and 11 reviews, comments, and letters. Finally, 14 studies were included in the qualitative and quantitative syntheses [8,16–28]. Figure 1 represented the flow chart of study selection.
Figure 1. The flow chart of study selection.
General characteristics and quality of included studies
All included studies were published from 2012 to 2016. Samples of all studies were from serum. All study patients were confirmed by pathologic biopsy. All studies used the enzyme-linked immunosorbent method to detect decarboxylation prothrombin, and most of studies applied ECL immunoassay to detect AFP. The sample size ranged from 126 to 635, with a total number of 8084. Of all the included studies, 14 studies solely evaluated the diagnostic ability of DCP for HCC, and 14 studies used AFP combined with DCP for diagnostic. The sensitivity of single studies ranged from 58 to 97%, and specificity ranged from 56 to 97%. The detailed characteristics were presented in Tables 1 and 2. All the included studies received moderately high scores from the QUADAS-2 quality assessments. The assessment of quality was presented in Supplementary materials S2 and S3.
Table 1. General characteristics included in the meta-analysis.
| Author | Year of publication | Source of sample | Reagent kits and machine | Cancer stage | Methods of examination | Gold standard | ||
|---|---|---|---|---|---|---|---|---|
| DCP | AFP | DCP | AFP | |||||
| Gao [18] | 2012 | Serum | Alisei | Elecsys2010 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Liu [19] | 2012 | Serum | Human DCP kit | Human-L3 kit | All stages | ELISA | ELISA | Pathologic biopsy |
| Li [20] | 2013 | Serum | – | ABBOTT i2000SR | – | ELISA | ELISA | Pathologic biopsy |
| Song [21] | 2014 | Serum | ED036, Eissai | – | – | ELISA | ECLIA | Pathologic biopsy |
| Pu [22] | 2014 | Serum | LUMI-PULSE G1200 | Cobase 601 | – | ELISA | ECLIA | Pathologic biopsy |
| Zhu [23] | 2014 | Serum | LUMI-PULSE G1200 | Cobase 601 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Fu [24] | 2015 | Serum | ELISA | ECLIA | Pathologic biopsy | |||
| Lin [8] | 2015 | Serum | LUMI-PULSE G1200 | Cobase 601 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Yu [25] | 2016 | Serum | LUMI-PULSE G1200 | AFP Reagent kit, ARTHITECT i2000 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Shen [16] | 2016 | Serum | LUMI-PULSE G1200 | – | – | ELISA | ELISA | Pathologic biopsy |
| Zheng [26] | 2016 | Serum | Sigma RS-232 | Sigma-RS-232 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Huang [17] | 2016 | Serum | LUMI-PULSE G1200 | Cobase 601 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Lu [27] | 2016 | Serum | LUMI-PULSE G1200 | Cobase 601 | All stages | ELISA | ECLIA | Pathologic biopsy |
| Huang [28] | 2016 | Serum | LUMI-PULSE G1200 | Cobase 601 | All stages | ELISA | ECLIA | Pathologic biopsy |
Abbreviation: ECLIA, electrochemiluminescence immunoassay.
Table 2. Parameter of included studies in the meta-analysis.
