Highlights
-
•
There are few reports about pancreatic MANEC with cystic features.
-
•
Mixed tumor of the pancreas may arise from totipotent stem cells.
-
•
The treatment strategy is unclear, but surgery may be the first choice if possible.
Abbreviations: MANEC, mixed adenoneuroendocrine carcinoma; CT, computed tomography; NET, neuroendocrine tumor; MRI, magnetic resonance imaging
Keywords: Pancreas, Mixed adenoneuroendocrine carcinoma, Cystic tumor
Abstract
Introduction
Pancreatic mixed adenoneuroendocrine carcinoma (MANEC) is a rare tumor. We report herein a case of pancreatic MANEC with cystic features.
Presentation of case
A 67-year-old woman presented with jaundice. A CT scan revealed an 18-mm mass at the pancreatic head that obstructed the common bile duct and another 35-mm cystic lesion containing a mural nodule in the pancreatic body, which was suspected to be an intraductal papillary mucinous carcinoma. A biopsy of the head mass led to the diagnosis of adenocarcinoma. The patient underwent pancreatoduodenectomy, and the body cyst was resected with the head mass. A histopathological analysis revealed that the body cyst had two components, ductal adenocarcinoma and neuroendocrine tumor. We diagnosed the cystic tumor as MANEC.
Discussion
Cases of MANEC have been reported as originating from the stomach, small intestine, and colon, but pancreatic MANEC is rare. The histogenesis and the therapeutic strategy for pancreatic MANEC are controversial.
Conclusion
The clinicopathological features of pancreatic MANEC remain unclear; therefore, more reports of cases of pancreatic MANEC are necessary for a complete analysis.
1. Introduction
Mixed adenoneuroendocrine carcinoma (MANEC) is a rare tumor composed of an adenocarcinoma and a neuroendocrine tumor (NET). Some MANEC cases are reported to originate from the stomach, the small intestine, and the colon [1], but pancreatic MANEC is rare. Therefore, the biological and oncological behavior of pancreatic MANEC is unknown. Furthermore, few reports are available on pancreatic MANEC with cystic features. Herein we report a case of pancreatic MANEC with a cystic tumor and provide a short review of the literature.
This work has been reported in line with the SCARE criteria [2].
2. Presentation of case
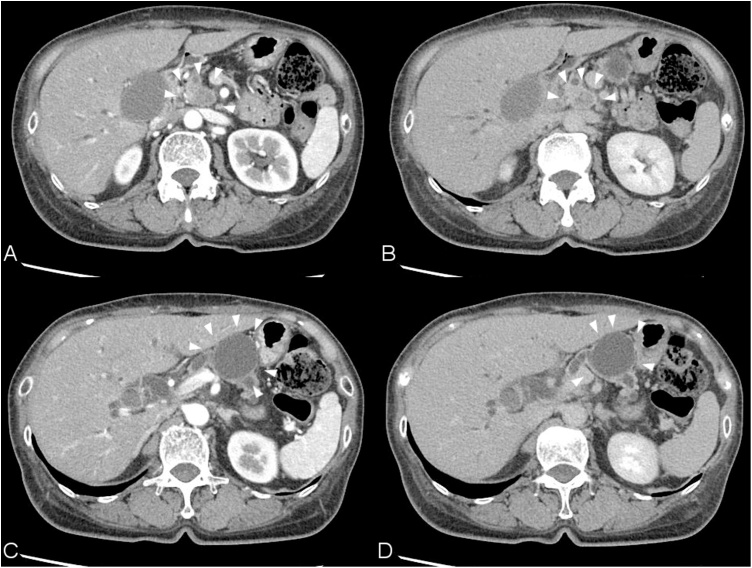
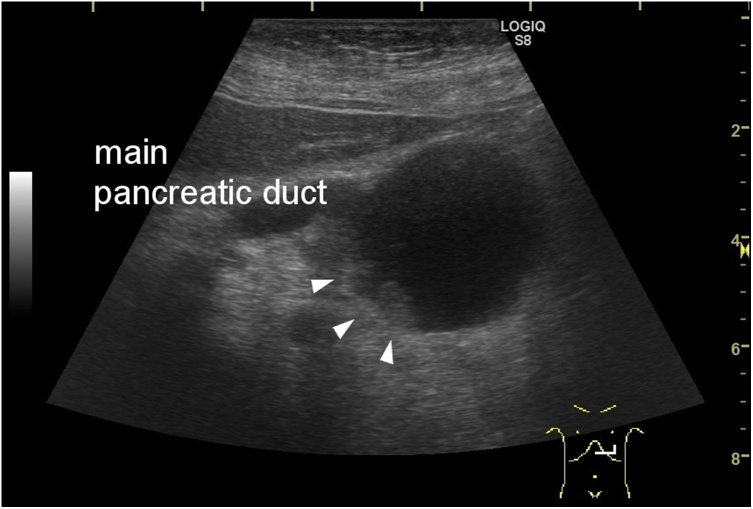
