Abstract
The data of root anatomical structure and the formation of aerenchyma tissues of five varieties of tobacco under waterlogging stress were obtained by modified paraffin method. Each tobacco varieties performed distinct anatomical adaptation response, including changes of cortical tissue, stele diameter, xylem diameter and the formation of aerenchyma under periodic waterlogging stress.
Keywords: Nicotiana tabacum, Periodic Waterlogging Stress, Anatomy of the roots
Specifications table
| Subject area | Biology |
|---|---|
| More specific subject area | Anatomy plant biology |
| Type of data | Figures and text |
| How data was acquired | Periodic waterlogging Method, paraffin method, data and image analysis |
| Data format | Analyzed |
| Experimental factors | Five tobacco varieties were traited under periodic waterlogging stress for 14 days, including 7 days with waterlogging conditions and followed by 7 days treatment of flooding conditions. |
| Experimental features | Tobacco varieties used in this study include var. Jepon Palakean, Srumpung, Morakot, Somporis and Manilo. The observation of root anatomy was conducted using modified paraffin method. |
| Data source location | Department of Biology, Institut Teknologi Sepuluh Nopember, Surabaya, Indonesia |
| Data accessibility | The data are available with this article |
Value of the data
-
•
The tobacco plant performs anatomical adaptation response of the roots under periodic waterlogging stress conditions through changes in cortical tissue, stele diameter, xylem diameter and the formation of aerenchyma.
-
•
Data on root anatomical responses might be useful for further study on tobacco plant breeding.
-
•
Data provided in this article could be combined with physiological and molecular study to elucidate the tobacco response mechanism against waterlogging and flooding stress.
1. Data
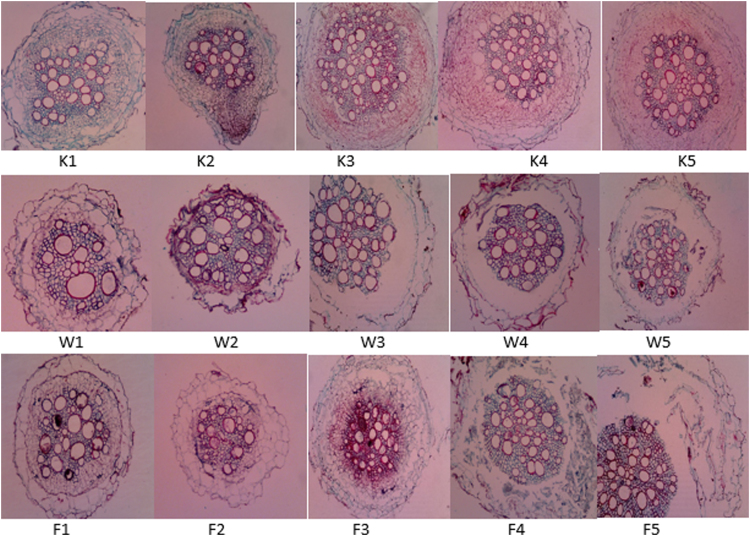
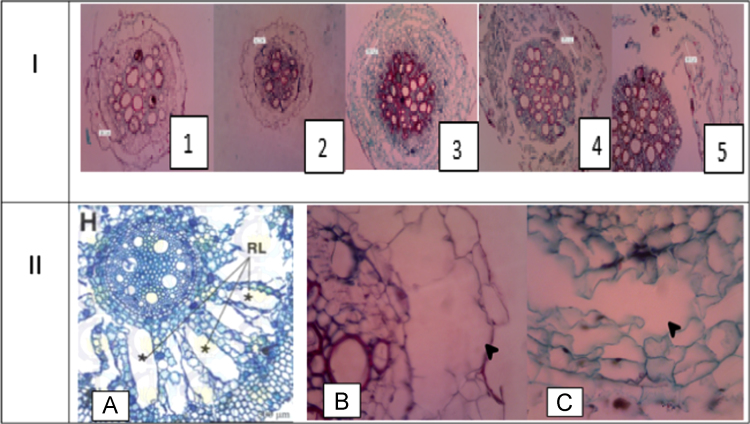
The data on Fig. 1 shows the waterlogging stress treatment and Fig. 2 shows the cross-section of fifth variety of tobacco root under periodic waterlogging stress. Our data clearly showed the anatomical differences between treated plant and control. All of treated plants have bigger size of all root parameter and much number in aerenchyma, epidermal and endodermal cells. All varieties showed the formation of aerenchyma tissue after being treated with waterlogging and flooding stress (Fig. 2, Fig. 3). During treatment, tobacco varieties exhibited different root anatomical responses. Tobacco var. Jepon Palakean, Marakot and Manilo showed an increase of cortex thickness (more than 60% in waterlogging and more than 100% in flooding), diameter of stele and xylem (more than 75% in waterlogging and more than 40% in flooding). In contrast, var. Srumpung and Somporis exhibited a decrease of cortex thickness, diameter of stele and xylem (Table 1).