| Author | Year | Sample size (case/control) | Total | Index | TP | FP | FN | TN | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Gao [18] | 2012 | 76/96 | 173 | DCP | 57 | 2 | 19 | 94 | 75 | 97 |
| Liu [19] | 2012 | 66/60 | 126 | DCP | 58 | 3 | 8 | 57 | 88 | 95 |
| Li [20] | 2013 | 198/414 | 612 | DCP | 139 | 82 | 59 | 332 | 70 | 80 |
| Song [21] | 2014 | 550/85 | 635 | DCP | 393 | 10 | 157 | 75 | 71 | 88 |
| Pu [22] | 2014 | 100/265 | 365 | DCP | 74 | 27 | 26 | 238 | 74 | 89 |
| Zhu [23] | 2014 | 136/192 | 328 | DCP | 115 | 18 | 21 | 174 | 84 | 90 |
| Fu [24] | 2015 | 100/100 | 200 | DCP | 76 | 11 | 24 | 89 | 76 | 88 |
| Lin [8] | 2015 | 100/190 | 290 | DCP | 94 | 21 | 6 | 169 | 94 | 88 |
| Yu [25] | 2016 | 45/138 | 183 | DCP | 26 | 10 | 19 | 128 | 58 | 92 |
| Shen [16] | 2016 | 103/156 | 259 | DCP | 72 | 11 | 31 | 145 | 70 | 92 |
| Zheng [26] | 2016 | 70/150 | 220 | DCP | 63 | 4 | 7 | 146 | 90 | 97 |
| Huang [17] | 2016 | 100/281 | 381 | DCP | 80 | 20 | 20 | 261 | 80 | 93 |
| Lu [27] | 2016 | 82/95 | 177 | DCP | 59 | 32 | 23 | 63 | 72 | 66 |
| Huang [28] | 2016 | 80/188 | 268 | DCP | 72 | 28 | 8 | 160 | 90 | 85 |
| Liu [19] | 2012 | 66/60 | 126 | AFP + DCP | 66 | 22 | 0 | 38 | 100 | 63 |
| Li [20] | 2013 | 198/414 | 612 | AFP + DCP | 172 | 97 | 26 | 317 | 87 | 76 |
| Song [21] | 2014 | 550/85 | 635 | AFP + DCP | 456 | 13 | 94 | 72 | 82 | 84 |
| Pu [22] | 2014 | 100/265 | 365 | AFP + DCP | 81 | 63 | 19 | 202 | 81 | 76 |
| Zhu [23] | 2014 | 136/192 | 328 | AFP + DCP | 126 | 20 | 10 | 172 | 92 | 89 |
| Fu [24] | 2015 | 100/100 | 200 | AFP + DCP | 61 | 1 | 39 | 99 | 61 | 99 |
| Lin [8] | 2015 | 100/190 | 290 | AFP + DCP | 98 | 39 | 2 | 151 | 98 | 79 |
| Yu [25] | 2016 | 45/138 | 183 | AFP + DCP | 40 | 20 | 5 | 118 | 88 | 85 |