A 67-year-old woman presented with jaundice and fatigue. She had a 20-mm cyst in the pancreas that had been identified 12 years prior. She underwent CT scan each year since, and the cyst had not changed significantly. A laboratory examination revealed increased levels of bilirubin and hepatic enzymes (AST, ALT, LDH, γGTP, and ALP). Her serum amylase levels were not elevated. Her CA19-9 levels were slightly elevated, but other tumor markers, such as CEA and AFP, were within normal levels. A CT scan showed another 18-mm solid mass in the pancreatic head that obstructed the common bile duct, and the main pancreatic duct was dilated (Fig. 1). The cyst in the pancreatic body was connected to the pancreatic duct and had grown to 35 mm. Ultrasonography showed that the cyst contained a mural node; intraductal papillary mucinous carcinoma was suspected (Fig. 2). The patient underwent endoscopic retrograde cholangiopancreatography; a biopsy of the pancreatic head mass revealed the possibility of pancreatic adenocarcinoma. After biliary drainage, pancreatoduodenectomy was performed, and the cyst within the pancreatic body was resected together with the tumor. The patient’s postoperative course was uneventful.
Fig. 1.
Abdominal enhanced CT images, A and C: Early phase. B and D: Delayed phase. A and B: There is an 18-mm solid mass in the pancreatic head. The mass was poorly enhanced in the delayed phase and obstructed the common bile duct and the main pancreatic duct. C and D: A 35-mm cystisin the pancreatic body and is connected to the main pancreatic duct.
Fig. 2.
Ultrasonography of the cyst connected to the main pancreatic duct, which contained a small solid component (arrows).
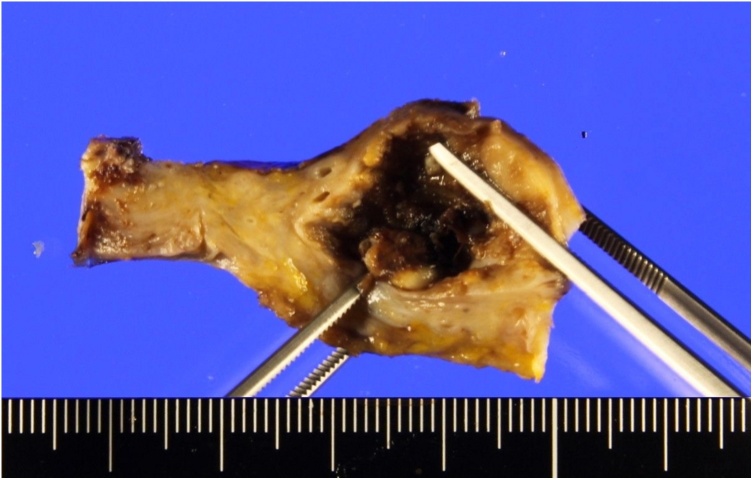
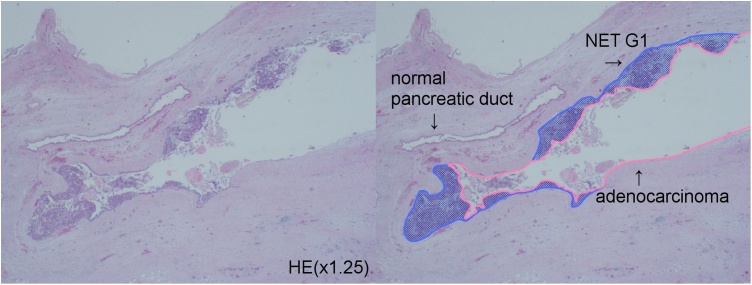
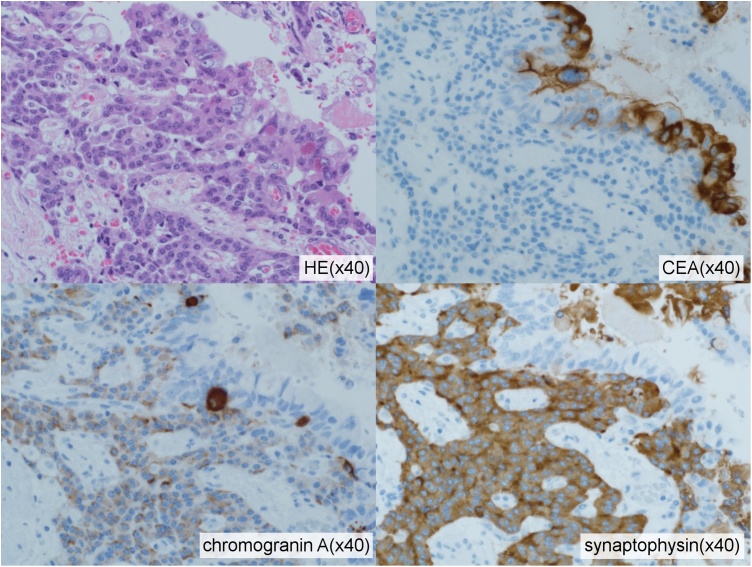
A histopathological examination of the specimen confirmed that the solid mass in the pancreatic head was a well tomoderately differentiated adenocarcinoma measuring 30 mm. Vascular invasion and direct infiltration into a lymph node were observed, but lymph node metastasis was not detected. The pathological stage was T3 N1 M0 stage III according to the General Rules for the Study of Pancreatic Cancer (seventh edition) in Japan. In contrast, a 25-mm cystic mass was observed in the pancreatic body (Fig. 3). The wall of the cyst consisted of two components (Fig. 4, Fig. 5). The inside of the cyst was lined with glandular structures containing large irregular nuclei and prominent nucleoli in the mucous cells that stained positive for CEA. The other component was represented by outer solid islands consisting of nests of small, uniform cells with round to oval nuclei thatstained negative for CEA and were immunoreactive for synaptophysin and chromogranin A. The Ki-67 index of this region was less than 2%, corresponding to NET G1. The final diagnosis was MANEC. The two malignant tumors were discontinuous and independent of each other.
Fig. 3.
A resected 25-mm cystic mass from the pancreatic body that was continuous with the main pancreatic duct.