Fig. 1.
Treatment of periodic waterloging stress in Tobacco (Nicotiana tabacum): A.Waterlogging condition and B. Flooding condition.
Fig. 2.
Cross-section of Fifth Root Varieties of Tobacco Plants under Periodic waterlogging (K: Tobacco Control Plants W: Tobacco Plants In Waterlogging Stress F: Tobacco Plants In Flooding Cash: 1. Var Jepon Palakean 2. Var Somporis3. Var, Marakot, 4. Var Srumpung, 5. Var Manilo).Observations were done using the Olympus CX 21 Light Microscope With Optilab Camera At Magnification (100×).
Fig. 3.
I: Aerenchyma In Cross Sliced Root of Tobacco Crops under periodic waterlogging stress Periodically / Flooding: (1. Var Jepon Palakean 2. Var Somporis; 3. Var. Marakot; 4. Var Srumpung; 5. Var.Marakot: Observations Using the Olympus CX 21 Light Microscope With Optilab Camera At 100× Magnification); II:II: A: Typa latifolia Root with radial type lisogeny [1] was used as literature standard;Arrows indicate aerenchyme: (B: aerenchyme Var Somporis And C: Var.Marakot: Observation Using the Olympus CX 21 Light Microscope With Optilab Camera At 400× Magnification).
Table 1.
Various root anatomical characters of Tobacco Varieties under periodic waterlogging stress.
| Parameter | Treatment | Tobacco Plant Varieties |
||||
|---|---|---|---|---|---|---|
| Palakean | Somporis | Marakot | Srumpung | Manilo | ||
| Root Diameter (µm) | W0 | 1356,9 ± 1,43ab | 784,47 ± 0,05ab | 1618,37 ± 0,36ab | 1302,8 ± 0,23ab | 1638,77 ± 0,27ab |
| W1 | 1639,6 ± 0,43ab | 705,93 ± 0,42ab | 1585,53 ± 0,54ab | 4410 ± 0,71ab | 1274 ± 0,88ab | |
| F0 | 721,67 ± 0,30ab | 1246,1 ± 0,48ab | 1281,87 ± 0,45ab | 1648,3 ± 0,17ab | 1627,37 ± 0,42ab | |
| F1 | 1256,57 ± 0,22ab | 846,4 ± 0,42ab | 1470,33 ± 0,26ab | 1421,4 ± 1,40ab | 1670,13 ± 0,07ab | |
| Stele Diameter (µm) | W0 | 484,13 ± 3,75ad | 616,93 ± 0,36ad | 981,7 ± 0,14ce | 904,57 ± 0,11be | 715,57 ± 0,91ce |
| W1 | 833,33 ± 0,17be | 562,83 ± 0,12ad | 1016,57 ± 0,44ce | 658,8 ± 0,21ad | 892,67 ± 1,14ad | |
| F0 | 348,13 ± 0,19ad | 721,67 ± 0,22ad | 672,8 ± 0,13ad | 842,07 ± 0,46ae | 733 ± 0,37ad | |
| F1 | 760,07 ± 0,13bd | 433,73 ± 0,49ad | 751,33 ± 0,68ad | 800,2 ± 0,60ad | 1332,9 ± 0,52ce | |
| Epidermal thickness(µm) | W0 | 71,43 ± 0,03bd | 107,7 ± 0,05bd | 171,9 ± 0,07bd | 168,5 ± 0,02bd | 177,4 ± 0,04bd |
| W1 | 41,13 ± 0,33ac | 28,23 ± 0,09ac | 45,43 ± 0,19ac | 35,53 ± 0,05ac | 66,87 ± 0,23bd | |
| F0 | 71,43 ± 0,22bd | 107,7 ± 0,10bd | 171,9 ± 0,01bd | 168,5 ± 0,12bd | 177,4 ± 0,12bd | |
| F1 | 30,23 ± 0,01ac | 23,73 ± 0,05ac | 37,93 ± 0,06 ac | 32,13 ± 0,09ac | 31,23 ± 0,07ac | |
| Cortex thickness (µm) | W0 | 158,2 ± 0,24ade | 65,53 ± 0,17ad | 79,13 ± 0,62ad | 110,33 ± 0,17ad | 155,8 ± 0,16ad |