| Shen [16] | 2016 | 103/156 | 259 | AFP + DCP | 87 | 18 | 16 | 138 | 84 | 88 |
| Zheng [26] | 2016 | 70/150 | 220 | AFP + DCP | 67 | 53 | 3 | 97 | 95 | 64 |
| Huang [17] | 2016 | 100/281 | 381 | AFP + DCP | 90 | 24 | 10 | 257 | 90 | 91 |
| Huang [28] | 2016 | 80/188 | 268 | AFP + DCP | 78 | 82 | 2 | 106 | 97 | 56 |
Pooled diagnostic accuracy of DCP for HCC
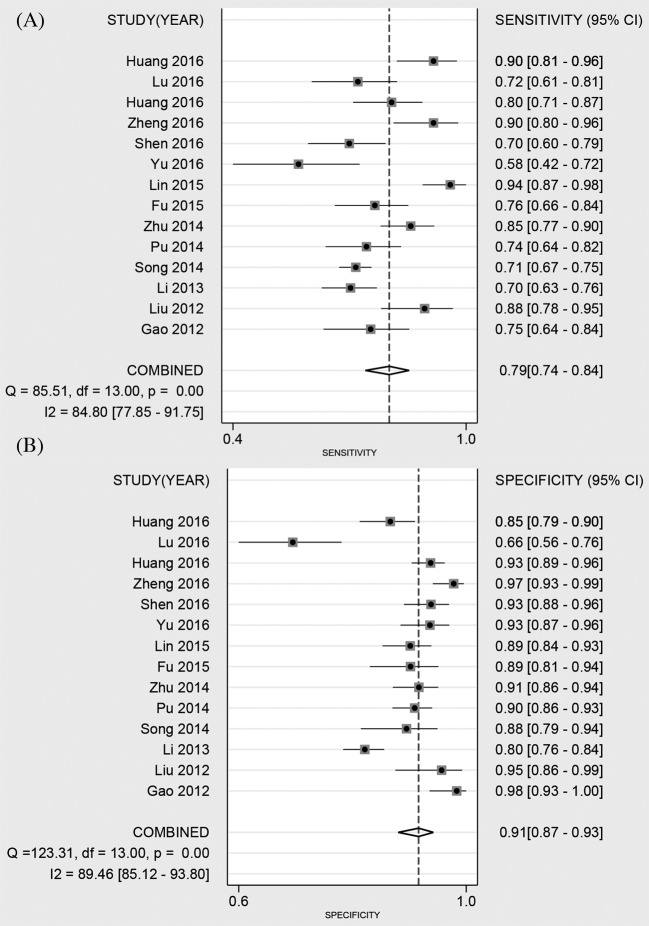
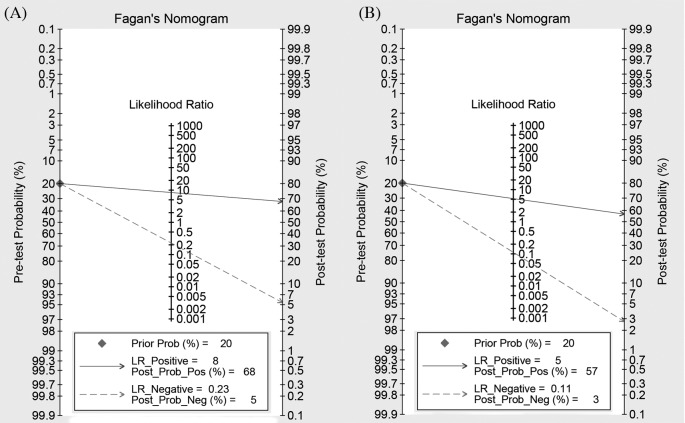
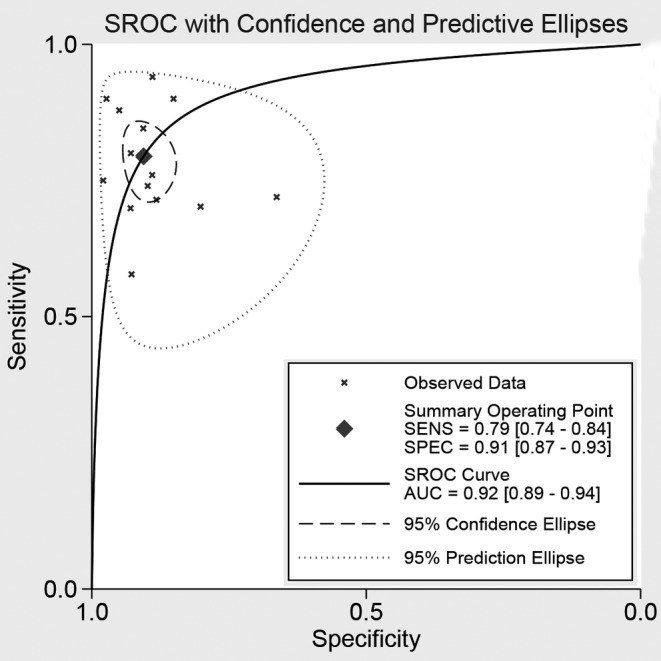
Fourteen studies reported the diagnostic ability of DCP for HCC, including 1806 positive subjects and 2410 negative subjects. The Spearman test showed no threshold effect (r = −0.134, P=0.647). The heterogeneity within studies was high (I2 = 84.80, P<0.001), and the random-effect model was used. The pooled sensitivity was 79% (95%CI: 74–84%, Figure 2A), and the specificity was 91% (95%CI: 87–93%, Figure 2B). The pooled PLR and NLR were 8.42 (95%CI: 5.79–12.23) and 0.23 (95%CI: 0.17–0.30), respectively. The DOR was 37.09 (95%CI: 21.37–64.36). The Fagan diagram for evaluating the diagnostic ability of DCP for HCC was shown in Figure 6A. The pre-test probability was ~20%, and the post-test probability was 69% with a PLR of 8. The Figure 4 given the summary receiver operating characteristic (SROC) curve. The pooled AUC was 0.92 (95%CI: 0.89–0.94), which suggested that DCP had a relatively high diagnostic ability for HCC.
Figure 2. Forest plot of pooled sensitivity (A) and specificity (B) of DCP for primary HCC.
Figure 6. Fagan diagram evaluating the overall diagnostic value of DCP combined with AFP for cancer ((A) DCP; (B) DCP and AFP).
Figure 4. The symmetric ROC curve of DCP for cancer.