Fig. 4.
Low-power view of the MANEC (left) (hematoxylin and eosin stain). A pink line outlines the adenocarcinoma in situ and the blue area contains the NEN (NET G1) (right).
Fig. 5.
High-power view of the two components of the MANEC(hematoxylin and eosin stain). The internal glandular material expresses CEA but not chromogranin Aor synaptophysin. The outer solid tumor islands were negative for CEA but positive for chromogranin A and synaptophysin.
3. Discussion
Neuroendocrine neoplasm (NEN) is a rare tumor with an incidence of 2.5–5 cases per 100,000 individuals per year [3] and mainly originates from tissues within the gastrointestinal tract [4,5]. The pancreas is one of the most common sites of NENs, accounting for 9%–34% of gastroenteropancreatic NENs [4,5]. MANECs comprise 3.0% of pancreatic NENs [4].
The World Health Organization published a new classification for NENs in the digestive system in 2010 that divided NENs into four main categories: NET G1, NET G2, neuroendocrine carcinoma, and MANEC. MANEC is defined as a tumor composed of both an adenocarcinoma and a NEN, with each component accounting for more than 30% of the tumor in the classification. MANEC was called a mixed exocrine-endocrine carcinoma (MEEC) prior to 2010. Many reports about pancreatic MEEC considered it a combination of an acinar cell tumor and an endocrine neoplasm [6]. More than 75% of all pancreatic tumors arise from the ductal cells, approximately 7% arise from the endocrine cells, and approximately 1% arise from the acinar cells [7]. Although acinar cell carcinoma is rare, approximately one-third of acinar cell carcinomas express endocrine markers [6]. However, this case was a combination of ductal adenocarcinoma and NET. Some reports of mixed ductal-endocrine carcinomas of the pancreas have been published. Chatelain [8] and Ballas [7] reported that mixed ductal-endocrine carcinomas of the pancreas tended to occur in middle-aged patients (mean age, in the 60 s) and were equally distributed between men and women. These tumors were usually large (mean, 60–70 mm in the greatest dimension) and located in the head of the pancreas (60%–70% of cases). Patients typically complained of jaundice, weight loss, or abdominal pain, while endocrine symptoms such as Zollinger-Ellison syndrome or Verner-Morrison syndrome were rarely observed. Recently, others reported that these tumors were not large (approximately 20 mm), and many patients remained asymptomatic [9], perhaps because the tumors in these cases were detected at earlier stages than in previous reports. Imaging technologies such as CT, magnetic resonance imaging, and endoscopic ultrasonography have been developed, making early diagnosis possible.