| W1 | 244,47 ± 0,21bde | 42,57 ± 0,12ad | 140,83 ± 0,37ad | 108,9 ± 0,37ad | 224,6 ± 0,04bde | |
| F0 | 86,27 ± 0,05ad | 157,97 ± 0,22ade | 201,7 ± 0,24bde | 257,83 ± 0,58bde | 225,37 ± 0,45bde | |
| F1 | 156,4 ± 0,39ade | 155,27 ± 0,22ade | 300,8 ± 0,61ce | 249,53 ± 0,28bde | 514,53 ± 1,14ce | |
| Endodermal thickness (µm) | W0 | 156,03 ± 0,31bd | 51,07 ± 0,20ac | 227,03 ± 0,63bd | 85,2 ± 0,22acd | 120,5 ± 0,13ad |
| W1 | 141,2 ± 0,21ad | 50,17 ± 0,02ac | 126,87 ± 0,63ad | 172,73 ± 0,59bd | 111,47 ± 0,15acd | |
| F0 | 44,67 ± 0,07ac | 122,23 ± 0,10ad | 92,27 ± 0,13acd | 92,73 ± 0,34acd | 207,7 ± 1,03bd | |
| F1 | 55,03 ± 0,34ac | 45,4 ± 0,11ac | 88,33 ± 0,04acd | 53,9 ± 0,18ac | 168,97 ± 0,44bd | |
| Xylem thickness (µm) | W0 | 20,3 ± 0,02ab | 30,8 ± 0,07ab | 28,8 ± 0,03ab | 28,73 ± 0,05ab | 28,17 ± 0,03ab |
| W1 | 29,7 ± 0,06ab | 25,23 ± 0,05ab | 36,5 ± 0,08ac | 29,87 ± 0,05ab | 30,47 ± 0,04abc | |
| F0 | 41,9 ± 0,04ac | 35,33 ± 0,01ac | 31,1 ± 0,03ab | 30,37 ± 0,05ab | 21 ± 0,06ab | |
| F1 | 35,37 ± 0,06ac | 29,57 ± 0,01ab | 36,9 ± 0,12ac | 29,23 ± 0,01ab | 34,67 ± 0,02ac | |
| Xylem Diameter (µm) | W0 | 99,97 ± 0,03ad | 116,8 ± 0,01bd | 108,87 ± 0,02bc | 135,27 ± 0,02bd | 87,97 ± 0,18ac |
| W1 | 121,17 ± 0,01bd | 97,4 ± 0,02ac | 148,1 ± 0,01bd | 102,33 ± 0,07ad | 116,2 ± 0,17ad | |
| F0 | 92,77 ± 0,02ac | 102,37 ± 0,01ad | 90,87 ± 0,01ac | 102,27 ± 0,01ad | 102,37 ± 0,01ad | |
| F1 | 119,53 ± 0,05bd | 76,97 ± 0,03ac | 93,6 ± 0,01ac | 98,27 ± 0,02ac | 87,03 ± 0,02ac | |
| The number of aerenchyma cells | W0 | 3 ± 0,00ac | 1 ± 0,01ab | 2 ± 0,02ab | 2 ± 0,01ab | 3 ± 0,01ac |
| W1 | 8 ± 0,05ac | 5 ± 0,03ab | 4 ± 0,02ab | 5 ± 0,01ab | 6 ± 0,02ac | |
| F0 | 2 ± 0,01ab | 2 ± 0,02ab | 1 ± 0,01ab | 2 ± 0,01ab | 1 ± 0,01ab | |
| F1 | 12 ± 0,01ad | 10 ± 0,02ad | 11 ± 0,02ad | 8 ± 0,03ac | 7 ± 0,00ac | |
| Aerenchym Cell Length (µm) | W0 | 34,55 ± 0,06ac | 41,4 ± 0,07ad | 33,67 ± 0,05ac | 45,62 ± 0,06ac | 48,97 ± 0,03ac |
| W1 | 115,16 ± 0,04be | 97,23 ± 0,09bd | 93,37 ± 0,03bd | 123,17 ± 0,03be | 80,93 ± 0,10ad | |
| F0 | 75,2 ± 0,02ad | 45,32 ± 0,01ac | 42,12 ± 0,03ac | 82,45 ± 0,10ad | 67,82 ± 0,02ad | |
| F1 | 137,76 ± 0,23be | 138,63 ± 0,06be | 166,1 ± 0,13bf | 160,1 ± 0,45bf | 168,17 ± 0,34be | |
Description: 1. numbers followed by the same letters in the same row and column for the measured parameters do not significantly different by Tukey Test at 5%; 2. Treatment Code: W0: Control 1; W1: Waterlogging; F0: Control 2; F1: Flooding
2. Experimental design, materials, and methods
2.1. Periodic waterlogging stress treatment