Pooled diagnostic accuracy of DCP combined AFP for HCC
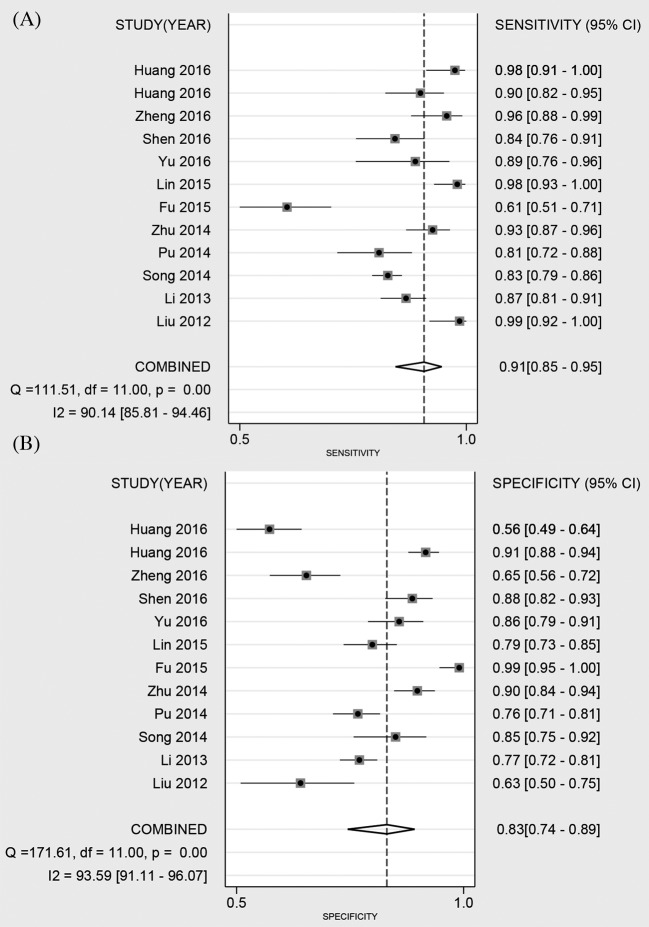
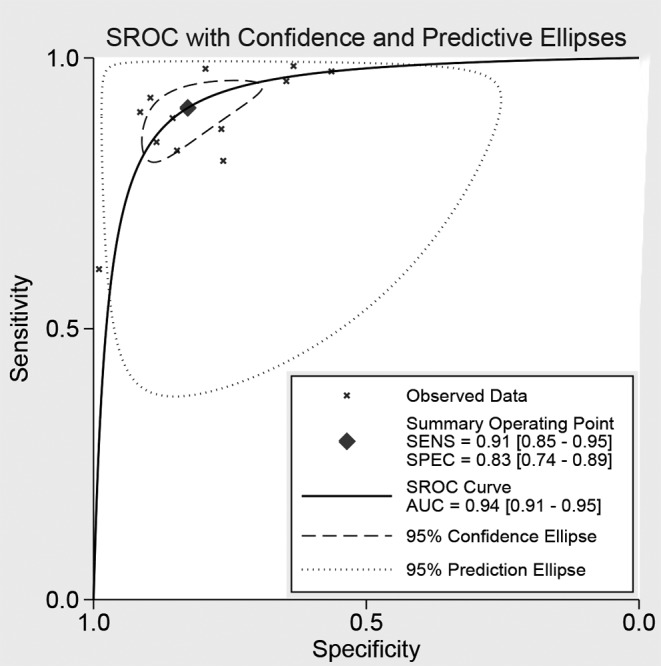
Twelve studies reported the diagnostic ability of DCP for HCC, including 1649 positive subjects and 2219 negative subjects. The Spearman test found no threshold effect (r = 0.510, P=0.089). The heterogeneity within studies was high (I2 = 90.14–93.59%), the random effect model was used. The pooled sensitivity was 91% (95%CI: 85–95%, Figure 3A), and the specificity was 83% (95%CI: 74–89%, Figure 3B). The pooled PLR and NLR were 5.26 (95%CI: 3.53–7.83) and 0.11 (95%CI: 0.07–0.18), respectively. The DOR was 47.14 (95%CI: 30.09–73.85). The Fagan diagram for evaluating the diagnostic ability of DCP for HCC was presented in Figure 6B. The pre-test probability was ~20%, and the post-test probability was 59% with a PLR of 5. Figure 5 gave the SROC curve. The pooled AUC was 0.94 (95%CI: 0.91–0.95), which suggested that DCP combined with AFP had a high diagnostic ability for HCC.
Figure 3. Forest plot of pooled sensitivity (A) and specificity (B) of DCP combined with AFP for primary HCC.
