Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease affecting apocrine gland areas leading to recurrent abscesses and nodules, forming scars and sinus tracts. Adalimumab, a tumor necrosis factor (TNF)-α monoclonal antibody is the first treatment approved by the US Food and Drug Administration for HS. TNF-α plays a primary and critical role in the mechanism of autoimmune disease by binding to TNF-α receptors, and initiating the immune response. Biological therapies have dramatically changed the management of chronic inflammatory skin disorders. The association between cervical dysplasia and TNF-α blocking therapy is well documented. TNF-α plays a critical role in the control of viral infection, including human papilloma virus (HPV). Therefore, the therapeutic TNF-α inhibition may increase the risk of HPV reactivation and lead to cervical dysplasia and carcinoma. We report, for the first time to our knowledge, the impressive case of an HS patient who went on to have a cervical epidermoid carcinoma 6 months after starting adalimumab therapy.
Case presentation
We report a case of a 48-year-old woman who had HS diagnosed 5 years ago. Her medical history included hypothyroidism treated by levothyroxine and hypertension treated by bisoprolol fumarate.
She underwent a sleeve gastrectomy 3 years previously. She had no active smoking or alcohol consumption habits but a past smoking history of 15 pack-years. Her body mass index value was evaluated at 33.7 kg/m2. The HS skin lesions were located along the axillae, pubis, and groin with involvement of the vulva and genital labia. The flares were characterized by recurrence of inflammatory nodules and abscesses. The disease was classified as Hurley II.1
Adalimumab was proposed as therapeutic option in January 2017 with a loading dose of 160 mg followed by a maintenance dose of 40 mg/wk.
Previous treatment included oral clindamycin for 10 months at 300 mg/d. She also received a combination of clindamycin/rifampicin but rifampicin was interrupted because of intestinal side effects. Topical steroids and antibiotics were applied during the relapses of the disease.
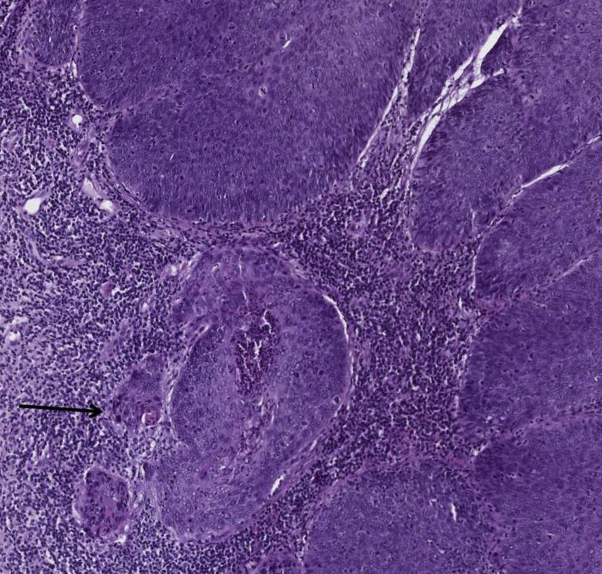
HS lesions were well controlled under adalimumab, and the therapy was well tolerated by the patient. Adalimumab was interrupted 6 months after initiation because the patient had a high-grade squamous intraepithelial lesion1 discovered on her annual Papanicolaou (Pap) smear. Surprisingly, the previous Pap smear performed 3 years ago was strictly normal. She was treated by conisation, and the histologic analysis diagnosed an in situ cervical epidermoid carcinoma with positive resection margins. A surgical re-excision was performed, but histologic analysis found an invasive cervical carcinoma classified as pT1a according to the TNM classification (8th edition) (Fig 1). She underwent a total hysterectomy, and HPV31 DNA was detected on the surgical specimen. Adalimumab was not reintroduced.
Fig 1.
Cervical epidermoid carcinoma. Histologic picture shows multiple microinvasive foci (arrow).
Discussion
We report for the first time, to our knowledge, the case of a patient who had an invasive cervical epidermoid carcinoma after the initiation of adalimumab therapy prescribed for HS. The cancer diagnosis came 6 months after starting adalimumab, highlighting the potential involvement of TNF-α inhibition in the occurrence of the carcinoma.
The literature regarding the risk of cervical dysplasia and carcinoma in patients receiving biological therapies for chronic skin inflammatory conditions is not well documented. Association between cervical dysplasia and the use of anti-TNF therapy is reported for other chronic inflammatory conditions including rheumatoid arthritis (RA) and Crohn's disease.2, 3
Wadström et al2 analyzed the risk of occurrence of cervical dysplasia in patients suffering from RA. This study included 9629 patients treated with anti-TNF therapy, 34,984 biologics-naive patients, and 300,331 general population comparators. The study clearly found that women treated by TNF-α blocking therapies were at higher risk for high-grade cervical dysplasia (hazard ratio, 1.36, CI 1.01 to 1.82) and invasive cervical cancer (hazard ratio, 2.10, CI 1.04 to 4.23) compared with biologics-naive women with RA. This is the main study focusing on the subject to our knowledge.
