Introduction
Non-Hodgkin lymphoma (NHL), including chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), is the seventh most common form of cancer in the United States in 2016. Patients with NHL not only have a higher risk of nonmelanoma skin cancer development, particularly squamous cell carcinoma (SCC), they are prone to having more aggressive SCCs.1, 2
Although these relationships are well established, few cases have been reported in which a patient had NHL diagnosed or a recurrence detected as a result of being treated for a SCC.3, 4 We report such a case in which the treatment of an aggressive SCC led to the diagnosis of NHL and a second case in which the treatment of an aggressive SCC led to the discovery that a patient's previously known low-grade CLL had transformed to a high-grade CLL.
Case reports
Case 1
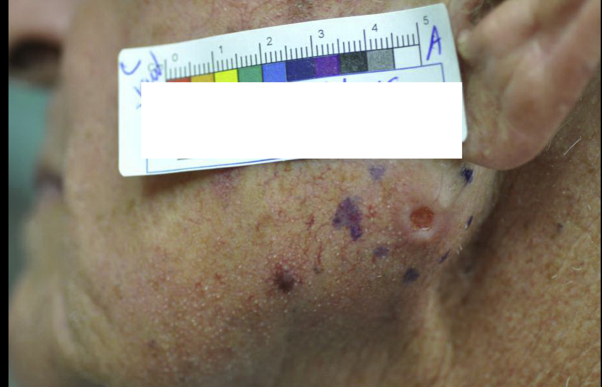
A 75-year-old white man presented to the Mohs micrographic surgical clinic for treatment of a 1.7-cm poorly differentiated SCC on his left jaw (Fig 1). Notably, he had a medical history of anal cancer 13 years prior treated with surgery, chemotherapy, and localized radiation. He also had a history of several basal cell carcinomas.
Fig 1.
SCC, left jaw.
During Mohs micrographic surgery (MMS), a dense lymphocytic infiltrate was noted intraoperatively (Fig 2, A), and permanent sections of the first stage found SCC (Fig 2, B) cleared after 5 stages of MMS. Because of the aggressive nature of the tumor, a computed tomography scan of the head and neck was performed which found numerous 1.5- to 2.1-cm lymph nodes in levels 1 through 5 bilaterally. A positron emission tomography scan showed extensive cervical, supraclavicular, axillary, small bowel mesentery, and bulky retroperitoneal lymphadenopathy with mild fludeoxyglucose F 18 activity concerning for a lymphoproliferative disease. Fine-needle aspiration of the left neck and axillary lymph nodes was consistent with a CD5+ B-cell lymphoma. Excisional biopsy confirmed the diagnosis to be CLL/SLL without evidence of large cell transformation. His CLL/SLL was treated with rituximab and bendamustine, but he unfortunately had progression of his disease and died of CLL.
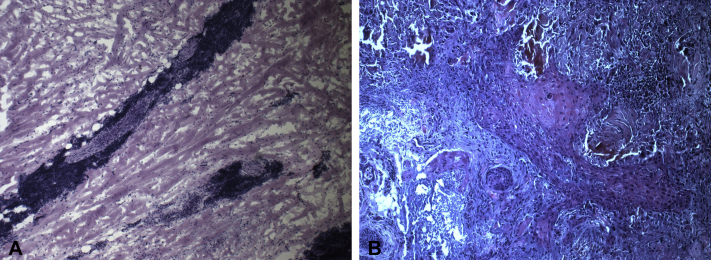
Fig 2.
A, Mohs intraoperative frozen section shows a dense lymphocytic infiltrate without clear visualization of SCC. B, Permanent section of the first stage shows SCC (horizontally cut).
Case 2
A 74-year-old white man presented to the Mohs surgical clinic for treatment of a 4- x 3-cm recurrent, invasive SCC of the left temple. He had a 5-year history of B-cell follicular lymphoma, which was treated with localized radiation therapy to his pelvis and multiple courses of chemotherapy, including bendamustine-rituximab and R-CVP (rituximab, cyclophosphamide, vincristine sulfate, prednisone). He finished his most recent course of R-CVP 5 months prior and was in remission. He also had a medical history of several basal cell carcinomas and a single SCC in situ.