Figure 5. The symmetric ROC curve of DCP combined with AFP for cancer.
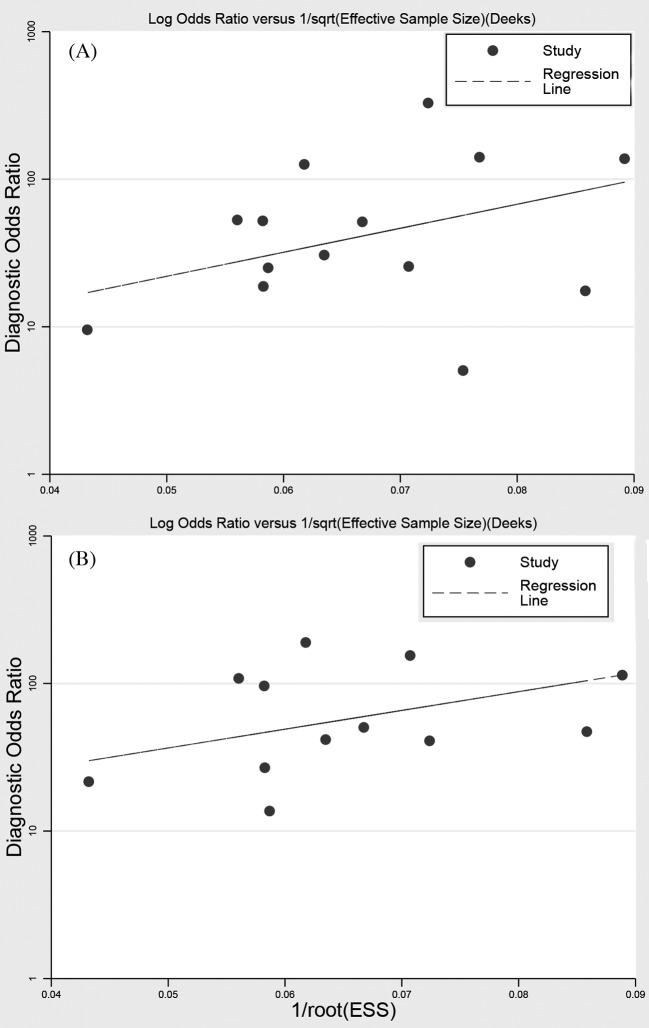
Publication bias
For DCP and DCP combined with AFP, we used the linear regression to evaluate the publication bias. As shown in Figure 7, the P-value of slope coefficient of publication bias was 0.133 for DCP, and 0.184 for DCP combined with AFP. No publication bias was found, and the present results were stable.
Figure 7. Line regression plot of publication bias ((A) DCP; (B) DCP, and AFP).
Discussion
This is the first meta-analysis to evaluate the clinical utility of decarboxylation prothrombin combined with AFP for diagnosing primary HCC. All studies included in this analysis are diagnostic tests with moderately high quality. The data could give greater power to assess the diagnostic accuracy of AFP and combined DCP for primary HCC. We pooled the sensitivity, specificity, and summary AUC that used DCP and combined AFP for diagnosing HCC through bivariate random effect models and found that both DCP and DCP combined AFP have relatively high diagnostic ability for HCC. The combined diagnostic can provide greater sensitivity, and the DCP have higher specificity.
Single tumor biomarker has some limitations for clinical diagnostic. In the past, AFP was a prior option for diagnosing primary HCC. Previous studies reported AFP could appear at 8–11 months before symptoms [29]. However, the sensitivity of AFP was low for early screening, and 30–35% of patients with primary HCC cannot detect AFP. Besides, patients with chronic hepatitis or liver cirrhosis also have higher level of AFP. It was estimated that 85% patients with HCC were followed by chronic hepatitis [30]. Missed diagnostic can happen easily under these situations. Thrombin is the precursor of the inactive thrombin, which is synthesized by the liver and converted into an activated thrombin form through vitamin K, as the auxiliary factor γ carboxylation. When the liver becomes cancerous, the synthesis of γ carboxylic acid, which is less than normal structure, forms an abnormal prothrombin [26]. The DCP of normal blood serum is very low in content and cannot be detected in general. However, the content of DCP in patients with liver disease increases, which can be detected by enzyme-linked immunoassay [31]. The dickkopf-1 or DCP alone has shown less than satisfactory sensitivity and specificity for the detection of HCC [32].