Another study was performed by Kane et al3 on a group of patients suffering from Crohn's disease. Pap smears were recorded in relation to exposure to immunomodulators including prednisone, purine analogs, methotrexate, and infliximab. They compared their Pap smears for 2 years with that of a control population. Women exposed to immunosuppressive therapy were more likely to have an abnormal Pap smear (P < .001) than control population. Pap smears done 6 months after the initiation of the immunosuppressive treatment resulted in higher risk of abnormalities (odds ratio, 1.5, CI 1.2–4.1; P = .021).
Interestingly, to follow the course of diagnosed cervical lesions (low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, and in situ carcinoma of the cervix) during biotherapy, Cordtz et al4 followed up with a population of 806 patients with chronic arthritis (RA, ankylosing spondylitis, psoriatic arthritis) treated with biological therapy (mostly anti-TNF therapy) for 3.5 years, and none of them experienced a progression to cervical carcinoma.
A causal link between cervical dysplasia and anti-TNF therapy remains unclear. The role of TNF-α in the control of viral infection is well known. TNF-α is involved in apoptosis signal in infected cells to stop viral replication and spread. We know that E6 protein of HPV-16 binds directly to TNF receptor 1 and leads to TNF-induced apoptosis of the host cell.5 Therefore, TNF-α blockade may increase the risk of HPV reactivation and ultimately lead to cervical dysplasia and cervical carcinoma.6
Recently, Chirch et al7 proposed guidelines of screening and vaccination for dermatologic patients on TNF-α antagonists. Concerning HPV prevention, they recommend an annual Pap smear for women and HPV vaccination, 3 doses through age 26, for females and males. Wine Lee et al8 confirmed these recommendations in an article concerning vaccination in adult patients receiving systemic therapy for psoriasis.
The high dosage of adalimumab schema proposed for HS has to be taken into consideration in the potential occurrence of cervical dysplasia or carcinoma. Interestingly, our patient combined smoking and obesity history, which are known risk factors for cervical cancer in the general population and major triggering factors related to HS.9, 10
Further studies are needed to better evaluate the occurrence of cervical dysplasia and carcinoma in HS patients treated by adalimumab. Additional data on the subject will identify modifiable risk factors and clarify the recommendations for gynecologic follow-up with Pap smear and HPV vaccination in HS patients on anti-TNF therapy.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
References
- 1.Jemec G.B. Clinical practice: hidradenitis suppurativa. N Engl J Med. 2012;366:158. doi: 10.1056/NEJMcp1014163. [DOI] [PubMed] [Google Scholar]
- 2.Wadström H. Do RA or TNF inhibitors increase the risk of cervical neoplasia or of recurrence of previous neoplasia? A nationwide study from Sweden. Ann Rheum Dis. 2016;75:1272–1278. doi: 10.1136/annrheumdis-2015-208263. [DOI] [PubMed] [Google Scholar]
- 3.Kane S. Higher incidence of abnormal Pap smears in women with inflammatory bowel disease. Am J Gastroenterol. 2008;103:631–636. doi: 10.1111/j.1572-0241.2007.01582.x. [DOI] [PubMed] [Google Scholar]
- 4.Cordtz R. Progression of precancerous lesions of the uterine cervix following biological DMARD therapy in patients with arthritis. Ann Rheum Dis. 2015;74:7. doi: 10.1136/annrheumdis-2014-206909. [DOI] [PubMed] [Google Scholar]
- 5.Filippova M. The human papillomavirus 16 E6 binds to tumor necrosis factor R1 and protects cell from TNF-induced apoptosis. J Biol Chem. 2002;277:9621–9624. doi: 10.1074/jbc.M200113200. [DOI] [PubMed] [Google Scholar]
- 6.Kim S., Salomon D. Tumor necrosis factor blockade and the risk of viral infection. Nat Rev Rheumatol. 2010;6:165–174. doi: 10.1038/nrrheum.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chirch L.M. Proactive infectious disease approach to dermatologic patients who are taking tumor necrossis factor-alfa antagonists. J Am Acad Dermatol. 2015;71:1. doi: 10.1016/j.jaad.2014.01.879. [DOI] [PubMed] [Google Scholar]
- 8.Lee L. Wine. From the medical board of the national psoriasis foundation: vaccination in adult patients on systemic therapy for psoriasis. J Am Acad Dermatol. 2013;69:1003–1013. doi: 10.1016/j.jaad.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 9.Sartorius K. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831–839. doi: 10.1111/j.1365-2133.2009.09198.x. [DOI] [PubMed] [Google Scholar]
- 10.Danaei G. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;36:1784–1793. doi: 10.1016/S0140-6736(05)67725-2. [DOI] [PubMed] [Google Scholar]