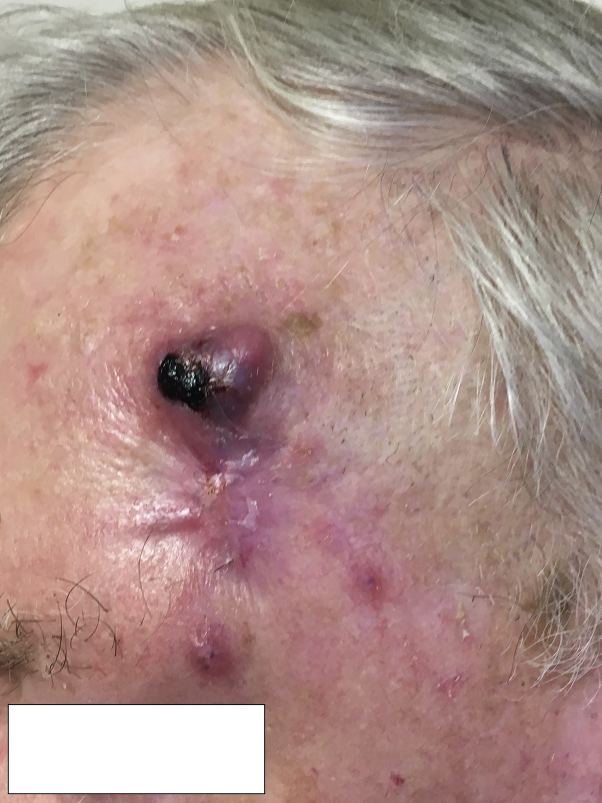
At consultation, 2 distinct erythematous 1-cm papules were noted in close proximity to the primary tumor (Fig 3), with same-day frozen biopsy confirming diagnosis of in-transit SCC metastases. On examination, he was found to have enlarged and tender parotid glands bilaterally and an enlarged left cervical lymph node. He stated that these areas had been enlarged for approximately 2 weeks. He denied any B symptoms. Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy in the head and neck, chest, abdomen and pelvis, which was most prominent in the cervical region bilaterally. The SCC on the left temple and both cutaneous metastases were cleared with MMS. Biopsy of the parotid and left clavicular nodes showed diffuse large B-cell lymphoma, a notable transformation from his prior diagnosis of follicular lymphoma.
Fig 3.
SCC, left temple.
He took rituximab and lenalidomide to treat the NHL and received radiation therapy for treatment of the both the left temple SCC and the lymphoma in his neck, parotids, and left axilla. Despite this, numerous, rapidly progressive regional and distant SCC metastases developed. He was put on palliative therapy with the PD-1 inhibitor pembrolizumab, but he was taken off it after just 5 weeks because both the SCC and NHL continued to progress. He died shortly thereafter of disease secondary to SCC.
Discussion
The relationship between SCC and NHL has been well described, but few cases have reported instances in which a diagnosis of NHL or a Richter's transformation (which describes the transformation of a CLL/SLL into a fast-growing diffuse large B-cell lymphoma) was made as a result of treating aggressive SCC.3, 4
Patients with a history of NHL are at least 5 times more likely to go on to have SCC than those without.5 Moreover, the disease course of their SCCs tends to be more aggressive as evidenced by a higher incidence of recurrence, even after MMS; higher rate of metastasis; and higher rate of death.1, 2
Although this aggressive disease course is primarily thought to be caused by the immunosuppressive effect of NHL,1 the presence of lymphocytic infiltration of the SCC can obscure visualization of the tumor, making margin control difficult and making it more challenging to clear these tumors with MMS. Roughly, one-third of patients with CLL treated with MMS for SCC or basal cell carcinoma show such an infiltrate as noted in Case 1.6
When treating patients with aggressive SCC, it is important to consider an underlying immunosuppressive condition such as NHL. Although an extensive workup does not need to be performed for every patient that presents with an aggressive SCC, a thorough review of systems and lymph node examination should be performed to evaluate for metastasis and tip off the physician in case there is another underlying disease such as CLL. Furthermore, when a dense lymphocytic infiltrate is encountered on histology, it may be prudent to ensure the patient has follow-up with their primary care physician so a more thorough workup can be performed.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
This project was presented as a poster at the 2017 American Society for Dermatology Surgery Annual Meeting, October 5-8, 2017, Chicago, IL.
References
- 1.Onajin O., Brewer J.D. Skin cancer in patients with chronic lymphocytic leukemia and non-Hodgkin lymphoma. Clin Adv Hematol Oncol. 2012;10(9):571–576. [PubMed] [Google Scholar]
- 2.Brewer J.D., Shanafelt T.D., Khezri F. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: a Rochester Epidemiology Project population-based study in Minnesota. J Am Acad Dermatol. 2015;72(2):302–309. doi: 10.1016/j.jaad.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padgett J.K., Parlette H.L., English J.C. A diagnosis of chronic lymphocytic leukemia prompted by cutaneous lymphocytic infiltrates present in Mohs micrographic surgery frozen sections. Dermatol Surg. 2003;29(7):769–771. doi: 10.1046/j.1524-4725.2003.29194.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilson M.L., Elston D.M., Tyler W.B., Marks V.J., Ferringer T. Dense lymphocytic infiltrates associated with non-melanoma skin cancer in patients with chronic lymphocytic leukemia. Dermatol Online J. 2010;16(3):4. [PubMed] [Google Scholar]
- 5.Adami J., Frisch M., Yuen J. Evidence of an association between non-Hodgkin's lymphoma and skin cancer. BMJ. 1995;310(6993):1491–1495. doi: 10.1136/bmj.310.6993.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrany K., Byrd D.R., Roenigk R.K., Glimelius B., Melbye M. Lymphocytic infiltrates and subclinical epithelial tumor extension in patients with chronic leukemia and solid-organ transplantation. Dermatol Surg. 2003;29(2):129–134. doi: 10.1046/j.1524-4725.2003.29034.x. [DOI] [PubMed] [Google Scholar]