In the present comprehensive analyses, we found that DCP achieved the overall pooled sensitivity of 79% (95%CI: 74–84%) and specificity of 91% (95%CI: 87–93%). This result suggested that the diagnostic ability of DCP for HCC was moderate, but still lower than combined AFP with sensitivity of 91% (95%CI: 85–95%), specificity of 83% (95%CI: 74–89%). For AUC, the AUC of DCP was 0.92 (95%CI: 0.89–0.94) while the combined AFP was 0.94 (95%CI: 0.91–0.95). Moreover, the single DCP had higher NLR (0.23 compared with 0.11) than combined AFP but lower PLR (37.09 compared with 47.14). Higher positive likelihood meant higher specificity and lower NLR meant lower FN rate. These results indicated that combined AFP has higher diagnostic ability. In fact, many previous studies also showed combined application can improve diagnostic ability of cancer. Lu et al. [33] found that combined detection of plasma miR-127-3p and HE4 improves the diagnostic efficacy of breast cancer. Some indictors achieved higher diagnostic ability when combined with other biomarkers. Lubowicka reported that MMP-9 had shown the usefulness in the diagnosis of cervical cancer, but only in the combined analysis with CA 125 [34]. The AUC revealed that DCP had a better accuracy than AFP in diagnosis of HCC (0.891 compared with 0.813, P<0.05). The analysis of indicators by logistic regression model indicated that the receiver operator characteristic (ROC) result of joint predictor (DCP, AFP joint detection) was 0.932, superior to the DCP or AFP alone [18]. The combined diagnostic ability had obvious advantage.
The application of serum markers is still important for HCC diagnostic. The AFP alone has some limitations, the AFP level of 30–40% patients with HCC was not significantly elevated while the increased AFP level was found in normal health [35]. Some patients with cirrhosis and/or hepatic inflammation can have an elevated AFP, even without the presence of a tumor. The test had a sensitivity of 39–65%, a specificity of 76–94%, and a positive predictive value of 9–50% for the presence of HCC in previously published studies [36]. Therefore, new serum markers are required. The combined diagnostic ability has obvious advantage. Though the diagnostic meaning of some serum markers of HCC has been downgraded and the importance of imaging had been highlighted, the serum markers still can be used in the clinical diagnostic of HCC in the absence of sensitive imaging methods. For early screening, serum markers and imaging should be considered together.
Our study still has some limitations. First, our study calculated the differences of examination methods, it was reported that diagnostic of DCP from ELSIA was different electrochemiluminescence immunoassay (ECLIA). Second, the control population of some studies may include some patients with hepatitis B infection, this may generate some bias. Third, the structure of population in different studies was different such as age, gender ratio. This could be one of the reasons that caused heterogeneity. Finally, the results showed there was no threshold effect. But the heterogeneity within studies were high, which meant the heterogeneity caused by other sources. The available information was limited, and meta-regression and subgroup analyses cannot be further conducted.
In conclusion, the serum DCP has a relatively higher diagnostic specificity for HCC. The combined diagnostic of AFP and DCP can improve sensitivity for HCC than any of the biomarkers alone. The tests are convenient and inexpensive, and may serve as a valuable addition to current options for the diagnostic of HCC.
Supporting information
supplementary Figure.
Acknowledgments
We thank the colleagues of Department of Oncology, Xiangya Hospital, Central South University.
Abbreviations
- AFP
α-fetoprotein
- AUC
area under the curve
- CI
confidence interval
- DCP
des-γ-carboxy-prothrombin
- DOR
diagnostic odds ratio
- ELISA
enzyme linked immunosorbent assay
- FN
false negative
- FP
false positive
- HCC
hepatocellular carcinoma
- HE4
human epididymis protein
- MMP-9
matrix metalloprotein-9
- NLR
negative likelihood ratio
- PIVKA-II
protein induced by Vitamin K absence or antagonist-II
- PLR
positive likelihood ratio
- QUADAS-2
quality assessment of diagnostic accuracy study 2
- ROC
receiver operating characteristic
- SROC
summary ROC
- TP
true positive
- TN
true negative
Author contribution
Z.L. and J.F. conceived and designed the research. Y.L. and Z.L. analyzed the data. N.L. and Z.L. created all tables and figures. J.F. drafted the manuscript. Z.L. and N.L. made critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the China Postdoctoral Science Foundation [grant number 2017M612597]; and the National Key Clinical Program (Department of Oncology, Xiangya Hospital Central South) [grant number 26].