The histogenesis of pancreatic MANEC remains controversial. Some investigators support the hypothesis that exocrine and endocrine neoplasms of the pancreas arise from a common neoplastic progenitor [7,10]. It is well known that acinar cell carcinoma may express endocrine markers and show ductal differentiation [11]. In contrast, a case report showed the presence of endocrine cells expressing exocrine markers, suggesting that endocrine neoplasms can undergo neoplastic progression of the exocrine tumor cells [7]. These findings suggest that mixed tumors can be derived from totipotent pancreatic stem cells. Chang et al. [10] classified the patterns of pancreatic mixed tumors into five groups: amphicrine type, mixed type, collision type, solitary concomitant type, and multiple concomitant type. The pattern of the mixed tumors of the pancreas differ with respect to the timing of the differentiation. However, our patient's tumor could not be classified using this classification system. The internal surface of this cystic MANEC was covered with adenocarcinoma and NET-formed nodules under the adenocarcinoma. As the two components were separated clearly, this tumor may be a solitary concomitant type, and this mixed tumor was believed to have arisen from the same precursor clone, with some cells differentiating into the adenocarcinoma and others into the NEN.
Furthermore, the mixed tumor of our patient showed a simple cyst. Pancreatic cysts are not rare because they are identified in 2.4%–19.6% of all examinations by CT or MRI [12]. Such cysts are mainly pseudocysts, and approximately 10%–15% of pancreatic cysts are estimated to be neoplasms [13]. Intraductal papillary mucinous neoplasms (IPMNs) represent approximately 50% of resected cystic neoplasms of the pancreas. Approximately 11%–18% of resected cystic tumors of the pancreas are mucinous cystic neoplasms, and 4%–7% are pancreatic NENs [12]. Ductal adenocarcinomas with cystic features are considered rare, but recent studies reported the incidence of ductal adenocarcinomas with cystic features to be 8% in pancreatic ductal adenocarcinoma [14]. We suspected that our patient’s cystic tumor was a malignant IPMN, so we performed a resection. Some cases of mixed IPMN and NEN tumor were recorded. One report of mixed IPMN and NEN tumors revealed prevalence between 2.8% and 4.6% in patients who underwent surgery for pancreas NENs or IPMNs [15].
Because limited data about pancreatic MANEC are available, the biological behavior of such tumors is unclear, and the optimal course of treatment is controversial. As with pancreatic adenocarcinoma, surgery may be the first line of treatment for cases with resectable tumors [9,11]. Other reports were published about the efficacy of systemic chemotherapy for pancreatic MANEC [16].
4. Conclusion
We reported a rare case of pancreatic MANEC with cystic features. The biological and oncological behavior of pancreatic MANEC remains unclear, and the gold standard treatment is controversial. Therefore, more reports are necessary to clarify the management strategy for this disease.
Conflict of interest
The authors declare that they have no competing interests.
Funding sources
This research did not receive any specific grant(s) from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
The ethical approval for the publication of this case was exempted by our institution because all of the data were collected from clinical records and imaging systems for routine perioperative planning.
Consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Author contribution
NS acquired and interpreted the data and drafted the manuscript. TA performed the operation and perioperative management of the patient. NK, SA, and NI participated in the operation, perioperative management of the patient, and revision of the manuscript. SM participated in the operation and postoperative-patient care, and gave final approval of the version of the manuscript to be published. KI diagnosed the tumor pathologically and participated in revision of the manuscript. All authors read and approved the final manuscript.
Registration of research studies
NA.
Guarantor
Shiro Miwa and Nao Shimada.
Provenance and peer review
Not commissioned, externally peer-reviewed.