Tobacco seedlings aged 65 DAS (days after sowing) were grown under the periodic waterlogging condition in a plastic container measuring 40 cm × 30 cm × 20 cm (Fig. 1). Five tobacco varieties were used in this study including var. Jepon Palakean, Srumpung, Marakot, Somporis and Manilo. Periodic waterlogging stress treatment with a total 14 days was divided into 7 days in waterlogging conditions and 7 days under flooding conditions.
2.2. Sample preservation tobacco׳s roots
Root samples were firstly washed before being used for further analysis. Samples were prepared as ± 2 cm size. Subsequently, samples were fixed in FAA solution (formalin: acetic acid: 95% alcohol = 50 ml: 50 ml: 900 ml for every 1 l of solution) in a desiccator tool. Hydration process is then performed in a desiccator for 3 × 30 min. The FAA solution was then removed, and the sample was stored in a 70% alcohol solution [2].
2.3. Observation of root anatomical structure
The cross-sectional root anatomical structure was prepared using modified paraffin method. Procedures of the modified paraffin method used in this study were: (1) gradual dehydration with alcohol; (2) redehydration; (3) immersion through paraffin: dehydrant 1: 1;(4) Embedding;(5) Cutting using microtome;(6) Staining; (8) mounting using entelan. Root anatomical observation was conducted using light microscope with a camera Olympus CX 21 OPTILAB. Quantitative observations of root anatomical roots include total root diameter, stele diameter, epidermal thickness, cortical thickness, endodermic thickness, xylem thickness, xylem diameter, aerenchyme cell count and aerenchyme cell length. The data were analyzed by analysis of variance two way followed by Tukey test.
Acknowledgments
We thank to PT. Sadhana for providing planting material and field, and reviewers for the valuable comments.
Acknowledgments
Funding sources
This research was supported by the Ministry of Research, Technology and Higher Education, Republic of Indonesia through research Grant No: 1500/UN3.14/LT/2017
Footnotes
Transparency data associated with this article can be found in the online version at https://doi.org/10.1016/j.dib.2018.09.046.
Transparency document. Supplementary material
Supplementary material
References
- 1.Jung Jongduk, Cho Lee Seung, Choi Hong-Keun. Anatomical patterns of aerenchyma in aquatic and wetland plants. Journal of Plant Biology. 2008;51(6):428–439. [Google Scholar]
- 2.Purnobasuki Hery, Suzuki Mitsuo. Aerenchyma tissue development and gas pathway structure in root of Avicennia marina (Forsk.) Vierh. Journal of Plant Research. 2005;118(4):285–294. doi: 10.1007/s10265-005-0221-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material