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Elserag H.B. (2012) Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142, 1246–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E. and Forman D. (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 3.Kew M.C. (2014) Hepatocellular carcinoma: epidemiology and risk factors. J. Hepatocell. Carcinoma 1, 115–125 10.2147/JHC.S44381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mi F. and Gong L. (2017) Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci. Rep. 37, 10.1042/BSR20170181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohamed A.A., Elbedewy T.A., El-Serafy M., El-Toukhy N., Ahmed W. and Ali E.D.Z. (2015) Hepatitis C virus: a global view. World J. Hepatol. 7, 2676–2680 10.4254/wjh.v7.i26.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xue J., Chen L., Li Z., Hu Y., Yan S. and Liu L. (2015) MicroRNA-433 inhibits cell proliferation in hepatocellular carcinoma by targeting p21 activated kinase (PAK4). Mol. Cell Biochem. 399, 77–86 10.1007/s11010-014-2234-9 [DOI] [PubMed] [Google Scholar]
- 7.Long L.L., Peng P. and Huang Z.K (2017) Early imaging diagnosis of primary liver cancer. Zhonghua Gan Zang Bing Za Zhi 25, 329–332 [DOI] [PubMed] [Google Scholar]
- 8.Lin Y., Chen Y., Chen Y., Hu M., Zhou X. and Luo L. (2015) Clinical value of PIVKA-II in detecting hepatocellular carcinoma. Modern Immunol. 35, 328–333 [Google Scholar]
- 9.Whiting P.F., Rutjes A.W., Westwood M.E., Mallett S., Deeks J.J, Reitsma J.B. et al. (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 155, 529–536 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 10.Hamza T.H., Arends L.R., van Houwelingen H.C. and Stijnen T. (2009) Multivariate random effects meta-analysis of diagnostic tests with multiple thresholds. BMC Med. Res. Methodol. 9, 73 10.1186/1471-2288-9-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins J.P. and Thompson S.G (2002) Quantifying heterogeneity in a meta-analysis. STAT Med. 21, 1539–1558 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 12.Li Z., Zhou Q., Li Y., Fu J., Huang X. and Shen L. (2016) Growth hormone replacement therapy reduces risk of cancer in adult with growth hormone deficiency: a meta-analysis. Oncotarget 7, 81862–81869 10.18632/oncotarget.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Zhou Q., Li Y., Yan S., Fu J., Huang X. et al. (2017) Mean cerebral blood volume is an effective diagnostic index of recurrent and radiation injury in glioma patients: a meta-analysis of diagnostic test. Oncotarget 8, 15642–15650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z., Shen L., Li Y., Chen P. and Chen L. (2016) Clinical utility of microRNA-378 as early diagnostic biomarker of human cancers: a meta-analysis of diagnostic test. Oncotarget 7, 58569–58578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 16.Shen C. and Zheng L. (2016) Application of combined tests of serum PIVKA-II and AFP in the diagnosis of liver cancer. China Modern Doctor 54, 21–23 [Google Scholar]
- 17.Huang C. (2016) Diagnostic value of combination of DCP and AFP for primary hepatocellular carcinoma. Liver 21, 372–374 [Google Scholar]
- 18.Gao M., Chen H., Lu F. and Zhu J. (2012) The diagnostic value of des-gamma-carboxyprothrombin and α-fetoprotein in primary hepatocellular carcinoma. J. Pract. Med. 28, 2516–2519 [Google Scholar]
- 19.Liu C. and Xu J. (2012) The expressions of serum tumor biomakers DCP, AFP-L3 and AFP in patients with hepatocellular carcinoma and its clinical significance. J. Bengbu. Med. Coll. 37, 1031–1033 [Google Scholar]
- 20.Li S., Lin Y., Pan Y. and Pan A. (2013) Value of combined determination of AFP༌AFU and DCP in the diagnosis of primary hepatic cancer. Anhui Med. Pharm. J. 17, 1720–1722 [Google Scholar]