References
- 1.Gurzu S., Kadar Z., Bara T., Tamasi A., Azamfirei L., Jung I. Mixed adenoneuroendocrine carcinoma of gastrointestinal tract: report of two cases. World J. Gastroenterol. 2015;21:1329–1333. doi: 10.3748/wjg.v21.i4.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agha R.A., Fowler A.J., Saetta A., Barai I., Rajmohan S., Orgill D.P., SCARE Group The SCARE statement: consensus-based surgical case report guidelines. Int. J. Surg. 2016;34:180–186. doi: 10.1016/j.ijsu.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 3.La Rosa S., Marando A., Sessa F., Capella C. Mixed adenoneuroendocrine carcinomas (MANECs) of the gastrointestinal tract: an update. Cancers. 2012;4:11–30. doi: 10.3390/cancers4010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y.H., Lin Y., Xue L., Wang J.H., Chen M.H., Chen J. Relationship between clinical characteristics and survival of gastroenteropancreatic neuroendocrine neoplasms: a single-institution analysis (1995–2012) in South China. BMC Endocr. Disord. 2012;12:30. doi: 10.1186/1472-6823-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J.Y., Hong S.M. Recent updates on neuroendocrine tumors from the gastrointestinal and pancreatobiliary tracts. Arch. Pathol. Lab. Med. 2016;140:437–448. doi: 10.5858/arpa.2015-0314-RA. [DOI] [PubMed] [Google Scholar]
- 6.Ohike N., Kosmahl M., Kloppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004;445:231–235. doi: 10.1007/s00428-004-1037-x. [DOI] [PubMed] [Google Scholar]
- 7.Ballas K.D., Rafailidis S.F., Demertzidis C., Alatsakis M.B., Pantzaki A., Sakadamis A.K. Mixed exocrine-endocrine tumor of the pancreas. JOP. 2005;6:449–454. [PubMed] [Google Scholar]
- 8.Chatelain D., Parc Y., Christin-Maitre S., Parc R., Flejou J.F. Mixed ductal-pancreatic polypeptide-cell carcinoma of the pancreas. Histopathology. 2002;41:122–126. doi: 10.1046/j.1365-2559.2002.01447.x. [DOI] [PubMed] [Google Scholar]
- 9.Imaoka K., Fukuda S., Tazawa H., Kuga Y., Mochizuki T., Hirata Y., Fujisaki S., Takahashi M., Nishida T., Sakimoto H. A mixed adenoneuroendocrine carcinoma of the pancreas: a case report. Surg. Case Rep. 2016;2:133. doi: 10.1186/s40792-016-0263-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang S.M., Yan S.T., Wei C.K., Lin C.W., Tseng C.E. Solitary concomitant endocrine tumor and ductal adenocarcinoma of pancreas. World J. Gastroenterol. 2010;16:2692–2697. doi: 10.3748/wjg.v16.i21.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z., Dong C., Wang C., Liu Q., Sun D., Wang L. Mixed acinar-endocrine carcinoma of pancreas: a case report and brief review of the literature. Oncol. Ther. 2015;8:1633–1642. doi: 10.2147/OTT.S87406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stark A., Donahue T.R., Reber H.A., Hines O.J. Pancreatic cyst disease: a review. JAMA. 2016;315:1882–1893. doi: 10.1001/jama.2016.4690. [DOI] [PubMed] [Google Scholar]
- 13.Bektas M., Krishna S.G., Ross W.A., Weston B., Katz M.H., Fleming J.B., Lee J.H., Bhutani M.S. Prevalence of extra-pancreatic cysts in patients with cystic pancreatic lesions detected by endoscopic ultrasound. Endosc. Ultrasound. 2015;4:219–224. doi: 10.4103/2303-9027.163001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosmahl M., Pauser U., Anlauf M., Kloppel G. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod. Pathol. 2005;18:1157–1164. doi: 10.1038/modpathol.3800446. [DOI] [PubMed] [Google Scholar]
- 15.Kadota Y., Shinoda M., Tanabe M., Tsujikawa H., Ueno A., Masugi Y., Oshima G., Nishiyama R., Tanaka M., Mihara K., Abe Y., Yagi H., Kitago M., Itano O., Kawachi S., Aiura K., Tanimoto A., Sakamaoto M., Kitagawa Y. Concomitant pancreatic endocrine neoplasm and intraductal papillary mucinous neoplasm: a case report and literature review. World J. Surg. Oncol. 2013;11:75. doi: 10.1186/1477-7819-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajima H., Kitagawa H., Shoji M., Watanabe T., Nakanuma S., Okamoto K., Sakai S., Kinoshita J., Makino I., Furukawa H., Nakamura K., Hayashi H., Oyama K., Inokuchi M., Nakagawara H., Miyashita T., Itoh H., Takamura H., Ninomiya I., Fushida S., Fujimura T., Ohta T., Satoh H., Ikeda H., Harada K., Nakanuma Y. Pancreatic body adenocarcinoma with neuroendocrine tumor characteristics: a case report. Oncol. Lett. 2014;7:1049–1052. doi: 10.3892/ol.2014.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]