- 21.Song P., Feng X., Inagaki Y., Song T., Zhang K., Wang Z. et al. (2014) Clinical utility of simultaneous measurement of α-fetoprotein and des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinoma in China: a multi-center case-controlled study of 1,153 subjects. Biosci. Trends 8, 266–273 10.5582/bst.2014.01116 [DOI] [PubMed] [Google Scholar]
- 22.Pu J., Wang X. and Peng Y. (2014) Application of serum abnormal prothrombin in clinical diagnosis of primary hepatocellular carcinoma. Lab. Med. 29, 270–273 [Google Scholar]
- 23.Zhu Y., Wang H., Wang H., Wang M., Han B., Zhang C. et al. (2014) Study on the significance of serum PIVKA-II in diagnosis of hepatocellular carcinoma. J. Clin. Exp. Med. 13, 513–516 [Google Scholar]
- 24.Fu M. (2015) Application of serum abnormal prothrombin in clinical diagnosis of patients with liver cancer. China Foreign Med. Treat., 2015, 28, 116–118 [Google Scholar]
- 25.Yu R., Xiang X., Tan Z., Zhou Y., Wang H. and Deng G. (2016) Corrigendum: efficacy of PIVKA-II in prediction and early detection of hepatocellular carcinoma: a nested case-control study in Chinese patients. Sci. Rep. 6, 39184 10.1038/srep39184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng H., Zhao R., Li D., Wang Q., Wang J. and Wang Q. (2016) Utility of DCP and AFP in diagnosis of primary liver cancer. Chin. J. General Pract. 14, 29–31 [Google Scholar]
- 27.Lu L. and Huang Y. (2016) Value of serum AFP, GPC3, DCP and VEGF concentration in the early diagnosis of primary hepatocellular carcinoma. Pract. J. Cancer 31, 1399–1401 [Google Scholar]
- 28.Huang S., Jiang F., Wang Y., Yu Y. and Lou J. (2016) Diagnostic value of joint detection of AFP and PIVKA-II in hepatocellular carcinoma. Labeled Immunoassays Clin. Med. 23, 1134–1138 [Google Scholar]
- 29.Gonzalez S.A. (2014) Novel biomarkers for hepatocellular carcinoma surveillance: has the future arrived. Hepatobiliary Surg. Nutr. 3, 410–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blendis L.M., Beilby J.O., Wilson J.P., Coles M.J. and Hadley G.D. (1967) Carcinoma of stomach: evaluation of individual and combined diagnostic accuracy of radiology, cytology, and gastrophotography. Br. Med. J. 1, 656–659 10.1136/bmj.1.5541.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin Q.F., Weng J., Xu G.X., Chen C.M. and Jia C.K. (2017) Combination of serum tumor markers dickkopf-1, DCP and AFP for the diagnosis of primary hepatocellular carcinoma. Asian Pac. J. Trop. Med. 10, 409–413 10.1016/j.apjtm.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Bertino G., Ardiri A.M., Calvagno G.S., Bertino N. and Boemi P.M. (2010) Prognostic and diagnostic value of des-gamma-carboxy prothrombin in liver cancer. Drug News Perspect. 23, 498–508 [DOI] [PubMed] [Google Scholar]
- 33.Lu M., Ju S., Shen X., Wang X., Jing R., Yang C. et al. (2017) Combined detection of plasma miR-127-3p and HE4 improves the diagnostic efficacy of breast cancer. Cancer Biomark. 18, 143–148 10.3233/CBM-160024 [DOI] [PubMed] [Google Scholar]
- 34.Lubowicka E., Gacuta E., Zajkowska M., Glazewska E.K., Przylipiak A., Chrostek L. et al. (2017) The plasma levels and diagnostic utility of matrix metalloproteinase-9 and CA 125 in cervical cancer patients. Pol. Merkur. Lekarski. 43, 10–14 [PubMed] [Google Scholar]
- 35.Kimura A., Sogawa K., Satoh M., Kodera Y., Yokosuka O., Tomonaga T. et al. (2012) The application of a three-step serum proteome analysis for the discovery and identification of novel biomarkers of hepatocellular carcinoma. Int. J. Proteomics 2012, 623190 10.1155/2012/623190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daniele B., Bencivenga A., Megna A.S. and Tinessa V. (2004) α-fetoprotein and ultrasonography screening for hepatocellular carcinoma. Gastroenterology 127 (Suppl. 1), S108–S112 10.1053/j.gastro.2004.09.023 [DOI] [PubMed] [Google Scholar]